Abstract
Civinini Morton’s Syndrome (CMS), better known as Morton’s Neuroma, is a benign enlargement that typically affects the third common digital branch of the plantar nerve. It is a common cause of metatarsalgia leading to debilitating pain. It prefers the female gender, with a female to male ratio of 5:1 and an average age of 50 years at time of surgery. Precise aetiology remains under debate, with four etiopathogenetic theories often cited in the literature. Clinical symptoms, physical exam and instrumental evidence are important in assessing and grading the disease. Biomechanics seem to play an important role, especially regarding the usefulness of correct footwear. The first approach in the early stages of this condition usually begins with shoe modifications and orthotics, designed to limit the nerve compression. In order to prevent or delay the development of CMS, shoes should be sufficiently long, comfortable, broad toe-boxed, should bear a flat heel and a sufficiently thick external sole which should not be excessively flexible. Most authors suggested that an insole with medial arch support and a retrocapital bar or pad, just proximal to the metatarsal heads, displaces the pressure sites and can be beneficial to relieve the pain from the pinched nerve. A threshold period of 4.5 months appears to emerge from the results of the analysed studies, indicating that, beyond this period and in neuromas larger than 5-6 mm, orthotics and/or shoes modifications do not seem to give convincing results, proving to be more a palliation for the clinical condition to allow an acceptable life with pain rather than a real treatment. (www.actabiomedica.it)
Keywords: Morton neuroma, Conservative treatment, Civinini-Morton syndrome, Non-surgical treatment, Orthotics
Introduction
Civinini Morton’s Syndrome (CMS), better known as Morton’s Neuroma, is a benign enlargement that typically affects the third common digital branch of the plantar nerve (1).
The magnitude of the problem is still unknown, although the condition has documented predisposing factors. It affects about 30% of the population and prefers the female sex, with a female to male ratio of 5:1 (2) and an average age of 50 years at time of surgery (3).
The pathology is bilateral in 21% of cases, affects the third intermetatarsal space (IMS) in 66% of cases, the second in 32%, known as Hauser’s Neuroma, and the fourth in 2%. Multiple locations are almost rare (1, 4).
It was first reported by Filippo Civinini in 1835 and later by Durlacher in 1845, who described it as a neuralgic condition. Finally, in 1876 Thomas George Morton wrongly described the pathology as a subluxation of the fourth metatarsophalangeal (MTP) joint (5). Hoadley, in 1883, first excised an interdigital neuroma from the third IMS of a foot (6). In 1940, Betts introduced the notion of “neuroma” and compression of the plantar nerve below the intermetatarsal ligament (7).
Histology shows that it is not really a neuroma, but a perineural fibrosis with intraneural sclerohyalinosis (8); therefore, authors as Weinfield and Myerson proposed the more correct term of “interdigital neuritis” (9). Classical symptoms are a tingling and burning sensation in the forefoot with an irregular numbness in the affected toe (1). It is a common cause of metatarsalgia (10), along with hallux rigidus (11,12) and hallux valgus (13), in the overload typology.
Anatomy and pathophysiology
Neuroma consists of a bulge in the interdigital nerve just distal to the metatarsal transverse ligament and proximal to the forking of the digital nerves (Figure 1). Entrapment of the interdigital nerve between the intermetatarsal ligaments is the principal reason in the occurrence of CMS (14). Macroscopically it has a typically glossy fusiform shape, from white to yellowish appearance and a relatively soft texture (1). The common plantar digital nerves are final boughs of the medial and lateral plantar nerves passing in the IMS, under the intermetatarsal ligaments. Every common digital nerve goes through the plantar aponeurosis and splits into 2 branches supplying the plantar skin of the toes. Smaller ramifications give innervations to the adjacent metatarsals, MTP joints and plantar skin, under the metatarsal heads (15).
Fig. 1.
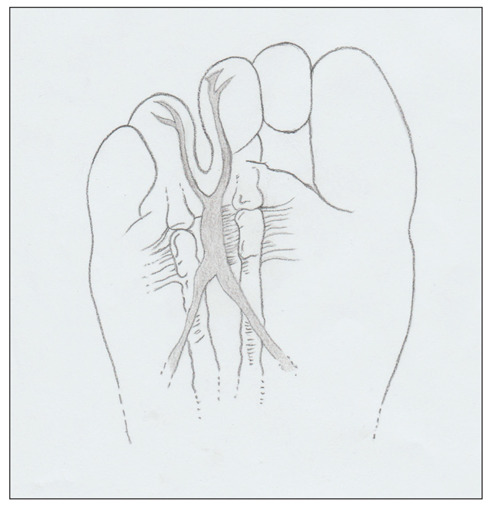
The schematic drawing of the neuroma in the typical third intermetatarsal space, just distal to the metatarsal transverse ligament and before the forking of the digital nerves.
Usually, the third common digital nerve, arising from the medial plantar nerve, receives a communicating bough from the lateral plantar nerve, which passes deep to the transverse metatarsal ligament. This is the narrowest space, and for that reason, the nerve there is less mobile during weight bearing. This might explain why it is a common location for the pathology (16). Female sex is usually the most affected, suggesting that the high heeled and tight footwear are contributing factors in the aetiology of this disorder (17).
The precise cause is still not clear. Until today, four etiopathogenetic theories have been propounded (18): chronic traction damage (16), inflammatory environment due to intermetatarsal bursitis (19), compression by the deep transverse intermetatarsal ligament (20) and ischemia of vasa nervorum (21).
Nevertheless, some of these biomechanical convictions are still debated. The sideboard that CMS should more commonly affected the third IMS in a more pronated foot, because of hyper mobility of the lateral column, is not supported anymore from the last studies. Even the rational hypothesis that subjects with a high body mass index (BMI) should increase pressure in the forefoot during the propulsive phase of walk, which could traumatize plantar IMS nerves, was not confirmed by recent studies on gait analysis. However, a strong association between CMS and restriction of ankle dorsiflexion was proved (22).
It was proposed that the common digital nerve of the third IMS is thicker than the others, as it is the result of an anastomosis between two nerve trunks (23). Another possible anatomical consideration is the increased mobility of the fourth ray (moving on the cuboid), compared to the third (fixed to the cuneiform), which could predispose to inflammation. In addition, some authors affirm that the distal metatarsal transverse ligament may compress the interdigital nerve (24).
In this view, the use of high heels is another predisposing factor, as it can further increase compression on it (17). Finally, some studies also described trauma as a possible cause (25).
Signs and symptoms
When CMS is suspected, medical history and physical examination play a pivotal role in the diagnostic process. It is important to listen the patient’s symptoms, often described in a very suggestive way. The foot inspection should search for the exact trigger points, distinguishing between intermetatarsal and metatarsal pain (1).
The typical symptom is a burning pain between the metatarsal heads, often radiating to the two corresponding toes, with cramps and hyperesthesia/dysesthesia (26). The pain is intense and so debilitating that the patients become afraid and anxious about walking or even putting their foot to the ground. The disorder is that of a severe, sharp, sometimes piercing pain that occurs abruptly while walking. At onset, relief of pain can be obtained by massaging the foot or manipulating the toes. In the worst cases, pain becomes debilitating and patients are timorous about walking. In other cases, patients describe milder symptoms of burning or tingling sensations (27).
Description is not always typical, and it is important to thoroughly interrogate the patient; it is to be underlined however, as a discriminating element, the disappearance of the pain at rest and the reappearance under weight-bearing. Pathognomonic of the syndrome is the so-called “sign of the shop window”, a curious and picturesque expression used by some specialists (28) to indicate the urgent need to remove the shoe that covers the affected foot: the patient (frequently a woman), to be unnoticed in the manoeuvre, stops in front of a shop window pretending to observe it.
Diagnosis
Clinical evaluation
CMS evaluation is mostly clinical and requires an accurate physical examination, carried out with the patient in the supine position. On inspection, foot is apparently normal, with absence of any deformity. Rarely a diastasis of the digital fornix is not present, however it is a common sign to all pathologies that create tension in the IMS, such as bursitis and MTP capsulitis (28).
The shape of the forefoot, the sub-metatarsal plantar skin and the position of the toes are carefully assessed. The metatarsal motility, plantar and dorsal flexion of the MTP joints are gently tested. Then, the attention moves on to the palpatory exploration of the IMS, where a tenderness and a dorsal bulging with a possible enlargement of the IMS might be appreciated (1). It’s very important to identify the pain, usually not located on the metatarsal heads, for a differential diagnosis (4). In these cases, it will be important to exclude other factors such as joint instabilities, forefoot conditions (first ray insufficiency, second metatarsal syndrome, bursitis), mid-hindfoot deformities (pes cavus), arthritis in the MTP joint, remote causes that act mechanically under the forefoot (retraction of gastrocnemius or Achilles tendon) or even to investigate some specific bone condition as Frieberg’s disease (1).
In presence of an atypical presentations such as a localization in the second space or the presence of multiple “neuromas” on the same foot, a mechanical “overload” disorder must be ruled out (29).
To help give an accurate clinical evaluation, a full foot and ankle examination should be performed with particular attention to gait, footwear, over-pronation, sensory disturbances and soft-tissue changes (16).
Subsequently, an accurate examination of digital sensitivity should be performed, both with a pointed instrument and with a vibratory tuning fork. The vibratory sensitivity of the tip of the toe is generally the most compromised (30). A characteristic sign is the “numbness of the toes”, when the opposite surfaces of the adjacent fingers show a reduced sensation (31); some authors call it “book page hypoesthesia” (28).
Various clinical tests are described in literature. The pressure can be practiced while tightening the metatarsal heads with the other hand, and this may be associated with a painful and palpable “click” sensation (Mulder’s sign) (29). This test demonstrated a 94–98% sensitivity (32). Another useful test is the “Thumb index finger squeeze” that appears to have the highest specificity and sensitivity (33).
Among provocative tests, Cloke and Greiss (34) described the “digital nerve stretch test” with 100% of sensitivity: the toes on either side of the affected IMS are passively fully extended, with the ankles in dorsiflexion and the foot on the examiner’s knees. Discomfort or pain in the IMS of the affected foot indicates a positive result.
Other authors (29, 32, 35) describe a similar test, the “Lasègue sign of the toes”, in which pain and hypoesthesia are evoked by forcing dorsal flexion of the toes and reduced by proximal interphalangeal joints flexion.
Imaging
As is often the case in medicine, the diagnosis of the disease is mainly clinical but rarely physical examination is considered sufficient; therefore, complementary exams are required. The most dependable method to clarify the diagnosis is a local anaesthetic injection, not always accepted by the patient (1).
X-rays appears to be essential as a first line imaging approach, to investigate other possible causes of metatarsalgia such as (36): tarsal–metatarsal joint stiffness, metatarsal hypermetria, Frieberg’s disease, toe deformities and MTP instabilities. However, in order to eliminate possible doubts, sonographic (US) confirmation is usually the instrumental investigation mostly utilized (14), certainly reliable and easy to prescribe, as it is fast and inexpensive for the patient. The imaging must be carried out with plantar and back, transversal and longitudinal US scans with a 7.5 MHz high frequency probe. The neuroma appears as homogeneously hypoechoic mass, well recognizable by the adjacent hyperechoic fat and by the shadow of the metatarsal cortex. Shapiro (37) and Quinn (38) state a diagnostic reliability around 95% for lesions larger than 5 mm, being 2 mm the maximum limit of the normal nerve.
Currently, US should be performed with a dynamic technique (dynamic US), as proposed by Torriani (39) and then by Perini (40), recreating the “click” described by Mulder during the examination (29). The dynamic US would be very effective for recognizing masses larger than 3.5 mm using a 10MHz probe (40). The most recent studies appear to confirm that the high sensitivity of US (0.91) is equal to (p = 0.88) that of the Magnetic Resonance Imaging (MRI) (0.90) for identification of neuroma and its thickness (41). MRI offers additional advantages in the diagnosis of CMS, but it must be considered a second level investigation with the limit, as in the US, to recognize lesions smaller than 4 mm (42).
Certainly, MRI is superior in the differential diagnosis, for its sensitivity on pathologies such as stress fractures, capsulo-synovitis of the MTP, synovial articular forms, or other pathologies of the soft tissues such as lipomas, angiomas, tendon ganglia or connective malignancies of the forefoot (43). Main indications for MRI are unclear clinical assessment and cases when more than one IMS is affected (1). Finally, it must be bear in mind that a negative result does not exclude the diagnosis (false negative 17%) (44).
Shoe modifications
Shoe modifications and orthotics can play an important role in the nonsurgical management of forefoot pathology. Therapeutic footwear may improve patient gait and increase the level of ambulation; on the contrary, inadequate footwear can worsen the symptoms and be a contributing cause for the development of the pathology. (45).
Already in 1897 Bradford (46) noted alterations caused by incorrect shoes through an analysis of historical art. Nowadays, women’s shoes continue to cause deformity and predispose to injury, even more so than in the past. Poorly fitting shoes are a major contributing factor to the difference in incidence of foot disorders between men and women, mostly for those over 61 years of age (47).
Traditionally, men’s shoes tend to be wider and have lower heels than women’s, and this could contribute to explain the different incidence of CMS in men compared to women, as described in some studies with a female-to-male ratio as high as 18 to 1 (48). As widely showed in pathophysiology, the prevalence of several musculoskeletal conditions in women are largely the result of biomechanical changes caused by ill-fitting shoes. In particular, the altered biomechanics (associated with shoes with a narrowed toe box and high-heeled shoes) has been linked to the genesis of Freiberg disease, hallux valgus, Haglund’s syndrome, hammer toe deformity, metatarsal stress fracture and, above all, CMS (17, 48).
Thus, the first approach in CMS should consist in patients’ education to avoid narrow and high-heeled shoes (4). The objective of shoes modifications is to deliver the pressures uniformly over the sole of foot. Shoes should be sufficiently long, comfortable, broad toe-boxed, flat heeled and should bear a sufficiently thick external sole, not excessively flexible (Figure 2) (2). A rocker-bottom sole may be helpful (45). Some authors showed that footwear and padding may be successful in relieving symptoms in 32% of cases after a mean of 4.5 months (17, 49) but they seem to achieve lower satisfaction rates when compared with other more invasive methods such as steroid injections (50).
Fig. 2.

Predisposed and comfortable shoe for orthotics, with a round toe-box and slight heel.
In most cases, clinicians will also have to educate the patient on lifestyle changes, such as losing weight and starting a regular physical activity; both being useful in most pathologies (51, 52).
Orthotics
Over the years, numerous studies have been carried out to highlight the different effects of plantar inserts for the foot comfort. Robbins and Gouw in 1991 (53) proposed that surface irregularities should be added to the insoles of running shoes to improve correct sensory inputs. In 1993,
Villeneuve (54) used a small insert to maintain postural equilibrium, by stimulating the mechanoreceptors in the plantar surface of the foot. Hayda et al. (55), the following year, found that placing a pad just proximal to the metatarsal heads provided significant reductions in forefoot plantar pressures around the first and second metatarsal heads. Burgess in 1997 (56) evaluated a series of 10 non-pathological male patients during walking, after one day wearing a pair of oxfords (hard) and running shoes (soft), containing an insert of 4 mm in height placed on a 0.8 mm EVA insole. He noted that the insert was successful in both shifting peak pressures from the medial to the lateral forefoot, whilst reducing peak pressures simultaneously. This was only evident in the hard shoe condition however, suggesting that the footbed of the running shoe was perhaps too soft to allow the insert to influence sensory input sufficiently.
More recent studies show that the perceived feel is best using wedge angles of 4 degrees and 5 degrees at a heel height of 25 mm, 10 degrees and 11 degrees at a heel height of 50 mm and 16 degrees and 18 degrees at a heel height of 75 mm (57).
Footbed shapes appear then to be essential to enhanced footwear comfort, regardless of the underlying disease. Thus, when footwear modifications alone are not enough, custom-made insoles could be designed to correct hindfoot malalignments as varus or valgus, support the medial arch, transfer the pressure just proximal to the metatarsal heads, and reduce the weights on pressure sites.
By decreasing metatarsal head loading and redeploying plantar pressures in a harmonious manner (58, 59), insoles, in selected cases, may alleviate forefoot problems (60).
In the case of CMS, some authors prefers a custom orthotics through foam impression methods, in a neutral subtalar position, with a prolonged longitudinal vault to support the first metatarsal, with a flat metatarsal support (without olive or bar), in order to favor the physiological pattern of the metatarsal weight bearing, from lateral to medial, before the pressure on the big toe (28).
Other authors suggested that a retrocapital bar or pad, just proximal to the metatarsal heads displaces the pressure sites and can be beneficial for symptoms (Figure 3). Metatarsal padding helps to spread and cushion metatarsal heads to relieve the pain from the pinched nerve. If needed, a cup can be added beneath the painful metatarsal head or heads. Custom-made toe inserts modeled in silicone rubber can be added in patients having associated claw-toe deformity (Figure 4) (60, 61).
Figure 3.
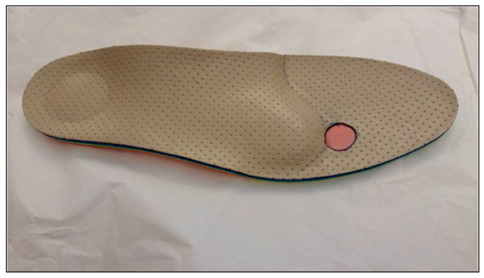
Insole with medial arch support, retrocapital bar just proximal to the metatarsal heads with targeted shock absorber insert in third intermetatarsal space.
Figure 4.
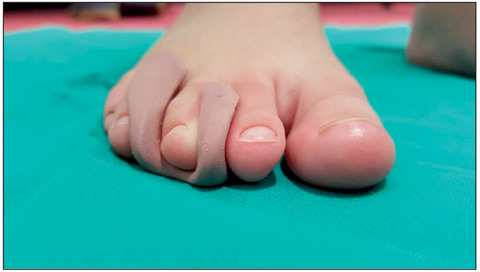
Custom-made toe insert modeled in silicone rubber added in patient affected by Civinini-Morton syndrome and associated with claw deformity of the third toe.
Bennett (17) reports that 41% of patients treated conservatively (shoes suitable for extra volume, unloading orthoses, soft metatarsal pads) demonstrate significant improvements with these non-invasive procedures. However, other authors (28) declare that these treatments, in case of CMS confirmed by “imaging” and larger than 5-6 mm, do not seem to give convincing results, proving them to be more a clinical condition to live acceptably with pain rather than a real treatment.
Kilmartin (62) examined 21 patients and states that pain associated with CMS was not significantly altered by changing the position of the foot with the compressed felt orthosis (14% of cases).
Notwithstanding, this has not been confirmed by latest studies. De Oliveira (63) in his randomized, controlled, double-blind clinical trial analyzed 72 patients and proved that customized insole with metatarsal and arch support relieved pain during walking (P = 0.048) and improved patient-reported measures of function (in general health domains (P < 0.001) and physical activity (P = 0.025)).
In any cases, when modifications fail or if affected individuals are no longer willing to make adjustments to their lifestyle or shoe wear (64, 65), patients may always choose to undergo surgery or other non-operative treatments such as US guided percutaneous radiofrequency (66), alcohol or corticosteroids injection and percutaneous electrostimulation-guided alcoholization with phenol (67-69).
Conclusion
During the years, many therapies have been utilized to treat symptomatic CMS. Among conservative treatments, orthotics and shoes modifications have been used to off-load the forefoot and thus reduce pain from weight-bearing pressure. In order to prevent or retard the development of CMS, shoes should be sufficiently long, comfortable, broad toe-boxed, they should have a flat heel and a sufficiently thick external sole that is not excessively flexible.
A threshold period of 4.5 months appears to emerge from the results of the studies, indicating that beyond this period and in larger neuromas than 5-6 mm, orthotics and/or shoes modification do not seem to give convincing results, proving to be more a clinical condition for living acceptably with pain than a real treatment.
Despite this, use of orthotics may be considered a safe and rational treatment before more invasive interventions, without any complications. Notwithstanding a good quality of the selected articles, further studies with a longer follow-up period and high quality randomized controlled trials are needed to provide more solid and accurate proofs.
Conflict of interest:
Each author declares that he or she has no commercial associations (e.g. consultancies, stock ownership, equity interest, patent/licensing arrangement etc.) that might pose a conflict of interest in connection with the submitted article
Compliance with Ethical Standards:
This article does not contain any studies with human participants or animals performed by any of the authors. Informed consent: Not applicable.
References
- 1.Di Caprio F, Meringolo R, Shehab Eddine M, Ponziani L. Morton’s interdigital neuroma of the foot: A literature review. Foot Ankle Surg. 2018 Apr;24(2):92–98. doi: 10.1016/j.fas.2017.01.007. doi: 10.1016/j.fas.2017.01.007. Epub 2017 Feb 4. [DOI] [PubMed] [Google Scholar]
- 2.Bradley N, Miller WA, Evans JP. Plantar neuroma: analysis of results following surgical excision in 145 patients. South Med J. 1976 Jul;69(7):853–4. [PubMed] [Google Scholar]
- 3.Kasparek M, Schneider W. Surgical treatment of Morton’s neuroma: clinical results after open excision. Int Orthop. 2013 Sep;37(9):1857–61. doi: 10.1007/s00264-013-2002-6. doi: 10.1007/s00264-013-2002-6. Epub 2013 Jul 13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Valisena S, Petri GJ, Ferrero A. Treatment of Morton’s neuroma: A systematic review. Foot Ankle Surg. 2018 Aug;24(4):271–281. doi: 10.1016/j.fas.2017.03.010. doi: 10.1016/j.fas.2017.03.010. Epub 2017 Apr 5. [DOI] [PubMed] [Google Scholar]
- 5.Pisani G. Is it Morton’s or Civinini’s syndrome. Foot Ankle Surg. 2010 Sep;16(3):105–6. doi: 10.1016/j.fas.2009.07.006. doi: 10.1016/j.fas.2009.07.006. Epub 2009 Sep 6. [DOI] [PubMed] [Google Scholar]
- 6.Hoadley A. Six cases of metatarsalgia. Chicago Med Rec. 1893;5(32) [Google Scholar]
- 7.Delmi M. Métatarsalgies de Morton. Chirurgie de l’avant-pied. 2005. Cahiers d’enseignement de la SOFCOT. In: Valtin B, Leemrijsee T, editors. Elsevier: Paris; [Google Scholar]
- 8.Giannini S, Bacchini P, Ceccarelli F, Vannini F. Interdigital neuroma: clinical examination and histopathologic results in 63 cases treated with excision. Foot Ankle Int. 2004 Feb;25(2):79–84. doi: 10.1177/107110070402500208. [DOI] [PubMed] [Google Scholar]
- 9.Weinfeld SB, Myerson MS. Interdigital Neuritis: Diagnosis and Treatment. J Am Acad Orthop Surg. 1996 Nov;4(6):328–335. doi: 10.5435/00124635-199611000-00005. [DOI] [PubMed] [Google Scholar]
- 10.Besse JL. Metatarsalgia. Orthop Traumatol Surg Res. 2017 Feb;103(1S):S29–S39. doi: 10.1016/j.otsr.2016.06.020. doi: 10.1016/j.otsr.2016.06.020. Epub 2017 Jan 18. [DOI] [PubMed] [Google Scholar]
- 11.Colò G, Alessio-Mazzola M, Dagnino G, Felli L. Long-Term Results of Surgical Treatment of Valenti Procedures for Hallux Rigidus: A Minimum Ten-Year Follow-Up Retrospective Study. J Foot Ankle Surg. 2019 Mar;58(2):291–294. doi: 10.1053/j.jfas.2018.08.055. doi: 10.1053/j.jfas.2018.08.055. [DOI] [PubMed] [Google Scholar]
- 12.Colò G, Samaila EM, Magnan B, Felli L. Valenti resection arthroplasty for hallux rigidus: A systematic review. Foot Ankle Surg. 2019 Dec 5 doi: 10.1016/j.fas.2019.11.009. pii: S1268-7731(19)30209-7. doi: 10.1016/j.fas.2019.11.009. (Epub ahead of print) [DOI] [PubMed] [Google Scholar]
- 13.Magnan B, Bortolazzi R, Samaila E, Pezzè L, Rossi N, Bartolozzi P. Percutaneous distal metatarsal osteotomy for correction of hallux valgus. Surgical technique. J Bone Joint Surg Am. 2006 Mar;88(Suppl 1 Pt 1):135–48. doi: 10.2106/JBJS.E.00897. [DOI] [PubMed] [Google Scholar]
- 14.Sofka CM, Adler RS, Ciavarra GA, Pavlov H. Ultrasound-guided interdigital neuroma injections: short-term clinical outcomes after a single percutaneous injection--preliminary results. HSS J. 2007 Feb;3(1):44–9. doi: 10.1007/s11420-006-9029-9. doi: 10.1007/s11420-006-9029-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Young G, Lindsey J. Etiology of symptomatic recurrent interdigital neuromas. J Am Podiatr Med Assoc. 1993 May;83(5):255–8. doi: 10.7547/87507315-83-5-255. [DOI] [PubMed] [Google Scholar]
- 16.Jain S, Mannan K. The diagnosis and management of Morton’s neuroma: a literature review. Foot Ankle Spec. 2013 Aug;6(4):307–17. doi: 10.1177/1938640013493464. doi: 10.1177/1938640013493464. Epub 2013 Jun 27. [DOI] [PubMed] [Google Scholar]
- 17.Bennett GL, Graham CE, Mauldin DM. Morton’s interdigital neuroma: a comprehensive treatment protocol. Foot Ankle Int. 1995 Dec;16(12):760–3. doi: 10.1177/107110079501601204. [DOI] [PubMed] [Google Scholar]
- 18.Hassouna H, Singh D. Morton’s metatarsalgia: pathogenesis, aetiology and current management. Acta Orthop Belg. 2005 Dec;71(6):646–55. [PubMed] [Google Scholar]
- 19.Bossley CJ, Cairney PC. The intermetatarsophalangeal bursa--its significance in Morton’s metatarsalgia. J Bone Joint Surg Br. 1980 May;62-B(2):184–7. doi: 10.1302/0301-620X.62B2.7364832. [DOI] [PubMed] [Google Scholar]
- 20.Gauthier G. Thomas Morton’s disease: a nerve entrapment syndrome. A new surgical technique. Clin Orthop Relat Res. 1979 Jul-Aug;142:90–2. [PubMed] [Google Scholar]
- 21.Nissen KI. Plantar digital neuritis; Morton’s metatarsalgia. J Bone Joint Surg Br. 1948 Feb;30B(1):84–94. [PubMed] [Google Scholar]
- 22.Naraghi R, Bremner A, Slack-Smith L, Bryant A. The relationship between foot posture index, ankle equinus, body mass index and intermetatarsal neuroma. J Foot Ankle Res. 2016 Dec;9:46. doi: 10.1186/s13047-016-0179-9. eCollection 2016. doi: 10.1186/s13047-016-0179-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Diez EM, Mas SM, Pi JF, Aramburo F. Comparative results of two different techniques in the treatment of the Morton’s neuroma. The Foot. 1999 Sep;9(3):134–7. [Google Scholar]
- 24.Alexander IJ, Johnson KA, Parr JW. Morton’s neuroma: a review of recent concepts. Orthopedics. 1987 Jan;10(1):103–6. doi: 10.3928/0147-7447-19870101-18. [DOI] [PubMed] [Google Scholar]
- 25.Litchman MH, Silver CM, Simon SD. Morton’s metatarsalgia and its relationship to trauma. R I Med J. 1964 Jul;47:328–31. [PubMed] [Google Scholar]
- 26.Ruiz Santiago F, Prados Olleta N, Tomás Muñoz P, Guzmán Álvarez L, Martínez Martínez A. Short term comparison between blind and ultrasound guided injection in morton neuroma. Eur Radiol. 2019 Feb;29(2):620–627. doi: 10.1007/s00330-018-5670-1. doi: 10.1007/s00330-018-5670-1. Epub 2018 Jul 31. [DOI] [PubMed] [Google Scholar]
- 27.Thomson CE, Gibson JN, Martin D. Interventions for the treatment of Morton’s neuroma. Cochrane Database Syst Rev. 2004;(3):CD003118. doi: 10.1002/14651858.CD003118.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Volpe A. La sindrome di Morton. LO SCAL. 2011;25:60–73. doi: 10.1007/s11639-011-0098-2. [Google Scholar]
- 29.Mulder JD. The causative mechanism in Morton’s metatarsalgia. J Bone Joint Surg Br. 1951 Feb;33-B(1):94–5. doi: 10.1302/0301-620X.33B1.94. [DOI] [PubMed] [Google Scholar]
- 30.Jarde O. Morton’s metatarsalgia. Foot Ankle Surg. 1998;4:187–191. doi: 10.1046/j.1460-9584.1998.00116.x. [Google Scholar]
- 31.Gougoulias N, Lampridis V, Sakellariou A. Morton’s interdigital neuroma: instructional review. EFORT Open Rev. 2019 Jan;4(1):14–24. doi: 10.1302/2058-5241.4.180025. doi: 10.1302/2058-5241.4.180025. eCollection 2019 Jan. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Pastides P, El-Sallakh S, Charalambides C. Morton’s neuroma: A clinical versus radiological diagnosis. Foot Ankle Surg. 2012 Mar;18(1):22–4. doi: 10.1016/j.fas.2011.01.007. doi: 10.1016/j.fas.2011.01.007. Epub 2011 Feb 16. [DOI] [PubMed] [Google Scholar]
- 33.Thomas JL, Blitch EL, 4th, Chaney DM, et al. Diagnosis and treatment of forefoot disorders. Section 3. Morton’s intermetatarsal neuroma. J Foot Ankle Surg. 2009;48(2):251–6. doi: 10.1053/j.jfas.2008.12.005. [DOI] [PubMed] [Google Scholar]
- 34.Cloke DJ, Greiss ME. (2006) The digital nerve stretch test: a sensitive indicator of Morton’s neuroma and neuritis. Foot Ankle Surg. 2006;12:201–203. doi: 10.1016/j.fas.2006.04.002. [Google Scholar]
- 35.Owens R, Gougoulias N, Guthrie H, Sakellariou A. (2011) Morton’s neuroma: clinical testing and imaging in 76 feet, compared to a control group. Foot Ankle Surg. 2011 Sep;17(3):197–200. doi: 10.1016/j.fas.2010.07.002. doi: 10.1016/j.fas.2010.07.002. Epub 2010 Sep 17. [DOI] [PubMed] [Google Scholar]
- 36.Read JW, Noakes JB, Kerr D, Crichton KJ. Morton’s metatarsalgia: sonographic findings and correlated histopathology. Foot Ankle Int. 1999 Mar;20(3):153–61. doi: 10.1177/107110079902000303. [DOI] [PubMed] [Google Scholar]
- 37.Shapiro PP, Shapiro SL. Sonographic evaluation of interdigital neuromas. Foot Ankle Int. 1995 Oct;16(10):604–6. doi: 10.1177/107110079501601003. [DOI] [PubMed] [Google Scholar]
- 38.Quinn TJ, Jacobson JA, Craig JG, van Holsbeeck MT. Sonography of Morton’s neuromas. AJR Am J Roentgenol. 2000 Jun;174(6):1723–8. doi: 10.2214/ajr.174.6.1741723. [DOI] [PubMed] [Google Scholar]
- 39.Torriani M, Kattapuram SV. Technical innovation. Dynamic sonography of the forefoot: The sonographic Mulder sign. AJR Am J Roentgenol. 2003 Apr;180(4):1121–3. doi: 10.2214/ajr.180.4.1801121. [DOI] [PubMed] [Google Scholar]
- 40.Perini L, Del Borrello M, Cipriano R, Cavallo A, Volpe A. (2006) Dynamic sonography of the forefoot in Morton’s syndrome: correlation with magnetic resonance and surgery. Radiol Med. 2006 Oct;111(7):897–905. doi: 10.1007/s11547-006-0088-2. Epub 2006 Oct 11. [DOI] [PubMed] [Google Scholar]
- 41.Bignotti B, Signori A, Sormani MP, Molfetta L, Martinoli C, Tagliafico A. (2015) Ultrasound versus magnetic resonance imaging for Morton neuroma: systematic review and meta-analysis. Eur Radiol. 2015 Aug;25(8):2254–62. doi: 10.1007/s00330-015-3633-3. doi: 10.1007/s00330-015-3633-3. Epub 2015 Mar 26. [DOI] [PubMed] [Google Scholar]
- 42.Sharp RJ, Wade CM, Hennessy MS, Saxby TS. The role of MRI and ultrasound imaging in Morton’s neuroma and the effect of size of lesion on symptoms. J Bone Joint Surg Br. 2003 Sep;85(7):999–1005. doi: 10.1302/0301-620x.85b7.12633. [DOI] [PubMed] [Google Scholar]
- 43.Zanetti M, Strehle JK, Kundert HP, Zollinger H, Hodler J. Morton neuroma: effect of MR imaging findings on diagnostic thinking and therapeutic decisions. Radiology. 1999 Nov;213(2):583–8. doi: 10.1148/radiology.213.2.r99nv06583. [DOI] [PubMed] [Google Scholar]
- 44.Torres-Claramunt R, Ginés A, Pidemunt G, Puig L, De Zabala S. MRI and ultrasonography in Morton’s neuroma: Diagnostic accuracy and correlation. Indian J Orthop. 2012 May;46(3):321–5. doi: 10.4103/0019-5413.96390. doi: 10.4103/0019-5413.96390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Janisse DJ, Janisse E. Shoe modification and the use of orthoses in the treatment of foot and ankle pathology. J Am Acad Orthop Surg. 2008 Mar;16(3):152–8. doi: 10.5435/00124635-200803000-00006. [DOI] [PubMed] [Google Scholar]
- 46.Bradford EH. The human foot in art. J Bone Joint Surg Am. 1897;(s1-s10):148–161. [Google Scholar]
- 47.Frey C. Foot health and shoewear for women. Clin Orthop Relat Res. 2000 Mar;(372):32–44. doi: 10.1097/00003086-200003000-00005. [DOI] [PubMed] [Google Scholar]
- 48.Goud A, Khurana B, Chiodo C, Weissman BN. Women’s musculoskeletal foot conditions exacerbated by shoe wear: an imaging perspective. Am J Orthop (Belle Mead NJ) 2011 Apr;40(4):183–91. [PubMed] [Google Scholar]
- 49.Saygi B, Yildirim Y, Saygi EK, Kara H, Esemenli T. Morton neuroma: comparative results of two conservative methods. Foot Ankle Int. 2005 Jul;26(7):556–9. doi: 10.1177/107110070502600711. [DOI] [PubMed] [Google Scholar]
- 50.Park HJ, Kim SS, Rho MH, Hong HP, Lee SY. Sonographic appearances of Morton’s neuroma: differences from other interdigital soft tissue masses. Ultrasound Med Biol. 2011 Aug;37(8):1204–9. doi: 10.1016/j.ultrasmedbio.2011.05.008. doi: 10.1016/j.ultrasmedbio.2011.05.008. Epub 2011 Jun 16. [DOI] [PubMed] [Google Scholar]
- 51.Colò G, Massarini M, Cavagnaro L, Felli L, Ferracini R. Exercise therapy indications in metastatic bone patients. Minerva Ortop e Traumatol. 2020;71:000–000. DOI: 10.23736/S0394-3410.19.03960-2. [Google Scholar]
- 52.Colò G, Cavagnaro L, Alessio-Mazzola M, Zanirato A, Felli L, Formica M. Incidence, diagnosis and management of sacroiliitis after spinal surgery: a systematic review of the literature. Musculoskelet Surg. 2019 May 7 doi: 10.1007/s12306-019-00607-0. doi: 10.1007/s12306-019-00607-0. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 53.Robbins SE, Gouw GJ. Athletic footwear: unsafe due to perceptual illusions. Med Sci Sports Exerc. 1991 Feb;23(2):217–24. [PubMed] [Google Scholar]
- 54.Villeneuve P. Foot and posture. 1993. In 3rd European and First World Congress of Podiatry Programme. :14. [Google Scholar]
- 55.Hayda R, Tremaine MD, Tremaine K, Banco S, Teed K. Effect of metatarsal pads and their positioning: a quantitative assessment. Foot Ankle Int. 1994 Oct;15(10):561–6. doi: 10.1177/107110079401501008. [DOI] [PubMed] [Google Scholar]
- 56.Burgess S, Jordan C, Bartlett R. The influence of a small insert, in the footbed of a shoe, upon plantar pressure distribution. Clin Biomech (Bristol, Avon) 1997 Apr;12(3):S5–S6. doi: 10.1016/s0268-0033(97)88313-1. [DOI] [PubMed] [Google Scholar]
- 57.Witana CP, Goonetilleke RS, Au EY, Xiong S, Lu X. Footbed shapes for enhanced footwear comfort. Ergonomics. 2009 May;52(5):617–28. doi: 10.1080/00140130802419503. doi: 10.1080/00140130802419503. [DOI] [PubMed] [Google Scholar]
- 58.Chang AH, Abu-Faraj ZU, Harris GF, Nery J, Shereff MJ. Multistep measurement of plantar pressure alterations using metatarsal pads. Foot Ankle Int. 1994 Dec;15(12):654–60. doi: 10.1177/107110079401501205. [DOI] [PubMed] [Google Scholar]
- 59.Holmes GB, Jr, Timmerman L. A quantitative assessment of the effect of metatarsal pads on plantar pressures. Foot Ankle. 1990 Dec;11(3):141–5. doi: 10.1177/107110079001100304. [DOI] [PubMed] [Google Scholar]
- 60.Kang JH, Chen MD, Chen SC, Hsi WL. Correlations between subjective treatment responses and plantar pressure parameters of metatarsal pad treatment in metatarsalgia patients: a prospective study. BMC Musculoskelet Disord. 2006 Dec 5;7:95. doi: 10.1186/1471-2474-7-95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Hinz A. Nerve disorders. In: DiGiovanni CW, Greisberg J, editors. Foot and ankle: core knowledge in orthopaedics. Philadelphia: Elsevier Mosby; 2007. pp. 171–6. [Google Scholar]
- 62.Kilmartin TE, Wallace WA. Effect of pronation and supination orthosis on Morton’s neuroma and lower extremity function. Foot Ankle Int. 1994 May;15(5):256–62. doi: 10.1177/107110079401500505. [DOI] [PubMed] [Google Scholar]
- 63.de Oliveira HAV, Natour J, Vassalli M, Rosenfeld A, Jennings F, Jones A. Effectiveness of customized insoles in patients with Morton’s neuroma: a randomized, controlled, double-blind clinical trial. Clin Rehabil. 2019 Dec;33(12):1898–1907. doi: 10.1177/0269215519873949. doi: 10.1177/0269215519873949. Epub 2019 Sep 11. [DOI] [PubMed] [Google Scholar]
- 64.DiPreta JA. Metatarsalgia, lesser toe deformities, and associated disorders of the forefoot. Med Clin North Am. 2014 Mar;98(2):233–51. doi: 10.1016/j.mcna.2013.10.003. doi: 10.1016/j.mcna.2013.10.003. Epub 2013 Dec 10. [DOI] [PubMed] [Google Scholar]
- 65.Mischitz M, Zeitlinger S, Mischlinger J, Rab M. Nerve decompression according to A.L. Dellon in Morton’s neuroma - A retrospective analysis. J Plast Reconstr Aesthet Surg. 2020 Jan 22 doi: 10.1016/j.bjps.2020.01.008. pii: S1748-6815(20)30027-9. doi: 10.1016/j.bjps.2020.01.008. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 66.Masala S, Cuzzolino A, Morini M, Raguso M, Fiori R. Ultrasound-Guided Percutaneous Radiofrequency for the Treatment of Morton’s Neuroma. Cardiovasc Intervent Radiol. 2018 Jan;41(1):137–144. doi: 10.1007/s00270-017-1786-y. doi: 10.1007/s00270-017-1786-y. Epub 2017 Sep 27. [DOI] [PubMed] [Google Scholar]
- 67.Perini L, Perini C, Tagliapietra M, et al. Percutaneous alcohol injection under sonographic guidance in Morton’s neuroma: follow-up in 220 treated lesions. Radiol Med. 2016 Jul;121(7):597–604. doi: 10.1007/s11547-016-0622-9. doi: 10.1007/s11547-016-0622-9. Epub 2016 Feb 16. [DOI] [PubMed] [Google Scholar]
- 68.Samaila EM, Ambrosini C, Negri S, Maluta T, Valentini R, Magnan B. Can percutaneous alcoholization of Morton’s neuroma with phenol by electrostimulation guidance be an alternative to surgical excision? Long-term results. Foot Ankle Surg. 2019 Apr 17 doi: 10.1016/j.fas.2019.04.004. pii: S1268-7731(19)30057-8. doi: 10.1016/j.fas.2019.04.004. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 69.Magnan B, Marangon A, Frigo A, Bartolozzi P. Local phenol injection in the treatment of interdigital neuritis of the foot (Morton’s neuroma) Chir Organi Mov. 2005 Oct-Dec;90(4):371–7. [PubMed] [Google Scholar]


