Abstract
Subdermal contraceptive implant is approved in more than 60 countries and used by millions of women around the world. Although relatively safe in nature, their implantation and removal may be associated with potential complications, some of which may require surgical intervention. Two types of peripheral neurological complications are reported: complications related to compressive neuropathy caused by device decubitus and complications related to device improper removal. An healthy 35-year-old woman come to our attention for paresthesia from medial side of right elbow to fourth and fifth fingers. Tinel sign was positive on medial side of distal third of right arm, above the elbow, as well. Clinical history of patients revealed a subcutaneous placement of a etonogestrel implant 3 years before. Patients reported disappearing of tactile feeling of subcutaneous contraceptive implant since two months. At clinical examination, implant was not felt in its original subcutaneous place. X-rays control revealed its proximal and deep migration. Surgical exploration for subcutaneous contraceptive implant removal revealed it lying on the ulnar nerve. Patient referred immediate paresthesia disappearing after surgery. At 1 month follow up no motor or sensory alteration were evident. Removal of implants inserted too deeply must be carefully performed to prevent damages to nervous and vascular structures and it should be performed by operators who are very familiar with the anatomy of the arm. In case of chronic neuropathy caused by implant nerve compression only an appropriate patients information about rare but possible neuropathic symptoms related to device migration and a careful medical history collecting can avoid a mistaken diagnosis of canalicular syndrome. (www.actabiomedica.it)
Keywords: neuropathy, subcutaneous contraceptive implant, implant migration, ulnar nerve
Introduction
Subdermal implantable devices are commonly used for long-acting contraception in the United States and Europe (1, 2). Although relatively safe, their implantation and removal may be associated with potential complications, some of which may require surgical intervention (3-7). Despite migrations is a rare complication associated with contraceptive implants, widespread use of this well-established method of contraception makes it a misleading complication to know.
Case presentation
An healthy 35-year-old woman presented to the our hospital with complaint of paresthesia in her right upper extremity. Paresthesia was referred from medial side of right elbow to fourth and fifth fingers. A cubital tunnel syndrome was immediately suspected. Physical examination was negative for tenderness or pain. The only clinical sign different from a classic cubital tunnel syndrome was a weak Tinel sign at cubital tunnel; indeed Tinel sign was positive on medial side of distal third of right arm, above the elbow, as well. Clinical history of patients revealed a subcutaneous placement of a etonogestrel implant 3 years before. Patients reported disappearing of tactile feeling of subcutaneous contraceptive implant since two months. At clinical examination, Implant was not felt in its original subcutaneous place. X-rays control revealed its proximal and deep migration (Fig 1). Motor nerve conduction study revealed a reduced conduction velocity, delayed latency, and decreased amplitude and area, which were consistent with ulnar nerve compression at the elbow joint. Ultrasonography showed a hyperechogenic structure near the ulnar nerve. Surgical treatment was attempted because the symptoms were progressively worsened. A little longitudinal incision slightly wider than the diameter of the small finger of the operator so that the finger was introduced to check implant positioning. Then brachial fascia between the biceps and triceps muscle was opened by scissors and the ulnar nerve was visualized. Surgical exploration of the sulcus revealed migrated contraceptive implant lying on the ulnar nerve (Fig 2). Implant was removed (Fig 3). Patient referred immediate paresthesia disappearing. At 1 month follow up no motor or sensory alteration were evident.
Figure 1.
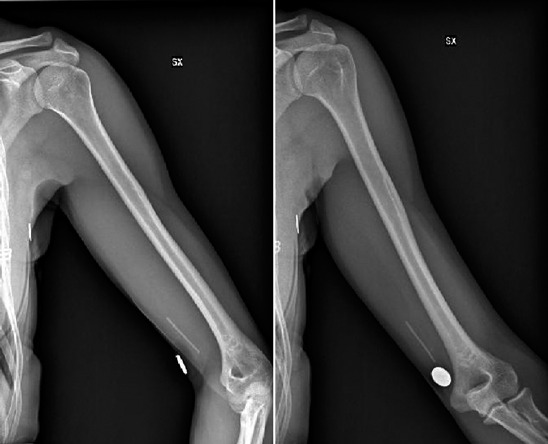
Pre-operative x-rays control: evidence of implant device proximally and deeply migrated, near to ovalar radiopaque marker (correct position).
Figure 2.
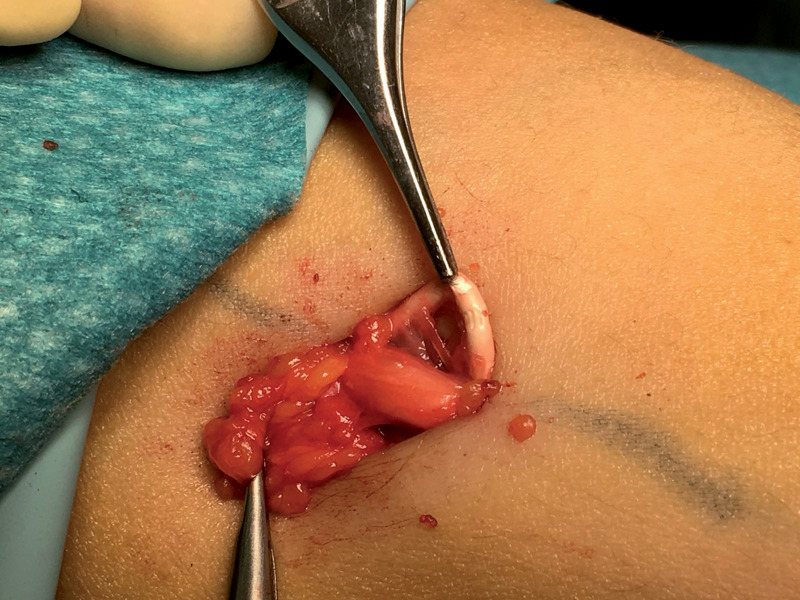
Intraoperative find of migrated contraceptive implant device lying on the ulnar nerve
Figure 3.
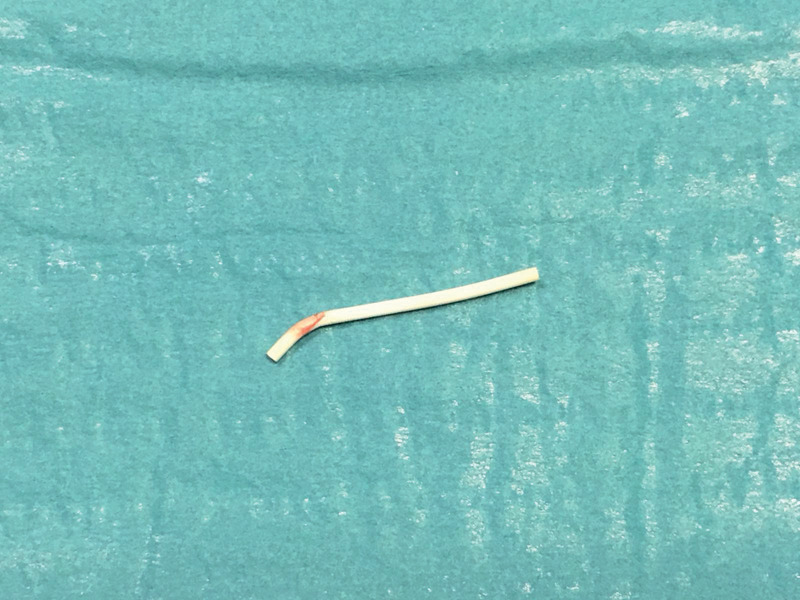
Removed implant device
Discussion
Contraceptive subdermal implants were specifically designed to provide contraceptive efficacy by inhibiting ovulation. They provide long-acting, highly effective reversible contraception. All subdermal implants for clinical use in humans release synthetic progestin from polymers for extended duration. These methods offer an excellent contraceptive option for women who have contraindications to combined hormonal methods and an option for any woman who desires long term protection against pregnancy that is rapidly reversible. Typical use of this implant achieves a contraceptive protection exceeding 99% (8-10).
The most common subdermal contraceptive devices are etonogestrel (Implanon, Nexplanon) and levonorgestrel (Norplant) implants. They consist of a 2mm x 4 cm single and multiple rod-shaped implants of ethylene vinylacetate copolymer, containing 68 mg of etonostrogel that are inserted under the skin by physician or health care professionals under local anesthetic. Newer models, such as Nexplanon, consist of a rigid tube preloaded in the needle of a disposable applicator for ease of release. Its suggested positioning is 6-8 cm above the elbow, in the non dominant arm (5, 10, 11).
According to injection technique, the tip of the applicator must be angled at less than 30 degrees in order to reach the skin, subcutaneous tissue, dermis, and sub-dermal tissue to avoid complications of deep insertion and endovascular insertion (12).
The subdermal etonogestrel contraceptive implant has a current approved duration of 3 years. Studies about its effectiveness for 2 additional years during which no pregnancies were recently published (13).
Before the removal physician must locate the implant by palpation. Movements and migrations of the system are quite rare and usually of few millimetres. Once the device has been located, it is necessary to press the proximal end in order to cause a lifting of the distal end. Identified the distal portion, after local anaesthesia, a small skin incision is practiced on this zone (10).
Ideal positioning of the implant is surprising on the orthopaedic point of view. In fact, in the proximal arm region, the ulnar nerve runs on the triceps, immediately deep to the subcutaneous investing fascia and just behind the medial intermuscular septum. Medial intermuscular septum divides ulnar nerve from basilica vein and median nerve (14, 15). If the injection angle is not proper, it could lead to the insertion of the device in the muscle or fascia. Although the implant can be effective even if located in the muscle or fascia, complications may arise (10). For this reason, physician must locate the implant by palpation immediately after insertion. However, there is no absolute or necessary indication for the early removal of the device in case of failed or doubtful localization through palpation or in case of migration of the system because contraceptive function of the device is preserved (10, 16, 17).
A more frequent complication due to implant migration is difficulty in palpating it before its removal. If the location of the device is in doubt or if the device is deep and the operator is not sufficiently experienced, the most correct approach is to directly contact the manufacturer, who will provide guidance on reference centers experienced in the removal of implants difficult to locate (8,4).
In case of implant migration ultrasound represents preferable exam because it avoids exposure of women to radiation and it is the most accurate procedure because it provides a three dimensional image. In case of ultrasound implant detection, removal procedure may be performed under ultrasound control. Persaud et Al (18) reported 119 patients in which ultrasound guide was necessary to implant removal without significant complications.
The X-ray may be also used to confirm the presence of the implant and identify the area where it is located. In case of axillary migration magnetic resonance imaging (MRI) can be suggested. Motor nerve conduction study can confirm a nerve compression (10, 11, 18).
Significant migrations (>2 cm) are uncommon, and primarily occur caudally looking to the insertion site. Serious but very rare cardiopulmonary complications after a contraceptive implant migration in pulmonary artery (5,18,19), brachial artery (6,20), cephalic vein (21) are reported.
An orthopaedic surgeon or hand surgeon can be involved in case of implant migration in two cases: patient with peripheral neuropathy or difficulty to implant removal by gynaecologist. Multiple case reports have described implant-related injuries to the median (22-23), ulnar (7, 24-29), and medial antebrachial cutaneous nerves (30).
Two types of peripheral neurological complications are reported: complications related to compressive neuropathy due to device decubitus or complications related to device improper removal.
Acute peripheral neuropathy related to the insertion of a contraceptive is a rare complication associated with excessive injection angle. Saeed et Al (26) described a case of a woman presented one day post insertion of a contraceptive implant with paraesthesia along the ulnar distribution of her hand and forearm, as well as shooting pain on palpating the course of the ulnar nerve. Ultrasonography found the implant to be lying in the subfascial plane of the inner arm. During surgical removal the implant was found lying in the perineurium of the ulnar nerve, causing ulnar nerve neuropathy. Osman et Al (30) described a young woman with ulnar nerve paraesthesia post insertion that resolved spontaneously.
A chronic ulnar neuropathy was described in a patient experiencing intermittent left-hand numbness and weakness with associated claw-hand deformity over a 2-year period. Ultrasonography revealed a hyper echogenic structure impinging the ulnar nerve, which they attributed to a contraceptive implant inserted 10 years prior. The patient’s recovery was not described in the report (27).
As we described, in case of chronic compression, risk of wrong diagnosis is concrete. No cutaneous sign of implant positioning can be detected during arm examination. Only referred history of subcutaneous contraceptive implant can make suspicious orthopaedic surgeon and exclude a diagnosis of cubital ulnar syndrome.
The majority of complications cases have been associated with the removal of difficulty sited implants rather than insertion. Lefebvre et Al (15) reported a case of ulnar nerve injury caused by improper removal manoeuvres during an attempted in-office removal of a deeply implanted device. Accidental nerve grasp caused an ulnar nerve traumatic neuroma needing a surgical reconstruction of the ulnar nerve.
An acute ulnar nerve neuropathy was reported one day post implant (24). Patient referred to a Plastic Surgery Department and Ultrasonography found the implant to be lying in the subfascial plane. On exploration in the operation theatre, the implant was found lying in the perineurium, with the nerve itself intact. Three months after removal of the implant, all her ulnar nerve functions apart from a slight residual sensory alteration had returned to normal. Two cases of median nerve injury following inappropriate dissection of the arm to remove an “impalpable” device have been reported (23) highlighting the need, in case of impalpable device, to study the patient with imaging techniques and to try the removal by qualified surgeon.
Despite symptoms related to implant migration are rarely described, worldwide growing use of subdermal contraceptive implants makes this complications more and more studied and prevented (31-33). For this reason subcutaneous contraceptive implant migration represents a debated topic in current obstetrician and gynaecological oriented literature. A recent systematic review of literature (34) identified 63 papers describing implant migrations. This study systematically selected 12 patients with fourteen nerve injuries. Two injuries was reported during or before device insertion and 12 during removal. The medial antebrachial cutaneous and median nerves were primarily affected. The primary reasons for nerve injury were pulling or grasping of the nerve after mistaking it for the implant. Neurapraxia was the most common lesion and was treated primarily with implant removal and clinical surveillance.
Ismail et al (4) measured the distance between the skin wound and the caudal end of the implant following 100 implant insertion. Thirty-four patients showed migration caudally and only 3 demonstrated cranial migration, which in one case was over 2 cm. None demonstrated migration deep into subcutaneous tissues or muscle.
A recently published multicenter study (35) of 4294 practitioners demonstrated 357 removal-related events among the 5701 removal evaluation forms. Eight (0.1%) of the removal reports described referral to a surgeon or interventional radiologist for removal of an etonogestrel implant. Seven of these eight referrals led to successful surgical implant removal
Conclusion
Subdermal implantable devices are commonly used for long-acting contraception in all over the world. Nerve injuries related to subdermal contraceptive implant generally involved patients with non-palpable implants. For this reason removal of implants inserted too deeply must be carefully performed to prevent damages to nervous and vascular structures and it should be performed by operators who are very familiar with the anatomy of the arm. Therefore patients with non-palpable implant should be treated by a neurosurgeon, a plastic surgeon or, especially in Europe, by an orthopaedic surgeon.
Orthopaedic surgeon likewise must know possibility of peripheral neuropathy doe to implant migration and must suspect it throughout medical history collecting. Gynaecologist must provide an appropriate patients information about rare but possible neuropathic symptoms related to device migration to avoid a mistaken diagnosis of canalicular syndrome.
Conflict of interest:
Each author declares that he or she has no commercial associations (e.g. consultancies, stock ownership, equity interest, patent/licensing arrangement etc.) that might pose a conflict of interest in connection with the submitted article
References
- 1.Diedrich JT, Klein DA, Peipert JF. Long-acting reversible contraception in adolescents: a systematic review and meta-analysis. Am J Obstet Gynecol. 2017;216(4):364.e1–364.e12. doi: 10.1016/j.ajog.2016.12.024. [DOI] [PubMed] [Google Scholar]
- 2.Thaxton L, Lavelanet A. Systematic review of efficacy with extending contraceptive implant duration. Int J Gynaecol Obstet. 2019;144(1):2–8. doi: 10.1002/ijgo.12696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mourtialon P, Tixier H, Loffroy R, Maillart JC, Calmelet P, Dellinger P, Vanwymeersch S, El Hassani R, Douvier S, Sagot P. Vascular complication after insertion of a subcutaneous contraceptive implant. Acta Obstet Gynecol Scand. 2008;87(11):1256–8. doi: 10.1080/00016340802484974. [DOI] [PubMed] [Google Scholar]
- 4.Ismail H, Mansour D, Singh M. Migration of Implanon. J Fam Plann Reprod Health Care. 2006 Jul;32(3):157–9. doi: 10.1783/147118906777888413. [DOI] [PubMed] [Google Scholar]
- 5.Barlow-Evans R, Jaffer K, Balogun M. Migration of a contraceptive subcutaneous device to the pulmonary artery. BMJ Case Rep. 2017 doi: 10.1136/bcr-2017-219259. bcr-2017-219259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rowlands S, Mansour D, Walling M. Intravascular migration of contraceptive implants: two more cases. Contraception. 2017;95(2):211–214. doi: 10.1016/j.contraception.2016.07.015. [DOI] [PubMed] [Google Scholar]
- 7.Smith JM, Conwit RA, Blumenthal PD. Ulnar nerve injury associated with removal of Norplant implants. Contraception. 1998;57(2):99–101. doi: 10.1016/s0010-7824(98)00007-9. [DOI] [PubMed] [Google Scholar]
- 8.Hohmann H, Creinin MD. The contraceptive implant. Clin Obstet Gynecol. 2007;50(4):907–17. doi: 10.1097/GRF.0b013e318159c2f6. [DOI] [PubMed] [Google Scholar]
- 9.Graesslin O, Korver T. The contraceptive efficacy of Implanon: a review of clinical trials and marketing experience. Eur J Contracept Reprod Health Care. 2008;13(Suppl 1):4–12. doi: 10.1080/13625180801942754. [DOI] [PubMed] [Google Scholar]
- 10.Palomba S, Falbo A, Di Cello A, Materazzo C, Zullo F. Nexplanon: the new implant for long-term contraception. A comprehensive descriptive review. Gynecological Endocrinology. 2012;28(9):710–721. doi: 10.3109/09513590.2011.652247. [DOI] [PubMed] [Google Scholar]
- 11.Lefebvre R, Hom M, Leland H, Stevanovic M. Peripheral nerve injury with Nexplanon removal: case report and review of the literature. Contracept Reprod Med. 2018 Oct 22;3(15) doi: 10.1186/s40834-018-0070-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Evans R, Holman R, Lindsay E. Migration of Implanon: two case reports. J Fam Plann Reprod Health Care. 2005;31:71–72. doi: 10.1783/0000000052973068. [DOI] [PubMed] [Google Scholar]
- 13.Ribeiro BC, Nogueira-Silva C, Afonso H, Silva PO, Reis ID. Use of etonogestrel implant beyond approved duration: prolonged contraceptive effectiveness. Eur J Contracept Reprod Health Care. 2018;23(4):309–310. doi: 10.1080/13625187.2018.1501799. [DOI] [PubMed] [Google Scholar]
- 14.Rinker B. Nerve Transfers in the Upper Extremity: A Practical User’s Guide. Ann Plast Surg. 2015;74(Suppl 4):S222–8. doi: 10.1097/SAP.0000000000000373. [DOI] [PubMed] [Google Scholar]
- 15.Mazurek MT, Shin AY. Upper extremity peripheral nerve anatomy: current concepts and applications. Clin Orthop Relat Res. 2001;383:7–20. doi: 10.1097/00003086-200102000-00004. [DOI] [PubMed] [Google Scholar]
- 16.Grentzer J, McNicholas C, Peipert JF. Use of the etonogestrel-releasing contraceptive implant. Expert Rev Obstet Gynecol. 2013;8(4):337–344. [Google Scholar]
- 17.Shulman LP, Gabriel H. Management and localization strategies for the nonpalpable Implanon rod. Contraception. 2006;73(4):325–330. doi: 10.1016/j.contraception.2005.10.009. [DOI] [PubMed] [Google Scholar]
- 18.Persaud T, Walling M, Geoghean T, Buckley O, Stunnel H, Torregiani WC. Ultrasound guided removal of Implanon devices. Eur Radiol. 2008;18:2582–2585. doi: 10.1007/s00330-008-1055-1. [DOI] [PubMed] [Google Scholar]
- 19.Kang S, Niak A, Gada N, Brinker A, Jones SC. Etonogestrel implant migration to the vasculature, chest wall, and distant body sites: cases from a pharmacovigilance database. Contraception. 2017;96(6):439–445. doi: 10.1016/j.contraception.2017.08.009. [DOI] [PubMed] [Google Scholar]
- 20.D’Journo CB, Vidal V, Agostini A. Intravasculary pulmonary migration of a subdermal contraceptive implant. Ann Thoracic Surg. 2015;99(5):1898. doi: 10.1016/j.athoracsur.2014.12.049. [DOI] [PubMed] [Google Scholar]
- 21.Mourtialon P, Tixier H, Loffroy R, Maillart JC, Calmelet P, Dellinger P, Vanwymeersch S, El Hassani R, Douvier S, Sagot P. Vascular complication after insertion of a subcutaneous contraceptive implant. Acta Obstet Gynecol Scand. 2008;87(11):1256–8. doi: 10.1080/00016340802484974. [DOI] [PubMed] [Google Scholar]
- 22.Christensen JM, Caggiano NM, Giladi AM, Iorio ML. Median nerve injury after removal of subdermal implantable contraceptive. Hand. 2017;13(3):6–9. doi: 10.1177/1558944717744335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gillies R, Scougall P, Nicklin S. Etonogestrel implants: a case studies of median nerve injury following removal. Aust Fam Physician. 2011;40:799–800. [PubMed] [Google Scholar]
- 24.O’Grady EE, Power DM. Ulnar nerve injury on removal of a contraceptive implant. Practitioner. 2016;260:21–4. [PubMed] [Google Scholar]
- 25.Belyea C, Ernat J, Gumboc R. Removal of a contraceptive implant from the brachial neurovascular sheath. J Hand Surg [Am] 2017;42:e115–7. doi: 10.1016/j.jhsa.2016.10.013. [DOI] [PubMed] [Google Scholar]
- 26.Saeed A, Narayan N, Pandya A. Contraceptive Implant-Related Acute Ulnar Neuropathy: Prompt Diagnosis, Early Referral, and Management Are Key. Eplasty. 2018;18:e28. [PMC free article] [PubMed] [Google Scholar]
- 27.Ong J, Therimadasamy A, Wilder-Smith E. Teaching NeuroImages: ulnar neuropathy related to a contraceptive subdermal implant. Neurology. 2014;83:e147–8. doi: 10.1212/WNL.0000000000000854. [DOI] [PubMed] [Google Scholar]
- 28.Restrepo CE, Spinner RJ. Major nerve injury after contraceptive implant removal: case illustration. J Neurosurg. 2016;124:188–9. doi: 10.3171/2015.1.JNS142642. [DOI] [PubMed] [Google Scholar]
- 29.Wechselberger G, Wolfram D, Pulzl P, Soelder E, Schoeller T. Nerve injury caused by removal of an implantable hormonal contraceptive. Am J Obstet Gynecol. 2006;195:323–6. doi: 10.1016/j.ajog.2005.09.016. [DOI] [PubMed] [Google Scholar]
- 30.Osman N, Dinh A, Dubert T, Goubier J. A new cause for iatrogenic lesion of the ulnar nerve at the arm: contraceptive hormonal implant. Report of two cases. Chir Main. 2005;24:181–3. doi: 10.1016/j.main.2005.04.005. [DOI] [PubMed] [Google Scholar]
- 31.Voedisch A, Hugin M. Difficult implant removals. Curr Opin Obstet Gynecol. 2007;29(6):449–457. doi: 10.1097/GCO.0000000000000416. [DOI] [PubMed] [Google Scholar]
- 32.Chevreau J, Krief D, Abou Arab O, Zitoun M, Foulon A, Sergent F. Gondry Factors associated with removal difficulties of etonogestrel-containing contraceptive implants (Nexplanon®). J. Eur J Obstet Gynecol Reprod Biol. 2018;224:81–84. doi: 10.1016/j.ejogrb.2018.03.019. [DOI] [PubMed] [Google Scholar]
- 33.Vidin E, Garbin O, Rodriguez B, Favre R, Bettahar-Lebugle K. Removal of etonogestrel contraceptive implants in the operating theater: report on 28 cases. Contraception. 2007;76(1):35–9. doi: 10.1016/j.contraception.2007.03.012. [DOI] [PubMed] [Google Scholar]
- 34.Laumonerie P, Blasco L, Tibbo ME, Leclair O, Kerezoudis P, Chantalat E, Mansat P. Peripheral Nerve Injury Associated with a Subdermal Contraceptive Implant: Illustrative Cases and Systematic Review of Literature. World Neurosurg. 2018;111:317–325. doi: 10.1016/j.wneu.2017.12.160. [DOI] [PubMed] [Google Scholar]
- 35.Creinin MD, Kaunitz AM, Darney PD, Schwartz L, Hampton T, Gordon K. The US etonogestrel implant mandatory clinical training and active monitoring programs: 6 year experience. Contraception. 2017;95:205–210. doi: 10.1016/j.contraception.2016.07.012. [DOI] [PubMed] [Google Scholar]


