Abstract
Background
Digital technologies (such as smartphone applications, activity trackers, and e-learning platforms) have supported patients with long-term conditions to change their lifestyle health behaviours. The aim of this study was to examine the effectiveness of digital technologies in supporting patients undergoing elective surgery to change their health behaviours.
Methods
A systematic review was conducted of articles reporting a digital intervention supporting behaviour change in adult patients who underwent elective bariatric, oncological or orthopaedic surgery. MEDLINE, Embase, CINAHL, PsycINFO, Web of Science, and Scopus were searched from inception to March 2019 for quantitative intervention studies with a specific focus on physical activity, dietary intake, and weight loss in patients before and after surgery (PROSPERO: CRD42019127972). The Joanna Briggs Institute critical appraisal checklist was used to assess study quality.
Results
Of 3021 citations screened, 17 studies were included comprising 4923 surgical patients; these included experimental (pre–post design, feasibility studies, and RCTs) and observational studies. Three factors were identified as effective for supporting health behaviour change in elective surgical populations: digital technology delivery, implementation, and theoretical underpinning. Six of eight studies that referred to behaviour change theories observed significant improvements in health behaviour relating to reduced weight regain, and improved lifestyle choices for physical activity and diet. Meta-analysis was not possible because of heterogeneous outcome measures.
Conclusion
Digital technologies may effectively support behavioural change in patients undergoing elective surgery.
Resumen
Antecedentes
Las tecnologías digitales (como las aplicaciones para teléfonos inteligentes, los rastreadores de actividad y las plataformas de aprendizaje electrónico) han ayudado a los pacientes con enfermedades crónicas a cambiar sus hábitos de salud y estilo de vida. El objetivo de este estudio fue examinar la efectividad de las tecnologías digitales para ayudar a los pacientes sometidos a cirugía electiva a cambiar sus comportamientos de salud.
Métodos
Se realizó una revisión sistemática de los artículos que presentaban una intervención digital que apoyaba el cambio de comportamiento en pacientes adultos que se sometieron a cirugía bariátrica, oncológica u ortopédica electiva. Se realizaron búsquedas en las bases de datos Medline, Embase, CINAHL, PsycINFO, Web of Science y Scopus desde el inicio hasta marzo de 2019, para seleccionar estudios de intervención cuantitativos con un enfoque específico en la actividad física, la ingesta dietética y la pérdida de peso en pacientes antes y después de la cirugía (ver PROSPERO: CRD42019127972) Se utilizó la lista de verificación de evaluación crítica del Instituto Joanna Briggs para evaluar la calidad del estudio.
Resultados
De 3.021 referencias examinadas, se seleccionaron 17 estudios con un total de 4.923 pacientes quirúrgicos, incluyendo estudios experimentales (diseño antes y después, estudios de viabilidad y ensayos aleatorizados y controlados) y estudios observacionales. Se identificaron tres factores como efectivos para apoyar el cambio de comportamiento de salud en poblaciones quirúrgicas electivas, incluida la entrega, la implementación y una base teórica de tecnología digital. Las tasas de retención de las intervenciones variaron del 55% al 100%. Seis de los ocho estudios que se refirieron a las teorías de cambio de comportamiento observaron mejoras significativas en el cambio de comportamiento de salud relacionado con la reducción de la recuperación de peso y mejores opciones de estilo de vida para la actividad física y la dieta. El metaanálisis no fue posible debido a las medidas de resultado heterogéneas.
Conclusión
Las tecnologías digitales pueden apoyar eficazmente el cambio de comportamiento en pacientes sometidos a intervenciones quirúrgicas electivas.
Introduction
Digital technologies are becoming an integral part of modern-day life. Recent reports from the UK Office of Communications and the Statista and the Office of Communications estimate that 78 per cent of adults own a smartphone, 90 per cent of people regularly access the internet in their home, 58 per cent own a tablet device, and 20 per cent use wearable technology, such as smart watches and fitness trackers1,2. A recent US-based review3 found that almost 60 per cent of American smartphone users have reported downloading and using fitness or health-related applications, more commonly termed apps. There has been a successful shift towards the integration of digital technologies in healthcare systems too. For clinicians, digital technologies can improve communication and information transfer between clinical teams and healthcare sectors4,5. For healthcare providers and organizations, digital technologies can assist in reducing the burden associated with working at increased capacity, and managing patients with increasing numbers of co-morbidities6,7. For patients, digital technologies can enhance education provision, improve communication with clinicians, and empower them to play an active role in their own care4,8–10.
In a surgical context, recent evidence has linked better patient physical preparedness before surgery with improved outcomes and benefits after surgery11–13. More specifically, improvements in a patient’s dietary intake14, physical activity levels15, and smoking cessation16 have been linked to improved recovery, better tolerance of postoperative treatment, and prevention of related disease in the long term8,17–19. At present, variable amounts of support and education, however, are made available for patients undergoing elective surgery in order to motivate health behaviour changes. For instance, before weight loss surgery, patients are encouraged to change their diet and lose weight, but many feel unsupported20–22. Patients recovering from cancer surgery have previously reported poor lifestyle support and this has also been recognized by healthcare professionals23,24. To encourage changes to lifestyle behaviours, education and information needs to be better communicated to patients having elective surgery. Digital technologies (such as smartphone apps, tablets, activity trackers, and the internet) could support this. The aim of this review was to determine whether digital technologies are effective at supporting patients undergoing elective surgery to change their health behaviours, focusing on physical activity, weight, and dietary intake.
Methods
This systematic review was conducted in accordance with PRISMA guidelines25 and registered with PROSPERO (CRD42019127972).
Search strategy and study selection
A comprehensive and systematic literature search was conducted in March 2019 across six electronic databases including MEDLINE, Embase, CINAHL, PsycINFO, Web of Science, and Scopus. No limit on the publication date was applied. Experimental and observational studies that evaluated a digital intervention supporting behaviour change(s) in adult patients undergoing elective surgery (aged over 18 years), of any sex, ethnicity or nationality, during the preoperative or postoperative period, were included. The studies must have conducted an initial baseline measurement of participants and (at least) one follow-up measure, so as to evaluate whether a change in behaviour (physical activity levels, weight, and/or dietary habits) took place in the population group. Any study where the intervention focused on healthcare professionals, family and/or caregivers, or patients more than 2 years after surgery were excluded. Any studies that evaluated digital interventions from a psychological or quality-of-life point of view, or where the behaviour change related to disease screening (rather than active surgical care), were excluded. Qualitative studies, editorials, reviews, conference abstracts, and study protocols were also excluded. This review focused on elective surgical procedures, specifically bariatric, cancer and orthopaedic procedures, where patients require preoperative and postoperative lifestyle and health behaviour changes; abdominal, cardiac, gastrointestinal, gynaecological, and trauma operations were excluded.
Additional papers were identified via the grey literature within personal libraries of the authors, professional research networks, and by reference checking. Search terms are described in Appendix S1.
Titles and abstracts from the database search were reviewed by one author. Full texts were retrieved for articles that met the inclusion criteria for further evaluation, and for those that could not be rejected with certainty. Full-text articles were screened independently by two authors. Disagreements were resolved by discussion with a third reviewer where necessary.
Data extraction and quality appraisal
Data extraction was carried out by two authors, using a customized data extraction form containing the following headings: study, intervention, population, behavioural change outcome, key findings, and study limitations. Quality and risk-of-bias assessment was conducted by two authors using the Joanna Briggs Institute critical appraisal tools26. This checklist includes questions relating to sampling, inclusion criteria, confounding, outcomes, and statistical analysis. All studies were assigned a methodological quality (bias) score for ease of reporting, expressed as a percentage. Interventions were grouped into three delivery time points for analysis: preoperative interventions (implemented before the surgical procedure); postoperative interventions (implemented after the surgical procedure); and preoperative and postoperative interventions (implemented before and continued after operation).
Analysis and synthesis
A narrative synthesis describing studies thematically was undertaken. Studies reported heterogeneous measures so a meta-analysis was not possible. Overall effectiveness in supporting behavioural change in surgical patients was reported in terms of the delivery method, timing of intervention delivery, and theoretical underpinning of the digital interventions.
Results
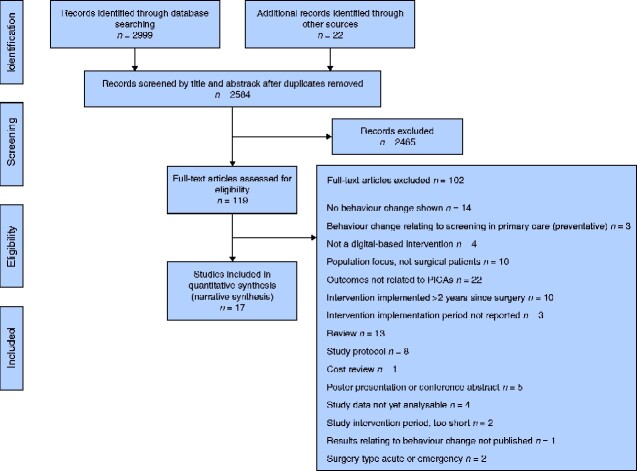
Initially 2999 citations were screened. An additional 22 studies were identified by hand-searching and grey literature search. After removal of duplicates and applying the eligibility criteria, 17 studies were included (Fig. 1). Ten of these were RCTs; the remaining seven included feasibility and efficacy studies, controlled observational studies, and a study employing a pre–post-test design.
Fig. 1.
PRISMA flow chart showing selection of articles for review
PICO, Population, Intervention, Comparison, Outcomes.
These studies were published between 2011 and 2019. They were conducted in seven different countries, including the USA (5)27–31, the Netherlands (4)32–35, Canada (3)15,36,37, New Zealand (2)38,39, South Korea (1)40, Australia (1)41, and Spain (1)42. The studies included a total of 4923 surgical patients.
The studies covered three different surgery types: bariatric surgery (10 studies), cancer surgery (5), and orthopaedic surgery (2). Further study characteristics, including the timing and behaviours targeted for change, are detailed in Table 1. The studies varied in intervention delivery method, duration, and frequency of use. Two articles35,42 did not report any statistical analysis of the results. Of the remaining 15 papers, nine reported a significant effect indicating a change in health behaviours (P ≤ 0.050).
Table 1.
Study characteristics
| Reference | Type of surgery | Intervention target |
Behaviour change target |
Population size in intervention group | Participant sex | Control or comparator group | ||||
|---|---|---|---|---|---|---|---|---|---|---|
| Before surgery | After surgery | Before and after surgery | Physical activity | Weight | Diet | |||||
| Baillot et al.36 | Bariatric | × | × | 6 | F | Yes | ||||
| Bradley et al.27 | Bariatric | × | × | 20 | F + M | No | ||||
| Coleman et al.28 | Bariatric | × | × | 26 | F + M | Yes | ||||
| Doiron-Cadrin et al.15 | Orthopaedic, TKA + THA | × | × | 12 | F + M | Yes | ||||
| Kanera et al.32 | Cancer, mixed | × | × | × | 265 | F + M | Yes | |||
| Kanera et al.33 | Cancer, mixed | × | × | × | 231 | F + M | Yes | |||
| King et al.29 | Bariatric | × | × | 310 | F + M | Yes | ||||
| Lauti et al.38 | Bariatric | × | × | 47 | F + M | Yes | ||||
| Lee et al.40 | Cancer, breast | × | × | × | 29 | n.r. | Yes | |||
| Lemanu et al.39 | Bariatric | × | × | 44 | F + M | Yes | ||||
| Mayer et al.30 | Cancer, colon | × | × | 144 | F + M | Yes | ||||
| Mundi et al.31 | Bariatric | × | × | × | 30 | F + M | No | |||
| Ormel et al.34 | Cancer, mixed | × | × | 16 | F + M | Yes | ||||
| Padwal et al.37 | Bariatric | × | × | 225 | F + M | Yes | ||||
| Russell et al.41 | Orthopaedic, TKA | × | × | 31 | F + M | Yes | ||||
| Tenhagen et al.35 | Bariatric | × | × | 14 | F + M | No | ||||
| Vilallonga et al.42 | Bariatric | × | × | 10 | F + M | Yes | ||||
TKA, total knee arthroscopy; THA, total hip arthroscopy; n.r., not reported.
Study quality
The overall methodological quality of included studies was good, with a mean quality score of 69 per cent (Appendix S2). Scores ranged from 54 per cent30,39 to 100 per cent34,36.
Delivery of intervention
Different digital technologies were used to deliver the interventions, including internet-based interventions (telemedicine, emails, and e-platforms)15,32,33,36,37,40,41, phone-based interventions (text messaging and apps)30,34,38,39, wearable interventions (activity monitors)29, and combination interventions (more than 1 form of digital technology to support health behaviour change)27,28,31,35,42. Appendix S3 provides an overview of the method of delivery, target, and engagement rate of interventions.
Internet-based interventions
Seven studies used internet-based interventions to promote health behaviour change, three15,36,41 of which employed telemedicine, and the remaining four32,33,37,40 used an e-platform system, made up of educational modules. None of the three telemedicine studies led to change in health behaviours, although the authors did recognize the potential benefits of using this method of delivery to overcome provision and geographical barriers41.
The e-platform approach produced health behaviour change across three of the four studies32,33,40. Two studies32,33 employed the Kanker Nazorg Wijzer e-platform to provide personalized educational modules concerning physical activity (in minutes of exercise per week) and diet (vegetable consumption in grams per day) to patients after cancer surgery. Kanera and colleagues32 reported how the intervention group improved moderate physical activity (by 150.73 min/week; P = 0.037) compared with control over a 6-month period, and this improvement was sustained over 12 months (P = 0.011). However, the increased vegetable consumption (grams per day; P = 0.027) over the 6-month period was not sustained at 12 months (P = 0.132). They also demonstrated that improvements in physical activity were significantly more successful in younger patients (aged less than 57 years) than older ones, over 6 months (minutes per week; P = 0.04) and 12 months (minutes per week; P < 0.010)33. This echoes findings from previous work that showed how younger cancer survivors were more likely to improve their physical activity levels compared with older survivors, possibly owing to their perceptions of future risk43,44.
Another study40 focused on a web-based self-management exercise and diet intervention e-platform to support patients to improve exercise and dietary intake health behaviours after breast cancer surgery. The results demonstrated an improvement in diet (servings of fruit and vegetables per day; P = 0.001) and physical activity levels (minutes of exercise per week; P < 0.001) compared with the control.
Phone-based interventions
Four studies delivered health behaviour change interventions using phone-based methods, two38,39 through text messaging services and two30,34 through smartphone apps. Lemanu and colleagues39 found that text message delivery over a 4–6-week period was successful in improving bariatric patient adherence to preoperative exercise (median days of exercise per week; P < 0.050), although this improvement was not sustained at 6 weeks’ postoperative follow-up. Ormel et al.34 showed significant improvements in physical activity in patients before and after cancer surgery with app use, which was not maintained at the 12-week follow-up. Mayer and co-workers30 also reported an improvement in physical activity in patients after surgery for colonic cancer with the SurvivorCHESS app. However, this improvement was no different from that in control patients (minutes of moderate and vigorous physical activity per week; P = 0.122) and only lasted as long as the intervention.
Wearable interventions
King and colleagues29 provided participants with a wearable digital activity monitor (which tracked physical activity, including daily step counts and active minutes) to use alongside self-reporting physical activity levels in a paper diary, from 1 week before to 1 year after surgery. More participants changed from inactive to active, than from active to inactive, over the intervention period (minutes of exercise per week; P < 0.001). By using the diary, more participants self-reported physical activity levels improving from less than 150 min/week before surgery to 150 min/week or more at 1 year after operation (P < 0.001). The activity monitor recorded an increase in the number of steps per day and active minutes per day from before to 1 year after surgery (both P < 0.001).
Combination interventions
Five studies used a combination of different digital approaches to motivate health behaviour change in patients undergoing bariatric surgery. One study27 used a combination of three digital elements (triple approach) and the other four28,31,35,42 used a dual approach. One study31 trialled a combination intervention before surgery and three studies27,28,42 after operation, and one study35 implemented the combined intervention both before and after operation across the surgical journey. Of the five combination interventions, three studies27,28,31 demonstrated behavioural change improvements, and two35,42 did not perform a statistical analysis.
In a triple-approach study, Bradley and co-workers27 implemented an e-platform in combination with an app and online log to investigate efficacy of reduced weight regain after bariatric surgery. Educational information was delivered through the e-platform and daily calorie intake calculated using an app. At completion of the intervention, 10 of 11 participants demonstrated weight loss or weight stabilization (in kilograms; P = 0.01). Weight loss was maintained at 3 months’ follow-up.
Coleman et al.28 implemented a dual approach, whereby participants used a form of wearable technology (pedometer) in combination with online activity logging to complement postoperative exercise programmes. An improvement was demonstrated in participants’ 6-min walk test (distance in metres; P = 0.001) during the intervention period and maintained at 6-month follow-up.
Mundi and colleagues31 employed a dual-approach intervention, consisting of an educational app and a daily text message service, for 12 weeks before bariatric surgery. At study completion there was a reduction in weight (in kilograms; P = 0.06) and BMI (kilograms per square metre; P < 0.001), and an increase in physical activity (minutes of vigorous activity per week; P = 0.04) in the intervention group.
Patients tracked their real-time weekly parameters before and after bariatric surgery, by using digital weighing scales (technology at home) connected to an online log in another dual-approach study35. On study completion, participant mean(s.d.) BMI decreased from 44.7(4.6) to 30.6(4.2) kg/m2; the mean estimated weight loss was 72(19.1) per cent and the mean BMI change was 32 per cent. Vilallonga and co-workers42 also employed a dual approach; WiFi-enabled weighing scales were used to log weight loss on an online account and members of the surgical team used e-mail to liaise with patients on postoperative weight-loss progress. The results demonstrated improvements in the mean percentage estimated weight loss, with the intervention group losing 65.3 per cent compared with 58.2 per cent for control. The mean postoperative BMI was 32.7 and 33.2 kg/m2 respectively.
Timing of intervention delivery
Appendix S3 shows details of the timing of each intervention in the studies, specifically how long patients used the interventions (intervention period) and their active engagement (retention rates). Four studies15,31,36,37 initiated interventions 12 weeks before surgery and one39 4–6 weeks before operation. Nine studies used postoperative interventions, with some patients beginning almost immediately after surgery with a rehabilitation focus41, some during follow-up monitoring27,32,33,40,42, and up to 2 years after surgery in three studies28,30,38. The overall intervention period in the included studies differed substantially, the shortest being 6 weeks41 and the longest continuing over 12 months40. The preoperative and postoperative intervention by Ormel et al.34 was initiated following the decision to undergo surgery, and was continued for 12 weeks after operation. Tenhagen and co-workers35 also initiated the intervention after the surgical decision had been made, but continued for 12 months after the procedure, whereas King and colleagues29 initiated the intervention for 7 days in the week before surgery and repeated the intervention for another 7-day interval 1 year after operation.
Overall retention rates over the intervention period were high; only one study27 had a retention rate below 60 per cent. Four studies reported 100 per cent retention rates, including two with preoperative interventions36,39, one with a postoperative intervention42, and one with intervention before and after surgery34.
Theoretical underpinning: behaviour change theories
Eight27,28,30,32–34,38,40 of the 17 studies referred to behaviour change theories or frameworks, either as a way of designing the intervention or for analysis of the results. Across these, social cognitive theory was used twice32,33, whereas theories such as acceptance and commitment therapy27, the trans-theoretical model40, self-determination theory30, the behaviour change wheel38, and goal-setting28 were used once. Ormel and colleagues34 did not specify which behavioural change theory informed the design of their app. Of the eight studies, six produced significant improvements in health behaviour changes (P ≤ 0.05) relating to reduced weight regain27, increased physical activity28,34, and improved lifestyle choices for physical activity and diet32,33,40.
Discussion
In patients undergoing elective surgery, various forms of digital technology can support behaviour change successfully, in particular physical activity, dietary intake, and weight loss. The duration of behaviour change has proven to be variable, with some technologies demonstrating more success on a short-term basis. Three factors were identified that could contribute to digital technology effectiveness in the elective surgical population: delivery of an intervention, timing of the intervention, and behavioural change theories underpinning the intervention design.
High overall retention rates across studies indicate the acceptability of modern technologies in surgical care. This is not an unusual finding, with previous research supporting the success of digital technology overlap from social to health-related purposes45–47. High satisfaction rates among intervention group participants were seen in the internet-based studies, with 100 per cent reporting their overall satisfaction with the delivery format15, and 96 per cent attendance recorded for the telemedicine intervention group compared with 80 per cent for the control36. Padwal and colleagues37 concluded that e-platforms were often more expensive and labour-intensive to produce and run.
Although none of the studies using telemedicine demonstrated improvements in health behaviours, the authors acknowledged many benefits underpinning this delivery method. These included reduced travel to face-to-face appointments32,33, increased accessibility to healthcare services for those who are geographically, economically or functionally disadvantaged36, and improved continuity of care with the same physician working to programme completion41. This adds to the already growing body of literature supporting the wide-ranging opportunities that telemedicine interventions present48. Specifically, in a surgical context, this can reduce the need for in-person consultations before and after surgery49,50. The benefits of phone-based interventions included convenience for the patient (accessible at any time), low cost, and user-friendliness30,38,39. A higher level of sophistication, such as text messages that allow a response, offers more personalized advice as well as the possibility to link with self-monitoring applications to track progress, which may produce superior results38. Newer forms of delivery, such as wearable technologies, have increased in popularity over recent years, yet only two studies used wearable technologies in this review. One wearable was successful in isolation29 and one in combination28.
There were no interventions that included digitally based peer support networks in this review. Peer forums supporting and motivating preoperative and postoperative lifestyle changes have demonstrated success in past studies51–53. Peer support has been found to enhance the effectiveness of behaviour change, with authors postulating how this may increase motivation and adoption of social-norm approaches through social interactions54–57.
The optimum value of intervention timings, specifically initiation, duration, and frequency, on outcomes is unclear. Other factors such as the surgical procedure (or the underlying disease triggering surgery) may contribute to variation in behavioural change, and may in fact determine the timing of when, for how long, and how often patients engage with digital technologies. It would appear that preoperative digital interventions are beneficial in cementing a culture of behaviour change for the patient at the earliest opportunity, capitalizing on the surgical teachable moment58–60. The challenge is continuing the intervention after surgery in an attempt to sustain health behaviour change and obtain greater improvements in outcomes61.
This review is subject to some limitations. Study outcome measures were heterogeneous, often adapted to the specific population rather than for undergoing surgery in general. This made it difficult to judge the optimum approach(es) responsible for contributing to significant behaviour change in each cohort. Although it was possible to identify elements of intervention delivery and timing that may be effective for supporting surgical patients, the most important and effective element could not be determined. It was also unclear which combination(s) of intervention delivery approaches would be optimal. In a world where digital technologies develop at rapid pace, and are implemented more than ever within healthcare systems, these components should be established in order to have maximal effectiveness in supporting behaviour change in patients undergoing elective surgery, thus improving surgical quality and safety.
Funding
Dr WE Harker PhD Studentship, Newcastle University.
Supplementary Material
Acknowledgements
This project was supported through the Dr W. E. Harker PhD Studentship, from Newcastle University.
Disclosure. The authors declare no conflict of interest.
Supplementary material
Supplementary material is available at BJS Open online.
References
- 1. Statista. Smartphone Usage among Mobile Phone Users in the United Kingdom (UK) in 2018. Newcastle upon Tyne: University of Newcastle, 2018
- 2. OFCOM. Communications Market Report. https://www.ofcom.org.uk/__data/assets/pdf_file/0022/117256/CMR-2018-narrative-report.pdf (accessed 2 August 2018)
- 3. Krebs P, Duncan DT.. Health app use among us mobile phone owners: a national survey. JMIR Mhealth Uhealth 2015;3:e101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Rathbone AP, Norris R, Parker P, Lindsley A, Robinson A, Baqir W. et al. Exploring the use of WhatsApp in out-of-hours pharmacy services: a multi-site qualitative study. Res Social Adm Pharm 2020;16:503–510 [DOI] [PubMed] [Google Scholar]
- 5. Grant PG, Loane RO, Davey AD.. G554(P) Whatsapp doc: social media as a quality improvement tool in perioperative fluid management. Arch Dis Child 2016;101(Suppl 1):A329 [Google Scholar]
- 6. Hamine S, Gerth-Guyette E, Faulx D, Green BB, Ginsburg AS.. Impact of mHealth chronic disease management on treatment adherence and patient outcomes: a systematic review. J Med Internet Res 2015;17:e52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Wolfstadt JI, Soong C, Ward SE.. Improving patient outcomes following total joint arthroplasty: is there an app for that? BMJ Qual Saf 2019;28:775–777 [DOI] [PubMed] [Google Scholar]
- 8. Pecorelli N, Fiore JF, Kaneva P, Somasundram A, Charlebois P, Liberman AS. et al. An app for patient education and self-audit within an enhanced recovery program for bowel surgery: a pilot study assessing validity and usability. Surg Endosc 2018;32:2263–2273 [DOI] [PubMed] [Google Scholar]
- 9. van der Meij E, Bouwsma EVA, van den Heuvel B, Bonjer HJ, Anema JR, Huirne JAF.. Using e-health in perioperative care: a survey study investigating shortcomings in current perioperative care and possible future solutions. BMC Surg 2017;17:61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Barker I, Steventon A, Williamson R, Deeny SR.. Self-management capability in patients with long-term conditions is associated with reduced healthcare utilisation across a whole health economy: cross-sectional analysis of electronic health records. BMJ Qual Saf 2018;27:989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Arora RC, Brown CH, Sanjanwala RM, McKelvie R.. ‘NEW’ prehabilitation: a 3-way approach to improve postoperative survival and health-related quality of life in cardiac surgery patients. Can J Cardiol 2018;34:839–849 [DOI] [PubMed] [Google Scholar]
- 12. Chughtai M, Shah NV, Sultan AA, Solow M, Tiberi JV, Mehran N. et al. The role of prehabilitation with a telerehabilitation system prior to total knee arthroplasty. Ann Transl Med 2019;7:68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Wynter-Blyth V, Moorthy K.. Prehabilitation: preparing patients for surgery. BMJ 2017;358:j3702. [DOI] [PubMed] [Google Scholar]
- 14. Beleigoli AM, de Andrade AQ, Diniz MDH, Alvares RS, Ribeiro AL.. Online platform for healthy weight loss in adults with overweight and obesity—the ‘POEmaS’ project: a randomized controlled trial. BMC Public Health 2018;18:945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Doiron-Cadrin P, Kairy D, Vendittoli PA, Lowry V, Poitras S, Desmeules F.. Feasibility and preliminary effects of a tele-prehabilitation program and an in-person prehabilitation program compared to usual care for total hip or knee arthroplasty candidates: a pilot randomized controlled trial. Disabil Rehabil 2020;42:989–998 [DOI] [PubMed] [Google Scholar]
- 16. Levett DZ, Edwards M, Grocott M, Mythen M.. Preparing the patient for surgery to improve outcomes. Best Pract Res Clin Anaesthesiol 2016;30:145–157 [DOI] [PubMed] [Google Scholar]
- 17. den Bakker CM, Huirne JA, Schaafsma FG, de Geus C, Bonjer HJ, Anema JR.. Electronic health program to empower patients in returning to normal activities after colorectal surgical procedures: mixed-methods process evaluation alongside a randomized controlled trial. J Med Internet Res 2019;21:e10674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. van der Meij E, Huirne JA, Ten Cate AD, Stockmann HB, Scholten PC, Davids PH. et al. A perioperative eHealth program to enhance postoperative recovery after abdominal surgery: process evaluation of a randomized controlled trial. J Med Internet Res 2018;20:e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. van Kasteren Y, Freyne J, Hussain MS.. Total knee replacement and the effect of technology on cocreation for improved outcomes and delivery: qualitative multi-stakeholder study. J Med Internet Res 2018;20:e95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Stewart F, Avenell A.. Behavioural interventions for severe obesity before and/or after bariatric surgery: a systematic review and meta-analysis. Obes Surg 2016;26:1203–1214 [DOI] [PubMed] [Google Scholar]
- 21. Koball AM, Jester DJ, Domoff SE, Kallies KJ, Grothe KB, Kothari SN.. Examination of bariatric surgery Facebook support groups: a content analysis. Surg Obes Relat Dis 2017;13:1369–1375 [DOI] [PubMed] [Google Scholar]
- 22. Anderson AS, Caswell S, Wells M, Steele RJ.. Obesity and lifestyle advice in colorectal cancer survivors—how well are clinicians prepared? Colorectal Dis 2013;15:949–957 [DOI] [PubMed] [Google Scholar]
- 23. Williams K, Beeken RJ, Fisher A, Wardle J.. Health professionals’ provision of lifestyle advice in the oncology context in the United Kingdom. Eur J Cancer Care 2015;24:522–530 [DOI] [PubMed] [Google Scholar]
- 24. Anderson AS, Steele R, Coyle J.. Lifestyle issues for colorectal cancer survivors—perceived needs, beliefs and opportunities. Support Care Cancer 2013;21:35–42 [DOI] [PubMed] [Google Scholar]
- 25. Moher D, Liberati A, Tetzlaff J, Altman DG.. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. BMJ 2009;339:b2535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Briggs J. The Joanna Briggs Institute Critical Appraisal Tools: Checklists. https://joannabriggs.org/critical_appraisal_tools (accessed 26 March 2019).
- 27. Bradley LE, Forman EM, Kerrigan SG, Goldstein SP, Butryn ML, Thomas JG. et al. Project HELP: a remotely delivered behavioral intervention for weight regain after bariatric surgery. Obes Surg 2017;27:586–598 [DOI] [PubMed] [Google Scholar]
- 28. Coleman KJ, Caparosa SL, Nichols JF, Fujioka K, Koebnick C, McCloskey KN. et al. Understanding the capacity for exercise in post-bariatric patients. Obes Surg 2017;27:51–58 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. King WC, Hsu JY, Belle SH, Courcoulas AP, Eid GM, Flum DR. et al. Pre- to postoperative changes in physical activity: report from the longitudinal assessment of bariatric surgery-2 (LABS-2). Surg Obes Relat Dis 2012;8:522–532 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Mayer DK, Landucci G, Awoyinka L, Atwood AK, Carmack CL, Demark-Wahnefried W. et al. SurvivorCHESS to increase physical activity in colon cancer survivors: can we get them moving? J Cancer Surviv 2018;12:82–94 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Mundi MS, Lorentz PA, Grothe K, Kellogg TA, Collazo-Clavell ML.. Feasibility of smartphone-based education modules and ecological momentary assessment/intervention in pre-bariatric surgery patients. Obes Surg 2015;25:1875–1881 [DOI] [PubMed] [Google Scholar]
- 32. Kanera IM, Bolman CA, Willems RA, Mesters I, Lechner L.. Lifestyle-related effects of the web-based Kanker Nazorg Wijzer (Cancer Aftercare Guide) intervention for cancer survivors: a randomized controlled trial. J Cancer Surviv 2016;10:883–897 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Kanera IM, Willems RA, Bolman CA, Mesters I, Verboon P, Lechner L.. Long-term effects of a web-based cancer aftercare intervention on moderate physical activity and vegetable consumption among early cancer survivors: a randomized controlled trial. Int J Behav Nutr Phys Act 2017;14:19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Ormel HL, van der Schoot GGF, Westerink NDL, Sluiter WJ, Gietema JA, Walenkamp AME.. Self-monitoring physical activity with a smartphone application in cancer patients: a randomized feasibility study (SMART-trial). Support Care Cancer 2018;26:3915–3923 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Tenhagen M, van Ramshorst GH, Demirkiran A, Hunfeld MAJM, Cense HA.. Perioperative online weight monitoring in bariatric surgery with a digital internet-connected scale. Obes Surg 2016;26:1120–1126 [DOI] [PubMed] [Google Scholar]
- 36. Baillot A, Boissy P, Tousignant M, Langlois MF.. Feasibility and effect of in-home physical exercise training delivered via telehealth before bariatric surgery. J Telemed Telecare 2017;23:529–535 [DOI] [PubMed] [Google Scholar]
- 37. Padwal RS, Klarenbach S, Sharma AM, Fradette M, Jelinski SE, Edwards A. et al. The evaluating self-management and educational support in severely obese patients awaiting multidisciplinary bariatric care (EVOLUTION) trial: principal results. BMC Med 2017;15:46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Lauti M, Kularatna M, Pillai A, Hill AG, MacCormick AD.. A randomised trial of text message support for reducing weight regain following sleeve gastrectomy. Obes Surg 2018;28:2178–2186 [DOI] [PubMed] [Google Scholar]
- 39. Lemanu DP, Singh PP, Shao RY, Pollock TT, MacCormick AD, Arroll B. et al. Text messaging improves preoperative exercise in patients undergoing bariatric surgery. ANZ J Surg 2018;88:733–738 [DOI] [PubMed] [Google Scholar]
- 40. Lee MK, Yun YH, Park HA, Lee ES, Jung KH, Noh DY.. A Web-based self-management exercise and diet intervention for breast cancer survivors: pilot randomized controlled trial. Int J Nurs Stud 2014;51:1557–1567 [DOI] [PubMed] [Google Scholar]
- 41. Russell TG, Buttrum P, Wootton R, Jull GA.. Internet-based outpatient telerehabilitation for patients following total knee arthroplasty: a randomized controlled trial. J Bone Joint Surg Am 2011;93:113–120 [DOI] [PubMed] [Google Scholar]
- 42. Vilallonga R, Lecube A, Fort JM, Boleko MA, Hidalgo M, Armengol M.. Internet of things and bariatric surgery follow-up: comparative study of standard and IoT follow-up. Minim Invasive Ther Allied Technol 2013;22:304–311 [DOI] [PubMed] [Google Scholar]
- 43. Niu C, Eng L, Qiu X, Shen X, Espin-Garcia O, Song Y. et al. Lifestyle behaviors in elderly cancer survivors: a comparison with middle-age cancer survivors. J Oncol Pract 2015;11:e450–e459 [DOI] [PubMed] [Google Scholar]
- 44. Mullens AB, McCaul KD, Erickson SC, Sandgren AK.. Coping after cancer: risk perceptions, worry, and health behaviors among colorectal cancer survivors. Psychooncology 2004;13:367–376 [DOI] [PubMed] [Google Scholar]
- 45. Agarwal S, Vasudevan L, Tamrat T, Glenton C, Lewin S, Bergman H. et al. Digital tracking, provider decision support systems, and targeted client communication via mobile devices to improve primary health care. Cochrane Database Syst Rev 2018;CD012925 [Google Scholar]
- 46. Gordon CR, Rezzadeh KS, Li A, Vardanian A, Zelken J, Shores JT. et al. Digital mobile technology facilitates HIPAA-sensitive perioperative messaging, improves physician–patient communication, and streamlines patient care. Pat Saf Surg 2015;9:21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Jenssen BP, Mitra N, Shah A, Wan F, Grande D.. Using digital technology to engage and communicate with patients: a survey of patient attitudes. J Gen Int Med 2016;31:85–92 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Asiri A, AlBishi S, AlMadani W, ElMetwally A, Househ M.. The use of telemedicine in surgical care: a systematic review. Acta Inform Med 2018;26:201–206 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Sudan R, Salter M, Lynch T, Jacobs DO.. Bariatric surgery using a network and teleconferencing to serve remote patients in the Veterans Administration Health Care System: feasibility and results. Am J Surg 2011;202:71–76 [DOI] [PubMed] [Google Scholar]
- 50. Schroeder C. Pilot study of telemedicine for the initial evaluation of general surgery patients in the clinic and hospitalized settings. Surg Open Sci 2019;1:9799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Bradford TW, Grier SA, Henderson GR.. Weight loss through virtual support communities: a role for identity-based motivation in public commitment. J Interact Mark 2017;40:9–23 [Google Scholar]
- 52. Myneni S, Cobb N, Cohen T.. In pursuit of theoretical ground in behavior change support systems: analysis of peer-to-peer communication in a health-related online community. J Med Internet Res 2016;18:e28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Graham YNH, Hayes C, Mahawar KK, Small PK, Attala A, Seymour K. et al. Ascertaining the place of social media and technology for bariatric patient support: what do allied health practitioners think? Obes Surg 2017;27:1691–1696 [DOI] [PubMed] [Google Scholar]
- 54. Elaheebocus SMRA, Weal M, Morrison L, Yardley L.. Peer-based social media features in behavior change interventions: systematic review. J Med Internet Res 2018;20:e20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Poirier J, Cobb NK.. Social influence as a driver of engagement in a web-based health intervention. J Med Internet Res 2012;14:e36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Latkin CA, Knowlton AR.. Social network assessments and interventions for health behavior change: a critical review. Behav Med 2015;41:90–97 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Petosa RL, Smith LH.. Peer mentoring for health behavior change: a systematic review. Am J Health Educ 2014;45:351–357 [Google Scholar]
- 58. Bluethmann SM, Basen-Engquist K, Vernon SW, Cox M, Gabriel KP, Stansberry SA. et al. Grasping the ‘teachable moment’: time since diagnosis, symptom burden and health behaviors in breast, colorectal and prostate cancer survivors. Psychooncology 2015;24:1250–1257 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Warner DO. Surgery as a teachable moment: lost opportunities to improve public health. Arch Surg 2009;144:1106–1107 [DOI] [PubMed] [Google Scholar]
- 60. Robinson A, Slight R, Husband A, Slight S.. The value of teachable moments in surgical patient care and the supportive role of digital technologies. Perioper Med 2020;9:2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Shi Y, Warner DO.. Surgery as a teachable moment for smoking cessation. Anesthesiology 2010;112:102–107 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.