Abstract
Background
Strong implementation strategies are critical to the success of Enhanced Recovery after Surgery (ERAS®) guidelines, though little documentation exists on effective strategies, especially in complex clinical situations and unfamiliar contexts. This study outlines the process taken to adopt a novel neonatal ERAS® guideline.
Methods
The implementation strategy was approached in a multi-pronged, concurrent but asynchronous fashion. Between September 2019 and January 2020, healthcare providers from various disciplines and different specialties as well as parents participated in the strategy. Multidisciplinary teams were created to consider existing literature and local contexts including potential facilitators and/or barriers. Task forces worked collaboratively to develop new care pathways. An audit system was developed to record outcomes and elicit feedback for revision.
Results
32 healthcare providers representing 9 disciplines and 5 specialties as well as 8 parents participated. Care pathways and resources were created. Elements recommended for a successful implementation strategy included identification of champions, multidisciplinary stakeholder involvement, consideration of local contexts and insights, patient/family engagement, education, and creation of an audit system.
Conclusion
A multidisciplinary and structured process following principles of implementation science was used to develop an effective implementation strategy for initiating ERAS® guidelines.
The authors' implementation model is presented as a reference to guide future healthcare teams as they embark on implementing similar complex healthcare interventions.
Resumen
Antecedentes
Unas estrategias de implementación sólidas son fundamentales para el éxito de las guías de recuperación intensificada tras cirugía (Enhanced Recovery after Surgery, ERAS®), aunque existe poca documentación sobre estrategias efectivas, especialmente en situaciones clínicas complejas y contextos desconocidos. Este estudio describe el proceso seguido para adoptar una nueva directriz ERAS® neonatal.
Métodos
La estrategia de implementación se abordó de una manera múltiple, concurrente pero asincrónica. Entre septiembre de 2019 y enero de 2020 participaron en la estrategia profesionales de diversas disciplinas y diferentes especialidades, así como los padres de los neonatos. Se crearon equipos multidisciplinarios para tener en cuenta la literatura existente y los contextos locales, incluidos los posibles factores facilitadores y/o barreras. Los equipos de trabajo funcionaron en colaboración para desarrollar nuevas vías de atención. Se desarrolló un sistema de auditoría para registrar los resultados y obtener comentarios para su revisión.
Resultados
Participaron
32 profesionales de atención médica que representaban 9 disciplinas y 5 especialidades, así como 8 padres. Se crearon vías de atención y recursos. Los elementos recomendados para una estrategia de implementación exitosa incluyeron la identificación de líderes, la participación multidisciplinar de las partes interesadas, la consideración de los contextos y las perspectivas locales, la participación del paciente y la familia, la educación y la creación de un sistema de auditoría.
Conclusión
Se utilizó un proceso multidisciplinar y estructurado siguiendo los principios de la ciencia de la implementación, a fin de desarrollar una estrategia de implementación eficaz para iniciar las directrices ERAS®.
Introduction
Enhanced Recovery After Surgery (ERAS®) guidelines are holistic, multidisciplinary tools used to deliver collaborative care to surgical patients throughout their perioperative journey1–8. ERAS programmes have successfully improved outcomes in multiple subspecialties by shortening length of stay, decreasing complications and improving patient satisfaction6,7,9–11. Multiple factors contribute to the success of ERAS in practice, including the nature of the guidelines themselves as well as structured implementation plans and audit systems that support their use1,8.
It is becoming increasingly evident that methods of adopting, applying and sustaining an intervention, are just as important in realizing potential outcomes as the interventions themselves12–15. Attention to implementation science is particularly important when adopting complex healthcare interventions, such as ERAS® guidelines. ERAS protocols have become an accepted part of many adult surgical practices and successful strategies of implementation have built on an understanding and acceptance of standard elements of the ERAS® guidelines8,16.
Few attempts have been made to create paediatric specific guidelines and none have targeted the neonatal population17–19; a unique group of patients and caregivers largely unfamiliar with ERAS principles20,21. The implementation of these guidelines required a novel approach to both neonatal surgical care and the application of ERAS.
Neonates have high rates of postoperative complications relating to a variety of physiologic and sociologic factors22–24. Significant practice variation exists and inconsistencies in care may contribute to the complication profile1. To address these concerns, this international team developed a neonatal intestinal resection ERAS® guideline through rigorous collaborative methods using best available evidence from the literature25,26. The published neonatal intestinal resection ERAS® guideline has been adopted at the Alberta Children’s Hospital (ACH) after creating a robust implementation plan.
The aim of the present study was to outline the structured process used to implement the neonatal intestinal resection ERAS® guideline. It was anticipated that this study may act as a model to guide future strategies and other institutions as they embark on implementing ERAS, particularly among specialties less familiar with the concepts involved.
Methods
Principles of effective implementation science and adherence to the Standards for Quality Improvement Reporting Excellence guidelines were followed27. The model for implementation of the neonatal intestinal resection ERAS® guideline followed a planned-action framework and normalization process theory (NPT). Steps in the planned-action framework included identifying a problem, analyzing existing knowledge and evidence to solve the problem, adapting insights to local settings, reviewing potential barriers, tailoring interventions to the local setting, monitoring and evaluating the use and outcomes, and sustaining the change15,28–30. The NPT describes how agents (individuals or groups), objects (procedures or protocols) and contexts (physical or organizational structures) interact with each other to explain how interventions become, or fail to become, normalized in daily practice12,15,31. It consisted of four constructs; coherence, cognitive participation, collective action and reflexive monitoring32.
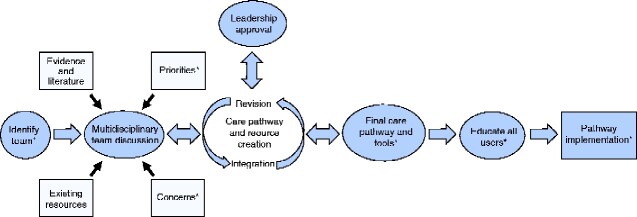
The implementation design occurred in a step-by-step, concurrent but asynchronous process. Between September 2019 and January 2020, the overall strategy included identifying champions, creating multi-disciplinary task forces, creating new care pathways, producing tools and resources, engaging parents/families, educating users and developing an audit system. An overview of the process is depicted in Fig. 1.
Fig. 1.
Overview of the Alberta Children’s Hospital neonatal Enhanced Recovery After Surgery® guideline implementation strategy *denotes points of parent/family engagement
Champions were identified to lead the initiative within their respective specialties based on their previous experience in using or developing ERAS protocols, their interest in the neonatal intestinal resection guideline and their clinical roles. Champions were responsible for overseeing the overall implementation process, obtaining support from their leaders, appointing representatives from their specialty for different task forces (discussed below), educating colleagues on ERAS principles prior to and throughout roll-out, modelling adoption and supporting operationalization of the guideline.
As the neonatal intestinal resection ERAS® guideline consists of 17 recommendations distributed into 10 major topics, individual multidisciplinary task forces approached each recommendation by identifying existing knowledge, local infrastructure and resources and barriers or facilitators of implementation. Each task force consisted of representation from all relevant stakeholders. Some topics were combined and tackled by a single task force. Individual task forces met in an asynchronous but concurrent manner throughout the process.
After evaluating available literature, existing resources and local contexts, each task force worked collaboratively to develop solutions to barriers of implementation. New care pathways and protocols were created through iterative consensus processes informed by multidisciplinary teams that satisfied all team members’ priorities and concerns.
After new care pathways were created, task force members developed methods to integrate and embed new pathways into existing infrastructure. Subsequently, production of various resources ensued, including checklists, protocols and infographics to assist clinicians in modifying their practice patterns. Resources were reviewed, revised and approved for final use by all team members.
Parent stakeholders were engaged at multiple times throughout the guideline development and implementation process25. Parent advisors provided feedback on the initial proposal and feedback on the key topics that would require parental involvement. They also contributed to the determination and development of the final recommendations.
After ethics approval and individual participant consent, a focus group of parents who had an infant with a surgical diagnosis was convened within the neonatal intensive care unit (NICU). Parents discussed priorities in their child’s care and shared experiences and perspectives of care. Parents were educated on ERAS principles and presented with the neonatal ERAS® guideline to provide input on how best to involve them as active partners in their child’s care. Field notes and an audio recording were taken. Parents reviewed and offered feedback on ERAS parent materials and handouts.
After infants had been discharged, follow-up phone calls were conducted with participants by a member of the implementation team using a semi-structured script. Discussions surrounded the education and instructions provided to families at discharge. Areas of inquiry included parent perspectives on communication and transfer of information, strategies to prepare families for discharge as well as reflections on aspects that were performed well and areas requiring improvement. Field notes and audio recordings were taken.
A thematic analysis using N Vivo 12™ (QSR International) was completed on field notes and transcripts using an inductive approach. Codes were applied to concepts using a constant comparative approach by 2 reviewers to ensure reliability and agreement on codes.
Prior to roll-out, all healthcare providers were educated on the principles of ERAS as well as specifics of the neonatal ERAS® guideline and its impact on their clinical practice, using a variety of different education modalities. Poster and print materials were made widely available along with regular email updates and podcasts.
Print materials and handouts were created specifically for parental use and education. A parental education booklet was developed considering institutional contexts. It described ERAS principles, counseled parents of their key role as partners in their child’s care, presented expectations of the typical perioperative journey and provided written discharge information.
Ongoing evaluation and tailoring of the guideline and implementation strategy is fundamental to ensuring continued success. As such, task forces identified objective measures for each recommendation to monitor user compliance with the guidelines (Table S1). Information was gathered on relevant patient outcome measures to evaluate overall guideline efficacy.
Separate surveys and interviews for parents and clinicians were developed to assess personal experiences and opinions of using the guideline and served to elicit suggestions for enhancing the guideline and implementation process. Review and revision of the strategy has been planned once 6 months of data and feedback has been acquired.
Results
Healthcare providers represented 9 separate disciplines and 5 specialties with a total of 32 individuals participating in at least one multidisciplinary task force (Table S2). There were 8 family advisors.
Champions and multidisciplinary task forces
Individuals acted as champions for surgery, anesthesia, neonatology, nursing and implementation (Table S2). Champions sat on multiple task forces and appointed representatives from their discipline for other task forces. In total, there were 8 task forces, each consisting of multidisciplinary representation from all relevant stakeholders (Table S2)
Care pathway creation
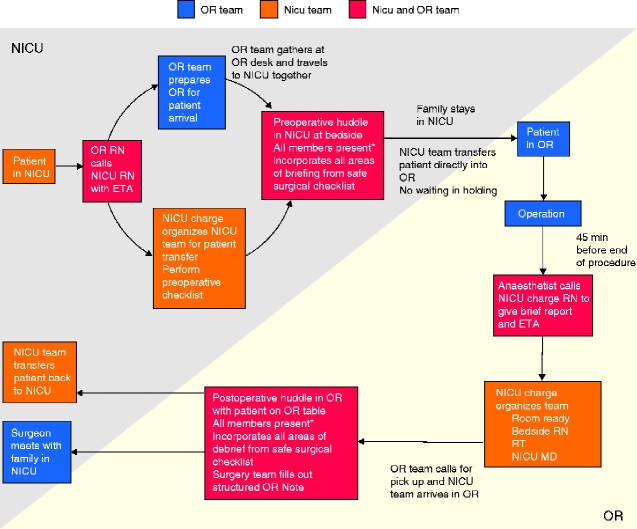
New care pathways were created by 7 of the 8 task forces. The most extensive change occurred in the team communication topic. An evaluation of the existing patient transfer and handover process identified patient care issues relating to potential miscommunication, patient/family privacy and patient safety. An example of the different priorities and concerns that were recognized by the separate disciplines is shown in Table 1. The multidisciplinary task force created a new perioperative patient and information transfer process (Fig. 2). The implementation champion ensured integration of the various new care pathways created by providing perspective on work being done by separate task forces.
Table 1.
Priorities and concerns identified by multidisciplinary task force members for the patient transfer process
| Priorities | Concerns | |
|---|---|---|
| Anaesthesia | Communication of patient information | Time/delay getting patient into the OR if NICU team not ready for transfer |
| Surgery |
Full team present for huddles Teamwork |
Previous ‘silo’ handover model |
| Neonatology | NICU team to continue to provide transport | Responsibility for other potentially sick babies in the NICU if waiting in OR holding to do preoperative huddle |
| OR RN |
Communication Teamwork |
Time/delay in patient transfer |
| NICU RN | Remain part of patient transfer team | Time provided to prepare patient for transfer |
| NICU RT | Remain part of patient transfer team | Equipment/technology issues, especially with HFO/HFJV |
| TM | Blood product distribution | Transfer of blood products between units |
| Transfer Process |
Patient/family confidentiality/privacy Anxiety for other families in holding |
Hypothermia risk Equipment/Technology issues |
OR- operating room; NICU- neonatal intensive care unit; RN- registered nurse; RT- respiratory therapist; HFO- high frequency oscillator; HFJV- high frequency jet ventilation; TM- transfusion medicine
Fig. 2.
Process map of new patient transfer process between the neonatal intensive care unit and the operating room
Resource production
Task forces identified areas where users were considered likely to require support in integrating the ERAS® guidelines into daily workflow. Resources (tools, checklists, infographics) were created to act as reminders and support clinicians with successful adoption. An ERAS baby logo was created and attached to the patient chart, the unit patient board and door to the patient room to remind clinicians to manage the patient under the ERAS protocol.
Each recommendation had at least 1 resource associated with it. Some resources incorporated multiple recommendations and most recommendations were included in multiple resources. For example, separate checklists were created for the pre- and post-operative huddles. These incorporated all important and relevant information for the patient transfer as well as all elements from the briefing and de-briefing sections of the institutional safe surgery checklist as well as incorporating aspects of preventing hypothermia, antibiotic prophylaxis and perioperative analgesia recommendations (Fig. 3). All other resources created are shown in (Table S3).
Fig. 3.
Alberta Children’s Hospital neonatal Enhanced Recovery After Surgery pre-operative huddle checklist tool ERAS- enhanced recovery after surgery; NICU- neonatal intensive care unit; OR- operating room; RN- registered nurse; RT- respiratory therapist; PMHx- past medical history; DI- diagnostic imaging; ETT- endotracheal tube; HFO- high frequency oscillator; HFJV- high frequency jet ventilation; IV- intravenous; NPO- nil per os; SSI- surgical site infection.
Family engagement
There were 5 parent participants in the focus group. After reviewing the ERAS parent materials, feedback for improvements included information on pain management, available support for families and how parents can be more involved. These aspects were incorporated into the parent education booklet.
Follow-up phone calls were conducted 2 months after the focus group with 2 parent participants. The other 3 parent participants were ineligible as their infants were still in-patients Themes included the importance of communication and transmission of knowledge and the opportunity for hands-on practice of skills, both as simulation and actual performance, under direct expert observation.
Discussion
A rigorous process has been outlined to create a robust and diversely informed implementation strategy for the initiation of neonatal intestinal resection ERAS® guideline at this institution. This may serve as a model to develop future implementation strategies of ERAS® guidelines at this centre and other institutions.
Engagement of the entire neonatology team was particularly important to the success of the implementation plan. To date, ERAS® guidelines have mainly found success in the adult world and are only starting to gain traction in paediatric surgery1,33. Introduction of an ERAS programme presents a number of challenges for specialties generally unfamiliar with this approach. Paediatric subspecialty colleagues (such as nursing or pharmacy) had never had exposure to ERAS® guidelines, in contrast to surgeons and anaesthetists. Most existing ERAS® guidelines are developed with the expectation that patients are admitted on a surgical ward under the operating surgeon rather than an intensive care unit. A further component to the successful adoption of paediatric specific ERAS protocols is the partnership with parents1. A fundamental tenet of ERAS protocols is the commitment to providing care via a patient and family centered approach17,33. Involving parent stakeholders enabled the development of care pathways and educational materials geared specifically towards what mattered most for parents. These considerations highlight the importance of considering local contexts and factors and how they contribute to the overall success of an implementation strategy.
The main purpose of this paper was to provide the major elements and framework that seemed necessary to successfully implement a complex healthcare intervention (Table 2) and offer examples of the approach used in order to aid future teams looking to adopt ERAS® guidelines in complex clinical settings or novel environments.
Table 2.
Major elements necessary to successfully implement complex healthcare interventions
| Recommended elements |
|---|
| Identification of Champions |
| Multidisciplinary Stakeholder Involvement |
| Examination, Consideration and Tailoring of Local Contexts and Insights |
| Patient/Parental/Family Engagement |
| Education for all Providers |
| Audit and Evaluation System for Guideline and Implementation Strategy Revision |
ERAS® guidelines should be implemented as a whole, as individual elements work synergistically to further improve outcomes. It is accepted, nevertheless, that this may not always be feasible. Teams who have little experience with ERAS protocols may begin by identifying a single or a few recommendations that will most benefit their environment. An option, termed horizontal adoption, identifies a single recommendation that may be broadly adopted across all patient populations.
The implementation of complex healthcare interventions requires a structured and intensive strategy to ensure that the intervention is adopted and integrated successfully into existing infrastructure and daily workflow12,31,32. A multidisciplinary and systematic approach with careful planning, following known elements of implementation science, can assist teams in addressing all factors and aspects necessary to develop successful implementation strategies for their complex interventions12,15,31. The strategy used here for implementing a neonatal ERAS® guideline may be a useful model to guide implementation of similar complex healthcare interventions.
Funding
Brian and Brenda MacNeill Chair in Pediatric Surgery through Alberta Children's Hospital Foundation
Maternal Newborn Child and Youth Strategic Clinical Network Health Outcomes Improvement Fund
Acknowledgements
This work was funded by the Brian and Brenda MacNeill Chair in Pediatric Surgery through the Alberta Children's Hospital Foundation as well as the Maternal Newborn Child and Youth Strategic Clinical Network Health Outcomes Improvement Fund.
Disclosure. The authors declare no conflicts of interest.
Supplementary material
Supplementary material is available at BJS Open online.
Supplementary Material
References
- 1. Brindle ME, Heiss K, Scott MJ, Herndon CA, Ljunqgvist O, Koyle MA.. Embracing change: the era for pediatric ERAS is here. Pediatr Surg Int 2019;35:631–634. doi:10.1007/s00383-019-04476-3 [DOI] [PubMed] [Google Scholar]
- 2. Ljungqvist O. ERAS—enhanced recovery after surgery: moving evidence-based perioperative care to practice. JPEN J Parenter Enteral Nutr 2014;38:559–566. doi:10.1177/0148607114523451 [DOI] [PubMed] [Google Scholar]
- 3. Gramlich LM, Sheppard CE, Wasylak T, Gilmour LE, Lyungqvist O, Basualdo-Hammond C. et al. Implementation of enhanced recovery after surgery: a strategy to transform surgical care across a health system. Implement Sci 2017;12:67. doi:10.1186/s13012-017-0597-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Nelson G, Kiyang LN, Crumley ET, Chuck A, Nguyen T, Faris P. et al. Implementation of enhanced recovery after surgery (ERAS) across a provincial healthcare system: The ERAS Alberta colorectal surgery experience. World J Surg 2016;40:1092–1103. doi:10.1007/s00268-016-3472-7 [DOI] [PubMed] [Google Scholar]
- 5. Shinnick JK, Short HL, Heiss KF, Santore MT, Blakely ML, Raval MV.. Enhancing recovery in pediatric surgery: a review of the literature. J Surg Res 2016;202:165–176. doi:10.1016/j.jss.2015.12.051 [DOI] [PubMed] [Google Scholar]
- 6. Sibbern T, Bull Sellevold V, Steindal SA, Dale C, Watt-Watson J, Dihle A.. Patients’ experiences of enhanced recovery after surgery: a systematic review of qualitative studies. J Clin Nurs 2017;26:1172–1188. doi:10.1111/jocn.13456 [DOI] [PubMed] [Google Scholar]
- 7. Nelson G, Kiyang LN, Chuck A, Thanh NX, Gramlich LM.. Cost impact analysis of Enhanced Recovery After Surgery program implementation in Alberta colon cancer patients. Curr Oncol 2016;23:221. doi:10.3747/co.23.2980 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Ljungqvist O, Scott M, Fearon KC.. Enhanced recovery after surgery: a review. JAMA Surg 2017;152:292. doi:10.1001/jamasurg.2016.4952 [DOI] [PubMed] [Google Scholar]
- 9. Khan S, Wilson T, Ahmed J, Owais A, MacFie J.. Quality of life and patient satisfaction with enhanced recovery protocols: ERAS pathways and HQoL and patient satisfaction. Colorectal Dis 2010;12:1175–1182. doi:10.1111/j.1463-1318.2009.01997.x [DOI] [PubMed] [Google Scholar]
- 10. Varadhan KK, Neal KR, Dejong CHC, Fearon KCH, Ljungqvist O, Lobo DN.. The enhanced recovery after surgery (ERAS) pathway for patients undergoing major elective open colorectal surgery: a meta-analysis of randomized controlled trials. Clin Nutr 2010;29:434–440. doi:10.1016/j.clnu.2010.01.004 [DOI] [PubMed] [Google Scholar]
- 11. Coolsen MME, van Dam RM, van der Wilt AA, Slim K, Lassen K, Dejong CHC.. Systematic review and meta-analysis of enhanced recovery after pancreatic surgery with particular emphasis on pancreaticoduodenectomies. World J Surg 2013;37:1909–1918. doi:10.1007/s00268-013-2044-3 [DOI] [PubMed] [Google Scholar]
- 12. Ross J, Stevenson F, Dack C, Pal K, May C, Michie S et al. Developing an implementation strategy for a digital health intervention: an example in routine healthcare. BMC Health Serv Res 2018;18:794. doi:10.1186/s12913-018-3615-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Nilsen P. Making sense of implementation theories, models and frameworks. Implement Sci 2015;10:53. doi:10.1186/s13012-015-0242-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Tabak RG, Khoong EC, Chambers DA, Brownson RC.. Bridging research and practice: models for dissemination and implementation research. Am J Prev Med 2012;43:337–350. doi:10.1016/j.amepre.2012.05.024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Grol R, Wensing M, Eccles M, Davis D (eds). Improving Patient Care: The implementation of Change in Health Care, 2nd edn.Hoboken, NJ: John Wiley & Sons, Ltd, 2013 [Google Scholar]
- 16. Liu VX, Rosas E, Hwang J, Cain E, Foss-Durant A, Clopp M. et al. Enhanced recovery after surgery program implementation in 2 surgical populations in an integrated health care delivery system. JAMA Surg 2017;152:e171032. doi:10.1001/jamasurg.2017.1032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Short HL, Heiss KF, Burch K, Tavers C, Edney J, Venable C. et al. Implementation of an enhanced recovery protocol in pediatric colorectal surgery. J Pediatr Surg 2018;53:688–692. doi:10.1016/j.jpedsurg.2017.05.004 [DOI] [PubMed] [Google Scholar]
- 18. Phillips MR, Adamson WT, McLean SE, Hance L, Lupa MC, Pittenger SL. et al. Implementation of a pediatric enhanced recovery pathway decreases opioid utilization and shortens time to full feeding. J Pedtr Surg 2020;55:101–105. doi:10.1016/j.jpedsurg.2019.09.065 [DOI] [PubMed] [Google Scholar]
- 19. Raval MV, Heiss KF.. Development of an enhanced recovery protocol for children undergoing gastrointestinal surgery. Curr Opin Pediatr 2018;30:399–404. doi:10.1097/MOP. 0000000000000622 [DOI] [PubMed] [Google Scholar]
- 20. Kain ZN, Caldwell-Andrews AA, Mayes LC, Weinberg ME, Wang SM, MacLaren JE. et al. Family-centered preparation for surgery improves perioperative outcomes in children: a randomized controlled trial. Anesthesiology 2007;106:65–74. doi:10.1097/00000542-200701000-00013 [DOI] [PubMed] [Google Scholar]
- 21. Gabriel MG, Wakefield CE, Vetsch J, Karpelowsky JS, Darlington ASE, Grant DM. et al. The psychosocial experiences and needs of children undergoing surgery and their parents: a systematic review. J Pediatr Health Care 2018;32:133–149. doi:10.1016/j.pedhc.2017.08.003 [DOI] [PubMed] [Google Scholar]
- 22. Segal I, Kang C, Albersheim SG, Skarsgard ED, Lavoie PM.. Surgical site infections in infants admitted to the neonatal intensive care unit. J Pediatr Surg 2014;49:381–384. doi:10.1016/j.jpedsurg.2013.08.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Matlow AG, Baker GR, Flintoft V, Cochrane D, Coffey M, Cohen E. et al. Adverse events among children in Canadian hospitals: the Canadian Paediatric Adverse Events Study. Can Med Assoc J 2012;184:E709–E718. doi:10.1503/cmaj.112153 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Mamie C, Habre W, Delhumeau C, Barazzone Argiroffo C, Morabia A.. Incidence and risk factors of perioperative respiratory adverse events in children undergoing elective surgery. Pediatr Anesth 2004;14:218–224. doi:10.1111/j.1460-9592.2004.01169.x [DOI] [PubMed] [Google Scholar]
- 25. Gibb ACN, Crosby MA, McDiarmid C, Urban D, Lam JYK, Wales PW. et al. Creation of an Enhanced Recovery After Surgery (ERAS) Guideline for neonatal intestinal surgery patients: a knowledge synthesis and consensus generation approach and protocol study. BMJ Open 2018;8:e023651. doi:10.1136/bmjopen-2018-023651 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Brindle ME, McDiarmid C, Short K, Miller K, MacRobie A, Lam JYK. et al. Consensus guidelines for perioperative care in neonatal intestinal surgery: Enhanced Recovery After Surgery (ERAS®) Society recommendations. World J Surg 2020;44:2482–2492 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Ogrinc G, Davies L, Goodman D, Batalden P, Davidoff F, Stevens D.. SQUIRE 2.0 (Standards for QUality Improvement Reporting Excellence): revised publication guidelines from a detailed consensus process. BMJ Qual Saf 2016;25:986–992. doi:10.1136/bmjqs-2015-004411 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Graham ID, Tetroe J.. Some theoretical underpinnings of knowledge translation. Acad Emerg Med 2007;14:936–941. doi:10.1197/j.aem.2007.07.004 [DOI] [PubMed] [Google Scholar]
- 29. Field B, Booth A, Ilott I, Gerrish K.. Using the knowledge to action framework in practice: a citation analysis and systematic review. Implement Sci 2014;9:172. doi:10.1186/s13012-014-0172-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Graham ID, Tetroe JM.. Getting evidence into policy and practice: perspective of a health research funder. J Can Acad Child Adolesc Psychiatry 2009;18:46–50 [PMC free article] [PubMed] [Google Scholar]
- 31. May CR, Cummings A, Girling M, Bracher M, Mair FS, May CM. et al. Using normalization process theory in feasibility studies and process evaluations of complex healthcare interventions: a systematic review. Implement Sci 2018;13:80. doi:10.1186/s13012-018-0758-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. May C, Finch T.. Implementing, embedding, and integrating practices: an outline of normalization process theory. Sociology 2009;43:535–554. doi:10.1177/0038038509103208 [Google Scholar]
- 33. Rove KO, Brockel MA, Brindle ME, Scott MJ, Herndon CDA, Ljungqvist O. et al. Embracing change—the time for pediatric enhanced recovery after surgery is now. J Pediatr Urol 2019;15:491–493. doi:10.1016/j.jpurol.2019.04.005 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.