Abstract
Objective
Severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2) is the etiologic agent of the current, world-wide coronavirus disease 2019 (COVID-19) pandemic. Angiotensin-converting enzyme 2 (ACE2) is the SARS-CoV-2 host entry receptor for cellular inoculation and target organ injury. We reviewed ACE2 expression and the role of ACE2-angiotensin 1-7-Mas receptor axis activity in abdominal aortic aneurysm (AAA) pathogenesis to identify potential COVID-19 influences on AAA disease pathogenesis.
Methods
A comprehensive literature search was performed on PubMed, National Library of Medicine. Key words included COVID-19, SARS-CoV-2, AAA, ACE2, ACE or angiotensin II type 1 (AT1) receptor inhibitor, angiotensin 1-7, Mas receptor, age, gender, respiratory diseases, diabetes, and autoimmune diseases. Key publications on the epidemiology and pathogenesis of COVID-19 and AAAs were identified and reviewed.
Results
All vascular structural cells, including endothelial and smooth muscle cells, fibroblasts, and pericytes express ACE2. Cigarette smoking, diabetes, chronic obstructive pulmonary disease, lupus, certain types of malignancies, and viral infection promote ACE2 expression and activity, with the magnitude of response varying by sex and age. Genetic deficiency of AT1 receptor, or pharmacologic ACE or AT1 inhibition also increases ACE2 and its catalytic product angiotensin 1-7. Genetic ablation or pharmacologic inhibition of ACE2 or Mas receptor augments, whereas ACE2 activation or angiotensin 1-7 treatment attenuates, progression of experimental AAAs. The potential influences of SARS-CoV-2 on AAA pathogenesis include augmented ACE-angiotensin II-AT1 receptor activity resulting from decreased reciprocal ACE2-angiotensin 1-7-Mas activation; increased production of proaneurysmal mediators stimulated by viral spike proteins in ACE2-negative myeloid cells or by ACE2-expressing vascular structural cells; augmented local or systemic cross-talk between viral targeted nonvascular, nonleukocytic ACE2-expressing cells via ligand recognition of their cognate leukocyte receptors; and hypoxemia and increased systemic inflammatory tone experienced during severe COVID-19 illness.
Conclusions
COVID-19 may theoretically influence AAA disease through multiple SARS-CoV-2-induced mechanisms. Further investigation and clinical follow-up will be necessary to determine whether and to what extent the COVID-19 pandemic will influence the prevalence, progression, and lethality of AAA disease in the coming decade.
Keywords: Angiotensin-converting enzyme 2, Severe acute respiratory syndrome (SARS), Coronavirus disease 2019 (COVID-19), Abdominal aortic aneurysm (AAA)
Abdominal aortic aneurysm (AAA) is a common and lethal disease of mature adults worldwide. The prevalence and progression of the disease is influenced by the age, sex, race, tobacco consumption, exercise habits, comorbidities, and medication regimens of affected individuals.1, 2, 3, 4, 5 Although the implementation of targeted screening programs for at-risk individuals and decreased surgical risk associated with endovascular repair has substantially reduced aneurysm-specific mortality,6 nearly 10,000 Americans died owing to rupture or other AAA-related complications in 2017 (https:/www.cdc.org).
Multiple pathogenic mechanisms promote AAA disease initiation and progression, including the hemodynamic consequences of sedentary existence or asymmetric iliac arterial flow; imbalances between proteinase and antiproteinase activity; renin-angiotensin (Ang) system (RAS) activation; accelerated innate and adaptive effector immunity; impaired immunoregulatory and tissue reparative mechanisms; increased oxidative stress; disturbed fibrinolytic pathways; and destabilized extracellular matrix architecture (Table I ).68, 69, 70, 71
Table I.
Evidence summarizing potential mechanistic links between severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2) and abdominal aortic aneurysms (AAAs)
| Evidence category | Evidence | References |
|---|---|---|
| Common risk factors | Male sex, advanced age, cigarette smoking Chronic obstructive pulmonary disease and obesity |
7, 8, 9, 10, 11, 12, 13, 14, 15 |
| Effects on/consequences of RAS dysregulation. | ACE-Ang II-AT1 receptor activity promotes, whereas ACE2-Ang 1-7-Mas receptor activity inhibits, experimental AAAs Reduced clinical AAA expansion associated with ARB use ACE2 expression is reduced in aneurysmal aorta. Apelin and resveratrol, protective against experimental AAA, increases ACE2 expression. Circulating ACE2 inversely associated with AAA risk and operative mortality following repair. Cell surface ACE2 internalization and shedding following SARS-CoV-2 entry impairs Ang II degradation and Ang 1-7 formation. Increased Ang II and reduced Ang 1-7 serum levels in patients with COVID-19. |
1,16, 17, 18, 19, 20, 21, 22, 23, 24, 25, 26, 27, 28, 29, 30, 31, 32, 33, 34, 35, 36, 37, 38, 39, 40, 41, 42, 43, 44 |
| Influences on/effects of inflammatory mediator expression | SARS-CoV-2-infected cells (respiratory epithelial cells, vascular endothelial cells, smooth muscle cells and fibroblasts) secrete chemokines and cytokines such as CCL2, CXCL12, MIF, IL-1β, TNF-α, IL-6, IL-8, type 1 interferons Recognition of chemokines and cytokines by their myeloid cell receptors mediates myeloid cell migration and inflammatory activity |
45, 46, 47, 48 |
| Biologic response to viral spike protein exposure. | Spike protein exposure increases IL-1β, IL-6, IL-8, IL-12, TNF-α, MHC II, and costimulatory molecule (CD80 and CD86) expression by ACE2-neagtive macrophages and dendritic cells, augmenting their inflammatory and T cell-stimulatory activity | 49, 50, 51, 52, 53, 54, 55 |
| SARS-CoV-2 RNA recognized by TLR7 and TLR8 | TLR signaling triggers proinflammatory type 1 interferon production by infected ACE2-expressing cells and impact leukocyte activity by interacting with their leukocyte type 1 receptor Directly or indirectly influence interferon regulatory factor expression on T cells and macrophages promoting the differentiation or activation of proinflammatory Th1 cells, Th17 cells and M1 macrophages |
49,56 |
| Viral pulmonary injury | Pulmonary injury creates hypoxemia stabilizing and increasing proaneurysmal HIF-1 levels Increases LPS owing to secondary pulmonary bacterial infection |
13,56, 57, 58, 59, 60, 61, 62, 63, 46 |
| Other | Intracranial and coronary arterial aneurysms have been reported in adults and children, respectively, with COVID-19 | 64, 65, 66, 67 |
ACE, Angiotensin-converting enzyme; Ang, angiotensin; COVID-19, coronavirus disease-2019; HIF, hypoxia-inducible factor; LPS, lipopolysaccharide; MHC, major histocompatibility complex; RAS, renin-angiotensin system; Th, T helper cell; TLR, Toll-like receptor; TNF, tumor necrosis factor.
Of these, substantial experimental evidence supporting RAS modulation of AAA risk and progression has been developed in murine modeling systems incorporating genetic deficiencies of angiotensin-converting enzyme (ACE) or Ang II type 1 (AT1) receptors, or wild-type mice treated with either ACE inhibitor (ACEi) or AT1 receptor blockers (ARB).16, 17, 18, 19, 20, 21, 22, 23 Retrospective clinical evidence has linked the presence of ARB therapy with decreased enlargement rate of small AAAs,1 , 24 although recently completed, prospective, randomized clinical trials failed to confirm their efficacy.72 , 73 Despite lingering uncertainty as to the effect size and specific mechanism(s) of action, the preponderance of clinical and experimental evidence clearly links RAS activity with AAA pathogenesis.
The ACE2 receptor serves three major recognized biological functions.74 As a surface carboxypeptidase enzymatic agent, ACE2 converts Ang I and Ang II to Ang 1-9 and Ang 1-7, respectively. Ang 1-7 binds to its surface receptor Mas on target cells, initiating anti-inflammatory and antiproliferative responses in target cells, decreasing oxidative stress and vasoconstriction, and generally offsetting reciprocal responses engendered via AT-1 receptor activation by Ang II. In this regard ACE2 activity is felt to be critical for maintaining cardiovascular health.75
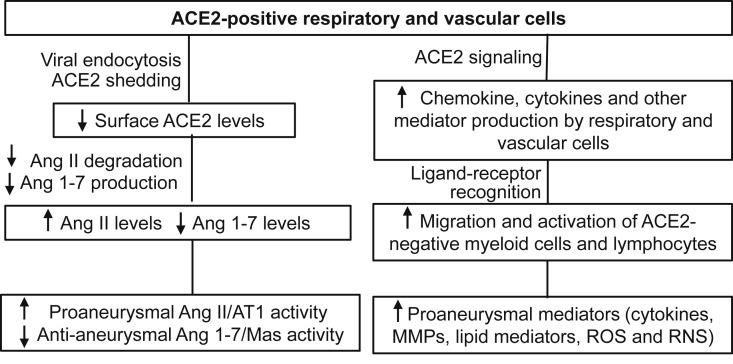
In conjunction with the amino acid transporter SLC6A19, ACE2 activity is required for amino acid uptake in the renal and intestinal epithelia.76 , 77 Coronaviruses, including severe acute respiratory syndrome coronavirus (SARS-CoV), severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), and human coronavirus NL63 (HCoV-NL63), also use ACE2 as their entry portal for infecting host cells.78, 79, 80, 81, 82 Subsequent endocytosis of viruses through, and proteinase-mediated shedding of, surface ACE2 have been shown to downregulate surface ACE2 (Table I; Fig 1 ). Although the involvement of ACE2 in promoting COVID19-related cardiovascular complications is the focus of intense investigation currently,75 , 83, 84, 85 the potential impacts on AAA pathogenesis and progression have yet to undergo comprehensive review.
Fig 1.
Angiotensin-converting enzyme 2 (ACE-2)-dependent potential influences of COVID-19 on abdominal aortic aneurysm (AAA) disease. AT1, Angiotensin II type receptor; EC, endothelial cells; HIF, hypoxia inducible factor; Mas, receptor for Ang 1-7; MMPs, matrix metalloproteinases; RNS, reactive nitrogen species; ROS, reactive oxygen species; SMCs, smooth muscle cells
With this monograph, we review the influence of COVID-19-and AAA-shared risk factors on ACE2 expression, the significance of the ACE2-Ang 1-7-Mas axis in AAA pathogenesis, and the theoretical potential for SARS-CoV-2/COVID-19 to influence AAA disease prevalence and progression in the years after the COVID-19 pandemic.
ACE2 expression and activity in health and disease
Tissue expression patterns and cellular sources
ACE2 expression was originally recognized in heart, kidney, and testis tissues in the setting of advanced congestive heart failure.86 Subsequent studies identified constitutive ACE2 expression in healthy human lung, intestine, blood vessels, nasal and oral mucosa, nasopharynx, and adipose tissues, with low or rare expression in immune organs such as bone marrow, spleen, and lymph nodes.87 , 88 By cell type, expression is present in cardiomyocytes and endothelial and smooth muscle cells, as well as in intestinal, renal, respiratory, and testicular epithelial cells.87 With the identification of ACE2 as the entry receptor for SARS and SARS-CoV-2,26, 27, 28, 29, 30 studies using bulk tissue and single cell transcriptomic analysis have extended known tissue and cellular expression patterns to include pericytes, fibroblasts, and certain immune cells.45 , 89, 90, 91, 92, 93, 94, 95
Age and sex
Advanced age and male sex are nonmodifiable risk factors for COVID-19 infection and AAA disease (Table I). Although differences exist, it remains uncertain the degree to which age or sex differences influence ACE2 expression, and the degree to which observed changes, when present, are tissue or organ specific. Increased ACE2 mRNA expression is present in adult nasal mucosae compared with that of children.96 ACE2 protein levels, but not ACE2 mRNA expression, were noted to decrease in rodent lung and thoracic aorta with increasing age in both genders but more rapidly in males,7 , 89 , 97 , 98 Additionally, increased ACE2 expression and activity were present in renal but not lung or heart tissue of male rats.8 , 89 No similar data exist regarding vascular cell ACE2 expression as a function of age or sex.
Cigarette smoking
Cigarette smoking increases ACE2 mRNA expression in the respiratory system, more significantly in current as opposed to former smokers7 , 9, 10, 11, 12 and increasing with smoking intensity (packs per year).7 Expression is similarly increased in mice exposed to cigarette smoke.7 In addition to increased ACE2 expression per cell, smoking also stimulates respiratory secretory cell proliferation, both accounting for most of the increased ACE2 expression in smokers.7
Increased ACE2 expression and activity may serve to counteract pulmonary inflammation in response to cigarette smoke. In this scenario, ACE2 either converts Ang I to Ang 1-10 or degrades Ang II formed by ACE to Ang 1-7. At the same time, however, smoking-induced ACE2 upregulation also accelerates the entry of SARS-CoV-2 into host respiratory cells, exacerbating existing pulmonary pathologies and impairing gas exchange, even in convalescent patients with COVID-19.13 Cigarette smoking has long been recognized as the single most important modifiable risk factor for the prevalence and progression of AAA disease. Smoking-related respiratory diseases such as chronic obstructive pulmonary disease and asthma also promote AAA disease risk14 , 15 , 99; thus, the potential influence of smoking on COVID-19 susceptibility and related pulmonary pathologies may further enhance AAA disease risk.
Comorbidities
Some preexisting conditions relevant for COVID-19 and AAA disease risk also influence ACE2 expression.3 , 100 , 101 For instance, chronic obstructive pulmonary disease, lupus, inflammatory bowel disease, and certain malignancies are associated with augmented ACE2 expression (Table I).7 , 102, 103, 104, 105 Diabetes has also been associated with increased ACE2 expression in the proximal tubular epithelial cells in diabetes-induced chronic kidney disease as well as aortic endothelial cells.106, 107, 108 This latter finding potentially provides additional insight into the known inhibitory influence of diabetes on AAA disease while simultaneously explaining increased morbidity and mortality in COVID-19 illness. ACE2 expression has also been negatively associated with the degree of type 2 immunity or IgE levels in patients with allergic asthma, an association also potentially relevant to a decreased AAA disease risk in diabetics.109, 110, 111
Medications
Because ACE2 activation counteracts ACE activity in many pathophysiologic processes, the influence of ACEis or ARBs on ACE2, and its catalytic product Ang 1-7, have been extensively investigated in rodent models. A large body of experimental evidence, summarized recently by Kreutz and colleagues,112 suggests that ACE-Ang II-AT1 axis inhibition augments the expression and/or activity of ACE2 and Ang 1-7. For example, either genetic deficiency or pharmacologic inhibition of AT1 reversed the downregulation of arterial ACE2 mRNA and protein levels in mice following surgical manipulation.113 ACEi or ARB administration produced increased rat myocardial ACE2 mRNA levels, with or without surgical myocardial infarction, in conjunction with elevated Ang 1-7.114 , 115 In the mouse model of high fat diet-induced metabolic syndrome, treatment with the ARB telmisartan effectively reversed the downregulation of ACE2 in adipose tissue.116 Additionally, well-controlled hyperglycemia was associated with less mortality in patients with COVID-19 and type II diabetes.117 Metformin, the world's most commonly prescribed oral hypoglycemic agent for type II diabetes, may promote conformational changes in ACE2 via AMPK-mediated phosphorylation without influencing ACE2 expression.118
Clinical data on the influence of ACEi and ARBs on ACE2 expression and activity are somewhat more limited. In patients with either chronic congestive heart failure or atrial fibrillation, ACEi treatment increased circulating Ang 1-7 levels and ACE2 mRNA expression, respectively.119 , 120 Neither ACEi nor ARB treatment increased plasma ACE2 levels in patients with congestive heart failure, however.120 , 121 Similarly, no upregulation of ACE2 mRNA expression was noted in the lungs of patients treated with either ACEis or ARBs.122 Despite great interest regarding the influence of ARBs or ACEis on clinical outcomes in COVID-19, no data exist regarding plasma levels of ACE2 or Ang 1-7 in affected patients with or without either medication.101 , 123, 124, 125, 126, 127, 128
Other influences
IL-13, apelin, Ang II, hypoxia, resveratrol, and type 1 interferons have all been reported to upregulate ACE2 expression and/or activity.7 , 25, 26, 27 , 43 Conversely, IFN-γ, IL-4, and estrogen are all associated with downregulated ACE2 expression.28 Infection with pathogenic viruses, such as seasonal influenza, respiratory syncytial virus, SARS-CoV, and Middle East respiratory syndrome, also upregulate ACE2 mRNA expression in airway cells.7
Significance of the ACE2-ANG 1-7-Mas axis in AAA pathogenesis
ACE2 in AAA disease
Transmural aortic ACE2 expression, although present in human AAAs,29 is diminished compared with that present in control aorta obtained at the time of organ donation (Table I).25 Serum ACE2 activity is negatively associated with AAA diagnosis, and circulating ACE2 is an independent risk factor for postoperative mortality after open surgical repair of ruptured AAA (Table I).25 , 30 ACE2 mRNA expression is increased in human and mouse vascular smooth muscle cells after exposure to Ang II.43
Influence on exogenous Ang II-dependent experimental AAAs
In functional studies of Ang II-induced experimental AAAs (aneurysmal degeneration preceded and precipitated by focal, segmental aortic dissection), genetic deficiency of ACE2 accelerated, whereas pharmacologic activation of or adenovirus-mediated overexpression of ACE2 inhibited, AAA formation in hyperlipidemic mice (Table I and Fig 1).25 , 29 , 31 ACE2 expression was profoundly localized to the adventitia of aneurysmal, as compared with nonaneurysmal aortic segments.25 Furthermore, treatment with the Mas receptor ligand Ang 1-7 or its analogue ameliorated, whereas genetic deficiency or pharmacologic inhibition of the Mas receptor augmented, AAAs in this same modeling system (Table I; Fig 1).32 , 33 , 129 Apelin and resveratrol, agents known to be effective in inhibiting experimental AAA formation and progression in multiple modeling systems, mediate aneurysm suppression in part by increasing ACE2 expression (Table I),25 , 43 , 130 , 131 additionally underscoring the potential antianeurysmal effects of ACE2 activity in experimental modeling systems.
Influence on experimental AAAs in alternative modeling systems
Published results are inconsistent regarding the importance of ACE2 in the pathogenesis of experimental AAAs not initiated with exogenous Ang II supplementation (nondissection-related AAAs). One study unexpectedly reported no apparent influence of ACE2 deficiency on AAA formation in the porcine pancreatic elastase intra-aortic infusion model.29 This model demonstrates greater pathologic fidelity to the human condition than aneurysms that develop after focal aortic dissection in the Ang II infusion models. In a third modeling system, one dependent on abluminal application of calcium chloride to initiate aneurysm formation, ACE2 deficiency was again found to augment experimental AAA formation, a similar response noted to that demonstrated in the Ang II models (Table I; Fig 1).25
In the report describing the absence of AAA augmentation in ACE2-deficient mice after porcine pancreatic elastase infusion, the magnitude of aneurysmal enlargement varied substantially in ACE2-deficient mice; thus, the relatively small numbers of mice in each group may have increased the risk for a type II error. In our own studies, conducted in an elastase fusion model,132 pretreatment with Ang 1-7 suppressed, whereas pretreatment with a Mas receptor antagonist promoted, the formation and progression of AAA-related pathologic changes (Table I; Fig 1). The initiation of Ang 1-7 supplementation in mice after AAA initiation halted further enlargement, suggesting a therapeutic application for Ang 1-7 or its analogues for clinical disease. Additionally, treatment with a Mas receptor antagonist promoted further AAA expansion and “rescuing” the AAA phenotype in mice treated with the AT1 blocker telmisartan, implying that the demonstrated ability of telmisartan to suppress experimental AAA progression may be dependent on Ang 1-7/Mas activity.16 , 17
Altogether, although inconsistencies exist, the balance of experimental evidence suggests that the ACE2-Ang 1-7-Mas axis exerts a regulatory role in AAA pathogenesis.
Potential impact of COVID-19 on clinical AAA disease
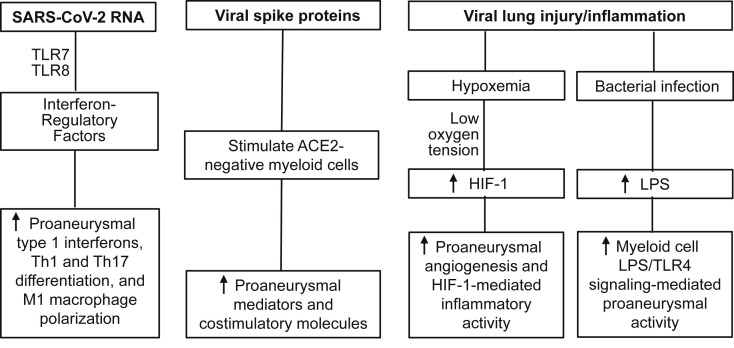
The natural history of AAA disease is progressive aortic diameter enlargement over the course of months to years to the point of symptomatic evolution or rupture. Although intracranial and coronary arterial aneurysms have developed in adults and children with COVID-19, respectively (Table I),64, 65, 66, 67 , 133, 134, 135 no similar relationship is yet recognized between SARS-CoV-2, COVID-19, and AAA disease. However, several COVID-19-related mechanisms, either ACE2 dependent or independent, could impact AAA disease risk and progression currently and in the years to come (Table I; Figs 1 and 2 ).
Fig 2.
angiotensin-converting enzyme 2 (ACE-2)-independent potential influence of COVID-19 on clinical adbominal aortic aneurysm (AAA) disease. HIF, Hypoxia inducible factor; LPS, lipopolysaccharide; M1, classically activated; Th, helper T cells; TLR, Toll-like receptor.
Influence on ACE2-Ang 1-7-Mas axis expression and activity
As noted elsewhere in this article, coronaviruses, including SARS, SARS-CoV-2, and HCoV-NL63, infect host cells via the binding of viral spike proteins to constitutively expressed ACE2 receptors. Viral spike protein endocytosis results in downregulation of cell surface ACE2 in infected cells (Table I; Fig 1).34 , 35 Cleavage of the ACE2 ectodomain by ADAM17/TACE releases soluble extracellular ACE2 (Table I; Fig 1).36 , 44 Both processes together decrease cell surface expression of ACE2, critical for optimal catalytic activity (although soluble ACE2 also remains active).
During the SARS pandemic, SARS-CoV infection was associated with decreased cardiac ACE2 protein levels in patients who succumbed to the disease.37 The administration of human SARS-CoV, or its surface spike protein, attenuates mRNA and protein levels of ACE2 in mouse lung and heart tissue, with an increase in Ang II levels in the lung.37 , 38 Exposure of human lung and kidney epithelial cells to SARS-CoV or its spike proteins also remarkably decrease ACE2 mRNA and protein expression.38 , 39 The SARS-CoV spike protein alone was sufficient for increasing pulmonary Ang II levels and promoting lung injury, although this effect could be blocked by ARB treatment.38
In patients with COVID-19, plasma Ang II is elevated, whereas Ang 1-7 was decreased, compared with healthy controls (Table I; Fig 1).40, 41, 42 Thus, it is reasonable to speculate that SARS-CoV-2 also downregulates systemic ACE2, potentially impairing Ang II degradation and Ang 1-7 formation. ACE2 is highly expressed in the lung, where epithelial ACE activity is the predominant source for systemic Ang II. Attenuation of ACE2 by SARS-CoV-2 increases systemic Ang II levels by reducing conversion of Ang II to Ang 1-7 or Ang 1 to Ang 1-9.
Alternatively, all vascular endothelial cells, smooth muscle cells, pericytes, fibroblasts, and certain immune cells also express ACE2. Downregulation of ACE2 by SARS-CoV-2 on these cells will increase vascular Ang II and AT1 activity. Shifting the Ang II/Ang 1-7 balance as a consequence of SARS-CoV-2 infection may increase AAA risk or accelerate the progression of early disease. To better assess this risk, Ang II or Ang 1-7 production should be investigated in a large cohort of active and convalescent patients with COVID-19.
Inflammatory mediator expression by ACE2-positive nonimmune cells
The binding of viral spike protein to ACE2 initiates the intracellular signaling cascade promoting inflammatory mediator production. SARS-CoV proteins (including spike proteins) augmented proaneurysmal chemokine CCL2 expression in human type II pneumocytes.136 In various tissues of patients with SARS-CoV-2, the expression of multiple mediators thought to promote (CCL2, IL-1β, IL-6, tumor necrosis factor [TNF]-α) and inhibit (transforming growth factor-β1) AAA disease progression were enhanced in ACE2-positive cells.137 In a recent single cell RNA analysis, CCL2 and CXCL12 were highly enriched in ACE2-positive type II alveolar epithelial cells and arterial vascular cells from healthy and diseased heart and lung as compared with ACE2-negative cells.90 Because essentially all constitutive vascular cells express ACE2,137 COVID-19 may promote AAA pathogenesis by increasing aortic recruitment of inflammatory leukocytes and/or enhancing aortic wall inflammation (Table I; Fig 1).
Inflammatory mediator expression by ACE2-negative immune cells
Immune cells, particularly macrophages and dendritic cells, modulate immune-mediated aneurysmal degradation despite a lack of surface ACE2 receptor expression. The SARS-CoV spike proteins induced IL-1β, IL-6, IL-8, and TNF-α mRNA and protein expression in human peripheral mononuclear cells via the nuclear factor-κB pathway without additional stimulus.50 Similarly, increased IL-6, IL-8, and TNF-α mRNA and protein expression were reported in mouse macrophages in the presence of the SARS-CoV spike protein.51 In the absence of intracellular replication, SARS-CoV upregulated the expression levels of costimulatory molecules (CD40 and CD86) and major histocompatibility complex II on the surface of macrophages and dendritic cells, augmented spontaneous and lipopolysaccharide-induced secretion of IL-6 and IL-12 by macrophages and dendritic cells, and enhanced the ability of dendritic cells to stimulate naive helper T cells to proliferate in vitro.52 Inflammasome NLRP3, critical for experimental AAAs, was activated and correlated with disease severity in COVID-19 patients.49 , 53, 54, 55 These findings suggest that SARS-CoV-2 infection may also enhance AAA pathogenesis by altering the production of proaneurysmal mediators by ACE2-negative myeloid cells present in aneurysmal tissue (Table I; Fig 2).
Cross-talk between ACE2-positive and -negative cells
ACE2-positive lung and vascular cells may promote AAA pathogenesis by cross-talk with ACE2-negative immune cells via ligand-receptor interaction (Table I; Fig 1). ACE2-positive cells produce ligands recognized by cognate receptors on immune cells across various tissues.45 The production of IL-6 and IL-8 by SARS-CoV-infected lung epithelial cells was effective in promoting the production of IL-1β, IL-6, IL-8, G-CSF, MIP-1α, MIP-1β, and TNF-α by macrophages, and IL-6, IL-8, and CCL2 by dendritic cells.47 Additional ligand-receptor pairs include macrophage migration inhibition factor, VEGF-A, GALS9, GRN, and SCGB31A on ACE2-positive cells and corresponding receptors CD74, NRP1/2, CD44, TNFSRF1B, and MARCO on macrophages, which enable SARS-CoV-2-targeted host cells to alter macrophage activity remotely or locally by secreting ligands. In bronchoalveolar lavage cells from patients with COVID-19, several proaneurysmal mediators, including CCL2, CCL7, HIF-1α, and type 1 interferons, were upregulated in myeloid cells, including macrophages and neutrophils, correlating with disease severity.48
ACE2 expression levels have been shown to correlate with type 1 interferon gene expression, which mediates AAA pathogenesis via its receptor activity and interferon regulatory factors (Table 1; Figs 1 and 2).57 , 89 , 138 , 139 Additionally, dysregulated lipid and amino acid metabolism, acute-phase amyloid A components, kynurenine pathway mediators, and complement system activation in patients with COVID-19 may also promote AAA disease risk,56 as well as attenuation of antianeurysmal mediators including HIF-1α-degrading enzyme inhibitors succinate and fumarate, serotonin-derived ant-aneurysmal melatonin and sphingosine-1-phosphate.58, 59, 60, 61, 62, 63 , 140
Additional influences
Enhanced IL-6, IL-8, IFN-γ, and CCL-2 expression in patients with COVID-19 may promote aneurysm pathogenesis by promoting aortic accumulation and activation of macrophages and neutrophils (Table I; Figs 1 and 2).46 Serum levels of lipopolysaccharide, the ligand for Toll-like receptor 1 and important for AAA pathogenesis, were also elevated in patients with severe COVID-19 or hospitalized at intensive care units as compared with healthy controls (Table I; Fig 2).141 , 142 Hypoxemia, a common consequence of COVID-19 pulmonary involvement, may reduce aortic mural oxygen tension (Table I; Fig 2).13 , 100 , 143, 144, 145, 146, 147, 148, 149 To the extent to which hypoxia reduces aortic mural HIF-1α ubiquination, the balance between ACE-Ang II and ACE2-Ang 1-7 may be tipped toward enhanced inflammation.150 Given the importance of HIF-1 in AAA pathogenesis,151, 152, 153, 154, 155 SARS-CoV-2 may influence AAA progression through its effects on the HIF-1 pathway.143 , 156
To combat the COVID-19 pandemic, several clinical trials for SARS-CoV-2 vaccines have completed or are ongoing worldwide.157 , 158 Immunity is achieved by injection of inactivated SARS-CoV-2, nonreplicating adenovirus expressing SARS-CoV-2 proteins (particularly spike protein 1), or stabilized spike protein 1 mRNA. Although potentially protective against SARS-CoV-2 infection, it remains to be seen whether vaccination per se, or the resultant humoral and cellular immunity after vaccination, may increase AAA disease risk via immunologic bystander effects.
Conclusions
Current concepts of SARS-CoV-2 infection and aneurysm pathogenicity suggest that COVID-19 may theoretically augment AAA disease progression. Table II prioritizes research questions on, and corresponding approaches for, the potential influence of COVID-19 on AAA disease. Time will tell whether this is indeed the case, and if so, how screening or surveillance protocols or intervention thresholds should be modified in AAA patients who have recovered from COVID-19.
Table II.
Prioritized research on the impact of coronavirus disease-2019 (COVID-19) on abdominal aortic aneurysm (AAA) disease
| Research question | Approaches |
|---|---|
| Does COVID-19 alter systemic levels of angiotensin II and Ang1-7? | Participants: COVID-19 convalescent patients with or without AAAs 3, 6, and 12 months after hospital discharge as well as matched non-COVID-19 controls with or without AAAs Approaches: Analyze plasma/serum; Ang II and Ang 1-7 via ELISA assays |
| Are systemic levels of selected proaneurysmal mediators modified following COVID-19? | Participants: COVID-19 convalescent patients with or without AAAs 3, 6, and 12 months after hospital discharge as well as matched non-COVID-19 controls with or without AAAs Approaches: Proteomic or specific protein arrays to determine plasma/serum key proaneurysmal mediators such as IL-1β, IL-6, IL-17, TNF-α, CCL2, CCL5, MMP2, MMP9 and VEGF-A |
| Is AAA enlargement rate, risk for rupture, or surgical repair at any given baseline aortic diameter increased following COVID-19? | Participants: All AAA patients (regardless of COVID-19), or convalescent COVID-19 AAA patients and non-COVID-19 AAA patients. Approaches: Compare AAA enlargement rate, rupture, or the need for surgical repair (primary end points) using retrospective case-control study, perform multi-variable analysis to determine whether COVID-19 is an independent factor for the development of a primary end point. |
| Is aneurysm prevalence increased following COVID-19? | Data source: Hospital electronic health record or Medicare database Compare AAA prevalence before, during and after the COVID-19 pandemic period |
| Does vaccination against SARS-CoV-2 modulate enlargement rate, risk for rupture or surgical repair of clinical AAAs? | Data source: Hospital electronic health record or Medicare database Approaches: Compare enlargement rate, rupture, or the need for AAA surgical repair in COVID-19 convalescent patients with non-COVID-19 convalescent patients or those who have received a vaccination |
Ang, angiotensin; SARS-CoV-2, severe acute respiratory syndrome coronavirus-2; TNF, tumor necrosis factor; VEGF, vascular endothelial growth factor.
Author contributions
Conception and design: BX, KK, RD
Analysis and interpretation: BX, KK, RD
Data collection: BX, GL, JG, TI, SZ
Writing the article: BX, RD
Critical revision of the article: BX, GL, JG, TI, KK, SZ, RD
Final approval of the article: BX, GL, JG, TI, KK, SZ, RD
Statistical analysis: Not applicable
Obtained funding: Not applicable
Overall responsibility: BX
Footnotes
Author conflict of interest: none.
The editors and reviewers of this article have no relevant financial relationships to disclose per the JVS policy that requires reviewers to decline review of any manuscript for which they may have a conflict of interest.
References
- 1.Itoga N.K., Rothenberg K.A., Suarez P., Ho T.V., Mell M.W., Xu B., et al. Metformin prescription status and abdominal aortic aneurysm disease progression in the U.S. veteran population. J Vasc Surg. 2019;69 doi: 10.1016/j.jvs.2018.06.194. 710-6.e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Fujimura N., Xiong J., Kettler E.B., Xuan H., Glover K.J., Mell M.W., et al. Metformin treatment status and abdominal aortic aneurysm disease progression. J Vasc Surg. 2016;64:46–54.e8. doi: 10.1016/j.jvs.2016.02.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kent K.C., Zwolak R.M., Egorova N.N., Riles T.S., Manganaro A., Moskowitz A.J., et al. Analysis of risk factors for abdominal aortic aneurysm in a cohort of more than 3 million individuals. J Vasc Surg. 2010;52:539–548. doi: 10.1016/j.jvs.2010.05.090. [DOI] [PubMed] [Google Scholar]
- 4.Bhak R.H., Wininger M., Johnson G.R., Lederle F.A., Messina L.M., Ballard D.J., et al. Factors associated with small abdominal aortic aneurysm expansion rate. JAMA Surg. 2015;150:44–50. doi: 10.1001/jamasurg.2014.2025. [DOI] [PubMed] [Google Scholar]
- 5.Thompson A., Cooper J.A., Fabricius M., Humphries S.E., Ashton H.A., Hafez H. An analysis of drug modulation of abdominal aortic aneurysm growth through 25 years of surveillance. J Vasc Surg. 2010;52:55–61.e2. doi: 10.1016/j.jvs.2010.02.012. [DOI] [PubMed] [Google Scholar]
- 6.Owens D.K., Davidson K.W., Krist A.H., Barry M.J., Cabana M., Caughey A.B., et al. Screening for abdominal aortic aneurysm: US Preventive Services Task Force recommendation statement. JAMA. 2019;322:2211–2218. doi: 10.1001/jama.2019.18928. [DOI] [PubMed] [Google Scholar]
- 7.Smith J.C., Sausville E.L., Girish V., Yuan M.L., Vasudevan A., John K.M., et al. Cigarette smoke exposure and inflammatory signaling increase the expression of the SARS-CoV-2 receptor ACE2 in the respiratory tract. Dev Cell. 2020;53 doi: 10.1016/j.devcel.2020.05.012. 514-29.e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Liu J., Ji H., Zheng W., Wu X., Zhu J.J., Arnold A.P., et al. Sex differences in renal angiotensin converting enzyme 2 (ACE2) activity are 17β-oestradiol-dependent and sex chromosome-independent. Biol Sex Differ. 2010;1:6. doi: 10.1186/2042-6410-1-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Brake S.J., Barnsley K., Lu W., McAlinden K.D., Eapen M.S., Sohal S.S. Smoking upregulates angiotensin-converting enzyme-2 receptor: a potential adhesion site for novel coronavirus SARS-CoV-2 (Covid-19) J Clin Med. 2020;9:841. doi: 10.3390/jcm9030841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Leung J.M., Yang C.X., Tam A., Shaipanich T., Hackett T.L., Singhera G.K., et al. ACE-2 expression in the small airway epithelia of smokers and COPD patients: implications for COVID-19. Eur Respir J. 2020;55:2000688. doi: 10.1183/13993003.00688-2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cai G., Bossé Y., Xiao F., Kheradmand F., Amos C.I. Tobacco smoking increases the lung gene expression of ACE2, the receptor of SARS-CoV-2. Am J Respir Crit Care Med. 2020;201:1557–1559. doi: 10.1164/rccm.202003-0693LE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zhang H., Rostami M.R., Leopold P.L., Mezey J.G., O'Beirne S.L., Strulovici-Barel Y., et al. Expression of the SARS-CoV-2 ACE2 receptor in the human airway epithelium. Am J Respir Crit Care Med. 2020;202:219–229. doi: 10.1164/rccm.202003-0541OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Li H., Zhao X., Wang Y., Lou X., Chen S., Deng H., et al. Damaged lung gas-exchange function of discharged COVID-19 patients detected by hyperpolarized (129)Xe MRI. Sci Adv. 2021;7:eabc8180. doi: 10.1126/sciadv.abc8180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lindholt J.S., Heickendorff L., Antonsen S., Fasting H., Henneberg E.W. Natural history of abdominal aortic aneurysm with and without coexisting chronic obstructive pulmonary disease. J Vasc Surg. 1998;28:226–233. doi: 10.1016/s0741-5214(98)70158-2. [DOI] [PubMed] [Google Scholar]
- 15.Takagi H., Umemoto T. A meta-analysis of the association of chronic obstructive pulmonary disease with abdominal aortic aneurysm presence. Ann Vasc Surg. 2016;34:84–94. doi: 10.1016/j.avsg.2015.12.023. [DOI] [PubMed] [Google Scholar]
- 16.Iida Y., Xu B., Schultz G.M., Chow V., White J.J., Sulaimon S., et al. Efficacy and mechanism of angiotensin II receptor blocker treatment in experimental abdominal aortic aneurysms. PLoS One. 2012;7:e49642. doi: 10.1371/journal.pone.0049642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Xuan H., Xu B., Wang W., Tanaka H., Fujimura N., Miyata M., et al. Inhibition or deletion of angiotensin II type 1 receptor suppresses elastase-induced experimental abdominal aortic aneurysms. J Vasc Surg. 2018;67 doi: 10.1016/j.jvs.2016.12.110. 573-84.e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Liao S., Miralles M., Kelley B.J., Curci J.A., Borhani M., Thompson R.W. Suppression of experimental abdominal aortic aneurysms in the rat by treatment with angiotensin-converting enzyme inhibitors. J Vasc Surg. 2001;33:1057–1064. doi: 10.1067/mva.2001.112810. [DOI] [PubMed] [Google Scholar]
- 19.Kaschina E., Schrader F., Sommerfeld M., Kemnitz U.R., Grzesiak A., Krikov M., et al. Telmisartan prevents aneurysm progression in the rat by inhibiting proteolysis, apoptosis and inflammation. J Hypertens. 2008;26:2361–2373. doi: 10.1097/HJH.0b013e328313e547. [DOI] [PubMed] [Google Scholar]
- 20.Fujiwara Y., Shiraya S., Miyake T., Yamakawa S., Aoki M., Makino H., et al. Inhibition of experimental abdominal aortic aneurysm in a rat model by the angiotensin receptor blocker valsartan. Int J Mol Med. 2008;22:703–708. [PubMed] [Google Scholar]
- 21.Sakaue T., Suzuki J., Hamaguchi M., Suehiro C., Tanino A., Nagao T., et al. Perivascular adipose tissue angiotensin II type 1 receptor promotes vascular inflammation and aneurysm formation. Hypertension. 2017;70:780–789. doi: 10.1161/HYPERTENSIONAHA.117.09512. [DOI] [PubMed] [Google Scholar]
- 22.Xu B., Xuan H., Iida Y., Miyata M., Dalman R.L. Pathogenic and therapeutic significance of angiotensin II type I receptor in abdominal aortic aneurysms. Curr Drug Targets. 2018;19:1318–1326. doi: 10.2174/1389450119666180122155642. [DOI] [PubMed] [Google Scholar]
- 23.Cassis L.A., Rateri D.L., Lu H., Daugherty A. Bone marrow transplantation reveals that recipient AT1a receptors are required to initiate angiotensin II-induced atherosclerosis and aneurysms. Arterioscler Thromb Vasc Biol. 2007;27:380–386. doi: 10.1161/01.ATV.0000254680.71485.92. [DOI] [PubMed] [Google Scholar]
- 24.Silverberg D., Younis A., Savion N., Harari G., Yakubovitch D., Sheick Yousif B., et al. Long-term renin-angiotensin blocking therapy in hypertensive patients with normal aorta may attenuate the formation of abdominal aortic aneurysms. J Am Soc Hypertens. 2014;8:571–577. doi: 10.1016/j.jash.2014.04.005. [DOI] [PubMed] [Google Scholar]
- 25.Moran C.S., Biros E., Krishna S.M., Wang Y., Tikellis C., Morton S.K., et al. Resveratrol inhibits growth of experimental abdominal aortic aneurysm associated with upregulation of angiotensin-converting enzyme 2. Arterioscler Thromb Vasc Biol. 2017;37:2195–2203. doi: 10.1161/ATVBAHA.117.310129. [DOI] [PubMed] [Google Scholar]
- 26.Clarke N.E., Belyaev N.D., Lambert D.W., Turner A.J. Epigenetic regulation of angiotensin-converting enzyme 2 (ACE2) by SIRT1 under conditions of cell energy stress. Clin Sci (Lond) 2014;126:507–516. doi: 10.1042/CS20130291. [DOI] [PubMed] [Google Scholar]
- 27.Sajuthi S.P., DeFord P., Jackson N.D., Montgomery M.T., Everman J.L., Rios C.L., et al. Type 2 and interferon inflammation strongly regulate SARS-CoV-2 related gene expression in the airway epithelium. bioRxiv. 2020 Apr 10 doi: 10.1038/s41467-020-18781-2. [E-pub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.de Lang A., Osterhaus A.D., Haagmans B.L. Interferon-gamma and interleukin-4 downregulate expression of the SARS coronavirus receptor ACE2 in Vero E6 cells. Virology. 2006;353:474–481. doi: 10.1016/j.virol.2006.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Thatcher S.E., Zhang X., Howatt D.A., Yiannikouris F., Gurley S.B., Ennis T., et al. Angiotensin-converting enzyme 2 decreases formation and severity of angiotensin II-induced abdominal aortic aneurysms. Arterioscler Thromb Vasc Biol. 2014;34:2617–2623. doi: 10.1161/ATVBAHA.114.304613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Nie W., Wang Y., Yao K., Wang Z., Wu H. Serum angiotensin-converting enzyme 2 is an independent risk factor for in-hospital mortality following open surgical repair of ruptured abdominal aortic aneurysm. Exp Ther Med. 2016;12:1412–1418. doi: 10.3892/etm.2016.3466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hao Q., Dong X., Chen X., Yan F., Wang X., Shi H., et al. Angiotensin-converting enzyme 2 inhibits angiotensin II-induced abdominal aortic aneurysms in mice. Hum Gene Ther. 2018;29:1387–1395. doi: 10.1089/hum.2016.144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ma H., Wang Y.L., Hei N.H., Li J.L., Cao X.R., Dong B., et al. AVE0991, a nonpeptide angiotensin-(1-7) mimic, inhibits angiotensin II-induced abdominal aortic aneurysm formation in apolipoprotein E knockout mice. J Mol Med (Berl) 2020;98:541–551. doi: 10.1007/s00109-020-01880-4. [DOI] [PubMed] [Google Scholar]
- 33.Stegbauer J., Thatcher S.E., Yang G., Bottermann K., Rump L.C., Daugherty A., et al. Mas receptor deficiency augments angiotensin II-induced atherosclerosis and aortic aneurysm ruptures in hypercholesterolemic male mice. J Vasc Surg. 2019;70 doi: 10.1016/j.jvs.2018.11.045. 1658-68.e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wang S., Guo F., Liu K., Wang H., Rao S., Yang P., et al. Endocytosis of the receptor-binding domain of SARS-CoV spike protein together with virus receptor ACE2. Virus Res. 2008;136:8–15. doi: 10.1016/j.virusres.2008.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Glowacka I., Bertram S., Herzog P., Pfefferle S., Steffen I., Muench M.O., et al. Differential downregulation of ACE2 by the spike proteins of severe acute respiratory syndrome coronavirus and human coronavirus NL63. J Virol. 2010;84:1198–1205. doi: 10.1128/JVI.01248-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Jia H.P., Look D.C., Tan P., Shi L., Hickey M., Gakhar L., et al. Ectodomain shedding of angiotensin converting enzyme 2 in human airway epithelia. Am J Physiol Lung Cell Mol Physiol. 2009;297:L84–L96. doi: 10.1152/ajplung.00071.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Oudit G.Y., Kassiri Z., Jiang C., Liu P.P., Poutanen S.M., Penninger J.M., et al. SARS-coronavirus modulation of myocardial ACE2 expression and inflammation in patients with SARS. Eur J Clin Invest. 2009;39:618–625. doi: 10.1111/j.1365-2362.2009.02153.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kuba K., Imai Y., Rao S., Gao H., Guo F., Guan B., et al. A crucial role of angiotensin converting enzyme 2 (ACE2) in SARS coronavirus-induced lung injury. Nat Med. 2005;11:875–879. doi: 10.1038/nm1267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Saheb Sharif-Askari N., Saheb Sharif-Askari F., Alabed M., Temsah M.H., Al Heialy S., Hamid Q., et al. Airways expression of SARS-CoV-2 receptor, ACE2, and TMPRSS2 is lower in children than adults and increases with smoking and COPD. Mol Ther Methods Clin Dev. 2020;18:1–6. doi: 10.1016/j.omtm.2020.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Liu Y., Yang Y., Zhang C., Huang F., Wang F., Yuan J., et al. Clinical and biochemical indexes from 2019-nCoV infected patients linked to viral loads and lung injury. Sci China Life Sci. 2020;63:364–374. doi: 10.1007/s11427-020-1643-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Henry B.M., Benoit J., Berger B., Pulvino C., Lavie C.J., Lippi G., et al. Coronavirus disease 2019 (COVID-19) is associated with low circulating plasma levels of angiotensin 1 and angiotensin 1,7. J Med Virol. 2020;93:678–680. doi: 10.1002/jmv.26479. [DOI] [PubMed] [Google Scholar]
- 42.Wu Z., Hu R., Zhang C., Ren W., Yu A., Zhou X. Elevation of plasma angiotensin II level is a potential pathogenesis for the critically ill COVID-19 patients. Crit Care. 2020;24:290. doi: 10.1186/s13054-020-03015-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wang W., Shen M., Fischer C., Basu R., Hazra S., Couvineau P., et al. Apelin protects against abdominal aortic aneurysm and the therapeutic role of neutral endopeptidase resistant apelin analogs. Proc Natl Acad Sci U S A. 2019;116:13006–13015. doi: 10.1073/pnas.1900152116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Haga S., Nagata N., Okamura T., Yamamoto N., Sata T., Yamamoto N., et al. TACE antagonists blocking ACE2 shedding caused by the spike protein of SARS-CoV are candidate antiviral compounds. Antiviral Res. 2010;85:551–555. doi: 10.1016/j.antiviral.2009.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Qi F., Qian S., Zhang S., Zhang Z. Single cell RNA sequencing of 13 human tissues identify cell types and receptors of human coronaviruses. Biochem Biophys Res Commun. 2020;526:135–140. doi: 10.1016/j.bbrc.2020.03.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Heurich A., Hofmann-Winkler H., Gierer S., Liepold T., Jahn O., Pöhlmann S. TMPRSS2 and ADAM17 cleave ACE2 differentially and only proteolysis by TMPRSS2 augments entry driven by the severe acute respiratory syndrome coronavirus spike protein. J Virol. 2014;88:1293–1307. doi: 10.1128/JVI.02202-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Yoshikawa T., Hill T., Li K., Peters C.J., Tseng C.T. Severe acute respiratory syndrome (SARS) coronavirus-induced lung epithelial cytokines exacerbate SARS pathogenesis by modulating intrinsic functions of monocyte-derived macrophages and dendritic cells. J Virol. 2009;83:3039–3048. doi: 10.1128/JVI.01792-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Bost P., Giladi A., Liu Y., Bendjelal Y., Xu G., David E., et al. Host-viral infection maps reveal signatures of severe COVID-19 patients. Cell. 2020;181 doi: 10.1016/j.cell.2020.05.006. 1475-88.e12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Rodrigues T.S., de Sá K.S.G., Ishimoto A.Y., Becerra A., Oliveira S., Almeida L., et al. Inflammasomes are activated in response to SARS-CoV-2 infection and are associated with COVID-19 severity in patients. J Exp Med. 2021;218:e20201707. doi: 10.1084/jem.20201707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Dosch S.F., Mahajan S.D., Collins A.R. SARS coronavirus spike protein-induced innate immune response occurs via activation of the NF-kappaB pathway in human monocyte macrophages in vitro. Virus Res. 2009;142:19–27. doi: 10.1016/j.virusres.2009.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Wang W., Ye L., Ye L., Li B., Gao B., Zeng Y., et al. Up-regulation of IL-6 and TNF-alpha induced by SARS-coronavirus spike protein in murine macrophages via NF-kappaB pathway. Virus Res. 2007;128:1–8. doi: 10.1016/j.virusres.2007.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Tseng C.T., Perrone L.A., Zhu H., Makino S., Peters C.J. Severe acute respiratory syndrome and the innate immune responses: modulation of effector cell function without productive infection. J Immunol. 2005;174:7977–7985. doi: 10.4049/jimmunol.174.12.7977. [DOI] [PubMed] [Google Scholar]
- 53.Usui F., Shirasuna K., Kimura H., Tatsumi K., Kawashima A., Karasawa T., et al. Inflammasome activation by mitochondrial oxidative stress in macrophages leads to the development of angiotensin II-induced aortic aneurysm. Arterioscler Thromb Vasc Biol. 2015;35:127–136. doi: 10.1161/ATVBAHA.114.303763. [DOI] [PubMed] [Google Scholar]
- 54.Wu D., Ren P., Zheng Y., Zhang L., Xu G., Xie W., et al. NLRP3 (Nucleotide oligomerization domain-like receptor family, pyrin domain containing 3)-caspase-1 inflammasome degrades contractile proteins: implications for aortic biomechanical dysfunction and aneurysm and dissection formation. Arterioscler Thromb Vasc Biol. 2017;37:694–706. doi: 10.1161/ATVBAHA.116.307648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Ren P., Wu D., Appel R., Zhang L., Zhang C., Luo W., et al. Targeting the NLRP3 Inflammasome with inhibitor MCC950 prevents aortic aneurysms and dissections in mice. J Am Heart Assoc. 2020;9:e014044. doi: 10.1161/JAHA.119.014044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Shen B., Yi X., Sun Y., Bi X., Du J., Zhang C., et al. Proteomic and metabolomic characterization of COVID-19 patient sera. Cell. 2020;182:59–72.e15. doi: 10.1016/j.cell.2020.05.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Yan H., Zhou H.F., Akk A., Hu Y., Springer L.E., Ennis T.L., et al. Neutrophil proteases promote experimental abdominal aortic aneurysm via extracellular trap release and plasmacytoid dendritic cell activation. Arterioscler Thromb Vasc Biol. 2016;36:1660–1669. doi: 10.1161/ATVBAHA.116.307786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Wang Q., Ding Y., Song P., Zhu H., Okon I., Ding Y.N., et al. Tryptophan-derived 3-hydroxyanthranilic acid contributes to angiotensin ii-induced abdominal aortic aneurysm formation in mice in vivo. Circulation. 2017;136:2271–2283. doi: 10.1161/CIRCULATIONAHA.117.030972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Tekin G., İsbir S., Şener G., Çevik Ö., Çetinel Ş., Dericioğlu O., et al. The preventive and curative effects of melatonin against abdominal aortic aneurysm in rats. J Vasc Surg. 2018;67:1546–1555. doi: 10.1016/j.jvs.2017.04.028. [DOI] [PubMed] [Google Scholar]
- 60.Tang L., Cong Z., Hao S., Li P., Huang H., Shen Y., et al. Protective effect of melatonin on the development of abdominal aortic aneurysm in a rat model. J Surg Res. 2017;209 doi: 10.1016/j.jss.2016.06.018. 266-78.e1. [DOI] [PubMed] [Google Scholar]
- 61.Metghalchi S., Vandestienne M., Haddad Y., Esposito B., Dairou J., Tedgui A., et al. Indoleamine 2 3-dioxygenase knockout limits angiotensin II-induced aneurysm in low density lipoprotein receptor-deficient mice fed with high fat diet. PLoS One. 2018;13:e0193737. doi: 10.1371/journal.pone.0193737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Kong J., Zhang Y., Liu S., Li H., Liu S., Wang J., et al. Melatonin attenuates angiotensin II-induced abdominal aortic aneurysm through the down-regulation of matrix metalloproteinases. Oncotarget. 2017;8:14283–14293. doi: 10.18632/oncotarget.15093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Dodd M.S., Sousa Fialho M.D.L., Montes Aparicio C.N., Kerr M., Timm K.N., Griffin J.L., et al. Fatty acids prevent hypoxia-inducible factor-1α signaling through decreased succinate in diabetes. JACC Basic Transl Sci. 2018;3:485–498. doi: 10.1016/j.jacbts.2018.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Whittaker E., Bamford A., Kenny J., Kaforou M., Jones C.E., Shah P., et al. Clinical characteristics of 58 children with a pediatric inflammatory multisystem syndrome temporally associated with SARS-CoV-2. JAMA. 2020;324:259–269. doi: 10.1001/jama.2020.10369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Davies P., Evans C., Kanthimathinathan H.K., Lillie J., Brierley J., Waters G., et al. Intensive care admissions of children with paediatric inflammatory multisystem syndrome temporally associated with SARS-CoV-2 (PIMS-TS) in the UK: a multicentre observational study. Lancet Child Adolesc Health. 2020;4:669–677. doi: 10.1016/S2352-4642(20)30215-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Feldstein L.R., Rose E.B., Horwitz S.M., Collins J.P., Newhams M.M., Son M.B.F., et al. Multisystem inflammatory syndrome in U.S. children and adolescents. N Engl J Med. 2020;383:334–346. doi: 10.1056/NEJMoa2021680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Hameed S., Elbaaly H., Reid C.E.L., Santos R.M.F., Shivamurthy V., Wong J., et al. Spectrum of imaging findings on chest radiographs, US, CT, and MRI images in multisystem inflammatory syndrome in children (MIS-C) associated with COVID-19. Radiology. 2020:202543. doi: 10.1148/radiol.2020202543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Davis F.M., Daugherty A., Lu H.S. Updates of recent aortic aneurysm research. Arterioscler Thromb Vasc Biol. 2019;39:e83–e90. doi: 10.1161/ATVBAHA.119.312000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Quintana R.A., Taylor W.R. Cellular mechanisms of aortic aneurysm formation. Circ Res. 2019;124:607–618. doi: 10.1161/CIRCRESAHA.118.313187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Golledge J. Abdominal aortic aneurysm: update on pathogenesis and medical treatments. Nat Rev Cardiol. 2019;16:225–242. doi: 10.1038/s41569-018-0114-9. [DOI] [PubMed] [Google Scholar]
- 71.Maegdefessel L., Dalman R.L., Tsao P.S. Pathogenesis of abdominal aortic aneurysms: microRNAs, proteases, genetic associations. Annu Rev Med. 2014;65:49–62. doi: 10.1146/annurev-med-101712-174206. [DOI] [PubMed] [Google Scholar]
- 72.Golledge J., Pinchbeck J., Tomee S.M., Rowbotham S.E., Singh T.P., Moxon J.V., et al. Efficacy of telmisartan to slow growth of small abdominal aortic aneurysms: a randomized clinical trial. JAMA Cardiol. 2020;5:1–9. doi: 10.1001/jamacardio.2020.3524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Kiru G., Bicknell C., Falaschetti E., Powell J., Poulter N. An evaluation of the effect of an angiotensin-converting enzyme inhibitor on the growth rate of small abdominal aortic aneurysms: a randomised placebo-controlled trial (AARDVARK) Health Technol Assess. 2016;20:1–180. doi: 10.3310/hta20590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Kuba K., Imai Y., Ohto-Nakanishi T., Penninger J.M. Trilogy of ACE2: a peptidase in the renin-angiotensin system, a SARS receptor, and a partner for amino acid transporters. Pharmacol Ther. 2010;128:119–128. doi: 10.1016/j.pharmthera.2010.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Gheblawi M., Wang K., Viveiros A., Nguyen Q., Zhong J.C., Turner A.J., et al. Angiotensin-converting enzyme 2: SARS-CoV-2 receptor and regulator of the renin-angiotensin system: celebrating the 20th anniversary of the discovery of ACE2. Circ Res. 2020;126:1456–1474. doi: 10.1161/CIRCRESAHA.120.317015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Fairweather S.J., Bröer A., Subramanian N., Tumer E., Cheng Q., Schmoll D., et al. Molecular basis for the interaction of the mammalian amino acid transporters B0AT1 and B0AT3 with their ancillary protein collectrin. J Biol Chem. 2015;290:24308–24325. doi: 10.1074/jbc.M115.648519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Singer D., Camargo S.M., Huggel K., Romeo E., Danilczyk U., Kuba K., et al. Orphan transporter SLC6A18 is renal neutral amino acid transporter B0AT3. J Biol Chem. 2009;284:19953–19960. doi: 10.1074/jbc.M109.011171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Li W., Moore M.J., Vasilieva N., Sui J., Wong S.K., Berne M.A., et al. Angiotensin-converting enzyme 2 is a functional receptor for the SARS coronavirus. Nature. 2003;426:450–454. doi: 10.1038/nature02145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Wan Y., Shang J., Graham R., Baric R.S., Li F. Receptor recognition by the novel coronavirus from Wuhan: an analysis based on decade-long structural studies of SARS coronavirus. J Virol. 2020;94:e00127–e00220. doi: 10.1128/JVI.00127-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Zhou P., Yang X.L., Wang X.G., Hu B., Zhang L., Zhang W., et al. A pneumonia outbreak associated with a new coronavirus of probable bat origin. Nature. 2020;579:270–273. doi: 10.1038/s41586-020-2012-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Shang J., Wan Y., Luo C., Ye G., Geng Q., Auerbach A., et al. Cell entry mechanisms of SARS-CoV-2. Proc Natl Acad Sci U S A. 2020;117:11727–11734. doi: 10.1073/pnas.2003138117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Letko M., Marzi A., Munster V. Functional assessment of cell entry and receptor usage for SARS-CoV-2 and other lineage B betacoronaviruses. Nat Microbiol. 2020;5:562–569. doi: 10.1038/s41564-020-0688-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Bourgonje A.R., Abdulle A.E., Timens W., Hillebrands J.L., Navis G.J., Gordijn S.J., et al. Angiotensin-converting enzyme 2 (ACE2), SARS-CoV-2 and the pathophysiology of coronavirus disease 2019 (COVID-19) J Pathol. 2020;251:228–248. doi: 10.1002/path.5471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Liu P.P., Blet A., Smyth D., Li H. The science underlying COVID-19: implications for the cardiovascular system. Circulation. 2020;142:68–78. doi: 10.1161/CIRCULATIONAHA.120.047549. [DOI] [PubMed] [Google Scholar]
- 85.Brojakowska A., Narula J., Shimony R., Bander J. Clinical implications of SARS-CoV-2 Interaction with renin angiotensin system: JACC review topic of the week. J Am Coll Cardiol. 2020;75:3085–3095. doi: 10.1016/j.jacc.2020.04.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Donoghue M., Hsieh F., Baronas E., Godbout K., Gosselin M., Stagliano N., et al. A novel angiotensin-converting enzyme-related carboxypeptidase (ACE2) converts angiotensin I to angiotensin 1-9. Circ Res. 2000;87:E1–E9. doi: 10.1161/01.res.87.5.e1. [DOI] [PubMed] [Google Scholar]
- 87.Hamming I., Timens W., Bulthuis M.L., Lely A.T., Navis G., van Goor H. Tissue distribution of ACE2 protein, the functional receptor for SARS coronavirus. A first step in understanding SARS pathogenesis. J Pathol. 2004;203:631–637. doi: 10.1002/path.1570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Harmer D., Gilbert M., Borman R., Clark K.L. Quantitative mRNA expression profiling of ACE 2, a novel homologue of angiotensin converting enzyme. FEBS Lett. 2002;532:107–110. doi: 10.1016/s0014-5793(02)03640-2. [DOI] [PubMed] [Google Scholar]
- 89.Li M.Y., Li L., Zhang Y., Wang X.S. Expression of the SARS-CoV-2 cell receptor gene ACE2 in a wide variety of human tissues. Infect Dis Poverty. 2020;9:45. doi: 10.1186/s40249-020-00662-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Guo J., Wei X., Li Q., Li L., Yang Z., Shi Y., et al. Single-cell RNA analysis on ACE2 expression provides insights into SARS-CoV-2 potential entry into the bloodstream and heart injury. J Cell Physiol. 2020;235:9884–9894. doi: 10.1002/jcp.29802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Chen L., Li X., Chen M., Feng Y., Xiong C. The ACE2 expression in human heart indicates new potential mechanism of heart injury among patients infected with SARS-CoV-2. Cardiovasc Res. 2020;116:1097–1100. doi: 10.1093/cvr/cvaa078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Tucker N.R., Chaffin M., Bedi K.C., Papangeli I., Akkad A.D., Arduini A., et al. Myocyte specific upregulation of ACE2 in cardiovascular disease: implications for SARS-CoV-2 mediated myocarditis. medRxiv. 2020 Apr 14 doi: 10.1161/CIRCULATIONAHA.120.047911. [E-pub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Aguiar J.A., Tremblay B.J., Mansfield M.J., Woody O., Lobb B., Banerjee A., et al. Gene expression and in situ protein profiling of candidate SARS-CoV-2 receptors in human airway epithelial cells and lung tissue. Eur Respir J. 2020;56:2001123. doi: 10.1183/13993003.01123-2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Zhao Y., Zhao Z., Wang Y., Zhou Y., Ma Y., Zuo W. Single-cell RNA expression profiling of ACE2, the receptor of SARS-CoV-2. Am J Respir Crit Care Med. 2020;202:756–759. doi: 10.1164/rccm.202001-0179LE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Dai Y.J., Hu F., Li H., Huang H.Y., Wang D.W., Liang Y. A profiling analysis on the receptor ACE2 expression reveals the potential risk of different type of cancers vulnerable to SARS-CoV-2 infection. Ann Transl Med. 2020;8:481. doi: 10.21037/atm.2020.03.61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Bunyavanich S., Do A., Vicencio A. Nasal Gene expression of angiotensin-converting enzyme 2 in children and adults. JAMA. 2020;323:2427–2429. doi: 10.1001/jama.2020.8707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Xie X., Chen J., Wang X., Zhang F., Liu Y. Age- and gender-related difference of ACE2 expression in rat lung. Life Sci. 2006;78:2166–2171. doi: 10.1016/j.lfs.2005.09.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Yoon H.E., Kim E.N., Kim M.Y., Lim J.H., Jang I.A., Ban T.H., et al. Age-associated changes in the vascular renin-angiotensin system in mice. Oxid Med Cell Longev. 2016;2016:6731093. doi: 10.1155/2016/6731093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Liu C.L., Wemmelund H., Wang Y., Liao M., Lindholt J.S., Johnsen S.P., et al. Asthma associates with human abdominal aortic aneurysm and rupture. Arterioscler Thromb Vasc Biol. 2016;36:570–578. doi: 10.1161/ATVBAHA.115.306497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Gupta S., Hayek S.S., Wang W., Chan L., Mathews K.S., Melamed M.L., et al. Factors associated with death in critically ill patients with coronavirus disease 2019 in the US. JAMA Intern Med. 2020 doi: 10.1001/jamainternmed.2020.3596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.de Lusignan S., Dorward J., Correa A., Jones N., Akinyemi O., Amirthalingam G., et al. Risk factors for SARS-CoV-2 among patients in the Oxford Royal College of General Practitioners Research and Surveillance Centre primary care network: a cross-sectional study. Lancet Infect Dis. 2020;20:1034–1042. doi: 10.1016/S1473-3099(20)30371-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Krzysztof N.J., Christoffer L.J., Rahul K., Ricanek P., Jonas H., Jack S. Age, inflammation and disease location are critical determinants of intestinal expression of SARS-CoV-2 receptor ACE2 and TMPRSS2 in inflammatory bowel disease. Gastroenterology. 2020;115(Suppl 1):S7–S8. doi: 10.1053/j.gastro.2020.05.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Pinto B.G.G., Oliveira A.E.R., Singh Y., Jimenez L., Gonçalves A.N.A., Ogava R.L.T., et al. ACE2 expression is increased in the lungs of patients with comorbidities associated with severe COVID-19. medRxiv. 2020 Mar 27 doi: 10.1093/infdis/jiaa332. [E-pub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Chai P., Yu J., Ge S., Jia R., Fan X. Genetic alteration, RNA expression, and DNA methylation profiling of coronavirus disease 2019 (COVID-19) receptor ACE2 in malignancies: a pan-cancer analysis. J Hematol Oncol. 2020;13:43. doi: 10.1186/s13045-020-00883-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Sawalha A.H., Zhao M., Coit P., Lu Q. Epigenetic dysregulation of ACE2 and interferon-regulated genes might suggest increased COVID-19 susceptibility and severity in lupus patients. medRxiv. 2020 Apr 4 doi: 10.1016/j.clim.2020.108410. [E-pub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Choi J.Y., Lee H.K., Park J.H., Cho S.J., Kwon M., Jo C., et al. Altered COVID-19 receptor ACE2 expression in a higher risk group for cerebrovascular disease and ischemic stroke. Biochem Biophys Res Commun. 2020;528:413–419. doi: 10.1016/j.bbrc.2020.05.203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Rao S., Lau A., So H.C. Exploring diseases/traits and blood proteins causally related to expression of ACE2, the putative receptor of SARS-CoV-2: a Mendelian randomization analysis highlights tentative relevance of diabetes-related traits. Diabetes Care. 2020;43:1416–1426. doi: 10.2337/dc20-0643. [DOI] [PubMed] [Google Scholar]
- 108.Menon R., Otto E.E., Sealfon R., Nair V., Wong A.K., Theesfeld C.L., et al. SARS-CoV-2 receptor networks in diabetic kidney disease, BK-Virus nephropathy and COVID-19 associated acute kidney injury. medRxiv. 2020 May 13 doi: 10.1016/j.kint.2020.09.015. [E-pub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Jackson D.J., Busse W.W., Bacharier L.B., Kattan M., O'Connor G.T., Wood R.A., et al. Association of respiratory allergy, asthma, and expression of the SARS-CoV-2 receptor ACE2. J Allergy Clin Immunol. 2020;146 doi: 10.1016/j.jaci.2020.04.009. 203-6.e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Camiolo M.J., Gauthier M., Kaminski N., Ray A., Wenzel S.E. Expression of SARS-CoV-2 receptor ACE2 and coincident host response signature varies by asthma inflammatory phenotype. J Allergy Clin Immunol. 2020;146 doi: 10.1016/j.jaci.2020.05.051. 315-24.e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Wang J., Lindholt J.S., Sukhova G.K., Shi M.A., Xia M., Chen H., et al. IgE actions on CD4+ T cells, mast cells, and macrophages participate in the pathogenesis of experimental abdominal aortic aneurysms. EMBO Mol Med. 2014;6:952–969. doi: 10.15252/emmm.201303811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Kreutz R., Algharably E.A.E., Azizi M., Dobrowolski P., Guzik T., Januszewicz A., et al. Hypertension, the renin-angiotensin system, and the risk of lower respiratory tract infections and lung injury: implications for COVID-19. Cardiovasc Res. 2020;116:1688–1699. doi: 10.1093/cvr/cvaa097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Iwai M., Nakaoka H., Senba I., Kanno H., Moritani T., Horiuchi M. Possible involvement of angiotensin-converting enzyme 2 and Mas activation in inhibitory effects of angiotensin II type 1 receptor blockade on vascular remodeling. Hypertension. 2012;60:137–144. doi: 10.1161/HYPERTENSIONAHA.112.191452. [DOI] [PubMed] [Google Scholar]
- 114.Ferrario C.M., Jessup J., Chappell M.C., Averill D.B., Brosnihan K.B., Tallant E.A., et al. Effect of angiotensin-converting enzyme inhibition and angiotensin II receptor blockers on cardiac angiotensin-converting enzyme 2. Circulation. 2005;111:2605–2610. doi: 10.1161/CIRCULATIONAHA.104.510461. [DOI] [PubMed] [Google Scholar]
- 115.Ishiyama Y., Gallagher P.E., Averill D.B., Tallant E.A., Brosnihan K.B., Ferrario C.M. Upregulation of angiotensin-converting enzyme 2 after myocardial infarction by blockade of angiotensin II receptors. Hypertension. 2004;43:970–976. doi: 10.1161/01.HYP.0000124667.34652.1a. [DOI] [PubMed] [Google Scholar]
- 116.Li H., Li M., Liu P., Wang Y., Zhang H., Li H., et al. Telmisartan ameliorates nephropathy in metabolic syndrome by reducing leptin release from perirenal adipose tissue. Hypertension. 2016;68:478–490. doi: 10.1161/HYPERTENSIONAHA.116.07008. [DOI] [PubMed] [Google Scholar]
- 117.Zhu L., She Z.G., Cheng X., Qin J.J., Zhang X.J., Cai J., et al. Association of blood glucose control and outcomes in patients with COVID-19 and pre-existing type 2 diabetes. Cell Metab. 2020;31 doi: 10.1016/j.cmet.2020.04.021. 1068-77.e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Cuschieri S., Grech S. COVID-19 and diabetes: the why, the what and the how. J Diabetes Complications. 2020:107637. doi: 10.1016/j.jdiacomp.2020.107637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Hu X.S., Xie X.D., Wang X.X., Zeng C.L., Ni Y.M., Yu G.W., et al. [Effects of angiotensin converting enzyme inhibitor on the expression of angiotensin converting enzyme 2 in atrium of patients with atrial fibrillation] Zhonghua Xin Xue Guan Bing Za Zhi. 2007;35:625–628. [PubMed] [Google Scholar]
- 120.Basu R., Poglitsch M., Yogasundaram H., Thomas J., Rowe B.H., Oudit G.Y. Roles of angiotensin peptides and recombinant human ACE2 in heart failure. J Am Coll Cardiol. 2017;69:805–819. doi: 10.1016/j.jacc.2016.11.064. [DOI] [PubMed] [Google Scholar]
- 121.Sama I.E., Ravera A., Santema B.T., van Goor H., Ter Maaten J.M., Cleland J.G.F., et al. Circulating plasma concentrations of angiotensin-converting enzyme 2 in men and women with heart failure and effects of renin-angiotensin-aldosterone inhibitors. Eur Heart J. 2020;41:1810–1817. doi: 10.1093/eurheartj/ehaa373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Milne S., Yang C.X., Timens W., Bossé Y., Sin D.D. SARS-CoV-2 receptor ACE2 gene expression and RAAS inhibitors. Lancet Respir Med. 2020;8:e50–e51. doi: 10.1016/S2213-2600(20)30224-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Chen Y., Yang D., Cheng B., Chen J., Peng A., Yang C., et al. Clinical characteristics and outcomes of patients with diabetes and COVID-19 in association with glucose-lowering medication. Diabetes Care. 2020;43:1399–1407. doi: 10.2337/dc20-0660. [DOI] [PubMed] [Google Scholar]
- 124.Zhang P., Zhu L., Cai J., Lei F., Qin J.J., Xie J., et al. Association of inpatient use of angiotensin-converting enzyme inhibitors and angiotensin II Receptor blockers with mortality among patients with hypertension hospitalized with COVID-19. Circ Res. 2020;126:1671–1681. doi: 10.1161/CIRCRESAHA.120.317134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.de Abajo F.J., Rodríguez-Martín S., Lerma V., Mejía-Abril G., Aguilar M., García-Luque A., et al. Use of renin-angiotensin-aldosterone system inhibitors and risk of COVID-19 requiring admission to hospital: a case-population study. Lancet. 2020;395:1705–1714. doi: 10.1016/S0140-6736(20)31030-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Mancia G., Rea F., Ludergnani M., Apolone G., Corrao G. Renin-angiotensin-aldosterone system blockers and the risk of Covid-19. N Engl J Med. 2020;382:2431–2440. doi: 10.1056/NEJMoa2006923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Reynolds H.R., Adhikari S., Pulgarin C., Troxel A.B., Iturrate E., Johnson S.B., et al. Renin-angiotensin-aldosterone system inhibitors and risk of Covid-19. N Engl J Med. 2020;382:2441–2448. doi: 10.1056/NEJMoa2008975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Yang G., Tan Z., Zhou L., Yang M., Peng L., Liu J., et al. Effects of angiotensin II receptor blockers and ACE (angiotensin-converting enzyme) inhibitors on virus infection, inflammatory status, and clinical outcomes in patients with COVID-19 and hypertension: a single-center retrospective study. Hypertension. 2020;76:51–58. doi: 10.1161/HYPERTENSIONAHA.120.15143. [DOI] [PubMed] [Google Scholar]
- 129.Xue F., Yang J., Cheng J., Sui W., Cheng C., Li H., et al. Angiotensin-(1-7) mitigated angiotensin II-induced abdominal aortic aneurysms in apolipoprotein E-knockout mice. Br J Pharmacol. 2020;177:1719–1734. doi: 10.1111/bph.14906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Chun H.J., Ali Z.A., Kojima Y., Kundu R.K., Sheikh A.Y., Agrawal R., et al. Apelin signaling antagonizes Ang II effects in mouse models of atherosclerosis. J Clin Invest. 2008;118:3343–3354. doi: 10.1172/JCI34871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Leeper N.J., Tedesco M.M., Kojima Y., Schultz G.M., Kundu R.K., Ashley E.A., et al. Apelin prevents aortic aneurysm formation by inhibiting macrophage inflammation. Am J Physiol Heart Circ Physiol. 2009;296:H1329–H1335. doi: 10.1152/ajpheart.01341.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Xu B., Li G., Deng H., Cabot A., Chen X., Yuan H., et al. Abstract 713: angiotensin 1-7 suppresses experimental abdominal aortic aneurysms. Arterioscleros Thrombos Vasc Biol. 2018;38(Suppl 1) A713-A. [Google Scholar]
- 133.Ma W., Li C., Zhang W., Ji Z., Li Y. Anterior wall myocardial infarction in a 16-year-old man caused by coronary artery aneurysm during the outbreak of COVID-19. BMC Cardiovasc Disord. 2020;20:314. doi: 10.1186/s12872-020-01593-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Rustemi O., Raneri F., Iannucci G., Volpin L., Segna A. Aneurysmal subarachnoid hemorrhage in a SARS-CoV-2 positive testing: casual or causal? Br J NeuroSurg. 2020:1–2. doi: 10.1080/02688697.2020.1787343. [DOI] [PubMed] [Google Scholar]
- 135.Sweid A., Hammoud B., Bekelis K., Missios S., Tjoumakaris S.I., Gooch M.R., et al. Cerebral ischemic and hemorrhagic complications of coronavirus disease 2019. Int J Stroke. 2020;15:733–742. doi: 10.1177/1747493020937189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Chen I.Y., Chang S.C., Wu H.Y., Yu T.C., Wei W.C., Lin S., et al. Upregulation of the chemokine (C-C motif) ligand 2 via a severe acute respiratory syndrome coronavirus spike-ACE2 signaling pathway. J Virol. 2010;84:7703–7712. doi: 10.1128/JVI.02560-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.He L., Ding Y., Zhang Q., Che X., He Y., Shen H., et al. Expression of elevated levels of pro-inflammatory cytokines in SARS-CoV-infected ACE2+ cells in SARS patients: relation to the acute lung injury and pathogenesis of SARS. J Pathol. 2006;210:288–297. doi: 10.1002/path.2067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Xu B., Li Y., Zheng X., Chen X., Zhao S., Guo J., et al. Abstract 269: myeloid cell-derived interferon regulatory factor 5 promotes experimental abdominal aortic aneurysms. Arterioscleros Thrombos Vasc Biol. 2019;39(Suppl 1) A269-A. [Google Scholar]
- 139.Shoji T., Guo J., Ge Y., Li Y., Ikezoe T., Wang W., et al. Abstract 431: type 1 interferon receptor deficiency limits the progression of experimental abdominal aortic aneurysms. Arterioscleros Thrombos Vasc Biol. 2020;40(Suppl 1) A431-A. [Google Scholar]
- 140.Weber A., Klocker H., Oberacher H., Gnaiger E., Neuwirt H., Sampson N., et al. Succinate accumulation is associated with a shift of mitochondrial respiratory control and HIF-1α upregulation in PTEN negative prostate cancer cells. Int J Mol Sci. 2018;19:2129. doi: 10.3390/ijms19072129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Arunachalam P.S., Wimmers F., Mok C.K.P., Perera R., Scott M., Hagan T., et al. Systems biological assessment of immunity to mild versus severe COVID-19 infection in humans. Science. 2020;369:1210–1220. doi: 10.1126/science.abc6261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Lai C.H., Wang K.C., Lee F.T., Tsai H.W., Ma C.Y., Cheng T.L., et al. Toll-like receptor 4 is essential in the development of abdominal aortic aneurysm. PLoS One. 2016;11:e0146565. doi: 10.1371/journal.pone.0146565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Codo A.C., Davanzo G.G., Monteiro L.B., de Souza G.F., Muraro S.P., Virgilio-da-Silva J.V., et al. Elevated glucose levels favor SARS-CoV-2 infection and monocyte Response through a HIF-1α/glycolysis-dependent axis. Cell Metab. 2020;32 doi: 10.1016/j.cmet.2020.07.015. 437-46.e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Bhatia P., Mohammed S. Severe hypoxemia in early COVID-19 pneumonia. Am J Respir Crit Care Med. 2020;202:621–631. doi: 10.1164/rccm.202004-1313LE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Rello J., Storti E., Belliato M., Serrano R. Clinical phenotypes of SARS-CoV-2: implications for clinicians and researchers. Eur Respir J. 2020;55:2001028. doi: 10.1183/13993003.01028-2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Fuglebjerg N.J.U., Jensen T.O., Hoyer N., Ryrsø C.K., Lindegaard B., Barrella Harboe Z. Silent hypoxia in patients with SARS CoV-2 infection before hospital discharge. Int J Infect Dis. 2020;99:100–101. doi: 10.1016/j.ijid.2020.07.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Coen M., Allali G., Adler D., Serratrice J. Hypoxemia in COVID-19; Comment on: "The neuroinvasive potential of SARS - CoV2 may play a role in the respiratory failure of COVID-19 patients". J Med Virol. 2020;92:1705–1706. doi: 10.1002/jmv.26020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Yang B.Y., Barnard L.M., Emert J.M., Drucker C., Schwarcz L., Counts C.R., et al. Clinical characteristics of patients with coronavirus disease 2019 (COVID-19) receiving emergency medical services in King County, Washington. JAMA Netw Open. 2020;3:e2014549. doi: 10.1001/jamanetworkopen.2020.14549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Xie J., Covassin N., Fan Z., Singh P., Gao W., Li G., et al. Association between hypoxemia and mortality in patients with COVID-19. Mayo Clin Proc. 2020;95:1138–1147. doi: 10.1016/j.mayocp.2020.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Zhang R., Wu Y., Zhao M., Liu C., Zhou L., Shen S., et al. Role of HIF-1alpha in the regulation ACE and ACE2 expression in hypoxic human pulmonary artery smooth muscle cells. Am J Physiol Lung Cell Mol Physiol. 2009;297:L631–L640. doi: 10.1152/ajplung.90415.2008. [DOI] [PubMed] [Google Scholar]
- 151.Erdozain O.J., Pegrum S., Winrow V.R., Horrocks M., Stevens C.R. Hypoxia in abdominal aortic aneurysm supports a role for HIF-1α and Ets-1 as drivers of matrix metalloproteinase upregulation in human aortic smooth muscle cells. J Vasc Res. 2011;48:163–170. doi: 10.1159/000318806. [DOI] [PubMed] [Google Scholar]
- 152.Strauss E., Waliszewski K., Oszkinis G., Staniszewski R. [Gene-environment interaction for the HIF1-A 1772C>T polymorphisms and cigarette smoking increase susceptibility to abdominal aortic aneurysm] Przegl Lek. 2012;69:744–749. [PubMed] [Google Scholar]
- 153.Tsai S.H., Huang P.H., Hsu Y.J., Peng Y.J., Lee C.H., Wang J.C., et al. Inhibition of hypoxia inducible factor-1α attenuates abdominal aortic aneurysm progression through the down-regulation of matrix metalloproteinases. Sci Rep. 2016;6:28612. doi: 10.1038/srep28612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Wang W., Xu B., Xuan H., Ge Y., Wang Y., Wang L., et al. Hypoxia-inducible factor 1 in clinical and experimental aortic aneurysm disease. J Vasc Surg. 2018;68 doi: 10.1016/j.jvs.2017.09.030. 1538-50.e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Yang L., Shen L., Li G., Yuan H., Jin X., Wu X. Silencing of hypoxia inducible factor-1α gene attenuated angiotensin Ⅱ-induced abdominal aortic aneurysm in apolipoprotein E-deficient mice. Atherosclerosis. 2016;252:40–49. doi: 10.1016/j.atherosclerosis.2016.07.010. [DOI] [PubMed] [Google Scholar]
- 156.Marchetti M. COVID-19-driven endothelial damage: complement, HIF-1, and ABL2 are potential pathways of damage and targets for cure. Ann Hematol. 2020;99:1701–1707. doi: 10.1007/s00277-020-04138-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Haynes B.F., Corey L., Fernandes P., Gilbert P.B., Hotez P.J., Rao S., et al. Prospects for a safe COVID-19 vaccine. Sci Transl Med. 2020;12:eabe0948. doi: 10.1126/scitranslmed.abe0948. [DOI] [PubMed] [Google Scholar]
- 158.Krammer F. SARS-CoV-2 vaccines in development. Nature. 2020;586:516–527. doi: 10.1038/s41586-020-2798-3. [DOI] [PubMed] [Google Scholar]