Abstract
Individuals infected with the severe acute respiratory syndrome (SARS)‐related coronavirus 2 (SARS‐CoV‐2) develop a critical and even fatal disease, called Coronavirus disease‐19 (COVID‐19), that eventually evolves into acute respiratory distress syndrome. The gravity of the SARS‐CoV‐2 pandemic, the escalating number of confirmed cases around the world, the many unknowns related to the virus mode of action, and the heterogenous outcome of COVID‐19 disease in the population ask for the rapid development of alternative approaches, including repurposing of existing drugs, that may dampen virus infectivity. SARS‐CoV‐2 infects human cells through interaction with sialylated receptors at the surface of epithelial cells, such as angiotensin‐converting enzyme 2 (ACE2). Glycan composition on virus entry receptors has been shown to influence the rate of infection of SARS‐CoV‐2 and spreading of virions has recently been linked to altered lysosomal exocytosis. These processes could concurrently involve the lysosomal system and its glycosidases. We hypothesize that modulating the activity of one of them, the lysosomal sialidase NEU1, could impinge on both the sialylation status of ACE2 and other host receptors as well as the extent of lysosomal exocytosis. Thus NEU1‐controlled pathways may represent therapeutic targets, which could impact on SARS‐CoV‐2 susceptibility, infectivity, and spread.
Keywords: ACE2, COVID‐19, lysosomal sialidase, NEU1, SARS‐CoV‐2, sialylation, spike protein
1. INTRODUCTION
Sialylation is a crucial posttranslational modification in mammalian cells, because it affects, among others, the half‐life, structural conformation, and ligand recognition of glycoconjugates. 1 This is particularly important in the field of host/pathogen interactions, where sialic acids are exploited by hosts to evade recognition by pathogens and by pathogens to escape the immune system response. 2 , 3 Given their terminal positions on glycan chains and electronegative charge, sialic acids influence the structural and biochemical properties of a wide range of sialo‐glycoconjugates, and, consequently, serve as important determinants in basic processes, including innate immunity, inflammation and viral recognition. 4 It is, therefore, not surprising that changes in the sialic acid content of host cell receptors as well as viral glycoproteins influence the tropism, pathogenicity, and infectivity of several viruses, including the novel SARS‐CoV‐2.
As of November 2020, SARS‐CoV‐2 has caused 58.425.681 confirmed cases of COVID‐19, including 1.385.218 deaths (https://covid19.who.int) worldwide. The clinical features of patients, with confirmed diagnosis of COVID‐19, include lower respiratory tract illness with fever, dry cough, and dyspnea, manifestations similar to those seen in two other diseases caused by coronaviruses, SARS and Middle East respiratory syndrome (MERS). 5 It is now established, that these symptoms are accompanied by a fulminant and damaging immune reaction, referred to as the “cytokine storm,” and involve not only the lungs but also multiple other organs, including the heart and the brain. 6 , 7 , 8 With regard to the severe lung disease that ensues upon SARS‐CoV‐2 infection, the most common lung manifestations in COVID‐19 patients are bilateral ground‐glass opacity with subpleural distribution and absence of pleural effusion, a typical lung injury caused by viral pneumonia, as previously seen in SARS and MERS. 9 Following the 2003 epidemic of SARS, it was noticed that many patients, who survived the severe illness, developed residual pulmonary fibrosis, as shown by clinical findings and radiography. 10 Varying degrees of fibrosis were also observed in autopsies of COVID‐19 fatal cases, and they appear to be a common feature in patients infected by SARS‐coronaviruses. 10
Based on data derived from the general population, it is clear that the pathogenesis and clinical outcome of coronavirus infection tend to be influenced by the age of the patients. The disease seems to affect more males than females, and older individuals with preexisting conditions, like hypertension, diabetes, obesity, and other cardiovascular diseases, have an increased risk of developing severe COVID‐19. 11 Despite this age‐related trend, SARS‐CoV‐2 infection does not spare young individuals, suggesting that genetic and/or epigenetic predisposition mechanisms might also play an important role. Here, we hypothesize that one such mechanism may involve the activity of the lysosomal sialidase NEU1. By modulating the levels of sialic acids on ACE2 (and other entry receptors), NEU1 may directly influence virus entry. We further postulate that NEU1 may contribute to virus spreading by directly controlling the process of lysosomal exocytosis.
1.1. SARS‐CoV‐2 entry receptors: role of sialylation
Similar to other coronaviruses, SARS‐CoV‐2 has been shown to infect host cells through interaction between the receptor binding domain (RBD) of the trimeric S1 subunit of its spike (S) glycoprotein (S1 and S2 subunit composition) and ACE2 on the surface of human epithelial cells of the lung, heart, kidney, intestine, and brain. 12 , 13 ACE2 is a sialylated, single‐pass, type I transmembrane enzyme that belongs to the angiotensin‐converting enzyme family of dipeptidyl carboxydipeptidases. 14 The external enzymatic domain of ACE2 binds SARS‐CoV‐2 prior to the fusion of the viral envelope with the host PM and endocytosis of the virus. This virus‐receptor interaction requires priming of the spike S1 protein by the host serine protease TMPRSS2 and furin. 12 , 15 Structural studies using cryo‐electron microscopy have shown that human ACE2, in complex with the amino acid transporter B0AT1, exists as a dimer and as such interacts with the RBD of the viral S glycoprotein of SARS‐CoV‐2. 13 Structural alignment of this receptor‐virus complex suggests that two S protein trimers can simultaneously bind to an ACE2 homodimer. 13 It is noteworthy that, besides its functional domains, also the glycans on ACE2, and in particular its sialic acids, play an active role in the infection process. 16 , 17
In addition to ACE2, Sigrist et al. have suggested that SARS‐CoV‐2 utilizes integrins (including β1‐ and β3‐integrins) as cell surface receptors in one or more host species; in this case, attachment of the virus to these receptors occurs through a conserved Arg‐Gly‐Asp motif embedded in the RBD of all SARS‐CoV‐2 that have been sequenced until now. 18 Given that the functions of integrins are known to be influenced by their glycosylation and sialylation status, 19 it is likely that these posttranslational modifications also impact virus binding and, in turn, infection efficacy. Recently, another glycosylated and sialylated membrane protein, Neuropilin‐1 (NRP1) has been shown to function as SARS‐CoV‐2 entry receptor, enhancing virus infectivity. 20 , 21 Furin cleavage of SARS‐CoV‐2 S1 protein allows binding to NRP1; subsequent processing of furin‐cleaved S1 by cathepsins and TMPRSS2 allows virus entry and infection. 20 , 21 These results suggest that the interaction between the viral spike protein and host NRP1 may represent a potential target for antiviral therapy.
Lastly, studies of MERS have proposed that the MERS‐CoV spike glycoprotein, which recognizes and binds to the dipeptidyl peptidase 4 (DPP4) entry receptor on respiratory epithelial cells, also uses sialic acids exposed at the cell surface as viral entry sites. In the case of MERS‐CoV the sialic acid binding site has been identified and found that it is homologous to a region upstream of the RBD of the SARS‐CoV‐2 spike protein (the highly variable region at the N‐terminus, NTD). 22 , 23 , 24 Thus, not only the functional activity of the entry receptors on the host cells but also the extent and type of their glycan chains, in particular their sialic acids, exposed at the cell surface may influence and modulate the rate of infection of the SARS‐CoV‐2, as demonstrated for other members of this virus family. 17 , 25 , 26
1.2. Chloroquine and hydroxychloroquine, pros and cons
Among the drugs used to treat SARS‐CoV‐2 infection, one well‐known antimalaria drug, chloroquine, and its less toxic hydroxychloroquine, have gained a lot of attention at the beginning of the pandemic but raised concerns and controversies later on. 27 , 28 , 29 , 30 , 31 , 32 , 33 , 34 , 35 , 36 , 37 When added in cultured cells, the non‐protonated portion of chloroquine enters the cell, where it becomes protonated and concentrated in acidic, low‐pH organelles, such as Golgi vesicles, endosomes, and lysosomes. 38 Intra‐vesicular pH is known to control basic cellular functions, including N‐glycosylation trimming/modification, organellar trafficking, and enzymatic activities. By increasing the pH of Golgi vesicles, chloroquine (and also NH4Cl and possibly hydroxychloroquine) is thought to interfere with terminal glycosylation, specifically sialylation, of the Golgi form of ACE2, likely by inhibiting Golgi sialyltransferases, 39 without affecting ACE2 expression levels at the cell surface. 40 , 41 , 42 , 43 In line with this finding is a recent modeling study showing that the sialylated portion of cell surface gangliosides, which functions as co‐receptor for ACE2 and binds to the NTD of the spike glycoprotein of SARS‐CoV‐2, is blocked by both chloroquine and hydroxychloroquine. 44 This hinders the host‐cell entry and subsequent virus replication, and, in turn, inhibits SARS‐CoV‐2 infection. 41 , 42 , 43 However, an opposite scenario could also be envisaged based on the effects of chloroquine on raising the lysosomal pH, 45 which inhibits the activity of lysosomal hydrolases, including NEU1. The latter could prevent desialylation of SARS‐CoV‐2 receptors that would recycle to the PM in their sialylated state and facilitate viral entry. These two opposing effects which may depend on the drug regimen and time of administration could explain the contradictory response to chloroquine/hydroxychloroquine treatment observed in COVID‐19 patients.
Collectively these examples point to sialylation as an important factor to be considered for the development of potential new treatments for COVID‐19 and for the design of new vaccines.
1.3. Multifactorial roles of the lysosomal sialidase NEU1
In eukaryotes, processing/removal of sialic acids on sialo‐glycoconjugates occurs via the action of 4 sialidases or neuraminidases, NEU1‐NEU4, that are differentially expressed in cells and tissues, and have a different pH optimum and substrate specificity. 46 These enzymes have been shown to participate in and regulate many biological and pathological processes, from cell‐cell communication and cell/tissue development and differentiation to cancer progression and metastasis. 47 , 48 Of the 4 mammalian sialidases, NEU1 is the most abundant and ubiquitously expressed, 49 and it is the only sialidase known to date that is deficient in two lysosomal storage diseases, sialidosis (NEU1 deficiency) and galactosialidosis (cathepsin A primary defect and secondary deficiency of NEU1). 49 , 50 These pediatric conditions, associated with severe systemic and neurological abnormalities, share clinical manifestations with common adult diseases of aging, such as Alzheimer's disease, diabetes, atherosclerosis, obesity, fibrosis, and cancer. 51 , 52 , 53
NEU1 catalyzes the removal of α2,3‐ or α2,6‐linked sialic acids from the non‐reducing end of glycan chains on sialo‐glycoconjugates, with a preference for sialo‐glycoproteins, an activity that is exerted at an acidic pH optimum. NEU1 localizes primarily in the endosomes/lysosomes but it is also found in the PM; both these cellular compartments are sites of desialylation of glycoproteins by NEU1. 49 , 54 , 55 Deficient or defective enzyme activity results in impaired processing/degradation of sialo‐glycoproteins, and in their accumulation in a sialylated state, a feature that alters their biochemical properties and function, and is at the basis of pathogenesis in sialidosis. 48 , 49 , 51 , 53 , 56 , 57 , 58 However, the pathological consequences of impaired desialylation downstream of NEU1 loss of function depend on the type of substrates the enzyme targets in vivo and on their subcellular distribution, which, in turn, affect specific cellular pathways. 48 , 66 For instance, by processing the sialic acids on the lysosomal associated membrane protein 1 (LAMP1), NEU1 negatively regulates the calcium‐dependent, physiological process of lysosomal exocytosis. 57 In cells with deficient or downregulated NEU1, lysosomes decorated with a sialylated, long‐lived, LAMP1 tend to preferentially dock at the PM, ready to engage in lysosomal exocytosis upon changes in calcium concentration. 57 The end result is excessive release of lysosomal contents, including soluble hydrolases and exosomes, into the extracellular space with deleterious consequences for PM and extracellular matrix integrity. 48 , 51 , 53 , 56 , 57 , 58 Importantly, in fibroblasts from sialidosis patients with different degrees of disease severity, the residual NEU1 activity inversely correlated with the levels of LAMP1 redistributed at the PM, identifying this feature as indicative of the extent of lysosomal exocytosis. 57
Based on this collective evidence, we hypothesize that reduced NEU1 activity in cells could have a dual effect: it could affect SARS‐COV‐2 infectivity because of impaired processing of the sialic acids in entry receptors and could promote virus release via LAMP1‐dependent, excessive lysosomal exocytosis. In support of this hypothesis is the recent finding that β‐coronaviruses use lysosomal exocytosis to egress into the extracellular environment. 67 These authors showed that the viral genome was enriched in lysosomes and that, upon viral infection, LAMP1 levels increased at the PM. They also demonstrated a de‐acidification of lysosomes, which affected the activity of lysosomal enzymes and inhibited antigen presentation by the host cells. 67
Lastly, a major downstream effect of NEU1 deficiency seen in the mouse model of sialidosis is a relentless expansion of the connective tissue, ultimately resulting in generalized fibrosis. 53 The underlying pathogenic process leading to this fibrotic disease is initiated and perpetuated by the excessive exocytosis by NEU1‐deficient myofibroblasts of soluble lysosomal contents and exosomes loaded with pro‐fibrotic signaling molecules, like TGFβ. 53 The fact that NEU1 is downregulated in a cohort of patient‐derived idiopathic pulmonary fibrosis (IPF) fibroblasts suggests a role for NEU1 deficiency/downregulation in causing or hastening the course of a fibrotic disease in the adult human population. It is, therefore, tempting to speculate that systemic downregulation of NEU1 coupled to excessive lysosomal exocytosis could represent a risk factor for the initiation and/or progression of the fibrotic disease associated with SARS‐CoV‐2. This is also supported by the failure of neuraminidase inhibitors, such as oseltamivir, peramivir, and zanamivir in the treatment of COVID‐19 patients, 68 , 69 while those are first‐line medications for the treatment and prophylaxis of influenza. 70 , 71
2. HYPOTHESIS
The premises outlined above have led us to design a workflow (Figure 1) aimed to identify additional functional biomarkers that will help to further characterize susceptibility to SARS‐CoV‐2 infection in the general population. We hypothesize that NEU1 levels inversely correlate with the extent of sialylation of the ACE2 receptor (and other entry receptors) and, in turn, the predisposition for entry of SARS‐CoV‐2 (Figure 1).
FIGURE 1.
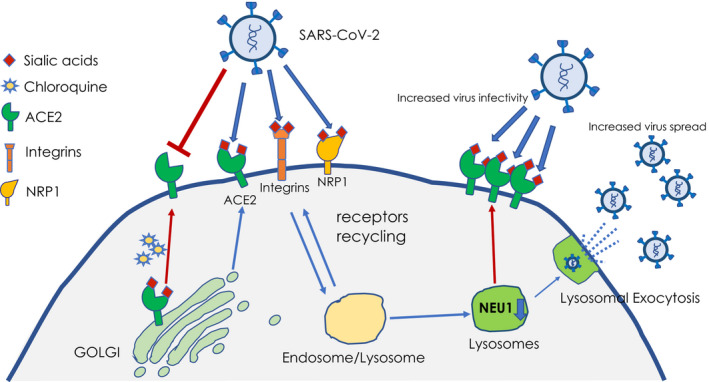
Proposed model of viral entry and spread. Low NEU1 activity results in excessive lysosomal exocytosis of viral particles and impaired desialylation of host receptors (ie, ACE2) recycled to the PM. These combined effects may lead to the higher infectivity and spreading of the SARS‐CoV‐2 virions. In contrast, chloroquine can block ACE2 sialylation in the Golgi, resulting in the exposure of undersialylated receptors at the PM and decreased susceptibility to the virus
Furthermore, low NEU1 levels may also result in LAMP1‐mediated excessive lysosomal exocytosis of lysosomal contents/exosomes and SARS‐CoV‐2 virions. Thus, NEU1 levels may also predict the tendency of an individual to become a SARS‐CoV‐2 “super spreader” (Figure 1).
In this context, it would be conceivable to:
-
IDENTIFY A RISK FACTOR FOR SARS‐CoV‐2 SUSCEPTIBILITY and SPREAD
by evaluating the expression levels of NEU1 mRNA in human samples (the lowest NEU1 levels should correspond to worst prognosis and fit our super spreader hypothesis).
by screening the general population for NEU1 synonymous variants known to lower NEU1 enzyme activity 72 as well as pathogenic mutations in carriers. These analyses will ascertain the involvement of NEU1 functional variants in the pathogenesis of COVID‐19.
APPLY UNCONVENTIONAL THERAPY TO INCREASE NEU1 LEVELS
Demonstrating a link between NEU1‐downregulation and SARS‐CoV‐2 infectivity will allow not only to better understand aspects of pathogenesis of the COVID‐19 disease, but also to test unconventional therapies aimed to raise NEU1 activity in patients. The need for these types of therapeutic approaches is dictated by the lack of any target therapy that could directly restore or increase NEU1 levels. 49 We have recently demonstrated that dietary supplements, like betaine, a natural amino acid derivative, as well as inhibitors of histone deacetylases, 73 , 74 increased the levels of mutant NEU1 when added to sialidosis fibroblasts or administered to mouse models of type I sialidosis with residual NEU1 activity. 73 , 74 Thus, it is conceivable that, if a correlation is found between NEU1 levels and extent and severity of viral infection, unconventional dietary compounds combined with conventional therapeutic approaches may prove beneficial for modulating NEU1 levels and ultimately for fighting infectious diseases like COVID‐19.
AUTHOR CONTRIBUTIONS
A. Bongiovanni and A. d’Azzo conceptualized the hypothesis. A. Bongiovanni, A. Cusimano, I. Annunziata, and A. d’Azzo wrote the paper.
ACKNOWLEDGMENTS
The authors acknowledge financial support from the VES4US project funded by the European Union’s Horizon 2020 research and innovation programme under grant agreement no. 801338 (A.B. and A.C.), from the National Institutes of Health grant no. GM104981 (A.d’A. and I.A.), from the Assisi Foundation of Memphis (A.d’A. and I.A.), and from the American Lebanese Syrian Associated Charities (ALSAC) (A.d’A.and I.A.). A.d’A. holds the Jewelers for Children Endowed Chair in Genetics and Gene Therapy.
REFERENCES
- 1. Varki A. Biological roles of glycans. Glycobiology. 2017;27:3‐49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Matrosovich M, Herrler G, Klenk HD. Sialic acid receptors of viruses. Top Curr Chem. 2015;367:1‐28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Pearce OM, Laubli H. Sialic acids in cancer biology and immunity. Glycobiology. 2016;26:111‐128. [DOI] [PubMed] [Google Scholar]
- 4. Varki A, Schauer R. Sialic acids. In: Varki A, Cummings RD, Esko JD, Freeze HH, Stanley P, Bertozzi CR, Hart GW, Etzler ME, eds. Essentials of glycobiology. New York, NY: Cold Spring Harbor; 2009. [PubMed] [Google Scholar]
- 5. Zhou P, Yang XL, Wang XG, et al. A pneumonia outbreak associated with a new coronavirus of probable bat origin. Nature. 2020;579:270‐273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Huang C, Wang Y, Li X, et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395:497‐506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Li YC, Bai WZ, Hashikawa T. The neuroinvasive potential of SARS‐CoV2 may play a role in the respiratory failure of COVID‐19 patients. J Med Virol. 2020;92(6):552‐555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Zheng YY, Ma YT, Zhang JY, Xie X. COVID‐19 and the cardiovascular system. Nat Rev Cardiol. 2020;17:259‐260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Wang H, Wei R, Rao G, Zhu J, Song B. Characteristic CT findings distinguishing 2019 novel coronavirus disease (COVID‐19) from influenza pneumonia. Eur Radiol. 2020;30(9):4910‐4917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Venkataraman T, Frieman MB. The role of epidermal growth factor receptor (EGFR) signaling in SARS coronavirus‐induced pulmonary fibrosis. Antiviral Res. 2017;143:142‐150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Severe Covid‐19 GWAS Group ; Ellinghaus D, Degenhardt F, et al. Genomewide association study of severe covid‐19 with respiratory failure. N Engl J Med. 2020;383:1522‐1534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Hoffmann M, Kleine‐Weber H, Schroeder S, et al. SARS‐CoV‐2 cell entry depends on ACE2 and TMPRSS2 and is blocked by a clinically proven protease inhibitor. Cell. 2020;181(271–280):e278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Yan R, Zhang Y, Li Y, Xia L, Guo Y, Zhou Q. Structural basis for the recognition of SARS‐CoV‐2 by full‐length human ACE2. Science. 2020;367:1444‐1448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Tipnis SR, Hooper NM, Hyde R, Karran E, Christie G, Turner AJ. A human homolog of angiotensin‐converting enzyme. Cloning and functional expression as a captopril‐insensitive carboxypeptidase. J Biol Chem. 2000;275:33238‐33243. [DOI] [PubMed] [Google Scholar]
- 15. Walls AC, Park YJ, Tortorici MA, Wall A, McGuire AT, Veesler D. Structure, function, and antigenicity of the SARS‐CoV‐2 spike glycoprotein. Cell. 2020;181(281–292):e286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Shajahan A, Supekar NT, Gleinich AS, Azadi P. Deducing the N‐ and O‐glycosylation profile of the spike protein of novel coronavirus SARS‐CoV‐2. Glycobiology. 2020;30:981‐988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Schwegmann‐Wessels C, Herrler G. Sialic acids as receptor determinants for coronaviruses. Glycoconj J. 2006;23:51‐58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Sigrist CJ, Bridge A, Le Mercier P. A potential role for integrins in host cell entry by SARS‐CoV‐2. Antiviral Res. 2020;177:104759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Pan D, Song Y. Role of altered sialylation of the I‐like domain of beta1 integrin in the binding of fibronectin to beta1 integrin: thermodynamics and conformational analyses. Biophys J. 2010;99:208‐217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Cantuti‐Castelvetri L, Ojha R, Pedro LD, et al. Neuropilin‐1 facilitates SARS‐CoV‐2 cell entry and infectivity. Science. 2020;370:856‐860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Daly JL, Simonetti B, Klein K, et al. Neuropilin‐1 is a host factor for SARS‐CoV‐2 infection. Science. 2020;370:861‐865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Park YJ, Walls AC, Wang Z, et al. Structures of MERS‐CoV spike glycoprotein in complex with sialoside attachment receptors. Nat Struct Mol Biol. 2019;26:1151‐1157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Qing E, Hantak M, Perlman S, Gallagher T. Distinct roles for sialoside and protein receptors in coronavirus infection. MBio. 2020;11(1):1‐18. 10.1128/mBio.02764-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Vandelli A, Monti M, Milanetti E, et al. Structural analysis of SARS‐CoV‐2 and preductions of the human interactome. Nucleic Acids Res. 2020;48:11270–11283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Tortorici MA, Walls AC, Lang Y, et al. Structural basis for human coronavirus attachment to sialic acid receptors. Nat Struct Mol Biol. 2019;26:481‐489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Li W, Hulswit RJG, Widjaja I, et al. Identification of sialic acid‐binding function for the Middle East respiratory syndrome coronavirus spike glycoprotein. Proc Natl Acad Sci U S A. 2017;114:E8508‐E8517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Gao J, Tian Z, Yang X. Breakthrough: chloroquine phosphate has shown apparent efficacy in treatment of COVID‐19 associated pneumonia in clinical studies. Biosci Trends. 2020;14:72‐73. [DOI] [PubMed] [Google Scholar]
- 28. Gautret P, Lagier JC, Parola P, et al. Clinical and microbiological effect of a combination of hydroxychloroquine and azithromycin in 80 COVID‐19 patients with at least a six‐day follow up: A pilot observational study. Travel Med Infect Dis. 2020;34:101663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Chen Z, Hu J, Zhang Z, et al. Efficacy of hydroxychloroquine in patients with COVID‐19: results of a randomized clinical trial. medRxiv, 2020.2003.2022.20040758. [Google Scholar]
- 30. Arshad S, Kilgore P, Chaudhry ZS, et al. Treatment with hydroxychloroquine, azithromycin, and combination in patients hospitalized with COVID‐19. Int J Infect Dis. 2020;97:396‐403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Molina JM, Delaugerre C, Le Goff J, et al. No evidence of rapid antiviral clearance or clinical benefit with the combination of hydroxychloroquine and azithromycin in patients with severe COVID‐19 infection. Med Mal Infect. 2020;50:384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Magagnoli J, Narendran S, Pereira F, et al. Outcomes of hydroxychloroquine usage in United States veterans hospitalized with COVID‐19. medRxiv. 2020;18:114–127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Geleris J, Sun Y, Platt J, et al. Observational study of hydroxychloroquine in hospitalized patients with covid‐19. N Engl J Med. 2020;382:2411‐2418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Mahevas M, Tran VT, Roumier M, et al. Clinical efficacy of hydroxychloroquine in patients with covid‐19 pneumonia who require oxygen: observational comparative study using routine care data. BMJ. 2020;369:m1844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Boulware DR, Pullen MF, Bangdiwala AS, et al. A randomized trial of hydroxychloroquine as postexposure prophylaxis for covid‐19. N Engl J Med. 2020;383:517‐525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Cavalcanti AB, Zampieri FG, Rosa RG, et al. Hydroxychloroquine with or without azithromycin in mild‐to‐moderate covid‐19. N Engl J Med. 2020;383(21):2041‐2052 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Liu J, Cao R, Xu M, et al. Hydroxychloroquine, a less toxic derivative of chloroquine, is effective in inhibiting SARS‐CoV‐2 infection in vitro. Cell Discov. 2020;6:16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Martin RE, Marchetti RV, Cowan AI, Howitt SM, Broer S, Kirk K. Chloroquine transport via the malaria parasite's chloroquine resistance transporter. Science. 2009;325:1680‐1682. [DOI] [PubMed] [Google Scholar]
- 39. Axelsson MA, Karlsson NG, Steel DM, Ouwendijk J, Nilsson T, Hansson GC. Neutralization of pH in the Golgi apparatus causes redistribution of glycosyltransferases and changes in the O‐glycosylation of mucins. Glycobiology. 2001;11:633‐644. [DOI] [PubMed] [Google Scholar]
- 40. Meyerowitz EA, Vannier AGL, Friesen MGN, et al. Rethinking the role of hydroxychloroquine in the treatment of COVID‐19. FASEB J. 2020;34:6027‐6037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Vincent MJ, Bergeron E, Benjannet S, et al. Chloroquine is a potent inhibitor of SARS coronavirus infection and spread. Virol J. 2005;2:69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Zhou Y, Vedantham P, Lu K, et al. Protease inhibitors targeting coronavirus and filovirus entry. Antiviral Res. 2015;116:76‐84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Wang M, Cao R, Zhang L, et al. Remdesivir and chloroquine effectively inhibit the recently emerged novel coronavirus (2019‐nCoV) in vitro. Cell Res. 2020;30:269‐271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Fantini J, Di Scala C, Chahinian H, Yahi N. Structural and molecular modelling studies reveal a new mechanism of action of chloroquine and hydroxychloroquine against SARS‐CoV‐2 infection. Int J Antimicrob Agents. 2020;55(5):105960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Homewood CA, Warhurst DC, Peters W, Baggaley VC. Lysosomes, pH and the anti‐malarial action of chloroquine. Nature. 1972;235:50‐52. [DOI] [PubMed] [Google Scholar]
- 46. Monti E, Bonten E, D'Azzo A, et al. Sialidases in vertebrates: a family of enzymes tailored for several cell functions. Adv Carbohydr Chem Biochem. 2010;64:403‐479. [DOI] [PubMed] [Google Scholar]
- 47. Forcella M, Mozzi A, Stefanini FM, et al. Deregulation of sialidases in human normal and tumor tissues. Cancer Biomark. 2018;21:591‐601. [DOI] [PubMed] [Google Scholar]
- 48. Machado E, White‐Gilbertson S, van de Vlekkert D, et al. Regulated lysosomal exocytosis mediates cancer progression. Sci Adv. 2015;1:e1500603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. d'Azzo A, Machado E, Annunziata I. Pathogenesis, emerging therapeutic targets and treatment in sialidosis. Expert Opin Orphan Drugs. 2015;3:491‐504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Annunziata I, d'Azzo A. Galactosialidosis: historic aspects and overview of investigated and emerging treatment options. Expert Opin Orphan Drugs. 2017;5:131‐141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Annunziata I, Patterson A, Helton D, et al. Lysosomal NEU1 deficiency affects amyloid precursor protein levels and amyloid‐beta secretion via deregulated lysosomal exocytosis. Nat Commun. 2013;4:2734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Platt FM, d'Azzo A, Davidson BL, Neufeld EF, Tifft CJ. Lysosomal storage diseases. Nat Rev Dis Primers. 2018;4:27. [DOI] [PubMed] [Google Scholar]
- 53. van de Vlekkert D, Demmers J, Nguyen XX, et al. Excessive exosome release is the pathogenic pathway linking a lysosomal deficiency to generalized fibrosis. Sci Adv. 2019;5:eaav3270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Bonten E, van der Spoel A, Fornerod M, Grosveld G, d'Azzo A. Characterization of human lysosomal neuraminidase defines the molecular basis of the metabolic storage disorder sialidosis. Genes Dev. 1996;10:3156‐3169. [DOI] [PubMed] [Google Scholar]
- 55. Bonten EJ, Annunziata I, d'Azzo A. Lysosomal multienzyme complex: pros and cons of working together. Cell Mol Life Sci. 2014;71:2017‐2032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Wu X, Steigelman KA, Bonten E, et al. Vacuolization and alterations of lysosomal membrane proteins in cochlear marginal cells contribute to hearing loss in neuraminidase 1‐deficient mice. Biochim Biophys Acta. 2010;1802:259‐268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Yogalingam G, Bonten EJ, van de Vlekkert D, et al. Neuraminidase 1 is a negative regulator of lysosomal exocytosis. Dev Cell. 2008;15:74‐86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Zanoteli E, van de Vlekkert D, Bonten EJ, et al. Muscle degeneration in neuraminidase 1‐deficient mice results from infiltration of the muscle fibers by expanded connective tissue. Biochim Biophys Acta. 2010;1802:659‐672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Starcher B, d'Azzo A, Keller PW, Rao GK, Nadarajah D, Hinek A. Neuraminidase‐1 is required for the normal assembly of elastic fibers. Am J Physiol Lung Cell Mol Physiol. 2008;295:L637‐647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Zhou X, Zhai Y, Liu C, et al. Sialidase NEU1 suppresses progression of human bladder cancer cells by inhibiting fibronectin‐integrin alpha5beta1 interaction and Akt signaling pathway. Cell Commun Signal. 2020;18:44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Abdulkhalek S, Szewczuk MR. Neu1 sialidase and matrix metalloproteinase‐9 cross‐talk regulates nucleic acid‐induced endosomal TOLL‐like receptor‐7 and ‐9 activation, cellular signaling and pro‐inflammatory responses. Cell Signal. 2013;25:2093‐2105. [DOI] [PubMed] [Google Scholar]
- 62. Amith SR, Jayanth P, Franchuk S, et al. Neu1 desialylation of sialyl alpha‐2,3‐linked beta‐galactosyl residues of TOLL‐like receptor 4 is essential for receptor activation and cellular signaling. Cell Signal. 2010;22:314‐324. [DOI] [PubMed] [Google Scholar]
- 63. Cross AS, Hyun SW, Miranda‐Ribera A, et al. NEU1 and NEU3 sialidase activity expressed in human lung microvascular endothelia: NEU1 restrains endothelial cell migration, whereas NEU3 does not. J Biol Chem. 2012;287:15966‐15980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Karmakar J, Roy S, Mandal C. Modulation of TLR4 sialylation mediated by a sialidase Neu1 and impairment of its signaling in leishmania donovani infected macrophages. Front Immunol. 2019;10:2360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Lillehoj EP, Hyun SW, Liu A, et al. NEU1 sialidase regulates membrane‐tethered Mucin (MUC1) ectodomain adhesiveness for pseudomonas aeruginosa and decoy receptor release. J Biol Chem. 2015;290:18316‐18331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Natori Y, Nasui M, Edo K, et al. NEU1 sialidase controls gene expression and secretion of IL‐6 and MCP‐1 through NF‐kappaB pathway in 3T3‐L1 adipocytes. J Biochem. 2017;162:137‐143. [DOI] [PubMed] [Google Scholar]
- 67. Ghosh S, Dellibovi‐Ragheb TA, Kerviel A, et al. Beta‐coronaviruses use lysosomes for egress instead of the biosynthetic secretory pathway. Cell. 2020;183(6):1520‐1535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Yousefi B, Valizadeh S, Ghaffari H, Vahedi A, Karbalaei M, Eslami M. A global treatments for coronaviruses including COVID‐19. J Cell Physiol. 2020;235:9133‐9142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Li G, De Clercq E. Therapeutic options for the 2019 novel coronavirus (2019‐nCoV). Nat Rev Drug Discov. 2020;19:149‐150. [DOI] [PubMed] [Google Scholar]
- 70. Adisasmito W, Chan PK, Lee N, et al. Effectiveness of antiviral treatment in human influenza A(H5N1) infections: analysis of a Global Patient Registry. J Infect Dis. 2010;202:1154‐1160. [DOI] [PubMed] [Google Scholar]
- 71. Wang CB, Chiu ML, Lin PC, et al. Prompt oseltamivir therapy reduces medical care and mortality for patients with influenza infection: an asian population cohort study. Medicine (Baltimore). 2015;94:e1070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Bonardi D, Ravasio V, Borsani G, et al. In silico identification of new putative pathogenic variants in the NEU1 sialidase gene affecting enzyme function and subcellular localization. PLoS One. 2014;9:e104229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Mosca R, van de Vlekkert D, Campos Y, et al. Conventional and unconventional therapeutic strategies for sialidosis type I. J Clin Med. 2020;9(3):695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Annunziata I, van de Vlekkert D, Wolf E, et al. MYC competes with MiT/TFE in regulating lysosomal biogenesis and autophagy through an epigenetic rheostat. Nat Commun. 2019;10:3623. [DOI] [PMC free article] [PubMed] [Google Scholar]


