Abstract
We examined the effect of intravenous ascorbate (VitC) administration on exercise-induced redox balance, inflammation, exertional dyspnea, neuromuscular fatigue, and exercise tolerance in patients with chronic obstructive pulmonary disease (COPD). Eight COPD patients completed constant-load cycling (∼80% of peak power output, 83 ± 10 W) to task failure after intravenous VitC (2 g) or saline (placebo, PL) infusion. All participants repeated the shorter of the two exercise trials (isotime) with the other infusate. Quadriceps fatigue was determined by pre- to postexercise changes in quadriceps twitch torque (ΔQtw, electrical femoral nerve stimulation). Corticospinal excitability before, during, and after exercise was assessed by changes in motor evoked potentials triggered by transcranial magnetic stimulation. VitC increased superoxide dismutase (marker for endogenous antioxidant capacity) by 129% and mitigated C-reactive protein (marker for inflammation) in the plasma during exercise but failed to alter the exercise-induced increase in lipid peroxidation (malondialdehyde) and free radicals [electron paramagnetic resonance (EPR)-spectroscopy]. Although VitC did, indeed, decrease neuromuscular fatigue (ΔQtw: PL −29 ± 5%, VitC −23 ± 6%, P < 0.05), there was no impact on corticospinal excitability and time to task failure (∼8 min, P = 0.8). Interestingly, in terms of pulmonary limitations to exercise, VitC had no effect on perceived exertional dyspnea (∼8.5/10) and its determinants, including oxygen saturation () (∼92%) and respiratory muscle work (∼650 cmH2O·s·min−1) (P > 0.3). Thus, although VitC facilitated indicators for antioxidant capacity, diminished inflammatory markers, and improved neuromuscular fatigue resistance, it failed to improve exertional dyspnea and cycling exercise tolerance in patients with COPD. As dyspnea is recognized to limit exercise tolerance in COPD, the otherwise beneficial effects of VitC may have been impacted by this unaltered sensation.
NEW & NOTEWORTHY We investigated the effect of intravenous vitamin C on redox balance, exertional dyspnea, neuromuscular fatigue, and exercise tolerance in chronic obstructive pulmonary disease (COPD) patients. Acute vitamin C administration increased superoxide dismutase (marker of antioxidant capacity) and attenuated fatigue development but failed to improve exertional dyspnea and exercise tolerance. These findings suggest that a compromised redox balance plays a critical role in the development of fatigue in COPD but also highlight the significance of exertional dyspnea as an important symptom limiting the patients’ exercise tolerance.
Keywords: central and peripheral fatigue, exercise, oxidative stress, respiratory muscle work, vitamin C
INTRODUCTION
Patients with chronic obstructive pulmonary disease (COPD) are characterized by increased skeletal muscle fatigue and, likely as a consequence, impaired exercise tolerance (1, 2). Fatigue, defined as a reversible reduction in force/power-generating capacity of an individual muscle or muscle group, can be peripheral and/or central in origin (3). Peripheral fatigue is the result of biochemical changes within skeletal muscle, yielding an attenuated response to neural stimulation. Central fatigue refers to a failure of the central nervous system (CNS) to maintain force/power, resulting in a reduction in descending neural drive and/or output from the spinal motoneurons (4). Interestingly, redox balance may play a role in both forms of fatigue. Specifically, reactive oxygen species (ROS) contribute to peripheral fatigue by impairing excitation-contraction coupling (5, 6) and stimulating central fatigue by augmenting the discharge rate of metabosensitive group IV afferents (7). Compared with their healthy counterparts, patients with COPD suffer from greater levels of ROS (8–11) and systemic inflammation (12, 13) even at rest, but particularly during exercise. Consequently, the contribution of free radicals to fatigue is likely greater in patients with COPD and could help to explain the faster development of fatigue during exercise in this population (14).
Recent studies, employing single-joint, small-muscle mass exercise and antioxidant interventions, identified ROS as a contributor to the impaired development of fatigue (using intravenous ascorbate, VitC) (15), and limited endurance capacity (utilizing N-acetylcysteine) (16), in patients with COPD. However, as limb and respiratory muscles are the main source of augmented ROS in COPD (8, 17, 18), exercise engaging only a small muscle mass, with low ventilatory requirements (19), likely evokes substantially less systemic oxidative stress compared with a locomotor activity, such as cycling, involving a large muscle mass and demanding significant respiratory muscle work. Of note, COPD-specific pulmonary limitations (14, 20, 21) have, in addition to altered intrinsic muscle characteristics (22–24), been identified as significant contributors to the greater exercise intolerance and compromised fatigue resistance in COPD patients. Indeed, although limb discomfort can limit exercise in most healthy individuals (25), there is considerable evidence that exertional dyspnea is key in limiting exercise in COPD (26, 27). Therefore, cycling exercise may be better suited to investigate the overall impact of exercise-induced redox balance, inflammation, and exertional dyspnea on fatigue in patients with COPD.
As noted above, metabosensitive group IV afferents can be stimulated by free radicals and have been documented to facilitate central fatigue (28, 29); however, this may be achieved, at least in part, by impairing corticospinal (CS) excitability, which negatively impacts neural muscle activation (30–33). Indeed, CS projections constitute the major motor pathway linking the brain to the working skeletal muscle (34), and limited research in this area has previously linked COPD to a dysfunctional CS pathway and compromised cortical excitability (35, 36). However, these findings were obtained during acute exacerbations of COPD and are limited to studies of muscles of the hand at rest. Presently, there are no data documenting the impact of ROS on CS excitability during locomotor exercise in patients with COPD.
Consequently, utilizing intravenous VitC, this study aimed to investigate the effect of exercise-induced changes in redox balance, inflammation, exertional dyspnea, neuromuscular fatigue, and cycling exercise tolerance in patients with COPD. We hypothesized that VitC administration would 1) increase antioxidant capacity, attenuating both oxidative stress and inflammation as a result of exercise, and 2) attenuate both exertional dyspnea and the development of fatigue, in part by increasing CS excitability, and therefore improve exercise tolerance in patients with COPD.
METHODS
Participants
Eight patients with COPD [GOLD classification 1–3; forced expiratory flow in 1 s (FEV1): 60 ± 4% predicted; peak O2 consumption (): 17 ± 2 mL·kg−1·min−1; peak work rate (Wpeak): 106 ± 12 W] volunteered to participate in the study. All patients had a history of smoking (1–3 packs/day); one participant was actively smoking at the time of the experiment but refrained from the use of tobacco products for 12 h before all data collection. The subjects were not engaged in regular physical activity and did not participate in a pulmonary rehabilitation program. All participants were utilizing inhaled bronchodilators before and throughout the study but refrained from using them the morning of the testing sessions. To avoid the potential interaction with ROS (37, 38), none of the participants used supplemental oxygen. Patient characteristics, including general hematological parameters obtained from a blood draw, are summarized in Table 1. All participants refrained from consuming supplemental vitamins for 1 wk before and during their participation in the study. Written informed consent was obtained from all participants. All experimental procedures were approved by the University of Utah and the Salt Lake City Department of Veterans Affairs Medical Center Institutional Review Boards and conformed to the Declaration of Helsinki, except for registration in a database.
Table 1.
Subject characteristics
| Age, yr | 65 ± 3 |
| Height, cm | 180 ± 1 |
| Weight, kg | 94 ± 3 |
| , % | 95 ± 0.4 |
| FVC, L | 3.6 ± 0.2 |
| FVC, % predicted | 76 ± 4 |
| SVC, L | 4.1 ± 0.3 |
| SVC, % predicted | 85 ± 5 |
| FEV1, L | 2.0 ± 0.2 |
| FEV1, % predicted | 60 ± 3 |
| FEV1/FVC | 60 ± 2 |
| FEV1/FVC, % predicted | 75 ± 3 |
| FEF25-75, L/s | 1.4 ± 0.3 |
| FEF25-75, % predicted | 44 ± 6 |
| IC, L | 3.4 ± 0.3 |
| IC, % predicted | 100 ± 9 |
| ERV, L | 0.65 ± 0.10 |
| ERV, % predicted | 47 ± 6.6 |
| Glucose, mg/dL | 115.0 ± 13.2 |
| Cholesterol, mg/dL | 179.8 ± 19.0 |
| Triglycerides, mg/dL | 119.5 ± 16.0 |
| HDL, mg/dL | 45.9 ± 3.5 |
| LDL, mg/dL | 112.4 ± 14.0 |
| WBC, K/μL | 6.9 ± 1.0 |
| RBC, M/μL | 4.7 ± 0.2 |
| Hemoglobin, g/dL | 14.5 ± 0.6 |
| Hematocrit, % | 43 ± 2 |
Values are means ± SE. ERV, expiratory reserve volume; FEF25-75, forced expiratory flow from 25 to 75% of forced vital capacity (FVC); FEV1, forced expiratory flow in 1 s; HDL, high-density cholesterol; IC, inspiratory capacity; LDL, low-density cholesterol; RBC, red blood cells; , oxygen saturation; SVC, slow vital capacity; WBC, white blood cells. N = 8.
Experimental Protocol
Exercise.
All participants were thoroughly familiarized with the experimental procedures during two preliminary visits; all exercise sessions were separated by a minimum of 48 h. During the first visit to the laboratory, participants performed a maximal incremental cycling exercise test to determine Wpeak and . After a 2-min warm-up at 20 W, the workload was increased by 5–15 W/min (14) until participants could no longer continue the exercise. Then, with the goal of minimizing the potential influence of learning effects, all participants practiced the constant-load cycling exercise (79 ± 7% Wpeak) to task failure (Tlim), at a self-selected constant pedal frequency (85 ± 10 rpm), several times. Task failure was defined as a >10% drop (in rpm) from the target cadence. Participants were enrolled in the experimental trials after a reproducible cycling time to task failure (coefficient of variation < 10%) was achieved during the practice sessions. This required two to four practice trials, performed on separate days. The two subsequent visits were single blinded and in random order: 1) with intravenous bolus infusion of saline (0.9% NaCl infused at 1 mL/min for 20 min, before any cycling exercise) (placebo trial, PL) and 2) with intravenous bolus infusion of ascorbate (100 mg/mL l-ascorbic acid dissolved in normal saline, infused at 1 mL/min for 20 min, before any cycling exercise) (experimental trial, VitC). A 20-gauge intravenous catheter was placed at the antecubital vein near the elbow and was used to infuse isotonic saline or ascorbate and to take blood samples before and immediately after exercise for later analysis. Finally, to allow “isotime” comparisons, all participants returned for a final visit during which the shorter of the two trials was repeated (isotime) with the other infusate (PL or VitC).
Cycle ergometer setup.
Participants were positioned on the cycle ergometer (Velotron, Elite Model; Racer Mate, Seattle, WA) with their feet fastened securely to the pedals. The participants’ preferred seat height was recorded during the initial visit and fixed for all remaining trials. A mouthpiece attached to a one-way nonrebreathe valve (Hans Rudolph Inc., Shawnee, KS) was fixed, with a clamp, directly in front of the participant at a comfortable height to ensure consistent posture and minimal head movement. The crank angle of the cycle ergometer was monitored continuously via a calibrated linear encoder mounted on the crankshaft. All stimulations during cycling were elicited at a fixed point of the crank cycle during the peak electromyogram (EMG) burst in the cycle, measured on the right vastus lateralis (VL) (knee angle ∼100°).
Measurements
Exercise responses.
Ventilation and pulmonary gas exchange were measured at rest and during exercise with a metabolic cart (Medgraphics Ultima CFX; MGC Diagnostics, St. Paul, MN). Heart rate was determined from the R-R interval of a 12-lead electrocardiogram (Nasiff Cardiocard, Central Square, NY), and arterial oxygen saturation () was estimated with a forehead pulse oximeter (Nellcor N-595, Pleasanton, CA). Ratings of exertional dyspnea were taken every minute during exercise with Borg’s modified CR10 scale (39). Esophageal pressure (Pes) was measured from a balloon placed, via the nares, in the lower third of the esophagus (40). Work of breathing (Wr) was estimated via the pressure-time products, calculated as described previously (41). The depth of the balloon within the esophagus was marked during the initial trial and kept constant through the remaining experiments.
Exercise flow volume measurements.
While participants were seated in a chair, baseline forced vital capacity (FVC) and slow vital capacity (SVC) maneuvers were performed, during each visit, before exercise testing and according to American Thoracic Society/European Respiratory Society standards (42). The largest FVC and SVC were used to determine the maximal flow-volume loop (MFVL) and vital capacity, respectively, for all flow limitation measurements before and during exercise (43). During rest and every other minute during exercise, participants were asked to perform a maximal inspiration to establish maximal inspiratory capacity (IC) needed to correctly place the exercise tidal flow-volume loop within the MFVL performed at the beginning of the trial (43). Expiratory reserve volume (ERV) was then determined from the four consecutive breaths immediately following the IC maneuver. Maximal inspiratory pressure (MIP) was defined as the lowest Pes established during the IC maneuver. After the IC assessment, ∼10 tidal flow-volume loops were recorded with commercial software (Breeze; MGC Diagnostics). The percentage of expiratory flow limitation (EFL) was determined as the percentage of the exercise tidal flow-volume loop that either met or exceeded the boundary of the MFVL (44). Dynamic hyperinflation (DH) was defined as an increase in ERV over functional residual capacity (FRC) (45). Of note, for reasons related to patient safety (i.e., dizziness/syncope following FVCs), MFVLs were obtained while participants were seated in a chair (vs. on the bike) and, to minimize the delay between the end of exercise and the assessment of postexercise neuromuscular function, only before the start of exercise. Consequently, because of postural differences between sitting in a chair and on a bike, the potential for a bronchodilation-induced widening of the MFVL during exercise, and the fact that the MFVL was obtained outside a body plethysmograph, the utilized preexercise MFVLs might not fully reflect the patients’ true MFVLs during upright cycling and could have influenced the quantification of expiratory flow limitations (46).
Torque and electromyogram recordings.
Torque and EMG recordings were measured as described previously (47). Briefly, quadriceps torque of the right leg was measured with a calibrated linear strain gauge (MLP 300; Transducer Techniques, Temecula, CA). Electromyogram recordings were recorded with surface electrodes (Ag-AgCl, 10-mm diameter) placed over the muscle belly of the vastus lateralis (VL) and biceps femoris (BF) in a bipolar configuration. Surface electrodes were placed according to SENIAM-recommended guidelines (48). EMG signals were amplified 1,000 times (Neurolog Systems; Digitimer, Welwyn Garden City, UK), band-pass filtered (20–1,000 Hz, NL-844; Digitimer), and analog to digitally converted at a sampling rate of 2,000 Hz with a 16-bit Micro 1401 mk-II and Spike 2 data collection software (Cambridge Electronic Design Ltd, Cambridge, UK).
Stimulations.
Two forms of stimulations were used during each session: 1) electrical motor nerve stimulation (MNS) and 2) transcranial magnetic stimulation (TMS).
electrical motor nerve stimulation.
The position of the stimulating electrode on the femoral nerve (located high in the femoral triangle) that elicited the greatest compound muscle action potential (M wave) in VL and quadriceps twitch torque was determined by delivering low-intensity single-pulse stimuli (200-µs pulse width; 100–150 mA) with a cathode probe (with the anode fixed between the greater trochanter and iliac crest) connected to a 400-V stimulator (model DS7AH; Digitimer). Once established, the cathode electrode was fixed in this optimal position. Thereafter, the stimulation intensity was increased in 30-mA increments until the size of the M wave exhibited no further increase (i.e., maximal M wave, Mmax) at rest and during a 50% quadriceps maximum voluntary contraction (MVC). The stimulation intensity was set at 130% of that which elicited Mmax and kept constant throughout the session. Optimal position for the MNS electrode and stimulator intensity were reestablished for all trials (PL: 640 ± 82 mA, VitC: 667 ± 94 mA).
transcranial magnetic stimulation.
A double-cone coil (diameter 130 mm) connected to a magnetic stimulator (Magstim 200; The Magstim Company Ltd, Dyfed, UK) was used to elicit motor evoked potentials (MEPs) in the VL. First, during a 20% quadriceps maximum voluntary contraction (MVC), the optimal coil position (posterior to anterior direction of current flow in the motor cortex) to preferentially activate the left motor cortex was determined by finding the location that elicited the greatest MEP and twitch torque for a given stimulator output (position relative to vertex: ∼2–3 cm lateral). This location was marked directly on the scalp for accurate placement throughout the session. Active motor threshold (AMT), defined as the smallest intensity of stimulation used to elicit a MEP, was then established during a VL contraction evoking an EMG response that corresponded to 20% of maximal VL EMG output. The stimulator intensity was set to 120% of AMT (72 ± 9% of maximum stimulator output) during the 20% of maximal EMG contraction to ensure a clear MEP. This established TMS intensity was used to measure CS excitability during the pre- and postexercise assessment of quadriceps function. TMS intensities were reestablished in every session. The output intensity of the stimulator was similar between sessions (P = 0.4).
Neuromuscular assessment of quadriceps function and corticospinal excitability.
Participants were seated comfortably on a custom-built chair with full back support, such that the hip and knee were at ∼120° and ∼90° of flexion, respectively. A cuff attached to the strain gauge was also attached ∼2 cm above the lateral malleolus of the right leg. Cuff position was marked at the ankle to ensure identical positioning between testing sessions. Then, three sets of contractions, separated by 45 s, were performed. In each set, participants performed a 3-s MVC during which a superimposed twitch was elicited via MNS to calculate the voluntary quadriceps activation (VA) (49). Immediately after the MVC, the femoral nerve was stimulated again to evoke a potentiated quadriceps resting twitch (Qtw,pot). Then, for the measurement of CS excitability, three single TMS pulses and one MNS pulse (randomized) were elicited during a sustained isometric knee extension corresponding to 20% of each participant’s maximal EMG output (obtained during MVC). Three sets were performed before infusion (Pre) and after infusion (Post-Inf). Two sets were performed immediately after exercise (Post).
Stimulations during cycling.
Three TMS and one MNS were elicited at the beginning of exercise (∼5 s after target workload was reached), every other minute during exercise, and within the final minute of the trial. Each stimulation was separated by at least five full pedal revolutions. Intensity of stimulator outputs remained constant from the established intensities for CS excitability before exercise. EMG during cycling was normalized to Mmax and served as a surrogate for central motor drive.
Analysis of neuromuscular data.
The twitch amplitude from both MNS- and TMS-evoked twitches was determined by taking the peak torque minus the onset torque. The peak-to-peak amplitude and area of MEPs and Mmax were measured between cursors placed to encompass all phases of evoked potentials in the VL. The area of each MEP was normalized to that of Mmax to account for changes in muscle sarcolemmal excitability. The cycling EMG signal was rectified and waveform average analysis was performed over a 20-s segment, immediately after the stimulation set had been completed. The reference point for overlaying and averaging was taken as the same point on the crank angle that was used to elicit stimulations (knee angle ∼100°). EMG during each revolution was averaged over a 100-ms window (50 ms before and 50 ms after the ∼100° knee angle). Root-mean-squared electromyogram (EMGrms) was also measured during the static contractions from a 100-ms segment before the point of stimulation.
The contractile properties of quadriceps muscle fibers were assessed from resting twitches elicited by MNS. Contraction time (CT) was represented by the time from the onset of the twitch to peak amplitude. Rate of force development (RFD) was determined as the twitch amplitude normalized to CT. Maximal rate of force development (MRFD) was calculated by measurement of the steepest rate of torque development. This was determined as the highest positive derivative of the torque for an interval of 10 ms between two cursors placed either side of the rise in torque. Maximal rate of relaxation (MRR) was calculated as the steepest rate of decline in torque, determined by the highest negative derivative of the torque for an interval of 10 ms between two cursors placed either side of the fall in torque. Half-relaxation time (RT0.5) was taken as the interval between the peak amplitude and the point at which torque was reduced by 50%.
Antioxidant capacity, inflammation, oxidative stress, and free radicals.
For all saline and VitC infusion trials, venous blood samples were obtained from the antecubital vein at rest (Pre) and immediately after exercise (Post). Plasma samples were stored at −80°C until later analysis. All samples were analyzed as described previously (15). Briefly, endogenous antioxidant activity was determined by quantifying plasma superoxide dismutase (SOD) and catalase (CAT) activity (50) (Cayman Chemical Company, Ann Arbor, MI). C-reactive protein level, a marker of inflammation, was determined by an immunoassay on plasma (R&D Systems, Minneapolis, MN). Lipid peroxidation, a marker of oxidant damage, was assessed by plasma malondialdehyde (MDA) levels (Bioxytech LPO-586, Foster City, CA). Oxygen-centered free radical levels were assessed by spin trapping and electron paramagnetic resonance (EPR). Three milliliters of venous blood was collected into a Vacutainer containing 1 mL of the spin trap α-phenyl-tert-butylnitrone (PBN) (0.0140 mol/L). After centrifugation, the PBN was extracted from the serum supernatant with toluene, and the adduct (200 µL) was pipetted into a precision-bore quartz EPR sample tube (Wilmad, Vineland, NJ) previously flushed with compressed N2. EPR was then performed at 21°C with an EMX X-band spectrometer (Bruker, Massachusetts) with commercially available software (version 2.11, Bruker Win EPR System).
Statistical Analysis
Data are presented as means ± SE. A two-way repeated-measures ANOVA (condition × time) was used to compare the effect of VitC on physiological parameters; a Tukey post hoc test was used to correct for multiple comparisons if a main effect was identified. A one-way repeated-measures ANOVA was used to compare the effect of treatment (VitC vs. PL) on physiological parameters averaged over the final minute of exercise and indexes of fatigue; a Tukey post hoc test was used to correct for multiple comparisons if a main effect was identified. Statistical significance was set at P < 0.05.
RESULTS
Impact of VitC on Exercise-Induced Changes in Blood-Borne Biomarkers
Neither the VitC nor the PL infusion evoked any side effects, and all participants remained unaware of the experimental condition. The effects of VitC infusion on the pre-/postexercise change in antioxidant capacity, inflammation, lipid peroxidation, and free radicals are documented in Table 2. VitC infusion significantly increased end-exercise plasma ascorbate levels, whereas there was no change in the PL condition. VitC infusion increased endogenous antioxidant capacity during exercise as reflected by the significant increase in end-exercise SOD levels. Furthermore, whereas C-reactive protein was significantly increased after exercise in the PL condition in all participants, this increase was prevented with the VitC infusion (P = 0.3).
Table 2.
Quantitative assessment of antioxidants, oxidative stress, and inflammation at baseline and after exercise following an intravenous saline or ascorbate infusion
| PL | VitC | |
|---|---|---|
| Ascorbate Pre, µg/mL | 8 ± 1 | 7 ± 3 |
| Ascorbate Post Ex, µg/mL | 8 ± 3 | 91 ± 30*† |
| CRP Pre, ng/mL | 1,674 ± 379 | 1,620 ± 357 |
| CRP Post Ex, ng/mL | 2,782 ± 660* | 1,757 ± 384† |
| MDA Pre, µM | 4.2 ± 0.4 | 3.9 ± 0.3 |
| MDA Post Ex, µM | 4.4 ± 0.4 | 4.3 ± 0.2 |
| SOD Pre, U/mL | 2.31 ± 0.36 | 1.95 ± 0.42 |
| SOD Post Ex, U/mL | 2.95 ± 0.35* | 4.46 ± 0.44*† |
| CAT Pre, μM | 113 ± 26 | 98 ± 18 |
| CAT Post Ex, μM | 163 ± 26* | 184 ± 21* |
| EPR (AUC) Pre | 2.7 ± 0.6 | 2.7 ± 0.6 |
| EPR (AUC) Post Ex | 9.5 ± 4.0* | 9.8 ± 3.4* |
Values are means ± SE. AUC, area under the curve; CAT, catalase; CRP, C-reactive protein; EPR, electron paramagnetic resonance; MDA, malondialdehyde; PL, placebo (saline); Post Ex, after exercise; Pre, baseline; SOD, superoxide dismutase; VitC, ascorbate. *P < 0.05 vs. Pre. †P < 0.05 vs. PL. N = 8.
Cycling Time to Task Failure and Exertional Dyspnea
There was no difference in cycling time to task failure between PL and VitC (P = 0.8; Table 3). Exertional dyspnea was not different between PL and VitC (P = 0.8).
Table 3.
Responses to final minute of constant-load exercise
| PL | VitC | |
|---|---|---|
| Work rate, W | 83 ± 10 | 83 ± 10 |
| Work rate, % of peak | 79 ± 3 | 79 ± 3 |
| Exercise time, min | 8.0 ± 1 | 8.0 ± 1 |
| , % | 92 ± 1 | 93 ± 1 |
| HR, beats/min | 126 ± 9 | 128 ± 10 |
| Exertional dyspnea (0–10) | 8.6 ± 0.8 | 8.4 ± 0.8 |
| Ti, s | 0.57 ± 0.06 | 0.57 ± 0.04 |
| Te, s | 1.07 ± 0.11 | 1.10 ± 0.06 |
| Ti/Ttot | 0.35 ± 0.01 | 0.34 ± 0.01 |
| Vt, L | 1.95 ± 0.2 | 1.97 ± 0.21 |
| fR, breaths/min | 39.4 ± 4.3 | 37.1 ± 2.1 |
| , L/min | 74 ± 8 | 72 ± 8 |
| , L/min | 1.59 ± 0.15 | 1.50 ± 0.18 |
| , % | 99 ± 3 | 97 ± 4 |
| , L/min | 2.03 ± 0.2 | 1.97 ± 0.23 |
| / | 49 ± 4.8 | 52 ± 6.3 |
| / | 37 ± 3 | 39 ± 4 |
| ICdyn, L | 2.7 ± 0.2 | 2.6 ± 0.2 |
| EFL, % | 72 ± 8 | 74 ± 9 |
| MIP, cmH2O | −40 ± 4 | −38 ± 4 |
Values are means ± SE. EFL, expiratory flow limitation; fR, respiratory frequency; HR, heart rate; ICdyn, dynamic inspiratory capacity; MIP, maximal inspiratory pressure; PL, placebo; , oxygen saturation; Te, expiratory time; Ti, inspiratory time; Ttot, respiratory cycle duration; , CO2 production; , minute ventilation; VitC, ascorbate; , oxygen consumption; , maximal ; Vt, tidal volume. N = 8.
Neuromuscular Function and Corticospinal Excitability
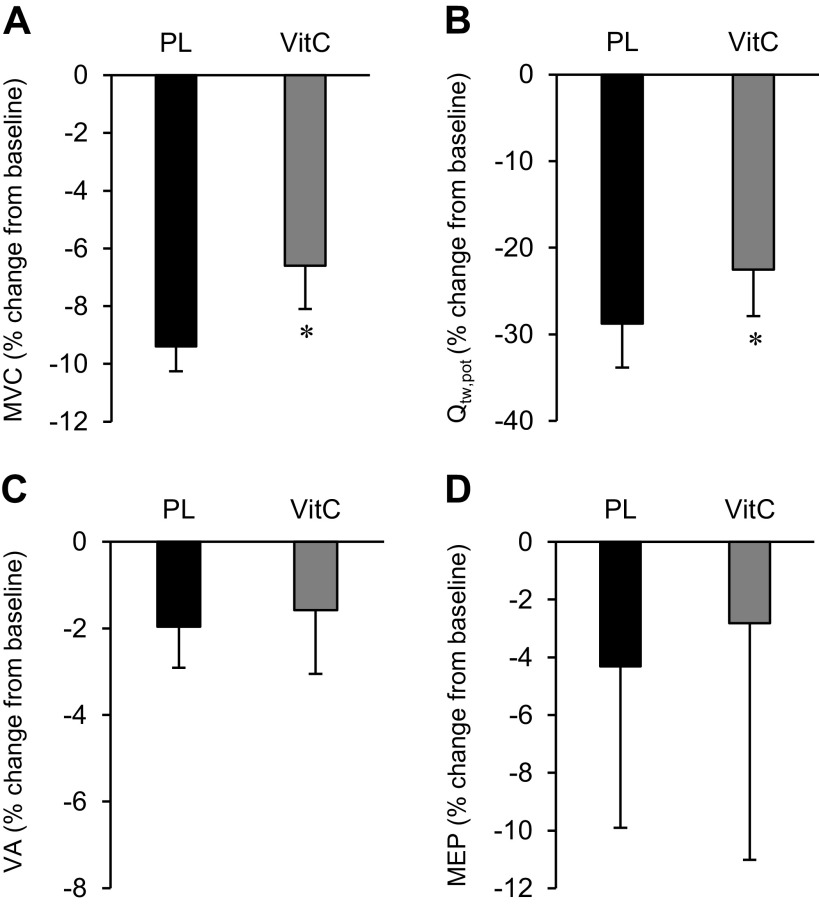
The indexes of neuromuscular function (MVC, Qtw,pot, VA, Mmax, MEP) were not affected by the infusion of VitC (P > 0.4) or saline (P > 0.5) and were similar between days (P > 0.4). Exercise-induced changes in neuromuscular function are presented in Table 4. Preexercise Mmax area (∼0.03 mVs) and peak-to-peak amplitude (∼4.4 mV) were similar between conditions before exercise (P > 0.3) and did not change after PL or VitC infusion (P > 0.3) or after exercise (P > 0.3). Exercise in the PL condition induced a significant degree of peripheral fatigue, as indicated by the reduction in both Qtw,pot and MVC (Fig. 1). VitC significantly reduced the pre-/postexercise fall in Qtw,pot (by ∼22%) and MVC (by ∼29%) but had no effect on VA (Fig. 1).
Table 4.
Exercise-induced changes in neuromuscular function
| PL | VitC | |
|---|---|---|
| RFD, Nm/s | 0.27 ± 0.04*† | 0.35 ± 0.06* |
| RFD, %c | −30 ± 6† | −17 ± 9 |
| CT, ms | 138 ± 18*† | 116 ± 13* |
| CT, % vs. Pre | 12 ± 3 | 5 ± 6 |
| MRFD, Nm/s | 594 ± 106*† | 706 ± 120* |
| MRFD, % vs. Pre | −33 ± 7† | −17 ± 10 |
| MRR, Nm/s | 305 ± 71* | 314 ± 41* |
| MRR, % vs. Pre | −16 ± 12 | −7 ± 10 |
| RT0.5, ms | 148 ± 19*† | 121 ± 13* |
| RT0.5, % vs. Pre | 11 ± 4 | 4 ± 5 |
| VL SP, ms | 106 ± 14 | 102 ± 3 |
| VL SP, % vs. Pre | −6 ± 6 | 4 ± 3 |
Values are means ± SE. CT, contraction time; MRFD, maximal rate of force development; MRR, maximal rate of relaxation; PL, placebo; Pre, baseline; RFD, rate of force development; RT0.5, half-relaxation time; VitC, ascorbate; VL SP, silent period of the vastus lateralis. *P < 0.05 vs. Pre. †P < 0.05 vs. PL. N = 8.
Figure 1.
Neuromuscular fatigue and corticospinal excitability represented as % change from pre- to postexercise. A: maximal voluntary contraction (MVC). B: potentiated quadriceps twitch torque (Qtw,pot). C: voluntary activation (VA). D: changes in motor evoked potentials (MEPs) were evoked during a quadriceps contraction corresponding to 20% of the electromyogram (EMG) obtained during the preexercise MVC. Data are presented as means ± SE. PL, placebo; VitC, ascorbate. *P < 0.05 vs. PL. N = 8.
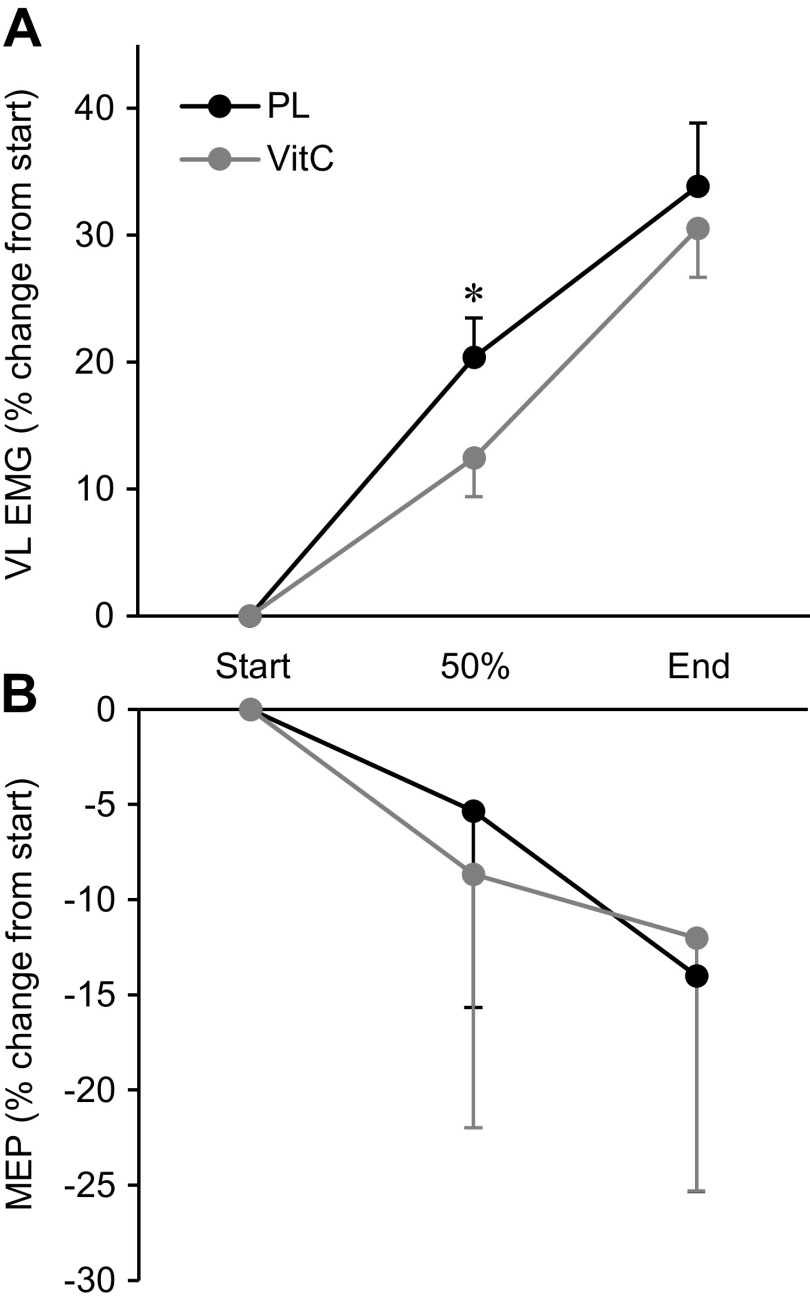
Various EMG data obtained during cycling exercise are presented as percent change from the first stimulation set, occurring ∼5 s after the onset of exercise (Fig. 2A). VitC attenuated the rise in EMG during exercise (P < 0.05). Mmax area, determined during cycling, remained unaltered in both conditions (PL 28 ± 3 µV·s; VitC 28 ± 4 µV·s; P > 0.4). MEPs were unchanged before, during, and after exercise in both conditions (Fig. 2B and Fig. 1D).
Figure 2.
Changes in electromyography (EMG, A) and motor evoked potentials (MEPs, B) during cycling exercise. Patients were stimulated during the first minute of cycling and every other minute thereafter until exhaustion. The 50% time point corresponds with the stimulation set closest to 50% of time to exhaustion. All MEPs were normalized to maximal M wave (Mmax) from the same stimulation set. Data are presented as means ± SE. PL, placebo; VitC, ascorbate; VL, vastus lateralis. *P < 0.05 vs. VitC. N = 8.
Pulmonary Responses
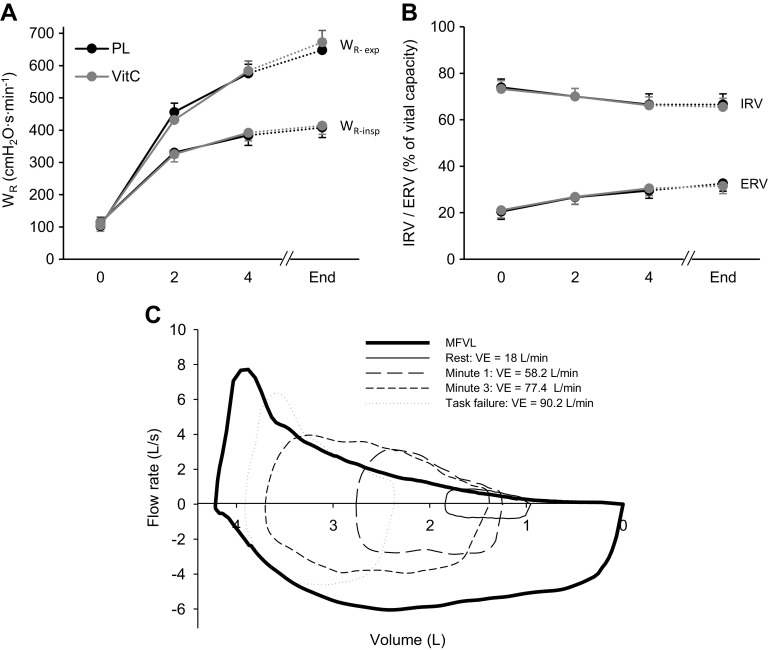
Ventilatory responses, pulmonary gas exchange, heart rate, and during the final minute of exercise are reported in Table 3. During the final minute of exercise, IC was, compared with rest, decreased in both conditions (PL 2.7 ± 0.2 L, VitC 2.6 ± 0.2 L; P < 0.001). Compared with the start of exercise, inspiratory reserve volume (IRV) progressively decreased whereas ERV increased during exercise (both P < 0.05; Fig. 3C). There was no effect of VitC on IRV and ERV (P > 0.5). Wr is illustrated in Fig. 3. MIPs were not affected by VitC (P > 0.6) (Table 3). EFL significantly increased throughout the exercise. VitC had no effect on EFL (Table 3).
Figure 3.
Pulmonary mechanics and flow-volume loops. All data points were recorded every other minute while there were no femoral or transcranial magnetic stimulations. End represents the final 30 s of exercise (uninterrupted by stimulations). A: respiratory muscle work (WR). B: inspiratory (IRV) and expiratory (ERV) reserve volume. C: representative example illustrating the maximum flow-volume loop (MFVL) and exercise-induced changes in tidal flow-volume loops. Data are presented as means ± SE. PL, placebo; , minute ventilation; VitC, ascorbate; WR-exp, expiratory WR; WR-insp, inspiratory WR N = 8.
DISCUSSION
This study sought to investigate the effect of VitC on exercise-induced redox balance, markers for inflammation, exertional dyspnea, neuromuscular fatigue, and cycling exercise tolerance in patients with COPD. Furthermore, the impact of ROS on CS excitability, which when attenuated hinders neural muscle activation and can contribute to fatigue during locomotor exercise, was assessed in this patient population. Acute VitC administration increased the patients’ antioxidant capacity, as suggested by the 129% increase in SOD, and attenuated the development of fatigue, a critical determinant of exercise tolerance, without affecting CS excitability during cycling exercise. However, VitC failed to improve exertional dyspnea, a key factor limiting cycle exercise performance in patients with COPD. Together, these findings suggest that a compromised redox balance plays a critical role in the exaggerated development of neuromuscular fatigue in patients with COPD but also highlight the significance of exertional dyspnea as an important symptom limiting endurance exercise tolerance in this population.
Impact of VitC on Selected Blood-Borne Biomarkers
Intravenous ascorbate improved the patients’ antioxidant defense system including CAT and SOD but did not affect the exercise-induced increases in oxygen-centered free radicals and lipid peroxidation assessed in the plasma (Table 2). As VitC is, generally, thought to attenuate oxidative stress by tipping the balance between pro- and antioxidant forces toward the latter (51), these observations were unexpected but not entirely surprising. Specifically, the current indexes of VitC-induced alterations in oxidative stress were limited to plasma MDA levels and mainly (52–54), but not exclusively, oxygen-centered free radicals (i.e., EPR) and do not exclude the potential positive effects on other free radical species and indexes of oxidative stress. Furthermore, the markers of oxidative stress employed are based on venous plasma samples and therefore may not exactly reflect the effect of the VitC on the cellular redox balance within the exercising muscle. Finally, high doses of exogenous antioxidants, such as vitamin C, have been suggested to display prooxidative effects (55, 56). In this case, the improved antioxidative capacity may have now faced elevated prooxidative activity, with the net effect of a similar exercise-induced increase in oxygen-centered free radicals and lipid peroxidation in the PL and VitC trials.
Interestingly, VitC prevented the exercise-induced increase in C-reactive protein observed during PL exercise (Table 2). This confirms earlier findings (57) and suggests that intravenous ascorbate may have attenuated the heightened inflammatory response characterizing patients with COPD (58). Importantly, since CRP is only one of many indexes of inflammation, the present observations do not allow for a definitive conclusion related to the effect of VitC on the inflammatory response to exercise. Moreover, our findings cannot determine whether the invariant level of C-reactive protein following exercise with VitC is secondary to the attenuated neuromuscular fatigue and, therefore, ameliorated muscle damage, or whether the attenuated inflammatory response actually contributed to the decrease in fatigue. This conundrum, although difficult to unravel, deserves additional evaluation.
Exercise-Induced Neuromuscular Fatigue and Corticospinal Excitability
Cycling exercise resulted in substantial peripheral quadriceps fatigue that was attenuated by ∼25% when the identical bout was repeated after the VitC infusion (Fig. 1). Interestingly, as limb and respiratory muscles are recognized to be the major source of augmented ROS in COPD (8, 17, 18, 59), VitC during cycling exercise (characterized by a large active muscle mass and high respiratory muscle work) attenuated peripheral fatigue to an extent (∼25%; Fig. 1) similar to that previously documented during single-leg knee extension exercise (characterized by small active muscle mass and low respiratory muscle work) (15, 60).
As ROS are recognized to impair cellular components that result in peripheral fatigue and lower myofibrillar Ca2+ sensitivity and Ca2+ reuptake by the sarcoplasmic reticulum (61, 62), the observed improvement in peripheral fatigue during VitC may be the consequence of a positive effect of VitC on the intramuscular redox balance (15). Indeed, CT, RFD, MRFD, RT0.5, and MRR, all variables directly influenced by myofibrillar Ca2+ sensitivity and SR Ca2+ cycling (63), were attenuated to a lesser extent during VitC compared with PL (Table 4). These findings highlight a potential role of ROS in determining the patients’ compromised peripheral fatigue resistance and suggest that VitC may attenuate peripheral fatigue in COPD, at least in part, by improved intramuscular Ca2+ handling. In addition to these intramuscular effects, VitC may have mediated the decrease in peripheral fatigue through its beneficial effects on limb blood flow. Indeed, endogenous antioxidants can improve exercising leg blood flow in COPD by alleviating the adverse impact of oxidative stress on various vascular control mechanisms (64). It is therefore reasonable to speculate that, given the sensitivity of peripheral fatigue to muscle O2 delivery (65), an increase in leg blood flow, secondary to the VitC infusion, could partially account for the lower level of end-exercise neuromuscular fatigue during VitC.
Maintaining or enhancing the functional integrity of the CS pathway during physical activity is key for the CNS to optimally “drive” muscle contraction required for a given task (29). While increases in EMG (i.e., surrogate for central motor drive) enhance CS excitability during cycling exercise, peripheral fatigue inhibits the motor pathway, with the net effect of no change in CS excitability in healthy individuals (31, 32, 47, 66). Furthermore, group III/IV muscle afferents, stimulated by ROS (7) and metabolites associated with peripheral fatigue (67), can alter CS excitability (68, 69). However, despite the positive effects of VitC on redox balance and peripheral fatigue in the patients with COPD, overall CS excitability and exercise-induced changes in VA were unaltered by this intervention. Although these findings suggest that oxidative stress and peripheral fatigue do not compromise the net excitability of the CS pathway during exercise in patients with COPD, the present data cannot address their specific effect on motor cortical and spinal motoneuronal excitability, the two compartments determining the net excitability of the CS motor pathway.
Pulmonary System Responses and Endurance Exercise Tolerance
All patients exhibited typical COPD-specific pulmonary responses to exercise, including, compared with healthy individuals (14, 70), an exaggerated Wr, exercise-induced arterial desaturation, evidence of expiratory flow limitation (see methods for critical consideration), and dynamic hyperinflation (Fig. 3, Table 3). As both exaggerated Wr and arterial desaturation limit peripheral hemodynamics and limb O2 delivery (71), these factors likely contributed, substantially, to the development of neuromuscular fatigue during exercise (14). Regardless, VitC did not alter Wr and arterial oxygenation during exercise, suggesting that the beneficial effect of intravenous ascorbate on the development of fatigue was predominantly the consequence of the aforementioned intracellular and/or vascular processes.
Exertional dyspnea has previously been identified as a key factor limiting exercise in COPD (26, 27). It may therefore be speculated that, despite a significant positive effect on neuromuscular fatigue, the lack of improvement in exercise tolerance with VitC was primarily due to the failure of this intervention to ameliorate the dyspnea exhibited by the patients. Specifically, as reflected in the patients’ similar derangements in pulmonary mechanics, high end-expiratory lung volumes, and exaggerated Wr during VitC and PL (Table 3, Fig. 3), VitC did not alter key determinants of the multifaceted mechanistic basis of exertional dyspnea (72). Regardless, the exact impact of antioxidant supplementation on endurance exercise in patients with COPD remains equivocal, with some studies reporting a positive effect (16), whereas others (60), including the present investigation, report no effect. Interestingly, in this context, even studies on healthy individuals are controversial, with some documenting an improvement in endurance performance following antioxidant supplementation (73) and others reporting no change (74).
It is important to note that the results of this study, although robust and similar in all participants, are based on a relatively small sample size. The present findings should therefore be considered a first step in investigating the utility of antioxidant supplementation for improving exercise capacity in COPD. Additional studies using larger sample sizes and perhaps different antioxidants are needed before the clinical relevance of this approach can be determined with certainty.
Conclusions
Although VitC raised markers for antioxidant capacity, diminished markers for inflammation, and improved neuromuscular fatigue resistance, VitC failed to improve exertional dyspnea and cycling exercise tolerance in patients with COPD. As dyspnea is recognized to limit exercise tolerance in COPD, the otherwise beneficial effects of VitC may have been impacted by this unaltered sensation. The outcome of this study therefore highlights the inability of VitC to improve exercise capacity in COPD and the significance of exertional dyspnea in limiting endurance exercise tolerance in this population.
GRANTS
This study was supported by National Heart, Lung, and Blood Institute Grants HL-116579 and HL-103786 as well as Ruth L. Kirschstein National Research Service Award T32 HL-139451 and by the Department of Veterans Affairs Office of Rehabilitation Research and Development (E6910-R, E1697-R, E1572-P, and E3207-R).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
T.J.H. and M.A. conceived and designed research; T.J.H., J.C.W., S.K.S., T.S.T., V.R.R., J.Z., A.D.N., and N.M.B. performed experiments; T.J.H., V.R.R. and J.Z. analyzed data; T.J.H., J.C.W., S.K.S., T.S.T., R.S.R., and M.A. interpreted results of experiments; T.J.H. prepared figures; T.J.H. drafted manuscript; T.J.H., J.C.W., S.K.S., T.S.T., R.S.R., and M.A. edited and revised manuscript; T.J.H., J.C.W., S.K.S., T.S.T., V.R.R., J.Z., A.D.N., N.M.B., R.S.R., and M.A. approved final version of manuscript.
REFERENCES
- 1.Oga T, Nishimura K, Tsukino M, Sato S, Hajiro T, Mishima M. Exercise capacity deterioration in patients with COPD: longitudinal evaluation over 5 years. Chest 128: 62–69, 2005. doi: 10.1378/chest.128.1.62. [DOI] [PubMed] [Google Scholar]
- 2.Saey D, Debigare R, LeBlanc P, Mador MJ, Cote CH, Jobin J, Maltais F. Contractile leg fatigue after cycle exercise: a factor limiting exercise in patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med 168: 425–430, 2003. doi: 10.1164/rccm.200208-856OC. [DOI] [PubMed] [Google Scholar]
- 3.Taylor JL, Amann M, Duchateau J, Meeusen R, Rice CL. Neural contributions to muscle fatigue: from the brain to the muscle and back again. Med Sci Sports Exerc 48: 2294–2306, 2016. [ doi: 10.1249/MSS.0000000000000923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Amann M. Central and peripheral fatigue: interaction during cycling exercise in humans. Med Sci Sports Exerc 43: 2039–2045, 2011. doi: 10.1249/MSS.0b013e31821f59ab. [DOI] [PubMed] [Google Scholar]
- 5.Andrade FH, Reid MB, Allen DG, Westerblad H. Effect of hydrogen peroxide and dithiothreitol on contractile function of single skeletal muscle fibres from the mouse. J Physiol 509: 565–575, 1998. doi: 10.1111/j.1469-7793.1998.565bn.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Westerblad H, Allen DC. Cellular mechanisms of skeletal muscle fatigue. In: Molecular and Cellular Aspects of Muscle Contraction, edited by Sugi H. Boston, MA: Springer, 2003, p. 563–570. [DOI] [PubMed] [Google Scholar]
- 7.Delliaux S, Brerro-Saby C, Steinberg JG, Jammes Y. Reactive oxygen species activate the group IV muscle afferents in resting and exercising muscle in rats. Pflugers Arch 459: 143–150, 2009. doi: 10.1007/s00424-009-0713-8. [DOI] [PubMed] [Google Scholar]
- 8.Couillard A, Koechlin C, Cristol JP, Varray A, Prefaut C. Evidence of local exercise-induced systemic oxidative stress in chronic obstructive pulmonary disease patients. Eur Respir J 20: 1123–1129, 2002. doi: 10.1183/09031936.02.00014302. [DOI] [PubMed] [Google Scholar]
- 9.Delample D, Durand F, Severac A, Belghith M, Mas E, Michel F, Cristol JP, Hayot M, Prefaut C. Implication of xanthine oxidase in muscle oxidative stress in COPD patients. Free Radic Res 42: 807–814, 2008. doi: 10.1080/10715760802429039. [DOI] [PubMed] [Google Scholar]
- 10.Remels AH, Gosker HR, Langen RC, Schols AM. The mechanisms of cachexia underlying muscle dysfunction in COPD. J Appl Physiol (1985) 114: 1253–1262, 2013. doi: 10.1152/japplphysiol.00790.2012. [DOI] [PubMed] [Google Scholar]
- 11.Wüst RC, Degens H. Factors contributing to muscle wasting and dysfunction in COPD patients. Int J Chron Obstruct Pulmon Dis 2: 289–300, 2007. [PMC free article] [PubMed] [Google Scholar]
- 12.Gan WQ, Man SF, Senthilselvan A, Sin DD. Association between chronic obstructive pulmonary disease and systemic inflammation: a systematic review and a meta-analysis. Thorax 59: 574–580, 2004. doi: 10.1136/thx.2003.019588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.van Helvoort HA, Heijdra YF, de Boer RC, Swinkels A, Thijs HM, Dekhuijzen PN. Six-minute walking-induced systemic inflammation and oxidative stress in muscle-wasted COPD patients. Chest 131: 439–445, 2007. doi: 10.1378/chest.06-1655. [DOI] [PubMed] [Google Scholar]
- 14.Amann M, Regan MS, Kobitary M, Eldridge MW, Boutellier U, Pegelow DF, Dempsey JA. Impact of pulmonary system limitations on locomotor muscle fatigue in patients with COPD. Am J Physiol Regul Integr Comp Physiol 299: R314–R324, 2010. doi: 10.1152/ajpregu.00183.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rossman MJ, Garten RS, Groot HJ, Reese V, Zhao J, Amann M, Richardson RS. Ascorbate infusion increases skeletal muscle fatigue resistance in patients with chronic obstructive pulmonary disease. Am J Physiol Regul Integr Comp Physiol 305: R1163–R1170, 2013. doi: 10.1152/ajpregu.00360.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Koechlin C, Couillard A, Simar D, Cristol JP, Bellet H, Hayot M, Prefaut C. Does oxidative stress alter quadriceps endurance in chronic obstructive pulmonary disease? Am J Respir Crit Care Med 169: 1022–1027, 2004. doi: 10.1164/rccm.200310-1465OC. [DOI] [PubMed] [Google Scholar]
- 17.Heunks LM, Viña J, van Herwaarden CL, Folgering HT, Gimeno A, Dekhuijzen PN. Xanthine oxidase is involved in exercise-induced oxidative stress in chronic obstructive pulmonary disease. Am J Physiol Regul Integr Comp Physiol 277: R1697–R1704, 1999. doi: 10.1152/ajpregu.1999.277.6.R1697. [DOI] [PubMed] [Google Scholar]
- 18.Wouters EF, Creutzberg EC, Schols AM. Systemic effects in COPD. Chest 121: 127S–130S, 2002. doi: 10.1378/chest.121.5_suppl.127S. [DOI] [PubMed] [Google Scholar]
- 19.Richardson RS, Sheldon J, Poole DC, Hopkins SR, Ries AL, Wagner PD. Evidence of skeletal muscle metabolic reserve during whole body exercise in patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med 159: 881–885, 1999. doi: 10.1164/ajrccm.159.3.9803049. [DOI] [PubMed] [Google Scholar]
- 20.Amann M. Pulmonary system limitations to endurance exercise performance in humans. Exp Physiol 97: 311–318, 2012. doi: 10.1113/expphysiol.2011.058800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Criner GJ, Celli BR. Ventilatory muscle recruitment in exercise with O2 in obstructed patients with mild hypoxemia. J Appl Physiol (1985) 63: 195–200, 1987. doi: 10.1152/jappl.1987.63.1.195. [DOI] [PubMed] [Google Scholar]
- 22.Maltais F, Decramer M, Casaburi R, Barreiro E, Burelle Y, Debigaré R, Dekhuijzen PN, Franssen F, Gayan-Ramirez G, Gea J, Gosker HR, Gosselink R, Hayot M, Hussain SN, Janssens W, Polkey MI, Roca J, Saey D, Schols AM, Spruit MA, Steiner M, Taivassalo T, Troosters T, Vogiatzis I, Wagner PD, ATS/ERS Ad Hoc Committee on Limb Muscle Dysfunction in COPD. An official American Thoracic Society/European Respiratory Society Statement: Update on limb muscle dysfunction in chronic obstructive pulmonary disease. Am J Respir Crit Care Med 189: e15–e62, 2014. doi: 10.1164/rccm.201402-0373ST. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Richardson RS, Leek BT, Gavin TP, Haseler LJ, Mudaliar SR, Henry R, Mathieu-Costello O, Wagner PD. Reduced mechanical efficiency in chronic obstructive pulmonary disease but normal peak VO2 with small muscle mass exercise. Am J Respir Crit Care Med 169: 89–96, 2004. doi: 10.1164/rccm.200305-627OC. [DOI] [PubMed] [Google Scholar]
- 24.Whittom F, Jobin J, Simard PM, Leblanc P, Simard C, Bernard S, Belleau R, Maltais F. Histochemical and morphological characteristics of the vastus lateralis muscle in patients with chronic obstructive pulmonary disease. Med Sci Sports Exerc 30: 1467–1474, 1998. doi: 10.1097/00005768-199810000-00001. [DOI] [PubMed] [Google Scholar]
- 25.Hamilton AL, Killian KJ, Summers E, Jones NL. Symptom intensity and subjective limitation to exercise in patients with cardiorespiratory disorders. Chest 110: 1255–1263, 1996. doi: 10.1378/chest.110.5.1255. [DOI] [PubMed] [Google Scholar]
- 26.Maltais F, Hamilton A, Marciniuk D, Hernandez P, Sciurba FC, Richter K, Kesten S, O’Donnell D. Improvements in symptom-limited exercise performance over 8 h with once-daily tiotropium in patients with COPD. Chest 128: 1168–1178, 2005. doi: 10.1378/chest.128.3.1168. [DOI] [PubMed] [Google Scholar]
- 27.O’Donnell DE, Flüge T, Gerken F, Hamilton A, Webb K, Aguilaniu B, Make B, Magnussen H. Effects of tiotropium on lung hyperinflation, dyspnoea and exercise tolerance in COPD. Eur Respir J 23: 832–840, 2004. doi: 10.1183/09031936.04.00116004. [DOI] [PubMed] [Google Scholar]
- 28.Amann M, Proctor LT, Sebranek JJ, Pegelow DF, Dempsey JA. Opioid-mediated muscle afferents inhibit central motor drive and limit peripheral muscle fatigue development in humans. J Physiol 587: 271–283, 2009. doi: 10.1113/jphysiol.2008.163303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gandevia SC. Spinal and supraspinal factors in human muscle fatigue. Physiol Rev 81: 1725–1789, 2001. doi: 10.1152/physrev.2001.81.4.1725. [DOI] [PubMed] [Google Scholar]
- 30.Butler JE, Taylor JL, Gandevia SC. Responses of human motoneurons to corticospinal stimulation during maximal voluntary contractions and ischemia. J Neurosci 23: 10224–10230, 2003. doi: 10.1523/JNEUROSCI.23-32-10224.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Martin PG, Smith JL, Butler JE, Gandevia SC, Taylor JL. Fatigue-sensitive afferents inhibit extensor but not flexor motoneurons in humans. J Neurosci 26: 4796–4802, 2006. doi: 10.1523/JNEUROSCI.5487-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Martin PG, Weerakkody N, Gandevia SC, Taylor JL. Group III and IV muscle afferents differentially affect the motor cortex and motoneurones in humans. J Physiol 586: 1277–1289, 2008. doi: 10.1113/jphysiol.2007.140426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.McNeil CJ, Giesebrecht S, Gandevia SC, Taylor JL. Behaviour of the motoneurone pool in a fatiguing submaximal contraction. J Physiol 589: 3533–3544, 2011. doi: 10.1113/jphysiol.2011.207191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Brouwer B, Ashby P. Corticospinal projections to lower limb motoneurons in man. Exp Brain Res 89: 649–654, 1992. doi: 10.1007/BF00229889. [DOI] [PubMed] [Google Scholar]
- 35.Mohamed-Hussein AA, Hamed SA, Abdel-Hakim N. Cerebral cortical dysfunction in chronic obstructive pulmonary disease: role of transcranial magnetic stimulation. Int J Tuberc Lung Dis 11: 515–521, 2007. [PubMed] [Google Scholar]
- 36.Oliviero A, Corbo G, Tonali PA, Pilato F, Saturno E, Dileone M, Versace V, Valente S, Di Lazzaro V. Functional involvement of central nervous system in acute exacerbation of chronic obstructive pulmonary disease: a preliminary transcranial magnetic stimulation study. J Neurol 249: 1232–1236, 2002. doi: 10.1007/s00415-002-0817-y. [DOI] [PubMed] [Google Scholar]
- 37.Carpagnano GE, Kharitonov SA, Foschino-Barbaro MP, Resta O, Gramiccioni E, Barnes PJ. Supplementary oxygen in healthy subjects and those with COPD increases oxidative stress and airway inflammation. Thorax 59: 1016–1019, 2004. doi: 10.1136/thx.2003.020768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.van Helvoort HA, Heijdra YF, Heunks LM, Meijer PL, Ruitenbeek W, Thijs HM, Dekhuijzen PN. Supplemental oxygen prevents exercise-induced oxidative stress in muscle-wasted patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med 173: 1122–1129, 2006. doi: 10.1164/rccm.200512-1957OC. [DOI] [PubMed] [Google Scholar]
- 39.Borg GA. Psychophysical bases of perceived exertion. Med Sci Sports Exerc 14: 377–381, 1982. [PubMed] [Google Scholar]
- 40.Baydur A, Behrakis PK, Zin WA, Jaeger M, Milic-Emili J. A simple method for assessing the validity of the esophageal balloon technique. Am Rev Respir Dis 126: 788–791, 1982. doi: 10.1164/arrd.1982.126.5.788. [DOI] [PubMed] [Google Scholar]
- 41.Amann M, Pegelow DF, Jacques AJ, Dempsey JA. Inspiratory muscle work in acute hypoxia influences locomotor muscle fatigue and exercise performance of healthy humans. Am J Physiol Regul Integr Comp Physiol 293: R2036–R2045, 2007. doi: 10.1152/ajpregu.00442.2007. [DOI] [PubMed] [Google Scholar]
- 42.Wanger J, Clausen JL, Coates A, Pedersen OF, Brusasco V, Burgos F, Casaburi R, Crapo R, Enright P, van der Grinten CP, Gustafsson P, Hankinson J, Jensen R, Johnson D, Macintyre N, McKay R, Miller MR, Navajas D, Pellegrino R, Viegi G. Standardisation of the measurement of lung volumes. Eur Respir J 26: 511–522, 2005. doi: 10.1183/09031936.05.00035005. [DOI] [PubMed] [Google Scholar]
- 43.Johnson BD, Weisman IM, Zeballos RJ, Beck KC. Emerging concepts in the evaluation of ventilatory limitation during exercise: the exercise tidal flow-volume loop. Chest 116: 488–503, 1999. doi: 10.1378/chest.116.2.488. [DOI] [PubMed] [Google Scholar]
- 44.Johnson BD, Saupe KW, Dempsey JA. Mechanical constraints on exercise hyperpnea in endurance athletes. J Appl Physiol (1985) 73: 874–886, 1992. doi: 10.1152/jappl.1992.73.3.874. [DOI] [PubMed] [Google Scholar]
- 45.O’Donnell DE, Laveneziana P. Physiology and consequences of lung hyperinflation in COPD. Eur Respir Rev 15: 61–67, 2006. doi: 10.1183/09059180.00010002. [DOI] [Google Scholar]
- 46.Guenette JA, Dominelli PB, Reeve SS, Durkin CM, Eves ND, Sheel AW. Effect of thoracic gas compression and bronchodilation on the assessment of expiratory flow limitation during exercise in healthy humans. Respir Physiol Neurobiol 170: 279–286, 2010. doi: 10.1016/j.resp.2010.01.017. [DOI] [PubMed] [Google Scholar]
- 47.Weavil JC, Sidhu SK, Mangum TS, Richardson RS, Amann M. Intensity-dependent alterations in the excitability of cortical and spinal projections to the knee extensors during isometric and locomotor exercise. Am J Physiol Regul Integr Comp Physiol 308: R998–R1007, 2015. doi: 10.1152/ajpregu.00021.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Hermens HJ, Freriks B, Disselhorst-Klug C, Rau G. Development of recommendations for SEMG sensors and sensor placement procedures. J Electromyogr Kinesiol 10: 361–374, 2000. doi: 10.1016/S1050-6411(00)00027-4. [DOI] [PubMed] [Google Scholar]
- 49.Merton PA. Voluntary strength and fatigue. J Physiol 123: 553–564, 1954. doi: 10.1113/jphysiol.1954.sp005070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Wheeler CR, Salzman JA, Elsayed NM, Omaye ST, Korte DW Jr.. Automated assays for superoxide dismutase, catalase, glutathione peroxidase, and glutathione reductase activity. Anal Biochem 184: 193–199, 1990. doi: 10.1016/0003-2697(90)90668-Y. [DOI] [PubMed] [Google Scholar]
- 51.Packer JE, Slater TF, Willson RL. Direct observation of a free radical interaction between vitamin E and vitamin C. Nature 278: 737–738, 1979. doi: 10.1038/278737a0. [DOI] [PubMed] [Google Scholar]
- 52.Connor HD, Thurman RG, Galizi MD, Mason RP. The formation of a novel free radical metabolite from CCl4 in the perfused rat liver and in vivo. J Biol Chem 261: 4542–4548, 1986. . [PubMed] [Google Scholar]
- 53.Poyer JL, McCay PB, Lai EK, Janzen EG, Davis ER. Confirmation of assignment of the trichloromethyl radical spin adduct detected by spin trapping during 13C-carbon tetrachloride metabolism in vitro and in vivo. Biochem Biophys Res Commun 94: 1154–1160, 1980. doi: 10.1016/0006-291X(80)90540-9. [DOI] [PubMed] [Google Scholar]
- 54.Samuni A, Krishna CM, Riesz P, Finkelstein E, Russo A. Superoxide reaction with nitroxide spin-adducts. Free Radic Biol Med 6: 141–148, 1989. doi: 10.1016/0891-5849(89)90111-1. [DOI] [PubMed] [Google Scholar]
- 55.Bouayed J, Bohn T. Exogenous antioxidants—double-edged swords in cellular redox state: health beneficial effects at physiologic doses versus deleterious effects at high doses. Oxid Med Cell Longev 3: 228–237, 2010. doi: 10.4161/oxim.3.4.12858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Podmore ID, Griffiths HR, Herbert KE, Mistry N, Mistry P, Lunec J. Vitamin C exhibits pro-oxidant properties. Nature 392: 559, 1998. doi: 10.1038/33308. [DOI] [PubMed] [Google Scholar]
- 57.Block G, Jensen CD, Dalvi TB, Norkus EP, Hudes M, Crawford PB, Holland N, Fung EB, Schumacher L, Harmatz P. Vitamin C treatment reduces elevated C-reactive protein. Free Radic Biol Med 46: 70–77, 2009. doi: 10.1016/j.freeradbiomed.2008.09.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Tetley TD. Inflammatory cells and chronic obstructive pulmonary disease. Curr Drug Targets Inflamm Allergy 4: 607–618, 2005. doi: 10.2174/156801005774912824. [DOI] [PubMed] [Google Scholar]
- 59.Couillard A, Maltais F, Saey D, Debigaré R, Michaud A, Koechlin C, LeBlanc P, Préfaut C. Exercise-induced quadriceps oxidative stress and peripheral muscle dysfunction in patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med 167: 1664–1669, 2003. doi: 10.1164/rccm.200209-1028OC. [DOI] [PubMed] [Google Scholar]
- 60.Rossman MJ, Groot HJ, Reese V, Zhao J, Amann M, Richardson RS. Oxidative stress and COPD: The impact of oral antioxidants on skeletal muscle fatigue. Med Sci Sports Exerc 45: 1235–1243, 2013. doi: 10.1249/MSS.0b013e3182846d7e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Allen DG, Lamb GD, Westerblad H. Skeletal muscle fatigue: cellular mechanisms. Physiol Rev 88: 287–332, 2008. doi: 10.1152/physrev.00015.2007. [DOI] [PubMed] [Google Scholar]
- 62.Moopanar TR, Allen DG. Reactive oxygen species reduce myofibrillar Ca2+ sensitivity in fatiguing mouse skeletal muscle at 37°C. J Physiol 564: 189–199, 2005. doi: 10.1113/jphysiol.2005.083519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Stehle R, Solzin J, Iorga B, Poggesi C. Insights into the kinetics of Ca2+-regulated contraction and relaxation from myofibril studies. Pflugers Arch 458: 337–357, 2009. doi: 10.1007/s00424-008-0630-2. [DOI] [PubMed] [Google Scholar]
- 64.Rossman MJ, Trinity JD, Garten RS, Ives SJ, Conklin JD, Barrett-O'Keefe Z, Witman MA, Bledsoe AD, Morgan DE, Runnels S, Reese VR, Zhao J, Amann M, Wray DW, Richardson RS. Oral antioxidants improve leg blood flow during exercise in patients with chronic obstructive pulmonary disease. Am J Physiol Heart Circ Physiol 309: H977–H985, 2015. doi: 10.1152/ajpheart.00184.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Amann M, Calbet JA. Convective oxygen transport and fatigue. J Appl Physiol (1985) 104: 861–870, 2008. doi: 10.1152/japplphysiol.01008.2007. [DOI] [PubMed] [Google Scholar]
- 66.Weavil JC, Sidhu SK, Mangum TS, Richardson RS, Amann M. Fatigue diminishes motoneuronal excitability during cycling exercise. J Neurophysiol 116: 1743–1751, 2016. doi: 10.1152/jn.00300.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Light AR, Hughen RW, Zhang J, Rainier J, Liu Z, Lee J. Dorsal root ganglion neurons innervating skeletal muscle respond to physiological combinations of protons, ATP, and lactate mediated by ASIC, P2X, and TRPV1. J Neurophysiol 100: 1184–1201, 2008. doi: 10.1152/jn.01344.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Sidhu SK, Weavil JC, Mangum TS, Jessop JE, Richardson RS, Morgan DE, Amann M. Group III/IV locomotor muscle afferents alter motor cortical and corticospinal excitability and promote central fatigue during cycling exercise. Clin Neurophysiol 128: 44–55, 2017. doi: 10.1016/j.clinph.2016.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Sidhu SK, Weavil JC, Thurston TS, Rosenberger D, Jessop JE, Wang E, Richardson RS, McNeil CJ, Amann M. Fatigue-related group III/IV muscle afferent feedback facilitates intracortical inhibition during locomotor exercise. J Physiol 596: 4789–4801, 2018. doi: 10.1113/JP276460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Smith JR, Cross TJ, Van Iterson EH, Johnson BD, Olson TP. Resistive and elastic work of breathing in older and younger adults during exercise. J Appl Physiol (1985) 125: 190–197, 2018. doi: 10.1152/japplphysiol.01105.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Harms CA, Babcock MA, McClaran SR, Pegelow DF, Nickele GA, Nelson WB, Dempsey JA. Respiratory muscle work compromises leg blood flow during maximal exercise. J Appl Physiol (1985) 82: 1573–1583, 1997. doi: 10.1152/jappl.1997.82.5.1573. [DOI] [PubMed] [Google Scholar]
- 72.Sheel AW, Foster GE, Romer LM. Exercise and its impact on dyspnea. Curr Opin Pharmacol 11: 195–203, 2011. doi: 10.1016/j.coph.2011.04.004. [DOI] [PubMed] [Google Scholar]
- 73.McKenna MJ, Medved I, Goodman CA, Brown MJ, Bjorksten AR, Murphy KT, Petersen AC, Sostaric S, Gong X. N-acetylcysteine attenuates the decline in muscle Na+,K+-pump activity and delays fatigue during prolonged exercise in humans. J Physiol 576: 279–288, 2006. doi: 10.1113/jphysiol.2006.115352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Medved I, Brown MJ, Bjorksten AR, Murphy KT, Petersen AC, Sostaric S, Gong X, McKenna MJ. N-acetylcysteine enhances muscle cysteine and glutathione availability and attenuates fatigue during prolonged exercise in endurance-trained individuals. J Appl Physiol (1985) 97: 1477–1485, 2004. doi: 10.1152/japplphysiol.00371.2004. [DOI] [PubMed] [Google Scholar]