Abstract
Physical inactivity and low aerobic capacity are primary drivers of chronic disease pathophysiology and are independently associated with all-cause mortality. Conversely, increased physical activity and exercise are central to metabolic disease prevention and longevity. Although these relationships are well characterized in the literature, what remains incompletely understood are the mechanisms by which physical activity/exercise prevents disease. Given methodological constraints of clinical research, investigators must often rely on preclinical rodent models to investigate these potential underlying mechanisms. However, there are several key barriers to applying exercise metabolism findings from rodent models to human health. These barriers include housing temperature, nutrient metabolism, exercise modality, exercise testing, and sex differences. Increased awareness and understanding of these barriers will enhance the ability to impact human health through more appropriate experimental design and interpretation of data within the context of these factors.
Keywords: metabolism, physical activity
INTRODUCTION
Aerobic capacity, an objective, physiological measure of physical fitness primarily determined by habitual physical activity (1, 2), is a strong predictor of all-cause and cardiovascular disease mortality (3–7). Independent of other known risk factors, low aerobic capacity is associated with higher mortality risk (8) and physical inactivity is estimated to cause 8%–9% of annual premature deaths in the United States and worldwide (9, 10). Furthermore, there is overwhelming evidence that physical inactivity is a primary driver of chronic disease pathophysiology (11, 12). Although clinically underappreciated, long-term physical inactivity (a sedentary lifestyle) is central to the cause of cardiorespiratory, skeletal muscle, nervous system, digestive, endocrine, immune, and reproductive diseases. More recently, studies with reduced step count interventions and prolonged sitting have drawn attention to the consequences of short-term physical inactivity on disease susceptibility and progression (13–15). High levels of societal physical inactivity and low aerobic capacity are largely driven by technological advances and the sedentary nature of our modern environment.
To counter these sedentary trappings of society today, physical activity and exercise are known therapies to prevent and slow chronic disease and increase longevity. In fact, even modest increases in step count, physical activity, or aerobic capacity lead to meaningful decreases in all-cause mortality (3, 16). Despite these known relationships, the mechanisms underlying disease prevention by physical activity/exercise remain incompletely understood. Therefore, researchers depend heavily on the use of preclinical rodent models to answer these mechanistic questions. However, as outlined in Fig. 1, key barriers exist in translating findings from rodent exercise models to human health—housing temperature, nutrient metabolism and availability, exercise modality, exercise testing, and sex differences. Therefore, this miniature review aims to discuss these barriers with the goal of enhancing future study design, data interpretation, and, ultimately, clinical relevance.
Fig. 1.
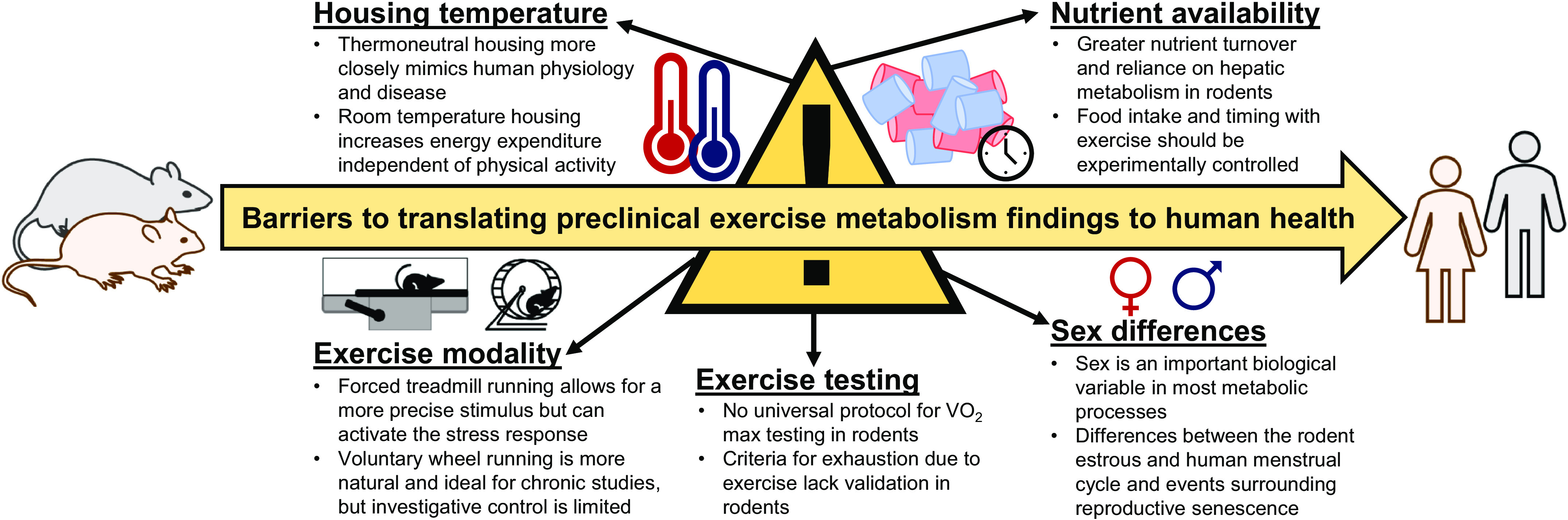
Housing temperature, nutrient availability, exercise modality, exercise testing, and sex differences exist as barriers in translating conclusions from preclinical exercise studies to human health. Careful experimental design and control of these variables paired with thorough reporting of methods and conclusions made within the proper context can maximize potential translation to humans.
HOUSING TEMPERATURE
Broadly, the thermoneutral zone is the range of ambient temperatures that do not require regulatory changes in heat loss or production and is functionally defined as 15°C–25°C for a clothed person completing light office work (17). However, biological factors such as age, sex, body composition, and energy expenditure can all influence a person’s thermoneutral zone by several degrees and clothing can shift the ambient temperature of the thermoneutral zone down by 9°C (compared with 26–33°C nude) (17). We will discuss how this wide range in TNZ and ambient temperatures presents a challenge for conducting metabolic studies and the translation from preclinical models. Importantly, unlike humans who primarily create living environments that are close to thermoneutrality, laboratory mice and rats are commonly housed at ambient room temperature (∼20°C), which falls below their thermoneutral zone of ∼26–34°C (18, 19). For reasons discussed below, we offer housing temperature as an important component of metabolic research experimental design and one that is vital to disclose in all written manuscripts. In doing so, the context and interpretation of data will be improved and so will the opportunity to more closely align rodent exercise modeling to the human condition.
The thermoneutral zone is defined by lower and upper critical temperatures; however, for this review we will focus on subthermoneutral temperatures at which in order to maintain thermal balance an animal must increase its metabolic heat production via shivering and/or nonshivering thermogenesis (20). These thermal maintenance mechanisms come at a high energetic cost. Compared with thermoneutral housing, room temperature housing elicits 30%–50% higher total energy expenditure (21, 22), resting metabolic rate (23), and oxygen consumption (24, 25), independent of known confounding variables such as diet, sex, or physical activity. These findings are not restricted to preclinical models, as mild cold exposure significantly increases resting energy expenditure in healthy men and women when compared with thermoneutral temperatures (26). Recent work by Fischer et al. (23) using high time-resolution calorimetry outlines that mice housed at thermoneutral had an energy expenditure: resting metabolic rate ratio that was 1.8 (compared with 3.1 at room temperature), closely mimicking that of free-living humans (1.6). Together, these data suggest that thermoneutral housing in rodents more closely models human energy metabolism, whereas room temperature housing can be used as a tool to increase energy expenditure independent of increasing exercise or physical activity levels.
Physical activity and exercise adherence are important considerations in selecting a housing temperature for preclinical experiments. Studies examining the effect of thermoneutral versus room temperature housing strongly suggest that ambient temperature does not significantly impact spontaneous physical activity (21, 25, 27). However, the data are less clear when utilizing voluntary wheel running (VWR) as some report decreased running distance at thermoneutral (28, 29), whereas others report that thermoneutral mice significantly outrun room temperature mice (30). These inconsistent data are similar to reports in humans on self-selected physical activity across a thermal gradient, with many showing increased physical activity when transitioning from colder winter temperatures to the spring or summer (31), and others reporting less activity in the warmer summer months (32). Despite inconclusive evidence and unknown mechanisms of activity along a thermal gradient, what remains important is that despite thermoneutral mice running 60% of the distance as their room temperature counterparts, both groups (with similar body weight) improved equally in running capacity, a surrogate marker of aerobic capacity and arguably one of the most clinically relevant exercise adaptations (28). In further support of utility of thermoneutral housing in exercise studies, McKie et al. (30) report that housing temperature did not impact time to exhaustion or run distance in a single bout of maximal treadmill exercise in C57BL/6 mice.
It is well appreciated that exercise and physical activity have profound effects on improving glucose homeostasis and insulin sensitivity. Thus, many preclinical rodent studies have been performed to examine the mechanism by which exercise improves these outcomes. Although not entirely consistent (25), mice housed at thermoneutral generally present with lower fasting blood glucose, lower glucose area under the curve (AUC) following a glucose challenge, and elevated fasting insulin and AUC glucose after an insulin tolerance test (24, 27, 28, 30). These outcomes are indicative of improved glucose tolerance with concurrently reduced insulin sensitivity. Importantly, animals housed at thermoneutral still experience hallmark metabolic impairments induced by high-fat feeding (decreased glucose tolerance, increased obesity) (24, 25). Further, the ability of exercise to improve these indices of metabolic health largely remains intact at thermoneutral (30), despite some recent evidence of blunted skeletal muscle insulin-stimulated glucose uptake in response to VWR (28). However, the report of diminished exercise-induced improvements in insulin action only collected data in female mice, pair housed VWR animals with only 4.5–7.5 km daily running distance per cage, and euthanized animals more than 24 h after wheels were locked (28), all which may help explain the unexpected lack of improvement with VWR at thermoneutrality. For example, it could well be that VWR mice housed at thermoneutral may have had improved insulin sensitivity and glucose tolerance if they had been studied at an earlier timepoint after their last bout of exercise (8–24 h). As a general cautionary note, many of the studies reported herein suggest that the effects of thermoneutral housing on metabolic exercise adaptations do appear to be both tissue- and sex-specific (21, 28).
Of great interest is leveraging thermoneutral housing for new experimental opportunities for investigating exercise in preclinical disease models. High-fat/sucrose diets induce greater pathology in existing rodent models held at thermoneutral, features especially important for disease conditions for which exercise is a primary treatment. There are several reports that thermoneutral housing leads to greater liver triglyceride content (steatosis) on both chow and a HFD (25, 33, 34), a clinically relevant finding as fatty liver disease develops in humans across multiple dietary conditions. Thermoneutral housing also exacerbates Western diet–induced hypercholesterolemia and contributed to the first report of atherosclerotic lesion development in WT mice (35). Similarly, thermoneutral housing increased atherosclerotic plaque burden in both chow- and Western diet–fed APOE−/− mice (36). Together, these data suggest that thermoneutral housing can be utilized to accelerate pathology toward what is seen clinically in human subjects by removing potential protection by room temperature-induced increases in energy expenditure. This in turn allows for new investigative opportunities to examine exercise-induced mechanisms for preventing and treating pathological disease states.
NUTRIENT METABOLISM AND SUBSTRATE AVAILABILITY
First, there are important inherent differences in substrate availability and storage between rodents and humans, which offer context to metabolic adaptations with exercise. These species differences have been excellently reviewed by Kowalski and Bruce previously (37). In short, rats and humans have similar fasting blood glucose and free fatty acid (FFA) levels, whereas mice have twofold higher circulating levels and also a basal glucose turnover rate that is 10–15 times that of humans (38–41). Additionally, the percent contribution of gluconeogenesis and glycogenolysis to endogenous glucose production varies by species. From gluconeogenesis/glycogenolysis, respectively, humans rely on these mechanisms 40%–50%/50%–60%, rats 50%/50%, and mice 80%/20% (37), highlighting a much greater role of the liver in murine glucose homeostasis and overall energy metabolism. Finally, mice intake food ∼36 times per day (42), a stark contrast to the human condition, and undoubtedly impactful to energy metabolism.
Classical clinical studies by Christensen and Hansen and Bergstrom and Hultman (43–45) not only highlighted the importance of carbohydrate utilization for exercise but also hinted at mechanisms by which physical activity improves glucose metabolism. There are notable similarities in skeletal muscle glucose metabolism in rodents and humans that encourage the use of rats and mice as a model system—glucose disposal and transport to the skeletal muscle is greater with exercise (46, 47), contraction restores insulin stimulated glucose uptake (48, 49), and carbohydrate feeding after exercise leads to glycogen re-synthesis (43, 50). However, a single bout of exercise in mice resulted in reduced liver glycogen and maintained skeletal muscle stores, a contrast to human physiology where skeletal muscle glycogen provides local substrate for the working muscle in an exercise intensity–specific manner (51) and is decreased following even moderate exercise (52, 53). As shown in a recent study using stable isotope tracers in mice, exercise training via voluntary wheel running leads to more efficient glucose uptake with a concomitant suppression of glucose output by the liver, further highlighting hepatic metabolism as a central player in the exercise adaptations that occur in mice (52). Relatedly, there is a large body of literature, suggesting that in mice and rats, diet-induced glucose intolerance stems from defects in hepatic glucose metabolism, whereas in humans skeletal muscle defects precede those in the liver (37). Given that dysregulated glucose metabolism is central to many of the metabolic diseases plaquing society today, and an important avenue by which exercise modulates disease prevention and treatment, barriers due to differences in nutrient supply and availability by species should be considered when translating between rodents and human health.
Beyond nutrient supply and metabolic flux, timing and control of food intake is a critical variable when designing research experiments. Energy demand increases during physical activity/exercise, pulling on the mitochondria to produce ATP from glucose, fatty acids, and/or triglycerides to support continued work. Because of this, the availability of these substrates in tissues or in circulation can dramatically impact metabolic responses to exercise. As an example, Philp et al. (54) report that pre-exercise skeletal muscle glycogen content modulates transcriptional activity of peroxisome proliferator-activated receptor delta following exercise, with implications to lipid utilization and hallmark mitochondrial training adaptations. Further, it is well accepted that the fasted state and postprandial condition elicit vastly different metabolic profiles. Fasting upregulates fuel production via glycogenolysis, gluconeogenesis, and lipolysis, and feeding elevates glycolysis, glycogenesis, and lipid synthesis. Although timing and frequency of food intake are not always well defined in clinical trials, research participants are often studied following an overnight fast (8–12 h) to equalize substrate availability and source, as well as liver and muscle glycogen stores, across treatment groups. In preclinical experiments, however, investigators often either forgo food intake controls or subject animals to an extended overnight fast. Given that food intake behavior and metabolic rate are greater in mice and rats than in humans, data support that a morning fast (5–6 h) is sufficient in rodents, and that prolonged fasting beyond 6 h does not result in any difference in intestinal emptying but rather induces stress and initiates survival mechanisms (55–57). Some recent evidence suggests that the routinely used 6-h fast model is suboptimal for assessing insulin responsiveness in mice, due to major weight loss (>5% at room temperature, thermoneutral housing resulted in less weight loss) and hepatic glycogen depletion (58). These authors instead suggest a 2-h fast during the light phase for insulin tolerance tests in mice (58), which would improve translation to humans by minimizing metabolic status not routinely present in the human condition. It is important to note that food restriction has its greatest effect in rodents during the dark phase, when the animals are most active.
Conversely, studying metabolic processes in the postprandial condition (fast-refeed) can be a tool to not only equalize nutrient availability across groups but also postprandial measures are strong predictors of metabolic disease risk (59). Whether data collection occurs in the fasted or fed state and timing of food intake with exercise should be based on the research goal, and importantly, always be consistent between experimental groups. Lack of controlling for food intake and failure to normalize between experimental groups can completely confound all major outcomes, as exemplified in a recent report by Sato et al. (60). Without food restriction before and following exercise, conclusions made by the authors that the time of day in which the exercise was performed resulted in large differences in hepatic and muscle glycogen and blood glucose levels are confounded by well-established differences in food intake behavior between the rest and active phases of the light cycle (60). Given the impact of substrate availability on study outcomes, it is necessary that preclinical studies carefully control food intake and timing around both exercise and/or data collection, to minimize inherent species differences mentioned above and maximize potential for translation to humans.
EXERCISE MODALITY
The two most commonly used rodent exercise modalities to mimic aerobic training in humans are forced treadmill training and volunteer wheel running. These approaches differ in not only researcher ease of use and level of experimental control but also rodent muscle activation, training adaptations, and stress response. Thus, we offer the following context and considerations for choosing which exercise modality is most appropriate for your research question and will yield the greatest potential translation to human health. Critically important with each modality is the variability due to age, sex, and strain of the animals (61, 62).
Volunteer wheel running offers many advantages both in utility and translatability. For starters, VWR more closely mirrors a rodent’s natural running pattern of spontaneous bouts of activity (63) and is generally accepted as a less stressful form of exercise compared with treadmill training (64). As an example of rodents’ desire to run on wheels, an experiment found that without any extrinsic reward, feral mice and rats will voluntarily run on wheels placed in the wild (65). However, despite animals improving aerobic capacity (66) via a high level of cumulative daily activity (∼3–7 h, 4–20 km) (63, 67), VWR’s activity pattern is intermittent in nature, which lends comparison to interval training (albeit greater volume and lower intensity) or high levels of physical activity in humans rather than continuous endurance exercise training. There is no doubt that total time and running volume is greater in VWR rodent models that that of self-selected physical activity in most humans, however as mice age the speed of VWR decreases and could become more analogous to high levels of ambulation in free-living humans. In contrast to treadmill training, which typically occurs without access to food, VWR bouts are interspersed with food intake. This constant supply of exogenous nutritional substrate from feeding, paired with the acute nature of VWR, limits the necessity for hepatic glucose output during running, as VWR does not pose a significant challenge to endogenous fuel production. As mentioned in the nutrient availability section above, these distinguishing features of exercise modality no doubt have impacts on metabolism and exercise adaptations.
Unfortunately, the spontaneous nature of VWR severely limits a researcher’s ability to uniformly prescribe a specific stimulus across all groups (intensity, duration, frequency) and thus run distance/time quickly emerges as a possible confounding variable for different experimental groups. It is also important to note that within an animal, distance and speed of running changes with age, typically peaking around 12–15 weeks old and slowly declining over time (61). However, to counter the lack of experimental control, VWR provides a unique opportunity to study how exercise or physical activity behavior may change under different experimental challenges (diet, age, transgenic modifications, etc.). Another strong critique against the use of VWR as an exercise intervention is that wheel running is not only a measure of physical activity but rather a complex behavior that encompasses motivation and reward seeking (68, 69). Despite these drawbacks, additional hallmark benefits of VWR as an exercise modality are that exercise occurs almost exclusively in the dark cycle (63), thereby minimizing disruptions to diurnal rhythm of laboratory rodents, the low level of investigator burden offers VWR as a useful modality for long-term studies, and the voluntary nature of VWR allows for exercise intervention without added stress to the animal. In fact, wheels are considered cage enrichment for mice and rats.
Owing to the high level of experimental control and continuous nature, forced treadmill training is extensively used in preclinical studies on metabolic adaptations to exercise. This exercise modality allows researchers to specifically program running speed, duration, incline, and intensity to recapitulate a wide range of human activity patterns—most commonly endurance exercise training, but also interval training or acute exercise tests. Compared with VWR, these features of treadmill training offer researchers a high level of consistency and reproducibility, which are critical to the field and our understanding of physiological responses to activity. However, forced treadmill training is not without limitations. On top of the physiological stress from running, forced treadmill running elicits an additional stress response due to a foreign environment, loss of control, and regularly used negative stimuli (electric shock, physical prodding, loud noise, etc.) (70). Reflective of stress response activity, when compared with VWR, treadmill running results in significant increases in mean arterial pressure, heart rate, and mesenteric blood flow immediately following activity of similar intensity and duration (71, 72). Researchers should aim to minimize the level of psychological stress induced by forced treadmill use by properly acclimatizing all animals before experimentation. Further, control animals should be placed on a stationary treadmill with the same negative stimuli as the running group to limit confounding due to stress response activation. An additional critique to forced treadmill training is that it typically takes place during the light cycle, when rodents are normally sleeping and inactive. Exercise during this time may disrupt clock genes, nutrient intake regulatory patterns, and other strong physiological mediators known to have circadian rhythmicity (73, 74), thereby confounding results of the exercise intervention. Despite these drawbacks, forced treadmill training is a potent tool for exercise-induced vascular improvements, mitochondrial changes, and enhanced cardiorespiratory fitness. Therefore, this modality remains an appropriate research tool for exercise studies, so long as conclusions are made within the context of potential confounders (stress response activation, circadian disruption, effects of fasting, etc.).
There are strong advantages and disadvantages to both VWR and forced treadmill running as exercise training modalities. In choosing the most appropriate modality to best translate findings to the human condition, it is important to understand how these considerations may impact the research goal and other experimental design factors. One example is how exercise modality and prescription may dictate skeletal muscles selection for sampling, as several studies in rodents suggest that high-intensity interval exercise tends to recruit larger motor units that include more fast-twitch white fibers (white vastus, gastroc) along with small motor units and red fibers, whereas lower intensity endurance exercise activates only smaller motor units and red fibers (soleus, red vastus, gastroc) (75, 76). When reported together, VWR and treadmill modalities often exhibit differential responses and conclusions (77, 78). However, a recent report suggests that although the intensity of exercise was higher with treadmill training, greater overall volume of exercise with VWR elicited similar responses on body weight, body composition, and adipocyte size (79). These data reinforce that exercise outcomes need to be evaluated based on specific stimuli rather than broad intervention. Finally, the paucity of data directly comparing VWR and forced treadmill training effects should be addressed.
EXERCISE TESTING
Like exercise modality, selecting methods for exercise testing in rodents for the evaluation of training effects or quantification of intervention intensity should be done with careful consideration. The gold standard examination of aerobic capacity in humans is maximal oxygen uptake (V̇O2max), a graded exercise test designed to test the cardiopulmonary system and be completed in 8–12 min (80–82). V̇O2max is achieved when VO2 plateaus despite increased work, with secondary criteria of a respiratory exchange ratio >1.15, postexercise blood lactate ≥8 mmol/L, and achievement of age-predicted maximum heart rate in lieu of a plateau (83). Exercise intensity is often prescribed as a percentage of V̇O2max, with 20%–40%, 40%–60%, and 60%–85% classified as light, moderate, and vigorous activity, respectively (84). There has been a great deal of research effort committed to testing V̇O2max in rodents in a comparable way to human testing. Similar to in the clinic, V̇O2max in rodents is highly dependent upon protocol (speed and time) and treadmill slope, with additional variation due to rodent strain and treadmill familiarization protocol (85, 86). Despite some groups recommending an RER of > 1.0 and blood lactate ≥6 mmol/L as cutoffs to confirm V̇O2max in rodents (87), we are unaware of standardized end point criteria. Given these limitations due to a lack of standardization, and the need for specialized metabolic chambers and gas analyzers, investigators often choose to rely on a low-cost maximum treadmill test to exhaustion as a quantitative measure of exercise capacity and/or training adaptations rather than directly measuring V̇O2max.
The utility of a time to exhaustion exercise test is that maximum run time and speed are touted as surrogate measures for maximum aerobic capacity and can also be used as a tool to characterize exercise intensity as a percentage of achieved maximum (88–90). However, the criterion for exhaustion is often ill-defined, based on practices that lack validation, or is excluded from written methodology altogether—concerns previously highlighted in a perspective by Booth et al. (91). Common approaches to defining exhaustion include an inability of rodents to right themselves, low blood glucose or dramatically reduced tissue glycogen levels, or a high number of contacts with an electrical shock apparatus (92–95). Unfortunately, unlike heart rate monitoring in humans, none of these measures represent limits of cardiovascular (central) contributors to V̇O2max. Furthermore, the lack of consideration of body weight differences (as in absolute and relative V̇O2max in humans) and inability to assess perceived exertion in rodents present other major problems with time to exhaustion tests in rodents.
Use of maximal lactate steady state, the highest exercise intensity at which blood lactate levels remain constant before increasing, is another widely used method for exercise testing in humans. Clinically, maximal lactate steady state equates to ∼70%–80% of maximal aerobic capacity and marks the transition between aerobic and anaerobic metabolism. However, the percentage of maximal aerobic capacity at which this transition occurs shifts to a higher percentage with exercise training (96). Given that blood lactate levels can be readily measured in rodents, this approach is used to assess exercise intensity and training adaptations in both rats and mice (97). Given that maximal lactate steady state is a nonexhaustive exercise test, it avoids many of the limitations mentioned above. However, there are some reports that maximal lactate steady state is independent from exercise training status in rats, limiting its ability to quantify training adaptations (98). Furthermore, recent reports question the reliability of this approach altogether as Lonbro et al. (99) report that gently prodding mice during running (a commonly used negative stimuli during forced treadmill running), resulted in a 30%–120% increase in blood lactate concentrations. Finally, glucose metabolism is profoundly different between mice and humans in ways that likely impact lactate production and catabolism, making it problematic as a comparative tool for clinical findings.
Cardiac hypertrophy, most commonly reported as an increase in heart mass relative to body mass, is used to confirm exercise training as chronic increases in hemodynamic and mechanical loads to the heart ultimately lead to a measurable structural and physiological change. Although this approach mimics an important human training response and has been successful in quantifying adaptations via multiple exercise modalities (VWR, forced treadmill running, swimming, etc.), its utility is limited by sex differences (exercise-induced cardiac hypertrophy is more pronounced in female rodents) and other variables such as diet-induced obesity that may disproportionately alter body weight of a treatment group (100, 101). Reporting heart mass relative to height (tibia length in rodents) has been shown to more accurately represent cardiac hypertrophy and should be considered by investigators using this approach to quantify exercise training adaptations (102).
Aerobic exercise training provokes many metabolic adaptations in skeletal muscle, and researchers have capitalized on the ability to phenotype skeletal muscle fibers to capture fiber-type switching resulting from exercise interventions. The most commonly used methods are phenotyping by SDS-PAGE separation of myosin heavy chain isoforms, myosin ATPase staining, and analysis via immunohistochemistry (103). Benefits to this approach include the ability to infer functional properties of the muscle by fiber phenotype as well as its utility with both aerobic and resistance training exercise interventions. However, like cardiac hypertrophy, this end point measurement only captures training adaptations and cannot measure exercise intensity. In addition, rodent skeletal muscles are primarily homogenous in nature regarding fiber type. For example, the extensor digitorum longus in mice and rats is primarily white (type II), fast-twitch fibers, although the soleus is primarily red (type I), slow-twitch fibers (104). Human skeletal muscle, in contrast, is organized such that red and white fibers are heterogeneously dispersed throughout the muscle beds. Lastly, in humans this approach requires a skeletal muscle biopsy, which is both invasive and requires specialized training by the investigator. Therefore, unless skeletal muscle fiber type is central to the research question, an alternative approach to exercise testing may be more appropriate for translation to human health.
SEX DIFFERENCES
There is an underrepresentation of females in exercise physiology and metabolism studies in the existing literature. This discrepancy stems from: 1) a historical lack of equal opportunity (many seminal studies were conducted in athletes before Title IX) and 2) avoidance of female inclusion owing to a concern or worry about the complexity of menstrual/estrous cycling to impact outcomes. It can be argued that this concern was outsized given that women regularly cycle and that information obtained at any part of the menstrual cycle is applicable to female health. In 2014, the National Institutes of Health implemented a policy requiring preclinical research (both vertebrate animal and human studies) to consider sex as an important biological variable. Since these policies were enacted, a large body of data has emerged highlighting a strong sexual dimorphism in exercise physiology, nutrient metabolism, and disease susceptibility. However, differences in reproductive physiology by species may challenge the translation of these findings and are also an important consideration for experimental design.
In general, there seems to be a good agreement between the sex differences reported in preclinical models of exercise metabolism and disease pathophysiology with those reported in human trials. For example, during endurance exercise both women and female rodents oxidize a greater proportion of lipid substrate and rely less on carbohydrate fuel sources compared with males (105, 106). Furthermore, administration of 17β-estradiol in both men and male rodents result in lower carbohydrate and greater fat oxidation during exercise (as seen in females) with menopause and rodent OVX studies showing the opposite effect (closer to a male phenotype) (107–110). Altogether, these data suggest not only a role of estrogen in this mechanism but also good translation of data on sex differences between rodent models and human studies. Furthermore, this elevated rate of fat oxidation is associated with greater adipocyte lipolysis, higher intramyocellular lipid, a higher proportion of oxidative types I and IIa skeletal muscle fibers, and more efficient mitochondria/greater maximal mitochondrial respiration in women (105, 111, 112). Again, these findings are generally reproducible between rodents and humans (33, 113, 114).
Given the overabundance of mechanistic evidence that estrogen is critical to sex differences reported in our field, paired with the epidemiological data of increased risk of metabolic disease with menopause, the ability to translate work in female rodent models to the clinic is of utmost importance. Women experience a menstrual cycle of specific hormonal changes and uterine events marked by an ovarian follicular (early and late) phase, ovulatory phase, and luteal phase each cycle. Rodents’ uterine lining is reabsorbed rather than shed during menstruation, a hallmark of the estrous cycle, which is defined by four distinct phases—proestrus, estrus, metestrus, and diestrus, roughly corresponding to the human ovarian phases listed above (115). The average menstrual cycle in women is 28 days (with regular cycles ranging from 21 to 40 days in length), whereas the average estrous cycle in rodents lasts only 4–5 days (116). The short length of the estrous cycle in rodents allows for investigation of changes that occur at each reproductive cycle stage, but also presents challenges when attempting to translate measures across several estrous cycles in rodents to only a portion of one menstrual cycle in women. Given mixed results in the literature as to whether menstrual/estrous cycle phase effects exercise metabolism (117–119), it is best to minimize potential confounders within a single study by collecting experimental data from all participants or animals during the same phase of the reproductive cycle. In women, self-report of menses is a low-cost and low-burden crude method of cycle staging, although ability to properly identify subphases is limited. More accurate methods include self-report in combination with basal body temperature, urinary luteinizing hormone (LH) levels, or circulating sex hormone levels, with the most accurate method being sonography (120). In rodents, estrous cycle stage can easily be established by the minimally invasive technique of vaginal cytology (119). At minimum, menstrual/estrous cycle stage could be recorded at the time of data collection, both as part of participant or animal characteristics and for potential covariate statistical analyses.
Given the data on estrogen’s role in metabolic health, another important factor related to sex differences in both rodent models and human research is age and reproductive status. Sexual maturity occurs at ∼15–20 yr and 6–8 wk with menopause or anestrous occurring around 45–55 yr or 15–18 mo, both in female rodents and women, respectively (121, 122). Age and menopausal status should be included as routine anthropometrics in clinical metabolic studies and preclinical work should be designed to match relative age and reproductive status of targeted study population. When considering models of menopause, a few critical differences between rodents and humans must be noted. First, reproductive senescence in humans occurs because of a depletion of immature ovarian follicles via normal ovulatory cycles and atresia (115). The transition to menopause, marked by both a declining follicle pool and dysregulation of the hypothalamic-pituitary-gonadal (HPG) axis, is a gradual process spanning 4–6 yr and typically occurs around the fifth decade of life (123). In menopause, women present with low levels of 17β-estradiol and progesterone, with elevated LH and follicle-stimulating hormone (FSH) (124). In contrast to the human condition, around 9–12 mo of age rats and mice experience dysregulated HPG axis activity (changes in estrogen responsiveness, hypothalamic release of gonadotropin-releasing hormone, etc.), which precede any observable changes in estrous cyclicity (125). Subsequently, rodents start to experience irregular estrous cycles, commonly marked by a persistence of estrus. Eventually, rodents transition to a cessation of cycling, anestrous, characterized by high circulating 17β-estradiol and moderate levels of progesterone, LH, and FSH (126). Unlike humans, rodents maintain the potential for mature ovulatory follicles in reproductive senescence (125). To put these differences in perspective, ovariectomy, which is commonly used to model menopause in rodents, accurately mimics the loss of ovarian follicles and steroid hormones in humans but is limited by its uncharacteristic sudden induction (rather than gradual loss) and removal of reproductive tract (115). Failure to consider these species differences may result in inappropriate translation of findings in rodents to the human condition.
Beyond those due to species differences, there exists a well-understood sexual dimorphism in the levels of voluntary activity and treadmill compliance/performance in rodents, serving as another important consideration for the translation of rodent exercise studies to human trials. As previously reviewed by Rosenfeld in 2017, most rodent studies suggest that female animals engage in more voluntary physical activity than their male counterparts, a contrast to data in humans where across the lifespan, males engage in more physical activity (127). Similarly, with forced treadmill running, male rodents often require negative stimuli, whereas female rats and mice complete running protocols without much investigator intervention (128). As described in the exercise modality section above, given the physiological response to negative stimuli these differences in compliance may confound results and thus warrant consideration.
Variances in body composition and body weight can also be integral to many of the reported sex differences in the field of exercise physiology. Given that women have ∼5%–10% more body fat than men, and diet and training interventions can alter body composition and body weight in a sex-specific manner, these measures are important contextual considerations for research conclusions (129). For example, both rodents and human studies report that males have higher maximal aerobic capacity and grip strength (117, 130–132). However, in most of the cited cases, normalizing these measures to either body weight, lean mass, or specific muscle mass eliminated sex differences reported. Analyzing important experimental outcome variables in relative terms can be a useful tool for matching males and females within an experimental group and may also identify potential mechanisms underlying sex differences.
CONCLUSIONS
Physical activity and exercise can prevent and treat many chronic metabolic diseases that plague human health, such as cardiovascular disease, diabetes, and nonalcoholic fatty liver disease. The mechanisms by which exercise mediates these effects are both multifactorial and incompletely understood. For these reasons, investigators rely on preclinical rodent models to investigate the relationship between activity and disease susceptibility/treatment as rodents provide an opportunity to study all tissues, and employee mechanistic methods that cannot take place in human participants. However, as shown in Fig. 1, housing temperature, nutrient availability, exercise modality, exercise testing, and sex differences exist as barriers in translating preclinical exercise metabolism findings to human health. Although some of these barriers can be highly minimized through thoughtful experimental design (housing temperature, exercise modality and testing, and food intake), intrinsic species differences in nutrient metabolism and reproductive physiology remain. Therefore, we suggest investigators recognize these barriers, select options that both maximize potential translation and best match the research goal, and thoroughly report methodology upon publication. Better designed experiments and conclusions made within the context of these barriers in translating rodent findings from preclinical exercise studies will not only enhance our understanding of the mechanisms of physical activity but improve our ability to impact human health.
GRANTS
K. N. Z. Fuller was supported by American Heart Association Grant 20PRE35120098. J. P. Thyfault was supported by a VA-Merit Grant 1I01BX002567 and NIH R01 KD121497, R01 AR071263, R01 AG069781, and U01 AG070928-01.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
J.P.T. conceived and designed research; K.N.Z.F. and J.P.T. drafted manuscript; K.N.Z.F. and J.P.T. edited and revised manuscript; and K.N.Z.F. and J.P.T. approved final version of manuscript.
REFERENCES
- 1.Church TS, Earnest CP, Skinner JS, Blair SN. Effects of different doses of physical activity on cardiorespiratory fitness among sedentary, overweight or obese postmenopausal women with elevated blood pressure: a randomized controlled trial. JAMA 297: 2081–2091, 2007. doi: 10.1001/jama.297.19.2081. [DOI] [PubMed] [Google Scholar]
- 2.Wang CY, Haskell WL, Farrell SW, Lamonte MJ, Blair SN, Curtin LR, Hughes JP, Burt VL. Cardiorespiratory fitness levels among US adults 20-49 years of age: findings from the 1999-2004 National Health and Nutrition Examination Survey. Am J Epidemiol 171: 426–435, 2010. doi: 10.1093/aje/kwp412. [DOI] [PubMed] [Google Scholar]
- 3.Blair SN, Kohl HW 3rd, Paffenbarger RS Jr, Clark DG, Cooper KH, Gibbons LW. Physical fitness and all-cause mortality. A prospective study of healthy men and women. JAMA 262: 2395–2401, 1989. doi: 10.1001/jama.262.17.2395. [DOI] [PubMed] [Google Scholar]
- 4.Kokkinos P, Myers J, Faselis C, Panagiotakos DB, Doumas M, Pittaras A, Manolis A, Kokkinos JP, Karasik P, Greenberg M, Papademetriou V, Fletcher R. Exercise capacity and mortality in older men: a 20-year follow-up study. Circulation 122: 790–797, 2010. doi: 10.1161/CIRCULATIONAHA.110.938852. [DOI] [PubMed] [Google Scholar]
- 5.Kokkinos P, Myers J, Kokkinos JP, Pittaras A, Narayan P, Manolis A, Karasik P, Greenberg M, Papademetriou V, Singh S. Exercise capacity and mortality in black and white men. Circulation 117: 614–622, 2008. doi: 10.1161/CIRCULATIONAHA.107.734764. [DOI] [PubMed] [Google Scholar]
- 6.Lyerly GW, Sui X, Lavie CJ, Church TS, Hand GA, Blair SN. The association between cardiorespiratory fitness and risk of all-cause mortality among women with impaired fasting glucose or undiagnosed diabetes mellitus. Mayo Clin Proc 84: 780–786, 2009. doi: 10.4065/84.9.780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sandvik L, Erikssen J, Thaulow E, Erikssen G, Mundal R, Rodahl K. Physical fitness as a predictor of mortality among healthy, middle-aged Norwegian men. N Engl J Med 328: 533–537, 1993. doi: 10.1056/NEJM199302253280803. [DOI] [PubMed] [Google Scholar]
- 8.Lee DC, Artero EG, Sui X, Blair SN. Mortality trends in the general population: the importance of cardiorespiratory fitness. J Psychopharmacol 24: 27–35, 2010. doi: 10.1177/1359786810382057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Carlson SA, Adams EK, Yang Z, Fulton JE. Percentage of deaths associated with inadequate physical activity in the United States. Prev Chronic Dis 15: E38, 2018. doi: 10.5888/pcd18.170354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lee IM, Shiroma EJ, Lobelo F, Puska P, Blair SN, Katzmarzyk PT; Lancet Physical Activity Series Working Group. Effect of physical inactivity on major non-communicable diseases worldwide: an analysis of burden of disease and life expectancy. Lancet 380: 219–229, 2012. doi: 10.1016/S0140-6736(12)61031-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Booth FW, Roberts CK, Laye MJ. Lack of exercise is a major cause of chronic diseases. Compr Physiol 2: 1143–1211, 2012. doi: 10.1002/cphy.c110025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Booth FW, Roberts CK, Thyfault JP, Ruegsegger GN, Toedebusch RG. Role of inactivity in chronic diseases: evolutionary insight and pathophysiological mechanisms. Physiol Rev 97: 1351–1402, 2017. doi: 10.1152/physrev.00019.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Healy GN, Wijndaele K, Dunstan DW, Shaw JE, Salmon J, Zimmet PZ, Owen N. Objectively measured sedentary time, physical activity, and metabolic risk: the Australian Diabetes, Obesity and Lifestyle Study (AusDiab). Diabetes Care 31: 369–371, 2008. doi: 10.2337/dc07-1795. [DOI] [PubMed] [Google Scholar]
- 14.Krogh-Madsen R, Thyfault JP, Broholm C, Mortensen OH, Olsen RH, Mounier R, Plomgaard P, van Hall G, Booth FW, Pedersen BK. A 2-wk reduction of ambulatory activity attenuates peripheral insulin sensitivity. J Appl Physiol (1985) 108: 1034–1040, 2010. doi: 10.1152/japplphysiol.00977.2009. [DOI] [PubMed] [Google Scholar]
- 15.Olsen RH, Krogh-Madsen R, Thomsen C, Booth FW, Pedersen BK. Metabolic responses to reduced daily steps in healthy nonexercising men. JAMA 299: 1261–1263, 2008. doi: 10.1001/jama.299.11.1259. [DOI] [PubMed] [Google Scholar]
- 16.Saint-Maurice PF, Troiano RP, Bassett DR Jr, Graubard BI, Carlson SA, Shiroma EJ, Fulton JE, Matthews CE. Association of daily step count and step intensity with mortality among US adults. JAMA 323: 1151–1160, 2020. doi: 10.1001/jama.2020.1382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kingma BR, Frijns AJ, Schellen L, van Marken Lichtenbelt WD. Beyond the classic thermoneutral zone: including thermal comfort. Temperature (Austin) 1: 142–149, 2014. doi: 10.4161/temp.29702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gordon CJ. Thermal physiology of laboratory mice: Defining thermoneutrality. J Therm Biol 37: 654–685, 2012. doi: 10.1016/j.jtherbio.2012.08.004. [DOI] [Google Scholar]
- 19.Poole S, Stephenson JD. Body temperature regulation and thermoneutrality in rats. Exp Physiol 62: 143–149, 1977. doi: 10.1113/expphysiol.1977.sp002384. [DOI] [PubMed] [Google Scholar]
- 20.Scholander PF, Hock R, Walters V, Johnson F, Irving L. Heat regulation in some arctic and tropical mammals and birds. Biol Bull 99: 237–258, 1950. doi: 10.2307/1538741. [DOI] [PubMed] [Google Scholar]
- 21.Morris EM, Noland RD, Allen JA, McCoin CS, Xia Q, Koestler DC, Shook RP, Lighton JRB, Christianson JA, Thyfault JP. Difference in housing temperature-induced energy expenditure elicits sex-specific diet-induced metabolic adaptations in mice. Obesity 28: 1922–1931, 2020. doi: 10.1002/oby.22925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Winn NC, Acin-Perez R, Woodford ML, Hansen SA, Haney MM, Ayedun LA, Rector RS, Vieira-Potter VJ, Shirihai OS, Sacks HS, Kanaley JA, Padilla JA. Thermogenic-like brown adipose tissue phenotype is dispensable for enhanced glucose tolerance in female mice. Diabetes 68: 1717–1729, 2019. doi: 10.2337/db18-1070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fischer AW, Cannon B, Nedergaard J. Optimal housing temperatures for mice to mimic the thermal environment of humans: an experimental study. Mol Metab 7: 161–170, 2018. doi: 10.1016/j.molmet.2017.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Dudele A, Rasmussen GM, Mayntz D, Malte H, Lund S, Wang T. Effects of ambient temperature on glucose tolerance and insulin sensitivity test outcomes in normal and obese C57 male mice. Physiol Rep 3: e12396, 2015. doi: 10.14814/phy2.12396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Small L, Gong H, Yassmin C, Cooney GJ, Brandon AE. Thermoneutral housing does not influence fat mass or glucose homeostasis in C57BL/6 mice. J Endocrinol 239: 313–324, 2018. doi: 10.1530/JOE-18-0279. [DOI] [PubMed] [Google Scholar]
- 26.Langeveld M, Tan CY, Soeters MR, Virtue S, Ambler GK, Watson LP, Murgatroyd PR, Chatterjee VK, Vidal-Puig A. Mild cold effects on hunger, food intake, satiety and skin temperature in humans. Endocr Connect 5: 65–73, 2016. doi: 10.1530/EC-16-0004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Uchida K, Shiuchi T, Inada H, Minokoshi Y, Tominaga M. Metabolic adaptation of mice in a cool environment. Pflugers Arch 459: 765–774, 2010. doi: 10.1007/s00424-010-0795-3. [DOI] [PubMed] [Google Scholar]
- 28.Raun SH, Henriquez-Olguín C, Karavaeva I, Ali M, Møller LLV, Kot W, Castro-Mejía JL, Nielsen DS, Gerhart-Hines Z, Richter EA, Sylow L. Housing temperature influences exercise training adaptations in mice. Nat Commun 11: 1560, 2020. doi: 10.1038/s41467-020-15311-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Takahashi H, Alves CRR, Stanford KI, Middelbeek RJW, Nigro P, Ryan RE, Xue R, Sakaguchi M, Lynes MD, So K, Mul JD, Lee MY, Balan E, Pan H, Dreyfuss JM, Hirshman MF, Azhar M, Hannukainen JC, Nuutila P, Kalliokoski KK, Nielsen S, Pedersen BK, Kahn CR, Tseng YH, Goodyear LJ. TGF-β2 is an exercise-induced adipokine that regulates glucose and fatty acid metabolism. Nat Metab 1: 291–303, 2019. doi: 10.1038/s42255-018-0030-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.McKie GL, Medak KD, Knuth CM, Shamshoum H, Townsend LK, Peppler WT, Wright DC. Housing temperature affects the acute and chronic metabolic adaptations to exercise in mice. J Physiol 597: 4581–4600, 2019. doi: 10.1113/JP278221. [DOI] [PubMed] [Google Scholar]
- 31.Shephard RJ, Aoyagi Y. Seasonal variations in physical activity and implications for human health. Eur J Appl Physiol 107: 251–271, 2009. doi: 10.1007/s00421-009-1127-1. [DOI] [PubMed] [Google Scholar]
- 32.Mora-Rodriguez R, Ortega JF, Fernandez-Elias VE, Kapsokefalou M, Malisova O, Athanasatou A, Husemann M, Domnik K, Braun H. Influence of physical activity and ambient temperature on hydration: the European Hydration Research Study (EHRS). Nutrients 8: 252, 2016.doi: 10.3390/nu8050252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.McCoin CS, Von Schulze A, Allen J, Fuller KNZ, Xia Q, Koestler DC, Houchen CJ, Maurer A, Dorn GW 2nd, Shankar K, Morris EM, Thyfault JP. Sex modulates hepatic mitochondrial adaptations to high-fat diet and physical activity. Am J Physiol Endocrinol Metab 317: E298–E311, 2019. doi: 10.1152/ajpendo.00098.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Von Schulze A, McCoin CS, Onyekere C, Allen J, Geiger P, Dorn GW 2nd, Morris EM, Thyfault JP. Hepatic mitochondrial adaptations to physical activity: impact of sexual dimorphism, PGC1α and BNIP3-mediated mitophagy. J Physiol 596: 6157–6171, 2018. doi: 10.1113/JP276539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Giles DA, Ramkhelawon B, Donelan EM, Stankiewicz TE, Hutchison SB, Mukherjee R, Cappelletti M, Karns R, Karp CL, Moore KJ, Divanovic S. Modulation of ambient temperature promotes inflammation and initiates atherosclerosis in wild type C57BL/6 mice. Mol Metab 5: 1121–1130, 2016. doi: 10.1016/j.molmet.2016.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Tian XY, Ganeshan K, Hong C, Nguyen KD, Qiu Y, Kim J, Tangirala RK, Tontonoz P, Chawla A. Thermoneutral housing accelerates metabolic inflammation to potentiate atherosclerosis but not insulin resistance. Cell Metab 23: 165–178, 2016. doi: 10.1016/j.cmet.2015.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kowalski GM, Bruce CR. The regulation of glucose metabolism: implications and considerations for the assessment of glucose homeostasis in rodents. Am J Physiol Endocrinol Metab 307: E859–E871, 2014. doi: 10.1152/ajpendo.00165.2014. [DOI] [PubMed] [Google Scholar]
- 38.Ayala JE, Bracy DP, McGuinness OP, Wasserman DH. Considerations in the design of hyperinsulinemic-euglycemic clamps in the conscious mouse. Diabetes 55: 390–397, 2006. doi: 10.2337/diabetes.55.02.06.db05-0686. [DOI] [PubMed] [Google Scholar]
- 39.Han BG, Hao CM, Tchekneva EE, Wang YY, Lee CA, Ebrahim B, Harris RC, Kern TS, Wasserman DH, Breyer MD, Qi Z. Markers of glycemic control in the mouse: comparisons of 6-h- and overnight-fasted blood glucoses to Hb A1c. Am J Physiol Endocrinol Metab 295: E981–E986, 2008. doi: 10.1152/ajpendo.90283.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Reaven GM, Hollenbeck C, Jeng CY, Wu MS, Chen YD. Measurement of plasma glucose, free fatty acid, lactate, and insulin for 24 h in patients with NIDDM. Diabetes 37: 1020–1024, 1988. doi: 10.2337/diabetes.37.8.1020. [DOI] [PubMed] [Google Scholar]
- 41.Turner N, Kowalski GM, Leslie SJ, Risis S, Yang C, Lee-Young RS, Babb JR, Meikle PJ, Lancaster GI, Henstridge DC, White PJ, Kraegen EW, Marette A, Cooney GJ, Febbraio MA, Bruce CR. Distinct patterns of tissue-specific lipid accumulation during the induction of insulin resistance in mice by high-fat feeding. Diabetologia 56: 1638–1648, 2013. doi: 10.1007/s00125-013-2913-1. [DOI] [PubMed] [Google Scholar]
- 42.Gannon KS, Smith JC, Henderson R, Hendrick P. A system for studying the microstructure of ingestive behavior in mice. Physiol Behav 51: 515–521, 1992. doi: 10.1016/0031-9384(92)90173-Y. [DOI] [PubMed] [Google Scholar]
- 43.Bergström J, Hultman E. Muscle glycogen synthesis after exercise: an enhancing factor localized to the muscle cells in man. Nature 210: 309–310, 1966. doi: 10.1038/210309a0. [DOI] [PubMed] [Google Scholar]
- 44.Christensen EH, Hansen O. Arbeitsfähigkeit und ernährung. Skand Arch Physiol 81: 160–171, 1939. doi: 10.1111/j.1748-1716.1939.tb01320.x. [DOI] [Google Scholar]
- 45.Christensen EH, Hansen O. Hypoglykame, arbeitsfähigkeit und ermudung. Skand Arch Physiol 81: 172–179, 1939. doi: 10.1111/j.1748-1716.1939.tb01321.x. [DOI] [Google Scholar]
- 46.Burstein R, Polychronakos C, Toews CJ, MacDougall JD, Guyda HJ, Posner BI. Acute reversal of the enhanced insulin action in trained athletes. Association with insulin receptor changes. Diabetes 34: 756–760, 1985. doi: 10.2337/diab.34.8.756. [DOI] [PubMed] [Google Scholar]
- 47.Kump DS, Booth FW. Alterations in insulin receptor signalling in the rat epitrochlearis muscle upon cessation of voluntary exercise. J Physiol 562: 829–838, 2005. doi: 10.1113/jphysiol.2004.073593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Christ-Roberts CY, Pratipanawatr T, Pratipanawatr W, Berria R, Belfort R, Mandarino LJ. Increased insulin receptor signaling and glycogen synthase activity contribute to the synergistic effect of exercise on insulin action. J Appl Physiol (1985) 95: 2519–2529, 2003. doi: 10.1152/japplphysiol.00605.2003. [DOI] [PubMed] [Google Scholar]
- 49.Thyfault JP, Cree MG, Zheng D, Zwetsloot JJ, Tapscott EB, Koves TR, Ilkayeva O, Wolfe RR, Muoio DM, Dohm GL. Contraction of insulin-resistant muscle normalizes insulin action in association with increased mitochondrial activity and fatty acid catabolism. Am J Physiol Cell Physiol 292: C729–C739, 2007. doi: 10.1152/ajpcell.00311.2006. [DOI] [PubMed] [Google Scholar]
- 50.Garcia-Roves PM, Han DH, Song Z, Jones TE, Hucker KA, Holloszy JO. Prevention of glycogen supercompensation prolongs the increase in muscle GLUT4 after exercise. Am J Physiol Endocrinol Metab 285: E729–E736, 2003. doi: 10.1152/ajpendo.00216.2003. [DOI] [PubMed] [Google Scholar]
- 51.Romijn JA, Coyle EF, Sidossis LS, Gastaldelli A, Horowitz JF, Endert E, Wolfe RR. Regulation of endogenous fat and carbohydrate metabolism in relation to exercise intensity and duration. Am J Physiol Endocrinol Metab 265: E380–E391, 1993. doi: 10.1152/ajpendo.1993.265.3.E380. [DOI] [PubMed] [Google Scholar]
- 52.Allerton TD, Kowalski G, Hang H, Stephens J. Dynamic glucose disposal is driven by reduced endogenous glucose production in response to voluntary wheel running: a stable isotope approach. Am J Physiol Endocrinol Metab 319: E2–E10, 2020. doi: 10.1152/ajpendo.00450.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Gollnick PD, Piehl K, Saltin B. Selective glycogen depletion pattern in human muscle fibres after exercise of varying intensity and at varying pedalling rates. J Physiol 241: 45–57, 1974. doi: 10.1113/jphysiol.1974.sp010639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Philp A, MacKenzie MG, Belew MY, Towler MC, Corstorphine A, Papalamprou A, Hardie DG, Baar K. Glycogen content regulates peroxisome proliferator activated receptor-∂ (PPAR-∂) activity in rat skeletal muscle. PLoS One 8: e77200, 2013. doi: 10.1371/journal.pone.0077200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Ayala JE, Samuel VT, Morton GJ, Obici S, Croniger CM, Shulman GI, Wasserman DH, McGuinness OP; NIH Mouse Metabolic Phenotyping Center Consortium. Standard operating procedures for describing and performing metabolic tests of glucose homeostasis in mice. Dis Model Mech 3: 525–534, 2010. doi: 10.1242/dmm.006239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Nowland MH, Hugunin KM, Rogers KL. Effects of short-term fasting in male Sprague-Dawley rats. Comp Med 61: 138–144, 2011. [PMC free article] [PubMed] [Google Scholar]
- 57.Vermeulen JK, de Vries A, Schlingmann F, Remie R. Food deprivation: common sense or nonsense? Animal Techn 48: 45–54, 1997. [Google Scholar]
- 58.Carper D, Coué M, Laurens C, Langin D, Moro C. Reappraisal of the optimal fasting time for insulin tolerance tests in mice. Mol Metab 42: 101058, 2020. doi: 10.1016/j.molmet.2020.101058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Saydah SH, Miret M, Sung J, Varas C, Gause D, Brancati FL. Postchallenge hyperglycemia and mortality in a national sample of U.S. adults. Diabetes Care 24: 1397–1402, 2001. doi: 10.2337/diacare.24.8.1397. [DOI] [PubMed] [Google Scholar]
- 60.Sato S, Basse AL, Schönke M, Chen S, Samad M, Altıntaş A, Laker RC, Dalbram E, Barrès R, Baldi P, Treebak JT, Zierath JR, Sassone-Corsi P. Time of exercise specifies the impact on muscle metabolic pathways and systemic energy homeostasis. Cell Metab 30: 92–110.e4, 2019. doi: 10.1016/j.cmet.2019.03.013. [DOI] [PubMed] [Google Scholar]
- 61.Goh J, Ladiges W. Voluntary wheel running in mice. Curr Protoc Mouse Biol 5: 283–290, 2015. doi: 10.1002/9780470942390.mo140295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Lerman I, Harrison BC, Freeman K, Hewett TE, Allen DL, Robbins J, Leinwand LA. Genetic variability in forced and voluntary endurance exercise performance in seven inbred mouse strains. J Appl Physiol (1985) 92: 2245–2255, 2002. doi: 10.1152/japplphysiol.01045.2001. [DOI] [PubMed] [Google Scholar]
- 63.De Bono JP, Adlam D, Paterson DJ, Channon KM. Novel quantitative phenotypes of exercise training in mouse models. Am J Physiol Regul Integr Comp Physiol 290: R926–R934, 2006. doi: 10.1152/ajpregu.00694.2005. [DOI] [PubMed] [Google Scholar]
- 64.Manzanares G, Brito-da-Silva G, Gandra PG. Voluntary wheel running: patterns and physiological effects in mice. Braz J Med Biol Res 52: e7830, 2018. doi: 10.1590/1414-431X20187830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Meijer JH, Robbers Y. Wheel running in the wild. Proc Biol Sci 281: 20140210, 2014. doi: 10.1098/rspb.2014.0210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Swallow JG, Garland T Jr, Carter PA, Zhan WZ, Sieck GC. Effects of voluntary activity and genetic selection on aerobic capacity in house mice (Mus domesticus). J Appl Physiol (1985) 84: 69–76, 1998. doi: 10.1152/jappl.1998.84.1.69. [DOI] [PubMed] [Google Scholar]
- 67.Allen DL, Harrison BC, Maass A, Bell ML, Byrnes WC, Leinwand LA. Cardiac and skeletal muscle adaptations to voluntary wheel running in the mouse. J Appl Physiol (1985) 90: 1900–1908, 2001. doi: 10.1152/jappl.2001.90.5.1900. [DOI] [PubMed] [Google Scholar]
- 68.Collier G, Hirsch E. Reinforcing properties of spontaneous activity in the rat. J Comp Physiol Psychol 77: 155–160, 1971. doi: 10.1037/h0031588. [DOI] [PubMed] [Google Scholar]
- 69.Greenwood BN, Foley TE, Le TV, Strong PV, Loughridge AB, Day HE, Fleshner M. Long-term voluntary wheel running is rewarding and produces plasticity in the mesolimbic reward pathway. Behav Brain Res 217: 354–362, 2011. doi: 10.1016/j.bbr.2010.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Moraska A, Deak T, Spencer RL, Roth D, Fleshner M. Treadmill running produces both positive and negative physiological adaptations in Sprague-Dawley rats. Am J Physiol Regul Integr Comp Physiol 279: R1321–R1329, 2000. doi: 10.1152/ajpregu.2000.279.4.R1321. [DOI] [PubMed] [Google Scholar]
- 71.Dunn AL, Reigle TG, Youngstedt SD, Armstrong RB, Dishman RK. Brain norepinephrine and metabolites after treadmill training and wheel running in rats. Med Sci Sports Exerc 28: 204–209, 1996. doi: 10.1097/00005768-199602000-00008. [DOI] [PubMed] [Google Scholar]
- 72.Yancey SL, Overton JM. Cardiovascular responses to voluntary and treadmill exercise in rats. J Appl Physiol (1985) 75: 1334–1340, 1993. doi: 10.1152/jappl.1993.75.3.1334. [DOI] [PubMed] [Google Scholar]
- 73.Mrosovsky N. Locomotor activity and non-photic influences on circadian clocks. Biol Rev Camb Philos Soc 71: 343–372, 1996. doi: 10.1111/j.1469-185X.1996.tb01278.x. [DOI] [PubMed] [Google Scholar]
- 74.Wolff G, Esser KA. Scheduled exercise phase shifts the circadian clock in skeletal muscle. Med Sci Sports Exerc 44: 1663–1670, 2012. doi: 10.1249/MSS.0b013e318255cf4c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Laughlin MH, Cook JD, Tremble R, Ingram D, Colleran PN, Turk JR. Exercise training produces nonuniform increases in arteriolar density of rat soleus and gastrocnemius muscle. Microcirculation 13: 175–186, 2006. doi: 10.1080/10739680600556829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Laughlin MH, Korthuis RJ, Sexton WL, Armstrong RB. Regional muscle blood flow capacity and exercise hyperemia in high-intensity trained rats. J Appl Physiol (1985) 64: 2420–2427, 1988. doi: 10.1152/jappl.1988.64.6.2420. [DOI] [PubMed] [Google Scholar]
- 77.Allen JM, Berg Miller ME, Pence BD, Whitlock K, Nehra V, Gaskins HR, White BA, Fryer JD, Woods JA. Voluntary and forced exercise differentially alters the gut microbiome in C57BL/6J mice. J Appl Physiol (1985) 118: 1059–1066, 2015. doi: 10.1152/japplphysiol.01077.2014. [DOI] [PubMed] [Google Scholar]
- 78.Jeneson JA, de Snoo MW, Verlinden NA, Joosten BJ, Doornenbal A, Schot A, Everts ME. Treadmill but not wheel running improves fatigue resistance of isolated extensor digitorum longus muscle in mice. Acta Physiol (Oxf) 190: 151–161, 2007. doi: 10.1111/j.1748-1716.2007.01680.x. [DOI] [PubMed] [Google Scholar]
- 79.Kim YJ, Kim HJ, Lee WJ, Seong JK. A comparison of the metabolic effects of treadmill and wheel running exercise in mouse model. Lab Anim Res 36: 3, 2020. doi: 10.1186/s42826-019-0035-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Balke B, Ware RW. An experimental study of physical fitness of Air Force personnel. U S Armed Forces Med J 10: 675–688, 1959. [PubMed] [Google Scholar]
- 81.Bruce RA, Blackmon JR, Jones JW, Strait G. Exercising testing in adult normal subjects and cardiac patients. Ann Noninvasive Electrocardiol 9: 291–303, 2004. doi: 10.1111/j.1542-474X.2004.93003.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Taylor HL, Buskirk E, Henschel A. Maximal oxygen intake as an objective measure of cardio-respiratory performance. J Appl Physiol 8: 73–80, 1955. doi: 10.1152/jappl.1955.8.1.73. [DOI] [PubMed] [Google Scholar]
- 83.Howley ET, Bassett DR Jr, Welch HG. Criteria for maximal oxygen uptake: review and commentary. Med Sci Sports Exerc 27: 1292–1301, 1995. [PubMed] [Google Scholar]
- 84.Pescatello LS; American College of Sports Medicine; Riebe D, Thompson PD. ACSM’s Guidelines for Exercise Testing and Prescription ( 9th ed.). Philadelphia, PA: Wolters Kluwer Health, 2014. [DOI] [PubMed] [Google Scholar]
- 85.Avila JJ, Kim SK, Massett MP. Differences in exercise capacity and responses to training in 24 inbred mouse strains. Front Physiol 8: 974, 2017. doi: 10.3389/fphys.2017.00974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Wisløff U, Helgerud J, Kemi OJ, Ellingsen O. Intensity-controlled treadmill running in rats: VO(2 max) and cardiac hypertrophy. Am J Physiol Heart Circ Physiol 280: H1301–H, 2001. doi: 10.1152/ajpheart.2001.280.3.H1301. [DOI] [PubMed] [Google Scholar]
- 87.Kemi OJ, Loennechen JP, Wisløff U, Ellingsen Ø. Intensity-controlled treadmill running in mice: cardiac and skeletal muscle hypertrophy. J Appl Physiol 93: 1301–1309, 2002. doi: 10.1152/japplphysiol.00231.2002. [DOI] [PubMed] [Google Scholar]
- 88.Fernando P, Bonen A, Hoffman-Goetz L. Predicting submaximal oxygen consumption during treadmill running in mice. Can J Physiol Pharmacol 71: 854–857, 1993. doi: 10.1139/y93-128. [DOI] [PubMed] [Google Scholar]
- 89.Rodrigues B, Figueroa DM, Mostarda CT, Heeren MV, Irigoyen MC, De Angelis K. Maximal exercise test is a useful method for physical capacity and oxygen consumption determination in streptozotocin-diabetic rats. Cardiovasc Diabetol 6: 38, 2007. doi: 10.1186/1475-2840-6-38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Schefer V, Talan MI. Oxygen consumption in adult and AGED C57BL/6J mice during acute treadmill exercise of different intensity. Exp Gerontol 31: 387–392, 1996. doi: 10.1016/0531-5565(95)02032-2. [DOI] [PubMed] [Google Scholar]
- 91.Booth FW, Laye MJ, Spangenburg EE. Gold standards for scientists who are conducting animal-based exercise studies. J Appl Physiol (1985) 108: 219–221, 2010. doi: 10.1152/japplphysiol.00125.2009. [DOI] [PubMed] [Google Scholar]
- 92.Fitts RH, Booth FW, Winder WW, Holloszy JO. Skeletal muscle respiratory capacity, endurance, and glycogen utilization. Am J Physiol 228: 1029–1033, 1975. doi: 10.1152/ajplegacy.1975.228.4.1029. [DOI] [PubMed] [Google Scholar]
- 93.Hickson RC, Rennie MJ, Conlee RK, Winder WW, Holloszy JO. Effects of increased plasma fatty acids on glycogen utilization and endurance. J Appl Physiol Respir Environ Exerc Physiol 43: 829–833, 1977. doi: 10.1152/jappl.1977.43.5.829. [DOI] [PubMed] [Google Scholar]
- 94.Terjung RL, Baldwin KM, Molé PA, Klinkerfuss GH, Holloszy JO. Effect of running to exhaustion on skeletal muscle mitochondria: a biochemical study. Am J Physiol 223: 549–554, 1972. doi: 10.1152/ajplegacy.1972.223.3.549. [DOI] [PubMed] [Google Scholar]
- 95.Totsuka Y, Nagao Y, Horii T, Yonekawa H, Imai H, Hatta H, Izaike Y, Tokunaga T, Atomi Y. Physical performance and soleus muscle fiber composition in wild-derived and laboratory inbred mouse strains. J Appl Physiol (1985) 95: 720–727, 2003.doi: 10.1152/japplphysiol.00946.2002. [DOI] [PubMed] [Google Scholar]
- 96.Billat VL, Sirvent P, Py G, Koralsztein JP, Mercier J. The concept of maximal lactate steady state: a bridge between biochemistry, physiology and sport science. Sports Med 33: 407–426, 2003. doi: 10.2165/00007256-200333060-00003. [DOI] [PubMed] [Google Scholar]
- 97.Ferreira JC, Rolim NP, Bartholomeu JB, Gobatto CA, Kokubun E, Brum PC. Maximal lactate steady state in running mice: effect of exercise training. Clin Exp Pharmacol Physiol 34: 760–765, 2007. doi: 10.1111/j.1440-1681.2007.04635.x. [DOI] [PubMed] [Google Scholar]
- 98.Gobatto CA, de Mello MA, Sibuya CY, de Azevedo JR, dos Santos LA, Kokubun E. Maximal lactate steady state in rats submitted to swimming exercise. Comp Biochem Physiol A Mol Integr Physiol 130: 21–27, 2001. doi: 10.1016/S1095-6433(01)00362-2. [DOI] [PubMed] [Google Scholar]
- 99.Lønbro S, Wiggins JM, Wittenborn T, Elming PB, Rice L, Pampo C, Lee JA, Siemann DW, Horsman MR. Reliability of blood lactate as a measure of exercise intensity in different strains of mice during forced treadmill running. PLoS One 14: e0215584, 2019. doi: 10.1371/journal.pone.0215584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Fernandes T, Baraúna VG, Negräo CE, Phillips MI, Oliveira EM. Aerobic exercise training promotes physiological cardiac remodeling involving a set of microRNAs. Am J Physiol Heart Circ Physiol 309: H543–H552, 2015. doi: 10.1152/ajpheart.00899.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Oláh A, Mátyás C, Kellermayer D, Ruppert M, Barta BA, Sayour AA, Török M, Koncsos G, Giricz Z, Ferdinandy P, Merkely B, Radovits T. Sex differences in morphological and functional aspects of exercise-induced cardiac hypertrophy in a rat model. Front Physiol 10: 889, 2019. doi: 10.3389/fpls.2019.00889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Yin FC, Spurgeon HA, Rakusan K, Weisfeldt ML, Lakatta EG. Use of tibial length to quantify cardiac hypertrophy: application in the aging rat. Am J Physiol Heart Circ Physiol 243: H941–H947, 1982. doi: 10.1152/ajpheart.1982.243.6.H941. [DOI] [PubMed] [Google Scholar]
- 103.Pandorf CE, Caiozzo VJ, Haddad F, Baldwin KM. A rationale for SDS-PAGE of MHC isoforms as a gold standard for determining contractile phenotype. J Appl Physiol (1985) 108: 222–225, 2010. doi: 10.1152/japplphysiol.01233.2009. [DOI] [PubMed] [Google Scholar]
- 104.Soukup T, Zacharová G, Smerdu V. Fibre type composition of soleus and extensor digitorum longus muscles in normal female inbred Lewis rats. Acta Histochem 104: 399–405, 2002.doi: 10.1078/0065-1281-00660. [DOI] [PubMed] [Google Scholar]
- 105.Carter SL, Rennie C, Tarnopolsky MA. Substrate utilization during endurance exercise in men and women after endurance training. Am J Physiol Endocrinol Metab 280: E898–E907, 2001. doi: 10.1152/ajpendo.2001.280.6.E898. [DOI] [PubMed] [Google Scholar]
- 106.Zhou W, Zeng G, Lyu C, Kou F, Zhang S, Wei H. The effect of exhaustive exercise on plasma metabolic profiles of male and female rats. J Sports Sci Med 18: 253–263, 2019. [PMC free article] [PubMed] [Google Scholar]
- 107.Ellis GS, Lanza-Jacoby S, Gow A, Kendrick ZV. Effects of estradiol on lipoprotein lipase activity and lipid availability in exercised male rats. J Appl Physiol (1985) 77: 209–215, 1994. doi: 10.1152/jappl.1994.77.1.209. [DOI] [PubMed] [Google Scholar]
- 108.Kendrick ZV, Steffen CA, Rumsey WL, Goldberg DI. Effect of estradiol on tissue glycogen metabolism in exercised oophorectomized rats. J Appl Physiol (1985) 63: 492–496, 1987. doi: 10.1152/jappl.1987.63.2.492. [DOI] [PubMed] [Google Scholar]
- 109.Maher AC, Akhtar M, Tarnopolsky MA. Men supplemented with 17beta-estradiol have increased beta-oxidation capacity in skeletal muscle. Physiol Genomics 42: 342–347, 2010.doi: 10.1152/physiolgenomics.00016.2010. [DOI] [PubMed] [Google Scholar]
- 110.Toth MJ, Gardner AW, Arciero PJ, Calles-Escandon J, Poehlman ET. Gender differences in fat oxidation and sympathetic nervous system activity at rest and during submaximal exercise in older individuals. Clin Sci (Lond) 95: 59–66, 1998. doi: 10.1042/cs0950059. [DOI] [PubMed] [Google Scholar]
- 111.Miotto PM, McGlory C, Holloway TM, Phillips SM, Holloway GP. Sex differences in mitochondrial respiratory function in human skeletal muscle. Am J Physiol Regul Integr Comp Physiol 314: R909–R915, 2018. doi: 10.1152/ajpregu.00025.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Roepstorff C, Donsmark M, Thiele M, Vistisen B, Stewart G, Vissing K, Schjerling P, Hardie DG, Galbo H, Kiens B. Sex differences in hormone-sensitive lipase expression, activity, and phosphorylation in skeletal muscle at rest and during exercise. Am J Physiol Endocrinol Metab 291: E1106–1114, 2006. doi: 10.1152/ajpendo.00097.2006. [DOI] [PubMed] [Google Scholar]
- 113.Haizlip KM, Harrison BC, Leinwand LA. Sex-based differences in skeletal muscle kinetics and fiber-type composition. Physiology (Bethesda) 30: 30–39, 2015.doi: 10.1152/physiol.00024.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Stubbins RE, Holcomb VB, Hong J, Núñez NP. Estrogen modulates abdominal adiposity and protects female mice from obesity and impaired glucose tolerance. Eur J Nutr 51: 861–870, 2012. doi: 10.1007/s00394-011-0266-4. [DOI] [PubMed] [Google Scholar]
- 115.Koebele SV, Bimonte-Nelson HA. Modeling menopause: the utility of rodents in translational behavioral endocrinology research. Maturitas 87: 5–17, 2016. doi: 10.1016/j.maturitas.2016.01.015. . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Caligioni CS. Assessing reproductive status/stages in mice. Curr Protoc Neurosci Appendix 4: Appendix 4I, 2009. doi: 10.1002/0471142301.nsa04is48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Aguiar AS Jr, Speck AE, Amaral IM, Canas PM, Cunha RA. The exercise sex gap and the impact of the estrous cycle on exercise performance in mice. Sci Rep 8: 10742, 2018. doi: 10.1038/s41598-018-29050-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Mattu AT, Iannetta D, MacInnis MJ, Doyle-Baker PK, Murias JM. Menstrual and oral contraceptive cycle phases do not affect submaximal and maximal exercise responses. Scand J Med Sci Sports 30: 472–484, 2020. doi: 10.1111/sms.13590. [DOI] [PubMed] [Google Scholar]
- 119.McLean AC, Valenzuela N, Fai S, Bennett SA. Performing vaginal lavage, crystal violet staining, and vaginal cytological evaluation for mouse estrous cycle staging identification. J Vis Exp 15: e4389, 2012. doi: 10.3791/4389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Allen AM, McRae-Clark AL, Carlson S, Saladin ME, Gray KM, Wetherington CL, McKee SA, Allen SS. Determining menstrual phase in human biobehavioral research: a review with recommendations. Exp Clin Psychopharmacol 24: 1–11, 2016. doi: 10.1037/pha0000057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Finch CE. The menopause and aging, a comparative perspective. J Steroid Biochem Mol Biol 142: 132–141, 2014. doi: 10.1016/j.jsbmb.2013.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Sengupta P. The laboratory rat: relating its age with human’s. Int J Prev Med 4: 624–630, 2013. [PMC free article] [PubMed] [Google Scholar]
- 123.Harlow SD, Gass M, Hall JE, Lobo R, Maki P, Rebar RW, Sherman S, Sluss PM, de Villiers TJ; STRAW + 10 Collaborative Group. Executive summary of the Stages of Reproductive Aging Workshop + 10: addressing the unfinished agenda of staging reproductive aging. Menopause 19: 387–395, 2012. doi: 10.1097/gme.0b013e31824d8f40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Santoro N, Randolph JF Jr.. Reproductive hormones and the menopause transition. Obstet Gynecol Clin North Am 38: 455–466, 2011. doi: 10.1016/j.ogc.2011.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Wise PM. Alterations in the proestrous pattern of median eminence LHRH, serum LH, FSH, estradiol and progesterone concentrations in middle-aged rats. Life Sci 31: 165–173, 1982. doi: 10.1016/0024-3205(82)90429-5. [DOI] [PubMed] [Google Scholar]
- 126.Lu KH, Hopper BR, Vargo TM, Yen SS. Chronological changes in sex steroid, gonadotropin and prolactin secretions in aging female rats displaying different reproductive states. Biol Reprod 21: 193–203, 1979. doi: 10.1095/biolreprod21.1.193. [DOI] [PubMed] [Google Scholar]
- 127.Rosenfeld CS. Sex-dependent differences in voluntary physical activity. J Neurosci Res 95: 279–290, 2017. doi: 10.1002/jnr.23896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Foright RM, Johnson GC, Kahn D, Charleston CA, Presby DM, Bouchet CA, Wellberg EA, Sherk VD, Jackman MR, Greenwood BN, MacLean PS. Compensatory eating behaviors in male and female rats in response to exercise training. Am J Physiol Regul Integr Comp Physiol 319: R171–R183, 2020. doi: 10.1152/ajpregu.00259.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Tarnopolsky MA. Sex differences in exercise metabolism and the role of 17-beta estradiol. Med Sci Sports Exerc 40: 648–654, 2008. doi: 10.1249/MSS.0b013e31816212ff. [DOI] [PubMed] [Google Scholar]
- 130.Leyk D, Gorges W, Ridder D, Wunderlich M, Rüther T, Sievert A, Essfeld D. Hand-grip strength of young men, women and highly trained female athletes. Eur J Appl Physiol 99: 415–421, 2007. doi: 10.1007/s00421-006-0351-1. [DOI] [PubMed] [Google Scholar]
- 131.Roepstorff C, Steffensen CH, Madsen M, Stallknecht B, Kanstrup IL, Richter EA, Kiens B. Gender differences in substrate utilization during submaximal exercise in endurance-trained subjects. Am J Physiol Endocrinol Metab 282: E435–E447, 2002. doi: 10.1152/ajpendo.00266.2001. [DOI] [PubMed] [Google Scholar]
- 132.Ueberschlag-Pitiot V, Stantzou A, Messéant J, Lemaitre M, Owens DJ, Noirez P, Roy P, Agbulut O, Metzger D, Ferry A. Gonad-related factors promote muscle performance gain during postnatal development in male and female mice. Am J Physiol Endocrinol Metab 313: E12–E25, 2017. doi: 10.1152/ajpendo.00446.2016. [DOI] [PubMed] [Google Scholar]


