Abstract
Obesity has become one of the most pressing public health issues of the 21st century and currently affects a substantial proportion of the older adult population. Although the cardiometabolic complications are well documented, research from the past 20 years has drawn attention to the detrimental effects of obesity on physical performance in older adults. Obesity-related declines in physical performance are due, in part, to compromised muscle strength and power. Recent evidence suggests there are a number of mechanisms potentially underlying reduced whole muscle function, including alterations in myofilament protein function and cellular contractile properties, and these may be related to morphological adaptations, such as shifts in fiber type composition and increased intramyocellular lipid content within skeletal muscle. To date, even less research has focused on how exercise and weight loss interventions for obese older adults affect these mechanisms. In light of this work, we provide an update on the current knowledge related to obesity and skeletal muscle contractile function and highlight a number of questions to address potential etiologic mechanisms as well as intervention strategies, which may help advance our understanding of how physical performance can be improved among obese older adults.
Keywords: adiposity, aging, cellular, myosin
INTRODUCTION
The United States is undergoing a dramatic demographic shift. The percentage of older adults (>65-yr) living in the United States was 13% of the total population in 2010, but is expected to reach 20% by 2030 (1). One of the most critical issues affecting older adults is the growing prevalence of obesity, which has emerged as a global epidemic over the last several decades, impacting every segment of the US population (2). The prevalence of obesity was 42.8% among adults ≥60 yr in 2017–2018 (3) and is associated with numerous chronic health conditions, including cardiovascular disease, diabetes mellitus, cancer, osteoarthritis, and back pain (4). One overlooked consequence of obesity is the impact on physical performance, which can be quite severe. In fact, obese older men and women (body mass index ≥ 30.0 kg/m2) had a 60% greater risk for functional decline compared to those with a normal body weight in a recent meta-analysis (5). Because the prevalence of physical limitations already increases with age, the combination of an aging population and widespread obesity will almost certainly lead to unprecedented levels of physical disability. To this end, research that advances our understanding of how obesity leads to poor physical performance in older adults has clear public health relevance.
Physical performance is influenced by a multitude of factors, but one of the strongest in older adults is skeletal muscle function (6). Among older adults, obesity often occurs in concert with the age-related loss of muscle mass (sarcopenia), resulting in a syndrome coined “sarcopenic obesity” (7), and these body composition changes are thought to synergistically compromise physical performance, partly due to impaired muscle function. Large epidemiological studies initially provided evidence that adiposity and/or body weight affected muscle quality (strength or power normalized to muscle size) (8,9). Other work showed that ectopic adipose tissue deposition, characterized by lipid infiltration into skeletal muscle, is related to impaired strength and power (10–12), slower thigh muscle contraction velocity (13), and reduced rate of torque development (14). Collectively, these findings indicate that excess body weight and/or adiposity has a negative impact on whole muscle strength and power.
The aim of the current review is to provide an update on our current understanding of obesity and skeletal muscle structure and function in the presence of aging by summarizing the contemporary literature. Although other mechanisms (e.g., inflammation, anabolic resistance, and muscle denervation) likely contribute to obesity-induced muscle weakness, the scope of this review is limited to the etiologic roles of a purported fiber type shift, single fiber contractile properties, and intramyocellular lipid (IMCL) content. Intervention approaches to remediate muscle function in obese older adults are also discussed.
POTENTIAL MECHANISMS LEADING TO OBESITY-INDUCED MUSCLE WEAKNESS
Measurement of Adipose Tissue
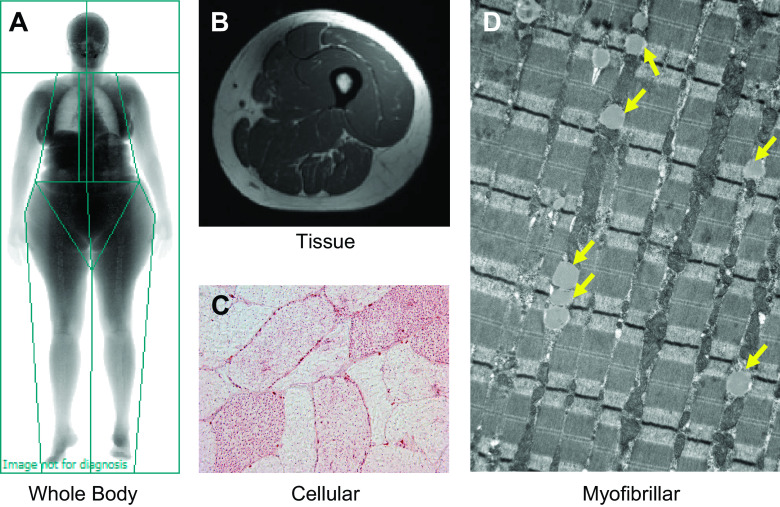
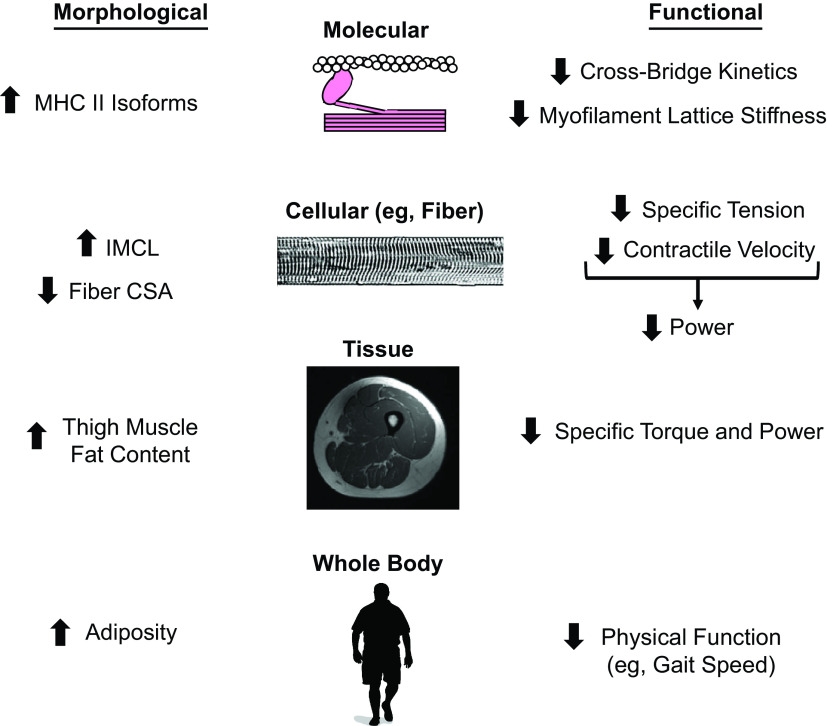
Adipose tissue accumulates in various anatomical depots, broadly classified as one of three main types: visceral (around internal organs), subcutaneous (beneath the skin), and ectopic (in organs/locations not classically associated with adipose tissue storage). Ectopic fat in skeletal muscle can be generally defined as intermuscular (between muscle fascicles/cells) and intramyocellular (within muscle cells) and different measurement techniques can quantify adiposity deposited in various locations (Fig. 1). Given the various locations where adipose tissue can be stored, this begs the question: does fat accumulation within different skeletal muscle depots affect contractile function? The available data suggest IMCL content can affect muscle contractility (Fig. 2) and we highlight studies suggesting this across the anatomic spectrum.
Figure 1.
Different measurement techniques can be used to quantify adiposity deposited at various anatomical locations. Briefly, adiposity can be assessed at the whole body (A), tissue (B), cellular (C), and subcellular (D) levels. At the whole body level (A), dual-energy X-ray absorptiometry scans yield measurements of overall adiposity (e.g., percent body fat) and regional estimates (android vs. gynoid deposition). Magnetic resonance (MR) imaging and computed tomography (not shown) scans can be used to characterize specific fat depots (B), such as visceral adipose tissue surrounding the organs of the abdomen and intermuscular adipose tissue of the thigh. Superconducting magnets also offer the capacity to examine the chemical composition of tissues, and MR spectroscopy can be used to provide a noninvasive estimate of intramyocellular lipid content. Finally, the number, size, and area of individual lipid droplets can be measured at the cellular (single fiber) level (C) using oil-red-o immunohistochemistry as well as at the myofibrillar level (D) with electron microscopy. Yellow arrows in electron micrograph indicate the presence of individual lipid droplets.
Figure 2.
Summary of obesity-induced alterations in skeletal muscle morphology and function. CSA, cross-sectional area; IMCL, intramyocellular lipid; MHC, myosin heavy chain.
Muscle Fiber Type Composition
Fiber type admixture of skeletal muscle tissue could influence IMCL content, which has clear implications for contractile function. Skeletal muscle contractile function scales up through various anatomical levels, from the molecular (myosin and actin) to the cellular (single fiber) to the whole muscle (quadriceps). As a result, whole muscle function is partly determined by the myosin heavy chain (MHC) isoform composition of single fibers and their contractile properties (15). Mammalian skeletal muscle contains four different MHC isoforms: one slow-contracting (MHC I) and three fast-contracting (MHC IIa, IIx, and IIb) (16). In humans, three of these isoforms are expressed (I, IIa, and IIx), whereas the IIb isoform is only seen in rodents. Importantly, the MHC isoform of a particular fiber is the predominant determinant of mechanical properties, including force, velocity, and power (force × velocity) [see reference (17) for a detailed discussion of single fiber mechanics]. Briefly, specific tension (isometric force normalized to fiber size), contraction velocity, and power output tend to be the lowest in MHC I fibers (oxidative) and greater in IIa (oxidative-glycolytic) and IIx (glycolytic) (15, 18).
Morphology
The temporal nature of the relationship between obesity and muscle fiber type is an active area of research. Currently, the prevailing hypothesis is that obesity is an antecedent to changes in MHC isoform composition (19); however, skeletal muscle, given its prominent role in metabolism, could also predispose an individual to obesity, although this theory has received less attention. For the purposes of this review, if obesity alters the MHC isoform composition of a particular muscle group (e.g., quadriceps), this could lead to changes in performance at the whole muscle level. Studies in humans and animals have frequently shown that obesity results in a slow-to-fast MHC isoform shift, characterized by a lower proportion of MHC I fibers and a greater proportion of MHC II fibers (19). However, most studies have examined younger to middle-aged adults or animals. Thus, data on MHC composition with obesity in older adults are lacking. In addition, obese older adults have lower physical activity levels than their normal weight counterparts (20), and the sedentary lifestyle may be responsible for the observed fiber type transition, rather than obesity. In support of this notion, skeletal muscle disuse is characterized by a shift towards a faster MHC isoform (21). Thus, whether obesity independently results in a fiber type shift is confounded by inactivity. This isoform shift has been purported to have functional consequences for skeletal muscle and suggested as a primary explanation for reduced force and power in obese older adults (19). However, an obesity-induced transition toward a faster MHC phenotype (i.e., greater percentage of MHC IIa fibers) should, in theory, increase force and power production at the whole muscle level, as power output for MHC II fibers is approximately three times that of MHC I fibers (22) and single fiber properties are the primary determinants of whole muscle function (17). Since most studies in humans and animals observe lower specific force and/or power at the whole muscle level with obesity, this highlights a notable disconnect between the widely reported MHC isoform shift and whole muscle phenotypes, and underscores the critical need for studies to evaluate physical activity and the possible role of disuse in fiber type shifts. In addition to an altered MHC isoform composition, skeletal muscle of obese individuals shows other morphological changes, such as greater IMCL content (10, 23) and a more central distribution of lipid droplets within fibers (23), compared to lean adults. Lipid accumulation may also be dependent on fiber type as MHC I fibers have a greater concentration of lipid droplets than IIa in humans (23), although this contrasts with murine soleus muscle showing the opposite pattern (24). None of these studies, however, have assessed the intrinsic contractility of skeletal muscle. That is, although fiber type admixture may suggest that obesity is associated with a more powerful phenotype, this assumes that function is preserved per unit muscle fiber with obesity, which may be incorrect.
Contractile Properties
Although there is a considerable literature detailing changes in skeletal muscle morphology with obesity, far less research has examined muscle fiber contractile properties. To date, only two studies (10, 12) have described the effects of obesity and/or adiposity on cellular and molecular function in older humans. Choi et al. (10) found that maximal shortening velocity was 25% slower in MHC I fibers from obese older adults relative to normal weight. Likewise, specific force was 28 and 25% lower in MHC I and IIa fibers from the obese group, respectively. They also found that single fiber shortening velocity and specific power were inversely associated with various measures of intramyocellular lipids in the obese group. Our recent work showed that lower quadriceps attenuation (greater lipid infiltration) was associated with reduced specific force in MHC I and IIa fibers in older women, but not men, suggesting fat may have sex-specific effects on muscle function (12). In this study, greater whole body adiposity was also associated with longer myosin attachment times, or the duration of time myosin is strongly bound to actin, indicating that fat may slow myosin-actin cross-bridge kinetics in both sexes. As myosin attachment time is inversely proportional to single fiber shortening velocity (25), this finding may explain the previous observation of slower shortening velocities in MHC I fibers from obese older adults (10). More broadly, these results provide molecular and cellular level mechanistic data showing impaired force production and velocity may limit contractility, which could explain the effects of adiposity on whole muscle contractile velocity (13), rate of torque development (14), and specific torque (9, 11) in older adults. However, whether obesity impacts other single fiber contractile properties, such as calcium sensitivity, has not been studied.
Lipid Intermediates
If IMCL promotes myofilament protein dysfunction, what mechanisms might underlie this effect? One possible explanation is increased amounts of lipid metabolites with greater IMCL (26). Studies focused on the effects of IMCLs to promote insulin resistance have shown that various metabolites can alter intracellular signaling pathways (27). Although less is known regarding the effects of these pathways on skeletal muscle myofilaments, some may alter contractility. For example, levels of ceramides are increased in skeletal muscle of obese individuals (28). In cardiac myocytes, increased ceramide levels activate protein kinase C, which, in turn, increases phosphorylation of myosin binding protein-C (MyBP-C) and depresses contractility (29). Accumulation of lipid intermediates resulting from obesity could alter MyBP-C posttranslational modifications (e.g, phosphorylation, oxidation, etc.), but whether this occurs with greater IMCL in human subjects to impact myofilament contractility requires additional research. Moreover, ceramides are tied to inflammation and insulin resistance, representing another pathway through which lipids may secondarily impair contractile function.
Another mechanism whereby lipid metabolites could impact myofilaments is via alterations in mitochondrial function. For instance, ceramides potentiate the production of reactive oxygen species (ROS). In mouse diaphragm muscle, exogenous sphingomyelinase, which produces ceramide from sphingomyelin, increases ROS production and caused a reduction in the maximal force and calcium sensitivity of permeabilized diaphragm fibers (30). Moreover, as increased dietary fat intake stimulates mitochondrial ROS production in humans (31), this mechanism may be present in both animals and humans. Indeed, the depressive effects of oxidative modification on myofilament contractility are well-described (32) and recent studies suggest that a similar mechanism may be operative in humans (33). However, the effects of sphingomyelinase on contractility could be prevented by coincubation with the antioxidant N-acetylcysteine. Notably, some basal level of ROS is necessary for optimal force output, with excessive ROS production (e.g., disease) causing a decline (34). Currently, the role of lipid intermediates in the pathophysiology of myofilament dysfunction, including a potential shift in the redox state, is not well understood, but preclinical animal studies could shed light on the link between IMCL and single fiber contractile properties in obese older adults.
Insights from Model Systems
Given the relative paucity of research on obesity and single fiber mechanics in older adults, much of our knowledge regarding the effects of adiposity on muscle contractility comes from animal models. In the last 5 years, a number of preclinical studies (35–39) have evaluated contractile function of an isolated muscle group [e.g., soleus, gastrocnemius, extensor digitorum longus (EDL), and diaphragm] following a period of high-fat feeding. Data from these studies show that obesity adversely affects muscle morphology and function, and that the effects are fiber type-specific, with more pronounced deficits among fast-twitch fibers (35), albeit these results are not unanimous (38). Moreover, the duration of a high-fat diet may be important, as changes in MHC isoform composition and contractile function were apparent after 12 but not 4 wk (37). The time course of morphological adaptations to obesity may also be dependent on age, as older mice on a high-fat diet show elevated levels of IMCL content earlier than young mice (38). In addition to changes in mechanical properties, impaired calcium handing in skeletal muscle was observed in aged mice following diet-induced obesity (36). Overall, results of recent animal studies suggest a number of morphological and functional changes in skeletal muscle following a high-fat diet, but these measures need to be examined in studies with older adults.
IMPLICATIONS FOR TREATMENT
What treatments can improve muscle function in the presence of obesity? To date, and to our knowledge, no studies have evaluated the effects of clinical weight loss alone on single muscle fiber contractile properties in obese older adults. In middle-aged obese women, weight loss, either due to caloric restriction via a specific diet or gastric bypass surgery, was greater in those with a larger percentage of MHC I fibers (40, 41). However, weight loss and/or exercise may not change fiber type proportion (42). In contrast, adding exercise to a weight loss intervention confers benefits for muscle mass preservation. For example, a combination of resistance and aerobic exercise was more effective than either exercise strategy alone for improving myocellular quality in obese older adults undergoing weight loss (43). In particular, there was increased expression of myocyte-specific enhancer factor 2A, an important regulator of myogenesis, as well as downregulation of atrogenes (e.g., those that mediate cellular autophagy), for the group that performed both modes of exercise. In terms of molecular and cellular contractile function, a different study (44) showed that resistance training, with or without the addition of caloric restriction for weight loss, similarly improved specific force in MHC I and II fibers from overweight and obese older men and women. Single fiber contractile properties (force and power) correlated with changes in thigh fat volume and in whole muscle function (gait speed and lower leg exercises), suggesting improvements at the single fiber level scaled to the whole muscle.
Regarding the aforementioned potential role of lipid intermediates in myofilament dysfunction, ceramides, which are greater in older adults than young (45, 46), may have a role in the attenuation of anabolic signaling molecules following a single bout of resistance training (45). Others have shown that 6 weeks of high-intensity interval training can reduce saturated ceramide content in overweight and obese older adults (46), but whether this translated to improved single fiber properties was not investigated. As ceramides may interfere with myofilament function, additional work is needed to determine if different modes of exercise can reduce lipid metabolites, and, in turn, improve molecular and cellular contractility.
The efficacy of different lifestyle therapies has been examined in animal studies, albeit with mixed results. For example, neither weight loss nor aerobic exercise corrected obesity-induced muscle dysfunction in zebrafish (39, 47). In contrast, 6 wk of aerobic exercise training improved contractile function of the EDL and intracellular Ca2+ flux of the flexor digitorum brevis in a diabetic mouse model, although the animals in this study were young and not classically obese (48). Nonetheless, a combination of therapies may be needed to facilitate improvements in skeletal muscle function in the presence of obesity. In accordance with this notion, 7 wk of caloric restriction plus exercise completely restored muscle mechanics and mitochondrial function in diet-induced obese rats, whereas muscle function was only partially restored with either therapy alone (49). Collectively, the limited evidence from human and animal studies suggest that lifestyle therapies have the potential to address some of the morphological (e.g., MHC isoform shift) and functional (e.g., reduced single fiber force) adaptations that have been reported to accompany obesity. However, there are notable gaps in this body of literature, including a need for human studies to evaluate the efficacy of these intervention strategies in randomized controlled trials involving obese older adults.
FUTURE DIRECTIONS
Preliminary results show that obesity alters skeletal muscle morphology and function in older adults. Given the limited knowledge base in this area, we propose a number of potential avenues for future research. Importantly, with a recent call by the National Institutes of Health to account for sex as a biological variable in research (50), the potential impact of sex should be examined in the context of these questions:
Mechanistic Insights
Does lipid infiltration into skeletal muscle affect specific steps of the myosin-actin cross-bridge cycle (e.g., ADP release rate)?
Does obesity affect single fiber contractile properties, such as force-velocity relationships or calcium sensitivity, in older adults?
Does obesity affect the posttranslational modifications of contractile proteins in skeletal muscle?
Do lipid metabolites mediate the effects of excessive IMCL content on myofilament protein dysfunction?
Is the slow-to-fast MHC isoform shift seen with obesity actually secondary to muscle disuse characterized by sedentary behavior?
What other etiologic factors (e.g., inflammation, oxidative stress, regulatory light chain phosphorylation) contribute to the pathophysiology of obesity-induced weakness?
Are the pathways that lead to muscle weakness unique to the location of adipose tissue deposition (e.g., visceral vs. intermuscular vs. intramyocellular)?
Intervention Effects
How do exercise interventions (aerobic vs. resistance) impact whole muscle, cellular, and molecular muscle function in the presence of obesity?
Does weight loss for obesity have positive or negative effects on single muscle fiber structure (size, lipid content) and function (force, velocity, power)?
Does fiber type composition affect the magnitude of weight loss in obese patients?
CONCLUSIONS
In conclusion, obesity reduces skeletal muscle contractility in older adults. The limited evidence from human studies suggests that reduced function is due, in part, to altered cellular (single fiber) and molecular (myosin-actin interactions) properties. As a result, many questions remain unanswered regarding the ways in which adiposity affects fundamental skeletal muscle structure and function. Studies aimed at uncovering the pathophysiology of obesity-induced weakness, as well as studies evaluating different therapeutic approaches, have the potential to advance the field and improve the physical function and health of older adults.
GRANTS
This work was supported in part by a Charles A. King Trust Fellowship to C. R. Straight.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
C.R.S. conceived and designed research; C.R.S., M.J.T., and M.S.M. prepared figures; C.R.S. drafted manuscript; C.R.S., M.J.T., and M.S.M. edited and revised manuscript; C.R.S., M.J.T., and M.S.M. approved final version of manuscript.
REFERENCES
- 1.Ortman JM, Velkoff VA, Hogan H. An Aging Nation: The Older Population in the United States. Washington, DC: United States Census Bureau, , 2014. [Google Scholar]
- 2.Wang Y, Beydoun MA, Liang L, Caballero B, Kumanyika SK. Will all Americans become overweight or obese? Estimating the progression and cost of the US obesity epidemic. Obesity (Silver Spring) 16: 2323–2330, 2008. doi: 10.1038/oby.2008.351. [DOI] [PubMed] [Google Scholar]
- 3.Hales CM, Carroll MD, Fryar CD, Ogden CL. Prevalence of obesity and severe obesity among adults: United States, 2017–2018. NCHS Data Brief. 360: 1–8, 2020. [PubMed] [Google Scholar]
- 4.Guh DP, Zhang W, Bansback N, Amarsi Z, Birmingham CL, Anis AH. The incidence of co-morbidities related to obesity and overweight: a systematic review and meta-analysis. BMC Public Health 9: 88, 2009. doi: 10.1186/1471-2458-9-88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Schaap LA, Koster A, Visser M. Adiposity, muscle mass, and muscle strength in relation to functional decline in older persons. Epidemiol Rev 35: 51–65, 2013. doi: 10.1093/epirev/mxs006. [DOI] [PubMed] [Google Scholar]
- 6.Reid KF, Fielding RA. Skeletal muscle power: a critical determinant of physical functioning in older adults. Exerc Sport Sci Rev 40: 4–12, 2012. doi: 10.1097/JES.0b013e31823b5f13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Batsis JA, Villareal DT. Sarcopenic obesity in older adults: aetiology, epidemiology and treatment strategies. Nat Rev Endocrinol 14: 513–537, 2018. doi: 10.1038/s41574-018-0062-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fabbri E, Chiles Shaffer N, Gonzalez-Freire M, Shardell MD, Zoli M, Studenski SA, Ferrucci L. Early body composition, but not body mass, is associated with future accelerated decline in muscle quality. J Cachexia Sarcopenia Muscle 8: 490–499, 2017. doi: 10.1002/jcsm.12183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Newman AB, Haggerty CL, Goodpaster B, Harris T, Kritchevsky S, Nevitt M, Miles TP, Visser M; Health Aging And Body Composition Research Group. Strength and muscle quality in a well-functioning cohort of older adults: the Health, Aging and Body Composition Study. J Am Geriatr Soc 51: 323–330, 2003. doi: 10.1046/j.1532-5415.2003.51105.x. [DOI] [PubMed] [Google Scholar]
- 10.Choi SJ, Files DC, Zhang T, Wang ZM, Messi ML, Gregory H, Stone J, Lyles MF, Dhar S, Marsh AP, Nicklas BJ, Delbono O. Intramyocellular lipid and impaired myofiber contraction in normal weight and obese older adults. J Gerontol A Biol Sci Med Sci 71: 557–564, 2016. doi: 10.1093/gerona/glv169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Goodpaster BH, Carlson CL, Visser M, Kelley DE, Scherzinger A, Harris TB, Stamm E, Newman AB. Attenuation of skeletal muscle and strength in the elderly: the Health ABC Study. J Appl Physiol (1985) 90: 2157–2165, 2001. doi: 10.1152/jappl.2001.90.6.2157. [DOI] [PubMed] [Google Scholar]
- 12.Straight CR, Voigt TB, Jala AV, Chase JD, Ringham OR, Ades PA, Toth MJ, Miller MS. Quadriceps lipid content has sex-specific associations with whole-muscle, cellular, and molecular contractile function in older adults. J Gerontol A Biol Sci Med Sci 74: 1879–1886, 2019. doi: 10.1093/gerona/gly235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Reid KF, Pasha E, Doros G, Clark DJ, Patten C, Phillips EM, Frontera WR, Fielding RA. Longitudinal decline of lower extremity muscle power in healthy and mobility-limited older adults: influence of muscle mass, strength, composition, neuromuscular activation and single fiber contractile properties. Eur J Appl Physiol 114: 29–39, 2014. doi: 10.1007/s00421-013-2728-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Frank-Wilson AW, Chalhoub D, Figueiredo P, Jónsson PV, Siggeirsdóttir K, Sigurdsson S, Eiriksdottir G, Guðnason V, Launer L, Harris TB; the AGES-Reykjavik Study. Associations of quadriceps torque properties with muscle size, attenuation, and intramuscular adipose tissue in older adults. J Gerontol A Biol Sci Med Sci 73: 931–938, 2018. doi: 10.1093/gerona/glx262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Harridge SD, Bottinelli R, Canepari M, Pellegrino MA, Reggiani C, Esbjörnsson M, Saltin B. Whole-muscle and single-fibre contractile properties and myosin heavy chain isoforms in humans. Pflugers Arch 432: 913–920, 1996. doi: 10.1007/s004240050215. [DOI] [PubMed] [Google Scholar]
- 16.Schiaffino S, Reggiani C. Myosin isoforms in mammalian skeletal muscle. J Appl Physiol (1985) 77: 493–501, 1994. doi: 10.1152/jappl.1994.77.2.493. [DOI] [PubMed] [Google Scholar]
- 17.Miller MS, Toth MJ. Myofilament protein alterations promote physical disability in aging and disease. Exerc Sport Sci Rev 41: 93–99, 2013. doi: 10.1097/JES.0b013e31828bbcd8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Miller MS, Bedrin NG, Ades PA, Palmer BM, Toth MJ. Molecular determinants of force production in human skeletal muscle fibers: effects of myosin isoform expression and cross-sectional area. Am J Physiol Cell Physiol 308: C473–484, 2015. doi: 10.1152/ajpcell.00158.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tallis J, James RS, Seebacher F. The effects of obesity on skeletal muscle contractile function. J Exp Biol 221: jeb163840, 2018. doi: 10.1242/jeb.163840. [DOI] [PubMed] [Google Scholar]
- 20.Cohen SA, Greaney ML, Sabik NJ. Assessment of dietary patterns, physical activity and obesity from a national survey: Rural-urban health disparities in older adults. PLoS One 13: e0208268, 2018. doi: 10.1371/journal.pone.0208268. . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Brooks NE, Myburgh KH. Skeletal muscle wasting with disuse atrophy is multi-dimensional: the response and interaction of myonuclei, satellite cells and signaling pathways. Front Physiol 5: 99, 2014. doi: 10.3389/fphys.2014.00099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bottinelli R, Canepari M, Pellegrino MA, Reggiani C. Force-velocity properties of human skeletal muscle fibres: myosin heavy chain isoform and temperature dependence. J Physiol 495: 573–586, 1996. doi: 10.1113/jphysiol.1996.sp021617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Malenfant P, Joanisse DR, Thériault R, Goodpaster BH, Kelley DE, Simoneau JA. Fat content in individual muscle fibers of lean and obese subjects. Int J Obes Relat Metab Disord 25: 1316–1321, 2001. doi: 10.1038/sj.ijo.0801733. [DOI] [PubMed] [Google Scholar]
- 24.Komiya Y, Sawano S, Mashima D, Ichitsubo R, Nakamura M, Tatsumi R, Ikeuchi Y, Mizunoya W. Mouse soleus (slow) muscle shows greater intramyocellular lipid droplet accumulation than EDL (fast) muscle: fiber type-specific analysis. J Muscle Res Cell Motil 38: 163–173, 2017. doi: 10.1007/s10974-017-9468-6. [DOI] [PubMed] [Google Scholar]
- 25.Piazzesi G, Reconditi M, Linari M, Lucii L, Bianco P, Brunello E, Decostre V, Stewart A, Gore DB, Irving TC, Irving M, Lombardi V. Skeletal muscle performance determined by modulation of number of myosin motors rather than motor force or stroke size. Cell 131: 784–795, 2007. doi: 10.1016/j.cell.2007.09.045. [DOI] [PubMed] [Google Scholar]
- 26.Goodpaster BH, Wolf D. Skeletal muscle lipid accumulation in obesity, insulin resistance, and type 2 diabetes. Pediatr Diabetes 5: 219–226, 2004. doi: 10.1111/j.1399-543X.2004.00071.x. [DOI] [PubMed] [Google Scholar]
- 27.Chaurasia B, Summers SA. Ceramides - lipotoxic inducers of metabolic disorders. Trends Endocrinol Metab 26: 538–550, 2015. doi: 10.1016/j.tem.2015.07.006. [DOI] [PubMed] [Google Scholar]
- 28.Adams JM 2nd, Pratipanawatr T, Berria R, Wang E, DeFronzo RA, Sullards MC, Mandarino LJ. Ceramide content is increased in skeletal muscle from obese insulin-resistant humans. Diabetes 53: 25–31, 2004. doi: 10.2337/diabetes.53.1.25. [DOI] [PubMed] [Google Scholar]
- 29.Previs MJ, Mun JY, Michalek AJ, Previs SB, Gulick J, Robbins J, Warshaw DM, Craig R. Phosphorylation and calcium antagonistically tune myosin-binding protein C's structure and function. Proc Natl Acad Sci USA 113: 3239–3244, 2016. doi: 10.1073/pnas.1522236113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ferreira LF, Moylan JS, Stasko S, Smith JD, Campbell KS, Reid MB. Sphingomyelinase depresses force and calcium sensitivity of the contractile apparatus in mouse diaphragm muscle fibers. J Appl Physiol (1985) 112: 1538–1545, 2012. doi: 10.1152/japplphysiol.01269.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Anderson EJ, Lustig ME, Boyle KE, Woodlief TL, Kane DA, Lin CT, Price JW 3rd, Kang L, Rabinovitch PS, Szeto HH, Houmard JA, Cortright RN, Wasserman DH, Neufer PD. Mitochondrial H2O2 emission and cellular redox state link excess fat intake to insulin resistance in both rodents and humans. J Clin Invest 119: 573–581, 2009. doi: 10.1172/JCI37048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Dutka TL, Mollica JP, Lamb GD. Differential effects of peroxynitrite on contractile protein properties in fast- and slow-twitch skeletal muscle fibers of rat. J Appl Physiol (1985) 110: 705–716, 2011. doi: 10.1152/japplphysiol.00739.2010. [DOI] [PubMed] [Google Scholar]
- 33.Callahan DM, Miller MS, Sweeny AP, Tourville TW, Slauterbeck JR, Savage PD, Maugan DW, Ades PA, Beynnon BD, Toth MJ. Muscle disuse alters skeletal muscle contractile function at the molecular and cellular levels in older adult humans in a sex-specific manner. J Physiol 592: 4555–4573, 2014. doi: 10.1113/jphysiol.2014.279034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Reid MB. Invited Review: Redox modulation of skeletal muscle contraction: what we know and what we don't. J Appl Physiol (1985) 90: 724–731, 2001. doi: 10.1152/jappl.2001.90.2.724. [DOI] [PubMed] [Google Scholar]
- 35.Ciapaite J, van den Berg SA, Houten SM, Nicolay K, van Dijk KW, Jeneson JA. Fiber-type-specific sensitivities and phenotypic adaptations to dietary fat overload differentially impact fast- versus slow-twitch muscle contractile function in C57BL/6J mice. J Nutr Biochem 26: 155–164, 2015. doi: 10.1016/j.jnutbio.2014.09.014. [DOI] [PubMed] [Google Scholar]
- 36.Eshima H, Tamura Y, Kakehi S, Kakigi R, Hashimoto R, Funai K, Kawamori R, Watada H. A chronic high-fat diet exacerbates contractile dysfunction with impaired intracellular Ca2+ release capacity in the skeletal muscle of aged mice. J Appl Physiol (1985) 128: 1153–1162, 2020. doi: 10.1152/japplphysiol.00530.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Eshima H, Tamura Y, Kakehi S, Kurebayashi N, Murayama T, Nakamura K, Kakigi R, Okada T, Sakurai T, Kawamori R, Watada H. Long-term, but not short-term high-fat diet induces fiber composition changes and impaired contractile force in mouse fast-twitch skeletal muscle. Physiol Rep 5: e13250, 2017. doi: 10.14814/phy2.13250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Messa GAM, Piasecki M, Hurst J, Hill C, Tallis J, Degens H. The impact of a high-fat diet in mice is dependent on duration and age, and differs between muscles. J Exp Biol 223: jeb217117, 2020. doi: 10.1242/jeb.217117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Seebacher F, Tallis J, McShea K, James RS. Obesity-induced decreases in muscle performance are not reversed by weight loss. Int J Obes (Lond) 41: 1271–1278, 2017. doi: 10.1038/ijo.2017.81. [DOI] [PubMed] [Google Scholar]
- 40.Gerrits MF, Ghosh S, Kavaslar N, Hill B, Tour A, Seifert EL, Beauchamp B, Gorman S, Stuart J, Dent R, McPherson R, Harper ME. Distinct skeletal muscle fiber characteristics and gene expression in diet-sensitive versus diet-resistant obesity. J Lipid Res 51: 2394–2404, 2010. doi: 10.1194/jlr.P005298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Tanner CJ, Barakat HA, Dohm GL, Pories WJ, MacDonald KG, Cunningham PR, Swanson MS, Houmard JA. Muscle fiber type is associated with obesity and weight loss. Am J Physiol Endocrinol Metab 282: E1191–1196, 2002. doi: 10.1152/ajpendo.00416.2001. [DOI] [PubMed] [Google Scholar]
- 42.Chomentowski P, Dubé JJ, Amati F, Stefanovic-Racic M, Zhu S, Toledo FG, Goodpaster BH. Moderate exercise attenuates the loss of skeletal muscle mass that occurs with intentional caloric restriction-induced weight loss in older, overweight to obese adults. J Gerontol A Biol Sci Med Sci 64: 575–580, 2009. doi: 10.1093/gerona/glp007. [19276190]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Colleluori G, Aguirre L, Phadnis U, Fowler K, Armamento-Villareal R, Sun Z, Brunetti L, Hyoung Park J, Kaipparettu BA, Putluri N, Auetumrongsawat V, Yarasheski K, Qualls C, Villareal DT. Aerobic plus resistance exercise in obese older adults improves muscle protein synthesis and preserves myocellular quality despite weight loss. Cell Metab 30: 261–273.e6, 2019. doi: 10.1016/j.cmet.2019.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wang ZM, Leng X, Messi ML, Choi SJ, Marsh AP, Nicklas B, Delbono O. Relationship of physical function to single muscle fiber contractility in older adults: Effects of resistance training with and without caloric restriction. J Gerontol A Biol Sci Med Sci 74: 412–419, 2019. doi: 10.1093/gerona/gly047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Rivas DA, Morris EP, Haran PH, Pasha EP, Morais MdS, Dolnikowski GG, Phillips EM, Fielding RA. Increased ceramide content and NFκB signaling may contribute to the attenuation of anabolic signaling after resistance exercise in aged males. J Appl Physiol (1985) 113: 1727–1736, 2012. doi: 10.1152/japplphysiol.00412.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Søgaard D, Baranowski M, Larsen S, Taulo Lund M, Munk Scheuer C, Vestergaard Abildskov C, Greve Dideriksen S, Dela F, Wulff Helge J. Muscle-saturated bioactive lipids are increased with aging and influenced by high-intensity interval training. Int J Mol Sci 20: 1240, 2019. [0.3390/ijms20051240] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Seebacher F, James RS. Increased physical activity does not improve obesity-induced decreases in muscle quality in zebrafish (Danio rerio). J Appl Physiol (1985) 127: 1802–1808, 2019. doi: 10.1152/japplphysiol.00433.2019. [DOI] [PubMed] [Google Scholar]
- 48.Eshima H, Tamura Y, Kakehi S, Nakamura K, Kurebayashi N, Murayama T, Kakigi R, Sakurai T, Kawamori R, Watada H. Dysfunction of muscle contraction with impaired intracellular Ca2+ handling in skeletal muscle and the effect of exercise training in male db/db mice. J Appl Physiol (1985) 126: 170–182, 2019. doi: 10.1152/japplphysiol.00048.2018. [DOI] [PubMed] [Google Scholar]
- 49.Pattanakuhar S, Sutham W, Sripetchwandee J, Minta W, Mantor D, Palee S, Pratchayasakul W, Chattipakorn N, Chattipakorn SC. Combined exercise and calorie restriction therapies restore contractile and mitochondrial functions in skeletal muscle of obese-insulin resistant rats. Nutrition 62: 74–84, 2019. doi: 10.1016/j.nut.2018.11.031. [DOI] [PubMed] [Google Scholar]
- 50.Arnegard ME, Whitten LA, Hunter C, Clayton JA. Sex as a biological variable: a 5-year progress report and call to action. J Womens Health (Larchmt) 29: 858–864, 2020. doi: 10.1089/jwh.2019.8247. [DOI] [PMC free article] [PubMed] [Google Scholar]