Abstract
Cerebral blood flow (CBF) becomes pulsatile in response to the pulsatile change in perfusion pressure that is regulated by cerebrovascular impedance. In this study, we aimed to characterize age-related differences in cerebrovascular impedance across the adult lifespan. Carotid artery pressure [(CAP), via applanation tonometry] and CBF velocity (CBFV) in the middle cerebral artery (via transcranial Doppler) were measured in 148 healthy adults (21–79 yr, 62% women). Cerebrovascular impedance was quantified using transfer function analysis. Coherence between changes in CBFV and CAP was >0.90 in the frequency range of 0.78–2.73 Hz, suggesting a linear dynamic relationship between these two variables. Impedance modulus at the first harmonics (0.78–1.56 Hz) of CBFV and CAP oscillations (Z1), reflecting mainly heart rate frequency, was 20% higher in the old (>64 yr, P = 0.002) and 13% higher in the middle-aged (45–64 yr, P = 0.08) than in young individuals (<45 yr). In addition, Z1 was 24% higher in men than in women (P < 0.001). Multiple linear regression analysis revealed that Z1 is negatively associated with systolic (β = –0.470), diastolic (β = –0.418), pulsatile (β = –0.374), and mean CBFV (β = –0.473; P < 0.001 for all) after adjustment for age, sex, and body mass index (BMI). These results suggest that older age and male sex are associated with higher cerebrovascular impedance than young individuals, which may contribute to brain hypoperfusion.
NEW & NOTEWORTHY Impedance modulus at the first harmonics of cerebral blood flow velocity (CBFV) and carotid artery pressure oscillations (Z1) was higher in the old (>64 yr) than in the young individuals (<45 yr), and it was higher in men than in women. Z1 is negatively associated with CBFV after adjustment for age, sex, and body mass index. Increases in cerebrovascular impedance with age may buffer systemic arterial pressure fluctuations at the cost of increased brain hypoperfusion risk.
Keywords: aging, cerebral blood flow, Fourier analysis, sex-difference, transcranial Doppler
INTRODUCTION
The brain is continuously perfused with a high volume of blood flow because of its high metabolic rate and relatively low vascular resistance. Cerebral blood flow (CBF) has been shown to decrease with age and is associated with an increase in cerebrovascular resistance (CVR) (1). Normally, large elastic conduit arteries such as the proximal aorta and carotid arteries dampen arterial pressure pulsatility to ensure a nearly steady flow in the microvascular bed of the brain (2, 3). However, such dampening ability (Windkessel function) may become less effective with advancing age due to “central arterial stiffening” (4–6). Thus, age-related central arterial stiffening may facilitate penetration of pulsatile blood pressure and flow into the cerebral vasculature, which may lead to brain structural and functional abnormalities (7–10).
In this regard, intracranial arteries and arterioles also buffer arterial pressure and CBF pulsatilites (11). The Windkessel function of the cerebral vasculature is partly determined by the smooth muscle tone that is often evaluated by vascular resistance or resistance index (i.e., mean arterial pressure/mean blood flow volume or velocity). Most studies to date evaluated age-related increases in cerebrovascular resistance, which reflect only the steady-state pressure-flow relationship of the cerebral circulation (12–14). A few studies quantified the intracranial pulsatile pressure-flow relationship using transcranial Doppler (TCD) (15, 16) and MRI (17) to estimate arterial compliance in healthy young subjects. However, at present, aging effects on the cerebral Windkessel function are not well understood, which may be quantified by the estimation of cerebrovascular impedance, a frequency-domain analysis to characterize pulsatile hemodynamics in the local vascular beds.
We previously demonstrated the feasibility of assessing cerebrovascular impedance by the recordings of noninvasive cerebral blood flow velocity (CBFV) from the middle cerebral artery [(MCA), via TCD] and arterial pressure at the carotid artery [(CAP), via applanation tonometry] (18). Furthermore, we observed a significant difference in cerebrovascular impedance modulus between young and elderly individuals and a significant correlation between cerebrovascular impedance modulus and CBFV (18). However, these findings were derived from a preliminary analysis of only six young (three women) and nine elderly (seven women) individuals. The sample size of this study was small, and the potential influence of sex on cerebrovascular impedance could not be determined (19–22). Accordingly, the purpose of this study was to determine age-related differences in cerebrovascular impedance across the adult lifespan. Based on our previous studies, we tested the hypothesis that the cerebrovascular impedance modulus is higher in older individuals and that higher cerebrovascular impedance is associated with lower CBFV. We also investigated the potential sex-related differences in cerebrovascular impedance.
METHODS
Subjects
In total, 148 participants aged between 21 and 79 yr (62% women) were recruited. Exclusion criteria were body mass index (BMI) > 40 kg/m2, smoking, the presence or a history of ischemic or structural heart disease screened by 12-lead ECG and echocardiography, carotid artery atherosclerotic plaque or stenosis with >50% occlusion evaluated by ultrasound image, use of antidiabetic drugs or fasting blood glucose >6.9 mmol/L, active alcohol or drug abuse, brain damage or trauma, and the presence or a history of cerebrovascular (e.g., stroke), neurological, psychiatric, or chronic inflammatory diseases. Physically active individuals who participated in regular moderate- to high-intensity aerobic exercise training (>30 min/session, three times per week over the past two years) and pregnant or breastfeeding women were also excluded. This study was approved by the Institutional Review Board of the University of Texas Southwestern Medical Center and Texas Health Presbyterian Hospital Dallas, and was performed in accordance with the guidelines of the Declaration of Helsinki and Belmont Report. A written informed consent was signed by all subjects for study participation.
Study Protocol and Measurements
All data were collected in an ambient temperature-controlled laboratory (∼22°C). Subjects abstained from consuming caffeinated beverages or alcohol and performing vigorous exercise at least 24 h before the study. After quiet supine resting for ≥10 min, all vascular measurements were performed under the supine position with normal breathing. We recorded brachial cuff pressure (via electrosphygmomanography, SunTech) and arterial pressure waveform (via applanation tonometry, SphygmoCor 8.0; AtCor Medical) three times and calculated the averaged systolic blood pressure (SBP) and diastolic blood pressure (DBP). Brachial mean arterial pressure (MAP) was defined as the time-averaged area under the brachial arterial pressure waveform over 10 s and corrected by the cuff SBP and DBP. Simultaneously, ECG (via three-lead system, Hewlett-Packard), end-tidal CO2 (ETCO2, via a nasal cannula, using capnography, Capnogard; Novametrix), CAP waveform of the right carotid artery (via applanation tonometry), and CBFV in the MCA (via TCD ultrasonography, Multi-Dop X2; DWL) were acquired as previously reported (18). During the tonometric CAP measurements, a pressure sensor was directly placed on the skin and pressed on the arteries at a location where the strongest pulse was felt and the vessel was well supported by an underlying bone structure (23). CAP and CBFV waveforms were recorded continually for 10 s for spectral analysis. All signals were stored on a computer using a commercial software package for data acquisition (AcqKnowledge 4.2, Biopac Systems, Inc.) with a sampling frequency of 1 kHz. Central arterial stiffness was measured with carotid-femoral pulse wave velocity (cfPWV) (24, 25). Sequential arterial tonometry recordings (∼13 s) of pulse pressure waveforms gated with ECG were performed on the right common carotid and the left femoral arteries (SphygmoCor 8.0; AtCor Medical, West Ryde, NSW, Australia) to measure cfPWV (12).
Data Analysis
Time-domain analysis.
The CAP waveform was calibrated with the assumption that the mean and diastolic CAP are equal to those of brachial arterial pressure (26, 27). Steady-state ETCO2, heart rate, CAP, and CBFV were obtained as the average of breath-by-breath or beat-by-beat values. Cerebrovascular resistance index (CVRi) was calculated as a ratio of mean CAP to mean CBFV.
Frequency-domain analysis.
Fast Fourier transform was used to obtain harmonic components of CAP and CBFV (Fig. 1). Transfer function method was used to estimate cerebrovascular impedance from pulsatile changes in CBFV and CAP (28). For this calculation, changes in CBFV were used as an “input,” whereas changes in arterial pressure were used as an “output” signal. Auto-spectra and cross-spectra of CBFV and CAP were estimated using the Welch algorithm (29). Briefly, time series of CBFV and CAP waveforms were resampled at 100 Hz, and the mean values of time series were removed. Then, these data were subdivided into 256-point segments (2.56 s), with 50% overlap for spectral estimation. To reduce the potential effects of including fractional cardiac cycles in these data segments on spectral estimation, each data segment was multiplied by a Hamming window before the periodogram estimation and average (30). This process resulted in a spectral resolution of 0.39 Hz for impedance estimation.
Figure 1.
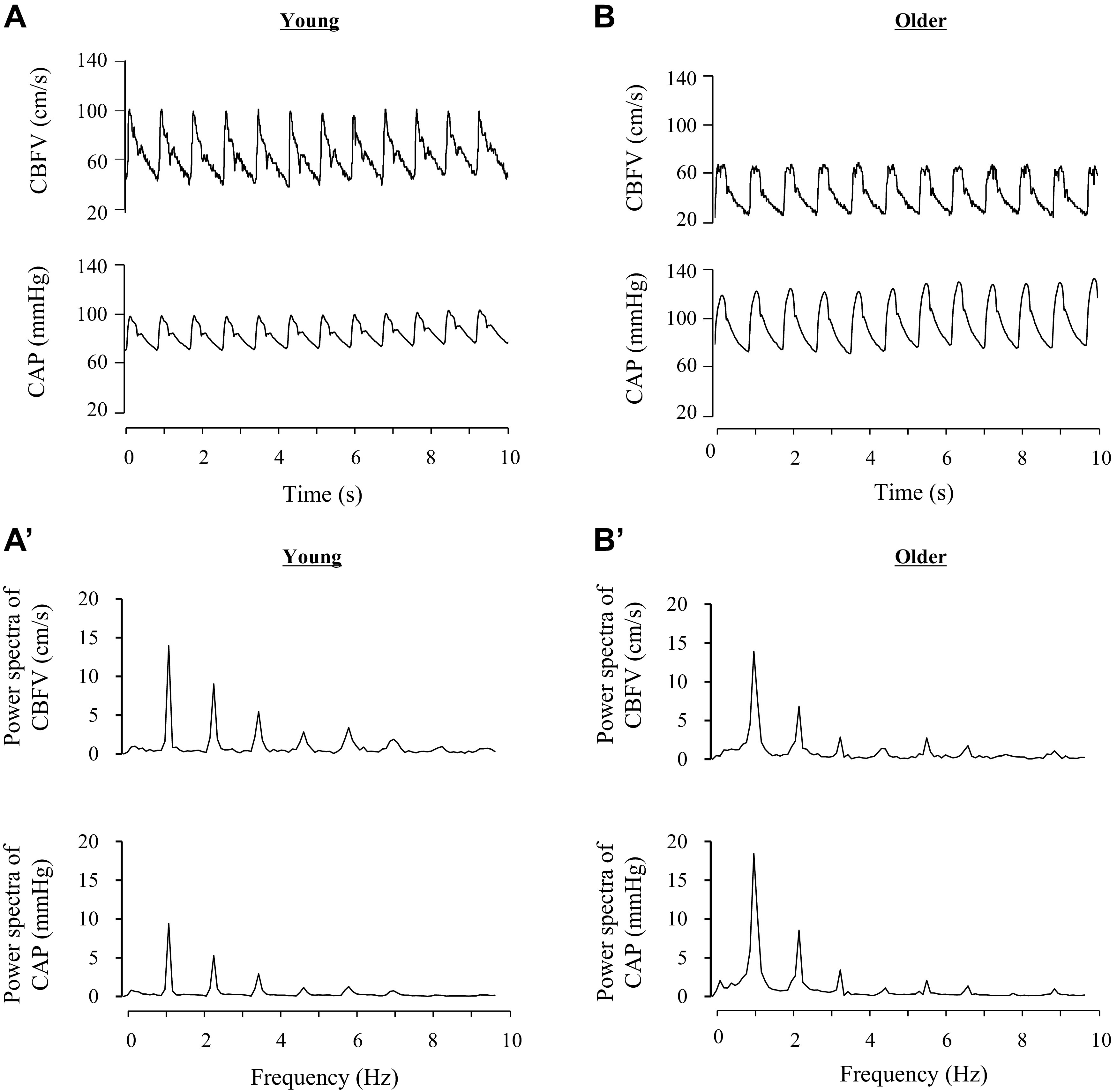
Representative data of cerebral blood flow velocity (CBFV) and carotid arterial pressure (CAP) in young (left) and older (right) individuals. A and B: time series of CBFV and CAP. A’ and B’: corresponding spectra of A and B.
The modulus of cerebrovascular impedance quantifies the magnitude relationship between pulsatile changes in CBFV and CAP. The coherence function quantifies the linear relationship between these two variables. Since the first harmonic of CBFV and CAP showed the highest value around 1.0 Hz (i.e., heart rate frequency of 60 beats/min, Fig. 1), areas under the curve of CBFV and CAP power spectrum in the range of 0.78–1.56 Hz (AUCPSV and AUCPSP, respectively) were calculated as measures of cerebrovascular hemodynamic oscillations. Averaged impedance modulus in this frequency range (Z1) was obtained for group comparisons.
Statistics Analysis
Subjects were divided into three age groups: young (21–44 yr), middle-aged (45–64 yr), and old (65–80 yr). χ2 Test was used to compare distributions of men and women among groups. Two-way analysis of variance (ANOVA) (age group × sex) was performed for comparisons of participant’s demographic characteristics and steady-state hemodynamic data. Mixed ANOVA was also used to examine the effects of age, sex, and frequency on the impedance modulus and coherence with Bonferroni’s post hoc tests when statistical significance was observed for the main effect and interaction. Pearson’s product-moment correlation was used to determine the relationship between steady-state and frequency-domain hemodynamic variables. Stepwise multiple linear regression analysis was used to test the relationship between cerebrovascular impedance and CBFV, with age, sex, and BMI forced to enter as covariates. Sex was coded as “0” for women and “1” for men. Additional covariates were chosen based on simple correlation (P < 0.10, Supplemental Table S1; all Supplemental material is available at https://doi.org/10.6084/m9.figshare.13151177.v1). CVRi was not included as a covariate to avoid multicollinearity. All statistical analyses were performed using SPSS 20.0 (Chicago, IL). Data are presented as means and SD for continuous variables, whereas frequency was used for categorical variables. The statistical significance level was set to P < 0.05.
RESULTS
Table 1 summarizes subject characteristics and steady-state hemodynamic variables for each age group. χ2 Test showed no significant sex difference in the numbers of each age group. Advancing age was associated with higher brachial and carotid systolic, pulsatile, mean blood pressure, CVRi, cfPWV, and BMI, but lower ETCO2 and systolic, diastolic, pulsatile, and mean CBFV (P < 0.05). In addition, men exhibited higher brachial blood pressure and CVRi but lower CBFV than women.
Table 1.
Subjects’ demographic characteristics
|
P Values |
||||||
|---|---|---|---|---|---|---|
| Variable | Young | Middle Age | Old | Sex | Age Group | Interaction |
| Sex (men/women) | 17/28 | 22/35 | 17/29 | |||
| Age, yr | ||||||
| Men | 33 ± 7 | 54 ± 6 | 71 ± 4 | 0.393 | <0.001 | 0.091 |
| Women | 32 ± 7 | 58 ± 6 | 70 ± 4 | |||
| Height, cm | ||||||
| Men | 175 ± 7 | 176 ± 7 | 175 ± 6 | <0.001 | 0.509 | 0.719 |
| Women | 162 ± 7 | 163 ± 10 | 160 ± 6 | |||
| Weight, kg | ||||||
| Men | 80 ± 10 | 86 ± 14 | 85 ± 13 | <0.001 | 0.009 | 0.823 |
| Women | 61 ± 11 | 69 ± 10 | 69 ± 9 | |||
| BMI, kg/m2 | ||||||
| Men | 26 ± 3 | 28 ± 4 | 27 ± 4 | 0.008 | 0.006 | 0.410 |
| Women | 23 ± 4 | 26 ± 4 | 27 ± 4 | |||
| Heart rate, beats/min | ||||||
| Men | 64 ± 10 | 64 ± 10 | 59 ± 8 | 0.082 | 0.034 | 0.263 |
| Women | 68 ± 10 | 64 ± 8 | 63 ± 7 | |||
| ETCO2, mmHg | ||||||
| Men | 39 ± 3 | 38 ± 4 | 35 ± 3 | 0.466 | 0.002 | 0.432 |
| Women | 38 ± 4 | 37 ± 4 | 36 ± 4 | |||
| Brachial BP, mmHg | ||||||
| Systolic | ||||||
| Men | 114 ± 11 | 115 ± 11 | 120 ± 17 | 0.001 | 0.013 | 0.476 |
| Women | 105 ± 8 | 111 ± 10 | 114 ± 12 | |||
| Mean | ||||||
| Men | 87 ± 10 | 91 ± 9 | 89 ± 10 | 0.002 | 0.011 | 0.384 |
| Women | 80 ± 7 | 87 ± 8 | 87 ± 9 | |||
| Diastolic | ||||||
| Men | 71 ± 9 | 74 ± 8 | 70 ± 8 | <0.001 | 0.024 | 0.391 |
| Women | 65 ± 6 | 70 ± 8 | 68 ± 8 | |||
| Pulse | ||||||
| Men | 43 ± 9 | 41 ± 6 | 50 ± 13 | 0.232 | 0.001 | 0.468 |
| Women | 40 ± 6 | 41 ± 9 | 46 ± 10 | |||
| Carotid BP, mmHg | ||||||
| Systolic | ||||||
| Men | 109 ± 15 | 113 ± 15 | 115 ± 14 | 0.101 | 0.001 | 0.174 |
| Women | 100 ± 9 | 112 ± 10 | 115 ± 13 | |||
| Pulse | ||||||
| Men | 38 ± 11 | 38 ± 10 | 45 ± 11 | 0.516 | <0.001 | 0.352 |
| Women | 36 ± 7 | 42 ± 11 | 47 ± 12 | |||
| CBFV, cm/s | ||||||
| Systolic | ||||||
| Men | 83 ± 18 | 73 ± 12 | 72 ± 14 | <0.001 | <0.001 | 0.543 |
| Women | 98 ± 16 | 85 ± 15 | 79 ± 17 | |||
| Mean | ||||||
| Men | 56 ± 14 | 49 ± 8 | 45 ± 8 | <0.001 | <0.001 | 0.445 |
| Women | 67 ± 13 | 57 ± 10 | 50 ± 10 | |||
| Diastolic | ||||||
| Men | 37 ± 10 | 33 ± 5 | 26 ± 6 | 0.001 | <0.001 | 0.277 |
| Women | 45 ± 10 | 36 ± 7 | 29 ± 6 | |||
| Pulse | ||||||
| Men | 46 ± 13 | 40 ± 8 | 46 ± 10 | <0.001 | 0.049 | 0.696 |
| Women | 53 ± 10 | 49 ± 9 | 51 ± 14 | |||
| CVRi, mmHg·s/cm | ||||||
| Men | 1.7 ± 0.5 | 1.9 ± 0.4 | 2.1 ± 0.4 | <0.001 | <0.001 | 0.571 |
| Women | 1.2 ± 0.2 | 1.6 ± 0.4 | 1.8 ± 0.4 | |||
| cfPWV, m/s | ||||||
| Men | 6.4 ± 0.8 | 7.9 ± 1.2 | 9.6 ± 2.3 | 0.065 | <0.001 | 0.774 |
| Women | 5.7 ± 0.7 | 7.5 ± 1.3 | 9.2 ± 1.8 | |||
Values are means ± SD.
BMI, body mass index; BP, blood pressure; CBFV, cerebral blood flow velocity; cfPWV, carotid-femoral pulse wave velocity; CVRi, cerebrovascular resistance index; ETCO2, end-tidal CO2.
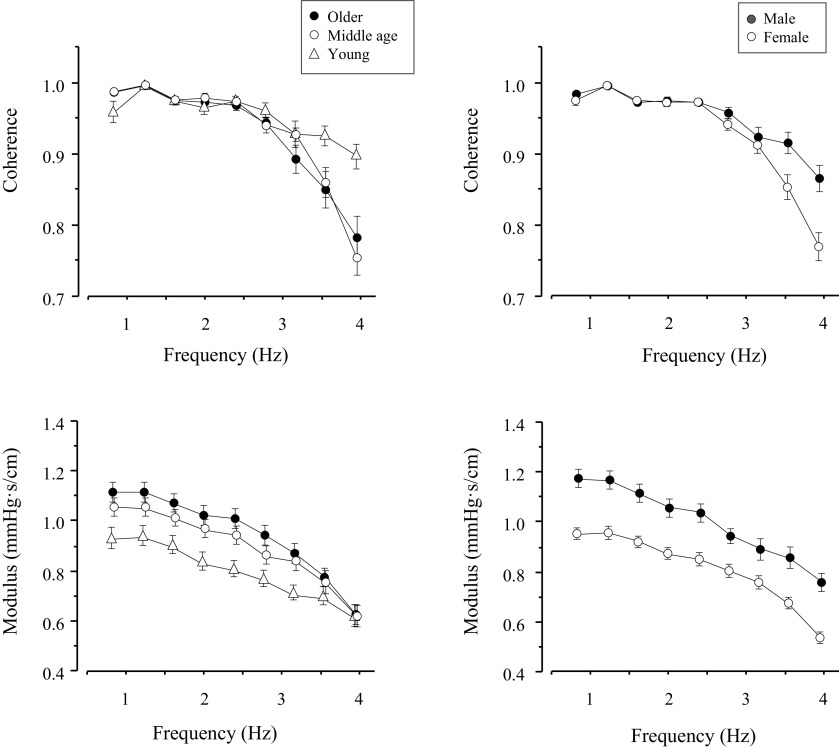
Figure 2 represents group average data of cerebrovascular impedance modulus and coherence in the range from 0.78 to 4.29 Hz. In this frequency range, the impedance modulus was 20% higher in the old (P = 0.002) and 13% higher in the middle-aged (45–64 yr, P = 0.08) than in young individuals. In addition, the impedance modulus was 24% higher in men than in women (P < 0.001). However, no age-sex interaction was observed (P = 0.622). In all groups, coherence between changes in CBFV and CAP was >0.90 in the frequency range of 0.78–2.73 Hz, suggesting a linear dynamic relationship between these two variables.
Figure 2.
Effects of aging (left) and sex (right) on cerebrovascular impedance modulus and coherence. CAP, carotid arterial pressure; CBFV, cerebral blood flow velocity.
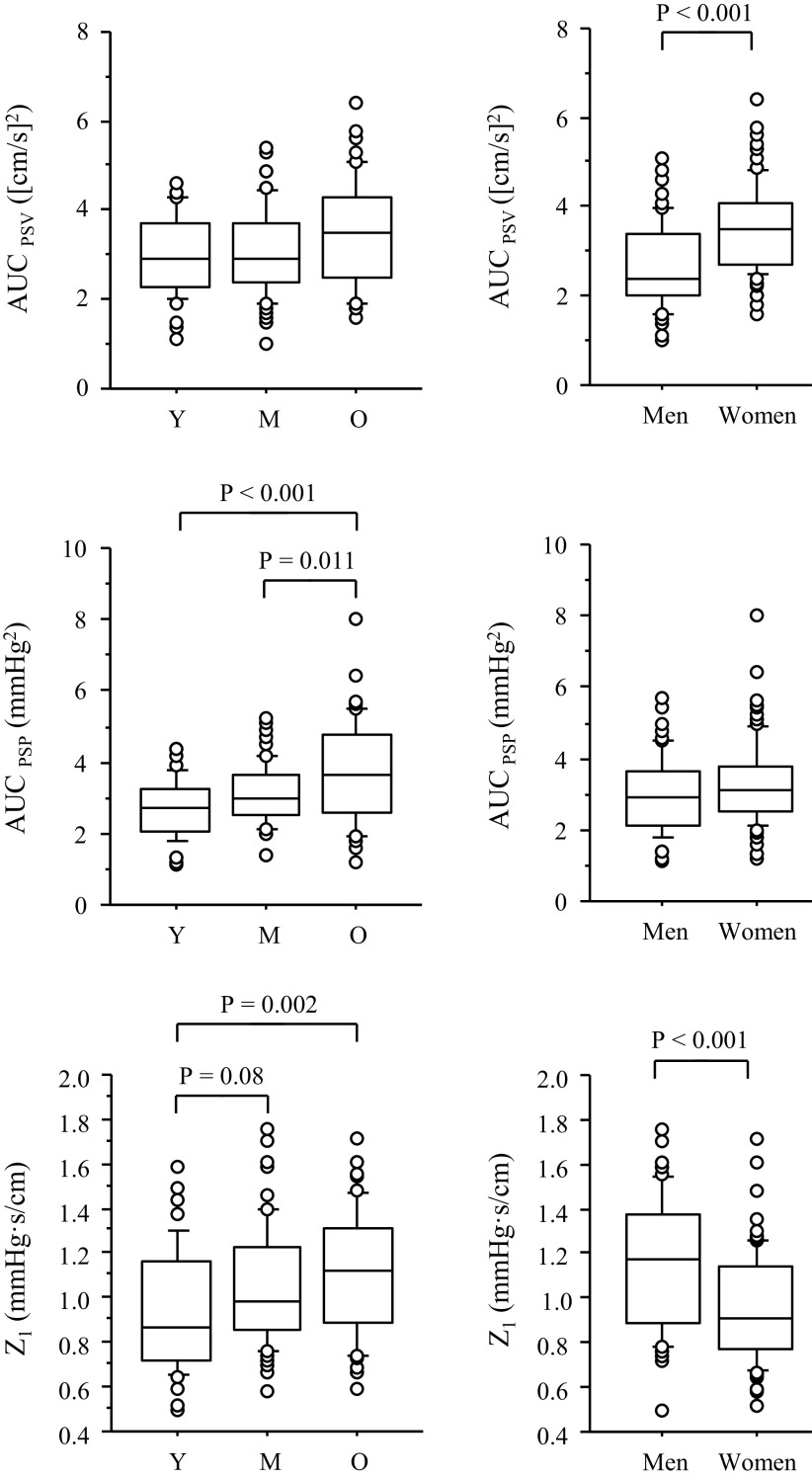
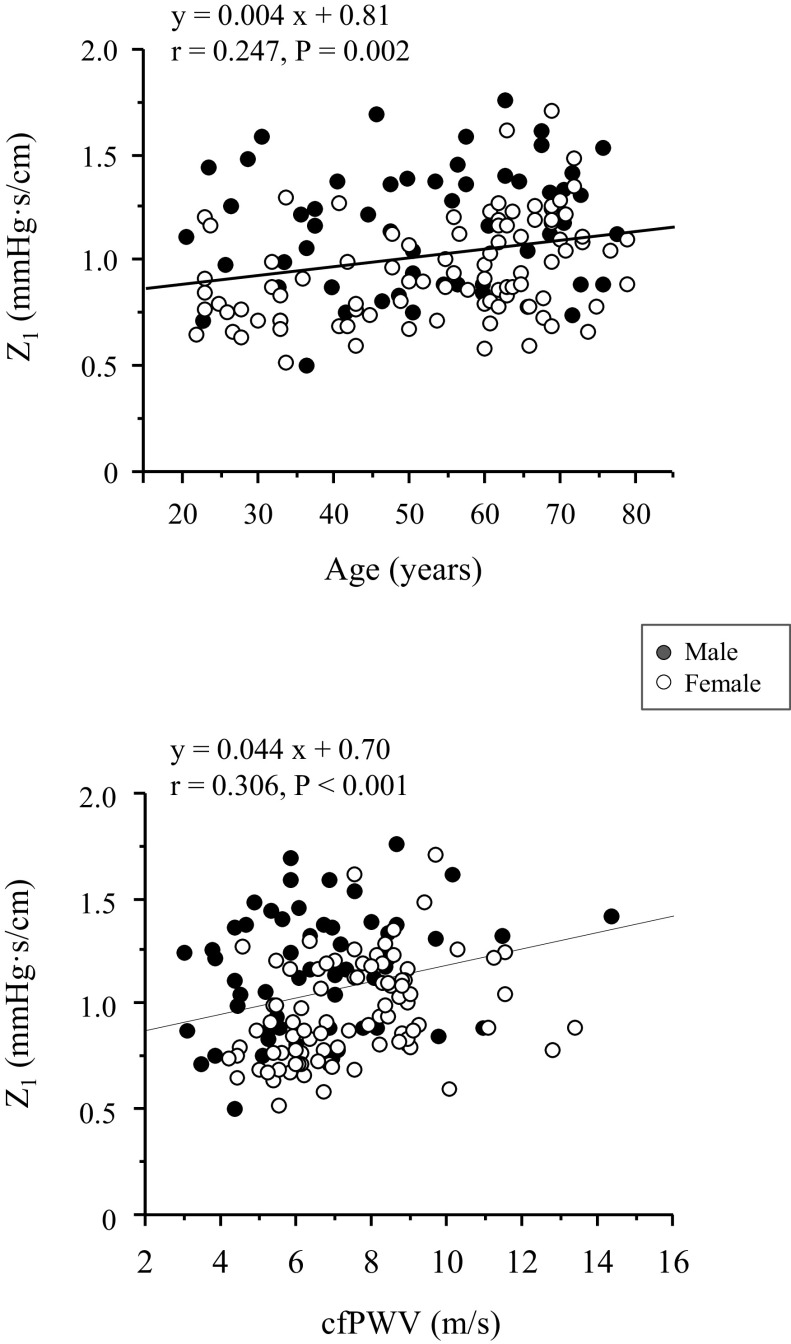
Figure 3 shows comparisons of AUCPSV, AUCPSP, and the impedance modulus at the first harmonics (Z1). AUCPSV was higher in women than in men by 31.9% (P < 0.0001). Aging effect was significant (P = 0.048), but there was no significant difference in pairwise comparison. AUCPSP was greater in the old group than in the young (by 38.5%, P < 0.001) and middle-aged (by 20.1%, P = 0.01) groups, but there was no significant sex difference (P = 0.133). Z1 was higher by 19.6% in the old than in the young group (P = 0.002). In addition, Z1 was 18.2% lower in women than in men (P < 0.0001). In the pooled subjects, Z1 showed weak but significant correlations with age (r = 0.247, P = 0.002) and cfPWV (r = 0.321, P < 0.001; Fig. 4). Correlation between Z1 and cfPWV remained significant after adjusting for age (r = 0.218, P = 0.009).
Figure 3.
The box-and-whisker plots of hemodynamic oscillation and the impedance modulus (Z1) at the first harmonic among age groups (left) and sex (right). AUCPSV and AUCPSP represent the spectral power of cerebral blood flow velocity and carotid arterial pressure oscillations in the frequency range of 0.78–1.56 Hz, respectively. Y, M, and O indicate young, middle age, and old, respectively.
Figure 4.
Simple linear correlations of cerebrovascular impedance modulus at the first harmonics (Z1) with age and carotid-femoral pulse wave velocity (cfPWV).
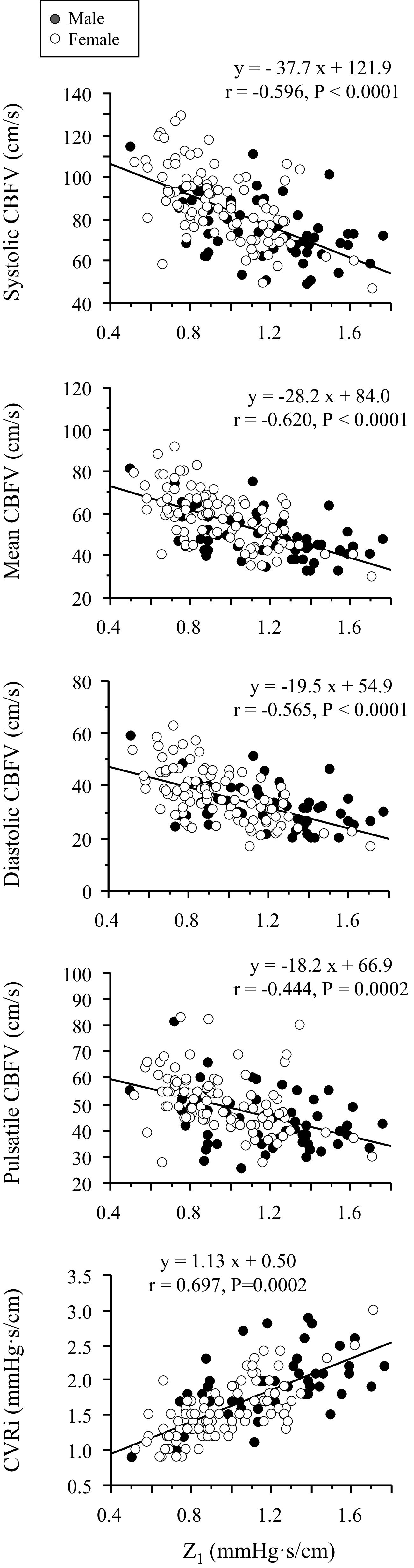
Figure 5 shows that Z1 is negatively associated with CBFV measures, but positively with CVRi. Multiple linear regression analysis showed that the negative associations between Z1 and CBFV remain after adjustment for age, sex, and BMI (Table 2).
Figure 5.
Simple linear correlations of cerebrovascular impedance modulus at the first harmonics (Z1) with cerebral blood flow velocity (CBFV) and resistance index (CVRi). Closed and open circles represent men and women, respectively.
Table 2.
Multiple linear regression analysis of cerebral hemodynamics
| Overall Model | Model Improvement | 95% CI |
||||
|---|---|---|---|---|---|---|
| Model | Variable | R2 (P Value) | ΔR2 (P Value) | β | Lower | Upper |
| Dependent variable: Mean cerebral blood flow velocity (cm/s) | ||||||
| 1 | Age | 0.337 (<0.001) | 0.337 (<0.001) | –0.450* | –0.46 | –0.24 |
| Sex | –0.305* | –11.57 | –4.37 | |||
| BMI | –0.108 | –0.79 | 0.11 | |||
| 2 | Z1 | 0.513 (<0.001) | 0.176 (<0.001) | –0.469* | –27.94 | –15.89 |
| 3 | MAP | 0.553 (<0.001) | 0.041 (<0.001) | 0.233* | 0.15 | 0.51 |
| Dependent variable: Systolic cerebral blood flow velocity (cm/sec) | ||||||
| 1 | Age | 0.266 (<0.001) | 0.266 (<0.001) | –0.353* | –0.54 | –0.22 |
| Sex | –0.300* | –16.09 | –5.60 | |||
| BMI | –0.132 | –1.23 | 0.08 | |||
| 2 | Z1 | 0.436 (<0.001) | 0.171 (<0.001) | –0.462* | –38.82 | –20.89 |
| Dependent variable: Diastolic cerebral blood flow velocity (cm/sec) | ||||||
| 1 | Age | 0.385 (<0.001) | 0.385 (<0.001) | –0.556* | –0.41 | –0.25 |
| Sex | –0.222* | –3.96 | –1.78 | |||
| BMI | –0.076 | –0.51 | –0.15 | |||
| 2 | Z1 | 0.524 (<0.001) | 0.139 (<0.001) | –0.417* | –19.16 | –10.18 |
| 3 | Carotid PP | 0.565 (<0.001) | 0.056 (<0.001) | 0.311* | 0.14 | 0.38 |
| Dependent variable: Pulsatile cerebral blood flow velocity (cm/sec) | ||||||
| 1 | Age | 0.122 (<0.001) | 0.122 (<0.001) | –0.078 | –0.17 | 0.06 |
| Sex | –0.276* | –10.18 | –2.75 | |||
| BMI | –0.139 | –0.86 | 0.07 | |||
| 2 | Z1 | 0.227 (<0.001) | 0.105 (<0.001) | –0.363* | –21.98 | –8.34 |
| 3 | Brachial DBP | 0.314 (<0.001) | 0.087 (<0.001) | –0.315* | –0.65 | –0.24 |
β, standardized coefficient; BMI, body mass index; CI, confidence interval; DBP, diastolic blood pressure; MAP, mean arterial pressure; PP, pulse pressure; R2, coefficient of determination; Z1, the averaged impedance modulus corresponding to the first harmonic oscillations of cerebral blood flow velocity and carotid arterial pressure. Age, sex, and BMI were forced to enter sequentially. Stepwise method was used to enter hemodynamic variables.
DISCUSSION
The main findings of the present study are as follows. First, aging was associated with higher cerebrovascular impedance, particularly at the first harmonic (Z1). Specifically, old (>64 yr) and middle-aged (45–64 yr) groups showed 20% and 13% higher values of Z1, respectively, when compared with young participants (<45 yr). Second, higher Z1 with age was associated with lower CBFV and higher CVRi. Third, men had higher Z1 and lower CBFV than women. Fifth, central arterial stiffness was associated positively with Z1. Taken together, these findings suggest that increases in cerebrovascular impedance may buffer systemic arterial pressure pulsatility at the cost of increased risks of brain hypoperfusion.
Mounting evidence suggests that greater, undampened systemic hemodynamic pulsatility generated by intermittent cardiac ejection coupled with central arterial stiffening may lead to brain damage in older adults (3, 9). A community-based cohort study showed that higher levels of carotid arterial pressure and blood flow pulsatility were associated with diffused microvascular brain lesions, including subcortical infarcts and white matter hyperintensities (WMH) (8). Likewise, we observed that a higher CBFV pulsatility index of the MCA was associated with greater total volume of brain WMH in older adults (12). However, few studies, to date, have examined the dynamic relationship between pulsatile changes in central arterial pressure (i.e., carotid arterial pressure) and CBF or CBFV (18).
The modulus of cerebrovascular impedance quantifies the magnitude relationship between pulsatile changes in CBFV and CAP. As depicted in Fig. 1, the largest energy of CAP and CBF signals was contained at the first harmonic (<1.56 Hz), which is associated with heart rate frequency at rest. Therefore, impedance modulus at this frequency range reflects the buffering ability of the cerebral vasculature against pulsatile pressure, which is partly related to cerebral arterial stiffness.
Our preliminary observations from a small sample of six young and nine elderly subjects showed that cerebrovascular impedance modulus in the frequency range of 0.78–4.0 Hz was higher in the elderly than in the young individuals (18). To extend and confirm this observation, in the present study, we examined the impact of aging on cerebrovascular hemodynamics in a wider age range of 148 healthy individuals including middle age. As shown in Fig. 2, cerebrovascular impedance modulus curve shifted upward gradually across the adult lifespan. This result is consistent with our previous findings (18). In particular, Z1 was elevated with age, and older adults showed significantly higher impedance values when compared with the young group. Z1 was also correlated positively with central arterial stiffness measured with cfPWV even after adjustment for age. These results suggest a role of central arterial stiffening in increases in cerebrovascular impedance.
We found that Z1 was negatively correlated with systolic, mean, diastolic, and pulsatile CBFV and that this relationship remained after adjustment for age, sex, and BMI. Of note, although CBFV does not measure volumetric CBF directly, mean CBFV measured from the MCA is correlated with total CBF (12). It also should be acknowledged that although CBFV pulsatility index, defined as (systolic CBFV − diastolic CBFV)/mean CBFV, is likely to be increased with age (12), the absolute magnitude of pulsatile changes in CBFV (i.e., systolic CBFV − diastolic CBFV) was not elevated corresponding to the elevations in CAP pulsatility, consistent with the increases in Z1 (Table 1 and Fig. 3). Collectively, these findings suggest that higher cerebrovascular impedance may contribute to a reduction in brain perfusion.
Cerebral hypoperfusion has been associated with brain hypometabolism, impaired neuronal protein synthesis, and increased neuronal toxins (e.g., amyloid-β protein and hyperphosphorylated τ) (31, 32). Furthermore, global and regional reductions in CBF have been associated with impairment of neurovascular coupling and cognitive function in patients with mild cognitive impairment and Alzheimer’s disease (33, 34). In this regard, it has been shown that women have a higher risk of Alzheimer’s disease than men (20, 22) albeit they have higher cerebral perfusion (1, 13). Besides, the incidence rate of subarachnoid hemorrhage was higher among women (19). In the present study, we found that women had ∼31.9% greater spectral power of CBFV, in the range of 0.78–1.56 Hz, than men (Fig. 3). This observation is concordant with the measurement of the absolute magnitude of pulsatile CBFV (Table 1) and our previous findings using pulsatility index (12). Z1 was 18.2% lower in women than in men. Since higher CBFV pulsatility may reflect greater mechanical stress to the cerebral blood vessels, the findings of the present study support the hypothesis that female sex is an important risk factor for the development of cerebral small vessel disease and other vascular abnormalities (35, 36). Future studies that determined whether lower cerebrovascular impedance in women than in men is related to the greater prevalence of neurodegenerative diseases among women are required (14, 19, 20, 22).
Cardiorespiratory fitness is considered to have beneficial impacts on systemic (37) and cerebrovascular (38, 39) health. In this regard, we and the others have demonstrated that cardiorespiratory fitness is associated with higher cerebrovascular compliance (17) and lower cerebrovascular impedance in young and middle-aged individuals (40). Future studies should be conducted to further understand the effect of regular physical activity and cardiorespiratory fitness on cerebrovascular impedance and CBF in the older population.
Several limitations should be mentioned. First, “vascular impedance” usually refers to vascular input impedance, which quantifies the pressure-flow relationship at the input site of a given vascular bed; thus, changes in pressure and blood flow should be measured simultaneously and at the same site. Because of the challenges in recording intracranial arterial pressure directly in human subjects, we measured arterial pressure at the carotid artery noninvasively using applanation tonometry (18, 23). Even though CAP is likely to be a good estimate of MCA pressure given the fact that MCA is a direct extension of the internal carotid artery (ICA) and the distance between the ICA and MCA is relatively short (≈10 cm) (41), a time delay of pressure wave propagation from the carotid artery to the MCA would be a confounding factor for the impedance phase estimation. Second, we measured unilateral CBFV at the MCA. The waveform contour of CBFV should be similar to that of CBF (2), which supports the evaluation of the dynamic relationship between CAP and CBFV in this study (15, 16). Third, we did not assess either vertebral arterial pressure or CBFV at the posterior cerebral artery. Thus, our findings of the higher cerebrovascular impedance at the MCA in older subjects may not apply to the posterior cerebral circulation. Fourth, we did not collect menopausal or menstrual cycle information in women. Therefore, we cannot rule out the possibility that our results are potentially confounded by the effect of sex hormones. Finally, for the cross-sectional study design, we cannot infer a causal relationship between age and elevated cerebrovascular impedance. For example, the age-related elevation of cerebrovascular impedance may reflect either vascular adaptations to increases in systemic pulsatile hemodynamics or age-related decreases in brain volume (i.e., atrophy) and metabolism. Moreover, the underlying biological mechanisms of the age-related increase in cerebrovascular impedance cannot be determined in this study.
In conclusion, we found that cerebrovascular impedance is progressively elevated across the adult lifespan, which is associated with reductions in systolic, diastolic, pulsatile, and mean CBFV with age. Furthermore, we found that men had higher cerebrovascular impedance than women. These findings provide new insights into the regulation of cerebral hemodynamics under dynamic conditions, which may be valuable for understanding brain aging and age-related cerebrovascular disease in clinical studies.
GRANTS
This study was supported in part by the National Institutes of Health Grant R01HL102457 (to R. Zhang) and Japan Society for the Promotion of Science Grant 16KK0011 (to J. Sugawara).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
T.Tarumi and R.Z. conceived and designed research; T.Tarumi, C.X., J.L., T.Tomoto, E.P.P., and R.Z. performed experiments; J.S., T.Tarumi, C.X., T.Tomoto, and E.P.P. analyzed data; J.S., T.Tomoto, and R.Z. interpreted results of experiments; J.S. prepared figures; J.S. drafted manuscript; J.S., E.P.P., and R.Z. edited and revised manuscript; J.S., T.Tarumi, C.X., J.L., T.Tomoto, E.P.P., and R.Z. approved final version of manuscript.
ACKNOWLEDGMENTS
The authors thank all the study participants for willingness, time, and effort devoted to this study.
REFERENCES
- 1.Xing CY, Tarumi T, Liu J, Zhang Y, Turner M, Riley J, Tinajero CD, Yuan LJ, Zhang R. Distribution of cardiac output to the brain across the adult lifespan. J Cereb Blood Flow Metab 37: 2848–2856, 2017. doi: 10.1177/0271678X16676826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Nichols WW, O'Rourke M, Vlachopoulos C. McDonald’s Blood Flow in Arteries Theoretical, Experimental and Clinical Principles ( 6th ed.). London, UK: Hodder Arnold, 2011. [Google Scholar]
- 3.O'Rourke MF, Hashimoto J. Mechanical factors in arterial aging: a clinical perspective. J Am Coll Cardiol 50: 1–13, 2007. doi: 10.1016/j.jacc.2006.12.050. [DOI] [PubMed] [Google Scholar]
- 4.Avolio AP, Chen SG, Wang RP, Zhang CL, Li MF, O'Rourke MF. Effects of aging on changing arterial compliance and left ventricular load in a northern Chinese urban community. Circulation 68: 50–58, 1983. doi: 10.1161/01.CIR.68.1.50. [DOI] [PubMed] [Google Scholar]
- 5.Sugawara J, Tomoto T, Noda N, Matsukura S, Tsukagoshi K, Hayashi K, Hieda M, Maeda S. Effects of endothelin-related gene polymorphisms and aerobic exercise habit on age-related arterial stiffening: a 10-yr longitudinal study. J Appl Physiol (1985) 124: 312–320, 2018. doi: 10.1152/japplphysiol.00697.2017. [DOI] [PubMed] [Google Scholar]
- 6.Tanaka H, Dinenno FA, Monahan KD, Clevenger CM, DeSouza CA, Seals DR. Aging, habitual exercise, and dynamic arterial compliance. Circulation 102: 1270–1275, 2000. doi: 10.1161/01.CIR.102.11.1270. [DOI] [PubMed] [Google Scholar]
- 7.Ding J, Mitchell GF, Bots ML, Sigurdsson S, Harris TB, Garcia M, Eiriksdottir G, van Buchem MA, Gudnason V, Launer LJ. Carotid arterial stiffness and risk of incident cerebral microbleeds in older people: the Age, Gene/Environment Susceptibility (AGES)-Reykjavik study. Arterioscler Thromb Vasc Biol 35: 1889–1895, 2015. doi: 10.1161/ATVBAHA.115.305451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mitchell GF, van Buchem MA, Sigurdsson S, Gotal JD, Jonsdottir MK, Kjartansson Ó, Garcia M, Aspelund T, Harris TB, Gudnason V, Launer LJ. Arterial stiffness, pressure and flow pulsatility and brain structure and function: the Age, Gene/Environment Susceptibility–Reykjavik study. Brain 134: 3398–3407, 2011. doi: 10.1093/brain/awr253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.O'Rourke MF, Safar ME. Relationship between aortic stiffening and microvascular disease in brain and kidney: cause and logic of therapy. Hypertension 46: 200–204, 2005. doi: 10.1161/01.HYP.0000168052.00426.65. [DOI] [PubMed] [Google Scholar]
- 10.Pase MP, Beiser A, Himali JJ, Tsao C, Satizabal CL, Vasan RS, Seshadri S, Mitchell GF. Aortic Stiffness and the risk of incident mild cognitive impairment and dementia. Stroke 47: 2256–2261, 2016. doi: 10.1161/STROKEAHA.116.013508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tzeng YC, MacRae BA, Ainslie PN, Chan GS. Fundamental relationships between blood pressure and cerebral blood flow in humans. J Appl Physiol 117: 1037–1048, 2014. doi: 10.1152/japplphysiol.00366.2014. [DOI] [PubMed] [Google Scholar]
- 12.Tarumi T, Ayaz Khan M, Lui J, Tseng BY, Tseng BM, Parker R, Riley J, Tinajero C, Zhang R. Cerebral hemodynamics in normal aging: central artery stiffness, wave reflection, and pressure pulsatility. J Cereb Blood Flow Metab 34: 971–978, 2014. doi: 10.1038/jcbfm.2014.44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Xing CY, Tarumi T, Meijers RL, Turner M, Repshas J, Xiong L, Ding K, Vongpatanasin W, Yuan LJ, Zhang R. Arterial pressure, heart rate, and cerebral hemodynamics across the adult life span. Hypertension 69: 712–720, 2017. doi: 10.1161/HYPERTENSIONAHA.116.08986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yew B, Nation DA; Alzheimer’s Disease Neuroimaging Initiative. Cerebrovascular resistance: effects on cognitive decline, cortical atrophy, and progression to dementia. Brain 140: 1987–2001, 2017. doi: 10.1093/brain/awx112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Brassard P, Ferland-Dutil H, Smirl JD, Paquette M, Le Blanc O, Malenfant S, Ainslie PN. Evidence for hysteresis in the cerebral pressure-flow relationship in healthy men. Am J Physiol Heart Circ Physiol 312: H701–H704, 2017. doi: 10.1152/ajpheart.00790.2016. [DOI] [PubMed] [Google Scholar]
- 16.Moir ME, Klassen SA, Zamir M, Shoemaker JK. Rapid changes in vascular compliance contribute to cerebrovascular adjustments during transient reductions in blood pressure in young, healthy adults. J Appl Physiol (1985) 129: 27–35, 2020. doi: 10.1152/japplphysiol.00272.2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Furby HV, Warnert EAH, Marley CJ, Bailey DM, Wise RG. Cardiorespiratory fitness is associated with increased middle cerebral arterial compliance and decreased cerebral blood flow in young healthy adults: A pulsed ASL MRI study. J Cerebr Blood Flow Metab, 2019. doi: 10.1177/0271678X19865449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zhu YS, Tseng BY, Shibata S, Levine BD, Zhang R. Increases in cerebrovascular impedance in older adults. J Appl Physiol (1985) 111: 376–381, 2011. doi: 10.1152/japplphysiol.01418.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Appelros P, Stegmayr B, Terent A. Sex differences in stroke epidemiology: a systematic review. Stroke 40: 1082–1090, 2009. doi: 10.1161/STROKEAHA.108.540781. [DOI] [PubMed] [Google Scholar]
- 20.Ferretti MT, Iulita MF, Cavedo E, Chiesa PA, Schumacher Dimech A, Santuccione Chadha A, Baracchi F, Girouard H, Misoch S, Giacobini E, Depypere H, Hampel H; the Women’s Brain Project and the Alzheimer Precision Medicine Initiative. Sex differences in Alzheimer disease – the gateway to precision medicine. Nat Rev Neurol 14: 457–469, 2018. doi: 10.1038/s41582-018-0032-9. [DOI] [PubMed] [Google Scholar]
- 21.Haberman S, Capildeo R, Rose FC. Sex differences in the incidence of cerebrovascular disease. J Epidemiol Community Health 35: 45–50, 1981. doi: 10.1136/jech.35.1.45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lee J, Cho H, Jeon S, Kim HJ, Kim YJ, Lee J, Kim ST, Lee JM, Chin J, Lockhart SN, Lee AY, Na DL, Seo SW. Sex-related reserve hypothesis in Alzheimer's disease: changes in cortical thickness with a five-year longitudinal follow-up. J Alzheimers Dis 65: 641–649, 2018. doi: 10.3233/JAD-180049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kelly R, Hayward C, Avolio A, O'Rourke M. Noninvasive determination of age-related changes in the human arterial pulse. Circulation 80: 1652–1659, 1989. doi: 10.1161/01.CIR.80.6.1652. [DOI] [PubMed] [Google Scholar]
- 24.Townsend RR, Wilkinson IB, Schiffrin EL, Avolio AP, Chirinos JA, Cockcroft JR, Heffernan KS, Lakatta EG, McEniery CM, Mitchell GF, Najjar SS, Nichols WW, Urbina EM, Weber T; American Heart Association Council on Hypertension. Recommendations for improving and standardizing vascular research on arterial stiffness: a scientific statement from the American Heart Association. Hypertension 66: 698–722, 2015. doi: 10.1161/HYP.0000000000000033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Van Bortel LM, Laurent S, Boutouyrie P, Chowienczyk P, Cruickshank JK, De Backer T, Filipovsky J, Huybrechts S, Mattace-Raso FU, Protogerou AD, Schillaci G, Segers P, Vermeersch S, Weber T; Artery Society; European Society of Hypertension Working Group on Vascular Structure and Function; European Network for Noninvasive Investigation of Large Arteries. Expert consensus document on the measurement of aortic stiffness in daily practice using carotid-femoral pulse wave velocity. J Hypertens 30: 445–448, 2012. doi: 10.1097/HJH.0b013e32834fa8b0. [DOI] [PubMed] [Google Scholar]
- 26.Armentano R, Megnien JL, Simon A, Bellenfant F, Barra J, Levenson J. Effects of hypertension on viscoelasticity of carotid and femoral arteries in humans. Hypertension 26: 48–54, 1995, doi: 10.1161/01.hyp.26.1.48. [DOI] [PubMed] [Google Scholar]
- 27.Laurent S, Cockcroft J, Van Bortel L, Boutouyrie P, Giannattasio C, Hayoz D, Pannier B, Vlachopoulos C, Wilkinson I, Struijker-Boudier H; European Network for Non-invasive Investigation of Large Arteries. Expert consensus document on arterial stiffness: methodological issues and clinical applications. Eur Heart J 27: 2588–2605, 2006. doi: 10.1093/eurheartj/ehl254. [DOI] [PubMed] [Google Scholar]
- 28.Bendat JS, Piersol AG. Engineering Application of Correlations and Spectral Analysis. New York: Wiley-Interscience, 1980. [Google Scholar]
- 29.Welch P. The use of fast Fourier transform for the estimation of power spectra: a method based on time averaging over short, modified periodograms. IEEE Trans Audio Electroacoust 15: 70–73, 1967. doi: 10.1109/TAU.1967.1161901. [DOI] [Google Scholar]
- 30.Marple S Jr. Digital Spectral Analysis With Applications. Englewood Cliffs, NJ: Prentice Hall, Inc., 1987. [Google Scholar]
- 31.Iturria-Medina Y, Sotero RC, Toussaint PJ, Mateos-Pérez JM, Evans AC; Alzheimer’s Disease Neuroimaging Initiative. Early role of vascular dysregulation on late-onset Alzheimer’s disease based on multifactorial data-driven analysis. Nat Commun 7: 11934, 2016. doi: 10.1038/ncomms11934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zlokovic BV. Neurovascular pathways to neurodegeneration in Alzheimer's disease and other disorders. Nat Rev Neurosci 12: 723–738, 2011.doi: 10.1038/nrn3114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Leeuwis AE, Benedictus MR, Kuijer JPA, Binnewijzend MAA, Hooghiemstra AM, Verfaillie SCJ, Koene T, Scheltens P, Barkhof F, Prins ND, van der Flier WM. Lower cerebral blood flow is associated with impairment in multiple cognitive domains in Alzheimer's disease. Alzheimers Dement 13: 531–540, 2017. doi: 10.1016/j.jalz.2016.08.013. [DOI] [PubMed] [Google Scholar]
- 34.Liu J, Zhu YS, Khan MA, Brunk E, Martin-Cook K, Weiner MF, Cullum CM, Lu H, Levine BD, Diaz-Arrastia R, Zhang R. Global brain hypoperfusion and oxygenation in amnestic mild cognitive impairment. Alzheimers Dement 10: 162–170, 2014. doi: 10.1016/j.jalz.2013.04.507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Habes M, Sotiras A, Erus G, Toledo JB, Janowitz D, Wolk DA, Shou H, Bryan NR, Doshi J, Völzke H, Schminke U, Hoffmann W, Resnick SM, Grabe HJ, Davatzikos C. White matter lesions: Spatial heterogeneity, links to risk factors, cognition, genetics, and atrophy. Neurology 91: e964–e975, 2018. doi: 10.1212/WNL.0000000000006116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Jabbarli R, Dinger TF, Darkwah Oppong MD, Pierscianek D, Dammann P, Wrede KH, Kaier K, Köhrmann M, Forsting M, Kleinschnitz C, Sure U. Risk factors for and clinical consequences of multiple intracranial aneurysms: a systematic review and meta-analysis. Stroke 49: 848–855, 2018. doi: 10.1161/STROKEAHA.117.020342. [DOI] [PubMed] [Google Scholar]
- 37.Seals DR, Walker AE, Pierce GL, Lesniewski LA. Habitual exercise and vascular ageing. J Physiol 587: 5541–5549, 2009. doi: 10.1113/jphysiol.2009.178822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bailey DM, Marley CJ, Brugniaux JV, Hodson D, New KJ, Ogoh S, Ainslie PN. Elevated aerobic fitness sustained throughout the adult lifespan is associated with improved cerebral hemodynamics. Stroke 44: 3235–3238, 2013. doi: 10.1161/STROKEAHA.113.002589. [DOI] [PubMed] [Google Scholar]
- 39.Tarumi T, Zhang R. Cerebral blood flow in normal aging adults: cardiovascular determinants, clinical implications, and aerobic fitness. J Neurochem 144: 595–608, 2018. doi: 10.1111/jnc.14234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sugawara J, Tomoto T, Repshas J, Zhang R, Tarumi T. Middle-aged endurance athletes exhibit lower cerebrovascular impedance than sedentary peers. J Appl Physiol 129: 335–342, 2020. doi: 10.1152/japplphysiol.00239.2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Giller CA, Aaslid R. Estimates of pulse wave velocity and measurement of pulse transit time in the human cerebral circulation. Ultrasound Med Biol 20: 101–105, 1994. doi: 10.1016/0301-5629(94)90074-4. [DOI] [PubMed] [Google Scholar]