Abstract
Objective:
To assess plasma and vaginal inflammation in 3 antenatal groups (HIV-uninfected women, HIV-infected women entering care on preconceptional ART, and HIV-infected women not on preconceptional ART) and whether these measures are associated with spontaneous preterm birth (sPTB).
Design:
Case-control study nested within a pregnancy cohort in Lusaka, Zambia.
Methods:
We analyzed 11 pro- and 2 anti-inflammatory markers in 207 women with paired plasma and vaginal specimens collected between 16–20 gestational weeks. Among 51 HIV-infected women, we repeated the assays in 24–34 week samples. We used confirmatory factor analysis to create inflammation scores and compared them among the 3 groups.
Results:
At baseline, HIV-infected women not on ART had higher vaginal pro-inflammatory scores than HIV-uninfected women (mean 0.37 [95%CI −0.06, 0.80] vs. −0.02 [−0.32, 0.27], p=0.02). In repeat testing, women not on preconceptional ART had an increase in vaginal inflammation between the baseline and 24–34 week visits compared to those continuing preconceptional ART (mean 0.62 [95%CI −0.80,4.20] vs. −0.07 [−2.78,2.11], p=0.04). In multivariate analyses, baseline vaginal inflammation predicted sPTB (aOR 1.5; 95%CI 1.0–2.3; p=0.02). Plasma inflammation did not differ by HIV or ART exposure and was not associated with sPTB.
Conclusions:
Women not receiving ART at entry into pregnancy care had more vaginal inflammation than women entering on treatment. They also experienced an increase in vaginal inflammation between the 2 sampling timepoints, possibly as a consequence of ART initiation. Vaginal (but not systemic) inflammation was associated with sPTB and offers a potential mechanistic insight into this important adverse birth outcome.
Keywords: Cytokines, vaginal inflammation, preterm birth, spontaneous preterm birth, HIV, Zambia
Introduction:
Preterm birth (PTB) is the world’s leading cause of neonatal death[1, 2]. The highest burden of PTB is borne by women living in Africa and south Asia, where more than 60% of cases occur and clinical infrastructure is often ill-equipped to care for premature newborns[3–6]. In sub-Saharan Africa, where HIV is endemic[7], PTB rates are among the highest in the world. Maternal HIV and exposure to antiretroviral therapy (ART) have been associated with elevations in prematurity risk[8, 9], with some studies suggesting that women initiating ART prior to conception have a higher risk of delivering prematurely than those initiating ART during pregnancy[10, 11].
PTB is a complex syndrome with several distinct phenotypes[12]. At the broadest level, PTB phenotypes can be divided into those arising spontaneously and those initiated by a provider. Intrauterine infection, which may be overt or subclinical, has long been associated with the spontaneous PTB phenotype (sPTB)[12–18]. Chronic inflammation and immune activation occurring in the absence of infection are also correlated with sPTB. For instance, rates of sPTB are higher in settings of obesity[19] and autoimmune disease[20–22]. The mechanisms by which intrauterine infection and inflammation lead to sPTB may be related to innate immune system activation[23], which, through a complex network of cytokines, chemokines, and other signaling molecules, promotes cervical ripening, uterine contractions, and amniotic membrane rupture[24–26]. The finding that women who become pregnant on ART (and whose HIV is better-controlled) have higher rates of PTB than women who enter antenatal care not on therapy is a counterintuitive observation that may be related to selection bias[27, 28].
Although the relationship between inflammatory cytokines and sPTB has been previously reported[29–31], few studies have been conducted in HIV-infected or African cohorts[32–34]. In this study, we sought to characterize local and systemic inflammation through the evaluation of vaginal and plasma cytokine profiles in a Zambian antenatal cohort, stratified by HIV serostatus. We hypothesized that the elevated risk of sPTB in HIV-infected women and those with preconceptional ART exposure may be related to measurable differences in the local or systemic immune milieu.
Materials and Methods:
Patient Population:
The Zambian Preterm Birth Prevention Study (ZAPPS) is an ongoing prospective obstetrical cohort at the Women and Newborn Hospital of the University Teaching Hospital (UTH) in Lusaka, Zambia[35, 36]. Written informed consent for collection of clinical data and biological specimens is obtained prior to study enrollment. Women are enrolled in early pregnancy, and antenatal care is provided at a study-run clinic. Ultrasounds are performed to determine gestational age, to measure cervical length at 20–24 weeks, and to monitor fetal growth in the third trimester. At delivery, birth phenotypes are assigned based on maternal and fetal factors as well as pathway to delivery. Using these phenotypes, parturition is classified as either spontaneous (e.g. cervical dilation, contractions) or provider-initiated (no signs of spontaneous labor prior to initiation of induction of labor or cesarean) [36, 37].
For the current nested case-control study, we defined a 3-level exposure variable that characterizes participants by HIV serostatus at enrollment and, among the HIV-infected, by the timing of ART exposure (preconceptional or not) (Supplemental File).
Specimen Collection and Storage:
Vaginal swabs and plasma were collected and stored according to standard operating procedures (Supplemental File). Study specimens are periodically shipped from Zambia to a sister biorepository in Chapel Hill, NC where we maintain a duplicate archive.
Sample Selection:
Eligibility for this study was limited to ZAPPS participants with paired plasma and vaginal specimens collected between 16–20 weeks of gestation available in the initial May 2017 biorepository shipment (Figure 1). We excluded women with an unknown delivery outcome (n=86), provider-initiated PTB (n=7), and unknown PTB phenotype (n=2). Additionally, HIV-infected women with unknown ART exposure status (n=2) were excluded. To maximize information from available specimens, we used a double sampling technique of eligible specimens that included all participants who were HIV-infected and all participants with sPTB. Among remaining HIV-uninfected participants delivering at term, we selected specimens at random, at a proportion dictated by available budget. We applied inverse probability sampling weights to our factor analyses as well as all subsequent univariate and multivariate analyses using the probability of selection among the baseline samples. Weighted regression models were estimated with robust standard errors computed by the linear variance estimator[41], using the probability svy function in STATA.[42] Additionally, for all HIV-infected women with matched repeat vaginal and plasma specimens obtained between 24–34 gestational weeks, we repeated assays. Further, as twin pregnancy and short midtrimester cervix (<2.5cm) are strong independent predictors of sPTB and rare in this study, women with these risk factors were excluded from PTB analyses (Figure 1).
Figure 1:
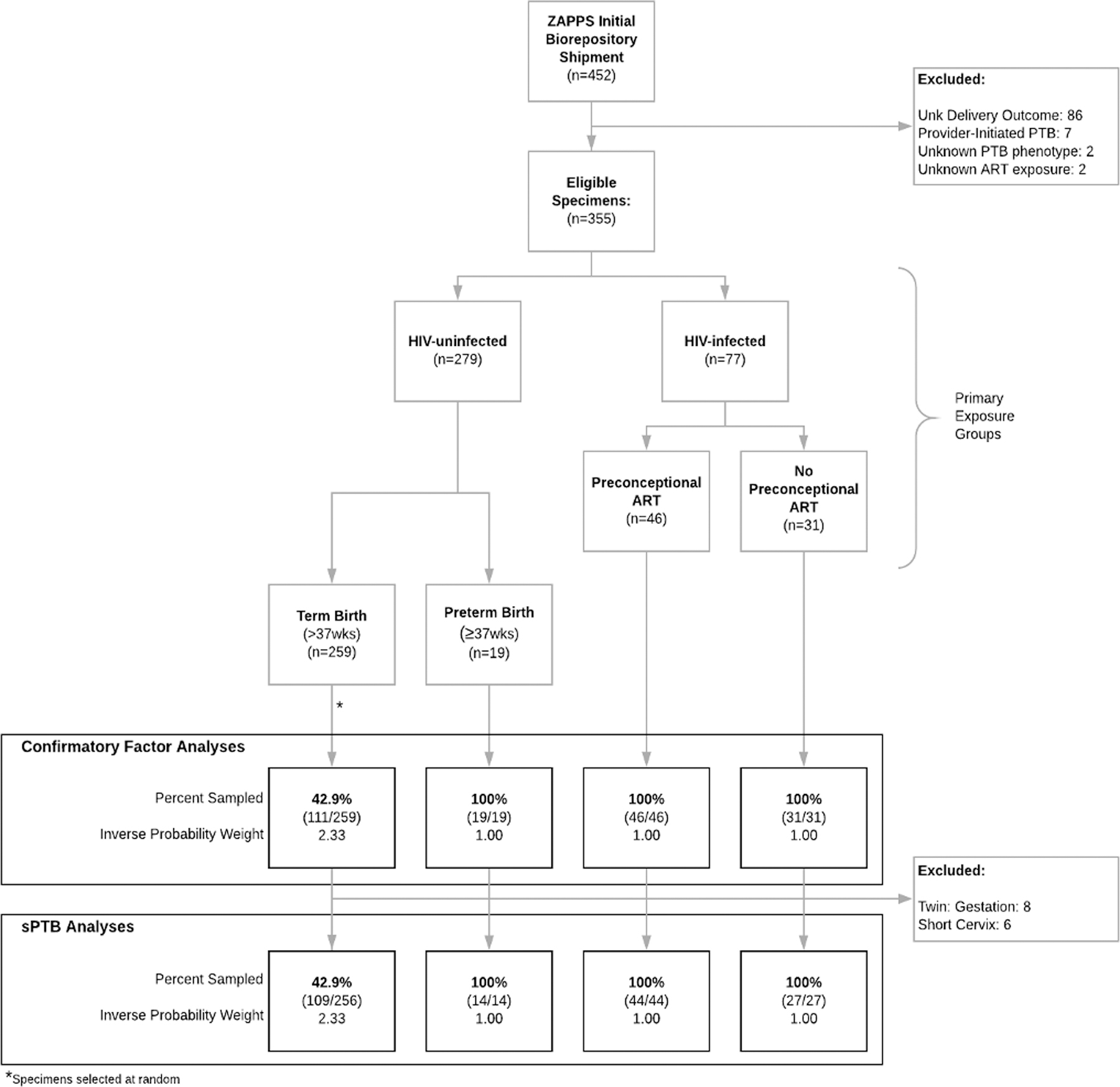
Study Sampling Technique
Cytokine Array Analysis:
Plasma and vaginal swab samples were analyzed by multiparameter bead array for interleukin-1β (IL-1β), IL-2, IL-6, IL-8, IL-10, IL-12p70, interferon-ɣ (IFN-ɣ), IFN-ɣ-induced protein 10 (IP-10), tumor necrosis factor-α (TNF-α), macrophage inflammatory protein-1α (MIP-1α), MIP-1β, and transforming growth factor-β (TGF-β) using a commercially available Milliplex-kit (Millipore-Sigma, Minneapolis, MN). In addition, we measured soluble CD14 (sCD14) by ELISA (R&D Systems, Darmstadt, Germany). Cytokine concentrations were calculated according to the manufacturer’s protocols. For values below the limit of detection, we imputed the value at half of the lower limit of detection. We did not apply newer methods for handling limits of detection[43, 44] because they cannot currently be applied to factor analysis. All concentrations were logarithmically transformed prior to analysis.
Data Analyses:
We analyzed baseline demographic data of the sub-study cohort, calculating median and interquartile range (IQR) for each continuous variable, and frequency and percentage for each categorical variable. We analyzed differences in continuous independent variables between exposure groups using Wilcoxon ranksum and Kruskal-Wallis test for comparisons between two or more than two groups, respectively.
From a biostatistical perspective, the analysis of cytokines is challenging due to strong correlations between cytokines, particularly those with similar underlying biological processes. Previous work by Genser et al[45] found that most immunological studies use basic statistical methods that do not account for correlations or relationships among study variables, such as cytokines. Thus, they recommend that more advanced and sophisticated multivariate statistical techniques be used to assess complex immunological data. Factor analysis is one such multivariable statistical technique that reduces a large number of intercorrelated variables into a smaller set of independent clusters underlying physiological relationships. As the biologic actions of cytokines can be broadly classified as either pro- or anti-inflammatory, we performed a confirmatory factor analysis, which is a hypothesis-driven approach developed to assess whether associations among many variables confirm hypothesized underlying factors. [20, 46] This approach contrasts an exploratory factor analysis, which lacks a hypothesis and a priori assumptions (and was not used in this study). We assigned cytokines as either pro-inflammatory (MIP-1α, MIP-1β, IL-1β, IL-2, IL-6, IL-8, IL-12p70, IFN-ɣ, IP-10, TNF-α, and sCD14) or anti-inflammatory (IL-10 and TGF-β). Consequently, pro- and anti-inflammatory activity were the hypothesized underlying factors or latent variables. Cytokines with standardized loading or R2 values (coefficient of determination) with p-values >0.01 were excluded to reduce estimated parameters and improve model fit, as assessed by Root Mean Square Error of Approximation (RMSEA), Comparative Fit Index (CFI) and Tucker-Lewis Index (TLI). Additionally, as previous studies have strongly hypothesized that immunological processes and expression of cytokines evolve differently in blood versus vaginal fluid[29, 31], confirmatory factor analyses were conducted with separate models for blood and vaginal fluid.
Factor scores from the confirmatory factor analysis at baseline were estimated for each patient. A factor score is a relative value to a latent variable (i.e. the factor: pro- or anti-inflammatory activity) for a particular patient as compared with other women included in the analysis. By default, the estimated factor scores have a mean of zero. Thus, factor scores cannot be interpreted in absolute terms but instead are relative values (e.g. a factor score of 0 does not indicate an absence of inflammation). Based on this model, an individual with a positive vaginal pro-inflammatory factor score is assumed to have increased vaginal inflammation as compared with the mean. Associations between underlying pro- and anti-inflammatory activities in both blood and vaginal fluid were assessed by plotting factor scores and calculating Spearman correlations coefficients.
We modeled factor scores versus HIV and ART exposure groups using linear regression with inverse-probability weighting, controlling for maternal age, parity, BMI, and prior PTB. As the pro- and anti-inflammatory factor scores were highly correlated within the plasma and vaginal compartments, only the pro-inflammatory factors were included in subsequent modeling, owing to their representation of the interaction and diversity of more cytokines and their improved model fit statistics. Associations between baseline factor scores and sPTB were assessed using logistic regression to calculate unadjusted and adjusted case-control prevalence odds ratios.
In the subset of HIV-infected participants with repeat specimens (Table 1), we repeated the model developed from our baseline factor analysis. In this analysis, we included specimens from baseline (16–20 weeks) and repeat (24–34 weeks) visits to estimate factor scores for each participant at both time points. We then produced a delta pro-inflammatory score by subtracting the baseline factor score from the repeat factor score. Consequently, a positive delta pro-inflammatory score represents an increase in relative inflammation from baseline to repeat, and a negative delta pro-inflammatory score represents a decrease. Delta pro-inflammatory scores were compared by preconception ART exposure groups using Wilcoxon ranksum test.
Table 1:
Baseline characteristics of participants with specimens analyzed
| Characteristic | Baseline | Repeat | |||||
|---|---|---|---|---|---|---|---|
| Overall | HIV-uninfected | HIV-infected preconceptional ART |
HIV-infected no preconceptional ART |
Overall | HIV-infected preconceptional ART |
HIV-infected no preconceptional ART |
|
| N (%) or median (IQR) |
N (%) or median (IQR) |
N (%) or median (IQR) |
N (%) or median (IQR) |
N (%) or median (IQR) |
N (%) or median (IQR) |
N (%) or median (IQR) |
|
| Total number of women | 207 | 130 | 46 | 31 | 51 | 30 | 21 |
| EGA at collection, weeks | 18 (17, 19) | 18 (17, 19) | 18 (16, 18) | 18 (17, 19) | 18 (16, 19) | 18 (16, 18) | 18 (17, 19) |
| Age, years | 26 (22, 32) | 25 (22, 31) | 30 (27, 33) | 25 (21, 29) | 28 (25, 32) | 30 (27, 33) | 25 (21, 29) |
| <20 | 16 (8%) | 13 (10%) | 0 (0%) | 0 (0%) | 2 (4%) | 0 (0%) | 2 (10%) |
| 20–34 | 164 (80%) | 97 (76%) | 39 (84%) | 28 (90%) | 43 (86%) | 24 (83%) | 19 (90%) |
| ≥35 | 24 (12%) | 17 (13%) | 7 (15%) | 3 (10%) | 5 (10%) | 5 (17%) | 0 (0%) |
| missing | 3 | 3 | 7 | 3 | 1 | 1 | 0 |
| Married or cohabitating | 173 (84%) | 110 (85%) | 38 (84%) | 25 (83%) | 42 (82%) | 26 (87%) | 16 (76%) |
| missing | 2 | 0 | 1 | 1 | 1 | 0 | 1 |
| BMI, kg/m2 | 23 (21, 27) | 24 (21, 28) | 24 (22, 26) | 22 (20, 25) | 23 (21, 25) | 23 (22, 26) | 23 (21, 25) |
| <18.5 | 7 (4%) | 4 (3%) | 1 (2%) | 2 (7%) | 1 (2%) | 0 (0%) | 1 (5%) |
| 18.5–30 | 161 (80%) | 96 (77%) | 38 (84%) | 27 (90%) | 45 (90%) | 27 (90%) | 18 (90%) |
| >30 | 32 (16%) | 25 (20%) | 6 (13%) | 1 (3%) | 4 (8%) | 3 (10%) | 1 (5%) |
| missing | 7 | 5 | 1 | 1 | 1 | 0 | 1 |
| Parity | 1 (0,2) | 1 (0,2) | 2 (1,3) | 1 (1,2) | 2 (1,3) | 2 (1,3) | 1 (1,2) |
| Prior PTB | |||||||
| 0 (nulliparous) | 54 (26%) | 41 (32%) | 6 (13%) | 7 (23%) | 7 (14%) | 3 (10%) | 4 (19%) |
| 0 (parous) | 93 (45%) | 55 (42%) | 27 (59%) | 11 (35%) | 25 (49%) | 18 (60%) | 33 (7%) |
| 1 or more | 60 (29%) | 34 (26%) | 13 (28%) | 13 (42%) | 19 (37%) | 9 (30%) | 10 (48%) |
| Twins | 8 (4%) | 5 (4%) | 1 (2%) | 2 (6%) | 1 (2%) | 1 (3%) | 0 (0%) |
| Short Cervix < 2.5 cm | 5 (2%) | 2 (2%) | 2 (5%) | 1 (3%) | 2 (4%) | 1 (4%) | 1 (5%) |
| missing | 18 | 13 | 4 | 1 | 2 | 1 | 1 |
IQR, interquartile range; BMI, body mass index; PTB, preterm birth; EGA, estimated gestational age
Confirmatory factor analysis and generation of factor scores were performed with Mplus software (version 8.0; Muthen & Muthen, Los Angeles, CA). We used STATA® (version 15.0; StataCorp, College Station, TX) for data management, descriptive statistics, and analyses. ZAPPS is registered at ClinicalTrials.gov (NCT02738892), and has ongoing approval by the University of Zambia Biomedical Research Ethics Committee and the University of North Carolina Institutional Review Board.
Results:
Study Sample:
We performed cytokine assays on 207 (58.3%) of the study-eligible 355 participants (45.8% of available 452 participants in initial biorepository shipment) with paired vaginal and plasma baseline specimens, including all HIV-infected women with known ART exposure (77/77), all HIV-uninfected women with sPTB (19/19), and a random sample of HIV-uninfected women who delivered at term (111/259, 42.9%; Figure 1). Of the paired specimens available from HIV-infected participants with known ART exposure, 46 (59.7%) were on preconceptional ART, 31 (40.3%) were not (Table 1). Of women with preconceptional ART exposure, 6 (13.0%) had detectable viral loads at enrollment.
The median age of participants at enrollment was 26 (IQR: 22, 32). HIV-infected participants on preconceptional ART were older (median: 30; IQR: 27, 33) than uninfected women (median: 26; IQR: 22, 32) and HIV-infected women without preconceptional ART (median: 25; IQR: 22,31; p=0.01). HIV-infected participants without preconceptional ART exposure had lower BMIs (median: 22.4; IQR: 20.3, 25.5) than both uninfected participants (median: 24.2; IQR: 21.9, 28.8) and infected participants on preconceptional ART (median: 24.4; IQR: 22.1, 26.4; p<0.01). Parity also differed by HIV serostatus and ART exposure: HIV-infected participants with preconceptional ART exposure had higher parity (median: 2; IQR: 1, 3) compared to those without preconceptional ART exposure (median: 1; IQR: 1, 2) and uninfected participants (median: 1; IQR: 0, 2; p=0.03).
Of 207 women included in this nested case-control study, 29 (14.0%) had sPTB. The majority of these sPTB outcomes were in HIV-uninfected participants (19/29, 66.5%). Ten (34.4%) sPTB occurred in HIV-infected women: 6 were on preconceptional ART and 4 were not. Of the 29 women with sPTB, 4 (14%) delivered before 28 weeks and 10 (34%) delivered before 32 weeks.
Confirmatory Factor Analysis:
A confirmatory factor analysis with cytokines assigned to pro- or anti-inflammatory biological action was conducted for vaginal and plasma samples at baseline. Factor loadings and R2 values were calculated (Supplemental Table 1). In vaginal fluid, MIP-1α and IFN-ɣ had standardized loadings with p-values >0.01 in the pro-inflammatory latent variable and were excluded from the final model. In plasma, MIP-1α, IL-6, IL-8, IL-12p70, IP-10, and sCD14 were excluded from the pro-inflammatory latent variable for the same reason. Using the model developed in the baseline confirmatory factor analysis, a second model was run including only matched specimens (women with both baseline and repeat specimens) to generate comparable baseline and repeat factor scores.
Using estimated factor scores from the confirmatory factor analysis for each patient, the correlations between underlying pro- and anti-inflammatory activities in both plasma and vaginal fluid samples were quantified with Spearman correlation coefficients (Supplemental Figure 1). Within each compartment, strong correlations were observed between factor scores for pro- versus anti-inflammatory activity (vaginal fluid: r=0.83; plasma: r=0.99). Pro-inflammatory or anti-inflammatory factor scores between the two distinct anatomic compartments – vaginal fluid versus plasma – were not or were only weakly correlated with each other.
Inflammation & HIV:
When controlling for maternal age, parity, BMI, and prior PTB, vaginal baseline specimens of HIV-infected women without preconceptional ART exposure had higher pro-inflammatory scores (mean 0.37 [95%CI −0.06, 0.80] vs. −0.02 [−0.32,0.27]; p=0.01) and anti-inflammatory scores (mean 0.33 [95%CI 0.11,0.55] vs. −0.03 [−0.18,0.12]; p<0.01) compared to HIV-uninfected women (Figure 2a,b). Pro-inflammatory scores (mean 0.21; 95%CI −0.21,0.62) and anti-inflammatory scores (mean 0.05; 95%CI −0.16,0.27) of HIV-infected women with preconceptional ART exposure did not differ from those of either their HIV-infected counterparts without preconceptional ART exposure or from HIV-uninfected women. In contrast to vaginal scores, no differences between HIV and ART sub-groups were observed for pro- or anti-inflammatory plasma scores (Figures 2c,d).
Figure 2:
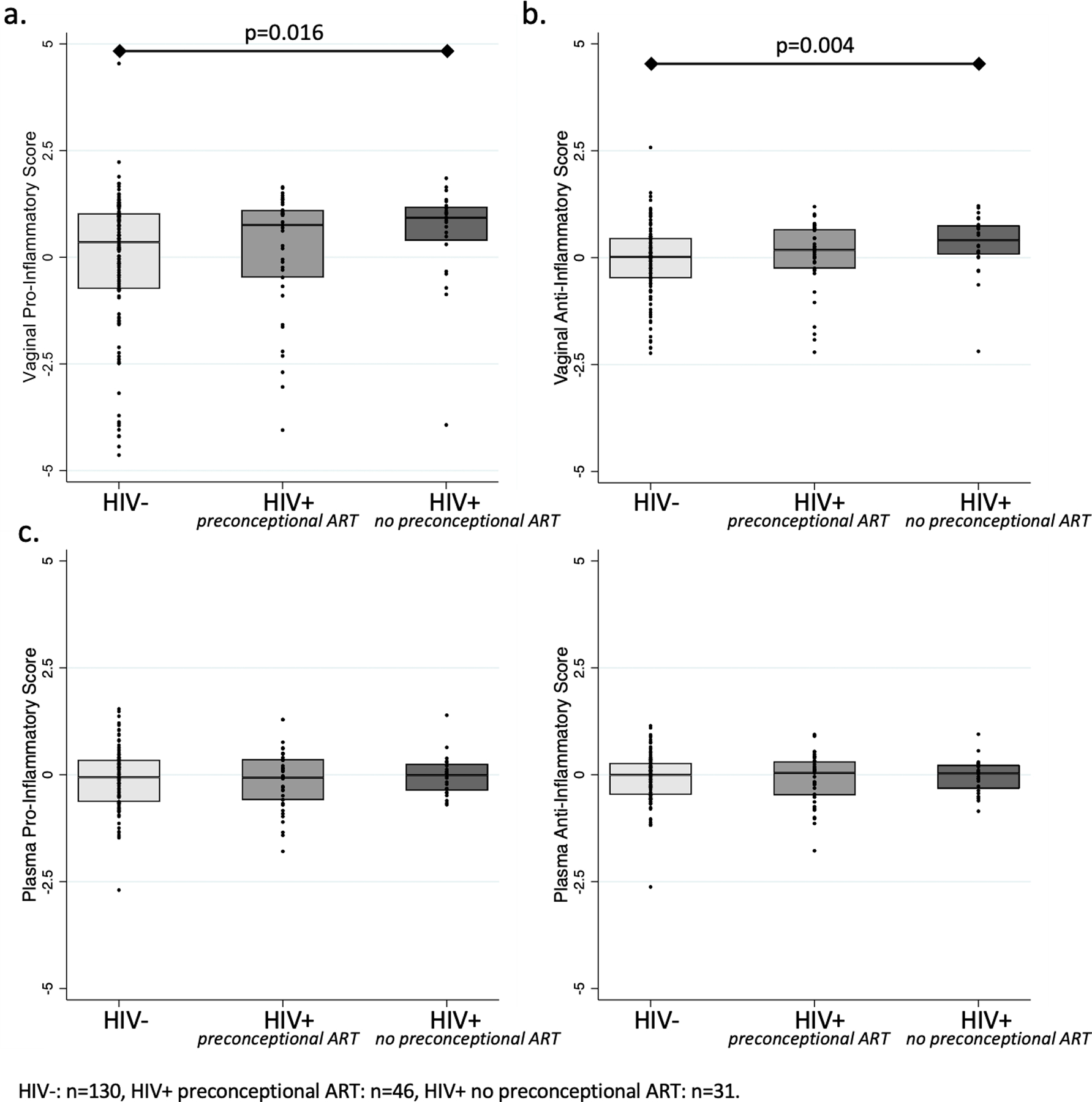
Pro- and anti-inflammatory scores in vaginal fluid and plasma between 16 and 20 gestational weeks by HIV serostatus and preconceptional ART exposure
In analyses of matched baseline and repeat specimens of HIV-infected participants, women without preconceptional ART (mean 0.62; 95%CI −0.80,4.20) had a larger increase in vaginal pro-inflammatory scores than women with preconceptional ART (mean −0.07; 95%CI −2.78,2.11; p=0.04; Figure 3). No differences were observed with respect to changes in plasma pro-inflammatory scores by ART timing sub-groups.
Figure 3:
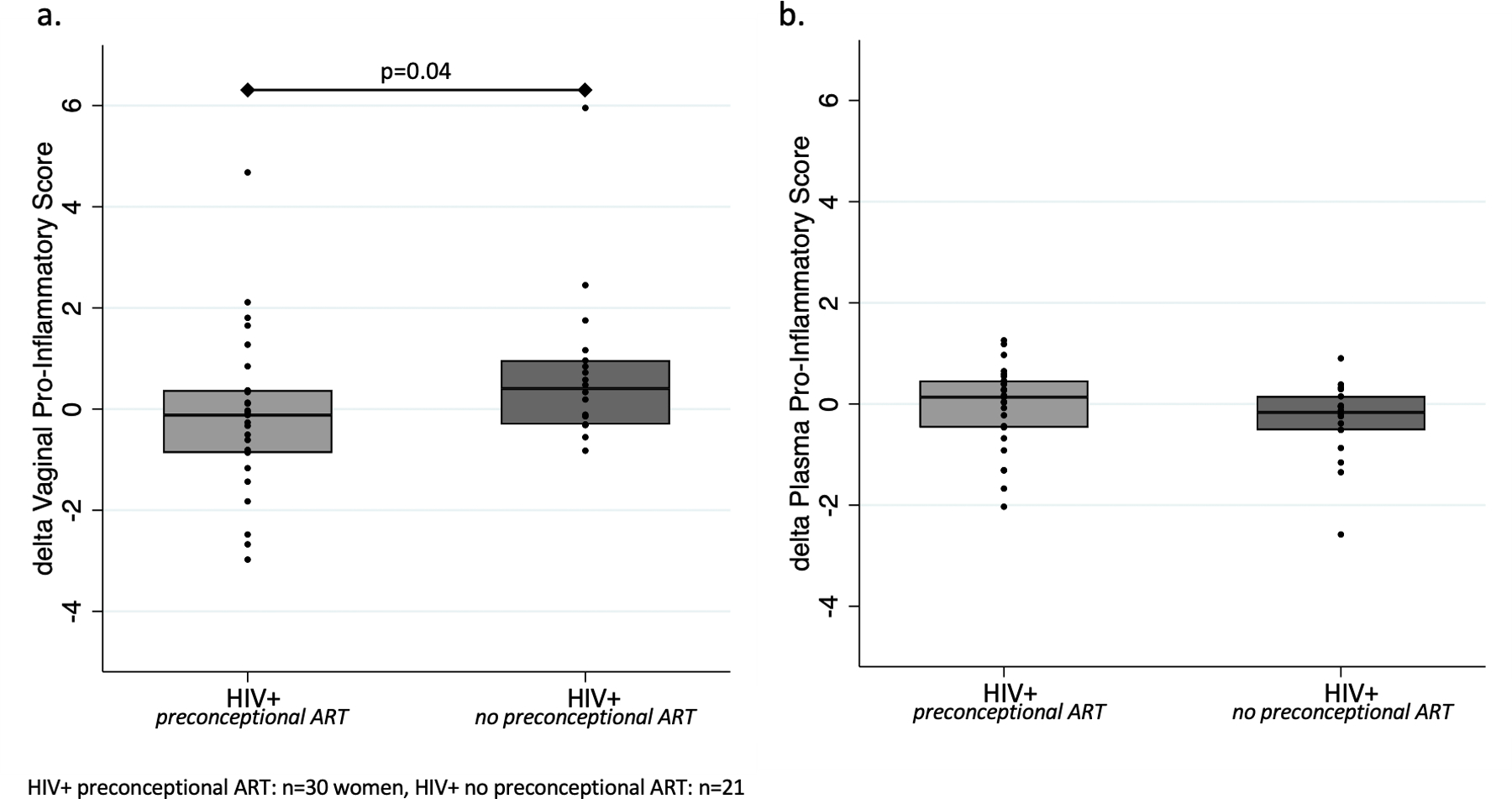
Delta pro-inflammatory scores in (a.) vaginal fluid and (b.) plasma from baseline to repeat by preconconceptional ART exposure in HIV-infected women.
Inflammation & sPTB:
In our study population of 207 women, 8 had twin gestation and 5 had short cervix, both known predictors of sPTB. In fact, 88% (7/8) of women with twins and 60% (3/5) with short cervix had sPTB. Given the small populations with these strong sPTB predictors, these 13 women were excluded from analyses of sPTB (Figure 1).
Women with sPTB had higher vaginal pro-inflammatory scores (mean 0.57 [95%CI 0.14, 0.99] vs. 0.02 [−0.26,0.23], p=0.02) and anti-inflammatory scores (mean 0.22 [95%CI 0.02,0.41] vs. −0.01 [−0.14,0.11]; p=0.05) in the midtrimester than their counterparts with term birth. When including vaginal pro-inflammatory score as a continuous variable in prevalence analyses, it was associated with increased sPTB in both unadjusted (OR 1.4; 95%CI 1.0,1.9; p=0.05) and adjusted (AOR 1.5; 95%CI 1.0,2.3; p=0.02; Table 2) analyses. Vaginal anti-inflammatory score also demonstrated an association with sPTB in adjusted analyses (AOR 1.5, 95%CI 1.0–2.3, p=0.03). Neither plasma pro- nor anti-inflammatory factor scores were associated with sPTB in unadjusted or adjusted analyses.
Table 2:
Prevalence odds ratios of spontaneous PTB in participants with baseline specimens analyzed
| Exposure | Spontaneous PTB <37 weeks | |||
|---|---|---|---|---|
| OR (95% CI) | p-value | AOR (95% CI) | p-value | |
| Vaginal Pro-Inflammatory Factor Score | 1.4 (1.0–1.9) | 0.05 | 1.6 (1.1–2.3) | 0.02 |
| Vaginal Anti-Inflammatory Factor Score | 1.3 (0.9–1.8) | 0.07 | 1.5 (1.0–2.3) | 0.03 |
| Plasma Pro-Inflammatory Factor Score | 1.4 (0.6–3.0) | 0.39 | 1.3 (0.6–3.1) | 0.53 |
| Plasma Anti-Inflammatory Factor Score | 1.2 (0.6–2.8) | 0.60 | 1.1 (0.5–2.6) | 0.87 |
OR: odds ratio; AOR: adjusted odds ratio
Multivariable model estimates of adjusted odds ratios are adjusted for HIV serostatus, ART exposure, prior PTB, maternal age, and BMI
Models exclude women with twin pregnancies (n=8) or short cervix (n=6)
Discussion:
Using confirmatory factor analysis, we identified pro- and anti-inflammatory cytokines that reflect the underlying local (vaginal fluid) and systemic (maternal plasma) immune milieu during the midtrimester in a Zambian cohort with high HIV and PTB prevalence. Our results demonstrate a relationship between a pro-inflammatory vaginal environment and the risk of subsequent sPTB. Further, HIV-infected women without preconceptional ART exposure were found to have a greater increase in vaginal inflammation between baseline and repeat sampling as compared to their HIV-infected counterparts with preconceptional ART exposure. These data support lower genital tract inflammation as a mechanism of sPTB, and further imply a potential link between timing of ART initiation and the elevated PTB risk among HIV-infected women.
Infection and inflammation are well-defined risk factors for sPTB[12]; thus, many cytokines in serum, amniotic fluid, or cervical fluid have been implicated as mediators of sPTB[29, 30, 47–55]. As cytokines function as part of an elaborate and complex network of molecules that regulate the immune response, we characterized the systemic and local immune milieu through confirmatory factor analysis, categorizing cytokines as pro- or anti-inflammatory. This approach is well-suited to assess the association and correlation between pro- or anti-inflammatory activities expressed biologically by cytokines and has been used in other diseases for this purpose[56–59]. Previously, Simhan and colleagues identified an association between elevated cervical cytokine inflammation at <16 weeks gestation and increased risk of sPTB using exploratory factor analyses[31]. Our study supports this finding by demonstrating a relationship between increased lower genital tract inflammation in the midtrimester and subsequent sPTB.
Consistent with current literature evaluating local and systemic biomarkers in pregnancy[47, 60], inflammatory scores between vaginal and venous compartments were poorly correlated. We also found no association between plasma inflammatory scores and sPTB. Although some prior studies have identified an association between specific plasma cytokines and sPTB[29, 52, 60, 61], our data are supported by multiple studies[62, 63] including a systematic review[47] disputing this relationship. We do highlight that while our vaginal pro-inflammatory latent variable included factor loadings from 9 cytokines, our plasma pro-inflammatory latent variable included factor loadings from only 5 cytokines, following our exclusion criteria to optimize fit. This finding may indicate that pro-inflammatory cytokines in serum are less strongly correlated, their relationships are more complex than in vaginal fluid, or they are impacted by a greater variety of stimuli than the cytokines in vaginal fluid.
Our results support the important role of lower genital tract inflammation as a risk factor for HIV- and ART-associated sPTB. Previously, Kyongo and colleagues[32] demonstrated elevated cervicovaginal pro-inflammatory cytokines in HIV-infected women on preconceptional ART in the first trimester of pregnancy, as compared to HIV-uninfected women. Our comparison of lower genital tract inflammation in HIV-infected women stratified by preconceptional ART exposure is novel. This is particularly important for pregnant women in sub-Saharan Africa as 91% of the 1.4 million HIV-infected pregnant women not currently on ART reside in this region[7, 64, 65]. We found that women without preconceptional ART have elevated vaginal inflammation at their initial prenatal encounter that was further increased later in pregnancy. While most HIV-infected women in Zambia who are not already taking ART do initiate during pregnancy, this study did not explicitly confirm initiation of ART. Despite this limitation, these data suggest that immune reconstitution after ART initiation may result in increased local inflammation, a finding that conflicts with epidemiologic reports that those initiating ART prior to pregnancy have a higher PTB risk than untreated women[10, 11]. These data further support recent work proposing selection bias in observational studies to be responsible for the posited association between preconceptional ART and elevated PTB risk[27]. Specifically, women who deliver preterm before initiating ART are systematically excluded from analyses, resulting in a falsely lowered risk of preterm birth among women remaining and inflating the preterm birth risk when compared to women on preconceptional ART. Further, multiple studies have identified a link between the use of protease inhibitors and elevated PTB risk[66–69]. However, first-line ART does not include protease inhibitors in Zambia. As the majority of HIV-infected women in Lusaka are prescribed a fixed-dose combination of tenofovir, emtricitabine, and efavirenz, analyses by drug regimen were not conducted. However, future studies should consider the effect measure modification of therapeutic regimens on inflammation.
Strengths of this study include early ultrasound gestational age dating and careful phenotyping of each PTB outcome. We are confident in our categorization of gestational age at delivery and exclusion of non-spontaneous phenotypes (e.g., provider-initiated delivery for preeclampsia), short cervix, and twin gestation. We chose a sampling approach that deliberately enriched for HIV and sPTB.
In the ZAPPS cohort, HIV-infected women represent 24% of all participants[35]; whereas they accounted for 37% in the current study. Although we adjusted all analyses to account for our sample selection, characterizing the inflammatory profiles of all ZAPPS participants after all women complete their pregnancies would provide more generalizable population-level data. Additionally, it is generally recommended that factor analysis models obtain fit statistics of RMSEA <0.06 as well as CFI and TFI >0.95[70]. We recognize that our factor scores do not meet all of these thresholds. Logarithmic transformation and a limited sample size may have influenced model fit. Additionally, the categorization of cytokines as pro- or anti-inflammatory does not fully reflect their dynamic functions and complex interactions[71]. Despite this limitation, the fits of our models are comparable or better than previously published factor analysis models of cytokines[56, 72–74]. Lastly, we would expect the association between lower genital inflammatory milieu and sPTB to be strongest with parturition resulting from intrauterine infection or inflammation. Placental histopathology, culture, or molecular pathogen detection methods would be necessary to confirm these processes. Unfortunately, these data are not yet available for the ZAPPS cohort and were a limitation of this analysis.
In conclusion, our study demonstrates a relationship between increased genital tract inflammation and subsequent sPTB, offering a potential mechanistic insight into this important adverse birth outcome. Women not receiving ART at initiation of pregnancy care had more vaginal inflammation than women entering on treatment. Further, they experienced an increase in vaginal inflammation between the two sampling timepoints, possibly as a consequence of ART initiation. As many African settings offer near universal coverage of HIV testing in pregnant women, antenatal care provides a unique opportunity to connect HIV-infected women with treatment. Initiation of ART in pregnancy in previously untreated HIV-infected women is an important component of prevention of mother to child transmission and optimization of maternal health. However, further research is needed to characterize the nuanced relationship between ART initiation and sPTB as well as inflammation and sPTB.
Supplementary Material
Supplemental Figure 1: Correlations between factor scores from the confirmatory factor analysis
Reference List:
- 1.Liu L, Oza S, Hogan D, Perin J, Rudan I, Lawn JE, et al. Global, regional, and national causes of child mortality in 2000–13, with projections to inform post-2015 priorities: an updated systematic analysis. Lancet 2015; 385(9966):430–440. [DOI] [PubMed] [Google Scholar]
- 2.Chawanpaiboon S, Vogel JP, Moller AB, Lumbiganon P, Petzold M, Hogan D, et al. Global, regional, and national estimates of levels of preterm birth in 2014: a systematic review and modelling analysis. Lancet Glob Health 2019; 7(1):e37–e46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Preterm birth - World Health Organization Fact Sheet In: World Health Organization; 2018. [Google Scholar]
- 4.Liu L, Oza S, Hogan D, Chu Y, Perin J, Zhu J, et al. Global, regional, and national causes of under-5 mortality in 2000–15: an updated systematic analysis with implications for the Sustainable Development Goals. Lancet 2016; 388(10063):3027–3035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Born too soon: the global action report on preterm birth. In. Geneva, Switzerland: March of Dimes; 2012. [Google Scholar]
- 6.Lawn JE, Blencowe H, Oza S, You D, Lee AC, Waiswa P, et al. Every Newborn: progress, priorities, and potential beyond survival. Lancet 2014; 384(9938):189–205. [DOI] [PubMed] [Google Scholar]
- 7.The gap report 2014. In: UN Joint Programme on HIV/AIDS (UNAIDS); 2014. [Google Scholar]
- 8.Wedi CO, Kirtley S, Hopewell S, Corrigan R, Kennedy SH, Hemelaar J. Perinatal outcomes associated with maternal HIV infection: a systematic review and meta-analysis. Lancet HIV 2016; 3(1):e33–48. [DOI] [PubMed] [Google Scholar]
- 9.Brocklehurst P, French R. The association between maternal HIV infection and perinatal outcome: a systematic review of the literature and meta-analysis. Br J Obstet Gynaecol 1998; 105(8):836–848. [DOI] [PubMed] [Google Scholar]
- 10.Fowler MG, Qin M, Fiscus SA, Currier JS, Makanani B,. Martinson F, Chipato T, Browning R, Shapiro D, Mofenson L. PROMISE: Efficacy and safety of 2 strategies to prevent perinatal HIV transmission, in Conference on Retroviruses and Opportunistic Infections. In. Seattle, Washington; 2015. [Google Scholar]
- 11.Uthman OA, Nachega JB, Anderson J, Kanters S, Mills EJ, Renaud F, et al. Timing of initiation of antiretroviral therapy and adverse pregnancy outcomes: a systematic review and meta-analysis. Lancet HIV 2017; 4(1):e21–e30. [DOI] [PubMed] [Google Scholar]
- 12.Goldenberg RL, Culhane JF, Iams JD, Romero R. Epidemiology and causes of preterm birth. Lancet 2008; 371(9606):75–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Velez DR, Fortunato SJ, Morgan N, Edwards TL, Lombardi SJ, Williams SM, et al. Patterns of cytokine profiles differ with pregnancy outcome and ethnicity. Hum Reprod 2008; 23(8):1902–1909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Simmons LE, Rubens CE, Darmstadt GL, Gravett MG. Preventing preterm birth and neonatal mortality: exploring the epidemiology, causes, and interventions. Semin Perinatol 2010; 34(6):408–415. [DOI] [PubMed] [Google Scholar]
- 15.Thaxton JE, Nevers TA, Sharma S. TLR-mediated preterm birth in response to pathogenic agents. Infect Dis Obstet Gynecol 2010; 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kolte L, Gaardbo JC, Skogstrand K, Ryder LP, Ersboll AK, Nielsen SD. Increased levels of regulatory T cells (Tregs) in human immunodeficiency virus-infected patients after 5 years of highly active anti-retroviral therapy may be due to increased thymic production of naive Tregs. Clin Exp Immunol 2009; 155(1):44–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mikyas Y, Aziz N, Harawa N, Gorre M, Neagos N, Nogueira M, et al. Immunologic activation during pregnancy: serial measurement of lymphocyte phenotype and serum activation molecules in HIV-infected and uninfected women. J Reprod Immunol 1997; 33(2):157–170. [DOI] [PubMed] [Google Scholar]
- 18.Kalk E, Schubert P, Bettinger JA, Cotton MF, Esser M, Slogrove A, et al. Placental pathology in HIV infection at term: a comparison with HIV-uninfected women. Trop Med Int Health 2017; 22(5):604–613. [DOI] [PubMed] [Google Scholar]
- 19.Cnattingius S, Villamor E, Johansson S, Edstedt Bonamy AK, Persson M, Wikstrom AK, et al. Maternal obesity and risk of preterm delivery. JAMA 2013; 309(22):2362–2370. [DOI] [PubMed] [Google Scholar]
- 20.RA Johnson DW. Applied multivariate statistical analysis. 6 ed. Saddle River, NJ: Pearson Prentice Hall. [Google Scholar]
- 21.Chen YH, Lin HL, Lin HC. Does multiple sclerosis increase risk of adverse pregnancy outcomes? A population-based study. Mult Scler 2009; 15(5):606–612. [DOI] [PubMed] [Google Scholar]
- 22.Danchenko N, Satia JA, Anthony MS. Epidemiology of systemic lupus erythematosus: a comparison of worldwide disease burden. Lupus 2006; 15(5):308–318. [DOI] [PubMed] [Google Scholar]
- 23.Romero R, Espinoza J, Kusanovic JP, Gotsch F, Hassan S, Erez O, et al. The preterm parturition syndrome. BJOG 2006; 113 Suppl 3:17–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Orsi NM, Tribe RM. Cytokine networks and the regulation of uterine function in pregnancy and parturition. J Neuroendocrinol 2008; 20(4):462–469. [DOI] [PubMed] [Google Scholar]
- 25.Challis JR, Lockwood CJ, Myatt L, Norman JE, Strauss JF 3rd, Petraglia F. Inflammation and pregnancy. Reprod Sci 2009; 16(2):206–215. [DOI] [PubMed] [Google Scholar]
- 26.Mendelson CR. Minireview: fetal-maternal hormonal signaling in pregnancy and labor. Mol Endocrinol 2009; 23(7):947–954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Stoner MCD, Cole SR, Price J, Winston J, Stringer JSA. Timing of Initiation of Antiretroviral Therapy and Risk of Preterm Birth in Studies of HIV-infected Pregnant Women: The Role of Selection Bias. Epidemiology 2018; 29(2):224–229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Stringer JS, Stoner MC, Kasaro MP, Vwalika B, Cole SR. Preconception ART and preterm birth: real effect or selection bias? Lancet HIV 2017; 4(4):e150. [DOI] [PubMed] [Google Scholar]
- 29.Gargano JW, Holzman C, Senagore P, Thorsen P, Skogstrand K, Hougaard DM, et al. Mid-pregnancy circulating cytokine levels, histologic chorioamnionitis and spontaneous preterm birth. J Reprod Immunol 2008; 79(1):100–110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Puchner K, Iavazzo C, Gourgiotis D, Boutsikou M, Baka S, Hassiakos D, et al. Mid-trimester amniotic fluid interleukins (IL-1beta, IL-10 and IL-18) as possible predictors of preterm delivery. In Vivo 2011; 25(1):141–148. [PubMed] [Google Scholar]
- 31.Simhan HN, Bodnar LM, Kim KH. Lower genital tract inflammatory milieu and the risk of subsequent preterm birth: an exploratory factor analysis. Paediatr Perinat Epidemiol 2011; 25(3):277–282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kyongo JK, Crucitti T, Menten J, Hardy L, Cools P, Michiels J, et al. Cross-Sectional Analysis of Selected Genital Tract Immunological Markers and Molecular Vaginal Microbiota in Sub-Saharan African Women, with Relevance to HIV Risk and Prevention. Clin Vaccine Immunol 2015; 22(5):526–538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Morrison C, Fichorova RN, Mauck C, Chen PL, Kwok C, Chipato T, et al. Cervical inflammation and immunity associated with hormonal contraception, pregnancy, and HIV-1 seroconversion. J Acquir Immune Defic Syndr 2014; 66(2):109–117. [DOI] [PubMed] [Google Scholar]
- 34.Maharaj NR, Phulukdaree A, Nagiah S, Ramkaran P, Tiloke C, Chuturgoon AA. Pro-Inflammatory Cytokine Levels in HIV Infected and Uninfected Pregnant Women with and without Preeclampsia. PLoS One 2017; 12(1):e0170063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Castillo MC, Fuseini NM, Rittenhouse K, Price JT, Freeman BL, Mwape H, et al. The Zambian Preterm Birth Prevention Study (ZAPPS): Cohort characteristics at enrollment. Gates Open Res 2018; 2:25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Price JT, Vwalika B, Rittenhouse KJ, Mwape H, Winston J, Freeman BL, et al. Adverse birth outcomes and their clinical phenotypes in an urban Zambian cohort. Gates Open Res 2019; 3:1533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Villar J, Papageorghiou AT, Knight HE, Gravett MG, Iams J, Waller SA, et al. The preterm birth syndrome: a prototype phenotypic classification. Am J Obstet Gynecol 2012; 206(2):119–123. [DOI] [PubMed] [Google Scholar]
- 38.Dumond JB, Yeh RF, Patterson KB, Corbett AH, Jung BH, Rezk NL, et al. Antiretroviral drug exposure in the female genital tract: implications for oral pre- and post-exposure prophylaxis. AIDS 2007; 21(14):1899–1907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Cottrell ML, Yang KH, Prince HM, Sykes C, White N, Malone S, et al. A Translational Pharmacology Approach to Predicting Outcomes of Preexposure Prophylaxis Against HIV in Men and Women Using Tenofovir Disoproxil Fumarate With or Without Emtricitabine. J Infect Dis 2016; 214(1):55–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Dumond JB, Yang KH, Kendrick R, Reddy YS, Kashuba AD, Troiani L, et al. Pharmacokinetic Modeling of Lamivudine and Zidovudine Triphosphates Predicts Differential Pharmacokinetics in Seminal Mononuclear Cells and Peripheral Blood Mononuclear Cells. Antimicrob Agents Chemother 2015; 59(10):6395–6401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Judkins D Fay’s method for variance estimation. Journal of Official Statistics 1990; 6:223–239. [Google Scholar]
- 42.Survey. STATA Survey Data Reference Manual: Release 13. College Station, TX: StataCorp LP.; 2013. [Google Scholar]
- 43.Cole SR, Chu H, Nie L, Schisterman EF. Estimating the odds ratio when exposure has a limit of detection. Int J Epidemiol 2009; 38(6):1674–1680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Richardson DB, Ciampi A. Effects of exposure measurement error when an exposure variable is constrained by a lower limit. Am J Epidemiol 2003; 157(4):355–363. [DOI] [PubMed] [Google Scholar]
- 45.Genser B, Cooper PJ, Yazdanbakhsh M, Barreto ML, Rodrigues LC. A guide to modern statistical analysis of immunological data. BMC Immunol 2007; 8:27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.J Wang XW. Structural equation modeling: applications using Mplus. In. West Sussex, England: John Wiley/Higher Education Press; 2012. pp. 453. [Google Scholar]
- 47.Wei SQ, Fraser W, Luo ZC. Inflammatory cytokines and spontaneous preterm birth in asymptomatic women: a systematic review. Obstet Gynecol 2010; 116(2 Pt 1):393–401. [DOI] [PubMed] [Google Scholar]
- 48.Kedzierska-Markowicz A, Krekora M, Biesiada L, Glowacka E, Krasomski G. [Evaluation of the correlation between IL-1beta, IL-8, IFN-gamma cytokine concentration in cervico-vaginal fluid and the risk of preterm delivery]. Ginekol Pol 2015; 86(11):821–826. [DOI] [PubMed] [Google Scholar]
- 49.Kacerovsky M, Musilova I, Jacobsson B, Drahosova M, Hornychova H, Janku P, et al. Vaginal fluid IL-6 and IL-8 levels in pregnancies complicated by preterm prelabor membrane ruptures. J Matern Fetal Neonatal Med 2015; 28(4):392–398. [DOI] [PubMed] [Google Scholar]
- 50.Kacerovsky M, Musilova I, Bestvina T, Stepan M, Cobo T, Jacobsson B. Preterm Prelabor Rupture of Membranes between 34 and 37 Weeks: A Point-of-Care Test of Vaginal Fluid Interleukin-6 Concentrations for a Noninvasive Detection of Intra-Amniotic Inflammation. Fetal Diagn Ther 2018; 43(3):175–183. [DOI] [PubMed] [Google Scholar]
- 51.Taylor BD, Holzman CB, Fichorova RN, Tian Y, Jones NM, Fu W, et al. Inflammation biomarkers in vaginal fluid and preterm delivery. Hum Reprod 2013; 28(4):942–952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Discacciati MG, Simoes JA, Silva MG, Marconi C, Brolazo E, Costa ML, et al. Microbiological characteristics and inflammatory cytokines associated with preterm labor. Arch Gynecol Obstet 2011; 283(3):501–508. [DOI] [PubMed] [Google Scholar]
- 53.Romero R, Espinoza J, Chaiworapongsa T, Kalache K. Infection and prematurity and the role of preventive strategies. Semin Neonatol 2002; 7(4):259–274. [DOI] [PubMed] [Google Scholar]
- 54.Romero R, Espinoza J, Goncalves LF, Kusanovic JP, Friel LA, Nien JK. Inflammation in preterm and term labour and delivery. Semin Fetal Neonatal Med 2006; 11(5):317–326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Goldenberg RL, Goepfert AR, Ramsey PS. Biochemical markers for the prediction of preterm birth. Am J Obstet Gynecol 2005; 192(5 Suppl):S36–46. [DOI] [PubMed] [Google Scholar]
- 56.Pripp AH, Stanisic M. The correlation between pro- and anti-inflammatory cytokines in chronic subdural hematoma patients assessed with factor analysis. PLoS One 2014; 9(2):e90149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Tziakas DN, Chalikias GK, Kaski JC, Kekes A, Hatzinikolaou EI, Stakos DA, et al. Inflammatory and anti-inflammatory variable clusters and risk prediction in acute coronary syndrome patients: a factor analysis approach. Atherosclerosis 2007; 193(1):196–203. [DOI] [PubMed] [Google Scholar]
- 58.Juntti H, Osterlund P, Kokkonen J, Dunder T, Renko M, Pokka T, et al. Cytokine responses in cord blood predict the severity of later respiratory syncytial virus infection. J Allergy Clin Immunol 2009; 124(1):52–58 e51–52. [DOI] [PubMed] [Google Scholar]
- 59.Mommersteeg PM, Vermetten E, Kavelaars A, Geuze E, Heijnen CJ. Hostility is related to clusters of T-cell cytokines and chemokines in healthy men. Psychoneuroendocrinology 2008; 33(8):1041–1050. [DOI] [PubMed] [Google Scholar]
- 60.Vogel I, Goepfert AR, Thorsen P, Skogstrand K, Hougaard DM, Curry AH, et al. Early second-trimester inflammatory markers and short cervical length and the risk of recurrent preterm birth. J Reprod Immunol 2007; 75(2):133–140. [DOI] [PubMed] [Google Scholar]
- 61.Lopez M, Figueras F, Coll O, Gonce A, Hernandez S, Lonca M, et al. Inflammatory Markers Related to Microbial Translocation Among HIV-Infected Pregnant Women: A Risk Factor of Preterm Delivery. J Infect Dis 2016; 213(3):343–350. [DOI] [PubMed] [Google Scholar]
- 62.Sozmen S, Mungan T, Micozkadioglu SD, Tapisiz OL. Predictive value of maternal serum and vaginal interleukin-6 levels in preterm labor. J Soc Gynecol Investig 2005; 12(4):e1–6. [DOI] [PubMed] [Google Scholar]
- 63.Tripathi R, Tyagi S, Singh N, Mala YM, Singh C, Bhalla P, et al. Can preterm labour be predicted in low risk pregnancies? Role of clinical, sonographic, and biochemical markers. J Pregnancy 2014; 2014:623269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Millennium Development Goals Report 2015. In: United Nations; 2015. [Google Scholar]
- 65.Lozano R, Wang H, Foreman KJ, Rajaratnam JK, Naghavi M, Marcus JR, et al. Progress towards Millennium Development Goals 4 and 5 on maternal and child mortality: an updated systematic analysis. Lancet 2011; 378(9797):1139–1165. [DOI] [PubMed] [Google Scholar]
- 66.Mesfin YM, Kibret KT, Taye A. Is protease inhibitors based antiretroviral therapy during pregnancy associated with an increased risk of preterm birth? Systematic review and a meta-analysis. Reprod Health 2016; 13:30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Favarato G, Townsend CL, Bailey H, Peters H, Tookey PA, Taylor GP, et al. Protease inhibitors and preterm delivery: another piece in the puzzle. AIDS 2018; 32(2):243–252. [DOI] [PubMed] [Google Scholar]
- 68.Kourtis AP, Schmid CH, Jamieson DJ, Lau J. Use of antiretroviral therapy in pregnant HIV-infected women and the risk of premature delivery: a meta-analysis. AIDS 2007; 21(5):607–615. [DOI] [PubMed] [Google Scholar]
- 69.Alemu FM, Yalew AW, Fantahun M, Ashu EE. Antiretroviral Therapy and Pregnancy Outcomes in Developing Countries: A Systematic Review. Int J MCH AIDS 2015; 3(1):31–43. [PMC free article] [PubMed] [Google Scholar]
- 70.L-T Hu PB. Cutoff criteria for fit indexes in covariance structure analysis: Conventional criteria versus new alternatives. Struct Equ Modeling 2009; 6:1–55. [Google Scholar]
- 71.Thomson A The cytokine handbook. 3 ed: Academic Press; 1998. [Google Scholar]
- 72.Smits MM, Woudstra P, Utzschneider KM, Tong J, Gerchman F, Faulenbach M, et al. Adipocytokines as features of the metabolic syndrome determined using confirmatory factor analysis. Ann Epidemiol 2013; 23(7):415–421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Chan JC, Cheung JC, Stehouwer CD, Emeis JJ, Tong PC, Ko GT, et al. The central roles of obesity-associated dyslipidaemia, endothelial activation and cytokines in the Metabolic Syndrome--an analysis by structural equation modelling. Int J Obes Relat Metab Disord 2002; 26(7):994–1008. [DOI] [PubMed] [Google Scholar]
- 74.Swardfager W, Yu D, Ramirez J, Cogo-Moreira H, Szilagyi G, Holmes MF, et al. Peripheral inflammatory markers indicate microstructural damage within periventricular white matter hyperintensities in Alzheimer’s disease: A preliminary report. Alzheimers Dement (Amst) 2017; 7:56–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Figure 1: Correlations between factor scores from the confirmatory factor analysis


