Abstract
Objectives:
The global pandemic of coronavirus disease 2019 (Covid-19) may disproportionately affect persons in congregate settings, including those in residential substance use treatment facilities. To limit the spread of SARS-CoV-2 through congregate settings, universal testing may be necessary. We aimed to determine the point prevalence of SARS-CoV-2 in a residential treatment program setting and to understand the unique challenges of Covid-19 transmission in this setting.
Methods:
We performed a case series of SARS-CoV-2 rT-PCR testing via nasopharyngeal in a residential substance use treatment program for women in Boston. Staff and residents of the treatment program were tested for SARS-CoV-2. The primary outcome was SARS-CoV-2 test result.
Results:
A total of 31 residents and staff were tested. Twenty-seven percent (6/22) of the residents and 44% (4/9) of staff tested positive for SARS-CoV-2. All of the SARS-CoV-2 positive residents resided in the same residential unit. Two positive cases resided together with two negative cases in a 4-person room. Two other positive cases resided together in a 2-person room. One positive case resided with two negative cases in a 3-person room. One positive case resided with a negative case in a 2-person room. Based on test results, residents were cohorted by infection status and continued to participate in addiction treatment on-site.
Conclusions:
SARS-CoV-2 infection was common among staff and residents within a residential substance use treatment program for women in Boston. Universal SARS-CoV-2 testing in residential substance use programs can be instituted to reduce the risk of further transmission and continue addiction treatment programming when accompanied by adequate space, supplies, and staffing.
Keywords: COVID-19, residential substance use treatment, coronavirus, universal testing
Background
The global pandemic of coronavirus disease 2019 (Covid-19) caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) has resulted in more than 1.7 million people having been diagnosed with Covid-19 and over 100,000 deaths in the United States.1 Recent studies have reported disproportionately high prevalence of Covid-19 disease in homeless shelters,2,3 skilled nursing facilities,4 and incarcerated settings.5 Persons in congregate settings are likely to have higher risk both of exposure to SARS-CoV-2 and severe disease from Covid-19 due to their limited ability to physically distance and medical comorbidities, respectively.6
Residential substance use treatment facilities face similar challenges to both residents and staff as other congregate settings. Residential substance use treatment facilities offer a safe, supportive and structured environment where participants build positive social support networks and relapse prevention skills that form a foundation for remission from substance use disorders.7,8 Residents are often experiencing homelessness,9 on probation or parole,10 placed as a requirement of child custody, or concerned that leaving care for any reason (including illness) may alter their recovery path. Because substance use disorders are life-threatening chronic conditions, inpatient substance use treatment programs are considered essential services including in the midst of the Covid-19 pandemic and thus, staff are essential workers. The burden of Covid-19 in such facilities has not yet been investigated. We, therefore, conducted a point prevalence study of residents and staff who were tested for SARS-CoV-2 from a single residential program in Boston. We aimed to understand the unique challenges of Covid-19 transmission in a residential treatment program setting and explore potential ways to mitigate further transmission in these settings.
Methods
On April 17, 2020, a provider team at Boston Medical Center (BMC) responded to a request from the director of a residential substance use treatment program to provide SARS-CoV-2 testing. The request came after one resident had been hospitalized eight days earlier with Covid-19 pneumonia, a staff member tested positive for SARS CoV-2, and another resident who was asymptomatic had also tested positive. Facility residents and staff available on that day were interviewed to ascertain information on travel history, symptoms, severity, coexisting medical conditions, and exposure to persons with known Covid-19. We also analyzed program administrative records to determine the number of roommates in each room, and number of persons who share a bathroom. Any residents or staff not available for onsite testing were offered testing at the BMC Influenza-Like Illness clinic. We retrospectively reviewed medical charts to confirm Covid-19 testing results.
Nasopharyngeal specimen collection and diagnostic testing were conducted in accordance with CDC guidelines11 and testing was performed at BMC Clinical Laboratory on an FDA approved SARS-CoV-2 rRT-PCR. Swabs and testing costs were covered by BMC.
This study was reviewed by the Boston University Medical Campus Institutional Review Board and determined to be exempt.
Results
Prevalence
A total of 31 residents and staff were tested. Thirteen residents and three staff were tested on site by our team. Nine additional residents and six additional staff were tested offsite (Figure). All residents were tested and one staff member was not tested. Twenty-seven percent (6/22) of the residents and 44% (4/9) of staff tested positive for SARS-CoV-2. Of the staff who agreed to testing, the median age was 41.5 (IQR=32.25, 52.25); 100% were female; 1 was Caucasian, 6 were black, and 2 were Asian. All but one staff member worked across all units. The median age of the residents was 42 (IQR=34.00, 50.25); 100% were female; 17 were Caucasian and 1 was black. Among the 13 residents who completed the symptom screening, the 5 who reported symptoms including cough, headache, sore throat, rhinorrhea, or fatigue were SARS-CoV-2 PCR negative. Both residents who were SARS-CoV-2 PCR positive and completed a symptom screen (n=2) were asymptomatic.
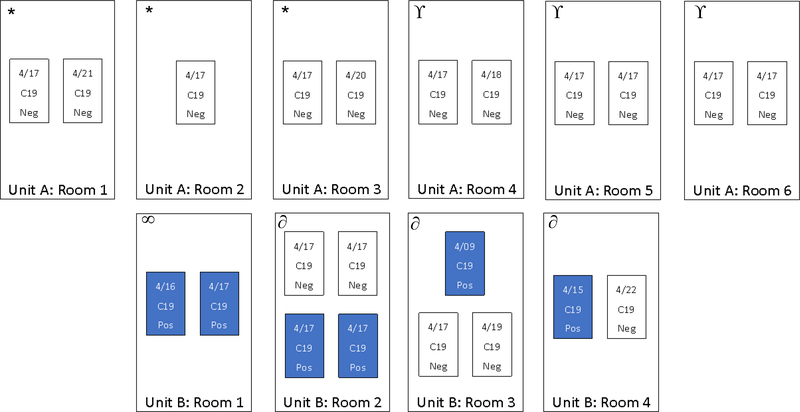
Figure. Schematic of Covid-19 test date and results of residents in two units of a residential substance use treatment program.
All Covid-19 positive cases resided in one unit of a residential substance use treatment facility in Boston, MA. All cases resided in four rooms. Covid-19 positive cases are denoted as “C19 Pos” in the figure. Testing date is denoted for each person. No cases occurred in individual rooms. Each box represents a room and each rectangle represents a resident in that room. Date and result of nasopharyngeal swab overlie each rectangle. The unit had a shared communal kitchen and two bathrooms. The residents use the bathrooms on their floor exclusively. Unit A Rooms 1, 2, and 3 shared a bathroom (denoted as * in the figure); Unit A Rooms 4, 5, and 6 shared a bathroom (denoted as ϒ in the figure); Unit B Rooms 2, 3, and 4 shared a bathroom (denoted as ∂ in the figure); and Unit B Room 1 used a different bathroom (denoted as ∞ in the figure). One Covid-19 negative person in Unit B Room 2 used a separate bathroom.
All of the SARS-CoV-2 positive residents resided in the same residential unit. Two positive cases resided together with two negative cases in a 4-person room. Two other positive cases resided together in a 2-person room. One positive case resided with two negative cases in a 3-person room. And one positive case resided with a negative case in a 2-person room (Figure). Bathroom sharing is outlined in the figure.
Cohorting within the program
All persons tested were notified of their test results by the provider team within 48 hours of the results being available, and, with HIPAA release, program staff were then notified of the persons who tested positive. This residential facility has three physically distinct units each with a varying number of rooms. One of the units had been unoccupied and was converted into a Covid-19 positive unit and the other two units were designated as isolation units for persons who had tested negative or who were waiting test results. Persons who tested positive were moved to the Covid-19 positive unit. The isolation units were decontaminated by a professional cleaning company and individuals in these units were provided masks and isolated as a unit for a fourteen-day period. The program also instituted daily monitoring among staff and residents for the development of symptoms and regular hand washing and sanitizing. Once the Covid-19 positive unit was established, the program accepted five residents who had recently tested positive from affiliated programs into their Covid-19 positive unit, allowing the affiliated programs to cohort their residents. Each resident who was cohorted to the COVID-negative units completed and discontinued her quarantine after 14 days, consistent with CDC guidance.12 None developed symptoms during the quarantine time. Each resident who was cohorted to the COVID-positive unit completed and discontinued her isolation after meeting either symptom or testing clearance criteria from CDC guidance.”
Staff who tested positive for SARS-CoV-2 quarantined at home for the full period of their illness, further reducing transmission. Staff who tested negative for SARS-CoV-2 and continued to work in the isolation units were provided with and instructed to wear face masks and gloves at all times. Staff who worked in the Covid-19 positive unit were instructed to wear face masks, gloves, gown, and face shield while working in the unit.
Discussion
SARS-CoV-2 infection was common among staff and residents within a residential substance use treatment program for women in Boston. Covid-19 was clustered among those residents who were roommates, though there were roommates who also tested negative. Additionally, nearly half of all staff tested positive for Covid-19. Universal testing and then cohorting by infection status likely reduced ongoing transmission in this residential substance use treatment program. We emphasized the need to limit bathroom sharing and shared spaces (e.g., kitchen).13 We recommended that persons could leave the Covid-19 unit and infected staff could return to work following the symptom-based or testing clearance approach outlined by the CDC. Furthermore, residents were still able to receive continued addiction treatment care on-site.
People who use drugs are often subject to negative consequences of social determinants of health (e.g., experiencing homelessness), have other chronic diseases, and have experienced stigma. Thus, it is especially important for treatment programs to be prepared to manage Covid-19, prevent people from needing to leave the program if infected and allow people to enter even if recently infected.
Our study is limited by the small sample size, our inability to perform follow up or repeat SARS-CoV-2 testing, and our inability to perform phylogenetic analysis on the specimens to understand transmission dynamics. Our findings add to the mounting evidence that persons who live and work in congregate settings are at higher risk for Covid-19 disease than the general population. Our findings demonstrate the feasibility of universal testing in residential addiction treatment programs and restructuring such facilities to enhance physical distancing and continue programming.
Acknowledgements
The authors acknowledge Julia Gunn, epidemiologist at the Boston Public Health Commission, for providing guidance on the cohorting strategy for the treatment program.
Funding statement
JAB is supported by the Charles A. King Trust. TCB is supported by the National Institutes of Health [grant number T32DA013911] and the Burroughs Wellcome Fund/American Society for Tropical Medicine and Hygiene Postdoctoral Fellowship in Tropical Infectious Diseases. JAB, SDK, AYW are supported by the National Institute on Drug Abuse [grant number 5UM1DA049412-02].
Footnotes
We report no conflicts of interest.
References
- 1.U.S. Centers for Disease Control and Prevention. Coronavirus Disease 2019 (COVID-19): Cases in the U.S. https://www.cdc.gov/coronavirus/2019-ncov/cases-updates/cases-in-us.html. 2020. Accessed May 29, 2020.
- 2.Mosites E, Parker EM, Clarke KE, et al. Assessment of SARS-CoV-2 Infection Prevalence in Homeless Shelters — Four U.S. Cities, March 27–April 15, 2020. MMWR Morb Mortal Wkly Rep. 2020. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Baggett TP, Keyes H, Sporn N, Gaeta JM. Prevalence of SARS-CoV-2 Infection in Residents of a Large Homeless Shelter in Boston. JAMA. 2020. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.McMichael TM, Currie DW, Clark S, et al. Epidemiology of Covid-19 in a Long-Term Care Facility in King County, Washington. N Engl J Med. 2020. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hawks L, Woolhandler S, McCormick D. COVID-19 in Prisons and Jails in the United States. JAMA Intern Med. 2020. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 6.Garg S, Kim L, Whitaker M, et al. Hospitalization Rates and Characteristics of Patients Hospitalized with Laboratory-Confirmed Coronavirus Disease 2019 - COVID-NET, 14 States, March 1–30, 2020. MMWR Morb Mortal Wkly Rep. 2020;69(15):458–464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.de Andrade D, Elphinston RA, Quinn C, Allan J, Hides L. The effectiveness of residential treatment services for individuals with substance use disorders: A systematic review. Drug Alcohol Depend. 2019;201:227–235. [DOI] [PubMed] [Google Scholar]
- 8.Reif S, George P, Braude L, et al. Residential treatment for individuals with substance use disorders: assessing the evidence. Psychiatr Serv. 2014;65(3):301–312. [DOI] [PubMed] [Google Scholar]
- 9.Stein MD, Anderson BJ, Bailey GL. Preferences for Aftercare Among Persons Seeking Short-Term Opioid Detoxification. J Subst Abuse Treat. 2015;59:99–103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Moore KE, Hacker RL, Oberleitner L, McKee SA. Reentry interventions that address substance use: A systematic review. Psychol Serv. 2020;17(1):93–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.U.S. Centers for Disease Control and Prevention. Interim Guidelines for Collecting, Handling, and Testing Clinical Specimens from Persons for Coronavirus Disease 2019 (COVID-19). 2020. Available at: https://www.cdc.gov/coronavirus/2019-ncov/hcp/clinical-criteria.html?CDC_AA_refVal=https%3A%2F%2Fwww.cdc.gov%2Fcoronavirus%2F2019-ncov%2Fclinical-criteria.html. Accessed May 7, 2020.
- 12.U.S. Centers for Disease Control and Prevention. Discontinuation of Isolation for Persons with COVID −19 Not in Healthcare Settings. https://www.cdc.gov/coronavirus/2019-ncov/hcp/disposition-in-home-patients.html. Published 2020. Accessed May 30, 2020.
- 13.Ong SWX, Tan YK, Chia PY, et al. Air, Surface Environmental, and Personal Protective Equipment Contamination by Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2) From a Symptomatic Patient. JAMA. 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]