The clinical course of coronavirus disease 2019 (Covid-19) is characterized by an initial stage with mild symptoms of the upper respiratory tract. During this stage, the viral load of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) peaks, with progressive lowering afterward.1 After 7 or 8 days, in 20% of patients the disease progresses to bilateral pneumonia with dyspnea, a reduction in oxygen saturation, and a need for supplemental oxygen. This second stage is characterized by a massive immune response with subsequent worsening of lung damage,2 respiratory failure that may require invasive mechanical ventilation, and multiorgan dysfunction.1-3 To date, the antiviral remdesivir and the antiinflammatory dexamethasone are considered the best treatment options for Covid-19 pneumonia.4,5
In the Adaptive Covid-19 Treatment Trial (ACTT-1)4 involving 1062 hospitalized patients with Covid-19 who had a score of 4 (not receiving supplemental oxygen) to 7 (receiving invasive mechanical ventilation or extracorporeal membrane oxygenation) on an 8-point ordinal scale, the time to recovery was significantly shorter with remdesivir than with placebo (median, 10 days vs. 15 days; rate ratio for recovery, 1.29; 95% confidence interval [CI], 1.12 to 1.49; P<0.001). The efficacy of the drug was highest among patients with a baseline ordinal score of 4 (not receiving oxygen) or 5 (receiving low-flow oxygen).
In the Randomized Evaluation of Covid-19 Therapy (RECOVERY) trial,5 2104 hospitalized patients receiving dexamethasone at a dose of 6 mg per day for up to 10 days were compared with 4321 controls receiving usual care. No stratification according to ordinal score was used. The incidence of death was lower in the dexamethasone group than in the usual-care group among patients receiving invasive mechanical ventilation (assumed ordinal score of 7) (29.3% vs. 41.4%; rate ratio, 0.64; 95% CI, 0.51 to 0.81) and, to a lesser extent, among those receiving oxygen only (23.3% vs. 26.2%; rate ratio, 0.82; 95% CI, 0.72 to 0.94). However, no indication of the level of oxygen support was provided; hence, it is unclear whether dexamethasone was effective in patients receiving low-flow oxygen (ordinal score of 5) or those receiving high-flow oxygen (ordinal score of 6).6
On the basis of this evidence, remdesivir is likely to be most effective in early Covid-19 (ordinal score of 4 or 5), whereas dexamethasone is likely to be most effective later in the disease course (ordinal score of 7).1 Additional therapeutic options for Covid-19 pneumonia in patients with an ordinal score of 5 or 6 (gray zone, Figure 1) are needed to reduce the likelihood of further progression to invasive procedures or death.
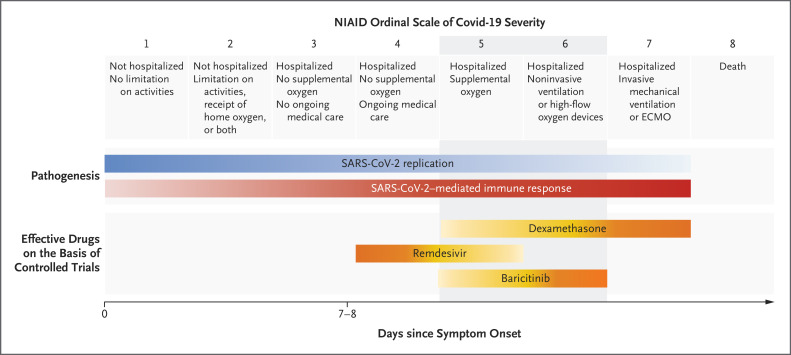
Figure 1. Evidence-based Efficacy of Covid-19 Therapies, According to Disease Severity.
The clinical course of coronavirus disease 2019 (Covid-19) is characterized at the outset by mild symptoms of the upper respiratory tract. During this stage, the viral load of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) peaks, with progressive lowering afterward. After 7 or 8 days, in 20% of patients the disease progresses to bilateral pneumonia. This second stage is characterized by a massive immune response, with subsequent worsening of the lung damage, respiratory failure often requiring invasive mechanical ventilation, and other organ dysfunction. To date, on the basis of controlled trials, the antiviral remdesivir, the antiinflammatory dexamethasone, and the Janus kinase inhibitor baricitinib are considered to be the best treatment options for Covid-19. Baricitinib fulfills the unmet need of providing efficacious therapies for the gray zone of treatments for pneumonia requiring oxygen support (ordinal scale of 5 or 6). The yellow gradation is from the lower expected beneficial effect of the drug (light yellow) to the highest (orange). The blue gradation is from the lower expected SARS-CoV-2 replication (light blue) to the highest (dark blue). The red gradation is from the lower SARS-CoV-2–mediated immune response (pink) to the highest (red). In the Randomized Evaluation of Covid-19 Therapy trial,5 mortality was lower with dexamethasone than with usual care among patients receiving invasive ventilation (assumed ordinal score of 7) and, to a lesser extent, among those receiving oxygen only (assumed score of 5 or 6). However, no indication of the level of oxygen support was provided (ordinal score of 5 or 6). We arbitrarily added it. ECMO denotes extracorporeal membrane oxygenation, and NIAID National Institute of Allergy and Infectious Diseases.
Kalil et al. report in the Journal the results of ACCT-2,7 a double-blind, randomized, placebo-controlled trial evaluating baricitinib, an inhibitor of Janus kinase 1 (JAK1) and JAK2, plus remdesivir in hospitalized adults with Covid-19. The primary outcome was the time to recovery. The key secondary outcome was clinical status at day 15.
At enrollment, 1033 patients were assigned to receive remdesivir and either baricitinib (combination group, 515 patients) or placebo (control group, 518 patients) and were stratified according to ordinal score (4, 5, 6, or 7). Remdesivir was administered intravenously at a loading dose of 200 mg on day 1, followed by 100 mg daily through day 10 or until hospital discharge or death. Baricitinib was given orally or through a nasogastric tube at a dose of 4 mg per day, or 2 mg per day if kidney function was reduced (estimated glomerular filtration rate, <60 ml per minute), for up to 14 days. Overall, the time to recovery was significantly shorter in the combination group than in the control group (median, 7 days vs. 8 days; rate ratio for recovery, 1.16; 95% CI, 1.01 to 1.32; P=0.03). The combination group also had 30% higher odds of improvement in clinical status at day 15 than the control group (odds ratio, 1.3; 95% CI, 1.0 to 1.6). The incidence of serious adverse events was lower in the combination group than in the control group (16.0% vs. 21.0%; difference, −5.0 percentage points; 95% CI, −9.8 to −0.3).
The efficacy of combination treatment was highest in patients with a baseline ordinal score of 6 (receiving high-flow oxygen or noninvasive ventilation). The time to recovery in such patients was 10 days in the combination group and 18 days in the control group (rate ratio for recovery, 1.51; 95% CI, 1.10 to 2.08). In addition, patients with this ordinal score who received combination treatment had the highest odds of improvement in clinical status (odds ratio vs. remdesivir alone, 2.2; 95% CI, 1.4 to 3.6). Similarly, patients with a baseline ordinal score of 5 had a median time to recovery of 5 days in the combination group and 6 days in the control group (rate ratio for recovery, 1.17; 95% CI, 0.98 to 1.39) and increased odds of clinical improvement (odds ratio, 1.2; 95% CI, 0.9 to 1.6). In the same ordinal-score categories, the greatest reduction in mortality at day 14 (score of 5: hazard ratio for death with combination treatment, 0.73 [95% CI, 0.16 to 3.26]; score of 6: hazard ratio, 0.21 [95% CI, 0.02 to 1.80]) and day 28 (score of 5: hazard ratio, 0.40 [95% CI, 0.14 to 1.14]; score of 6: hazard ratio, 0.55 [95% CI, 0.22 to 1.38]) was observed.
By confirming the results of the previous open-label studies showing the beneficial effects of baricitinib for Covid-19 treatment,8,9 ACTT-2 provides the highest grade of evidence on the efficacy of the drug, which acts through the inhibition of JAK1 and JAK2 and consequently blocks the immune cascade and reduces viral replication.10 The reported highest efficacy of baricitinib in patients with ordinal scores of 5 and 6 allows expansion of the therapeutic armamentarium against Covid-19 pneumonia, mainly in patients receiving oxygen support without invasive mechanical ventilation (Figure 1). Comparison between baricitinib and dexamethasone for the treatment of patients with Covid-19 pneumonia requiring supplemental oxygen would be an intriguing subject for future clinical research.
Disclosure Forms
Footnotes
Disclosure forms provided by the authors are available with the full text of this editorial at NEJM.org.
References
- 1.Gandhi RT, Lynch JB, Del Rio C. Mild or moderate Covid-19. N Engl J Med 2020;383:1757-1766. [DOI] [PubMed] [Google Scholar]
- 2.Falasca L, Nardacci R, Colombo D, et al. Postmortem findings in Italian patients with COVID-19: a descriptive full autopsy study of cases with and without comorbidities. J Infect Dis 2020;222:1807-1815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cantini F, Goletti D, Petrone L, Najafi Fard S, Niccoli L, Foti R. Immune therapy, or antiviral therapy, or both for COVID-19: a systematic review. Drugs 2020;80:1929-1946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Beigel JH, Tomashek KM, Dodd LE, et al. Remdesivir for the treatment of Covid-19 — final report. N Engl J Med 2020;383:1813-1826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.The RECOVERY Collaborative Group. Dexamethasone in hospitalized patients with Covid-19. N Engl J Med 2021;384:693-704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Matthay MA, Thompson BT. Dexamethasone in hospitalised patients with COVID-19: addressing uncertainties. Lancet Respir Med 2020;8:1170-1172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kalil AC, Patterson TF, Mehta AK, et al. Baricitinib plus remdesivir for hospitalized adults with Covid-19. N Engl J Med 2021;384:795-807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cantini F, Niccoli L, Matarrese D, Nicastri E, Stobbione P, Goletti D. Baricitinib therapy in COVID-19: a pilot study on safety and clinical impact. J Infect 2020;81:318-356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cantini F, Niccoli L, Nannini C, et al. Beneficial impact of baricitinib in COVID-19 moderate pneumonia; multicentre study. J Infect 2020;81:647-679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Stebbing J, Sánchez Nievas G, Falcone M, et al. JAK inhibition reduces SARS-CoV-2 liver infectivity and modulates inflammatory responses to reduce morbidity and mortality. Sci Adv 2020. November 13 (Epub ahead of print). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.