Abstract
Multiple myeloma (MM) involving the breast tissue is rare. We report the case of a 70-year-old woman with a background of previously treated MM in remission presenting with a breast lump. Histology showed a plasma cell neoplasm and subsequent staging investigations showed widespread extramedullary relapse of MM. Despite its rarity, this diagnosis should be considered within the differential diagnosis of breast masses as it can arise de novo or may be the first presenting feature of myeloma. The importance of the multidisciplinary team approach with triple assessment of the breast, as well as recent advances in knowledge regarding extramedullary disease in myeloma and novel treatment approaches in MM are discussed.
Keywords: haematology (incl blood transfusion), breast cancer, chemotherapy, pathology, radiology
Background
Plasma cell neoplasms are characterised by the malignant proliferation of plasma cells, usually producing a monoclonal immunoglobulin. Plasma cell neoplasms can present as a single lesion (solitary plasmacytoma) or as multiple myeloma (MM). Solitary plasmacytomas most frequently occur in bone but can also be found outside bone in soft-tissues.1 MM is usually confined to the bone marrow2; extramedullary disease (EMD) in MM is rare and occurs in 6%–8% of newly diagnosed MM patients and 10%–30% of MM patients later in the course of the disease.3 EMD can develop in any tissue, most commonly in the upper respiratory tract (oronasopharynx and paranasal sinuses).4 Extramedullary plasmacytoma of the breast is extremely rare. The first case of MM in the breast was reported in 1925. Since then, only 20 other patients with breast involvement in MM have been documented in medical literature.5 The diagnosis of breast plasmacytoma is challenging as the clinical and radiological features are similar to other breast diseases.6 We report a breast lesion in a patient with previously treated MM, which has several valuable clinical lessons. First, it demonstrates the importance of clinicians considering alternative diagnoses of breast lumps out of keeping with the primary breast pathology. Second, the case highlights the importance of triple assessment (clinical examination, imaging and biopsy) in patients with breast lesions. Finally, the case highlights the potential for breast involvement as a sign of extramedullary relapse of myeloma.
Case presentation
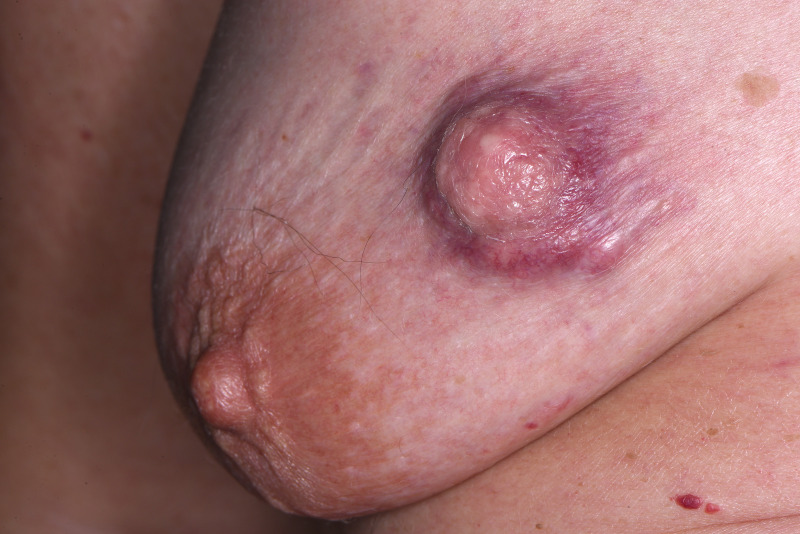
A 70-year-old postmenopausal femalewoman presented to the breast clinic with a 3-week history of a nodule in the right breast. The lesion was initially thought to be an insect bite, however, there was no resolution following a course of antibiotics. There was no associated nipple discharge, pain or trauma. The lump was in the right upper medial quadrant of the breast and measured 3×2 cm. It was firm and irregular in shape and non-tender (figure 1). No axillary lymph nodes were palpable and regional clinical examination findings elsewhere were normal.
Figure 1.
Clinical photo of right upper medial breast nodule.
Ten months earlier, she was diagnosed with MM after presenting with back pain, fatigue, confusion and polyuria. Subsequent investigations showed anaemia, hypercalcaemia, impaired renal function and multiple lytic lesions without evidence of EMD on a whole-body MRI scan. Protein electrophoresis demonstrated 19 g/L of IgA kappa paraprotein with a kappa: lambda serum free light chain ratio of 244.51. Bone marrow trephine confirmed MM with plasma cells accounting for 80%–90% of the bone marrow. Unfortunately, cytogenetic samples failed at baseline. The patient completed six cycles of Velcade (bortezomib), Cyclophosphamide and Dexamethasone chemotherapy to complete response (undetectable paraprotein, normal serum free light chain ratio and 1% clonal plasma cells on bone marrow trephine). An autologous stem cell transplant was planned to consolidate response, however, this was delayed due to the COVID-19 outbreak.
Her other medical history included hypertension, irritable bowel syndrome, lactose intolerance, partial thyroidectomy and hysterectomy. Her sister had breast cancer at the age of 35 which recurred at the age of 52. She does not smoke cigarettes or drink alcohol and had an Eastern Cooperative Oncology Group performance status of 1.
Investigations
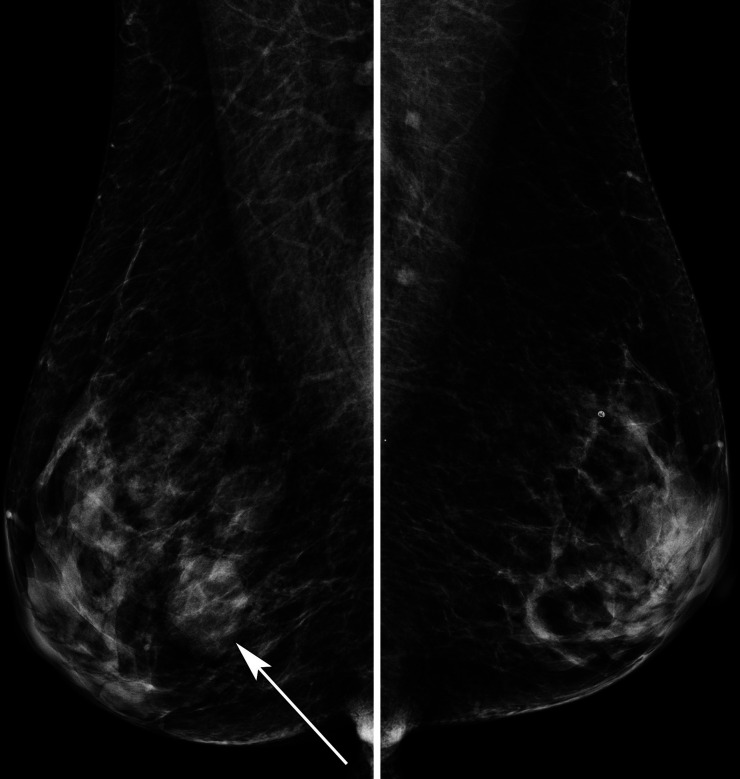
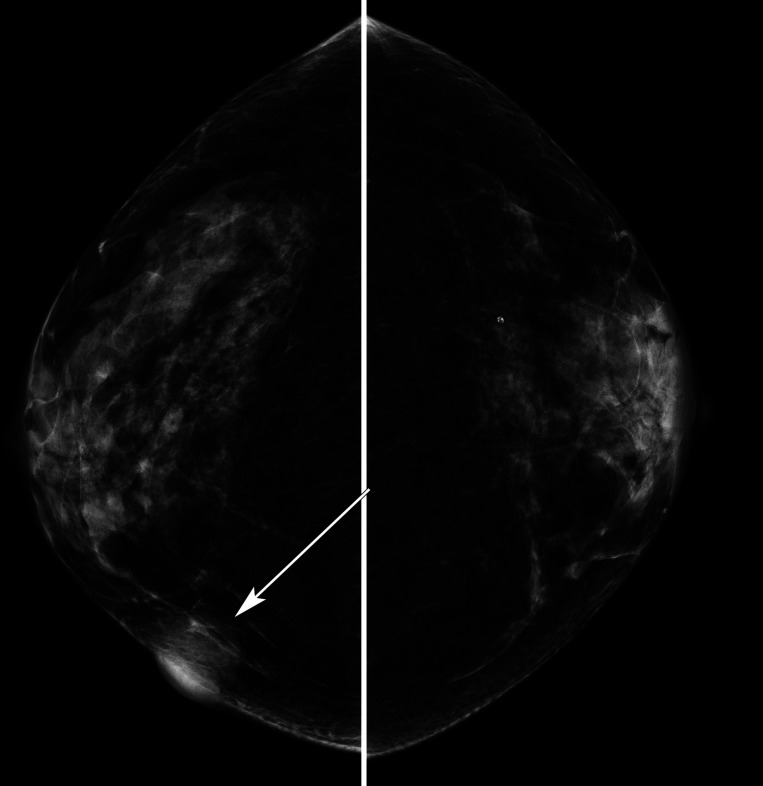
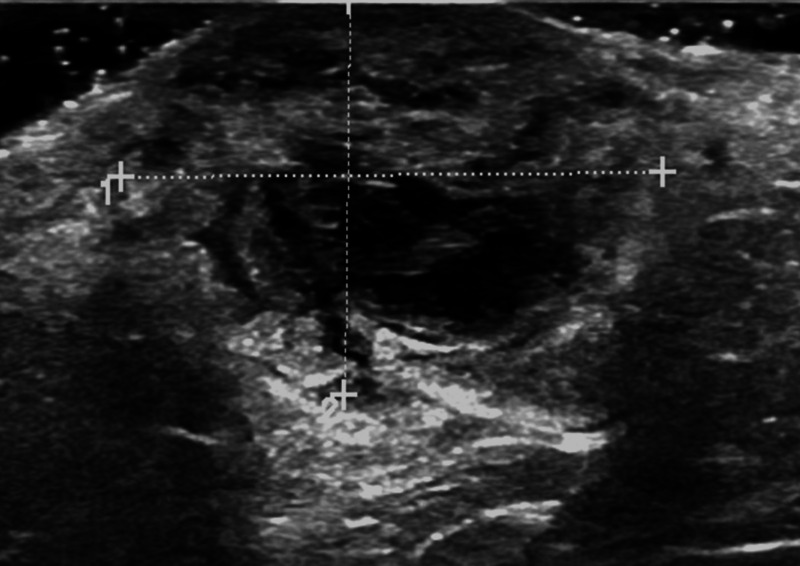
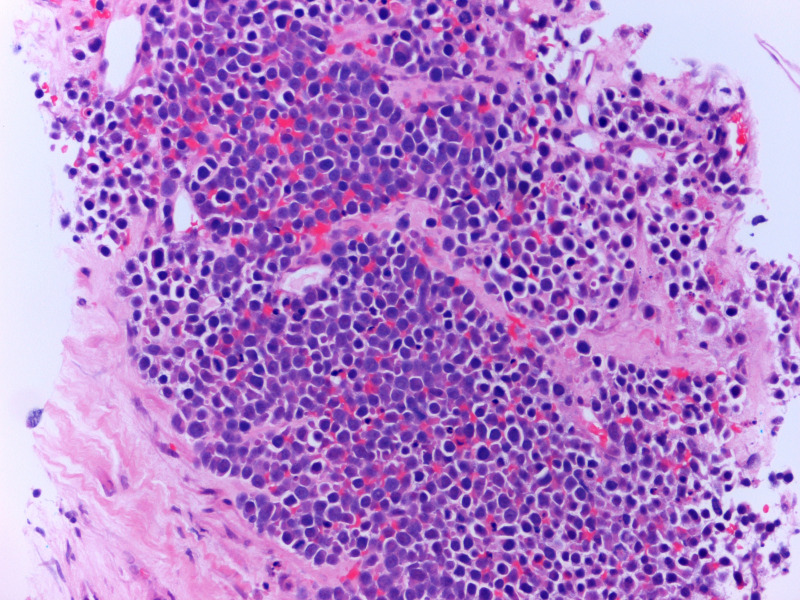
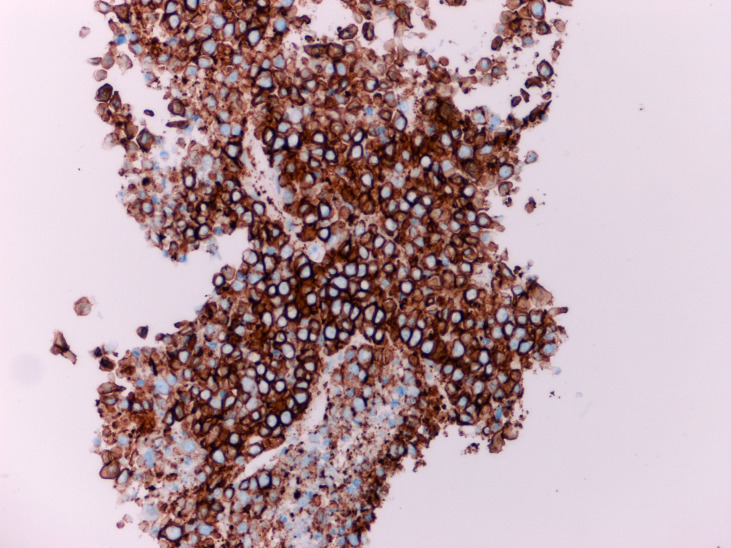
She proceeded to breast-specific investigations, including mammography and breast/axillary ultrasound. Mammography demonstrated an ill-defined opacity in the medial right breast (arrows, figures 2 and 3). Ultrasound of the palpable and mammographic abnormality confirmed a 45×32×22 mm lobulated mixed echogenicity mass (figure 4, callipers). There were no enlarged or malignant nodes in the axilla. The patient subsequently underwent an ultrasound-guided core needle biopsy of the lesion (18-gauge needle, 2 passes). Histology showed diffuse infiltration by sheets of atypical plasma cells (figures 5 and 6). The tumour cells showed positive staining for CD138, MUM1, EMA and negative staining for CK7, GATA3, oestrogen receptor, progesterone receptor, CD117, CD79a, CD56, CD45, CD20 and CD10. The histological appearance and immunohistochemical profile were those of a plasma cell neoplasm.
Figure 2.
Lateral oblique mammograms right and left breast showing a circumscribed opacity lower right breast, arrow.
Figure 3.
Craniocaudal mammograms right and left breast showing a lobulated opacity medial right breast with skin thickening, arrow.
Figure 4.
Sonogram right breast lesion confirms an ill-defined mixed texture mass, callipers, surrounding breast oedema and skin thickening.
Figure 5.
Core biopsy of breast showing infiltration by sheets of atypical plasma cells (H&E stain, high power).
Figure 6.
Immunohistochemical staining shows diffuse and strong positivity with malignant plasma cells marker CD138.
A staging CT scan showed disseminated disease with multiple deposits above and below the diaphragm, paraspinal deposits with spinal canal encroachment at C1/C2 and L4/L5 levels. There was also an obstructed biliary tree secondary to a pancreatic lesion. An MRI of the spine showed multilevel paravertebral soft-tissue lesions and epidural soft-tissue extension encroaching on the anterior thecal sac at thoracic and lumbar regions.
During workup, the patient developed double vision, protrusion of the left orbit and a left facial droop in keeping with a unilateral lower motor neuron facial nerve palsy. An MRI brain confirmed a left intra-orbital enhancing mass of predominantly low T1/T2 signal intensity, 3.2×2×3 cm, displacing the eye globe inferiorly and likely infiltrating the lacrimal gland, superior rectus and lateral rectus muscles. There was also intracranial extension to the left temporal lobe, pterygoid muscle and the sphenoid sinus and palpable subcutaneous lesions across the scalp. These findings are in keeping with disseminated relapsed myeloma with widespread EMD.
Differential diagnosis
A new-onset, hard, irregular and lobulated breast mass in a patient of this age group without signs of infection should be suspected to be malignant that is, primary malignancy (carcinoma, rarely sarcoma) or secondary/metastatic malignancy as well as melanoma or lymphoma. Other differentials include fibroadenoma or cysts although unlikely in this age group with this presentation.
Treatment
The patient received steroids (dexamethasone 8 mg two times a day) to reduce the risk of cord compression and had radiotherapy to the orbit and spinal lesions. She was started on an intensive inpatient combination chemotherapy regimen with dexamethasone, thalidomide, cisplatin, Adriamycin (doxorubicin), cyclophosphamide and Etoposide (DT-PACE).
Outcome and follow-up
The patient reported a clinical reduction in the size of the breast lump following the first cycle of chemotherapy. She also had improvement of her double vision and complete resolution of obstructive jaundice and subcutaneous lesions. She will complete further cycles of DT-PACE with a view to autologous stem cell transplant to consolidate her clinical response.
Discussion
Malignant plasma cell dyscrasias comprise of a heterogeneous group of disorders with a variable clinical course and prognosis. They are characterised by the monoclonal proliferation of plasma cells and include isolated plasmacytoma of the bone, solitary extramedullary plasmacytoma, MM, MM with extramedullary manifestations and plasma cell leukaemia. Unlike MM, isolated plasmacytoma of the bone and solitary plasmacytoma do not infiltrate the bone marrow.
Clinical manifestations of symptomatic myeloma are related to end-organ damage leading to CRAB features (hypercalcaemia, renal impairment, anaemia and bony lesions). The revised International Myeloma Working Group criteria in 2014 add three myeloma-defining events (MDEs) in addition to CRAB features for the diagnosis of active myeloma. MDEs include more than 60% clonal plasma cells in the bone marrow, serum involved/uninvolved free light chain ratio of more than 100 or one focal lesions on MRI that is at least 5 mm or greater in size.7
After a thorough history and examination, MM testing includes a full blood count, blood film, renal function, serum calcium, serum protein electrophoresis, immunoglobulin levels and serum free light chains. To confirm MM, a bone marrow aspirate and trephine biopsy is essential and will contain over 10% or more clonal plasma cells. Cytogenetics may show the presence of high risk MM, these abnormalities include del(17 p), t(4;14), t(14;16), t(14;20), gain 1q or p53 mutation. The presence of any two high-risk factors is considered double‐hit myeloma; three or more high-risk factors is triple‐hit myeloma. Cytogenetic information has important prognostic value and it is increasingly recognised that treatment options may be tailored to certain subgroups.8
Imaging is crucial for the assessment of patients with MM. Skeletal survey using plain radiographs has been superseded by more sensitive methods such as low-dose whole-body CT or whole-body MRI. A Positron emission tomography (PET)/CT scan is particularly useful to stage those with non-secretory myeloma or EMD.9
EMD in MM simultaneously involving several organs is unusual. Furthermore, myeloma affecting the breast and/or the female reproductive organs is very rare.6 EMD of the breast can be unilateral or bilateral. Clinically, plasmacytomas presenting as breast lumps can mimic more common causes such as primary breast cancer. Generally, radiological features are non-specific and can mimic other diseases such as primary breast carcinoma, lymphoproliferative diseases and even benign masses and therefore histology is crucial.
The histological differential diagnosis of a plasmacytoma in the breast includes some reactive processes such as plasma cell mastitis, a mixed chronic inflammatory infiltrate of non-clonal plasma cells; and pseudolymphoma, a reactive entity composed of mixed inflammatory cells. Malignant differentials include melanoma and non-Hodgkin’s lymphoma with plasmacytic features. However, immunohistochemistry helps to differentiate these conditions.10
Extramedullary relapse of MM is generally considered to be an aggressive manifestation. It indicates that a subclone of malignant plasma cells can survive independently of the bone marrow microenvironment, evade cell death and may be resistant to conventional treatment. It is theorised that extramedullary plasmacytomas in MM develop either via direct extension from skeletal tumours or via haematogenous spread. Mechanisms of such spread are thought to be due to a decreased expression of adhesion molecules and a loss of CD56 leading to dissociation of myeloma cells from the bone marrow endothelium.11
Usmani et al report their experience on extramedullary involvement of MM in a large series of 1965 patients. These patients had a baseline PET scan performed at diagnosis and at the time of disease progression. The frequency of EMD at initial diagnosis was 3.4% (66 of 1965) and around 5% at the time of relapse or progression. The most frequent location at diagnosis was the skin while disease progression, liver involvement was seen. The 5-year overall survival in patients with EMD at diagnosis was shorter than those without EMD (31% vs 59%).12
Treatment with triplet induction therapy using proteasome inhibitors (PIs), immunomodulatory drugs (IMiDs) and corticosteroids is the cornerstone of MM treatment. VDT-PACE followed by autologous stem cell transplant to consolidate response is used for more aggressive myeloma.13
For elderly patients not eligible for stem cell transplantation, bortezomib–melphalan–prednisone may be considered. Bortezomib based regimens, unlike thalidomide-based regimens, have proven efficacy against EMD.14 There is an emerging role of newer anti-myeloma drugs such as lenalidomide in EMD.15
Novel generation IMiDs (pomalidomide) and PIs (carfilzomib (CFZ), ixazomib) are at disposal, as well as monoclonal antibodies (daratumumab, isatuximab) for use in MM at relapse, however, further studies are needed to evaluate their role in EMD.
Daratumumab is the first‐in‐class monoclonal antibody targeting CD38 for the treatment of MM. Daratumuab is frequently used in the relapsed setting as a second line agent in combination with Velcade and dexamethasone in the UK. Recent studies have proven efficacy in the frontline setting. The phase 3 Cassiopeia trial evaluated daratumumab (D) in combination with bortezomib, thalidomide and dexamethasone (D-VTd) versus VTd alone. Patients treated with D-VTd achieved a more stringent complete remission compared with those treated with VTd at rates of 39% vs 26%, respectively.16 Additionally, the GRIFFIN study, evaluated daratumumab with the lenalidomide, bortezomib, dexamethasone backbone (RVd) in those who were candidates for autograft. Overall, patients treated with D-RVd achieved a more frequently stringent complete response compared with those receiving standard RVd (62.6% vs 45.4%, respectively).17 Quadruplet regimens of D-RVd and D-VTd have the potential to become the new standard of care for upfront therapy in MM.
Elotuzumab is a monoclonal antibody recognising the cell surface glycoprotein SLAMF7. It is Food and Drug administration (FDA) approved for relapsed/refractory MM. Data from the ELOQUENT-2 study shows that elotuzumab in combination with lenalidomide and dexamethasone performs well (ELd). Patients in the ELd arm had significantly higher overall response rates (79% vs 66%) and prolonged PFS (10.3 vs 4.7 months) compared with those who received Ld alone.18
CFZ is a potent, second-generation PI, with significant activity in combination with other antimyeloma agents in patients with relapsed or refractory MM. The CANDOR study compared the efficacy and safety of CFZ, dexamethasone and daratumumab (KdD) versus CFZ and dexamethasone (Kd) in patients with relapsed or refractory MM. The study showed that the median treatment duration was longer in the KdD versus the Kd group (70.1 vs 40.3 weeks).19
Other promising treatments on the horizon for MM treatment include Selinexor, a first-in-class nuclear export protein inhibitor and venetoclax, a bcl2 inhibitor.20 Additionally, belantamab–mafodotin, an antibody-drug conjugate represents a promising option for MM with an overall response rate of 31%–34% in the phase 2 DREAMM-2 trial with belantamab as a single agent. However, it can cause corneal keratopathy and thus regular ophthalmic monitoring is required.21
Chimeric antigen receptor T-cell (CAR-T) therapy is characterised by genetically modified T cells to induce powerful anticancer cytotoxic effects via specific tumour antigens. CAR-T therapy in myeloma principally targets the B cell maturation antigen (BCMA) on plasma cells. Results from the phase 2 study, KarMMa1 trial, who recruited heavily pretreated patients, showed an overall response rate of 73%, including 33% complete response, and median progression free survival of 8 months.22 Additionally, the CARTITUDE-1 study showed an overall response rate of 100%, with 69% achieving complete response.23 Even though CAR-T cell therapy may lead to deep responses, durability of response remains a major issue. Adverse effects of CAR-T cell therapy include cytokine-release syndrome, neurotoxicity and infection.24 Furthermore, CAR-T cell treatments are limited by the time taken to produce autologous CAR-T cells (several weeks) by which time MM may progress.
Bispecific T cell engagers (BiTEs) are dual-targeting antibody constructs which are designed to carry out an attack on MM cells. There is no need to extract cells from the patient, avoiding the current laborious process of removing, manufacturing, and reinfusing cells for treatment in CAR-T cell therapy. AMG 420 is a BiTE targeting BCMA and it is likely to be a safe and effective treatment for patients with relapsed/refractory MM. In a phase I trial, the drug elicited a response in 31% of patients and was associated with relatively manageable side effects.25
Learning points.
Extramedullary relapse of multiple myeloma involving the breast is extremely rare, however, is an important differential for any patient with a history of multiple myeloma presenting with a breast lump.
Imaging and triple assessment and accurate evaluation of histology are invaluable in obtaining the correct diagnosis of extramedullary myeloma, thereby aiding correct treatment and avoiding radical mastectomy.
Patients with extramedullary disease have shorter overall survival, intensive treatment is required for fit patients.
Eligible patients should be considered for combination chemotherapy with bortezomib, dexamethasone, thalidomide, cisplatin, doxorubicin, cyclophosphamide and etoposide, followed by autologous stem cell transplant to consolidate response.
Current options for antimyeloma treatment includes immunomodulatory drugs, proteasome inhibitors and antibody therapies. The future of antimyeloma therapy is likely to include chimeric antigen receptor t cells targeting B cell maturation antigen and bispecific T cell engagers.
Footnotes
Contributors: DS: wrote up the case report and the discussion. ZA: provided histolopathology images, report and figure legends. DH: provided radiology images and expert editing of material.
Funding: The authors have not declared a specific grant for this research from any funding agency in the public, commercial or not-for-profit sectors.
Competing interests: None declared.
Patient consent for publication: Obtained.
Provenance and peer review: Not commissioned; externally peer reviewed.
References
- 1.Kilciksiz S, Karakoyun-Celik O, Agaoglu FY. A review for solitary plasmacytoma of bone and extramedullary plasmacytoma. Sci World J 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bladé J, de Larrea CF, Rosiñol L. Extramedullary involvement in multiple myeloma. Haematologica 2012;97:1618–9. 10.3324/haematol.2012.078519 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Touzeau C, Moreau P. How I treat extramedullary myeloma. Blood 2016;127:971–6. 10.1182/blood-2015-07-635383 [DOI] [PubMed] [Google Scholar]
- 4.Dos Anjos Corvo MA, Granato L, Ikeda F. Extramedullary nasal plasmacytoma: literature review and a rare case report. Int Arch Otorhinolaryngol Published Online First 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Urano M, Denewar FA, Okabe R. Relapsed multiple myeloma manifesting as extramedullary plasmacytoma of the breast: imaging findings. Radiol Case Reports Published Online First 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ali HOE, Nasir Z, Marzouk AMSM. Multiple myeloma breast involvement: a case report. Case Rep Radiol 2019;2019:2079439. 10.1155/2019/2079439 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rajkumar SV. Multiple myeloma: 2016 update on diagnosis, risk-stratification, and management. Am J Hematol 2016;91:719–34. 10.1002/ajh.24402 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Walker BA, Mavrommatis K, Wardell CP, et al. A high-risk, Double-Hit, group of newly diagnosed myeloma identified by genomic analysis. Leukemia 2019;33:159–70. 10.1038/s41375-018-0196-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chantry A, Kazmi M, Barrington S, et al. Guidelines for the use of imaging in the management of patients with myeloma. Br J Haematol 2017;178:380–93. 10.1111/bjh.14827 [DOI] [PubMed] [Google Scholar]
- 10.Binesh F, Vahedian HA, Shabani M. Extramedullary plasmacytoma of the breast: a case report and literature review. Acta Med. Iran 2018. [Google Scholar]
- 11.Sher T, Miller KC, Deeb G, et al. Plasma cell leukaemia and other aggressive plasma cell malignancies. Br J Haematol 2010;150:418–27. 10.1111/j.1365-2141.2010.08157.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Usmani SZ, Heuck C, Mitchell A, et al. Extramedullary disease portends poor prognosis in multiple myeloma and is over-represented in high-risk disease even in the era of novel agents. Haematologica 2012;97:1761–7. 10.3324/haematol.2012.065698 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lakshman A, Singh PP, Rajkumar SV, et al. Efficacy of VDT PACE-like regimens in treatment of relapsed/refractory multiple myeloma. Am J Hematol 2018;93:179–86. 10.1002/ajh.24954 [DOI] [PubMed] [Google Scholar]
- 14.Rosiñol L, Cibeira MT, Uriburu C. Bortezomib: an effective agent in extramedullary disease in multiple myeloma. Eur J Haematol 2006. [DOI] [PubMed] [Google Scholar]
- 15.Jullien M, Trudel S, Tessoulin B, et al. Single-Agent daratumumab in very advanced relapsed and refractory multiple myeloma patients: a real-life single-center retrospective study. Ann Hematol 2019;98:1435–40. 10.1007/s00277-019-03655-5 [DOI] [PubMed] [Google Scholar]
- 16.Moreau P, Attal M, Hulin C, et al. Bortezomib, thalidomide, and dexamethasone with or without daratumumab before and after autologous stem-cell transplantation for newly diagnosed multiple myeloma (CASSIOPEIA): a randomised, open-label, phase 3 study. Lancet 2019;394:29–38. 10.1016/S0140-6736(19)31240-1 [DOI] [PubMed] [Google Scholar]
- 17.Voorhees PM, Kaufman JL, Laubach J, et al. Daratumumab, lenalidomide, bortezomib, and dexamethasone for transplant-eligible newly diagnosed multiple myeloma: the Griffin trial. Blood 2020;136:936–45. 10.1182/blood.2020005288 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dimopoulos MA, Lonial S, White D. Elotuzumab plus lenalidomide/dexamethasone for relapsed or refractory multiple myeloma: ELOQUENT-2 follow-up and post-hoc analyses on progression-free survival and tumour growth. Br J Haematol 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dimopoulos M, Quach H, Mateos M-V, et al. Carfilzomib, dexamethasone, and daratumumab versus carfilzomib and dexamethasone for patients with relapsed or refractory multiple myeloma (CANDOR): results from a randomised, multicentre, open-label, phase 3 study. Lancet 2020;396:186–97. 10.1016/S0140-6736(20)30734-0 [DOI] [PubMed] [Google Scholar]
- 20.Chim CS, Kumar SK, Orlowski RZ, et al. Management of relapsed and refractory multiple myeloma: novel agents, antibodies, immunotherapies and beyond. Leukemia 2018;32:252–62. 10.1038/leu.2017.329 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lonial S, Lee HC, Badros A, et al. Belantamab mafodotin for relapsed or refractory multiple myeloma (DREAMM-2): a two-arm, randomised, open-label, phase 2 study. Lancet Oncol 2020;21:207–21. 10.1016/S1470-2045(19)30788-0 [DOI] [PubMed] [Google Scholar]
- 22.Jagannath S, Lin Y, Goldschmidt H. A study of real-world treatment patterns in heavily pretreated patients with relapsed and refractory multiple myeloma (RRMM) and comparison of outcomes to KarMMa. J Clin Oncol 2020. [Google Scholar]
- 23.Madduri D, Usmani SZ, Jagannath S, et al. Results from CARTITUDE-1: a phase 1b/2 study of JNJ-4528, a CAR-T cell therapy directed against B-cell maturation antigen (BCMA), in patients with relapsed and/or refractory multiple myeloma (R/R Mm). Blood 2019;134:577. 10.1182/blood-2019-12173131416814 [DOI] [Google Scholar]
- 24.Kenderian SS, Porter DL, Gill S. Chimeric antigen receptor T cells and hematopoietic cell transplantation: how not to put the cart before the horse. Biol Blood Marrow Transplant 2017;23:235–46. 10.1016/j.bbmt.2016.09.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Topp MS, Duell J, Zugmaier G. Evaluation of AMG 420, an anti-BCMA bispecific T-cell engager (bite) immunotherapy, in R/R multiple myeloma (Mm) patients: updated results of a first-in-human (FIH) phase I dose escalation study. J Clin Oncol 2019. [Google Scholar]