Abstract
Purpose:
Approximately 25% of patients receiving weekly paclitaxel for breast cancer require treatment disruptions to avoid severe, irreversible peripheral neuropathy (PN). Vitamin insufficiencies are PN risk factors in many diseases, but their relevance to chemotherapy-induced PN is unknown.
Methods:
We investigated whether baseline insufficiency of vitamin D, vitamin B12, folate, or homocysteine increased PN in patients with breast cancer receiving weekly paclitaxel in a retrospective analysis of a prospective observational study. Patient-reported PN was collected at baseline and during treatment on the Quality of Life Questionnaire Chemotherapy-Induced Peripheral Neuropathy(CIPN20). The primary analysis tested associations between vitamin deficiency and the maximum increase from baseline in the CIPN20 sensory subscale (ΔCIPN8). Secondary analyses tested for association with PN-induced treatment disruptions and adjusted associations for treatment and clinical variables.
Results:
25-hydroxy-vitamin D was the only nutrient with sufficient deficiency (< 20 ng/mL) for analysis (15/37=41%). Vitamin D deficient patients had a greater mean PN increase than non-deficient patients (ΔCIPN8±SD, 36±23 vs. 16±16, p=0.003) and a non-significant, approximately 3-fold increase in risk of treatment disruption (OR = 2.98, 95% CI [0.72, 12.34], p=0.16). In multivariable models adjusted for clinical and treatment variables, baseline vitamin D level was inversely associated with PN (β = −0.04, p = 0.02).
Conclusions:
Pre-treatment vitamin D deficiency was associated with PN in women receiving weekly paclitaxel for breast cancer. Vitamin D deficiency may be an easily detected PN risk factor that could be resolved prior to treatment to prevent PN, avoid treatment disruptions, and improve treatment outcomes.
Keywords: chemotherapy-induced peripheral neuropathy, paclitaxel, breast cancer, nutrient deficiency, vitamin D
Background
Paclitaxel is a highly effective chemotherapy agent and is widely used in the treatment of breast cancer.[1] In the setting of early-stage breast cancer, a regimen of 80 mg/m2 administered weekly for 12 weeks is commonly used as part of the curative therapeutic plan.[2] Although weekly dosing has an acceptable risk-benefit profile, many patients suffer from dose-limiting toxicities, particularly peripheral neuropathy (PN).[2] Based on the National Cancer Institute (NCI) Common Terminology Criteria for Adverse Events (CTCAE) grading scale[3], grade 2 or higher PN occurs in approximately 25% of patients receiving weekly paclitaxel and symptoms continue beyond two years after treatment in approximately 10% of patients.[2, 4, 5] Mild PN is characterized by bothersome burning, tingling, and numbness which can progress to irreversible loss of fine motor movements and gait stability with continued taxane treatment.[6] Given the >80% five-year survival in early stage breast cancer, it is particularly desirable to limit PN that can cause a lasting negative impact on quality of life.[1, 7–9] To avoid long term morbidity, up to 25% of patients require paclitaxel treatment disruptions including dose decreases, dose delays, and cessation of therapy.[10, 11] These disruptions reduce further PN progression but place patients at risk for suboptimal treatment efficacy.[12, 13]
Discovery of effective preventative strategies to reduce paclitaxel-induced PN is a critical unmet need. Previous efforts to identify predictive biomarkers have focused extensively on patient’s genetics, but these have failed to be successfully replicated and may be challenging to translate into patient care.[14] Discovery and validation of a predictive, easily modifiable biomarker of PN could reduce patient suffering secondary to PN, prevent paclitaxel treatment disruptions and maximize treatment benefit.
Baseline nutrient deficiencies may be a modifiable predictive biomarker of paclitaxel induced PN but these relationships remain relatively unexplored. Deficiency of folic acid, B vitamins, and vitamin D are known risk factors for PN in other disease states including chronic alcoholism and diabetes mellitus.[14–19] Despite the known link across etiologies, the role of these deficiencies as risk factors for chemotherapy induced PN has not been well defined.[20] Deficiency of B vitamins and vitamin D have been associated with increased chemotherapy-induced PN and past case reports indicate supplementation may be an effective treatment option in deficient patients.[21–24] Nutritional deficiencies present an attractive option as a predictive biomarker because they are easily detected by commonly conducted clinical labs and are often modifiable through nutritional supplementation or dietary modification.
Our group has completed an observational study (NCT02338115) of patients with early stage breast cancer receiving paclitaxel for 12 weeks at 80 mg/m2. Previous work in this cohort has sought to discover pharmacokinetic, pharmacogenomic, and pharmacometabolomic biomarkers of paclitaxel-induced neuropathy for future validation and clinical translation.[25–27] The objective of this study was to conduct a secondary analysis to investigate associations between pre-treatment nutrient insufficiencies and severity of PN during weekly paclitaxel treatment in this cohort.
Methods
Study design and patient enrollment:
Full details regarding the study design, patient enrollment, and sample and data collection for this observational clinical study have been described previously (NCT02338115).[25] Briefly, this study enrolled adult, female patients scheduled to receive paclitaxel 80 mg/m2 weekly × 12 weeks for stage I–III or oligometastatic breast cancer. All patients were recruited from the University of Michigan Rogel Cancer Center. The primary objective of the clinical study was to confirm the relationship between systemic paclitaxel concentrations after the first infusion and PN severity throughout treatment. Blood samples were collected at baseline for planned, retrospective secondary analyses of genomics, pharmacometabolomics, and neuropathy-associated nutrient insufficiencies. The clinical study and secondary analyses were approved by University of Michigan IRBMED and all patients completed informed consent. The primary study and all subsequent analyses were conducted ethically according to relevant institutional and national guidelines and statutes and according to the Declaration of Helsinki and The Belmont Report.
Peripheral Neuropathy Assessment
Patients completed the European Organisation for Research and Treatment of Cancer (EORTC) Quality of Life Questionnaire Chemotherapy-Induced Peripheral Neuropathy (CIPN20) at baseline and weekly until the end of treatment.[28] The CIPN20 is a semi-validated patient-reported assessment tool used to quantify sensory, motor, and autonomic neuropathy experienced in the previous week on a scale of 1 (“not at all”) to 4 (“very much”). Because paclitaxel is known to cause a predominantly sensory PN, an 8-item sensory subscale (CIPN8) of the full survey was used in the primary and this secondary analysis.[25, 29, 30] The raw CIPN8 score at each time point is calculated by summing the responses to each of the eight items, yielding a raw score of 8–32. Raw CIPN8 scores are then converted to a linear scale ranging from 0 to 100 with higher scores indicating greater PN, as recommended by the EORTC [28]. All reports of CIPN8 in this manuscript refer to this linearly scaled score. Clinicians did not have access to the CIPN20 surveys during treatment, and as such all treatment decisions were blinded to CIPN20 information.
Sample collection and measurement of nutrients and paclitaxel concentrations
Blood samples were collected the morning of the first paclitaxel infusion, prior to initiation of treatment, for baseline measurement of neuropathy-related nutrients: 25-hydroxy-vitamin D (vitamin D), vitamin B12, folate, and homocysteine. Nutrient levels were measured by the clinical laboratory at Michigan Medicine and insufficiency defined by institutional standards.
Statistical analysis and modeling
Baseline characteristics were compared between groups using the Student’s t-test and Chi-Square or Fisher’s exact test as appropriate. To determine the relationship between baseline nutrient levels and paclitaxel-induced PN, the a priori primary endpoint was defined as the maximum increase from baseline to any time point during treatment in the scaled CIPN8 (Max CIPN8 reported – baseline CIPN8 = ΔCIPN8). For each nutrient, the ΔCIPN8 was compared between nutrient insufficient and sufficient patients using the Student’s t-test. Nutrients associated with PN in the primary univariate analysis were also tested in a secondary analysis by introducing nutrient status into our previously published PN multivariable repeated-measure linear regression model. [25] The base model includes baseline CIPN8 (0–100), cumulative dose [in mg/m2 according to actual body weight and adjusted for body-surface area (BSA)], relative dose intensity (proportion of cumulative planned dose received to expected cumulative dose, to account for delays and decreases) and other covariates previously found to be significant predictors of PN: age, alcohol status (self-reported alcohol intake, yes vs. no), and diabetes (determined through self-reported diagnosis, diagnosis abstracted from the medical record, or baseline HbA1C > 6.5%)). Vitamin status was introduced into this model in two ways, as a dichotomous variable (deficient vs. not) and as a continuous variable (raw vitamin level). A square root transformation was applied to scaled CIPN8 scores to meet model assumptions.
Any nutrients associated with ΔCIPN8 in the primary analysis were also tested for association with a secondary clinically relevant endpoint of PN-induced treatment disruption, defined previously as a paclitaxel dose decrease, delay, or discontinuation due to PN using Chi-Square or Fisher’s Exact tests, as appropriate.[25] All statistical analyses and modeling were conducted in SAS v9.4 (SAS Institute).
Results
Baseline nutrient levels:
Sixty participants completed the observational clinical study and were eligible for this secondary analysis. The initial pilot feasibility study did not include collection of nutrient data. After enrollment of the first 22 patients, an amendment was approved to expand enrollment and permit determination of baseline vitamin and nutrient levels of 38 subsequently enrolled patients (Figure 1). Vitamin D deficiency (defined as <20 ng/mL) was identified in 41% (15/37) of assessed patients. Vitamin B12 insufficiency (<211 pg/mL) was found in 3% (1/30) of patients, and no patients were identified with folate (<4 ng/mL) or homocysteine (normal range 5 to 15 μmol/L) abnormalities. Due to limited sample size for patients with deficiencies of vitamin B12, folate, and homocysteine, secondary analyses were conducted using a binary classification based on the median value of the nutrient (i.e., above vs. below median).
Figure 1.
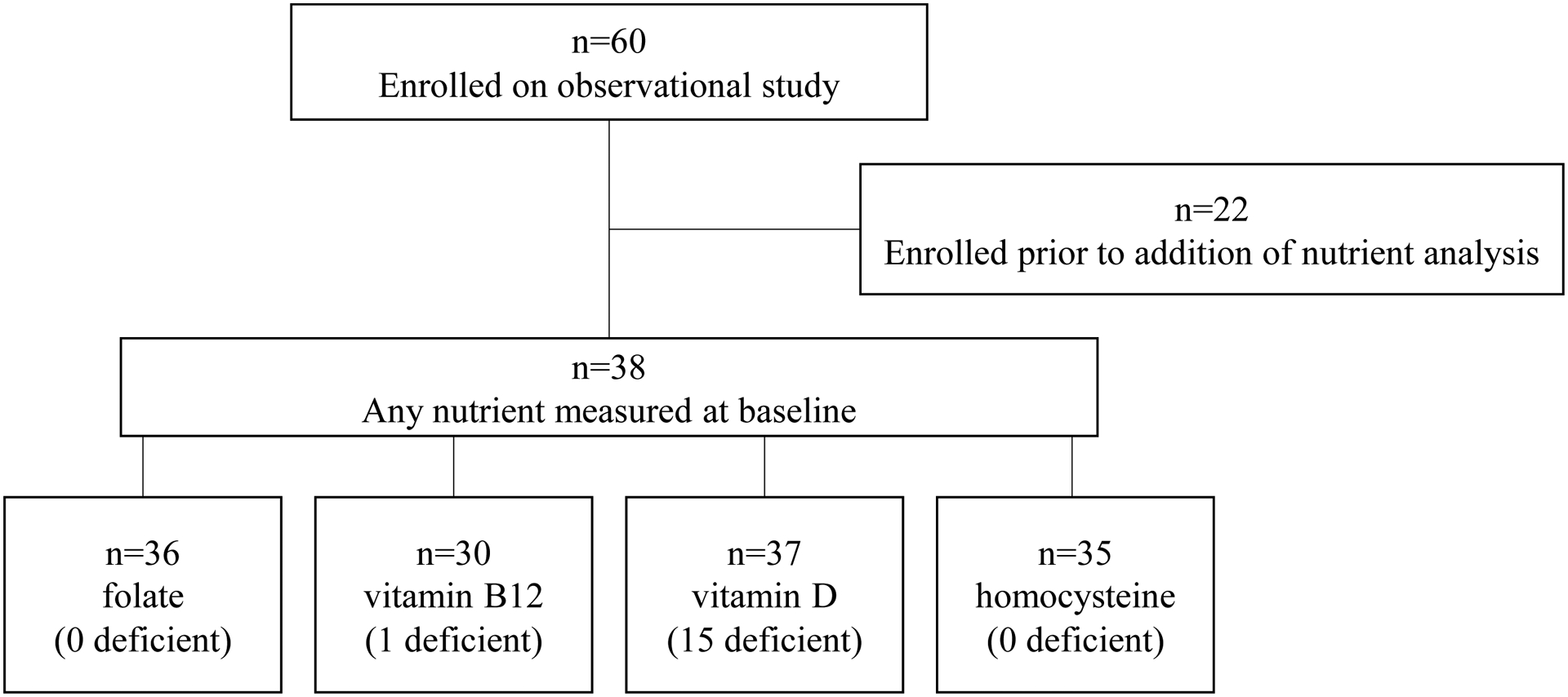
Patient flow diagram from the parent observational study to this secondary nutrient analysis.
Patient demographics and baseline characteristics:
Patient demographics and clinical characteristics for the 37 patients with measured vitamin D were stratified by vitamin D status: deficient (vit D−) or non-deficient (vit D+, Table 1). Patient demographics including age and race were similar between groups, as were BMI and other comorbidities known to be associated with PN (i.e., diabetes mellitus and alcohol intake). The patients included in this analysis are representative of the parent study cohort, except that patients included in this analysis were less likely to be taking concurrent HER2-directed treatment with trastuzamab and/or pertuzumab (32.4% vs. 73.9%, p =0.001).
Table 1.
Participant characteristics stratified by vitamin D status deficient (−) vs. non-deficient (+)
| Vitamin D− | Vitamin D+ | p-value | ||
|---|---|---|---|---|
| Participants | Number | 15 (40.5%) | 22 (59.5%) | NA |
| Age | Years | 47.6 [28, 59] | 54.6 [34, 71] | 0.051 |
| Self-reported race | White | 15 (100%) | 19 (86.4%) | 0.257 |
| Other | 0 (0%) | 3 (13.7%) | 0.257 | |
| BMI | kg/m2 | 28.2 ± 5.3 | 28.3 ± 5.1 | 0.953 |
| Treatment regimen | Prior AC | 14 (93.3%) | 21 (95.5%) | 1.000 |
| Concurrent H and/or P | 6 (40.0%) | 6 (27.3%) | 0.488 | |
| Alcohol consumption | Yes | 9 (60.0%) | 10 (45.5%) | 0.508 |
| Diabetes mellitus | Self-reported and/or HbA1C>6.5% | 1 (6.7%) | 7 (31.8%) | 0.108 |
| Peripheral neuropathy | Baseline CIPN8 | 1.94 ± 4.1 | 1.14 ± 2.6 | 0.471 |
Abbreviations: A = doxorubicin, C = cyclophosphamide, H = trastuzamab, P = pertuzumab; HbA1C = hemoglobin A1C, CIPN8 = Scaled score (range 0–100) of the 8-item sensory subscale of EORTC chemotherapy induced peripheral neuropathy 20 (CIPN20) questionnaire.
Continuous variables are presented as mean ± standard deviation except age which is presented as mean [minimum, maximum].
All continuous variables are compared by T-test. Categorical variables are presented as counts (%) and compared by chi-square or Fisher’s exact test as appropriate.
Association of vitamin D with peripheral neuropathy and treatment disruption
Baseline CIPN8 was low and similar between groups (Vit D− = 1.94 ± 4.1, Vit D+ =1.14 ± 2.6, p = 0.574). In the primary analysis, patients with baseline vitamin D deficiency experienced a greater change in CIPN8 (ΔCIPN8) than patients without vitamin D deficiency (Vit D− = 36.39 ±22.8 vs. Vit D+ = 16.29 ±16.3, p=0.003). Vitamin D deficiency as a binary variable did not retain significance in the CIPN8 model after adjusting for treatment and clinical covariates (β =0.40, SE = 0.25, p = 0.11, Table 2, Model 1). However, vitamin D measurement (ng/mL) as a continuous variable was inversely associated with ΔCIPN8 in the fully adjusted model (β = −0.04, SE = 0.02, p = 0.02, Table 2, Model 2). Due to substantial nutrient data-missingness and limited sample size we attempted to impute vitamin D for the entire cohort (n=60) and rerun the models. However, vitamin D was not significant in any imputed models (data not shown). For vitamin B12, folate, and homocysteine, there were no differences in ΔCIPN8 between patients above and below the median (data not shown).
Table 2.
Multivariable repeated-measure linear regression model of CIPN8 with previously published clinical variables[25] and vitamin D.
| Description | Fixed Effect | Coefficient Estimate | Standard Deviation | P-value |
|---|---|---|---|---|
| Model 1 N=37 | Baseline CIPN8 | 0.21 | 0.04 | <0.0001 |
| Cumulative dose | 0.48 | 0.06 | <0.0001 | |
| Relative dose intensity | 0.26 | 0.93 | 0.78 | |
| Age | 0.01 | 0.01 | 0.27 | |
| Alcohol vs. none | 0.46 | 0.23 | 0.05 | |
| Diabetes vs. no diabetes | 0.35 | 0.30 | 0.24 | |
| Vitamin D deficiency vs. nota | 0.40 | 0.25 | 0.11 | |
| Model 2 N=37 | Baseline CIPN8 | 0.21 | 0.04 | <0.0001 |
| Cumulative dose | 0.48 | 0.06 | <0.0001 | |
| Relative dose intensity | 0.10 | 0.93 | 0.91 | |
| Age | 0.01 | 0.01 | 0.24 | |
| Alcohol vs. none | 0.48 | 0.23 | 0.04 | |
| Diabetes vs. no diabetes | 0.28 | 0.29 | 0.33 | |
| Vitamin D level (ng/mL) | −0.04 | 0.02 | 0.02 |
Vitamin D deficiency defined as < 20 ng/mL. Acronyms: CIPN8, Scaled score (range 0–100) of the 8-item sensory subscale of EORTC chemotherapy induced peripheral neuropathy 20 (CIPN20) questionnaire.
Twelve out of 37 (32.4%) patients experienced a PN-induced treatment disruption. Patients with vitamin D deficiency had nominally greater risk for a PN-induced treatment disruption but this did not reach statistical significance (OR = 2.98, 95% CI [0.72, 12.34], p=0.16).
Discussion
PN is a dose-limiting toxicity of paclitaxel that can affect function and quality of life. With a lack of proven effective treatment or prevention strategies, there is a critical need to identify predictive biomarkers of paclitaxel-induced PN that can be translated into clinical practice.[31] In this retrospective analysis of a prospectively enrolled observational cohort of women receiving weekly adjuvant paclitaxel for breast cancer, baseline vitamin D deficiency was associated with greater patient-reported PN during treatment, and a corresponding 3-fold increased risk of PN-induced treatment disruption that was not confirmed statistically.
This study suggests vitamin D deficiency as a potential risk factor for paclitaxel induced PN and is consistent with a growing body of evidence that vitamin D may serve as a modifiable biomarker for PN across etiologies. Recent systematic reviews have found lower levels of vitamin D in diabetic patients with PN and that vitamin D deficiency increases the risk of PN secondary to diabetes.[18, 19] Lower serum levels of vitamin D have also been associated with increased PN and neurotoxicity in aging[32] and other disease states including immune-mediated PNs[33] and other autoimmune conditions[34, 35], though the mechanism for this has not been determined. Despite the association of vitamin D deficiency with increased PN across these disparate etiologies, there has been limited work conducted to determine whether vitamin D deficiency is a risk factor for chemotherapy-induced PN. A recent case-control study of patients who did and did not experience PN from paclitaxel found lower pre-treatment vitamin D levels in patients with PN (38.2 nmol/L vs. 25.6 nmol/L, p=0.008).[22] A separate cohort of patients with multiple myeloma treated with bortezomib and/or thalidomide demonstrated increased severity of motor (p=0.042) and sensory PN (p=0.009) in vitamin D deficient patients.[21] The direct association between vitamin D deficiency and PN has never been analyzed in a large prospective clinical trial, to our knowledge. However, a post-hoc analysis of SWOG 0221 (S0221), a phase III clinical trial of early stage breast cancer patients receiving paclitaxel, found decreased risk for PN in patients taking multivitamins (OR=0.78, 95% CI 0.61–1.00).[36] These findings, in concert with the findings of our analysis, present strong justification for confirmatory studies of the association between pre-treatment vitamin D deficiency and PN in large, independent prospectively accrued cohorts of patients receiving paclitaxel and other neurotoxic chemotherapy agents.
A modifiable predictive biomarker of paclitaxel induced PN has the potential to dramatically improve outcomes of patients with breast cancer. PN limits activities of daily life and causes sustained, long-term morbidity for patients.[5, 8] A PN biomarker could be used to identify patients who would benefit from alternative and less neurotoxic regimens, enhanced PN monitoring, or individualized dosing strategies. Deficiency of vitamin D or other nutrients are particularly attractive biomarkers as they are conveniently detected through standard clinical testing and easily corrected with supplementation or nutritional intervention. In diabetes, vitamin D supplementation has been shown to correct vitamin D deficiency and improve PN symptoms.[37, 38] A case of vitamin D supplementation improving PN symptoms in a multiple myeloma patient taking bortezomib has been reported, but the role of vitamin D supplementation in chemotherapy-induced PN has not been widely studied.[23] Many clinical studies have investigated the supplementation of other vitamins and nutraceuticals in preventing and/or treating PN with most studies failing to convincingly demonstrate efficacy of this approach (Vitamins B[39] and E[40], acetyl-l-carnitine[41], glutathione[42], and glutamine[43]). However, these studies tested supplementation of nutrients that have not been validated as risk factors for PN and tested supplementation in patients irrespective of their deficiency status. If lower pre-treatment circulating vitamin D level is confirmed as a PN risk factor, vitamin D supplementation could be explored as an effective precision medicine strategy to mitigate the risk of paclitaxel-induced PN in vitamin D deficient patients, thus reducing PN incidence and improving treatment outcomes. However, it is worth noting that vitamin D supplementation to correct underlying deficiencies and improve outcomes has been explored widely across cancer and other disease states with limited success.[44–47]
Limitations of the present study include a modestly sized cohort of predominately white patients with breast cancer treated at a single center. It is unknown whether these findings can be generalized to patients of other races or genders, those receiving alternative paclitaxel regimens and dosing schemes, and those receiving other neurotoxic chemotherapies. The vitamin D-deficient group in this cohort was comprised entirely of white women. Therefore, our findings are not due to higher risk of PN and vitamin D deficiency in black patients.[48–50] Nonetheless, the relationship between vitamin D deficiency and PN needs to be validated in more heterogeneous cohorts using a clinically meaningful endpoint such as PN-induced treatment disruption or persistent neuropathy to justify clinical translation. The relatively small cohort and substantial missingness in baseline vitamin levels limited the statistical power of the present analysis, particularly for the clinical endpoint of neuropathy-induced treatment disruption. This may have resulted in the non-significance of the finding that vitamin D-deficient patients had an approximate three-fold increase in risk of PN-induced treatment disruption. Finally, while our study did not adjust for multiple comparisons, there was a single a priori defined analysis with 4 independent variables (vitamin D, vitamin B12, folate, and homocysteine), therefore, our primary finding for vitamin D deficiency would have retained significance after multiple comparisons correction (α=0.0125).
Conclusion
The results of this secondary analysis of an observational clinical study demonstrated baseline vitamin D deficiency was associated with increased patient reported PN in patients with breast cancer receiving weekly paclitaxel. The results of this study add to a growing body of evidence supporting vitamin D as a predictive biomarker of paclitaxel-induced PN. These results require validation in large, independent cohorts followed by prospective demonstration that vitamin D supplementation in deficient patients prevents PN, avoids treatment disruption, and improves overall treatment outcomes to warrant translation of this modifiable biomarker into patient care.
Figure 2.
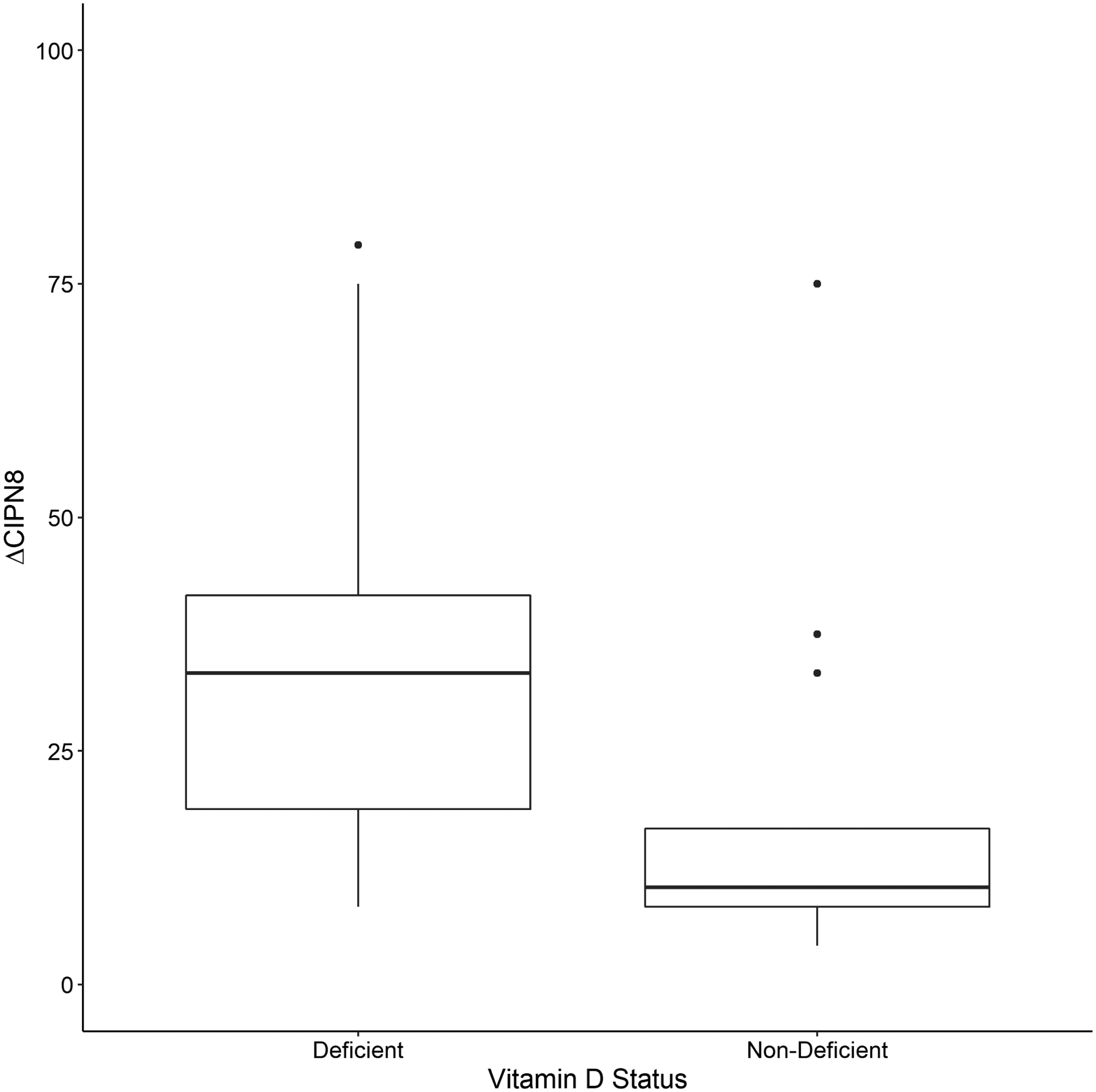
Patient reported PN (ΔCIPN8) stratified by Vitamin D status: deficient (<20 ng/mL, n=15) or non-deficient (≥ 20 ng/mL, n=22). The Y-axis represents the maximum change from baseline in the scaled CIPN8 score, ΔCIPN8, as described in the methods. Patients with baseline vitamin D deficiency experienced greater PN than those who were non-deficient (Vit D− = 36.39 ±22.8 vs. Vit D+ = 16.29 ±16.3, p=0.003).
Acknowledgements:
This work was supported by the National Center for Advancing Translational Sciences under award number KL2TR000434 and 2UL1TR000433 (D. Hertz) and the National Cancer Institutes under Award Number P30CA046592 (K. Kidwell). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. Additional support was provided by the Fashion Footwear Charitable Foundation of New York/QVC Presents Shoes on Sale ™ (D. Hayes).
Footnotes
Conflict of interest: Theodore Jennaro declares that he has no conflict of interest. Fang Fang declares that she has no conflict of interest. Kelley M Kidwell declares that she has no conflict of interest. Ellen M Lavoie Smith declares that she has no conflict of interest. Kiran Vangipuram declares that he has no conflict of interest. Monika Burness declares that she has no conflict of interest. Jennifer Griggs declares that she has no conflict of interest. Catherine Van Poznak has received research funding from Bayer for an unrelated project. Daniel Hayes declares that he has no conflict of interest. N. Lynn Henry is a local PI of a pharma-sponsored trial for Pfizer, Abbvie, and Innocrin Pharmaceuticals that are unrelated to this work. Daniel Hertz received research grants from The Michigan Institute for Clinical Health & Research (MICHR) to conduct this study.
Research involving human participants: All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Informed consent: Informed consent was obtained from all individual participants included in the study.
Data availability: The datasets during and/or analysed during the current study are available from the corresponding author on reasonable request.
This work was presented in part at the 2019 American Society of Clinical Oncology Annual Meeting.
References
- 1.Peto R, Davies C, Godwin J, Gray R, Pan HC, Clarke M, Cutter D, Darby S, McGale P, Taylor C et al. : Comparisons between different polychemotherapy regimens for early breast cancer: meta-analyses of long-term outcome among 100,000 women in 123 randomised trials. Lancet (London, England) 2012, 379(9814):432–444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Seidman AD, Berry D, Cirrincione C, Harris L, Muss H, Marcom PK, Gipson G, Burstein H, Lake D, Shapiro CL et al. : Randomized phase III trial of weekly compared with every-3-weeks paclitaxel for metastatic breast cancer, with trastuzumab for all HER-2 overexpressors and random assignment to trastuzumab or not in HER-2 nonoverexpressors: final results of Cancer and Leukemia Group B protocol 9840. Journal of clinical oncology : official journal of the American Society of Clinical Oncology 2008, 26(10):1642–1649. [DOI] [PubMed] [Google Scholar]
- 3.U.S. Department of Health and Human Services. Common Terminology Criteria for Adverse Events (CTCAE) Version 4.0. Bethesda, MD: NIH; 2010. [Google Scholar]
- 4.Hershman DL, Weimer LH, Wang A, Kranwinkel G, Brafman L, Fuentes D, Awad D, Crew KD: Association between patient reported outcomes and quantitative sensory tests for measuring long-term neurotoxicity in breast cancer survivors treated with adjuvant paclitaxel chemotherapy. Breast Cancer Research and Treatment 2011, 125(3):767–774. [DOI] [PubMed] [Google Scholar]
- 5.Bandos H, Melnikow J, Rivera DR, Swain SM, Sturtz K, Fehrenbacher L, Wade JL 3rd, Brufsky AM, Julian TB, Margolese RG et al. : Long-term Peripheral Neuropathy in Breast Cancer Patients Treated With Adjuvant Chemotherapy: NRG Oncology/NSABP B-30. Journal of the National Cancer Institute 2018, 110(2). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mielke S, Sparreboom A, Mross K: Peripheral neuropathy: a persisting challenge in paclitaxel-based regimes. European journal of cancer (Oxford, England : 1990) 2006, 42(1):24–30. [DOI] [PubMed] [Google Scholar]
- 7.Hershman DL, Lacchetti C, Dworkin RH, Lavoie Smith EM, Bleeker J, Cavaletti G, Chauhan C, Gavin P, Lavino A, Lustberg MB et al. : Prevention and management of chemotherapy-induced peripheral neuropathy in survivors of adult cancers: American Society of Clinical Oncology clinical practice guideline. Journal of clinical oncology : official journal of the American Society of Clinical Oncology 2014, 32(18):1941–1967. [DOI] [PubMed] [Google Scholar]
- 8.Tanabe Y, Hashimoto K, Shimizu C, Hirakawa A, Harano K, Yunokawa M, Yonemori K, Katsumata N, Tamura K, Ando M et al. : Paclitaxel-induced peripheral neuropathy in patients receiving adjuvant chemotherapy for breast cancer. International journal of clinical oncology 2013, 18(1):132–138. [DOI] [PubMed] [Google Scholar]
- 9.Shimozuma K, Ohashi Y, Takeuchi A, Aranishi T, Morita S, Kuroi K, Ohsumi S, Makino H, Katsumata N, Kuranami M et al. : Taxane-induced peripheral neuropathy and health-related quality of life in postoperative breast cancer patients undergoing adjuvant chemotherapy: N-SAS BC 02, a randomized clinical trial. Supportive care in cancer : official journal of the Multinational Association of Supportive Care in Cancer 2012, 20(12):3355–3364. [DOI] [PubMed] [Google Scholar]
- 10.Speck RM, Sammel MD, Farrar JT, Hennessy S, Mao JJ, Stineman MG, DeMichele A: Impact of chemotherapy-induced peripheral neuropathy on treatment delivery in nonmetastatic breast cancer. Journal of oncology practice 2013, 9(5):e234–240. [DOI] [PubMed] [Google Scholar]
- 11.Kim WY, Woo SU, Seo JH, Son GS, Lee JB, Bae JW: Toxicities, dose reduction and delay of docetaxel and paclitaxel chemotherapy in breast cancer without distant metastases. Journal of cancer research and therapeutics 2011, 7(4):412–415. [DOI] [PubMed] [Google Scholar]
- 12.de Morree ES, Vogelzang NJ, Petrylak DP, Budnik N, Wiechno PJ, Sternberg CN, Doner K, Bellmunt J, Burke JM, Ochoa de Olza M et al. : Association of Survival Benefit With Docetaxel in Prostate Cancer and Total Number of Cycles Administered: A Post Hoc Analysis of the Mainsail Study. JAMA oncology 2017, 3(1):68–75. [DOI] [PubMed] [Google Scholar]
- 13.Loibl S, Skacel T, Nekljudova V, Luck HJ, Schwenkglenks M, Brodowicz T, Zielinski C, von Minckwitz G: Evaluating the impact of Relative Total Dose Intensity (RTDI) on patients’ short and long-term outcome in taxane- and anthracycline-based chemotherapy of metastatic breast cancer- a pooled analysis. BMC cancer 2011, 11:131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.England JD, Gronseth GS, Franklin G, Carter GT, Kinsella LJ, Cohen JA, Asbury AK, Szigeti K, Lupski JR, Latov N et al. : Evaluation of distal symmetric polyneuropathy: the role of laboratory and genetic testing (an evidence-based review). Muscle & nerve 2009, 39(1):116–125. [DOI] [PubMed] [Google Scholar]
- 15.Chopra K, Tiwari V: Alcoholic neuropathy: possible mechanisms and future treatment possibilities. Br J Clin Pharmacol 2012, 73(3):348–362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Reynolds EH: The neurology of folic acid deficiency. Handbook of clinical neurology 2014, 120:927–943. [DOI] [PubMed] [Google Scholar]
- 17.Leishear K, Boudreau RM, Studenski SA, Ferrucci L, Rosano C, de Rekeneire N, Houston DK, Kritchevsky SB, Schwartz AV, Vinik AI et al. : Relationship between vitamin B12 and sensory and motor peripheral nerve function in older adults. Journal of the American Geriatrics Society 2012, 60(6):1057–1063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lv WS, Zhao WJ, Gong SL, Fang DD, Wang B, Fu ZJ, Yan SL, Wang YG: Serum 25-hydroxyvitamin D levels and peripheral neuropathy in patients with type 2 diabetes: a systematic review and meta-analysis. Journal of endocrinological investigation 2015, 38(5):513–518. [DOI] [PubMed] [Google Scholar]
- 19.Qu G-B, Wang L-L, Tang X, Wu W, Sun Y-H: The association between vitamin D level and diabetic peripheral neuropathy in patients with type 2 diabetes mellitus: An update systematic review and meta-analysis. J Clin Transl Endocrinol 2017, 9:25–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Armstrong T, Almadrones L, Gilbert MR: Chemotherapy-induced peripheral neuropathy. Oncology nursing forum 2005, 32(2):305–311. [DOI] [PubMed] [Google Scholar]
- 21.Wang J, Udd KA, Vidisheva A, Swift RA, Spektor TM, Bravin E, Ibrahim E, Treisman J, Masri M, Berenson JR: Low serum vitamin D occurs commonly among multiple myeloma patients treated with bortezomib and/or thalidomide and is associated with severe neuropathy. Supportive care in cancer : official journal of the Multinational Association of Supportive Care in Cancer 2016, 24(7):3105–3110. [DOI] [PubMed] [Google Scholar]
- 22.Grim J, Ticha A, Hyspler R, Valis M, Zadak Z: Selected Risk Nutritional Factors for Chemotherapy-Induced Polyneuropathy. Nutrients 2017, 9(6):535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Clement Z, Ashford M, Sivakumaran S: Vitamin D deficiency in a man with multiple myeloma. N Am J Med Sci 2011, 3(10):469–471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Schloss JM, Colosimo M, Airey C, Vitetta L: Chemotherapy-induced peripheral neuropathy (CIPN) and vitamin B12 deficiency. Supportive Care in Cancer 2015, 23(7):1843–1850. [DOI] [PubMed] [Google Scholar]
- 25.Hertz DL, Kidwell KM, Vangipuram K, Li F, Pai MP, Burness M, Griggs JJ, Schott AF, Van Poznak C, Hayes DF et al. : Paclitaxel Plasma Concentration after the First Infusion Predicts Treatment-Limiting Peripheral Neuropathy. Clinical cancer research : an official journal of the American Association for Cancer Research 2018, 24(15):3602–3610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sun Y, Kim JH, Vangipuram K, Hayes DF, Smith EML, Yeomans L, Henry NL, Stringer KA, Hertz DL: Pharmacometabolomics reveals a role for histidine, phenylalanine, and threonine in the development of paclitaxel-induced peripheral neuropathy. Breast Cancer Res Treat 2018, 171(3):657–666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Marcath LA, Kidwell KM, Robinson AC, Vangipuram K, Burness ML, Griggs JJ, Poznak CV, Schott AF, Hayes DF, Henry NL et al. : Patients carrying CYP2C8*3 have shorter systemic paclitaxel exposure. Pharmacogenomics 2018, 20(2):95–104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Postma TJ, Aaronson NK, Heimans JJ, Muller MJ, Hildebrand JG, Delattre JY, Hoang-Xuan K, Lanteri-Minet M, Grant R, Huddart R et al. : The development of an EORTC quality of life questionnaire to assess chemotherapy-induced peripheral neuropathy: the QLQ-CIPN20. European journal of cancer (Oxford, England : 1990) 2005, 41(8):1135–1139. [DOI] [PubMed] [Google Scholar]
- 29.Lavoie Smith EM, Barton DL, Qin R, Steen PD, Aaronson NK, Loprinzi CL: Assessing patient-reported peripheral neuropathy: the reliability and validity of the European Organization for Research and Treatment of Cancer QLQ-CIPN20 Questionnaire. Quality of life research : an international journal of quality of life aspects of treatment, care and rehabilitation 2013, 22(10):2787–2799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kieffer JM, Postma TJ, van de Poll-Franse L, Mols F, Heimans JJ, Cavaletti G, Aaronson NK: Evaluation of the psychometric properties of the EORTC chemotherapy-induced peripheral neuropathy questionnaire (QLQ-CIPN20). Quality of life research : an international journal of quality of life aspects of treatment, care and rehabilitation 2017, 26(11):2999–3010. [DOI] [PubMed] [Google Scholar]
- 31.Travis LB, Fossa SD, Sesso HD, Frisina RD, Herrmann DN, Beard CJ, Feldman DR, Pagliaro LC, Miller RC, Vaughn DJ et al. : Chemotherapy-induced peripheral neurotoxicity and ototoxicity: new paradigms for translational genomics. Journal of the National Cancer Institute 2014, 106(5). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Annweiler C, Schott AM, Berrut G, Chauviré V, Le Gall D, Inzitari M, Beauchet O: Vitamin D and Ageing: Neurological Issues. Neuropsychobiology 2010, 62(3):139–150. [DOI] [PubMed] [Google Scholar]
- 33.Elf K, Askmark H, Nygren I, Punga AR: Vitamin D deficiency in patients with primary immune-mediated peripheral neuropathies. Journal of the neurological sciences 2014, 345(1–2):184–188. [DOI] [PubMed] [Google Scholar]
- 34.Yesil H, Sungur U, Akdeniz S, Gurer G, Yalcin B, Dundar U: Association between serum vitamin D levels and neuropathic pain in rheumatoid arthritis patients: A cross-sectional study. International journal of rheumatic diseases 2018, 21(2):431–439. [DOI] [PubMed] [Google Scholar]
- 35.Agmon-Levin N, Kivity S, Tzioufas AG, Lopez Hoyos M, Rozman B, Efes I, Shapira Y, Shamis A, Amital H, Youinou P et al. : Low levels of vitamin-D are associated with neuropathy and lymphoma among patients with Sjogren’s syndrome. Journal of autoimmunity 2012, 39(3):234–239. [DOI] [PubMed] [Google Scholar]
- 36.Zirpoli GR, McCann SE, Sucheston-Campbell LE, Hershman DL, Ciupak G, Davis W, Unger JM, Moore HCF, Stewart JA, Isaacs C et al. : Supplement Use and Chemotherapy-Induced Peripheral Neuropathy in a Cooperative Group Trial (S0221): The DELCaP Study. Journal of the National Cancer Institute 2017, 109(12). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Shehab D, Al-Jarallah K, Abdella N, Mojiminiyi OA, Al Mohamedy H: Prospective evaluation of the effect of short-term oral vitamin d supplementation on peripheral neuropathy in type 2 diabetes mellitus. Medical principles and practice : international journal of the Kuwait University, Health Science Centre 2015, 24(3):250–256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Alam U, Fawwad A, Shaheen F, Tahir B, Basit A, Malik RA: Improvement in Neuropathy Specific Quality of Life in Patients with Diabetes after Vitamin D Supplementation. Journal of diabetes research 2017, 2017:7928083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Schloss JM, Colosimo M, Airey C, Masci P, Linnane AW, Vitetta L: A randomised, placebo-controlled trial assessing the efficacy of an oral B group vitamin in preventing the development of chemotherapy-induced peripheral neuropathy (CIPN). Support Care Cancer 2017, 25(1):195–204. [DOI] [PubMed] [Google Scholar]
- 40.Kottschade LA, Sloan JA, Mazurczak MA, Johnson DB, Murphy BP, Rowland KM, Smith DA, Berg AR, Stella PJ, Loprinzi CL: The use of vitamin E for the prevention of chemotherapy-induced peripheral neuropathy: results of a randomized phase III clinical trial. Support Care Cancer 2011, 19(11):1769–1777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hershman DL, Unger JM, Crew KD, Minasian LM, Awad D, Moinpour CM, Hansen L, Lew DL, Greenlee H, Fehrenbacher L et al. : Randomized double-blind placebo-controlled trial of acetyl-L-carnitine for the prevention of taxane-induced neuropathy in women undergoing adjuvant breast cancer therapy. J Clin Oncol 2013, 31(20):2627–2633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Cascinu S, Catalano V, Cordella L, Labianca R, Giordani P, Baldelli AM, Beretta GD, Ubiali E, Catalano G: Neuroprotective effect of reduced glutathione on oxaliplatin-based chemotherapy in advanced colorectal cancer: a randomized, double-blind, placebo-controlled trial. J Clin Oncol 2002, 20(16):3478–3483. [DOI] [PubMed] [Google Scholar]
- 43.Loven D, Levavi H, Sabach G, Zart R, Andras M, Fishman A, Karmon Y, Levi T, Dabby R, Gadoth N: Long-term glutamate supplementation failed to protect against peripheral neurotoxicity of paclitaxel. European Journal of Cancer Care 2009, 18(1):78–83. [DOI] [PubMed] [Google Scholar]
- 44.Keum N, Lee DH, Greenwood DC, Manson JE, Giovannucci E: Vitamin D supplementation and total cancer incidence and mortality: a meta-analysis of randomized controlled trials. Annals of oncology : official journal of the European Society for Medical Oncology 2019, 30(5):733–743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Bjelakovic G, Gluud LL, Nikolova D, Whitfield K, Krstic G, Wetterslev J, Gluud C: Vitamin D supplementation for prevention of cancer in adults. The Cochrane database of systematic reviews 2014(6):Cd007469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Bolland MJ, Grey A, Avenell A: Effects of vitamin D supplementation on musculoskeletal health: a systematic review, meta-analysis, and trial sequential analysis. The lancet Diabetes & endocrinology 2018, 6(11):847–858. [DOI] [PubMed] [Google Scholar]
- 47.Barbarawi M, Kheiri B, Zayed Y, Barbarawi O, Dhillon H, Swaid B, Yelangi A, Sundus S, Bachuwa G, Alkotob ML et al. : Vitamin D Supplementation and Cardiovascular Disease Risks in More Than 83000 Individuals in 21 Randomized Clinical Trials: A Meta-analysis. JAMA Cardiol 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Schneider BP, Shen F, Jiang G, O’Neill A, Radovich M, Li L, Gardner L, Lai D, Foroud T, Sparano JA et al. : Impact of Genetic Ancestry on Outcomes in ECOG-ACRIN-E5103. JCO precision oncology 2017, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Hertz DL, Roy S, Motsinger-Reif AA, Drobish A, Clark LS, McLeod HL, Carey LA, Dees EC: CYP2C8*3 increases risk of neuropathy in breast cancer patients treated with paclitaxel. Annals of oncology : official journal of the European Society for Medical Oncology 2013, 24(6):1472–1478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Harris SS: Vitamin D and African Americans. The Journal of nutrition 2006, 136(4):1126–1129. [DOI] [PubMed] [Google Scholar]


