Abstract
Aberrant performance of skilled action has long been noted in schizophrenia and relatedly, recent reports have demonstrated impaired use, performance, and perception of hand gestures in this group. Still, this deficit is not acknowledged as apraxia, which to the broader medical field, characterizes impairments in skilled actions. Understanding the relationship between apraxia and schizophrenia may shed an invaluable new perspective on disease mechanism, and highlight novel treatment opportunities as well. To examine this potential link, we reviewed the evidence for the types of praxis errors, associated psychopathology, and cerebral correlates of the praxis deficit in schizophrenia. Notably, the review indicated that gesture deficits are severe enough to be considered genuine apraxia in a substantial proportion of patients (about 25%). Further, other potential contributors (e.g., hypokinetic motor abnormalities, cognitive impairment) are indeed associated with gesture deficits in schizophrenia, but do not sufficiently explain the abnormality. Finally, patients with praxis deficits have altered brain structure and function including the left parieto-premotor praxis network and these neural correlates are specific to the praxis deficit. Therefore, we argue that the gestural disorder frequently observed in schizophrenia shares both the clinical and neurophysiological features of true apraxia, as in other neuropsychiatric disorders with impaired higher order motor control, such as Parkinson’s disease.
Keywords: praxis deficit, psychosis, motor abnormalities, non-verbal communication
1. Introduction
Schizophrenia is characterized by hallucinations, delusions, disorganized behavior, abnormal psychomotor behavior, impaired cognition, and negative symptoms. Collectively, these symptom domains contribute to problems in social interaction and community functioning. Early descriptions of schizophrenia report abnormal use of tools and inadequate or incomprehensive gestures, all of which are suggestive of apraxia. Therefore, we will review the evidence on disturbed praxis function in schizophrenia to explore, whether the deficit was a form of apraxia or a different clinical issue with similar phenomenology.
1.1. Historical view on praxis deficits in psychosis
Eugen Bleuler described odd behaviors including inappropriate use of objects or inability to perform skilled action, which he attributed to negativism or mannerisms (pages 76, 157, and 161)(Bleuler, 1911). These observations were shared by Emil Kraepelin, who noted disturbances of skilled actions and pointed to post-mortem findings of white matter fiber alterations (pages 60–61, 67)(Kraepelin, 1921). The most comprehensive description of deficits in action planning was written by Karl Kleist, a German psychiatrist and neurologist, who started his residency in the department of Carl Wernicke. In his 1908 book (Kleist, 1908), Kleist introduced the term “psychomotor apraxia” to describe the disturbance of skillful movements in patients with psychosis. Most of his psychosis patients would today qualify for a range of disorders such as schizophrenia, schizoaffective or bipolar disorder. Years before the advent of antipsychotic medications, and accompanying motor side-effects, Kleist first described patients who were unable to light a match, button their shirt, or write simple numbers. Despite similarities in the movements and types of errors with the “transcortical apraxia of Liepmann” and the “innervatory (cortical) apraxia”, Kleist considered psychomotor apraxia a distinct form, because the control of movements was the central impairment (pages 52–60 and 145–153)(Kleist, 1908). He observed that patients’ praxis deficits might change in a different context. He considered disturbances of the fronto-ponto-cerebellar pathways (“…Ursprungsgebiete der absteigenden fronto-ponto-cerebellaren Bahnen…”, page 149) to cause psychomotor apraxia, and he suspected the origin of the pathological process to localize within the frontal cortex.
1.2. Apraxia definition
Starting with Hugo Liepmann in 1900, the concept of apraxia has continuously evolved over the past century (Leiguarda & Marsden, 2000). Leiguarda and Marsden stated that apraxia resembles an impairment of skilled movements of the forelimbs that results from acquired brain disease and is not explained by problems in language comprehension, dementia, or an elementary motor-sensory deficit (Leiguarda & Marsden, 2000). Apraxia is defined by the types of errors made during gesture imitation of a seen movement or pantomime upon verbal command.
A recent definition of limb apraxia is “the inability to perform skilled and/or learned limb actions on request and/or imitation, independently of sensory-motor impairments and cognitive deficits preventing the understanding of the task or the processing of the stimulus material” (Osiurak & Rossetti, 2017). Patients with limb apraxia typically have difficulties using tools appropriately or efficiently. Furthermore, performing meaningful and meaningless gestures are impeded, depending on clinical examination. The question arises whether the definition of apraxia applies to schizophrenia? Schizophrenia is a heterogeneous syndrome that may present with motor disturbances or cognitive dysfunction. We will explore the current state of knowledge on the types of gesture errors, the psychopathology associated with praxis deficits, as well as the neural correlates of impaired gesture performance.
2. Types of errors in schizophrenia
The phenomenology of errors during gesture or tool use would inform on whether deficits in schizophrenia parallel those of patients with apraxia. Our group applied the well-validated test of upper limb apraxia (TULIA)(Vanbellingen et al., 2010) in schizophrenia spectrum disorders, ensuring identical assessment of gesture performance across disorders. In our studies, frequent gesture errors pertained to minor spatial and temporal errors (18%). The minor errors often include hesitant or sloppy, low amplitude performance, errors in timing movements, as well as inaccurate spatial configuration of the gestures. In a similar frequency (17.5%) severe errors occurred such as body-part-as-object errors, omissions, and errors in spatial orientation (Walther, Alexaki, Stegmayer, Vanbellingen, & Bohlhalter, 2020; Walther, Vanbellingen, Muri, Strik, & Bohlhalter, 2013b). Rarely did we notice incomprehensible movements or no movements at all. Figure 1 depicts the distribution of errors across subjects in a combined sample of 98 patients and 84 healthy controls, all right-handed, who performed the TULIA with their dominant right hand. As can be seen, following demonstration (imitation domain) patients show spatial and temporal errors in 30% and content errors in 12% of the items. Similarly, following verbal instructions (pantomime domain) patients present spatial and temporal errors in 30% and content errors in 21%. The frequency of errors in controls is much lower.
Figure 1.
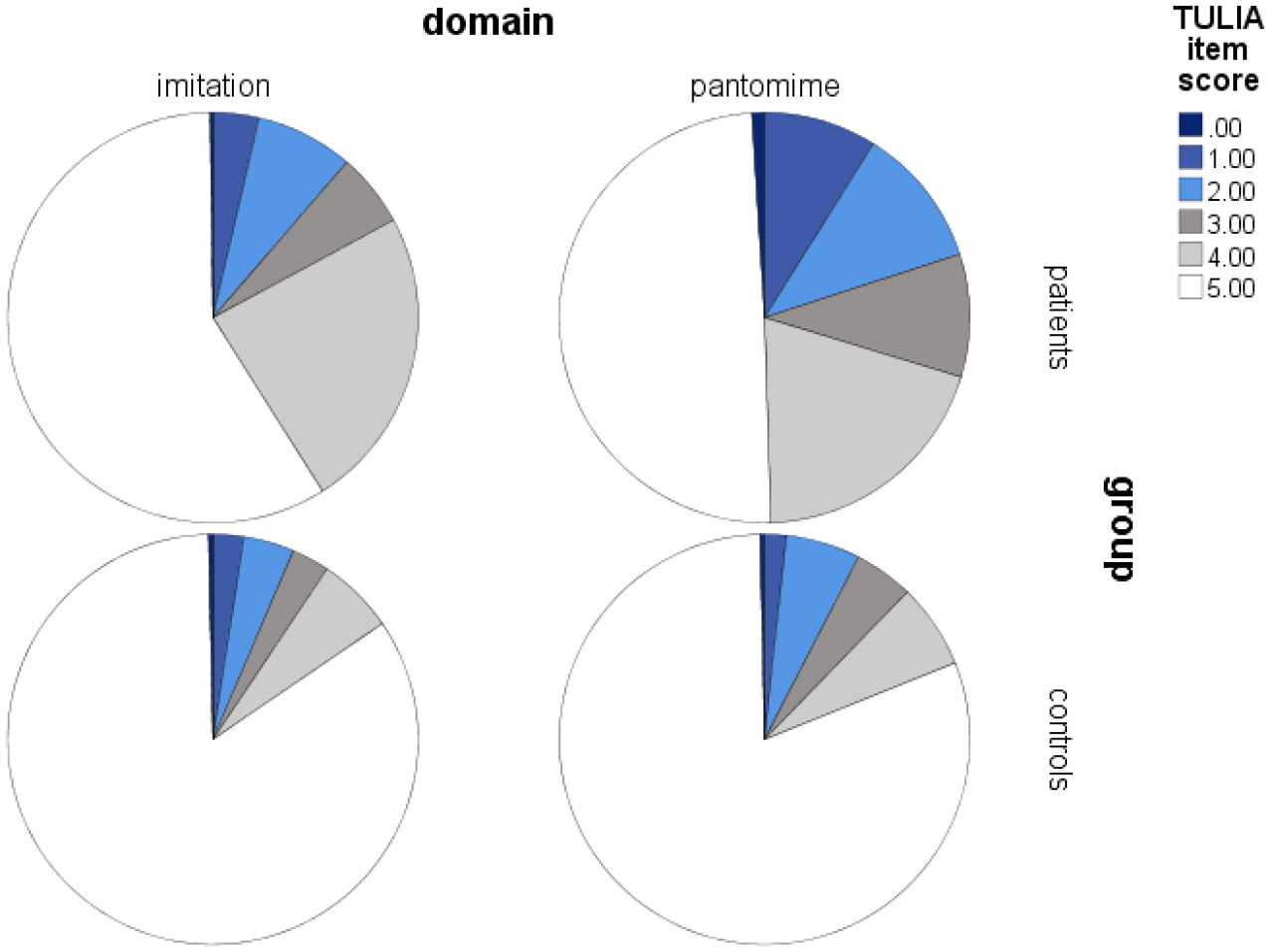
Frequency distribution of gesture performance errors in a combined sample of 98 schizophrenia patients and 84 controls.
Note that a score of 5 means correct performance, scores of 4 and 3 (grey fills) indicate minor spatial and temporal errors, while scores of 1 and 2 (blue fills) indicate major spatial or content errors, and lack of visible movement is rated 0. (Vanbellingen et al., 2010).
Praxis function, i.e. the use of gestures or skilled manual movements, has rarely been studied before in schizophrenia yielding conflicting results. One study noted coarse gestures, slowed and with low amplitudes, in a group of 21 patients with chronic schizophrenia, of whom 71% presented body-part-as-object errors when performing pantomimes (Martin, Tewesmeier, Albers, Schmid, & Scharfetter, 1994). While body-part-as-object errors constitute important deficits in apraxia (Leiguarda & Marsden, 2000; Raymer, Maher, Foundas, Heilman, & Rothi, 1997), authors stated that they were unable to find clear-cut apraxia in their schizophrenia patients according to the test of Kertesz and Ferro (Kertesz & Ferro, 1984). Thus, the interpretation of the finding is ambiguous: schizophrenia patients present errors suggestive of apraxia, but perform above the cut-off in an apraxia test (Martin et al., 1994). Along this line, an examination of apraxia in Parkinson’s syndromes included 10 chronic schizophrenia patients with neuroleptic induced parkinsonism (Leiguarda et al., 1997). Authors also noted that chronic schizophrenia patients had no signs of ideomotor apraxia. However, their evaluation disregarded content errors and unrecognizable movements as well as delayed initiation, timing or low amplitudes. In contrast, another report found ideo-kinetic apraxia in the majority of ten chronic schizophrenia patients, suggesting that patients have problems to use acquired motor knowledge (Portnoff, Golden, Snyder, & Gustavson, 1982). Likewise, two studies reported more spatial errors in schizophrenia compared to controls when imitating meaningless finger gestures (Matthews, Gold, Sekuler, & Park, 2013; Park, Matthews, & Gibson, 2008), however, they did not categorize subjects according to a performance cut-off.
We investigated gesturing in schizophrenia with the TULIA and demonstrated poor gesture performance in three different patient samples (Walther, Kunz, et al., 2020; Walther et al., 2015; Walther et al., 2013b). In all instances, the imitation performance of hand gestures following demonstration is less frequently impaired than the performance of gestures on verbal command, with 33% vs. 50–66% of the patients presenting deficits (Walther et al., 2015; Walther, Vanbellingen, Muri, Strik, & Bohlhalter, 2013a). Even when we apply the original apraxia cut-off of the TULIA, a substantial proportion (27%) of schizophrenia patients qualify for a praxis deficit (Walther, Alexaki, et al., 2020)(see also Table 1). Moreover, the gesture deficit did not occur in isolation. Instead, poor gesture performance in the TULIA was strongly correlated with impaired postural knowledge (r = .76) or poor tool use performance (r = .78), arguing for a generalized praxis deficit in schizophrenia (Walther et al., 2015). Finally, praxis deficits occurred in a proportion of first episode patients (21%), who performed significantly better than chronic patients in the TULIA (Stegmayer, Moor, et al., 2016).
Table 1.
Rates of praxis deficits in schizophrenia across samples.
| Original TULIA cut-off 194 for apraxia (Vanbellingen et al. 2010) | Cut-off for gesture deficits in younger adults 210 (Walther et al. 2013b) | |
|---|---|---|
| Walther et al. 2013a/b, n = 30 | 40% | 67% |
| Walther et al. 2015, n = 46 | 22% | 46% |
| Walther et al. 2020, n = 20 | 70% | 100% |
Note that Walther et al. 2020 selected patients for a clinical trial for having at least some signs of praxis deficit.
In sum, deficits in praxis function in schizophrenia have been detected in most of the studies that applied specific clinical tests. And the reported frequency of deficits varies as a function of the instruments used for assessments. In addition, video-analysis has enabled to investigate spontaneous gesture behavior in schizophrenia in common situations. For example, less frequent use of hand gestures has been observed during typical conversations in schizophrenia (Lavelle, Healey, & McCabe, 2013). Likewise, reduced gesturing during interactions with the psychiatrists indicates more symptoms (Lavelle, Dimic, Wildgrube, McCabe, & Priebe, 2015). Similarly, reduced gesture use or the use of incorrect mismatch gestures has been noted in subjects at risk for psychosis, who were videotaped during clinical interviews (Millman et al., 2014; Mittal et al., 2006). Thus, aberrant gesturing in schizophrenia is not a mere laboratory observation, but relevant for nonverbal communication.
How did this praxis deficit go unnoticed despite the vivid research on schizophrenia? First, for decades motor behavior has been largely neglected in schizophrenia research when the field preferably explored cognition or self-reported symptoms, instead of clinical signs (Kendler, 2016; Mittal & Walther, 2018; Strik, Stegmayer, Walther, & Dierks, 2017; Walther & Mittal, 2017). Second, lack of expressive gestures is a key feature of the negative syndrome in schizophrenia and is included in modern rating scales on negative symptoms (Andreasen, 1989; Forbes et al., 2010; Kirkpatrick et al., 2011). Thus, implicitly clinicians may have subsumed abnormal hand gestures as a component of the negative syndrome without further exploring the nature of gesture errors.
Finally, gesture perception and interpretation is also compromised in schizophrenia (Bucci, Startup, Wynn, Baker, & Lewin, 2008; Nagels, Kircher, Grosvald, Steines, & Straube, 2018; Walther et al., 2015; White, Borgan, Ralley, & Shergill, 2016). And in some instances, this perceptual deficit is linked to motor abnormalities and positive symptoms (Walther et al., 2015).
Are the gesture errors specific to schizophrenia or could this deficit occur in a range of psychiatric disorders, particularly those with neurodevelopmental origin? Very few studies explored gesture abnormalities in other psychiatric conditions. In patients with autism errors during the imitation of meaningless gestures include spatial orientation and the context of the environment, while gesture recognition was preserved in some studies (Fourie, Palser, Pokorny, Neff, & Rivera, 2019; Stieglitz Ham, Corley, Rajendran, Carletta, & Swanson, 2008). In patients with depression, less gesture use was observed, but a distinct analysis of hand gesture errors is missing (Annen, Roser, & Brune, 2012; Fiquer et al., 2018). Finally, in patients with obsessive compulsive disorder performed poorly in a test of meaningless gesture imitation (Rounis, Banca, & Voon, 2016). This set of single findings suggests that gesture deficits may occur in other psychiatric disorders, however, the pattern of impairment is quite distinct from the one observed in schizophrenia. Therefore, the current state of knowledge supports the view of a specific gesture deficit in schizophrenia and ties into a broader line of research exploring aberrant motor function as a transdiagnostic phenomenon (Bernard & Mittal, 2015; Mittal, Bernard, & Northoff, 2017; Walther, Bernard, Mittal, & Shankman, 2019).
3. Clinical correlates of gesture impairments
Gesture deficits are expected to occur more frequently in patients with a severe course of the illness. Still, impaired gesture use may also occur in less severe cases. For example, incongruent gesturing to the speech content has been noted in subjects at risk for psychosis before the actual onset of the disorder (Millman et al., 2014). Likewise, although we found gestures to be more severely impaired in chronic patients (Stegmayer, Moor, et al., 2016), some first episode patients clearly performed less accurate than healthy controls. Both age and illness chronicity are associated with more impaired gesture performance (Walther et al., 2015; Walther et al., 2013b). Furthermore, praxis deficits are strong predictors of poor outcome including impaired social and occupational functioning and poor functional capacity (Walther et al., 2016). Besides these general implications of illness severity, gesture deficits in schizophrenia are linked to specific domains of psychopathology.
Negative symptoms, particularly the domain of diminished expression, are expected to be linked to poor gesture performance. Domains such as blunted affect describe the decrease of expression of emotion and reactivity to events (Galderisi, Mucci, Buchanan, & Arango, 2018). When broad measures of negative symptoms were applied, a moderate association of poor gesturing with more severe negative symptoms is reported (Matthews et al., 2013; Millman et al., 2014; Park et al., 2008; Troisi, Spalletta, & Pasini, 1998; Walther, Alexaki, et al., 2020; Walther et al., 2016; Walther et al., 2013a). However, when more sophisticated measures of negative symptoms were applied, the associations were less specific, challenging a critical contribution of negative symptoms on poor gesture use (Walther et al., 2015). On the contrary, gesture performance predicted a subsequent decrease of negative symptom severity in the next six months (Walther et al., 2016). Thus, while gesture performance has modest association with current negative symptoms, it is still predictive of poor outcome. In the future, it will be important to test whether treatment changes in either negative symptoms or nonverbal skills does ameliorate the severity of gesture impairments or negative symptoms.
Positive symptoms, such as delusions, hallucinations, and agitation seem to be linked to gesture deficits. In fact, poor gesture performance was correlated with more severe positive symptoms across studies (Mittal et al., 2006; Walther et al., 2015; Walther et al., 2013b). Similarly, disorganization of thought and behavior has been linked to impaired gesture performance, although this association only appeared in a combined sample of 79 patients, indicating a small effect (Walther, Alexaki, et al., 2020).
Furthermore, cognitive impairment is a potential contributor to praxis deficits in schizophrenia. Most domains of cognitive function can be compromised in schizophrenia, particularly executive function, processing speed, working memory, episodic memory, verbal fluency, and attention (Schaefer, Giangrande, Weinberger, & Dickinson, 2013). Impaired gesture performance in schizophrenia has been repeatedly found to be associated with defective frontal lobe function, i.e. executive function, and impaired working memory (Matthews et al., 2013; Walther et al., 2015; Walther et al., 2013a, 2013b). However, this link is not exclusively driving the gesture deficit in schizophrenia. In our comprehensive study on nonverbal skills, performance in gesture tasks remained strongly impaired even when controlling for nonverbal intelligence and working memory deficits (Walther et al., 2015). Finally, motor abnormalities could hamper skillful action such as hand gestures. Indeed, in our studies, we found catatonia and parkinsonism to correlate with poor gesture performance in all samples, whereas dyskinesia was associated with gesture performance only in one study (Walther et al., 2015; Walther et al., 2013a, 2013b). Motor abnormalities were also associated with poor tool use and poor gesture perception (Walther et al., 2015). Likewise, infrequent use of beat gestures in subjects at risk for psychosis were correlated with abnormal postural control (Osborne et al., 2017). Thus, hypokinetic motor abnormalities are linked to the severity of gesture impairments in schizophrenia across studies. However, these motor abnormalities only partially account for the praxis deficit observed in schizophrenia.
Motor abnormalities in schizophrenia have long been merely viewed as consequences of antipsychotic medication, however, motor abnormalities were already reported in the pre-neuroleptic era and are frequently found in medication naïve first-episode patients (Pappa & Dazzan, 2009; Walther & Mittal, 2017; Walther & Strik, 2012; Whitty, Owoeye, & Waddington, 2009). Similarly, praxis deficits have been reported by authors prior to the introduction of antipsychotics in the 1950s (see 1.1.). While most of the patients currently are receiving such drugs, all reports on gesture in schizophrenia failed to find an association between the current antipsychotic dosage and gesture performance (Matthews et al., 2013; Stegmayer, Moor, et al., 2016; Walther et al., 2015; Walther et al., 2013b). Along with findings in subjects at risk for psychosis and first episode psychosis (Millman et al., 2014; Mittal et al., 2006; Osborne, Vargas, & Mittal, 2020; Stegmayer, Moor, et al., 2016; Walker, Savoie, & Davis, 1994), these results suggest that praxis deficits in schizophrenia may occur in the absence of antipsychotic medication and are not related to the current dosage of antipsychotics. Furthermore, in all our studies the statistical analyses were controlled for chlorpromazine equivalents (including age and disease duration). Therefore, antipsychotic medication is unlikely to account for the gestural deficits. Since gesture performance is a left-lateralized higher order motor function, hemispheric laterality could contribute to poor gesture in schizophrenia. Indeed, schizophrenia has been shown to have reduced hemispheric laterality, which is for example linked to language abnormalities (Berretz, Wolf, Gunturkun, & Ocklenburg, 2020; Wiberg et al., 2019). We focused on right-handed subjects exclusively and controlled the analyses for handedness (Walther et al., 2015; Walther et al., 2013). The laterality index in these right-handed subjects was not associated with gesture behavior. Therefore, in line with other brain disorders, handedness cannot explain gestural deficits in schizophrenia.
In sum, praxis deficits in schizophrenia are found more often in severely ill, chronic patients, who have more positive and negative symptoms. Furthermore, although cognitive impairment and abnormal psychomotor behavior is associated with gesture deficits in schizophrenia, they only partially explain impaired gestural skills. However, the associations have to be considered in the design of treatment trials, in that aiming at improving gesture may involve ameliorating cognitive impairment or hypokinetic motor abnormalities.
4. Neural correlates
Structural brain abnormalities in subjects with gesture impairments are localized within the praxis network (see figure 2). Grey matter volume is reduced in patients with gesture deficits compared to healthy subjects predominantly in the left inferior frontal gyrus (IFG), left superior parietal lobe, left middle temporal cortex, but also in the right insula, right temporal and parietal cortex (Stegmayer, Bohlhalter, et al., 2016). Interestingly, patients with gesture deficits had lower grey matter volumes than patients without gesture deficits in the right insula, right inferior parietal lobe (IPL), and the right superior temporal gyrus. In line with these findings, we also detected reduced cortical thickness within the entire praxis network in schizophrenia patients with gesture deficits (Viher et al., 2018). These findings suggest that in schizophrenia, gesture impairments are linked to grey matter structural alterations in bilateral components of the praxis network including both routes of action planning (Binkofski & Buxbaum, 2013). Indeed, altered brain structure in the praxis network is not generally found in schizophrenia, but specific to those patients who present praxis deficits. In addition to the categorical findings, there is also evidence for a dimensional association of brain structure and gesture performance. Indeed, a graph-theory approach demonstrated that the global efficiency of the praxis network and the local efficiency of network components contribute to gesture performance in healthy subjects and schizophrenia patients (Viher et al., in press). Moreover, the tract integrity of the superior longitudinal and the arcuate fascicles was linked to gesture performance. Interestingly, the structural abnormalities were predominantly found in the left anterior fronto-temporal components of the praxis network. These brain areas have been suggested to be specifically relevant for the communicative content of gestures, as demonstrated in several lesion mapping studies (Finkel, Hogrefe, Frey, Goldenberg, & Randerath, 2018; Goldenberg & Randerath, 2015; Mengotti et al., 2013; Pizzamiglio et al. 2019, Weiss et al., 2016). Thus, structural brain abnormalities in schizophrenia may be particularly affecting communicative gesture use.
Figure 2.
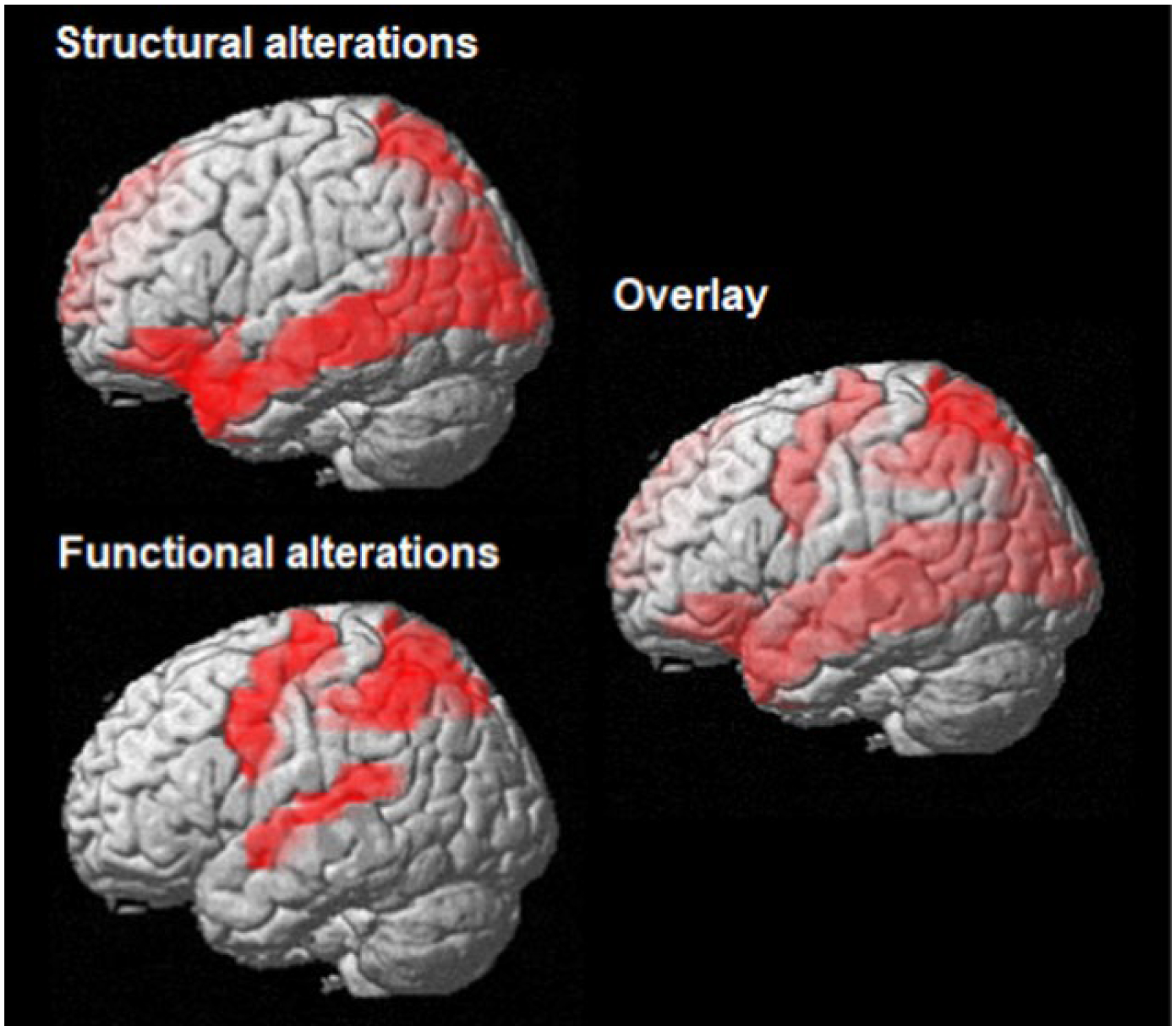
Cerebral alterations in schizophrenia patients with praxis deficits.
Top left: brain areas with reduced grey matter volume in schizophrenia patients with praxis deficits, modified from (Stegmayer, Bohlhalter, et al., 2016). Bottom left: brain areas with altered resting state functional connectivity in schizophrenia patients with praxis deficits, modified from (Wuthrich et al., 2020). Right side: overlay of brain areas with structural and functional alterations.
In order to study the neural processes involved in planning gestures on verbal command in schizophrenia, we adopted a paradigm previously tested in healthy subjects (Bohlhalter et al., 2009). Participants were instructed with visual verbal cues to plan and later perform distal arm gestures during functional MRI. Performance was video-taped. We found that schizophrenia patients engaged the praxis network including left IFG, IPL, supplementary motor area (SMA) and superior temporal gyrus (STG) less than healthy controls when planning familiar and novel hand gestures (Stegmayer et al., 2018). In addition, patients had reduced neural activation during the execution of familiar gestures in the SMA, but neural activity did not differ from controls when performing novel hand gestures. Finally, the actual performance of the gestures was linked to the neural activity within the left IPL during gesture planning in patients, with lower neural activity predicting less accurate performance. Thus, inappropriate activation of the praxis network, particularly the left IPL, during gesture planning is associated with poor gesture performance in schizophrenia.
In addition, blood oxygenation level dependent (BOLD) resting state functional connectivity within the praxis network also differed between schizophrenia patients and healthy subjects (Wuthrich et al., 2020). Patients had increased functional connectivity at rest between bilateral precentral gyrus and bilateral IPL and superior parietal lobes, while they had lower connectivity than controls between bilateral STG. Moreover, the functional connectivity at rest was linked to gesture performance outside the scanner, with lower IPL-precentral gyrus resting state connectivity predicting more accurate gesture performance.
These findings are corroborated by fMRI tasks testing finger gestures in schizophrenia. Thakkar and colleagues demonstrated reduced left IPL activation during the imitation of finger gestures in schizophrenia patients and reduced right IPL activation during action observation (Thakkar, Peterman, & Park, 2014). The only negative study most likely had too few stimulus repetitions to demonstrate a group difference in brain activation during gesture execution or observation between schizophrenia patients and controls (Horan et al., 2014).
Taken together, the neuroimaging findings summarized above suggest that the neural basis of praxis deficits in schizophrenia is similar to patients with limb apraxia following brain lesions (Lesourd et al., 2018; Sperber, Wiesen, Goldenberg, & Karnath, 2019; Weiss et al., 2016). Both structural abnormalities and aberrant brain function map on the praxis network, particularly the left hemispheric insula, IFG, and IPL.
Along this line, the use of noninvasive brain stimulation techniques, such as repetitive transcranial magnetic stimulation (rTMS) has supported the neuroimaging findings. Indeed, transcranial modulation of the praxis network alters gesture performance. In healthy subjects, imitation performance in the TULIA was improved with inhibitory stimulation of the right inferior parietal lobe (IPL) with continuous theta burst stimulation (cTBS) (Vanbellingen et al., 2020). Notably, this behavioral improvement was most evident in subjects with higher indices of structural connectivity in transcallosal white matter fibers. Thus, the improvement of gesture performance was suggested to stem from disinhibition of left IPL by diminishing the influence of interhemispheric rivalry. In line with this finding, a randomized, cross-over trial of rTMS in schizophrenia demonstrated an 7% increase in gesture performance following a single administration of cTBS over the right IPL, while facilitatory intermittent theta burst stimulation over left IFG failed to enhance gesture performance more than placebo stimulation (Walther, Kunz, et al., 2020). This parallel effect argues for interhemispheric rivalry also hampering gesture planning in schizophrenia, even though this would have to be tested in combined neurostimulation and neuroimaging studies. Given, that the positive cTBS effect over the right IPL could be sustained with repeated administration, cTBS might become a novel treatment option for praxis deficits in schizophrenia.
5. Apraxia or apraxia-like deficit?
As stated in the introduction, it remains unclear whether the deficits in skilled movements observed in schizophrenia patients are truly due to limb apraxia or whether these impairments resemble a group of apraxia-like deficits. We have summarized the evidence on the types of gesture and broader praxis errors, both of which are similar to those of patients with apraxia following brain lesions. A considerable proportion of schizophrenia patients scored below the cut-off of apraxia in the TULIA (see table 1). While our analyses support the notion of apraxia in schizophrenia as stated by Kleist, we have no means of distinguishing apraxia following brain lesions from severe praxis deficits in schizophrenia. Only for a proportion of schizophrenia patients did the severity of gesture errors remain lower than that observed in apraxia following stroke. Thus, our findings argue against a specific type of apraxia in schizophrenia, e.g. Kleist’s psychomotor apraxia.
Interestingly, schizophrenia is associated with another higher order motor control deficit, the purposeful, internally guided eye movements that are considered an endophenotype for the disorder (Thaker, 2008). Particularly, the compromised sensorimotor control of smooth pursuit eye movements is related to the familial risk of psychotic disorders (Lencer et al., 2015). Furthermore, schizophrenia is conceptualized as a neurodevelopmental disorder and aberrant motor development is linked to the risk of schizophrenia (Rosso et al., 2000). Likewise, altered communication skills at age 4–5 predicts adolescent social functioning in subjects at clinical high risk for psychosis (Osborne et al., 2020). More broadly, there is growing interest in studying motor signs across development (Mittal & Wakschlag, 2017). Given that movement skills are acquired during childhood neuromotor maturation, some of the gesture deficits seen in patients may have arisen in childhood. However, this link between motor development and adult gesture behavior in psychosis requires further study.
Certainly, schizophrenia patients lack circumscribed brain lesions. However, in a wide range of cortical and subcortical brain areas, structural alterations are common in schizophrenia. Remarkably, our own data demonstrated structural alterations in the praxis network in schizophrenia patients with gesture deficits, a finding that was not observed in the patients without gesture deficits. Finally, typical domains of psychopathology in schizophrenia correlate with gesture impairments, particularly hypokinetic motor abnormalities and cognitive impairment. Still, praxis deficits in schizophrenia also occur in the absence of relevant motor abnormalities or cognitive impairment. Thus, in absence of a brain lesion, roughly 25% of schizophrenia patients present a gesture deficit mimicking apraxia, that is associated with but not explained by hypokinetic motor abnormalities and cognitive impairment. Furthermore, about half of the patients with schizophrenia demonstrate a substantial gesture deficit without qualifying for apraxia according to cut-off scores. If apraxia requires a brain lesion, the deficit in schizophrenia would be one mimicking apraxia. If apraxia is conceptualized as a higher order motor disorder that may present with a variety of neuropsychiatric conditions, we could call the schizophrenia praxis deficit apraxia.
Similar to Parkinson’s disease, a substantial proportion of patients with schizophrenia has these higher order motor control deficits that feed into apraxia. Patients with Parkinson’s disease may present with apraxia, which is related to clinical stage and bradykinesia, but independent of dopaminergic medication (Leiguarda et al., 1997; Vanbellingen, Kersten, Bellion, et al., 2011). We would argue that as in Parkinson’s disease, the praxis deficit in schizophrenia may be termed apraxia. This is justified as the patients produce similar visual-spatial and content errors that are not explained by other neurocognitive or psychiatric deficits.
6. Schizophrenia as a model for apraxia
We have argued previously that schizophrenia might aid research in apraxia as a model disorder (Stegmayer, Bohlhalter, et al., 2016). In fact, schizophrenia patients present with behavioral deficits that mimic apraxia. Concurrently, schizophrenia is associated with aberrant brain structure in many brain regions, including the praxis network. This holds particularly true for grey matter of fronto-temporal cortices and white matter fiber tracts such as the uncinated fascicle, the superior longitudinal fascicle, and the external capsule (Bora et al., 2011; Cetin-Karayumak et al., 2019; Holleran et al., 2014; van Erp et al., 2018). In schizophrenia, multiple neuroimaging techniques can be readily applied allowing for precise localization of neural dysfunction. In contrast, patients with apraxia have varying multiple brain lesions associated with similar behavioral deficits (Binder et al., 2017; Dressing et al., 2018; Lesourd et al., 2018; Sperber et al., 2019), while healthy subjects can be scanned with modern MRI techniques but lack the behavioral abnormalities (Vry et al., 2015). Thus, schizophrenia may be an interesting model to study apraxia.
7. Outlook
Even though the praxis deficit in schizophrenia has been acknowledged by classical psychiatry literature, there was little progress until recently to unravel this deficit. We have shown that praxis deficits are important predictors of functional outcome (Walther et al., 2016). Furthermore, rTMS may alleviate the deficit in schizophrenia. Next, studies need to determine whether continued administration of rTMS or the combination with cognitive remediation would have beneficial effects in schizophrenia. In addition, we need in-depth analyses on structural and functional cerebral alterations associated with praxis deficits in schizophrenia. Moreover, the development of the deficit requires more attention. Are praxis deficits observable in the premorbid stage of psychosis during childhood, when no symptoms are to be noticed yet? Furthermore, the impact of cumulative antipsychotic exposure on gesture deficits needs to be explored. Finally, the development of bed-side tests may aid the detection of praxis deficits in clinical routine. A condensed version of the TULIA is available as short bed-side test of apraxia and has been tested in stroke, Parkinson’s, Alzheimer’s, and multiple sclerosis (Kamm et al., 2012; Ozkan, Adapinar, Elmaci, & Arslantas, 2013; Vanbellingen, Kersten, Bellion, et al., 2011; Vanbellingen, Kersten, Van de Winckel, et al., 2011; Vanbellingen et al., 2012). It might be worthwhile to evaluate its use in schizophrenia as well. In sum, gesture deficits and apraxia should be acknowledged in schizophrenia. The next endeavors are implementing valid and simple bed-side tests and probing effective treatments, e.g. training, psychotherapy, and non-invasive brain stimulation.
Funding
This work was supported by the Swiss National Science Foundation (grant #184717 to Dr. Walther) and the National Institute of Mental Health (R01 MH 118741 to Drs. Mittal and Walther, and R21 MH 119677, R21 MH115231 to Dr. Mittal). The funding sources had no influence on the content of the text.
References
- Andreasen NC (1989). The Scale for the Assessment of Negative Symptoms (SANS): conceptual and theoretical foundations. Br J Psychiatry Suppl(7), 49–58. Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/2695141 [PubMed] [Google Scholar]
- Annen S, Roser P, & Brune M (2012). Nonverbal behavior during clinical interviews: similarities and dissimilarities among schizophrenia, mania, and depression. J Nerv Ment Dis, 200(1), 26–32. doi: 10.1097/NMD.0b013e31823e653b [DOI] [PubMed] [Google Scholar]
- Bernard JA, & Mittal VA (2015). Updating the research domain criteria: the utility of a motor dimension. Psychol Med, 45(13), 2685–2689. doi: 10.1017/S0033291715000872 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berretz G, Wolf OT, Gunturkun O, & Ocklenburg S (2020). Atypical lateralization in neurodevelopmental and psychiatric disorders: What is the role of stress? Cortex, 125, 215–232. doi: 10.1016/j.cortex.2019.12.019 [DOI] [PubMed] [Google Scholar]
- Binder E, Dovern A, Hesse MD, Ebke M, Karbe H, Saliger J, … Weiss PH (2017). Lesion evidence for a human mirror neuron system. Cortex, 90, 125–137. doi: 10.1016/j.cortex.2017.02.008 [DOI] [PubMed] [Google Scholar]
- Binkofski F, & Buxbaum LJ (2013). Two action systems in the human brain. Brain Lang, 127(2), 222–229. doi: 10.1016/j.bandl.2012.07.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bleuler E (1911). Dementia praecox oder Gruppe der Schizophrenien. Leipzig and Wien: Franz Deuticke. [Google Scholar]
- Bohlhalter S, Hattori N, Wheaton L, Fridman E, Shamim EA, Garraux G, & Hallett M (2009). Gesture subtype-dependent left lateralization of praxis planning: an event-related fMRI study. Cereb Cortex, 19(6), 1256–1262. doi: 10.1093/cercor/bhn168 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bora E, Fornito A, Radua J, Walterfang M, Seal M, Wood SJ, … Pantelis C (2011). Neuroanatomical abnormalities in schizophrenia: a multimodal voxelwise meta-analysis and meta-regression analysis. Schizophr Res, 127(1–3), 46–57. doi:S0920–9964(11)00011–9 [pii] 10.1016/j.schres.2010.12.020 [DOI] [PubMed] [Google Scholar]
- Bucci S, Startup M, Wynn P, Baker A, & Lewin TJ (2008). Referential delusions of communication and interpretations of gestures. Psychiatry Res, 158(1), 27–34. doi: 10.1016/j.psychres.2007.07.004 [DOI] [PubMed] [Google Scholar]
- Cetin-Karayumak S, Di Biase MA, Chunga N, Reid B, Somes N, Lyall AE, … Kubicki M (2019). White matter abnormalities across the lifespan of schizophrenia: a harmonized multi-site diffusion MRI study. Mol Psychiatry. doi: 10.1038/s41380-019-0509-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dressing A, Nitschke K, Kummerer D, Bormann T, Beume L, Schmidt CSM, … Martin M (2018). Distinct Contributions of Dorsal and Ventral Streams to Imitation of Tool-Use and Communicative Gestures. Cereb Cortex, 28(2), 474–492. doi: 10.1093/cercor/bhw383 [DOI] [PubMed] [Google Scholar]
- Finkel L, Hogrefe K, Frey SH, Goldenberg G, & Randerath J (2018). It takes two to pantomime: Communication meets motor cognition. Neuroimage Clin, 19, 1008–1017. doi: 10.1016/j.nicl.2018.06.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fiquer JT, Moreno RA, Brunoni AR, Barros VB, Fernandes F, & Gorenstein C (2018). What is the nonverbal communication of depression? Assessing expressive differences between depressive patients and healthy volunteers during clinical interviews. J Affect Disord, 238, 636–644. doi: 10.1016/j.jad.2018.05.071 [DOI] [PubMed] [Google Scholar]
- Forbes C, Blanchard JJ, Bennett M, Horan WP, Kring A, & Gur R (2010). Initial development and preliminary validation of a new negative symptom measure: the Clinical Assessment Interview for Negative Symptoms (CAINS). Schizophr Res, 124(1–3), 36–42. doi: 10.1016/j.schres.2010.08.039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fourie E, Palser ER, Pokorny JJ, Neff M, & Rivera SM (2019). Neural Processing and Production of Gesture in Children and Adolescents With Autism Spectrum Disorder. Front Psychol, 10, 3045. doi: 10.3389/fpsyg.2019.03045 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galderisi S, Mucci A, Buchanan RW, & Arango C (2018). Negative symptoms of schizophrenia: new developments and unanswered research questions. Lancet Psychiatry, 5(8), 664–677. doi: 10.1016/S2215-0366(18)30050-6 [DOI] [PubMed] [Google Scholar]
- Goldenberg G, & Randerath J (2015). Shared neural substrates of apraxia and aphasia. Neuropsychologia, 75, 40–49. doi: 10.1016/j.neuropsychologia.2015.05.017 [DOI] [PubMed] [Google Scholar]
- Holleran L, Ahmed M, Anderson-Schmidt H, McFarland J, Emsell L, Leemans A, … Cannon DM (2014). Altered interhemispheric and temporal lobe white matter microstructural organization in severe chronic schizophrenia. Neuropsychopharmacology, 39(4), 944–954. doi: 10.1038/npp.2013.294 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horan WP, Iacoboni M, Cross KA, Korb A, Lee J, Nori P, … Green MF (2014). Self-reported empathy and neural activity during action imitation and observation in schizophrenia. Neuroimage Clin, 5, 100–108. doi: 10.1016/j.nicl.2014.06.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamm CP, Heldner MR, Vanbellingen T, Mattle HP, Muri R, & Bohlhalter S (2012). Limb apraxia in multiple sclerosis: prevalence and impact on manual dexterity and activities of daily living. Arch Phys Med Rehabil, 93(6), 1081–1085. doi: 10.1016/j.apmr.2012.01.008 [DOI] [PubMed] [Google Scholar]
- Kendler KS (2016). Phenomenology of Schizophrenia and the Representativeness of Modern Diagnostic Criteria. JAMA Psychiatry, 73(10), 1082–1092. doi: 10.1001/jamapsychiatry.2016.1976 [DOI] [PubMed] [Google Scholar]
- Kertesz A, & Ferro JM (1984). Lesion size and location in ideomotor apraxia. Brain, 107 (Pt 3), 921–933. doi: 10.1093/brain/107.3.921 [DOI] [PubMed] [Google Scholar]
- Kirkpatrick B, Strauss GP, Nguyen L, Fischer BA, Daniel DG, Cienfuegos A, & Marder SR (2011). The brief negative symptom scale: psychometric properties. Schizophr Bull, 37(2), 300–305. doi: 10.1093/schbul/sbq059 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kleist K (1908). Untersuchungen zur Kenntnis der psychomotorischen Bewegungsstörungen bei Geisteskranken. Leipzig: Verlag von Dr. Werner Klinkhardt. [Google Scholar]
- Kraepelin E (1921). Einführung in die Psychiatrische Klinik (Vol. Band I: Allgemeine Übersicht). Leipzig: Verlag von Johann Ambrosius Barth [Google Scholar]
- Lavelle M, Dimic S, Wildgrube C, McCabe R, & Priebe S (2015). Non-verbal communication in meetings of psychiatrists and patients with schizophrenia. Acta Psychiatr Scand, 131(3), 197–205. doi: 10.1111/acps.12319 [DOI] [PubMed] [Google Scholar]
- Lavelle M, Healey PG, & McCabe R (2013). Is nonverbal communication disrupted in interactions involving patients with schizophrenia? Schizophr Bull, 39(5), 1150–1158. doi: 10.1093/schbul/sbs091 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leiguarda RC, & Marsden CD (2000). Limb apraxias: higher-order disorders of sensorimotor integration. Brain, 123 (Pt 5), 860–879. doi: 10.1093/brain/123.5.860 [DOI] [PubMed] [Google Scholar]
- Leiguarda RC, Pramstaller PP, Merello M, Starkstein S, Lees AJ, & Marsden CD (1997). Apraxia in Parkinson’s disease, progressive supranuclear palsy, multiple system atrophy and neuroleptic-induced parkinsonism. Brain, 120 (Pt 1), 75–90. doi: 10.1093/brain/120.1.75 [DOI] [PubMed] [Google Scholar]
- Lencer R, Sprenger A, Reilly JL, McDowell JE, Rubin LH, Badner JA, … Sweeney JA (2015). Pursuit eye movements as an intermediate phenotype across psychotic disorders: Evidence from the B-SNIP study. Schizophr Res, 169(1–3), 326–333. doi: 10.1016/j.schres.2015.09.032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lesourd M, Osiurak F, Baumard J, Bartolo A, Vanbellingen T, & Reynaud E (2018). Cerebral correlates of imitation of intransitive gestures: An integrative review of neuroimaging data and brain lesion studies. Neurosci Biobehav Rev, 95, 44–60. doi: 10.1016/j.neubiorev.2018.07.019 [DOI] [PubMed] [Google Scholar]
- Martin P, Tewesmeier M, Albers M, Schmid G, & Scharfetter C (1994). Investigation of gestural and pantomime performance in chronic schizophrenic inpatients. Eur Arch Psychiatry Clin Neurosci, 244(2), 59–64. doi: 10.1007/BF02193520 [DOI] [PubMed] [Google Scholar]
- Matthews N, Gold BJ, Sekuler R, & Park S (2013). Gesture imitation in schizophrenia. Schizophr Bull, 39(1), 94–101. doi: 10.1093/schbul/sbr062 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mengotti P, Corradi-Dell’Acqua C, Negri GA, Ukmar M, Pesavento V, & Rumiati RI (2013). Selective imitation impairments differentially interact with language processing. Brain, 136(Pt 8), 2602–2618. doi: 10.1093/brain/awt194 [DOI] [PubMed] [Google Scholar]
- Millman ZB, Goss J, Schiffman J, Mejias J, Gupta T, & Mittal VA (2014). Mismatch and lexical retrieval gestures are associated with visual information processing, verbal production, and symptomatology in youth at high risk for psychosis. Schizophr Res, 158(1–3), 64–68. doi: 10.1016/j.schres.2014.06.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mittal VA, Bernard JA, & Northoff G (2017). What Can Different Motor Circuits Tell Us About Psychosis? An RDoC Perspective. Schizophr Bull, 43(5), 949–955. doi: 10.1093/schbul/sbx087 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mittal VA, Tessner KD, McMillan AL, Delawalla Z, Trotman HD, & Walker EF (2006). Gesture behavior in unmedicated schizotypal adolescents. J Abnorm Psychol, 115(2), 351–358. doi: 10.1037/0021-843X.115.2.351 [DOI] [PubMed] [Google Scholar]
- Mittal VA, & Wakschlag LS (2017). Research domain criteria (RDoC) grows up: Strengthening neurodevelopment investigation within the RDoC framework. J Affect Disord, 216, 30–35. doi: 10.1016/j.jad.2016.12.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mittal VA, & Walther S (2018). As Motor System Pathophysiology Returns to the Forefront of Psychosis Research, Clinical Implications Should Hold Center Stage. Schizophr Bull. doi: 10.1093/schbul/sby176 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagels A, Kircher T, Grosvald M, Steines M, & Straube B (2018). Evidence for gesture-speech mismatch detection impairments in schizophrenia. Psychiatry Res, 273, 15–21. doi: 10.1016/j.psychres.2018.12.107 [DOI] [PubMed] [Google Scholar]
- Osborne KJ, Bernard JA, Gupta T, Dean DJ, Millman Z, Vargas T, … Mittal VA (2017). Beat gestures and postural control in youth at ultrahigh risk for psychosis. Schizophr Res, 185, 197–199. doi: 10.1016/j.schres.2016.11.028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osborne KJ, Vargas T, & Mittal VA (2020). Early childhood social communication deficits in youth at clinical high-risk for psychosis: Associations with functioning and risk. Dev Psychopathol, 32(2), 559–572. doi: 10.1017/S0954579419000385 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osiurak F, & Rossetti Y (2017). Definition: Limb apraxia. Cortex, 93, 228. doi: 10.1016/j.cortex.2017.03.010 [DOI] [PubMed] [Google Scholar]
- Ozkan S, Adapinar DO, Elmaci NT, & Arslantas D (2013). Apraxia for differentiating Alzheimer’s disease from subcortical vascular dementia and mild cognitive impairment. Neuropsychiatr Dis Treat, 9, 947–951. doi: 10.2147/NDT.S47879 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pappa S, & Dazzan P (2009). Spontaneous movement disorders in antipsychotic-naive patients with first-episode psychoses: a systematic review. Psychol Med, 39(7), 1065–1076. doi:S0033291708004716 [pii] 10.1017/S0033291708004716 [DOI] [PubMed] [Google Scholar]
- Park S, Matthews N, & Gibson C (2008). Imitation, simulation, and schizophrenia. Schizophr Bull, 34(4), 698–707. doi: 10.1093/schbul/sbn048 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pizzamiglio G, Zhang Z, Kolasinski J, Riddoch JM, Passingham RE, Mantini D, Rounis E (2019). A Role for the Action Observation Network in Apraxia After Stroke. Frontiers in Human Neuroscience 10.3389/fnhum.2019.00422 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Portnoff LA, Golden CJ, Snyder TJ, & Gustavson JL (1982). Deficits of ideokinetic praxis in chronic schizophrenics on a modified version of the Luria-Nebraska Motor Scale. Int J Neurosci, 16(3–4), 151–158. doi: 10.3109/00207458209147142 [DOI] [PubMed] [Google Scholar]
- Raymer AM, Maher LM, Foundas AL, Heilman KM, & Rothi LJ (1997). The significance of body part as tool errors in limb apraxia. Brain Cogn, 34(2), 287–292. doi: 10.1006/brcg.1997.0919 [DOI] [PubMed] [Google Scholar]
- Rosso IM, Bearden CE, Hollister JM, Gasperoni TL, Sanchez LE, Hadley T, & Cannon TD (2000). Childhood neuromotor dysfunction in schizophrenia patients and their unaffected siblings: a prospective cohort study. Schizophr Bull, 26(2), 367–378. Retrieved from http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=10885637 [DOI] [PubMed] [Google Scholar]
- Rounis E, Banca P, & Voon V (2016). Deficits in Limb Praxis in Patients With Obsessive-Compulsive Disorder. J Neuropsychiatry Clin Neurosci, 28(3), 232–235. doi: 10.1176/appi.neuropsych.15090233 [DOI] [PubMed] [Google Scholar]
- Schaefer J, Giangrande E, Weinberger DR, & Dickinson D (2013). The global cognitive impairment in schizophrenia: consistent over decades and around the world. Schizophr Res, 150(1), 42–50. doi: 10.1016/j.schres.2013.07.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sperber C, Wiesen D, Goldenberg G, & Karnath HO (2019). A network underlying human higher-order motor control: Insights from machine learning-based lesion-behaviour mapping in apraxia of pantomime. Cortex, 121, 308–321. doi: 10.1016/j.cortex.2019.08.023 [DOI] [PubMed] [Google Scholar]
- Stegmayer K, Bohlhalter S, Vanbellingen T, Federspiel A, Moor J, Wiest R, … Walther S (2016). Structural brain correlates of defective gesture performance in schizophrenia. Cortex, 78, 125–137. doi: 10.1016/j.cortex.2016.02.014 [DOI] [PubMed] [Google Scholar]
- Stegmayer K, Bohlhalter S, Vanbellingen T, Federspiel A, Wiest R, Muri RM, … Walther S (2018). Limbic Interference During Social Action Planning in Schizophrenia. Schizophr Bull, 44(2), 359–368. doi: 10.1093/schbul/sbx059 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stegmayer K, Moor J, Vanbellingen T, Bohlhalter S, Muri RM, Strik W, & Walther S (2016). Gesture Performance in First- and Multiple-Episode Patients with Schizophrenia Spectrum Disorders. Neuropsychobiology, 73(4), 201–208. doi: 10.1159/000446116 [DOI] [PubMed] [Google Scholar]
- Stieglitz Ham H, Corley M, Rajendran G, Carletta J, & Swanson S (2008). Brief report: imitation of meaningless gestures in individuals with Asperger syndrome and High-Functioning Autism. J Autism Dev Disord, 38(3), 569–573. doi: 10.1007/s10803-007-0417-x [DOI] [PubMed] [Google Scholar]
- Strik W, Stegmayer K, Walther S, & Dierks T (2017). Systems Neuroscience of Psychosis: Mapping Schizophrenia Symptoms onto Brain Systems. Neuropsychobiology, 75(3), 100–116. doi: 10.1159/000485221 [DOI] [PubMed] [Google Scholar]
- Thaker GK (2008). Neurophysiological endophenotypes across bipolar and schizophrenia psychosis. Schizophr Bull, 34(4), 760–773. doi: 10.1093/schbul/sbn049 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thakkar KN, Peterman JS, & Park S (2014). Altered brain activation during action imitation and observation in schizophrenia: a translational approach to investigating social dysfunction in schizophrenia. Am J Psychiatry, 171(5), 539–548. doi: 10.1176/appi.ajp.2013.13040498 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Troisi A, Spalletta G, & Pasini A (1998). Non-verbal behaviour deficits in schizophrenia: an ethological study of drug-free patients. Acta Psychiatr Scand, 97(2), 109–115. Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/9517903 [DOI] [PubMed] [Google Scholar]
- van Erp TGM, Walton E, Hibar DP, Schmaal L, Jiang W, Glahn DC, … Turner JA (2018). Cortical Brain Abnormalities in 4474 Individuals With Schizophrenia and 5098 Control Subjects via the Enhancing Neuro Imaging Genetics Through Meta Analysis (ENIGMA) Consortium. Biol Psychiatry, 84(9), 644–654. doi: 10.1016/j.biopsych.2018.04.023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vanbellingen T, Kersten B, Bellion M, Temperli P, Baronti F, Muri R, & Bohlhalter S (2011). Impaired finger dexterity in Parkinson’s disease is associated with praxis function. Brain Cogn, 77(1), 48–52. doi: 10.1016/j.bandc.2011.06.003 [DOI] [PubMed] [Google Scholar]
- Vanbellingen T, Kersten B, Van de Winckel A, Bellion M, Baronti F, Muri R, & Bohlhalter S (2011). A new bedside test of gestures in stroke: the apraxia screen of TULIA (AST). J Neurol Neurosurg Psychiatry, 82(4), 389–392. doi: 10.1136/jnnp.2010.213371 [DOI] [PubMed] [Google Scholar]
- Vanbellingen T, Kersten B, Van Hemelrijk B, Van de Winckel A, Bertschi M, Muri R, … Bohlhalter S (2010). Comprehensive assessment of gesture production: a new test of upper limb apraxia (TULIA). Eur J Neurol, 17(1), 59–66. doi: 10.1111/j.1468-1331.2009.02741.x [DOI] [PubMed] [Google Scholar]
- Vanbellingen T, Lungu C, Lopez G, Baronti F, Muri R, Hallett M, & Bohlhalter S (2012). Short and valid assessment of apraxia in Parkinson’s disease. Parkinsonism Relat Disord, 18(4), 348–350. doi: 10.1016/j.parkreldis.2011.11.023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vanbellingen T, Pastore-Wapp M, Kubel S, Nyffeler T, Schupfer AC, Kiefer C, … Bohlhalter S (2020). Interhemispheric facilitation of gesturing: A combined theta burst stimulation and diffusion tensor imaging study. Brain Stimul, 13(2), 457–463. doi: 10.1016/j.brs.2019.12.013 [DOI] [PubMed] [Google Scholar]
- Viher PV, Abdulkadir A, Savadijev P, Stegmayer K, Kubicki M, Makris N, … Walther S (in press). Structural organization of the praxis network predicts gesture production: Evidence from healthy subjects and patients with schizophrenia. Cortex. [DOI] [PubMed] [Google Scholar]
- Viher PV, Stegmayer K, Kubicki M, Karmacharya S, Lyall AE, Federspiel A, … Walther S (2018). The cortical signature of impaired gesturing: Findings from schizophrenia. Neuroimage Clin, 17, 213–221. doi: 10.1016/j.nicl.2017.10.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vry MS, Tritschler LC, Hamzei F, Rijntjes M, Kaller CP, Hoeren M, … Weiller C (2015). The ventral fiber pathway for pantomime of object use. Neuroimage, 106, 252–263. doi: 10.1016/j.neuroimage.2014.11.002 [DOI] [PubMed] [Google Scholar]
- Walker EF, Savoie T, & Davis D (1994). Neuromotor precursors of schizophrenia. Schizophr Bull, 20(3), 441–451. Retrieved from http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=7526446 [DOI] [PubMed] [Google Scholar]
- Walther S, Alexaki D, Stegmayer K, Vanbellingen T, & Bohlhalter S (2020). Conceptual disorganization impairs hand gesture performance in schizophrenia. Schizophr Res, 215, 467–468. doi: 10.1016/j.schres.2019.09.001 [DOI] [PubMed] [Google Scholar]
- Walther S, Bernard JA, Mittal VA, & Shankman SA (2019). The utility of an RDoC motor domain to understand psychomotor symptoms in depression. Psychol Med, 49(2), 212–216. doi: 10.1017/S0033291718003033 [DOI] [PubMed] [Google Scholar]
- Walther S, Eisenhardt S, Bohlhalter S, Vanbellingen T, Muri R, Strik W, & Stegmayer K (2016). Gesture Performance in Schizophrenia Predicts Functional Outcome After 6 Months. Schizophrenia Bulletin, 42(6), 1326–1333. doi: 10.1093/schbul/sbw124 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walther S, Kunz M, Muller M, Zurcher C, Vladimirova I, Bachofner H, … Viher PV (2020). Single Session Transcranial Magnetic Stimulation Ameliorates Hand Gesture Deficits in Schizophrenia. Schizophr Bull, 46(2), 286–293. doi: 10.1093/schbul/sbz078 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walther S, & Mittal VA (2017). Motor System Pathology in Psychosis. Curr Psychiatry Rep, 19(12), 97. doi: 10.1007/s11920-017-0856-9 [DOI] [PubMed] [Google Scholar]
- Walther S, Stegmayer K, Sulzbacher J, Vanbellingen T, Muri R, Strik W, & Bohlhalter S (2015). Nonverbal social communication and gesture control in schizophrenia. Schizophr Bull, 41(2), 338–345. doi: 10.1093/schbul/sbu222 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walther S, & Strik W (2012). Motor Symptoms and Schizophrenia. Neuropsychobiology, 66(2), 77–92. doi:000339456 [pii] 10.1159/000339456 [DOI] [PubMed] [Google Scholar]
- Walther S, Vanbellingen T, Muri R, Strik W, & Bohlhalter S (2013a). Impaired gesture performance in schizophrenia: particular vulnerability of meaningless pantomimes. Neuropsychologia, 51(13), 2674–2678. doi: 10.1016/j.neuropsychologia.2013.08.017 [DOI] [PubMed] [Google Scholar]
- Walther S, Vanbellingen T, Muri R, Strik W, & Bohlhalter S (2013b). Impaired pantomime in schizophrenia: Association with frontal lobe function. Cortex, 49(2), 520–527. doi:S0010–9452(11)00333–9 [pii] 10.1016/j.cortex.2011.12.008 [DOI] [PubMed] [Google Scholar]
- Weiss PH, Ubben SD, Kaesberg S, Kalbe E, Kessler J, Liebig T, & Fink GR (2016). Where language meets meaningful action: a combined behavior and lesion analysis of aphasia and apraxia. Brain Struct Funct, 221(1), 563–576. doi: 10.1007/s00429-014-0925-3 [DOI] [PubMed] [Google Scholar]
- White TP, Borgan F, Ralley O, & Shergill SS (2016). You looking at me?: Interpreting social cues in schizophrenia. Psychol Med, 46(1), 149–160. doi: 10.1017/S0033291715001622 [DOI] [PubMed] [Google Scholar]
- Whitty PF, Owoeye O, & Waddington JL (2009). Neurological signs and involuntary movements in schizophrenia: intrinsic to and informative on systems pathobiology. Schizophr Bull, 35(2), 415–424. doi:sbn126 [pii] 10.1093/schbul/sbn126 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiberg A, Ng M, Al Omran Y, Alfaro-Almagro F, McCarthy P, Marchini J, … Furniss D (2019). Handedness, language areas and neuropsychiatric diseases: insights from brain imaging and genetics. Brain, 142(10), 2938–2947. doi: 10.1093/brain/awz257 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wuthrich F, Viher PV, Stegmayer K, Federspiel A, Bohlhalter S, Vanbellingen T, … Walther S (2020). Dysbalanced Resting-State Functional Connectivity Within the Praxis Network Is Linked to Gesture Deficits in Schizophrenia. Schizophr Bull. doi: 10.1093/schbul/sbaa008 [DOI] [PMC free article] [PubMed] [Google Scholar]


