Abstract
Foregut duplication is more common in girls, particularly if there is bronchopulmonary involvement. The incidence of oesophageal duplication cyst is estimated to be one in 8200 live births with male prevalence. Most duplications are benign, but the presence of ectopic gastric mucosa and the potential for malignant degeneration remain a concern. A newborn female, antenatally diagnosed with right-sided thoracic mass, was diagnosed with a foregut duplication cyst of size 4.1 cm × 3.7 cm × 8 cm in the posterior mediastinum. Thoracoscopic resection was done on day of life 14. The postoperative recovery was uneventful and histopathology confirmed the diagnosis. A literature search revealed only a few cases of an early thoracoscopic intervention, and ours is the earliest reported. Thoracoscopy in the neonatal period is safe and effective.
Keywords: Bronchogenic, oesophageal, foregut duplication cyst, gastric, thoracoscopy
INTRODUCTION
W.E. Ladd first introduced the term 'duplication' in 1934.[1] Oesophageal duplication cysts (EDCs) are mostly found in the distal one-third of the oesophagus in the middle or posterior mediastinum usually to the right side. Cysts in the upper or middle one-third present with mass effects like airway or oesophageal obstruction.
Various theories include the aberrant luminal recanalization theory by Bremer[2] and the diverticulum theory of Lewis and Thyng. Early total excision is the treatment of choice. We report the youngest thoracoscopically treated foregut duplication cyst in a neonate on 14th day after birth.
CASE REPORT
A 2.1 kg newborn female child was born with antenatally diagnosed right congenital cystic adenomatoid malformation (CCAM). It was a 6 cm × 3.8 cm × 3.1 cm anechoic right lung cystic lesion displacing the heart to the left.
The neonate was vitally stable, active, well-hydrated and acyanotic with reduced air entry on the right side of the chest. Chest radiograph showed a space-occupying lesion in the right hemithorax with mediastinal shift.
Ultrasonography (USG) of the chest showed a 6.9 cm × 4 cm × 4 cm cystic lesion in the posterolateral aspect of the right hemithorax to be a type 1 CCAM.
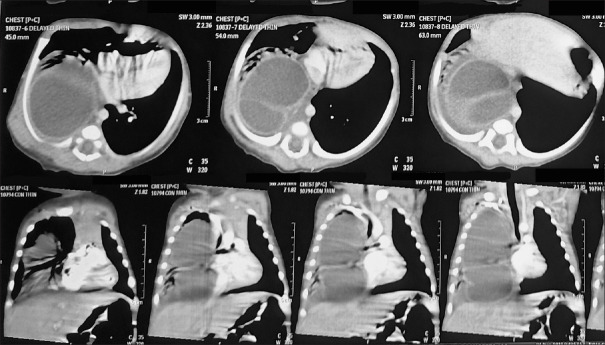
Computed tomography scan of chest revealed a 4.1 cm × 3.7 cm × 8cm, tubular cystic lesion in the posterior mediastinum in the right hemithorax (D2 to D11 vertebra) with mediastinal shift to left [Figure 1].
Figure 1.
Computed tomography scan of the chest showing cystic lesion in the right hemithorax
The neonate was operated on the day of life 14. Single-lung isolation was not required. Lateral position with a slight elevation of the right side of the thorax was given. The first 5 mm-camera port was inserted below the tip of the angle of scapula and positive pressure insufflation at 4mmHg was started with a flow rate of 1 L/min. The second 5-mm port was inserted in the posterior axillary line and the third 5-mm port was inserted at the anterior axillary line. The large cystic mass filled almost the entire pleural cavity [Figure 2], so the needle was inserted in the cyst and 60 ml of clear fluid was aspirated. Pleura was incised posterior to the mediastinal cyst which had no obvious communication with oesophagus or the neural canal. One port had to be increased by 2 cm for the complete cyst removal. The cyst was excised completely with adequate lung expansion. Intercostal drain (ICD) was kept in situ and wound closed. ICD was removed after 3 days and the patient was discharged.
Figure 2.
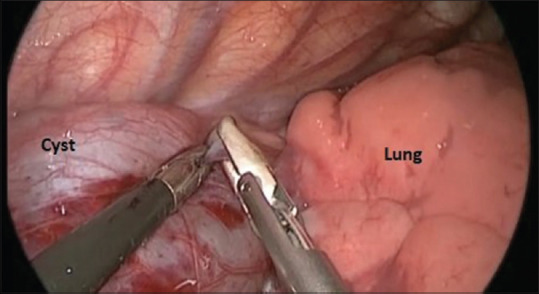
Intraoperative photograph showing intrathoracic cystic mass
Histopathology confirmed foregut duplication cyst (FDC) of immature oesophageal mucosa with ganglion cells and stomach mucosa with nerve plexus [Figure 3].
Figure 3.
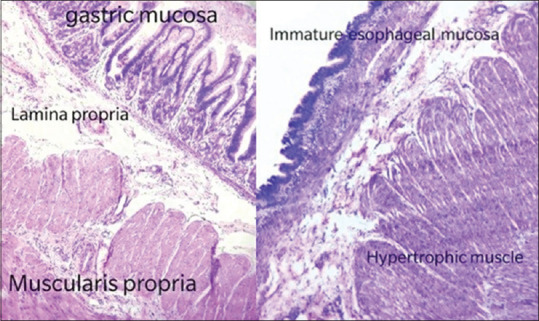
Histopathology showing oesophageal and stomach mucosa
DISCUSSION
FDC are duplications of the alimentary tract that form a cystic or spherical structure attached to a part of the bowel, sharing a wall of the smooth muscle and lined by a mucous membrane.
Associated congenital anomalies are vertebral (neuroenteric cysts), spinal cord, genitourinary malformations, intestinal malrotation and intestinal atresia.
Bentley and Smith's split notochord theory seems most plausible to explain the aetiopathogenesis.[3] FDCs develop from abnormal canalization of the solid tube, during the 5th to 8th week of embryonic life.
Most (80%) present before 2 years of age; prenatal USG can detect duplications as early as 16 weeks of gestational age.
Differential diagnoses include bronchogenic cysts, mature cystic teratomas, pericardial cysts, CCAM and neurogenic tumours.
Magnetic resonance imaging is most sensitive investigation and helps in diagnosing other associated anomalies.
If complete removal is not feasible, then all ectopic or heterotopic tissue should be removed, including the lining of the duplication.
There are three main criteria of an EDC: (1) It must be attached to or within the oesophageal wall, (2) It is covered by two muscle layers, and (3) It is lined with a squamous, cuboidal, columnar, pseudostratified or ciliated epithelium.
Oesophageal lumen integrity after cyst removal must be checked by inserting fluid or air in the lumen during surgery. An unrecognised oesophageal leak will lead to mediastinitis and severe morbidity.
Hirose et al., in a series of six cases of FDC, managed thoracoscopically report short operating time, improved cosmesis and less pain.[4] Thoracoscopic cyst excision in a neonate was earlier reported on day of life 15.[5] This is the youngest (day of life 14th) thoracoscopically treated foregut duplication cyst occupying the right hemithorax.
CONCLUSION
Early surgical excision is always recommended to prevent a symptomatic course of EDC. Technical progress in surgical instrumentation with its minimization and anaesthetic advances allowed for the use of minimally invasive techniques in the treatment of this mediastinal cyst.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent forms. In the form the patient(s) has/have given his/her/their consent for his/her/their images and other clinical information to be reported in the journal. The patients understand that their names and initials will not be published and due efforts will be made to conceal their identity, but anonymity cannot be guaranteed.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.Ladd WE. Duplications of the alimentary tract. South Med J. 1934;30:363–71. [Google Scholar]
- 2.Bremer JL. Diverticula and duplications of the intestinal tract. Arch Pathol. 1944;38:132–40. [Google Scholar]
- 3.Bentley JF, Smith JR. Developmental posterior enteric remnants and spinal malformations: The split notochord syndrome. Arch Dis Child. 1960;35:76–86. doi: 10.1136/adc.35.179.76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hirose S, Clifton MS, Bratton B, Harrison MR, Farmer DL, Nobuhara KK, et al. Thoracoscopic resection of foregut duplication cysts. J Laparoendosc Adv Surg Tech A. 2006;16:526–9. doi: 10.1089/lap.2006.16.526. [DOI] [PubMed] [Google Scholar]
- 5.Cuch B, Nachulewicz P, Wieczorek AP, Wozniak M, Pac-Kozuchowska E. Esophageal duplication cyst treated thoracoscopically during the neonatal period: clinical case report. Medicine (Baltimore) 2015;94:e2270. doi: 10.1097/MD.0000000000002270. [DOI] [PMC free article] [PubMed] [Google Scholar]