Abstract
In this study, we topically administered two antioxidant compounds, the manganese-porphyrin-derivatives BMX-001 and BMX-010, in a mouse model of allergic dermatitis and compared the efficacy for reduction of itch and inflammation. In vitro effects of BMX-001 and BMX-010 on keratinocytes, bone marrow derived dendritic cells (BMDCs) and T-cells were initially analysed. For assessment of scratching behaviour, BMX-001 and BMX-010 (0.01 and 0.1 %) were topically applied 16 h and/or 1 h before compound 48/80 or toluene-2,4,-diisocyanate (TDI) challenge in a TDI induced mouse dermatitis model. Additionally, assessment of allergic skin inflammation was performed in a similar manner in the TDI model. Posttreatment ear thickness was measured 24 h after TDI challenge and compared to basal values. The mice were sacrificed and the ear auricle was removed for further analysis. In vitro, both BMX substances significantly inhibited cytokine production of keratinocytes as well as of BMDC and T-cell proliferation. Topical treatment with BMX cream resulted in a significant decrease in scratching behaviour in the compound 48/80 model, but not in the TDI model. Mice treated with BMX-001 and BMX-010 showed a moderate dose dependent decrease in ear thickness, and interestingly, the concentration of the cytokines IL-1β and IL-4 in inflamed skin was reduced by 80–90 % by all treatment options. These first results suggest the potential benefit of a BMX-001 and BMX-010 cream for the treatment of allergic-inflammatory skin diseases.
Keywords: Superoxide dismutase mimics, BMX-010, BMX-001, Allergic dermatitis, Mice
Introduction
Allergic skin diseases like atopic dermatitis in humans and dogs are characterized by chronic relapsing skin lesions and severe itch. There is a need for an effective treatment of inflammatory dermatologic conditions, including itch, with a formulation lacking typical side effects associated with the use of e.g., glucocorticoids [15, 16]. To evaluate a novel therapeutic agent for allergic skin disorders, we focused on two different types of Mn(III) porphyrin-based superoxide dismutase (SOD) mimics, BMX-001 and BMX-010, which are described to have anti-inflammatory potential in a variety of models [6] including rodent models of irradiation induced proctitis [3].
Reactive oxygen species (ROS) are implicated in a broad spectrum of pathologies, including malignant diseases, type II diabetes, atherosclerosis, chronic inflammatory processes, ischemia/reperfusion injury, and several neurodegenerative diseases [1, 4, 14]. Oxidative stress is generally defined as an imbalance that favors the production of ROS over antioxidant defenses in eukaryotic cells and compounds able to reduce oxidative stress have been actively sought for over three decades [14]. It has been reported that SOD is a biological enzyme playing a key role in maintaining appropriate levels of ROS and oxidative stress. Manganese superoxide dismutase (MnSOD) is the key enzyme located in the mitochondria, which protects energy-generating mitochondria from oxidative damage. Superoxide anions play a pro-inflammatory part in many diseases. So, MnSOD is likely to be used as an anti-inflammatory agent because of its superoxide anion scavenging ability [20, 21]. However, possible anti-inflammatory and anti-itch properties in the context of allergic dermatitis have not been previously characterized.
In this study, we first elucidated whether two different SOD mimics, BMX-001 and BMX-010, have an impact on keratinocyte activation, dendritic cell activation and T-cell proliferation in vitro as keratinocytes, dendritic cells and T-cells are crucial players in allergic skin diseases, controlling the extent and quality of response to allergens [19, 22]. We then topically administered several formulations of BMX-001 and BMX-010 during the elicitation phase of the TH2 dominated toluene-2,4-diisocyanate (TDI) induced allergic dermatitis, and evaluated the efficacy of BMX-001 and BMX-010 by comparing anti-itch and anti-inflammatory responses.
Materials and methods
Experimental animals
BALB/cAnN (female seven-week-old) were purchased from Charles River Laboratories (Raleigh, NC) and housed in groups of four mice per cage under controlled lighting (a 12-h light–dark cycle), temperature (22 ± 3 °C), humidity (55 ± 15 %), and ventilation (at least ten complete freshair changes/h). Water and a standard diet were available ad libitum. All animals were acclimatized for 1 week before experimental procedures were commenced. All aspects of the current study were conducted in accordance with the Animal Care and Use Program of the North Carolina State University (IACUC Protocol No. 13-111-B).
Reagents
Mn(III) meso-tetrakis(N–n-hexylpyridinium-2-yl)porphyrin (BMX-001), Mn(III) meso-tetrakis (N-ethylpyridinium-2-yl)porphyrin (BMX-010), and the cream formulation without the active ingredients were provided by BioMimetix JV, LLC, and delivered within the week of the usage. The compositions of the cream are purified water (37 %), white petrolatum (25 %), stearyl alcohol (25 %), propylene glycol (12 %), and sodium lauryl sulfate (1 %). Compound 48/80, lipopolysaccharide (LPS; O127:B8), peptidoglycan (PGN), toluene-2,4,-diisocyanate (TDI), acetone, 2-mercaptoethanol and lipopolysaccharide (LPS; O111:B4) were obtained from Sigma (St. Louis, MO). Mycophenolic acid was purchased from MP Biomedicals, LLC (Solon, OH). DC protein assay kit was from BIO-RAD (Richmond, CA). Pefabloc was purchased from Roche (Basel, Switzerland). CellTrace™ CASE Cell Proliferation Kit, Alexa Fluor® 488 Annexin V/Dead cell Apoptosis kit, phosphate buffered saline (PBS), methylcellulose and tween 20 were ordered from Thermo Fisher Scientific Inc. (Waltham, MA, USA). RPMI1640 medium was from Mediatech Inc. (Manassas, VA, USA). MEM eagle (EMEM) medium was from Lonza Group Ltd. (Allendale, NJ, USA). Recombinant Mouse GM-CSF was obtained from Pepro Tech, Inc. (Rocky Hill, NJ, USA). ELISAs for IL-1β, −4, −6, −12, CXCL1/KC, TNFα and CCL17/TARC, TSLP were from R&D systems (Minneapolis, MN). KGM-Gold BulletKit media was from Lonza Biosciences (USA).
Murine and human keratinocyte cell line culture
The murine keratinocyte cell line Balb/MK2 previously used for cancer research [7, 17] was used in this study. Balb/MK2 cells were cultured in EMEM medium supplemented with 10 % fetal calf serum, 100 μg/ml streptomycin and 100 U/ml penicillin. Confluent cells (1.5 × 104 cells/ml) were exposed to BMX-001 (at 0.1, 1, or 10 μmol/l) and BMX-010 (at 0.1, 1, or 10 μmol/l) in FCS-free EMEM medium containing toll-like receptor two ligand, PGN (50 μg/ml) for 24 h. Pooled Neonatal Normal Human Epidermal Keratinocytes (NHEK-neo) (Lonza Biosciences) were cultured following the standard protocols. NHEK-neo were seeded from cryopreservation at 2.5 × 103 cells/cm2, and cells were passaged after reaching 60–70 % confluence. After the second passage, NHEK-neo were seeded into well plates and confluent cells were exposed to BMX-001 (at 0.1, 1, or 10 μmol/l) and BMX-010 (at 0.1, 1, or 10 pmol/l) in medium containing PGN (50 μg/ml) and LPS (10 μg/ml) for 24 h. NHEK-neo were always maintained with KGM-Gold BulletKit media and incubated at 37 °C in 5 % CO2 and 100 % humidity. The high concentration for each chemical (10 μmol/l) did not induce any cell toxicity. After exposure, cytokine production (murine CXCL1/KC, murine TSLP, human IL-8 and human TSLP) in supernatants were evaluated using the ELISA according to the manufacturer’s protocol. Three independent experiments were performed in different settings.
Preparation of BMDCs and cytokine assay
Bone marrow-derived dendritic cells (BMDCs) were generated from bone marrow cells of female BALB/cAnN mice, as described previously [9, 11, 13] with GM-CSF. Bone marrow was flushed from femurs with PBS and taken into BMDCs medium (RPMI 1640 with l-glutamine, 10 % heat-inactivated FCS, 50 μmol/l 2-mercaptoethanol, and 20 ng/ml GM-CSF). On day 3, another 10 ml of BMDC medium was added. At day 6, 10 ml of BMDC medium was replaced. At day 8, non-adherent and loosely adherent cells were collected and the numbers of viable cells were determined with a Cellometer (Nexcelom Bioscience LLC., Laurence, MA). Then, BMDCs (2.5 × 105 cells/ml) were exposed to BMX-001 (at 0.1, 1, or 10 μmol/l) and BMX-010 (at 0.1, 1, or 10 μmol/l) in BMDC medium containing LPS (25 ng/ml) for 24 h. The high concentration for each chemical (10 μmol/l) did not induce any cell toxicity. After exposure, cytokine production (IL-1β, IL-12 and TNFα) in BMDCs were evaluated using the ELISA. Three independent experiments were performed in DCs from different animals.
Mixed leukocyte reaction assay
Mixed leukocyte reaction assay was conducted according to the protocol of Bäumer et al. [10]. High-purity bone marrow-derived dendritic cells were generated and treated with same doses of BMX substances as mentioned above. A 10 μmol/l mycophenolic acid solution was also taken as positive control. For initiation of the mixed leukocyte reaction, T-cells were isolated from the spleen of female C57BL/6 mice. In short, single-cell suspensions were prepared from spleen by passage through a sterile 70-μm nylon cell strainer in 1 ml BMDC medium. Then spleen cells were incubated in an erythrocyte lysis buffer for 5 min followed by an enrichment of T-lymphocytes achieved by adhesion of non T-cells on cell? Petri dishes. This leads to a purity of approximately 70 % T-cells. T-cells were stained with 0.5 μmol/l carboxyfluorescein succinimidyl ester (CFSE) according to manufacturer’s protocol. The cells (1 × 105/well) were seeded in a U-bottom 96-well plate. Dendritic cells isolated from bone marrow of a BALB/c mouse (1 × 104, day 10 of culture) were added and incubated for five additional days. After 5 days, proliferation of T-cells was determined by flow cytometry. To exclude cytotoxic or apoptotic effects induced by the drugs, a portion of the T-cells were stimulated by concanavalin A (2 μg/ml) and incubated with varying doses of BMX-010 or BMX-001 for 3 days. There was a less than 10 % reduction of cell viability and no measurable induction of apoptosis tested by the Fluor® 488 Annexin V/Dead cell Apoptosis kit (data not shown).
Compound 48/80-induced acute pruritus model
The hair on the rostral part of the back region was shaved with a hair clipper 1 day before the start of the experiment. The 0.01 and 0.1 % cream formulation of BMX-010 were topically applied 1 h before intradermal injection of the compound 48/80 (50 μg/50 μl injection site) (n = 5 or 7 mice per group, Two independent experiments were performed). Scratching behaviour was evaluated for 30 min immediately after the injection of the compound 48/80.
TDI-induced allergic contact dermatitis model
TDI-induced allergic contact dermatitis model was performed with female mice as described earlier [8, 10, 11]. The abdominal skin of the mice was shaved and depilated with a commercially available depilating cream. Subsequently, the horny layers of the abdominal skin were stripped off ten times with adhesive tape. For active sensitization, 50 μl of 5 % TDI solution in acetone was applied to the stripped epidermis (day 1). On day 2 and 3, 50 μl of 5 % TDI in acetone was applied to the same site without tape stripping. Twenty-one days after the first sensitization, the allergic reaction was boosted by the administration of 50 μl 0.5 % TDI onto the shaved abdominal skin.
Monitoring of itch behaviour in TDI-induced allergic contact dermatitis model
A week after last sensitization of TDI, mice were treated with 50 μl of the 0.1 % cream formulation of BMX-001, BMX-010 and positive control (tofacitinib) 16 and 1 h before topical administration of TDI (30 μl, 0.5 %) onto the shaved rostral part of the neck. Directly after TDI application, the mice were video monitored for 1 h and scratching bouts (directed against the neck region) were determined (n = 7 mice per group, experiments were repeated at least two times in different settings).
Drug administration and mouse ear swelling test
In a first setting, BMX-001 and BMX-010 were dissolved in water:acetone (1:9) to a final concentration of 0.04, 0.1 and 0.4 mg/ml. The positive control triamcinolone acetonide was dissolved to a 0.1 mg/ml solution. Each solution was topically administered (20 μl) onto the ear skin of seven mice/group 16 and 1 h before topical challenge with TDI (0.5 %, 20 μl). The topical doses used in this study were selected based on our pilot experiments to avoid systemic toxicity or excessive local irritation. In a second setting, BMX-001 and BMX-010 were tested in a cream formulation provided by Bio-Mimetix JV, LLC, and delivered within the week of the challenge. The control received the same cream formulation without the active ingredients. This vehicle cream was also used to formulate a 0.1 % triamcinolone acetonide ream as a positive control: One part of 0.5 % triamcinolone acetonide solution in DMSO was mixed with four parts of vehicle cream, which resulted in an end concentration of 0.1 % triamcinolone acetonide. Just after measuring ear thickness, mice were anesthetized and sacrificed. The ear auricle was removed from each mouse and stored until used for histology and cytokine determination.
Histological evaluation
Samples from ear skin were collected and fixed in 4 % paraformaldehyde solution. The samples were sectioned and stained with haematoxylin-eosin and evaluated in a blinded manner in respect of cell influx and edema by a semi-quantitative examination (0, no influx, no edema; 1, mild; 2, moderate; and 3, severe influx, severe edema).
Determination of cytokines in ear tissue
One part of the ear tissue was shock-frozen in liquid nitrogen and stored at −80 °C until use. Cytokine determination for ear tissue was performed according to Bäumer et al. [8]. Briefly, mice ears were homogenized under liquid nitrogen, and the homogenates were taken in 200 μl RPMI 1640 medium containing 1 mmol/l Pefabloc. The samples were mixed intensively and stored for 1 h on ice. After centrifugation at 3000×g for 10 min at 4 °C, the supernatants were collected and the protein content was determined with DC protein assay kit. IL-1β, −4, −6, and CCL17/TARC were measured by ELISA.
Statistical analyses
Statistical significance of the difference between the vehicle control and treated groups was estimated at the 5 and 1 % levels of probability. Data from the vehicle-only control and the BMX-001, and BMX-010 treated groups were evaluated by Bartlett’s test for equality of variance. When group variances were homogeneous, a parametric one-way analysis of variance was conducted to determine statistical differences among groups. When the analysis of variance was significant, Dunnett’s multiple comparison test was applied. When group variances were heterogeneous, data were evaluated by Kruskal–Wallis non-parametric analysis of variance. When differences were significant, Dunnett’s mean rank-sum test was applied. Data are expressed as mean ± 1 SD. The data was analysed using Prism 4 (GraphPad Software, San Diego, CA, USA).
Results
Inhibitory effect of BMX-001 and BMX-010 on cytokine production of PGN-stimulated murine keratinocytes
To assess whether exposure to BMX-001 and BMX-010 affects an inflammatory response in vitro, we first focused on murine keratinocytes and measured PGN-induced cytokine production (CXCL1/KC and TSLP). We exposed BMX-001 or BMX-010 to keratinocytes for 24 h and stimulated with PGN to examine effects on keratinocytes. Statistically significant differences in CXCL1/KC and TSLP levels of each drug treated were found compared with the values for the vehicle-only control (*P < 0.05, **P < 0.01; Fig. 1a, b). Similar effects were seen in three independent experiments.
Fig. 1.
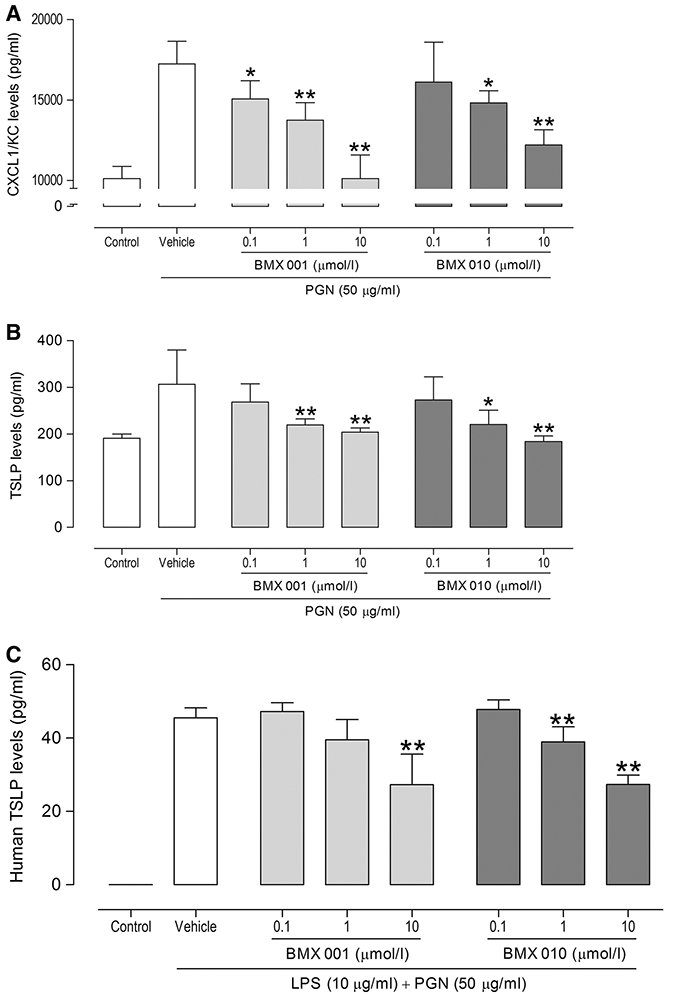
Effect of keratinocytes functions by BMX-001 or BMX-010 exposure: Suppression of PGN and/or LPS-induced production of a murine CXCL1/KC, b murine TSLP and, c human TSLP by BMX-001 or BMX-010 exposure. Results are expressed as mean ± 1 SD (pg/mL; n = 6 per group). *P < 0.05 and **P < 0.01 (Dunnett’s multiple comparison test) vs. vehicle-only control group
Inhibitory effect of BMX-001 and BMX-010 on cytokine production of PGN and LPS-stimulated human keratinocytes
We next confirmed the anti-inflammatory effects of BMX-001 and BMX-010 with human keratinocyte by measuring LPS and PGN-induced cytokine production (IL-8 and TSLP) in same setting as we used for murine keratinocytes. Whereas there were no effects in IL-8 secretion (data was not shown), statistically significant differences in TSLP levels of each drug treated were found compared with the values for the vehicle-only control (**P < 0.01; Fig. 1c).
Inhibitory effect of BMX-001 and BMX-010 on cytokine production of LPS-stimulated BMDCs
We next focused on murine BMDCs and measured LPS-induced cytokine production (IL-1β, IL-12 and TNFα) in vitro. We exposed BMX-001 or BMX-010 to BMDCs for 24 h and stimulated with LPS to examine effects on DCs. Statistically significant differences in IL-1β and IL-12 levels of each drug treated were found compared with the values for the vehicle-only control (*P < 0.05, **P < 0.01, Fig. 2a, b), whereas each drug had no effect on TNFα production (Fig. 2c). Similar results were obtained in three independent experiments.
Fig. 2.
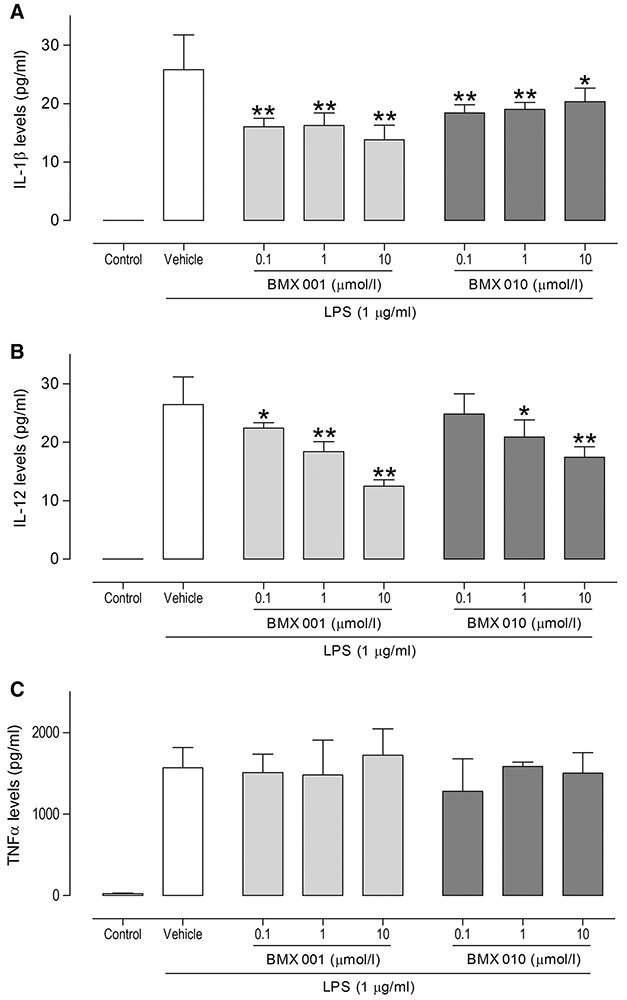
Effect of BMDC functions by BMX-001 or BMX-010 exposure: Suppression of LPS-induced production of a IL-1β, b IL-12 and c TNFα by BMX-001 or BMX-010 exposure. Results are expressed as mean ± 1 SD (pg/mL; n = 7 per group). *P < 0.05 and **P < 0.01 (Dunnett’s multiple comparison test) vs. vehicle-only control group
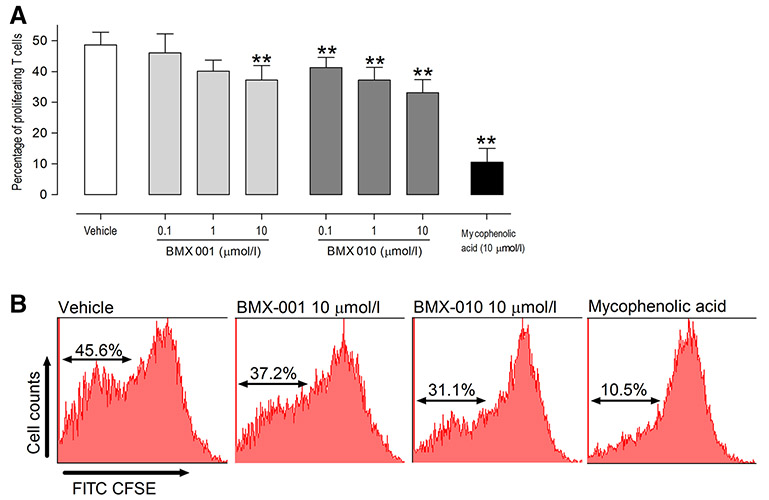
Inhibitory effect of BMX-010 and BMX-001 on T-cell proliferation in vitro
We then examined the effects of each drug on T-cell proliferation. Both BMX-001 and BMX-010 significantly reduced the proliferation of T-cells as compared with the values for the vehicle-only control (Fig. 3). Significant differences were found in 0.1 and 1 μmol/l concentration of BMX-010 (P < 0.01), 10 μmol/l concentration of each drug (P < 0.01) compared with the values for the vehicle-only control. Similar effects could be seen in three independent experiments. These concentrations of each drug were not cytotoxic to T-cells (data not shown).
Fig. 3.
Reduced proliferation of T-cell by exposure of BMX-001 or BMX-010. a Results are expressed as mean ± 1 SD (%; n = 8 per group). *P < 0.05 and **P < 0.01 (Dunnett’s multiple comparison test) vs. vehicle-only control group. b Representative histograms of mixed leukocyte reaction assay
Impact of topically administered acetone/water solution of BMX-001 and BMX-010 on scratching behaviour and ear swelling in the challenge phase of TDI-induced allergic contact dermatitis model
A topical administration of 0.04, 0.1 and 0.4 mg/ml BMX-001 or BMX-010 solution did not reduce the TDI induced ear swelling response determined 24 h after challenge, whereas the positive control triamcinolone acetonide (0.1 mg/ml) reduced the inflammatory response significantly (Table 1). In addition, the TDI induced scratching bouts were not significantly reduced by the topical administration of BMX-001 or BMX-010 (Table 1).
Table 1.
Impact of topically administered aceton/water solution of BMX-001 and BMX-010 on scratching behavior and ear swelling in the challenge phase of allergic contact dermatitis
| Drug | Dose (mg/ml) | Ear swelling (μm, mean ± SD, n = 7) |
Scratching bouts (60 min, mean ± SD, n = 7) |
|---|---|---|---|
| Vehicle | 0 | 363 ± 46 | 108 ± 28 |
| BMX-001 | 0.04 | 374 ± 48 | ND |
| 0.1 | 388 ± 28 | ND | |
| 0.4 | 385 ± 56 | 136 ± 29 | |
| BMX-010 | 0.04 | 327 ± 59 | ND |
| 0.1 | 356 ± 29 | ND | |
| 0.4 | 327 ± 43 | 130 ± 62 | |
| Triamcinolone | 0.1 | 58 ± 35** | ND |
Results are expressed as mean ± 1 SD (n = 7 per group)
P < 0.01 (Dunnett’s multiple comparison test) vs. vehicle-only control group ND not determined
Effects of topically applied cream formulation of BMX-010 on scratching behaviour in the compound 48/80-induced acute pruritus
When scratching behaviour was observed for 30 min directly after compound 48/80 injection, scratching bouts of BMX-010 treated mice decreased in a dose-dependent manner, and statistically significant differences were found compared with the values for the vehicle-only control mice (Fig. 4a).
Fig. 4.

Scratching behaviour of topical pre-treatment with a cream formulation of BMX substances on a compound and 48/80-induced acute pruritus and b TDI-induced allergic dermatitis model. Scratching behaviour experiments for both models were induced 1 h min after the application of each drug and then evaluated for 30 min and 1 h, respectively. Results are expressed as mean ± 1 SD (n = 5 or 7 per group). *P < 0.05 (Dunnett’s multiple comparison test) vs. vehicle-only control group
Effects of topically applied cream formulation of BMX-001 and BMX-010 on scratching behaviour in the challenge phase of allergic contact dermatitis
When scratching behaviour was observed for 60 min directly after TDI challenge, there was no inhibition by BMX-001 or BMX-010 0.1 % cream, whereas the JAK inhibitor tofacitinib reduced scratching bouts significantly (Fig. 4b).
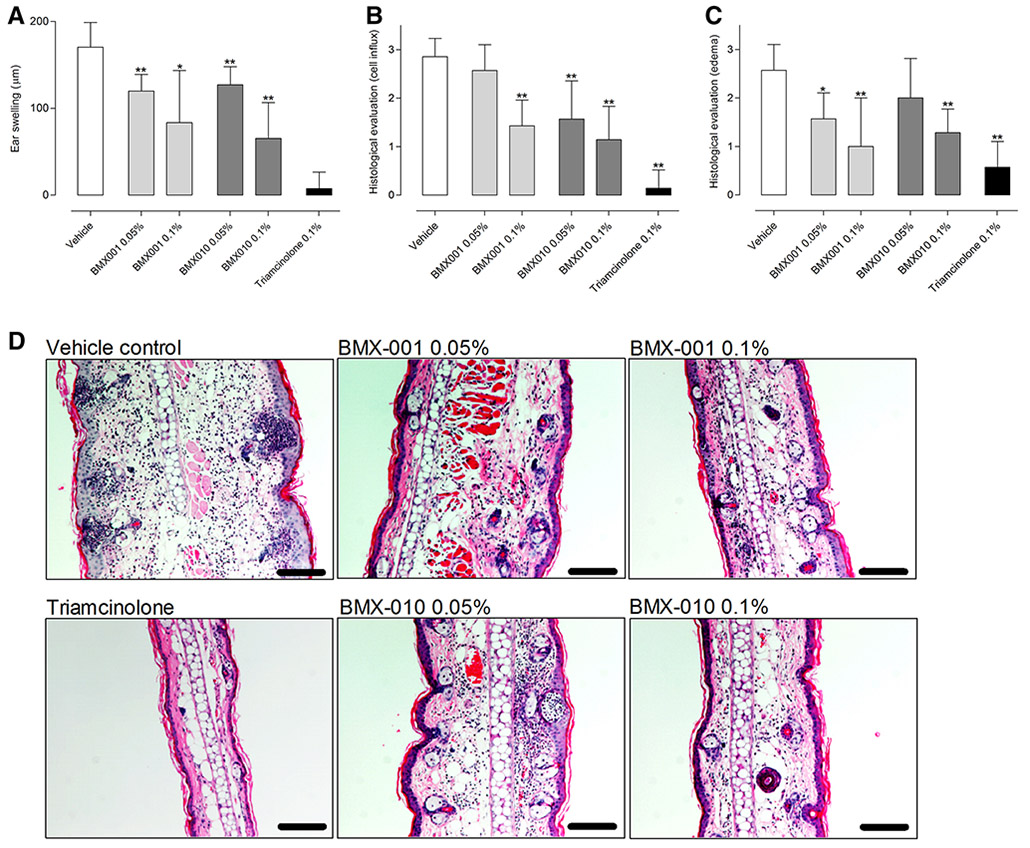
Inhibitory effects of topically applied cream formulation of BMX-001 and BMX-010 on skin inflammation in the challenge phase of TDI-induced allergic contact dermatitis
Administration of the vehicle cream had no effect on TDI induced ear swelling (a mean ear swelling response of 170 μm). BMX-001 0.05 % reduced this swelling to 117 μm and BMX-001 0.1 % to 83 μm. Correspondingly BMX-010 0.05 % reduced this swelling to 127 μm and the higher concentration (0.1 %) to 65 μm. The positive control triamcinolone reduced ear swelling to 7 μm (Fig. 5a). This reduction of ear swelling was corroborated by the blinded histological evaluation, where a dose dependent reduction of inflammatory cell influx (Fig. 5b) and edema (Fig. 5c) was observed (representative histological sections in Fig. 5d). Interestingly, the concentration of the cytokines IL-1β and IL-4 in inflamed skin was reduced by 80–90 % by all treatment options, irrespective of administered dose. The cytokines IL-1β, IL-4, IL-6, and CCL17/TARC were all significantly reduced by topical treatment with both BMX substances. The reduction was comparable to the reduction induced by triamcinolone acetonide (Fig. 6).
Fig. 5.
Ear swelling effects and Hematoxylin/eosin staining in ear skin of topical pre-treatment with a cream formulation of BMX-001 or BMX-010 on TDI-induced allergic dermatitis: a The ear swelling was calculated by a comparison of the ear thickness before and 24 h after TDI challenge. Each drug was applied topically 16 h and 1 h before TDI challenge. b, c Histological skin severity scores of edema (left) and cell influx (right). Ear skins were treated with nothing (untreated), vehicle, BMX-001 (0.05 and 0.1 %) or BMX-010 (0.05 and 0.1 %). Results are expressed as mean ± 1 SD (n = 7 per group). *P < 0.05 and **P < 0.01 (Dunnett’s multiple comparison test) vs. vehicle-only control group. d Typical microscopic features of skin with hematoxylin and eosin staining. Bar = 50 μm
Fig. 6.
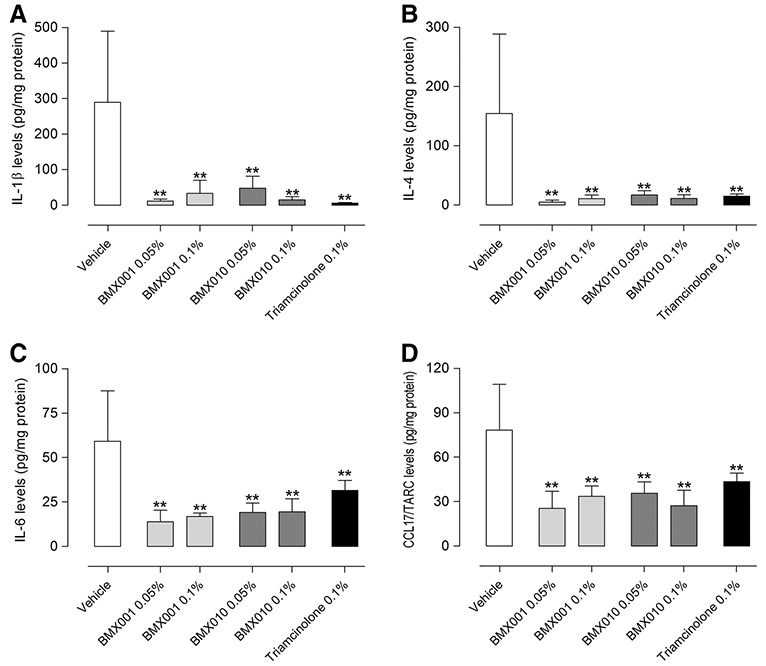
Down-regulated cytokine levels in ear skin 24 h following topical application of BMX-001 or BMX-010: Concentrations of a IL-1β, b IL-4, c IL-6, d CCL17/TARC were determined by ELISA. Results are expressed as mean ± SD (pg/mL; n = 7 per group). Designations of treatments are as in Fig. 3. *P < 0.05 and **P < 0.01 (Dunnett’s multiple comparison test) vs. vehicle control group
Discussion
In this study we were interested in characterizing the anti-inflammatory and anti-pruritic effects of the MnSOD mimics in a mouse model of allergic dermatitis. Our aim was to open up the options for topical treatment of inflammation and itch in allergic skin disease that can decrease the use of glucocorticoids or calcineurin inhibitors and to diminish their side effects seen in patients. The current data indicates that: (1) both BMX substances significantly inhibited the CXCL1/KC and TSLP production of activated keratinocyte in vitro, (2) both BMX substances had moderate effect on IL-1β and IL-12 production of BMDCs, and proliferation of T-cells in vitro, (3) topical treatment with BMX-010 cream resulted in a significant decrease in scratching behaviour in a compound 48/80-induced acute pruritus model, whereas no inhibition of itch by BMX-001 or BMX-010 was seen in a TDI-induced allergic contact dermatitis model and, (4) allergic skin inflammation were significantly reduced by topical treatment with both BMX formulations compared to the vehicle treatment group.
We first performed in vitro studies to see whether activated keratinocyte and immune cells produced less cytokines or proliferation in response to incubation with MnSOD mimics. Recent studies clearly point to an active participation of keratinocytes in the cutaneous immune response [18]. In addition, immune cells such as DCs and T-cells play an important role to both the initiation and maintenance phase of allergic inflammatory skin diseases. Thus, we can at least partly predict an inhibitory action of immunomodulatory substances by inhibition of keratinocytes, DCs as well as T-cell functions.
Activation of murine keratinocytes with the toll-like receptor agonist PGN induced cytokine production of the mouse IL-8 homologue CXCL1/KC and the TH2 cytokine TSLP which recently has been directly linked to itch [23]. We observed a robust dose-dependent inhibition for both cytokines CXCL1/KC and TSLP (Fig. 1). In addition, we could also observe a significant inhibition of TSLP secretion by both BMX substances in human keratinocytes activated by PGN and LPS. LPS induced a cytokine production in BMDC and we again saw significant dose-dependent decreases in cytokines IL-1β and IL-12, but interestingly we did not see reductions in TNF-α secretion (Fig. 2). In addition, both BMX substances were capable of suppressing the proliferative stimulus of T-cell in the mixed leukocyte reaction (Fig. 3). However, the most efficient reduction was seen in activated keratinocytes compared to the effect of DC and T-cells.
These in vitro data could be translated into an interesting in vivo profile in animal models of allergy and itch. In the next step, we attempt to examine whether topical exposure to each BMX substances affects both itching and allergic inflammation in a TDI-induced mouse model of allergic contact dermatitis. We found that scratching behaviour was not modulated by the two compounds in this model, whereas our positive control (tofacitnib) displayed an anti-pruritic effect as published by our group very recently [11]. However, it has to be kept in mind, that possible modulation of itch behaviour have been tested in different models of itch sensation as the pathways of itch are manifold and might not be reflected adequately by just one model of itch [12]. BMX-010 was evaluated in an induced acute pruritus model using compound 48/80. Mice treated topically with BMX-010 cream showed a significant decrease in scratching behaviour. Compound 48/80 induces mast cell degranulation but likely also direct sensory activation leading to scratching behaviour in mice and humans [2]. Interestingly BMX-010 displayed a dose dependent reduction of scratching bouts in this model, underscoring the necessity to test possible anti-pruritic substances in various models of itch.
The formulation of the two BMX substances is crucial to unfold their anti-inflammatory potential. It was shown in the rodent models of radiation-induced proctitis that systemic administration of the porphyrins via subcutaneous injections achieved an anti-inflammatory result [3], and it was interesting to note that an anti-inflammatory effect was still achieved via a topical preparation of the substances. Although no reduction of inflammatory response was observed in the acetone–water solution, both substances displayed a significant reduction of allergic inflammatory response to the TH2-allergen TDI in a dose dependent manner in a cream formulation. This was accompanied by a vast reduction of cytokines in inflamed tissue. The topical cream formulation most likely favours the dermal penetration of the manganese porphyrins in this formulation due to its lipophilic property, leading to increased absorption and to higher bioavailability of the manganese porphyrins [5]. Thus, limited penetration is likely the reason for lack of efficacy we observed with the acetone-water solution.
Based on our findings, we suggest that the cream formulations of BMX-001 and BMX-010 would be useful to patients as a supplement for treating allergic inflammatory skin disease. Our results demonstrate the porphyrins’ ability to significantly reduce the cytokine response in vitro and in vivo and reduce the allergic inflammatory response in a TH2-allergen model (Fig. 5). There was a comparable reduction to triamcinolone acetonide in cytokines IL1-β, IL-4, IL-6 and CCL17/TARC, a group of cytokines involved in the inflammation processes in the skin (Fig. 6). It is not clear why such a strong down-regulation of cytokines was observed in vivo, though perhaps keratinocytes could play an important role based on our in vitro results. We also saw a dose-dependent reduction in the ear tissue edema and cell infiltration (Fig. 5), which depicts that the porphyrins have the potential to reduce inflammation mechanisms when applied as a cream formulation to affected skin areas.
Future studies would be beneficial to continue characterizing these manganese porphyrins in their anti-inflammatory and anti-pruritic potentials. Evaluating both parameters in other models of allergic dermatitis and eventually in humans would be useful to further determine the anti-inflammatory mechanisms.
In summary, our results demonstrate that manganese porphyrins have the potential to be used therapeutically to reduce inflammation in the skin in a topical cream formulation. We suggest that this cream formulation can be used as a supplement in treating allergic-inflammatory skin disease to aid in decreasing topical glucocorticoid use and its negative effects.
Acknowledgments
This work was funded by BioMimetix JV, LLC. We deeply appreciate Dr. Sarah Ehling and Dr. Joy Rachel Ganchingco (North Carolina State College of Veterinary Medicine) for their variable suggestions and discussions.
Footnotes
Conflict of interest Authors K.S., T.F., M.A.D., K,T,Y., and W.B. have no conflicts of interest. J.D.C. is manager of BioMimetix JV, LLC, and holds equity. R.E.O.D. has equity in BioMimetix JV, LLC.
References
- 1.Aikawa T, Ito S, Shinohara M, Kaneko M, Kondo T, Yuasa M (2015) A drug formulation using an alginate hydrogel matrix for efficient oral delivery of the manganese porphyrin-based superoxide dismutase mimic. Biomater Sci 3(6):861–869 [DOI] [PubMed] [Google Scholar]
- 2.Akiyama T, Carstens E (2013) Neural processing of itch. Neuroscience 250:697–714 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Archambeau JO, Tovmasyan A, Pearlstein RD, Crapo JD, Batinic-Haberle I (2013) Superoxide dismutase mimic, MnTE-2-PyP(5+) ameliorates acute and chronic proctitis following focal proton irradiation of the rat rectum. Redox Biol 1:599–607 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Batinic-Haberle I, Rajic Z, Tovmasyan A, Reboucas JS, Ye X, Leong KW, Dewhirst MW, Vujaskovic Z, Benov L, Spasojevic I (2011) Diverse functions of cationic Mn(III) N-substituted pyridylporphyrins, recognized as SOD mimics. Free Radic Biol Med 51(5):1035–1053 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Batinic-Haberle I, Reboucas JS, Spasojevic I (2010) Superoxide dismutase mimics: chemistry, pharmacology, and therapeutic potential. Antioxid Redox Signal 13(6):877–918 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Batinic-Haberle I, Tovmasyan A, Roberts ER, Vujaskovic Z, Leong KW, Spasojevic I (2014) SOD therapeutics: latest insights into their structure-activity relationships and impact on the cellular redox-based signaling pathways. Antioxid Redox Signal 20(15):2372–2415 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Baumer W, Kietzmann M (2007) Effects of cyclosporin A and cilomilast on activated canine, murine and human keratinocytes. Vet Dermatol 18(2):107–114 [DOI] [PubMed] [Google Scholar]
- 8.Baumer W, Seegers U, Braun M, Tschernig T, Kietzmann M (2004) TARC and RANTES, but not CTACK, are induced in two models of allergic contact dermatitis. Effects of cilomilast and diflorasone diacetate on T-cell-attracting chemokines. Br J Dermatol 151(4):823–830 [DOI] [PubMed] [Google Scholar]
- 9.Baumer W, Tschernig T, Sulzle B, Seegers U, Luhrmann A, Kietzmann M (2003) Effects of cilomilast on dendritic cell function in contact sensitivity and dendritic cell migration through skin. Eur J Pharmacol 481(2–3):271–279 [DOI] [PubMed] [Google Scholar]
- 10.Baumer W, Wlaz P, Jennings G, Rundfeldt C (2010) The putative lipid raft modulator miltefosine displays immunomodulatory action in T-cell dependent dermal inflammation models. Eur J Pharmacol 628(1–3):226–232 [DOI] [PubMed] [Google Scholar]
- 11.Fukuyama T, Ehling S, Cook E, Baumer W (2015) Topically administered Janus-Kinase inhibitors tofacitinib and oclacitinib display impressive antipruritic and anti-inflammatory responses in a model of allergic dermatitis. J Pharmacol Exp Ther 354(3):394–405 [DOI] [PubMed] [Google Scholar]
- 12.Liu T, Ji RR (2013) New insights into the mechanisms of itch: are pain and itch controlled by distinct mechanisms? Pflugers Arch 465(12):1671–1685 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lutz MB, Kukutsch N, Ogilvie AL, Rossner S, Koch F, Romani N, Schuler G (1999) An advanced culture method for generating large quantities of highly pure dendritic cells from mouse bone marrow. J Immunol Methods 223(1):77–92 [DOI] [PubMed] [Google Scholar]
- 14.Orrenius S, Gogvadze V, Zhivotovsky B (2007) Mitochondrial oxidative stress: implications for cell death. Annu Rev Pharmacol Toxicol 47:143–183 [DOI] [PubMed] [Google Scholar]
- 15.Ring J, Alomar A, Bieber T, Deleuran M, Fink-Wagner A, Gelmetti C, Gieler U, Lipozencic J, Luger T, Oranje AP, Schafer T, Schwennesen T, Seidenari S, Simon D, Stander S, Stingl G, Szalai S, Szepietowski JC, Taieb A, Werfel T, Wollenberg A, Darsow U (2012) Guidelines for treatment of atopic eczema (atopic dermatitis) Part II. J Eur Acad Dermatol Venereol 26(9):1176–1193 [DOI] [PubMed] [Google Scholar]
- 16.Ring J, Alomar A, Bieber T, Deleuran M, Fink-Wagner A, Gelmetti C, Gieler U, Lipozencic J, Luger T, Oranje AP, Schafer T, Schwennesen T, Seidenari S, Simon D, Stander S, Stingl G, Szalai S, Szepietowski JC, Taieb A, Werfel T, Wollenberg A, Darsow U (2012) Guidelines for treatment of atopic eczema (atopic dermatitis) part I. J Eur Acad Dermatol Venereol 26(8):1045–1060 [DOI] [PubMed] [Google Scholar]
- 17.Shim M, Powers KL, Ewing SJ, Zhu S, Smart RC (2005) Diminished expression of C/EBPalpha in skin carcinomas is linked to oncogenic Ras and reexpression of C/EBPalpha in carcinoma cells inhibits proliferation. Cancer Res 65(3):861–867 [PubMed] [Google Scholar]
- 18.Steinhoff M, Brzoska T, Luger TA (2001) Keratinocytes in epidermal immune responses. Curr Opin Allergy Clin Immunol 1(5):469–476 [DOI] [PubMed] [Google Scholar]
- 19.Steinman RM (2007) Lasker Basic Medical Research Award. Dendritic cells: versatile controllers of the immune system. Nat Med 13(10):1155–1159 [DOI] [PubMed] [Google Scholar]
- 20.van de Wetering CI, Coleman MC, Spitz DR, Smith BJ, Knudson CM (2008) Manganese superoxide dismutase gene dosage affects chromosomal instability and tumor onset in a mouse model of T cell lymphoma. Free Radic Biol Med 44(8):1677–1686 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wang YH, Xu XJ, Li HL (2014) Hepatoprotective effects of mimic of manganese superoxide dismutase against carbon tetrachloride-induced hepatic injury. Int Immunopharmacol 22(1):126–132 [DOI] [PubMed] [Google Scholar]
- 22.Werfel T (2009) The role of leukocytes, keratinocytes, and allergen-specific IgE in the development of atopic dermatitis. J Invest Dermatol 129(8):1878–1891 [DOI] [PubMed] [Google Scholar]
- 23.Wilson SR, The L, Batia LM, Beattie K, Katibah GE, McClain SP, Pellegrino M, Estandian DM, Bautista DM (2013) The epithelial cell-derived atopic dermatitis cytokine TSLP activates neurons to induce itch. Cell 155(2):285–295 [DOI] [PMC free article] [PubMed] [Google Scholar]