Abstract
Background:
Prenatal phthalate exposure has been linked with altered neurodevelopment, including externalizing behaviors and attention-deficit hyperactivity disorder (ADHD). However, the implicated metabolite, neurobehavioral endpoint, and child sex have not always been consistent across studies, possibly due to heterogeneity in neurodevelopmental instruments. The complex set of findings may be synthesized using executive function (EF), a construct of complex cognitive processes that facilitate ongoing goal-directed behaviors. Impaired EF can be presented with various phenotypes of poor neurodevelopment, differently across structured conditions, home/community, or preschool/school. We evaluated the relationship between prenatal phthalate exposure and comprehensive assessment of preschool EF.
Methods:
Our study comprised 262 children with clinically significant/subthreshold ADHD symptoms and 78 typically developing children who were born between 2003 and 2008 and participated in the Preschool ADHD Substudy, which is nested within a population-based prospective cohort study, the Norwegian Mother, Father, and Child Cohort (MoBa). Twelve phthalate metabolites were measured from urine samples that their mothers had provided during pregnancy, at 17 weeks’ gestation. All children, at approximately 3.5-years, took part in a detailed clinical assessment that included parent-and teacher-rated inventories and administered tests. We used instruments that measured constructs related to EF, which include a parent-and teacher-reported Behavior Rating Inventory of Executive Function-Preschool (BRIEF-P) and three performance-based tests: A Developmental NEuroPSYchological Assessment (NEPSY), Stanford-Binet intelligence test V (SB5), and the cookie delay task (CDT). The standard deviation change in test score per interquartile range (IQR) increase in phthalate metabolite was estimated with multivariable linear regression. We applied weighting in all models to account for the oversampling of children with clinically significant or subthreshold symptoms of ADHD. Additionally, we assessed modification by child sex and potential co-pollutant confounding.
Results:
Elevated exposure to mono-benzyl phthalate (MBzP) during pregnancy was associated with poorer EF, across all domains and instruments, in both sex. For example, an IQR increase in MBzP was associated with poorer working memory rated by parent (1.23 [95% CI: 0.20, 2.26]) and teacher (1.13 [0.14, 2.13]) using BRIEF-P, and administered tests such as SB5 (no-verbal: 0.19 [0.09, 0.28]; verbal: 0.13 [0.01, 0.25]). Adverse associations were also observed for mono-n-butyl phthalate (MnBP) and mono-iso-butyl phthalate (MiBP), although results varied by instruments. EF domains reported by parents using BRIEF-P were most apparently implicated, with stronger associations among boys (e.g., MnBP and inhibition: 2.74 [1.77, 3.72]; MiBP and inhibition: 1.88 [0.84, 2.92]) than among girls (e.g., MnBP and inhibition: −0.63 [−2.08, 0.83], interaction p-value: 0.04; MiBP and inhibition: −0.15 [−1.04, 0.74], interaction p-value: 0.3). Differences by sex, however, were not found for the teacher-rated BRIEF-P or administered tests including NEPSY, SB5, and CDT.
Conclusion and relevance:
Elevated mid-pregnancy MBzP, MiBP, and MnBP were associated with more adverse profiles of EF among preschool-aged children across a range of instruments and raters, with some associations found only among boys. Given our findings and accumulating evidence of the prenatal period as a critical window for phthalate exposure, there is a timely need to expand the current phthalate regulations focused on baby products to include pregnancy exposures.
Keywords: Executive function, Phthalates, Neurodevelopment, MoBa, Butyl-phthalates, Benzyl-phthalates
1. Introduction
Over the past decade, prenatal exposure to endocrine disrupting chemicals (EDCs) has emerged as a possible threat to brain development (Bennett et al., 2016). Among the potentially neurotoxic EDCs, phthalates are one of the most universally detected in light of their popular use in cosmetics, personal care, consumer, and medical products (EPA, 2012). Since phthalates are used as plasticizers or fixatives that are not covalently bound to the original materials, they can easily leach into surrounding media, resulting in human exposures (EPA, 2012; Miodovnik 2011).
Phthalate exposure during pregnancy has been demonstrated to impact neurodevelopment in offspring (Radke et al. 2020), however, not all studies report adverse associations (Factor-Litvak et al. 2014; Gascon et al. 2015; Kim et al. 2017) and the specific phthalate metabolite(s) implicated is largely heterogeneous across previous studies. The existence of sex-specific associations is also controversial, although studies that observed sex-interaction frequently found associations among boys (Engel et al. 2010; Lien et al. 2015; Philippat et al., 2017; Whyatt et al. 2012). Evidence synthesis is complicated by substantial variability in the age at assessment and a lack of overlap in the neurobehavioral assessments, along with variability in the rater (e.g., parent-reported, teacher-reported, and performance-based). Nonetheless, multiple studies have linked prenatal phthalates exposure with small increases in the symptoms of hyperactivity (Engel et al. 2010; Kobrosly et al. 2014), inattention (Engel et al. 2010; Kobrosly et al. 2014; Lien et al. 2015), emotional control (Engel et al. 2010; Gascon et al. 2015; Kobrosly et al. 2014; Lien et al. 2015; Philippat et al., 2017; Whyatt et al. 2012), aggressive behaviors (Engel et al. 2010; Kobrosly et al. 2014; Lien et al. 2015; Singer et al. 2017), and more generally, problematic externalizing behaviors (Engel et al. 2010; Kobrosly et al. 2014; Lien et al. 2015). Externalizing behaviors are outwardly focused behaviors, typically including aggressive and hyperactive behaviors (Liu 2004), which are often found in children with ADHD (Willcutt et al. 2005).
The complex set of findings across previous studies could be synthesized using executive function (EF), a construct of complex cognitive processes that facilitate ongoing goal-directed behaviors. Key features of EF include emotional regulation, impulse control, working memory, and attentional flexibility, and their deficits can underlie ADHD (Clark et al. 2000) and problematic externalizing behaviors. EFs begin to develop early in life (Garon et al. 2008), with individual differences remaining relatively stable throughout the developmental trajectory (Miyake and Friedman 2012). EFs can be presented differently across multiple settings such as optimal under structured conditions, typical in-home/community, or preschool/school, depending on the raters: direct assessment, parent-rated, or teacher-rated measurements. However, no studies of phthalates and preschool neurodevelopment have accounted for differences in presentation across multiple settings/raters.
We sought to conduct a comprehensive investigation of the associations between prenatal phthalate exposure and EF during the preschool period, leveraging a standardized clinical assessment of preschool ADHD that included validated parent- and teacher- inventories as well as performance-based assessments of EF. By incorporating data from multiple raters, including parent, teacher, and performance-based, we can assess the consistency of associations across the child’s EF measured in different environments.
2. Materials and methods
2.1. Study population
The Norwegian Mother, Father and Child Cohort Study (MoBa) is a population-based pregnancy cohort study conducted by the Norwegian Institute of Public Health. Pregnant women across Norway were recruited from 1999 to 2008 (Schreuder and Alsaker 2014). An invitation was sent to women prior to their routine prenatal ultrasound, which up to 98% of pregnant women in Norway have before their 20th gestational week (Backe 1997). During the ultrasound visit, pregnant women consented to participation (41% of the pregnancies) and now includes 114,500 children, 95,200 mothers, and 75,200 fathers. Study participants completed questionnaires and provided biosamples during pregnancy (Paltiel et al. 2014). The current study is based on version 9 of the quality-assured data files released for research on preschool ADHD.
Our study population is nested within the MoBa Preschool ADHD Substudy (Øvergaard, 2018). Eligibility criteria for the Preschool ADHD Substudy included births after April 1, 2004, living within a direct flight to Oslo. Among the eligible population, children exhibiting possible ADHD symptoms were oversampled based on items in the 36-month questionnaire describing ADHD-like symptoms (approximately 62% of the MoBa participants completed the questionnaire (Magnus 2007)). From the 36-month questionnaire, these 11 items describing ADHD-like symptoms were summed into a quantitative index and included six items from the Child Behavior Checklist/1.5–5 (Achenbach, 2010) and five items from the DSM-IV-TR criteria for ADHD (Association 2000). All children with summed scores ≥90th percentile were invited to participate in the Preschool ADHD Substudy (n = 2798), along with a random sample of the remainder of children (n = 654). Of those invited, 1195 children took part in a one-day clinical assessment when they were aged between 3 and 4 years (Skogan et al. 2015). We further restricted the Preschool ADHD Substudy participants to 870 children whose mothers had provided urine samples during pregnancy (870 out of 1195; Supplementary Figure 1).
Our analytic sample includes 262 children with clinically significant or subthreshold symptoms of ADHD based on DSM-IV-TR criteria, hereafter referred to as the preschool ADHD group; and 78 typically developing children, who were clinically confirmed as neurotypical following the on-site assessment. Because this population deliberately oversamples children who were symptomatic for ADHD using the 36-month questionnaire, such a sampling structure must be considered in analysis (Richardson et al. 2007). We, therefore, estimated the sampling fraction and conducted weighted analyses as described in the statistical analysis section below.
2.2. Measurement of phthalate metabolites
Methods (Engel et al. 2018) and quality control procedures (Ye et al. 2009) associated with phthalate metabolite measurement have been previously described. Briefly, phthalate metabolites were measured from maternal urine samples collected at approximately 17 weeks gestation. The urine samples were assayed in randomized batches at the Norwegian Institute of Public Health, using on-line column switching liquid chromatography coupled with tandem mass spectrometry (Sabaredzovic et al. 2015). These include: monoethyl phthalate (MEP), a metabolite of diethyl phthalate; mono-iso-butyl phthalate (MiBP), a metabolite of di-iso-butyl phthalate; mono-n-butyl phthalate (MnBP), a metabolite of di-n-butyl phthalate; monobenzyl phthalate (MBzP), a metabolite of BBzP; mono-2-ethylhexyl phthalate (MEHP), mono-2-ethyl-5-hydroxyhexyl phthalate (MEHHP), mono-2-ethyl-5-oxoyhexyl phthalate (MEOHP), mono-2-ethyl-5-carboxypentyl phthalate (MECPP), and mono-2-methylcarboxyhexyl phthalate (MMCHP), metabolites of DEHP; and, mono-4-methyl-7-hydroxyoctyl phthalate (OHMiNP), mono-4-methyl-7oxooctyl phthalate (oxo-MiNP), and mono-4-methyl-7-carboxyheptyl phthalate (cx-MiNP), metabolites of di-iso-nonyl phthalate (DiNP). Specific gravity was measured with a pocket refractometer (PAL-10S) from Atago, to account for urine dilution.
2.3. Measurement of executive function
We selected the instruments in the clinical assessment that measured constructs related to EF (Supplementary Table 1). This includes a parent-and teacher-rated inventory, Behavior Rating Inventory of Executive Function-Preschool [BRIEF-P], and three performance-based assessments, Stanford Binet IV short version [SB5]; A Developmental NEuroPSYchological Assessment [NEPSY]; and cookie delay task [CDT].
Parents and teachers were requested to fill in BRIEF-P, four weeks prior to the one-day clinical exam, and return it by the clinical exam (Skogan et al. 2015). The BRIEF-P was originally developed to rate EF of children aged 2–5 within the context of everyday environments, at home and preschool (Gioia et al. 1996). The instrument consists of 63 behavioral descriptors that characterize five EF domains: emotional control, inhibition, working memory, planning/organization, and shift. Parents and teachers rated whether the 63 behavioral descriptors have been a problem for the child during the past 6 months, by checking never (1), sometimes (2), or often (3). For the current study, we restricted the EF domains to emotional control, inhibition, and working memory, excluding planning/organization and shift. At this early point in development, the ability to shift and plan/organize has yet to reach a stable functional level. Consequently, these domains and their relations with other aspects of behavioral or cognitive functions may not be meaningfully interpreted (Skogan et al. 2015). Domain-specific raw scores rated by parents and teachers were standardized by age and sex to calculate T-scores.
At the one-day clinical exam, performance-based assessments were carried out by a psychologist while one parent was present, described in detail previously (Rohrer-Baumgartner et al. 2014). The range and interpretation of the test scores varied by the instrument; therefore the test scores from all performance-based assessments were standardized to have a mean of 0, a standard deviation of 1, with higher scores indicating a worse EF, in order to facilitate comparison across instruments.
SB5 is an intellectual battery that can be used to measure nonverbal and verbal cognitive factors: fluid reasoning, knowledge, quantitative reasoning, visual-spatial processing, and working memory (Roid and Pomplun 2012). We obtained measures of working memory from SB5, and an earlier version of SB5 has been validated in 30-months-old toddlers (Robinson et al. 1990). Verbal working memory was measured by asking each child to repeat sentences of increasing length. Nonverbal working memory was measured using the subtasks “delayed response” and “block span”. In “delayed response”, children were asked to find a toy that was hidden under one of three cups after a few seconds delay, or after switching the arrangement of the cups. In “block span”, children were asked to tap blocks in the same order as demonstrated.
NEPSY was originally developed to evaluate attention/EF, language, sensorimotor, visuospatial, and memory/learning in children from 3 to 12 years (Kemp and Korkman 2010). We obtained a measure of inhibition from NEPSY. The subtask “statue” in the NEPSY characterizes motor persistence and inhibition (3–6 years), by asking the child to stand still with their eyes closed and inhibit the impulse to talk, move, or open their eyes in response to distracting sounds.
CDT was originally designed to evaluate children’s ability to delay in response to verbal direction by an adult (Golden et al. 1977). We obtained a measure of self-control from CDT. Children were asked to choose between an immediate but smaller reward (one cookie) or a delayed but larger reward (two cookies). This task is similar to the marshmallow test, which measures self-control.
2.4. Measurement of covariates
Covariates that could influence exposure and/or outcome were selected after conducting a literature review. Maternal characteristics prior to and during pregnancy were obtained from the MoBa questionnaires that mothers completed at baseline (17 weeks gestation): ADHD symptoms, parity, marital status, education, pre-pregnancy BMI, self-reported depression before or during pregnancy, smoking, and alcohol intake during pregnancy. Maternal ADHD symptoms were assessed with the ADHD Symptom Checklist, which is an instrument consisting of the eighteen DSM-IV-TR criteria (Adler et al. 2003). Maternal lifestyle characteristics during pregnancy were obtained from a questionnaire at the 22nd gestational week: fish intake and folate use. Birth characteristics such as maternal age at birth, childbirth year, and child sex were obtained via data linkage with the medical birth registry of Norway, and information on child age at clinical exam was obtained from the Preschool ADHD Substudy.
2.5. Approach
Phthalate metabolite concentrations were standardized for specific gravity and potential batch effects as previously described (Engel et al. 2018). For the secondary metabolites of DEHP and DiNP, we calculated and used molar sum as opposed to using the individual secondary metabolites. Sampling fractions (Hernán et al. 2004) were calculated separately in two groups, children with ADHD symptoms summed scores ≥90th and <90th percentile, and represent the probability of being selected into the study sample from the ADHD Substudy eligible population. Distributions of phthalate metabolites, covariates, and EF performances were examined in the study population weighted by the inverse of the sampling fraction.
As we did not identify non-linear relations between phthalates and EF using loess curves, we used weighted multiple linear regression to estimate the difference in EF scores per interquartile range (IQR) increase in phthalate metabolite concentrations. All models were adjusted for covariates identified through a directed acyclic graph (DAG). Following covariates were considered in the construction of the DAG (Supplementary Figure 2): maternal ADHD symptoms, parity, marital status, education, pre-pregnancy BMI, self-reported depression before or during pregnancy, smoking during pregnancy, alcohol intake during pregnancy, fish intake during pregnancy, folate use during pregnancy, maternal age at birth, childbirth year, child sex, and child age at the clinical exam. Since missing data was present, we considered omitting some potential confounders from the minimally sufficient adjustment set in order to improve variance and selection bias. The final adjustment variables included maternal age at delivery (linear), maternal ADHD symptoms (linear), pre-pregnancy BMI (–24;25–29;30–), parity (no previous delivery; 1 or more previous delivery), childbirth year (2004; 2005; and 2006–2007), and child sex (female; male). Model fit was examined with visual inspections of residual plots and Cook’s distance.
We explored various approaches to address weighting and examined the robustness and plausibility of findings. In the main text, we present the results using the approach that best reflected the sampling procedure: inverse probability weighting based on children’s ADHD symptoms summed scores. Results from alternative approaches are presented as a supplementary figure, and include a simple adjustment for the preschool ADHD group/typically developing child status, stratifying by preschool ADHD group/typically developing child status, applying an alternative weight based on population prevalence of preschool ADHD, stratifying by ADHD symptoms summed scores, and no adjustment or weighting. The plausible range of bias observed between estimates from the weighted approaches and no adjustment were further investigated with simulations under different selection scenarios. Details of the simulation are provided in the supplementary document.
Lastly, effect measure modification (EMM) by child sex was assessed by including an interaction term between sex and a phthalate metabolite. We considered EMM significant at an alpha level of 0.10. Amongst the implicated phthalate metabolites in our analysis of single-pollutant models, we additionally considered potential co-pollutant confounding by including one additional implicated phthalate metabolite into the model. We used this simple approach to adjust for potential co-pollutant confounding since the correlations across phthalate species were low to moderate, ranging from −0.07 to 0.64 (Villanger et al. 2020).
All analyses were conducted using SAS 9.4 and R statistical software version 4.0.0. This study was approved by Regional Committee for Medical Research Ethics in Norway and was reviewed and determined to be exempt from further review by the Office of Human Research Ethics at the University of North Carolina at Chapel Hill.
3. Results
The median child age at clinical assessment was 3.5 years, with boys accounting for 55.7% of preschool children with elevated ADHD symptoms and 53.8% of the typically developing children (Table 1). The median maternal age at delivery was 30 years, with a higher percentage of mothers scoring high on ADHD symptoms (12.4% versus 6.5%) or having less than college complete education (35.2% versus 24.4%) among the preschool ADHD group as compared to the typically developing group (Table 1). The summary statistics for all EF measures are provided in Supplementary Table 1.
Table 1.
Socio-demographic characteristics of preschool attention-deficit hyperactivity disorder (ADHD) cases and the typically developing children in the Norwegian Mother, Father and Child Cohort (MoBa), 2003–2008.
| Variable | Weighted population (N = 310) | Preschool ADHD group (N = 262) | Typically developing children (N = 78) | |||
|---|---|---|---|---|---|---|
| N | % | N | % | N | % | |
| Birth year | ||||||
| 2004 | 68 | 22 | 26 | 9.9 | 20 | 25.6 |
| 2005 | 111 | 36 | 63 | 24.0 | 30 | 38.5 |
| 2006 | 103 | 33.3 | 90 | 34.4 | 23 | 29.5 |
| 2007 | 27 | 8.7 | 83 | 31.7 | 5 | 6.4 |
| Child sex | ||||||
| Male | 176 | 56.9 | 146 | 55.7 | 42 | 53.8 |
| Female | 134 | 43.1 | 116 | 44.3 | 36 | 46.2 |
| Child age at clinical assessment | ||||||
| 38–41 months | 208 | 68 | 157 | 60.4 | 50 | 64.9 |
| 42–45 months | 98 | 32 | 103 | 39.6 | 27 | 35.1 |
| Missing | 3 | 2 | 1 | |||
| Maternal age at delivery | ||||||
| <=20 years | 3 | 1.1 | 2 | 0.8 | 1 | 1.3 |
| 21–30 years | 163 | 53.3 | 141 | 53.8 | 40 | 51.9 |
| 31–40 years | 134 | 43.6 | 118 | 45.0 | 35 | 45.5 |
| >40 years | 6 | 2.1 | 1 | 0.4 | 1 | 1.3 |
| Missing | 3 | 0 | 1 | |||
| Maternal education | ||||||
| Less than college completed | 78 | 25.2 | 92 | 35.2 | 19 | 24.4 |
| College completed | 146 | 47 | 110 | 42.1 | 34 | 43.6 |
| More than college completed | 76 | 24.6 | 56 | 21.5 | 22 | 28.2 |
| Other education | 10 | 3.1 | 3 | 1.1 | 3 | 3.8 |
| Missing | 0 | 1 | 0 | |||
| Marital status | ||||||
| Single/other | 10 | 3.4 | 15 | 5.7 | 3 | 3.8 |
| Cohabiting | 141 | 45.4 | 132 | 50.6 | 33 | 42.3 |
| Married | 159 | 51.3 | 114 | 43.7 | 42 | 53.8 |
| Missing | 0 | 1 | 0 | |||
| Maternal body mass index (kg/m2) | ||||||
| <25 (under to normal weight) | 191 | 62.9 | 169 | 66.5 | 52 | 67.5 |
| 25-<30 (overweight) | 89 | 29.3 | 25 | 9.8 | 6 | 7.8 |
| ≥30 (obese) | 24 | 7.8 | 60 | 23.6 | 19 | 24.7 |
| Missing | 7 | 8 | 1 | |||
| Parity | ||||||
| Nulliparous | 174 | 56.7 | 157 | 59.9 | 43 | 55.8 |
| Multiparous | 133 | 43.3 | 105 | 40.1 | 34 | 44.2 |
| Missing | 3 | 0 | 1 | |||
| Maternal ADHD score | ||||||
| Not indicative of ADHD | 289 | 94.1 | 226 | 87.3 | 72 | 93.5 |
| Indicative of ADHD | 18 | 5.9 | 33 | 12.7 | 5 | 6.5 |
| Missing | 3 | 3 | 1 | |||
| Maternal smoking at baseline (17 weeks) | ||||||
| No | 230 | 75.1 | 200 | 76.6 | 61 | 79.2 |
| Yes | 76 | 24.9 | 61 | 23.4 | 16 | 20.8 |
| Missing | 3 | 1 | 1 | |||
Phthalate metabolites were detected in all maternal urine samples collected at 17 weeks’ gestation (Table 2). The highest geometric mean was observed for MEP (129.71 ± 3.56 ng/ml) and the lowest for a secondary metabolite of DiNP, OH-MiNP (0.92 ± 1.79 ng/ml; Table 2).
Table 2.
Geometric means (standard deviation) of phthalate metabolite concentrations in the study population, measured from urine samples provided by mothers during mid-pregnancy (specific gravity adjusted).
| Phthalate | Phthalate metabolites | Weighted population (N = 310) | Preschool ADHD group (N = 262) | Typically developing children (N = 78) | |
|---|---|---|---|---|---|
| Benzylbutyl phthalate | Monobenzyl phthalate (ng/ml) | MBzP | 5.45 (2.36) | 5.38 (2.48) | 5.35 (2.40) |
| Di-iso-butyl phthalate | Monoisobutyl phthalate (ng/ml) | MiBP | 19.64 (2.23) | 19.86 (2.11) | 18.95 (2.31) |
| Di-n-butyl | Mono-n-butyl phthalate (ng/ml) | MnBP | 21.70 (2.11) | 20.03 (2.23) | 20.90 (2.07) |
| Di-ethyl phthalate | Monoethyl phthalate (ng/ml) | MEP | 129.71 (3.65) | 113.36 (4.33) | 126.49 (3.89) |
| Di (2-ethylhexyl) phthalate | Mono (2-ethylhexyl) phthalate | MEHP | 13.06 (1.77) | 12.02 (2.12) | 13.51 (1.80) |
| Mono (2-ethyl-5-hydroxyhexyl) phthalate | MEHHP | 16.11 (2.20) | 15.57 (2.53) | 16.11 (2.28) | |
| Mono (2-ethyl-5-oxohexyl) phthalate | MEOHP | 22.53 (1.88) | 23.03 (2.11) | 22.22 (1.91) | |
| Mono (2-ethyl-5-carboxypentyl) phthalate | MECPP | 11.03 (2.22) | 10.56 (2.54) | 10.98 (2.32) | |
| Mono-2-methylcarboxyhexyl phthalate | MCHP | 23.21 (1.84) | 22.59 (2.02) | 23.37 (1.92) | |
| Σ Di (2-ethylhexyl) phthalate metabolites (μmol/L) | Σ DEHP | 0.30 (1.85) | 0.29 (2.12) | 0.30 (1.89) | |
| Diisononyl phthalate | Mono-4-methyl-7-hydroxyoctyl phthalate | OH-MiNP | 0.92 (1.79) | 1.11 (2.22) | 0.91 (1.86) |
| Mono-4-methyl-7oxooctyl phthalate | oxo-MiNP | 1.01 (1.93) | 1.30 (2.58) | 0.99 (1.99) | |
| Mono-4-methyl-7-carboxyheptyl phthalate | oxo-MiNP | 3.14 (1.55) | 3.78 (1.83) | 3.12 (1.58) | |
| Σ Diisononyl phthalate metabolites (μmol/L) | Σ DiNP | 0.02 (1.60) | 0.02 (2.04) | 0.02 (1.65) |
All urine samples had detectable concentrations of all phthalate metabolites.
Elevated MBzP during pregnancy was associated with poorer offspring EF during preschool age, consistently across domains and raters (Table 3). Specifically, an IQR increase in MBzP was associated with poorer working memory rated by teacher (: 1.13 [95% confidence intervals: 0.14, 2.13]), parent (1.23 [0.20, 2.26]), and performance-based (verbal: 0.13 [0.01, 0.25]; nonverbal 0.19 [0.09, 0. 28]). We also observed worse inhibition as reported by parents (1.00 [0.03, 1.98]) and performance-based (0.18 [0.08, 0.28]); and reporter-based emotional control (teacher: 1.23 [0.31, 2.15]; parent: 1.67 [0.89, 2.45]). Parent-reported emotional control scores were worse among boys with elevated prenatal MBzP (interaction p-value: 0.02; Table 4).
Table 3.
Change in executive function scoresa with an interquartile range (IQR) increase in urinary phthalate metabolite concentrationb during pregnancy, from fully adjusted linear regression models with inverse probability weightsc (N = 310).
| Phthalate metabolite | Behavior Rating Inventory of Executive Function-Preschool (BRIEF-P) | ||||||
|---|---|---|---|---|---|---|---|
| Teacher report | Parent report | ||||||
| Emotional Control | Inhibition | Working Memory | Emotional Control | Inhibition | Working Memory | ||
| Name | IQR | (95% CI) | (95% CI) | (95% CI) | (95% CI) | (95% CI) | (95% CI) |
| MBzP | 7.17 (ng/ml) | 1.23 (0.31, 2.15) | 0.52 (−0.54, 1.57) | 1.13 (0.14, 2.13) | 1.67 (0.89, 2.45) | 1.00 (0.03, 1.98) | 1.23 (0.20, 2.26) |
| MiBP | 19.39 (ng/ml) | 0.07 (−0.58, 0.72) | −0.32 (−1.06, 0.42) | −0.16 (−0.86, 0.54) | 0.89 (0.34, 1.44) | 0.71 (0.03, 1.39) | 0.23 (−0.49, 0.96) |
| MnBP | 22.93 (ng/ml) | 0.37 (−0.43, 1.17) | −0.14 (−1.05, 0.78) | −0.05 (−0.91, 0.81) | 1.56 (0.88, 2.23) | 1.70 (0.88, 2.53) | 1.49 (0.60, 2.37) |
| MEP | 213.15 (ng/ml) | 0.11 (−0.32, 0.54) | −0.13 (−0.62, 0.36) | −0.13 (−0.60, 0.34) | 0.26 (−0.11, 0.63) | −0.25 (−0.70, 0.21) | −0.21 (−0.70, 0.27) |
| Σ DEHP | 0.26 (μmol/L) | 0.03 (−0.52, 0.57) | −0.60 (−1.22, 0.01) | −0.79 (−1.37, −0.21) | 0.30 (−0.17, 0.77) | −0.18 (−0.76, 0.40) | −0.52 (−1.13, 0.09) |
| Σ DiNP | 0.01 (μmol/L) | −0.28 (−0.63, 0.06) | −0.004 (−0.40, 0.39) | −0.11 (−0.49, 0.26) | 0.13 (−0.17, 0.43) | 0.35 (−0.02, 0.72) | 0.02 (−0.38, 0.41) |
| Phthalate metabolite | Stanford-Binet IV | Cookie Delay Task | A Developmental NEuroPSYchological Assessment - Statue Task | ||||
| Child assessment | Child assessment | Child assessment | |||||
| Non-verbal WM | Verbal WM | Self-control | Inhibition | ||||
| Name | IQR | (95% CI) | (95% CI) | (95% CI) | (95% CI) | ||
| MBzP | 7.17 (ng/ml) | 0.19 (0.09, 0.28) | 0.13 (0.01, 0.25) | 0.07 (−0.03, 0.17) | 0.18 (0.08, 0.28) | ||
| MiBP | 19.39 (ng/ml) | 0.04 (−0.03, 0.11) | 0.05 (−0.03, 0.13) | −0.004 (−0.07, 0.07) | −0.04 (−0.12, 0.04) | ||
| MnBP | 22.93 (ng/ml) | 0.06 (−0.02, 0.15) | 0.07 (−0.02, 0.15) | 0.20 (0.12, 0.28) | 0.13 (0.05, 0.22) | ||
| MEP | 213.15 (ng/ml) | 0.004 (−0.04, 0.05) | −0.07 (−0.12, −0.02) | −0.01 (−0.05, 0.04) | 0.05 (0.01, 0.10) | ||
| Σ DEHP | 0.26 (μmol/L) | −0.01 (−0.07, 0.04) | −0.03 (−0.09, 0.03) | −0.04 (−0.10, 0.02) | −0.03 (−0.09, 0.03) | ||
| Σ DiNP | 0.01 (μmol/L) | −0.02 (−0.06, 0.02) | −0.01 (−0.05, 0.03) | −0.02 (−0.06, 0.01) | −0.02 (−0.06, 0.01) | ||
All scores of SB5, CDT, and NEPSY Statue Test were modified to have a mean of 0, a standard deviation of 1, and higher scores to indicate worse executive function.
Specific gravity and batch effect adjusted.
All models adjusted for maternal ADHD, BMI, age at delivery, parity, childbirth year, and child sex.
Abbreviations: MEP: Monoethyl phthalate; MnBP: Mono-n-butyl phthalate; MiBP: Monoisobutyl phthalate; MBzP: Monobenzyl phthalate; DEHP: Di (2-ethylhexyl) phthalate; DiNP: Diisononyl phthalate.
∑DEHP: molar sum of mono (2-ethylhexyl) phthalate, mono (2-ethyl-5-hydroxyhexyl) phthalate, mono (2-ethyl-5-oxohexyl) phthalate, mono (2-ethyl-5-carboxypentyl) phthalate, and mono-2-methylcarboxyhexyl phthalate; ∑DiNP: molar sum of mono-4-methyl-7-hydroxyoctyl phthalate, mono-4-methyl-7oxooctyl phthalate, mono-4-methyl-7-carboxyheptyl phthalate, oxo-DiNP, and ch-DiNP.
Abbreviations: WM: Working Memory; MEP: Monoethyl phthalate; MnBP: Mono-n-butyl phthalate; MiBP: Monoisobutyl phthalate; MBzP: Monobenzyl phthalate; DEHP: Di (2-ethylhexyl) phthalate; DiNP: Diisononyl phthalate; ∑DEHP: molar sum of mono (2-ethylhexyl) phthalate, mono (2-ethyl-5-hydroxyhexyl) phthalate, mono (2-ethyl-5-oxohexyl) phthalate, mono (2-ethyl-5-carboxypentyl) phthalate, and mono-2-methylcarboxyhexyl phthalate; ∑DiNP: molar sum of mono-4-methyl-7-hydroxyoctyl phthalate, mono-4-methyl-7oxooctyl phthalate, mono-4-methyl-7-carboxyheptyl phthalate, oxo-DiNP, and ch-DiNP.
Table 4.
Sex-specific changes in executive function scoresa per with an interquartile range increase (IQR) increase in phthalate metabolitesb and the interaction term p-values from fully adjusted linear regression models with inverse probability weightsc (N = 310).
| MBzP (IQR: 7.17 ng/ml) | MiBP (IQR: 19.39 ng/ml) | MnBP (IQR: 22.93) | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Boys | Girls | p | Boys | Girls | p | Boys | Girls | p | |
| Behavior Rating Inventory of Executive Function Preschool (teacher) | |||||||||
| Emotional control | 1.50 (0.27, 2.73) | 0.90 (−0.47, 2.26) | 0.52 | −0.11 (−1.12, 0.90) | 0.20 (−0.65, 1.06) | 0.64 | 0.10 (−0.87, 1.06) | 0.98 (−0.44, 2.41) | 0.31 |
| Inhibition | 1.39 (−0.02, 2.79) | −0.55 (−2.12, 1.01) | 0.07 | 0.14 (−1.00, 1.28) | −0.65 (−1.62, 0.31) | 0.30 | 0.51 (−0.58, 1.60) | −1.57 (−3.19, 0.05) | 0.04 |
| Working memory | 0.92 (−0.41, 2.25) | 1.4 (−0.08, 2.87) | 0.64 | −1.33 (−2.40, −0.26) | 0.69 (−0.22, 1.59) | <0.01 | −0.52 (−1.56, 0.51) | 1.00 (−0.54, 2.54) | 0.11 |
| Behavior Rating Inventory of Executive Function Preschool (parent) | |||||||||
| Emotional control | 2.51 (1.47, 3.55) | 0.67 (−0.46, 1.81) | 0.02 | 2.16 (1.32, 2.99) | −0.03 (−0.74, 0.68) | <0.01 | 2.04 (1.23, 2.84) | 0.48 (−0.72, 1.67) | 0.03 |
| Inhibition | 1.50 (0.20, 2.81) | 0.41 (−1.02, 1.83) | 0.26 | 1.88 (0.84, 2.92) | −0.15 (−1.04, 0.74) | <0.01 | 2.74 (1.77, 3.72) | −0.63 (−2.08, 0.83) | <0.01 |
| Working memory | 1.52 (0.14, 2.90) | 0.88 (−0.62, 2.39) | 0.53 | 0.95 (−0.17, 2.06) | −0.29 (−1.24, 0.66) | 0.10 | 2.91 (1.89, 3.94) | −1.72 (−3.25, −0.19) | <0.01 |
| Stanford-Binet IV (child) | |||||||||
| Non-verbal working memory | 0.14 (0.01, 0.27) | 0.24 (0.09, 0.38) | 0.32 | 0.17 (0.06, 0.28) | −0.05 (−0.15, 0.04) | <0.01 | 0.16 (0.06, 0.27) | −0.16 (−0.31, −0.01) | <0.01 |
| Verbal working memory | 0.17 (0.03, 0.31) | 0.03 (−0.18, 0.25) | 0.28 | 0.06 (−0.05, 0.17) | 0.05 (−0.07, 0.16) | 0.87 | 0.04 (−0.07, 0.14) | 0.13 (−0.03, 0.29) | 0.37 |
| Cookie Delay Task (child) | |||||||||
| Self-control | 0.03 (−0.10, 0.17) | 0.11 (−0.03, 0.26) | 0.42 | 0.02 (−0.08, 0.13) | −0.02 (−0.11, 0.07) | 0.51 | 0.29 (0.19, 0.39) | −0.003 (−0.15, 0.15) | <0.01 |
| A Developmental NEuroPSYchological Assessment - Statue Task (child) | |||||||||
| Inhibition | 0.09 (−0.04, 0.23) | 0.27 (0.13, 0.42) | 0.07 | −0.18 (−0.29, −0.08) | 0.12 (0.01, 0.24) | <0.01 | 0.14 (0.04, 0.24) | 0.11 (−0.06, 0.27) | 0.71 |
| MEP (IQR: 213.15) | Σ DEHP metabolites (IQR: 0.26 mol/L) | Σ DiNP metabolites (IQR: 0.01 mol/L) | |||||||
| Boys | Girls | p | Boys | Girls | p | Boys | Girls | p | |
| Behavior Rating Inventory of Executive Function Preschool (parent) | |||||||||
| Emotional control | −0.01 (−0.48, 0.45) | 0.71 (0.12, 1.29) | 0.05 | 0.3 (−0.2, 0.79) | 0.29 (−1.21, 1.79) | 0.99 | 0.2 (−0.24, 0.63) | 0.07 (−0.34, 0.49) | 0.70 |
| Inhibition | −0.52 (−1.09, 0.05) | 0.2 (−0.52, 0.92) | 0.11 | −0.13 (−0.74, 0.48) | −0.62 (−2.47, 1.22) | 0.62 | 0.18 (−0.36, 0.71) | 0.51 (0, 1.01) | 0.38 |
| Working memory | −0.66 (−1.26, −0.07) | 0.52 (−0.23, 1.28) | 0.01 | −0.31 (−0.94, 0.33) | −2.46 (−4.39, −0.53) | 0.04 | 0.18 (−0.39, 0.75) | −0.13 (−0.67, 0.41) | 0.44 |
| Behavior Rating Inventory of Executive Function Preschool (teacher) | |||||||||
| Emotional control | −0.23 (−0.77, 0.3) | 0.67 (−0.01, 1.34) | 0.03 | 0.16 (−0.41, 0.73) | −1.21 (−2.94, 0.52) | 0.14 | −0.04 (−0.54, 0.46) | −0.5 (−0.98, −0.03) | 0.19 |
| Inhibition | 0.05 (−0.56, 0.66) | −0.42 (−1.19, 0.35) | 0.33 | −0.55 (−1.2, 0.09) | −1.04 (−2.99, 0.92) | 0.65 | 0.02 (−0.55, 0.59) | −0.03 (−0.57, 0.52) | 0.90 |
| Working memory | 0.04 (−0.54, 0.61) | −0.4 (−1.13, 0.33) | 0.35 | −0.77 (−1.38, −0.16) | −0.89 (−2.74, 0.95) | 0.90 | 0.06 (−0.48, 0.6) | −0.27 (−0.79, 0.24) | 0.38 |
| Stanford-Binet IV (child) | |||||||||
| Non-verbal working memory | 0.04 (−0.02, 0.1) | −0.05 (−0.13, 0.02) | 0.05 | −0.02 (−0.09, 0.04) | 0.06 (−0.13, 0.25) | 0.39 | −0.04 (−0.09, 0.02) | −0.01 (−0.06, 0.04) | 0.50 |
| Verbal working memory | −0.09 (−0.16, −0.03) | −0.04 (−0.12, 0.04) | 0.25 | −0.02 (−0.09, 0.04) | −0.05 (−0.26, 0.16) | 0.81 | −0.02 (−0.07, 0.04) | −0.001 (−0.05, 0.05) | 0.67 |
| Cookie Delay Task (child) | |||||||||
| Self-control | −0.01 (−0.07, 0.04) | 0.002 (−0.07, 0.07) | 0.74 | −0.06 (−0.12, −0.004) | 0.17 (−0.02, 0.35) | 0.02 | −0.01 (−0.06, 0.05) | −0.04 (−0.09, 0.01) | 0.40 |
| A Developmental NEuroPSYchological Assessment - Statue Task (child) | |||||||||
| Inhibition | 0.1 (0.04, 0.15) | −0.02 (−0.09, 0.05) | 0.007 | −0.04 (−0.1, 0.02) | 0.08 (−0.12, 0.28) | 0.24 | −0.02 (−0.08, 0.03) | −0.03 (−0.08, 0.03) | 0.94 |
All scores of child assessment modified to have a mean of 0, standard deviation of 1, and higher scores to indicate worse executive function.
Specific gravity and batch effect adjusted.
All models adjusted for maternal Attention-deficit hyperactivity disorder, BMI, age at delivery, parity, child birth year, and child sex.
Abbreviations: MBzP: Monobenzyl phthalate; MnBP: Mono-n-butyl phthalate; MiBP: Monoisobutyl phthalate.
Abbreviations: MEP: Monoethyl phthalate; DEHP: Di (2-ethylhexyl) phthalate; DiNP: Diisononyl phthalate; ∑DEHP: molar sum of mono (2-ethylhexyl) phthalate, mono (2-ethyl-5-hydroxyhexyl) phthalate, mono (2-ethyl-5-oxohexyl) phthalate, mono (2-ethyl-5-carboxypentyl) phthalate, and mono-2-methylcarboxyhexyl phthalate; ∑DiNP: molar sum of mono-4-methyl-7-hydroxyoctyl phthalate, mono-4-methyl-7oxooctyl phthalate, mono-4-methyl-7-carboxyheptyl phthalate, oxo-DiNP, and ch-DiNP.
Elevated MiBP and MnBP during pregnancy were also associated with poorer EF in offspring during preschool years, although we observed variability in associations by raters. Parents consistently reported worse EF across all domains (Table 3), particularly among boys (MiBP: 2.16 [1.32, 2.99], interaction p-value < 0.001; MnBP 2.04 [1.23, 2.84], interaction p-value: 0.03; Table 4). Teacher-rated inventories and performance-based assessments generated substantially fewer statistically significant sex-interactions than parents (Table 4), however, both teacher-and parent-reported measures of inhibition were modification by child sex. Specifically, poorer inhibition was reported by teachers and parents among boys (teacher: 0.51 [−0.58, 1.60]; parent: 2.74 [1.77, 3.72]); while fewer inhibition symptoms were rated among girls (teacher: −1.57 [−3.19, 0.05], interaction p-value: 0.04; parent: −0.63 [−2.08, 0.83], interaction p-value < 0.01).
For MEP, we observed potential sex-specific interactions, however, the most implicated sex was not always consistent across EF domains. We did not observe notable associations with DEHP or DiNP and EF.
In sensitivity analyses, we did not observe strong evidence of confounding by phthalate co-exposures (Fig. 1). We observed stronger associations in the primary analysis that used inverse probability of selection weighting, which accounted for the oversampling of children in the high ADHD symptoms group, although the confidence intervals of the alternative approaches overlapped in most instances (Supplementary Figure 3). Such deviation between the weighted and unweighted approaches shows the potential bias that can be introduced by not considering the over-sampling of the ADHD group. The observed bias appeared within a plausible range from our simulation study, where not accounting for selection into the study resulted in bias as large as 3 times the true association (Supplementary materials regarding simulation; Supplementary Table 2; Supplementary Figure 4).
Fig. 1.
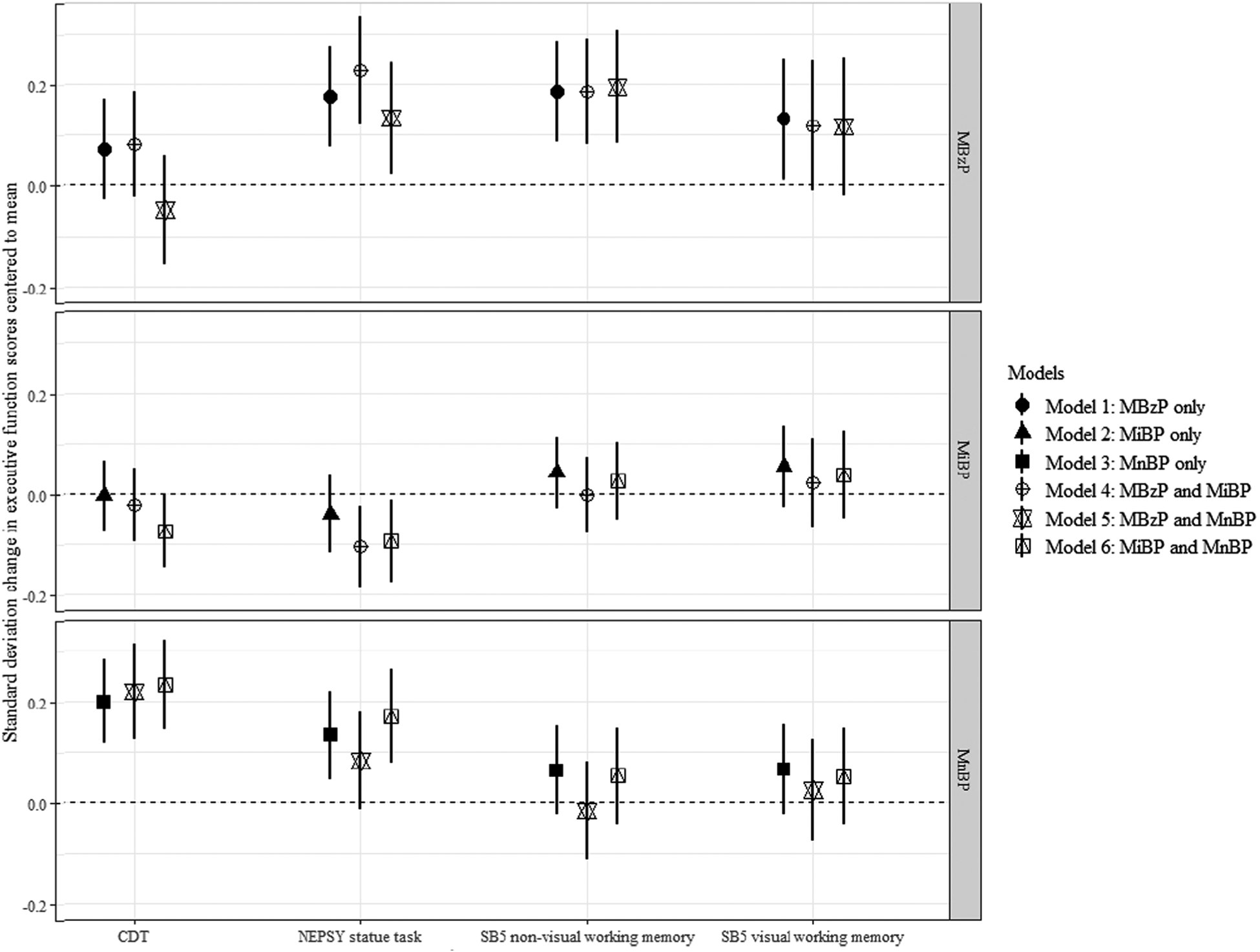
Changes in executive function scoresa ( and 95% confidence intervals, CI) per interquartile range increase in urinary phthalate metabolite concentrationb during pregnancy, from weighted linear regression modelsc with single phthalate (Models 1–3) or two phthalate metabolites (Models 4–6). aAll scores were modified to have a mean of 0, a standard deviation of 1, and higher scores to indicate worse executive function. b Specific gravity and batch effect adjusted. cAll models weighted for study selection and additionally adjusted for maternal ADHD, BMI, age at delivery, parity, child birth year, and child sex. Abbreviations: NEPSY: A Developmental NEuroPSYchological Assessment; SB5: Stanford-Binet IV; CDT: Cookie Delay Task; MnBP: Mono-n-butyl phthalate; MiBP: Monoisobutyl phthalate; MBzP: Monobenzyl phthalate.
4. Discussion
In this investigation of prenatal phthalate exposure and neurodevelopment, we leveraged a comprehensive assessment of EF that included both rater-based inventories and performance-based assessments, in order to evaluate the consistency in associations of phthalates and EF across multiple environments. We found that higher exposure to benzyl-butyl phthalate during pregnancy was consistently associated with poorer EF in all domains across most instruments. Sex-specific associations were observed for di-butyl phthalate metabolites, with adverse associations mostly found among boys. However, these associations varied somewhat by rater. We did not find strong evidence of confounding by phthalate co-exposures.
The poor EF in 3-year-olds associated with elevated MBzP is noteworthy given that the point estimates are consistent in the direction across domains (i.e., emotional control, inhibition, and working memory) and instruments (parent/teacher-rated and performance-based). We observed the strongest associations for parent-reported measures, particularly emotional control, which is consistent with previous studies of children under the age of 5. For children with elevated prenatal MBzP, parents reported more symptoms of emotional problems (Philippat et al., 2017; Whyatt et al. 2012), impulsive symptoms (Gascon et al. 2015), internalizing behaviors (Philippat et al., 2017), social fear (Singer et al. 2017), and peer relationship problems (Philippat et al., 2017), although some estimates were imprecise (Gascon et al. 2015; Philippat et al., 2017). Interestingly, in a study that examined behavioral problems at age 3 and 5, weaker and more imprecise associations were observed among older children (Philippat et al., 2017). Similarly across studies of older children, mixed findings have been reported (Kobrosly et al. 2014; Lien et al. 2015).
We also observed that prenatal MiBP and MnBP exposures were associated with poorer EF across parent-rated BRIEF-P. Particularly, the association between MnBP and working memory observed in our study is in line with the findings from the only other study that utilized BRIEF (Engel et al. 2010), despite the differences in gestational weeks at urine sample collection (current: 17; previous: 25–40) and age at outcome assessment (4–9; 3.5 years). In our study, MnBP mostly affected working memory among boys, along with worse emotional control and inhibition. Similarly, more emotional problems (Engel et al. 2010; Lien et al. 2015; Philippat et al., 2017; Whyatt et al. 2012), internalizing behaviour (Lien et al. 2015; Philippat et al., 2017; Whyatt et al. 2012), conduct problems (Philippat et al., 2017), and externalizing behavior (Engel et al. 2010; Lien et al. 2015) have been reported by parents, often among boys (Engel et al. 2010; Kobrosly et al. 2014; Philippat et al., 2017). Although we observed similar associations for MiBP, findings are more inconsistent across literature with only one study reporting notable associations with more externalizing behavior, aggression, rule-breaking behavior, and attention problems at age 8, mostly among boys (Kobrosly et al. 2014). The geometric mean of MiBP in Kobrosly’s population (2.34 ng/ml) is much lower compared to our study (19.64 ng/ml) or other studies (9–40 ng/ml; (Radke et al. 2020)), however, both our study and Kobrosly measured MiBP during 2nd trimester as compared to other studies, which estimated exposure during the 3rd trimester. Such differences in exposure window may have contributed to heterogeneous results, since MiBP has been reported with intraclass correlation coefficients slightly lower than that of MnBP (Casas et al. 2018; Sakhi et al. 2017).
In contrast with our prior research documenting associations of prenatal DEHP exposure with diagnosed ADHD in childhood (Engel et al. 2018), we did not observed deficits in preschool EF in relation to DEHP exposure. Associations with DEHP are mixed with a number of near-null findings in studies using performance-based (Factor-Litvak et al. 2014) or parent-rated (Engel et al. 2010; Gascon et al. 2015; Kim et al. 2017; Kim et al. 2018; Kobrosly et al. 2014; Lien et al. 2015; Philippat et al., 2017; Whyatt et al. 2012) instruments, however, some have found adverse associations (Engel et al. 2018; Lien et al. 2015). Discrepancies in DEHP associations with neurodevelopment across studies may in part be related to the notable temporal decline in population DEHP exposures that have been observed worldwide (Jensen et al. 2012; Shu et al. 2018; Zota et al. 2014). Given the temporal changes in DEHP exposure, births in more recent years would have had lower cumulative exposure to DEHP compared to those in earlier years. For example, our previous investigation had an earlier mean year at birth (Engel et al. 2018). In general, studies consisting of heterogeneous birth years due to longer enrollment periods are more likely to report null findings as compared to studies that enrolled pregnant women over a shorter period of time spanning earlier years. Another explanation may involve low intraclass correlation coefficients reported for DEHP (Braun et al. 2012; Casas et al. 2018).
The current study adds to a growing literature documenting substantial adverse neurodevelopmental impacts of prenatal phthalate exposure (Braun et al. 2017; Braun et al. 2014; Doherty et al. 2017; Engel et al. 2010; Engel et al. 2018; Engel et al. 2009; Factor-Litvak et al. 2014; Gascon et al. 2015; Huang et al. 2015; Huang et al., 2017; Ipapo et al. 2017; Kim et al. 2017; Kim et al. 2018; Kim et al. 2011; Kobrosly et al. 2014; Larsson et al. 2009; Lien et al. 2015; Messerlian et al. 2017; Miodovnik et al. 2011; Nakiwala et al., 2018; Olesen et al. 2018; Percy et al. 2016; Philippat et al. 2015; Philippat et al., 2017; Polanska et al. 2014; Qian et al. 2019; Singer et al. 2017; Tellez-Rojo et al. 2013; Whyatt et al. 2012; Yolton et al. 2011), and provides evidence that deficits in EF may be a unifying neurological mechanism tying together behavioral and cognitive endpoint. To our knowledge, no other study to date has incorporated both parent-and teacher- completed inventories along with performance-based assessments during the preschool period. It has long been known that parents and teachers report differently about child behavior (Achenbach et al. 1987), which has also been found in the ADHD Substudy with correlation up to 0.3 (Overgaard et al. 2019). Rater-based and performance-based instruments of EF are known to be poorly correlated (Toplak et al. 2013), in part because they assess different aspects of executive function. Performance-based assessments instantaneously evaluate a child’s optimal executive function under highly standardized conditions, whereas rater-based inventories are aggregated measures of the child’s functioning in typical everyday situations over a longer period of time, either at school or in the home/community setting. By incorporating all of these instruments, our reported association of MBzP with poorer EF can be said to be robust to the environment, rater, and condition. On the contrary, the impact of MiBP and MnBP on EF varied by raters, with more pronounced associations reported among parents but not as much with teacher-reported or performance-based measures of EF. Such phenotypical differences in implicated executive function may imply that the underlying neurological mechanism of MiBP and MnBP are different from that of MBzP and calls for further mechanistic research.
Our study leveraged an existing, comprehensive assessment of EF that enabled us to investigate our hypotheses at a level of detail not previously attempted. We also had access to important covariates unavailable in prior studies, including maternal ADHD symptoms, which allowed us to account for heritable aspects of executive function. Although some previous studies adjusted for maternal IQ (Braun et al. 2014; Factor-Litvak et al. 2014; Kim et al. 2017; Lien et al. 2015; Whyatt et al. 2012) or psychological difficulties during pregnancy (Philippat et al., 2017), these covariates may not accurately capture genetical drivers of executive function. We additionally assessed potential confounding by phthalate co-exposures and found no evidence; although it is possible that there may be potential confounding by non-phthalate co-exposures.
Our study also had several limitations. MoBa only collected one urine sample during pregnancy, and we were therefore limited to single spot urine to characterize phthalate exposure in our population (Adibi et al. 2008). Although phthalate metabolites have short half-lives, individuals’ exposure patterns to phthalates may be stable in the short-term, given that phthalates are included in consumer products that humans use on a daily basis. In general, the phthalates we found to be most strongly associated with EF have been previously reported with moderate to high intraclass correlation coefficients (Adibi et al. 2008; Braun et al. 2012; Casas et al. 2018), and there are very few studies in the literature that have utilized more than one urine during pregnancy to assess exposure (Braun et al. 2014; Gascon et al. 2015; Percy et al. 2016; Qian et al. 2019; Yolton et al. 2011). Another limitation is in the relatively small sample size and over-sampling of children with symptoms of ADHD. We accounted for this over-selection of children with ADHD symptoms by down-weighting the contribution of children with ADHD symptoms, and up-weighting the contribution of the children without ADHD symptoms, to reflect their selection probability in the eligible population. Although the point estimates from the weighted analysis were oftentimes overlapping with an alternative unweighted approach, in some cases unweighted estimates attenuated to the null. Finally, we were only able to account for selection due to the over-sampling of ADHD-like symptoms and not other factors that may influence attrition of study participants. For example, the ADHD Substudy participants’ mothers were older and had slightly higher education and fewer children than the general MoBa population.
Although experimental evidence showing toxicities of phthalates on neurogenesis exist (Lee et al. 2015), their underlying mechanism is not well-established. Multiple underlying pathways have been proposed, including alterations in sex steroid or thyroid hormone signaling (Miodovnik et al. 2014). Phthalates such as benzylbutyl and dibutyl phthalate have been reported to interact with genes regulating androgen/estrogen synthesis (Ghisari and Bonefeld-Jorgensen 2009; Singh and Li 2011), which are critical to neuronal maturation and growth (Gore et al. 2014; Matsumoto 1991; McEwen 2002). Additionally, benzylbutyl phthalate and dibutyl phthalate may interfere with the thyroid hormone signaling pathway (Ghisari and Bonefeld-Jorgensen 2009; Singh and Li 2011), which plays a key role in central nervous system development (Parent et al. 2011). Phthalates may also cross the placental barrier (Latini et al. 2003) and directly influence neurogenesis (Lee et al. 2015) through these or other pathways.
The phthalate metabolites implicated in our study continues to be highly prevalent in contemporary populations (CDC, 2019; Wu et al. 2020). Further, butyl-benzyl and di-butyl phthalates are considered substances of very high concern by the European Union and are regulated in toys and childcare products due to their endocrine disruption properties (Regulation 1999). These policies should be modified to target exposures in the general population, given the increasing scientific evidence that links prenatal phthalate exposures to neurobehavioral development as well as for other developmental endpoints (Lovekamp-Swan and Davis 2003).
5. Conclusion
We found that higher urinary concentrations of MBzP, MiBP, and MnBP during pregnancy were associated with poorer executive function in offspring. In some cases, associations were primarily found among boys. Exposures to pregnant women should be considered in policy regulations aimed at reducing exposures during critical windows of child development.
Supplementary Material
Acknowledgement
We thank all families who participated in this ongoing cohort study. GC was supported in part by a training grant from the National Institute of Environmental Health Sciences [T32ES007018]. This research was funded in part by National Institutes of Health/National Institute of Environmental Health Sciences (NIH/NIEHS) R01ES021777, P30 ES010126, and by the Intramural Research Program of the NIH/NIEHS. The Norwegian Mother, Father and Child (MoBa) Cohort Study is supported by the Norwegian Ministry of Health and Care Services and the Ministry of Education and Research, NIH/NIEHS (no. N01-ES-75558), NIH NIH/National Institute of Neurological Disorders and Stroke (NINDS) (no. 1 UO1 NS 047537-01 and no. 2 UO1 NS 047537-06A1). The Preschool ADHD study, a substudy to MoBa, is supported by funds and grants from the Norwegian Ministry of Health, The Norwegian Health Directorate, The South Eastern Health Region, the G&PJ Sorensen Fund for Scientific Research, and from The Norwegian Centre of Expertise for Neurodevelopmental Disorders and Hypersomnias, Oslo University Hospital.
Footnotes
Declaration of Competing Interest
None.
Appendix A. Supplementary material
Supplementary data to this article can be found online at https://doi.org/10.1016/j.envint.2021.106403.
References
- Achenbach TM, McConaughy SH, Howell CT, 1987. Child/adolescent behavioral and emotional problems: implications of cross-informant correlations for situational specificity. Psychol. Bull 101, 213. [PubMed] [Google Scholar]
- Achenbach TM, R. L, 2010. Multicultural supplement to the Manual for the ASEBA preschool forms & profiles: child behavior checklist for ages 1 1/2–5, language development survey, caregiver-teacher report form: an integrated system of multi-informant assessment ed. Burlington, VT: ASEBA. [Google Scholar]
- Adibi JJ, Whyatt RM, Williams PL, Calafat AM, Camann D, Herrick R, Nelson H, Bhat HK, Perera FP, Silva MJ, Hauser R, 2008. Characterization of phthalate exposure among pregnant women assessed by repeat air and urine samples. Environ Health Perspect. 116, 467–473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adler L, Kessler RC, Spencer T, 2003. Adult ADHD self-report scale-v1. 1 (ASRS-v1. 1) symptom checklist New York, NY: World Health Organization; 2003. [Google Scholar]
- Association AP, 2000. Diagnostic and statistical manual of mental disorders: DSM-IV-TR edêds. American Psychiatric Association, Washington, DC. [Google Scholar]
- Backe B, 1997. Routine ultrasonography in obstetric care in Norway, 1994. Tidsskrift for den Norske laegeforening: tidsskrift for praktisk medicin, ny raekke 117, 2314–2315. [PubMed] [Google Scholar]
- Bennett D, Bellinger DC, Birnbaum LS, Bradman A, Chen A, Cory-Slechta DA, Engel SM, Fallin MD, Halladay A, Hauser R, Hertz-Picciotto I, Kwiatkowski CF, Lanphear BP, Marquez E, Marty M, McPartland J, Newschaffer CJ, Payne-Sturges D, Patisaul HB, Perera FP, Ritz B, Sass J, Schantz SL, Webster TF, Whyatt RM, Woodruff TJ, Zoeller RT, Anderko L, Campbell C, Conry JA, DeNicola N, Gould RM, Hirtz D, Huffling K, Landrigan PJ, Lavin A, Miller M, Mitchell MA, Rubin L, Schettler T, Tran HL, Acosta A, Brody C, Miller E, Miller P, Swanson M, Witherspoon NO, American College of, O., Gynecologists, Child Neurology, S., Endocrine, S., International Neurotoxicology, A., International Society for Children’s, H., the, E., International Society for Environmental, E., National Council of Asian Pacific Islander, P., National Hispanic Medical, A., National Medical, A. Project TENDR: Targeting Environmental Neuro-Developmental Risks The TENDR Consensus Statement. Environ Health Perspect 2016,124:A118–122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braun JM, Bellinger DC, Hauser R, Wright RO, Chen A, Calafat AM, Yolton K, Lanphear BP, 2017. Prenatal phthalate, triclosan, and bisphenol A exposures and child visual-spatial abilities. Neurotoxicology 58, 75–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braun JM, Kalkbrenner AE, Just AC, Yolton K, Calafat AM, Sjodin A, Hauser R, Webster GM, Chen A, Lanphear BP, 2014. Gestational exposure to endocrine-disrupting chemicals and reciprocal social, repetitive, and stereotypic behaviors in 4- and 5-year-old children: the HOME study. Environ. Health Perspect 122, 513–520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braun JM, Smith KW, Williams PL, Calafat AM, Berry K, Ehrlich S, Hauser R, 2012. Variability of urinary phthalate metabolite and bisphenol A concentrations before and during pregnancy. Environ. Health Perspect 120, 739–745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casas M, Basagaña X, Sakhi AK, Haug LS, Philippat C, Granum B, Manzano-Salgado CB, Brochot C, Zeman F, de Bont J, 2018. Variability of urinary concentrations of non-persistent chemicals in pregnant women and school-aged children. Environ. Int 121, 561–573. [DOI] [PubMed] [Google Scholar]
- CDC, 2019. Fourth Report on Human Exposure to Environmental Chemicals, Updated Tables.
- Clark C, Prior M, Kinsella GJ, 2000. Do executive function deficits differentiate between adolescents with ADHD and oppositional defiant/conduct disorder? A neuropsychological study using the Six Elements Test and Hayling Sentence Completion Test. J. Abnorm. Child Psychol 28, 403–414. [DOI] [PubMed] [Google Scholar]
- Doherty BT, Engel SM, Buckley JP, Silva MJ, Calafat AM, Wolff MS, 2017. Prenatal phthalate biomarker concentrations and performance on the Bayley Scales of Infant Development-II in a population of young urban children. Environ. Res 152, 51–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engel SM, Miodovnik A, Canfield RL, Zhu C, Silva MJ, Calafat AM, Wolff MS, 2010. Prenatal phthalate exposure is associated with childhood behavior and executive functioning. Environ. Health Perspect 118, 565–571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engel SM, Villanger GD, Nethery RC, Thomsen C, Sakhi AK, Drover SS, Hoppin JA, Zeiner P, Knudsen GP, Reichborn-Kjennerud T, 2018. Prenatal Phthalates, Maternal Thyroid Function, and Risk of Attention-Deficit Hyperactivity Disorder in the Norwegian Mother and Child Cohort. Environ. Health Perspect 126, 057004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engel SM, Zhu C, Berkowitz GS, Calafat AM, Silva MJ, Miodovnik A, Wolff MS, 2009. Prenatal phthalate exposure and performance on the Neonatal Behavioral Assessment Scale in a multiethnic birth cohort. Neurotoxicology 30, 522–528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- EPA, 2012. Phthalates Action Plan.
- Factor-Litvak P, Insel B, Calafat AM, Liu X, Perera F, Rauh VA, Whyatt RM, 2014. Persistent Associations between Maternal Prenatal Exposure to Phthalates on Child IQ at Age 7 Years. PLoS ONE 9, e114003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garon N, Bryson SE, Smith IM, 2008. Executive function in preschoolers: a review using an integrative framework. Psychol. Bull 134, 31–60. [DOI] [PubMed] [Google Scholar]
- Gascon M, Valvi D, Forns J, Casas M, Martinez D, Julvez J, Monfort N, Ventura R, Sunyer J, Vrijheid M, 2015. Prenatal exposure to phthalates and neuropsychological development during childhood. Int. J. Hyg. Environ. Health 218, 550–558. [DOI] [PubMed] [Google Scholar]
- Ghisari M, Bonefeld-Jorgensen EC, 2009. Effects of plasticizers and their mixtures on estrogen receptor and thyroid hormone functions. Toxicol. Lett 189, 67–77. [DOI] [PubMed] [Google Scholar]
- Gioia GA, Andrwes K, Isquith PK, 1996. Behavior rating inventory of executive function-preschool version (BRIEF-P) edêds. Psychol. Assess. Resourc. Odessa, FL. [Google Scholar]
- Golden M, Montare A, Bridger W, 1977. Verbal control of delay behavior in two-year-old boys as a function of social class. Child Dev. 1107–1111. [Google Scholar]
- Gore AC, Martien KM, Gagnidze K, Pfaff D, 2014. Implications of prenatal steroid perturbations for neurodevelopment, behavior, and autism. Endocr. Rev 35, 961–991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hernán MA, Hernández-Díaz S, Robins JM, 2004. A structural approach to selection bias. Epidemiology 15, 615–625. [DOI] [PubMed] [Google Scholar]
- Huang HB, Chen HY, Su PH, Huang PC, Sun CW, Wang CJ, Chen HY, Hsiung CA, Wang SL, 2015. Fetal and Childhood Exposure to Phthalate Diesters and Cognitive Function in Children Up to 12 Years of Age: Taiwanese Maternal and Infant Cohort Study. PLoS ONE 10, e0131910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang PC, Tsai CH, Chen CC, Wu MT, Chen ML, Wang SL, Chen BH, Lee CC, Jaakkola JJK, Wu WC, Chen MK, Hsiung CA, Group R, 2017. Intellectual evaluation of children exposed to phthalate-tainted products after the 2011 Taiwan phthalate episode. Environ. Res 156:158–166. [DOI] [PubMed] [Google Scholar]
- Ipapo KN, Factor-Litvak P, Whyatt RM, Calafat AM, Diaz D, Perera F, Rauh V, Herbstman JB, 2017. Maternal prenatal urinary phthalate metabolite concentrations and visual recognition memory among infants at 27 weeks. Environ. Res 155, 7–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jensen MS, Norgaard-Pedersen B, Toft G, Hougaard DM, Bonde JP, Cohen A, Thulstrup AM, Ivell R, Anand-Ivell R, Lindh CH, Jonsson BA, 2012. Phthalates and perfluorooctanesulfonic acid in human amniotic fluid: temporal trends and timing of amniocentesis in pregnancy. Environ. Health Perspect 120, 897–903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kemp SL, Korkman M, 2010. Essentials of NEPSY-II assessment. John Wiley & Sons. [Google Scholar]
- Kim JI, Hong YC, Shin CH, Lee YA, Lim YH, Kim BN, 2017. The effects of maternal and children phthalate exposure on the neurocognitive function of 6-year-old children. Environ. Res 156, 519–525. [DOI] [PubMed] [Google Scholar]
- Kim S, Eom S, Kim HJ, Lee JJ, Choi G, Choi S, Kim S, Kim SY, Cho G, Kim YD, Suh E, Kim SK, Kim S, Kim GH, Moon HB, Park J, Kim S, Choi K, Eun SH, 2018. Association between maternal exposure to major phthalates, heavy metals, and persistent organic pollutants, and the neurodevelopmental performances of their children at 1 to 2years of age- CHECK cohort study. Sci. Total Environ 624, 377–384. [DOI] [PubMed] [Google Scholar]
- Kim Y, Ha EH, Kim EJ, Park H, Ha M, Kim JH, Hong YC, Chang N, Kim BN, 2011. Prenatal exposure to phthalates and infant development at 6 months: prospective Mothers and Children’s Environmental Health (MOCEH) study. Environ. Health Perspect 119, 1495–1500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kobrosly RW, Evans S, Miodovnik A, Barrett ES, Thurston SW, Calafat AM, Swan SH, 2014. Prenatal phthalate exposures and neurobehavioral development scores in boys and girls at 6–10 years of age. Environ. Health Perspect 122, 521–528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larsson M, Weiss B, Janson S, Sundell J, Bornehag C-G, 2009. Associations between indoor environmental factors and parental-reported autistic spectrum disorders in children 6–8 years of age. Neurotoxicology 30, 822–831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Latini G, De Felice C, Presta G, Del Vecchio A, Paris I, Ruggieri F, Mazzeo P, 2003. Exposure to di (2-ethylhexyl) phthalate in humans during pregnancy. Neonatology 83, 22. [DOI] [PubMed] [Google Scholar]
- Lee TW, Tumanov S, Villas-Bôas SG, Montgomery JM, Birch NP, 2015. Chemicals eluting from disposable plastic syringes and syringe filters alter neurite growth, axogenesis and the microtubule cytoskeleton in cultured hippocampal neurons. J. Neurochem 133, 53–65. [DOI] [PubMed] [Google Scholar]
- Lien YJ, Ku HY, Su PH, Chen SJ, Chen HY, Liao PC, Chen WJ, Wang SL, 2015. Prenatal exposure to phthalate esters and behavioral syndromes in children at 8 years of age: Taiwan Maternal and Infant Cohort Study. Environ. Health Perspect 123, 95–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu J, 2004. Childhood externalizing behavior: Theory and implications. J. Child Adolescent Psychiatric Nursing 17, 93–103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lovekamp-Swan T, Davis BJ, 2003. Mechanisms of phthalate ester toxicity in the female reproductive system. Environ. Health Perspect 111, 139–145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magnus P, 2007. The Norwegian Mother and Child Cohort Study (MoBa)–new research possibilities. Norsk Epidemiologi 17. [Google Scholar]
- Matsumoto A, 1991. Synaptogenic action of sex steroids in developing and adult neuroendocrine brain. Psychoneuroendocrinology 16, 25–40. [DOI] [PubMed] [Google Scholar]
- McEwen B, 2002. Estrogen actions throughout the brain. Recent Prog. Horm. Res 57, 357–384. [DOI] [PubMed] [Google Scholar]
- Messerlian C, Bellinger D, Mínguez-Alarcón L, Romano ME, Ford JB, Williams PL, Calafat AM, Hauser R, Braun JM, 2017. Paternal and maternal preconception urinary phthalate metabolite concentrations and child behavior. Environ. Res 158, 720–728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miodovnik A, 2011. Environmental neurotoxicants and developing brain. Mt Sinai J. Med 78, 58–77. [DOI] [PubMed] [Google Scholar]
- Miodovnik A, Edwards A, Bellinger DC, Hauser R, 2014. Developmental neurotoxicity of ortho-phthalate diesters: review of human and experimental evidence. Neurotoxicology 41, 112–122. [DOI] [PubMed] [Google Scholar]
- Miodovnik A, Engel SM, Zhu C, Ye X, Soorya LV, Silva MJ, Calafat AM, Wolff MS, 2011. Endocrine disruptors and childhood social impairment. Neurotoxicology 32, 261–267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyake A, Friedman NP, 2012. The nature and organization of individual differences in executive functions: Four general conclusions. Current Direct. Psychol. Sci 21, 8–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakiwala D, Peyre H, Heude B, Bernard JY, Beranger R, Slama R, Philippat C, Group EM-CS In-utero exposure to phenols and phthalates and the intelligence quotient of boys at 5 years. Environ. Health 2018;17:17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olesen TS, Bleses D, Andersen HR, Grandjean P, Frederiksen H, Trecca F, Bilenberg N, Kyhl HB, Dalsager L, Jensen IK, 2018. Prenatal phthalate exposure and language development in toddlers from the Odense Child Cohort. Neurotoxicol. Teratol 65, 34–41. [DOI] [PubMed] [Google Scholar]
- Overgaard KR, Oerbeck B, Friis S, Biele G, Pripp AH, Aase H, Zeiner P, 2019. Screening with an ADHD-specific rating scale in preschoolers: A cross-cultural comparison of the Early Childhood Inventory-4. Psychol. Assess 31, 985. [DOI] [PubMed] [Google Scholar]
- Øvergaard K.R.e.a., 2018. Attention-Deficit/Hyperactivity Disorder in Preschoolers: The Accuracy of a Short Screener. J. Am. Acad. Child Adolesc. Psychiatry 428–435. [DOI] [PubMed] [Google Scholar]
- Paltiel L, Anita H, Skjerden T, Harbak K, Bækken S, Kristin SN, Knudsen GP, Magnus P, 2014. The biobank of the Norwegian Mother and Child Cohort Study–present status.Norsk Epidemiologi. 24. [Google Scholar]
- Parent AS, Naveau E, Gerard A, Bourguignon JP, Westbrook GL, 2011. Early developmental actions of endocrine disruptors on the hypothalamus, hippocampus, and cerebral cortex. J. Toxicol. Environ. Health B Crit. Rev 14, 328–345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Percy Z, Xu Y, Sucharew H, Khoury JC, Calafat AM, Braun JM, Lanphear BP, Chen A, Yolton K, 2016. Gestational exposure to phthalates and gender-related play behaviors in 8-year-old children: an observational study. Environ. Health 15, 87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Philippat C, Bennett DH, Krakowiak P, Rose M, Hwang HM, Hertz-Picciotto I, 2015. Phthalate concentrations in house dust in relation to autism spectrum disorder and developmental delay in the CHildhood Autism Risks from Genetics and the Environment (CHARGE) study. Environ. Health 14, 56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Philippat C, Nakiwala D, Calafat AM, Botton J, De Agostini M, Heude B, Slama R, Group EM-CS Prenatal Exposure to Nonpersistent Endocrine Disruptors and Behavior in Boys at 3 and 5 Years. Environ. Health Perspect 2017;125:097014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polanska K, Ligocka D, Sobala W, Hanke W, 2014. Phthalate exposure and child development: the Polish Mother and Child Cohort Study. Early Hum. Dev 90, 477–485. [DOI] [PubMed] [Google Scholar]
- Qian X, Li J, Xu S, Wan Y, Li Y, Jiang Y, Zhao H, Zhou Y, Liao J, Liu H, Sun X, Liu W, Peng Y, Hu C, Zhang B, Lu S, Cai Z, Xia W, 2019. Prenatal exposure to phthalates and neurocognitive development in children at two years of age. Environ. Int 131, 105023. [DOI] [PubMed] [Google Scholar]
- Radke EG, Braun JM, Nachman RM, Cooper GS, 2020. Phthalate exposure and neurodevelopment: A systematic review and meta-analysis of human epidemiological evidence. Environ. Int 137, 105408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Regulation, E. No 1907/2006 of the European Parliament and of the Council of 18 December 2006 concerning the Registration. Evaluation, Authorisation and Restriction of Chemicals (REACH), establishing a European Chemicals Agency, amending Directive 1999;45:1–849. [Google Scholar]
- Richardson DB, Rzehak P, Klenk J, Weiland SK, 2007. Analyses of case-control data for additional outcomes. Epidemiology 18, 441–445. [DOI] [PubMed] [Google Scholar]
- Robinson NM, Dale PS, Landesman S, 1990. Validity of Stanford-Binet IV with linguistically precocious toddlers. Intelligence 14, 173–186. [Google Scholar]
- Rohrer-Baumgartner N, Zeiner P, Egeland J, Gustavson K, Skogan AH, Reichborn-Kjennerud T, Aase H, 2014. Does IQ influence Associations between ADHD Symptoms and other Cognitive Functions in young Preschoolers? Behav. Brain Funct 10, 16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roid GH, Pomplun M, 2012. The stanford-binet intelligence scales. The Guilford Press. [Google Scholar]
- Sabaredzovic A, Sakhi AK, Brantsaeter AL, Thomsen C, 2015. Determination of 12 urinary phthalate metabolites in Norwegian pregnant women by core-shell high performance liquid chromatography with on-line solid-phase extraction, column switching and tandem mass spectrometry. J. Chromatogr. B Analyt. Technol. Biomed. Life Sci 1002, 343–352. [DOI] [PubMed] [Google Scholar]
- Sakhi AK, Sabaredzovic A, Cequier E, Thomsen C, 2017. Phthalate metabolites in Norwegian mothers and children: Levels, diurnal variation and use of personal care products. Sci. Total Environ 599–600, 1984–1992. [DOI] [PubMed] [Google Scholar]
- Schreuder P, Alsaker E, 2014. The Norwegian Mother and Child Cohort Study (MoBa)–MoBa recruitment and logistics. Norsk Epidemiologi 24. [Google Scholar]
- Shu H, Jönsson BA, Gennings C, Svensson Å, Nånberg E, Lindh CH, Knutz M, Takaro TK, Bornehag C-G, 2018. Temporal trends of phthalate exposures during 2007–2010 in Swedish pregnant women. J. Eposure Sci. Environ. Epidemiol 28, 437. [DOI] [PubMed] [Google Scholar]
- Singer AB, Wolff MS, Silva MJ, Calafat AM, Engel SM, 2017. Prenatal phthalate exposures and child temperament at 12 and 24 months. NeuroToxicology 62, 248–257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh S, Li SS, 2011. Phthalates: toxicogenomics and inferred human diseases. Genomics 97, 148–157. [DOI] [PubMed] [Google Scholar]
- Skogan AH, Zeiner P, Egeland J, Urnes AG, Reichborn-Kjennerud T, Aase H, 2015. Parent ratings of executive function in young preschool children with symptoms of attention-deficit/-hyperactivity disorder. Behav. Brain Funct 11, 16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tellez-Rojo MM, Cantoral A, Cantonwine DE, Schnaas L, Peterson K, Hu H, Meeker JD, 2013. Prenatal urinary phthalate metabolites levels and neurodevelopment in children at two and three years of age. Sci. Total Environ 461–462, 386–390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toplak ME, West RF, Stanovich KE, 2013. Practitioner review: Do performance-based measures and ratings of executive function assess the same construct? J. Child Psychol. Psychiatry 54, 131–143. [DOI] [PubMed] [Google Scholar]
- Villanger GD, Drover SSM, Nethery RC, Thomsen C, Sakhi AK, Overgaard KR, Zeiner P, Hoppin JA, Reichborn-Kjennerud T, Aase H, Engel SM, 2020. Associations between urine phthalate metabolites and thyroid function in pregnant women and the influence of iodine status. Environ. Int 137, 105509. [DOI] [PubMed] [Google Scholar]
- Whyatt RM, Liu X, Rauh VA, Calafat AM, Just AC, Hoepner L, Diaz D, Quinn J, Adibi J, Perera FP, Factor-Litvak P, 2012. Maternal prenatal urinary phthalate metabolite concentrations and child mental, psychomotor, and behavioral development at 3 years of age. Environ. Health Perspect 120, 290–295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willcutt EG, Doyle AE, Nigg JT, Faraone SV, Pennington BF, 2005. Validity of the executive function theory of attention-deficit/hyperactivity disorder: a meta-analytic review. Biol. Psychiatry 57, 1336–1346. [DOI] [PubMed] [Google Scholar]
- Wu H, Kupsco AJ, Deierlein AL, Just AC, Calafat AM, Oken E, Braun JM, Mercado-Garcia A, Cantoral A, Tellez-Rojo MM, Wright RO, Baccarelli AA, 2020. Trends and Patterns of Phthalates and Phthalate Alternatives Exposure in Pregnant Women from Mexico City during 2007–2010. Environ. Sci. Technol 54, 1740–1749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ye X, Pierik FH, Angerer J, Meltzer HM, Jaddoe VW, Tiemeier H, Hoppin JA, Longnecker MP, 2009. Levels of metabolites of organophosphate pesticides, phthalates, and bisphenol A in pooled urine specimens from pregnant women participating in the Norwegian Mother and Child Cohort Study (MoBa). Int. J. Hyg. Environ. Health 212, 481–491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yolton K, Xu Y, Strauss D, Altaye M, Calafat AM, Khoury J, 2011. Prenatal exposure to bisphenol A and phthalates and infant neurobehavior. Neurotoxicol. Teratol 33, 558–566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zota AR, Calafat AM, Woodruff TJ, 2014. Temporal trends in phthalate exposures: findings from the National Health and Nutrition Examination Survey, 2001–2010. Environ. Health Perspect 122, 235–241. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


