Abstract
Purpose:
Prostate specific membrane antigen targeted 18F-DCFPyL positron emission tomography/computerized tomography may offer superior image quality and sensitivity for the detection of biochemically recurrent prostate cancer. We examined the association of Gleason sum, serum prostate specific antigen and prostate specific antigen doubling time with any detectable and pelvic confined disease in patients with biochemically recurrent prostate cancer.
Materials and Methods:
Data from 108 patients with biochemically recurrent prostate cancer after radical prostatectomy who underwent prostate specific membrane antigen targeted 18F-DCFPyL positron emission tomography/computerized tomography were analyzed. Data were collected on positive positron emission tomography findings as well as pelvic confined disease. Associations between Gleason sum, prostate specific antigen and prostate specific antigen doubling time were retrospectively explored.
Results:
Serum prostate specific antigen was associated with positive prostate specific membrane antigen targeted imaging as continuous (OR 3.08, 95% CI 1.60–7.95, p=0.005) and categorical values (ie prostate specific antigen greater than 2.0 to 5.0 vs 0.5 ng/ml or less, OR 16.92, 95% CI 3.13–315.81, p=0.008). No relationship between Gleason sum or prostate specific antigen doubling time with overall positive imaging was observed. Patients with a prostate specific antigen greater than 2.0 to 5.0 ng/ml were significantly less likely to be diagnosed with pelvic confined disease compared with the 0.5 ng/ml or less subgroup (OR 0.21, 95% CI 0.06–0.69, p=0.013). A prostate specific antigen doubling time of 9 months or more (OR 4.20, 95% CI 1.57–11.89, p=0.005) or prostate specific antigen doubling time of 12 months or more (OR 3.03, 95% CI 1.12–8.76, p=0.033) was significantly associated with pelvic confined disease. No relationship between Gleason sum and pelvic confined disease was observed.
Conclusions:
Absolute prostate specific antigen was positively associated with the presence of findings on prostate specific membrane antigen targeted imaging and negatively associated with pelvic confined disease. Prostate specific antigen doubling time did not predict for overall disease detection, but long prostate specific antigen doubling times were associated with pelvic confined prostate cancer.
Keywords: pelvis, recurrence, prostatic neoplasms, neoplasm staging
Nearly a third of men will experience biochemical recurrence following primary therapy for localized prostate cancer.1,2 For men with BCR who underwent initial treatment with radical prostatectomy, salvage radiotherapy, with or without androgen deprivation therapy, has been shown to improve clinical outcomes.3,4 Additionally, it is known that men with BCR who benefit most from salvage radiotherapy have low PSA values.5,6 Specifically, men with BCR and a PSA less than 0.5 ng/ml had a more than 2.5-fold likelihood of disease-free survival after salvage radiotherapy compared to higher PSA levels.5 More recent studies, based on new European Association of Urology guidelines, similarly found that patients with a PSA less than 0.5 ng/ml had the most protective benefit from salvage radiotherapy.7,8 Thus, in men with BCR it is of critical importance to use clinical variables, and potentially imaging, to define the location and extent of disease before implementing salvage treatment.
PET/CT with small molecules directed against PSMA has gained considerable attention for its enhanced ability to detect sites of metastatic disease not seen on conventional imaging.9-11 To date, PSMA targeted imaging has been explored in a number of clinical contexts, including staging, evaluation of response to therapy, determination of extent of systemic disease, and re-staging in patients with negative conventional imaging findings and an elevated PSA.12-18 The majority of those data have been generated with 68Ga-labeled agents, most notably 68Ga-PSMA-11.19-22 In this study we sought to investigate factors that predict the presence of abnormal uptake consistent with sites of recurrent prostate cancer detected by 18F-DCFPyL PSMA targeted PET/CT in men with BCR.
METHODS
This study is a retrospective analysis of men with BCR prostate cancer who were imaged with 18F-DCFPyL PET/CT as part of a larger, institutional review board approved prospective trial aimed at understanding the therapeutic impact of imaging findings on men with all stages of prostate cancer (ClinicalTrials.gov NCT02825875). PET/CT images were acquired on either a Siemens Biograph mCT 128-slice (Siemens Healthineers, Erlangen, Germany) or a GE Discovery RX 64-slice (GE Healthcare, Waukesha, Wisconsin) scanner operating in 3D emission mode with CT based attenuation correction. Scans were initiated 60 minutes after the intravenous infusion of 333 MBq (9 mCi) of 18F-DCFPyL with a field of view from the mid thighs through the skull vertex. Intravenous iodinated contrast was not used. Images were reconstructed with a manufacturer supplied ordered subset expectation maximization method.
Patients in the current analysis met the inclusion criteria of history of adenocarcinoma of the prostate treated with radical prostatectomy, a serum PSA 0.1 to 5.0 ng/ml within 45 days prior to study enrollment, and negative complete staging evaluation with a bone scan as well as CT of the abdomen and pelvis or magnetic resonance imaging of the pelvis within 45 days prior to study enrollment. Patients with a history of other malignancy and/or an intention to enroll in a blinded therapeutic clinical trial were excluded from the study. All 18F-DCFPyL PET/CT scans were interpreted by a single radiologist (SPR) with 5 years of experience in reading PSMA targeted PET studies. Radiotracer uptake outside of the normal biodistribution of 18F-DCFPyL was categorized according to the PSMA-RADS version 1.0 interpretive framework and lesions that were PSMA-RADS-3A-5 were considered positive for prostate cancer.18 A specific standardized uptake value was not used as a determinant of the presence or absence of disease; rather, qualitative focal uptake at a site typical for prostate cancer was used. The use of the PSMA targeted imaging result and subsequent clinical management of each patient was at the discretion of the treating providers.
Pelvic confined disease was defined by uptake of the 18F-DCFPyL radiotracer in the prostate bed, pelvic soft tissue and/or pelvic lymph nodes (sacral, external/internal iliac, obturator). Any patient with bone disease, even if within the pelvic girdle, was considered to have extrapelvic disease. Extrapelvic disease was defined as any detectable sites outside of the prostate bed, pelvic soft tissue or pelvic lymph nodes.
PSADT was calculated using the natural log of 2 (=0.693) divided by the slope of the linear regression of the natural log of PSA vs time (in months). This was calculated using the 3 most recent PSA values prior to PSMA targeted PET. If the slope of the linear regression was 0 (elevated but constant PSA) or negative (decreasing PSA after initial increase), the PSADT was set to 100 months, as previously described.4
A Fisher’s exact test was used to compare the number of positive/negative tests as well as pelvic confined/extrapelvic disease. Logistic regression analysis was used to estimate the association of Gleason sum, PSA and PSADT with positive 18F-DCFPyL PET/CT results, or pelvic confined disease in patients with positive PSMA targeted PET/CT. In addition, the performance of both PSA and PSADT for detecting any positive 18F-DCFPyL PET/CT imaging or pelvic confined disease was assessed using the area under the receiver operating characteristic curve. All values 2-sided p <0.05 were considered significant. All statistical analyses were performed using R version 3.5.3.
RESULTS
A total of 108 patients met the study inclusion criteria. Patient characteristics are described in table 1. The median patient age was 67 years (IQR 61–71) and 12.0% were of ethnic minority race. Patients had a median PSA of 0.7 ng/ml (IQR 0.3—1.8). 18F-DCFPyL PET/CT detected radiographic evidence of prostate cancer in 82 (75.9%) men. In those patients with a positive PET/CT, pelvic confined disease was observed in 61.0% of cases.
Table 1.
Patient characteristics and summarized PSMA PET findings
| Age: | ||
| Median | 67 | |
| IQR | 61–71 | |
| No. race (%): | ||
| White | 95 | (88.0) |
| Nonwhite | 13 | (12.0) |
| PSA (ng/ml): | ||
| Median | 0.7 | |
| IQR | 0.3–1.8 | |
| No. ng/ml PSA categorical (%): | ||
| 0.5 or Less | 46 | (42.6) |
| Greater than 0.5–2.0 | 23 | (21.3) |
| Greater than 2.0 | 39 | (36.1) |
| No. mos PSADT (%):* | ||
| Less than 9/9 or more | 43 | (46.7)/49 (53.3) |
| Less than 12/12 or more | 52 | (56.5)/40 (43.5) |
| No. Gleason sum (%): | ||
| 7 or Less | 76 | (70.4) |
| 8 or Greater | 29 | (26.8) |
| Unknown | 3 | (2.8) |
| No. PSMA imaging result (%): | ||
| Pos | 82 | (75.9) |
| Neg | 26 | (24.1) |
| No. pelvic confined disease (%):† | ||
| Yes | 50 | (61) |
| No | 32 | (39) |
In 92.
In 82, positive only.
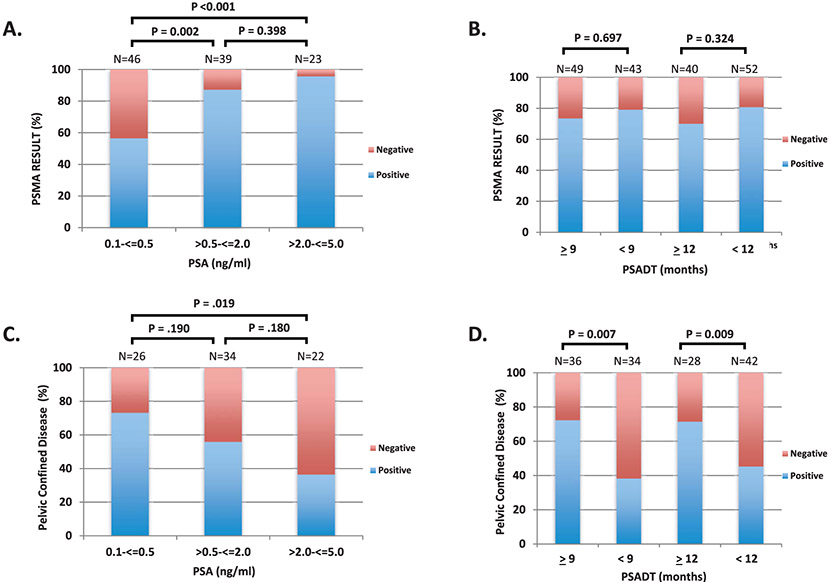
We explored the relationship of Gleason sum, PSA and PSADT with 18F-DCFPyL PET/CT result (ie dichotomized by positive or negative for the detection of prostate cancer). We observed improved detection of radiographic disease with increasing PSA values (fig. 1,A). For instance, 56.5% of PSMA targeted PET/CT scans were positive in the 0.5 ng/ml or less subgroup compared to 87.1% (p=0.002) and 95.7% (p < 0.001) in the greater than 0.5 to 2.0 ng/ml and greater than 2.0 to 5.0 ng/ml, respectively. Data that include more narrowly defined PSA subgroups are provided in the supplementary figure (https://www.jurology.com). Using logistic regression analysis, PSA as a continuous variable (OR 3.08, 95% CI 1.60–7.95, p=0.005) was significantly associated with a positive 18F-DCFPyL PET/CT (table 2). As a categorical variable, PSA greater than 2.0 to 5.0 vs 0.5 ng/ml or less (OR 16.92, 95% CI 3.13–315.81, p=0.008) and greater than 0.5 to 2.0 vs 0.5 ng/ml or less (OR 5.23, 95% CI 1.84–17.42, p=0.003) was also associated with a positive scan result. Gleason sum and PSADT were not associated with detectable disease on 18F-DCFPyL PET/CT (fig. 1, B; table 2).
Figure 1.
Prostate cancer disease detection and prevalence of pelvic confined disease by 18F-DCFPyL PSMA targeted imaging in patients with biochemically recurrent prostate cancer. Percentage of 18F-DCFPyL PSMA targeted PET scans that detected prostate cancer stratified by absolute PSA (A) and PSADT (B). Prevalence of pelvic confined disease decreased with higher PSA (C) and shorter PSADTs (D).
Table 2.
Association of Gleason sum, PSA and PSADT with positive PSMA PET using logistic regression analysis
| Value | Reference | OR | 95% CI | p Value | |
|---|---|---|---|---|---|
| PSA ng/ml (continous) | — | — | 3.08 | 1.60–7.95 | 0.005 |
| PSA ng/ml (categorical): | Greater than 2 | 0.5 or Less | 16.92 | 3.13–315.81 | 0.008 |
| Greater than 0.5–2 | 0.5 or Less | 5.23 | 1.84–17.42 | 0.003 | |
| Mos PSADT (categorical): | 9 or Greater | Less than 9 | 0.73 | 0.27-1.92 | 0.531 |
| 12 or Greater | Less than 12 | 0.56 | 0.21–1.46 | 0.233 | |
| Gleason sum (categorical) | 8 or Greater | 7 or Less | 1.05 | 0.40–2.99 | 0.927 |
We next investigated the association of Gleason sum, PSA and PSADT with pelvic confined disease in patients with a positive PSMA targeted PET/CT. The prevalence of pelvic confined disease decreased across higher PSA subgroups (0.5 ng/ml or less—19 of 26 [73.1%], greater than 0.5 to 2.0 ng/ml—19 of 34 [55.9%], greater than 2.0 to 5.0 ng/ml—8 of 22 [36.4%]) (fig. 1, C). As a continuous variable, PSA was nonsignificantly negatively associated with identifying pelvic confined disease on PSMA targeted PET/CT (OR 0.72, 95% CI 0.5–1.00, p=0.056, table 3). Using our defined PSA subgroups, a PSA greater than 2.0 to 5.0 ng/ml was significantly less likely to be associated with pelvic confined disease compared with the 0.5 ng/ml or less subgroup (OR 0.21, 95% CI 0.06–0.69, p=0.013). No difference between the greater than 0.5 to 2.0 ng/ml and 0.5 ng/ml or less subgroups was observed (OR 0.47, 95% CI 0.15–1.37, p=0.174). Next, we examined the association of PSADT with pelvic confined disease. We used the 2 cut points of 9 and 12 months for the analysis. Patients with a PSADT of 9 months or more had pelvic confined disease in 72.2% of cases vs 38.3% in the PSADT less than 9 months subgroup (p=0.007, fig. 1, D). Similar findings were observed in the PSADT 12 months or more (71.4%) vs less than 12 months (45.2%) cohorts (p=0.009). Using linear regression analysis, patients with a PSADT of 9 months or more (OR 4.20, 95% CI 1.57–11.89, p=0.005) or PSADT of 12 months or more (OR 3.03, 95% CI 1.12–8.76, p=0.033) were significantly associated with pelvic confined disease. We did not observe a significant association between Gleason sum (8 or more vs 7 or less) and pelvic confined disease (OR 1.21, 95% CI 0.45–3.36, p=0.706).
Table 3.
Association of Gleason sum, PSA and PSADT with PSMA PET confirmed pelvic confined disease using logistic regression analysis
| Value | Reference | OR | 95% CI | p Value | |
|---|---|---|---|---|---|
| PSA ng/ml (continous) | — | — | 0.72 | 0.5–1.00 | 0.056 |
| PSA ng/ml (categorical): | Greater than 2 | Less than 0.5 | 0.21 | 0.06–0.69 | 0.013 |
| Greater than 0.5–2 | Less than 0.5 | 0.47 | 0.15–1.37 | 0.174 | |
| Mos PSADT (categorical): | Greater than 9 | Less than 9 | 4.20 | 1.57–11.89 | 0.005 |
| Greater than 12 | Less than 12 | 3.03 | 1.12–8.76 | 0.033 | |
| Gleason sum (categorical) | Greater than 8 | Less than 7 | 1.21 | 0.44–3.36 | 0.706 |
ROC curves were generated to demonstrate the ability of PSA and PSADT to predict positive 18F-DCFPyL PET/CT imaging and pelvic confined disease (fig. 2). The AUC using PSA to predict positive 18F-DCFPyL PET/CT imaging and pelvic confined disease was 0.75 and 0.66, respectively (fig. 2, A and C). The PSA that generated the maximum Youden index was 1.15 ng/ml for positive imaging and 1.77 ng/ml for pelvic confined disease. Using PSADT resulted in an AUC of 0.53 for positive imaging (fig. 2, B). For pelvic confined disease an AUC of 0.67 for PSADT was observed (fig. 2, D). A PSADT of 10.45 months generated the maximum Youden index for pelvic confined disease.
Figure 2.

AUC for detection of prostate cancer and pelvic confined disease stratified by PSA and PSADT in biochemically recurrent prostate cancer using 18F-DCFPyL PSMA targeted imaging. AUC for radiographic detection of prostate cancer (A, B) and pelvic confined disease (C, D) using 18F-DCFPyL PSMA targeted imaging is shown based on absolute PSA and PSADT.
DISCUSSION
In this cohort of patients with BCR and PSA less than 5.0 ng/ml the percentage of patients with a positive scan increased with higher PSA values. Using discrete subgroups, nearly 90% of patients with a PSA greater than 0.5 ng/ml had detectable disease compared with approximately 50% of the 0.5 ng/ml or less cohort, consistent with prior studies using 68Ga-PSMA.22 This may suggest that the clinical utility of these scans is greatest when the PSA is greater than 0.5 ng/ml, although the detection rate at PSA levels between 0.1 and 0.5 ng/ml is still higher than other imaging modalities. We suspect that many patients may still have their treatment effectively guided by PSMA targeted PET despite low PSA. To this end, European Association of Urology guidelines for prostate cancer suggest the use of PSMA targeted PET in men with BCR after prostatectomy and PSA greater than 0.2 ng/ml.23 Larger randomized studies are still needed to demonstrate improved outcomes with the use of PSMA targeted imaging for patients with BCR with low serum PSA.
A standard of care approach to BCR post prostatectomy is to consider salvage radiotherapy with the premise being that early disease relapse will be confined to the pelvis.3,4 Using 68Ga-PSMA PET imaging, negative scans and prostate bed confined disease independently predicted for benefit from salvage radiotherapy suggesting PSMA targeted imaging modalities may better define ideal candidates for local versus systemic therapy.24,25 Similarly, PSA at the time of imaging was associated with disease outside of standard salvage radiation fields using PSMA targeted PET. In patients with a positive 18F-DCFPyL PET/CT, we observed a significant association with pelvic confined disease in those patients with a PSA 0.5 ng/ml or less. This finding may provide a radiographic rationale for improved outcomes observed following salvage radiotherapy in patients with BCR with a PSA 0.5 ng/ml or less.5,8 Those patients with an intermediate PSA (0.5 to 2.0 ng/ml) may benefit the most from having PSMA targeted imaging when considering salvage radiotherapy. More than half of patients with a PSA between 0.5 and 2.0 ng/ml had disease limited to the pelvis, suggesting PSMA targeted PET may help discriminate between truly pelvic confined versus distant M1 disease in this subset.
Prior studies have shown that PSADT was a strong predictor for developing metastatic disease using conventional imaging.26,27 However, our data with 18F-DCFPyL PSMA targeted PET found no association between PSADT and a positive PET. In addition, long PSADTs (eg greater than 9 or greater than 12 months) were associated with pelvic confined disease. These PSADT cut points have previously been associated with longer metastasis-free survival in patients with BCR.26,27 Since shorter PSADTs were associated with extra pelvic disease in this study, but not scan positivity, we speculate that a shorter PSADT may reflect a more aggressive biology rather than predicting the underlying volume of disease. We also did not find an association between pelvic confined disease or positive imaging with Gleason sum, similar to the data with 68Ga-PSMA imaging.22 In the absence of PSMA targeted imaging, PSA and PSADT should be taken into account when counseling patients with BCR after prostatectomy prior to salvage radiotherapy.
There are several limitations to our study. Gleason sum, PSA and PSADT were examined only in univariate analyses when investigating associations with positive imaging and pelvic confined disease. A larger number of patients would be needed to perform a more in-depth, multivariate analysis. Nearly 50% of patient with a PSA 0.5 ng/ml or less had a negative 18F-DCFPyL PET/CT. Only those patients with a positive scan were considered for the pelvic confined analysis. We cannot infer that those patients with negative PSMA imaging would have a similar prevalence of pelvic confined disease. Our analysis of Gleason sum may have been underpowered given the predominance of Gleason 7 or lower disease represented in the study population. Lastly, we did not collect prospective outcome data following PSMA targeted imaging. We do not know whether those patients with pelvic confined prostate cancer had more favorable outcomes versus those patients with distant M1 disease.
In conclusion, our results indicate that PSMA targeted PET may allow for the improved selection of salvage therapies following BCR. Distinguishing between a local recurrence in the pelvis and distant metastases is critical, as treatment may entail local salvage therapy to eradicate disease or systemic treatment to prevent disease progression. Currently, clinical decision making should be tailored based on clinical results (such as serum PSA and PSADT), conventional imaging and genomic characteristics. When appropriate and available, PSMA targeted PET may be used to provide additional clinical data.
Supplementary Material
Acknowledgments
No direct or indirect commercial, personal, academic, political, religious or ethical incentive is associated with publishing this article. Supported by the Sidney Kimmel Comprehensive Cancer Center at Johns Hopkins NIH grant P30 CA006973, Prostate Cancer Foundation Young Investigator Awards, a Patrick C. Walsh Foundation Award, and Progenics Pharmaceuticals, Inc. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Cancer Institute or the National Institutes of Health.
Abbreviations and Acronyms
- BCR
biochemically recurrent prostate cancer
- CT
computerized tomography
- PET
positron emission tomography
- PSA
prostate specific antigen
- PSADT
prostate specific antigen doubling time
- PSMA
prostate specific membrane antigen
Contributor Information
Mark C. Markowski, Department of Oncology, Sidney Kimmel Comprehensive Cancer Center at Johns Hopkins, Baltimore, Maryland.
Ramy Sedhom, Department of Oncology, Sidney Kimmel Comprehensive Cancer Center at Johns Hopkins, Baltimore, Maryland.
Wei Fu, Department of Biostatistics, Johns Hopkins Bloomberg School of Public Health, Baltimore, Maryland.
Javaughn Corey R. Gray, Department of Oncology, Sidney Kimmel Comprehensive Cancer Center at Johns Hopkins, Baltimore, Maryland
Mario A. Eisenberger, Department of Oncology, Sidney Kimmel Comprehensive Cancer Center at Johns Hopkins, Baltimore, Maryland
Martin G. Pomper, The Russel H. Morgan Department of Radiology and Radiological Science, Johns Hopkins University School of Medicine, Baltimore, Maryland.
Kenneth J. Pienta, The James Buchanan Brady Urological Institute and Department of Urology, Johns Hopkins University School of Medicine, Baltimore, Maryland.
Michael A. Gorin, The James Buchanan Brady Urological Institute and Department of Urology, Johns Hopkins University School of Medicine, Baltimore, Maryland.
Steven P. Rowe, The Russel H. Morgan Department of Radiology and Radiological Science, Johns Hopkins University School of Medicine, Baltimore, Maryland.
REFERENCES
- 1.Pound CR, Partin AW, Epstein JI et al. : Prostate-specific antigen after anatomic radical retropubic prostatectomy. Patterns of recurrence and cancer control. Urol Clin North Am 1997; 24: 395. [DOI] [PubMed] [Google Scholar]
- 2.Trapasso JG, deKernion JB, Smith RB et al. : The incidence and significance of detectable levels of serum prostate specific antigen after radical prostatectomy. J Urol 1994; 152: 1821. [DOI] [PubMed] [Google Scholar]
- 3.Shipley WU, Seiferheld W, Lukka HR et al. : Radiation with or without antiandrogen therapy in recurrent prostate cancer. N Engl J Med 2017; 376: 417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Trock BJ, Han M, Freedland SJ et al. : Prostate cancer-specific survival following salvage radiotherapy vs observation in men with biochemical recurrence after radical prostatectomy. JAMA 2008; 299: 2760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Stephenson AJ, Scardino PT, Kattan MW et al. : Predicting the outcome of salvage radiation therapy for recurrent prostate cancer after radical prostatectomy. J Clin Oncol 2007; 25: 2035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Stephenson AJ, Shariat SF, Zelefsky MJ et al. : Salvage radiotherapy for recurrent prostate cancer after radical prostatectomy. JAMA 2004; 291: 1325. [DOI] [PubMed] [Google Scholar]
- 7.Van den Broeck T, van den Bergh RCN, Briers E et al. : Biochemical recurrence in prostate cancer: the European Association of Urology Prostate Cancer Guidelines Panel Recommendations. Eur Urol Focus 2020; 6: 231. [DOI] [PubMed] [Google Scholar]
- 8.Tilki D, Preisser F, Graefen M et al. : External validation of the European Association of Urology biochemical recurrence risk groups to predict metastasis and mortality after radical prostatectomy in a European cohort. Eur Urol 2019; 75: 896. [DOI] [PubMed] [Google Scholar]
- 9.Byun Y, Pullambhatla M, Wang H et al. : Synthesis and biological evaluation of substrate-based imaging agents for the prostate-specific membrane antigen. Macromol Res 2013; 21: 565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Demirkol MO, Acar O, Ucar B et al. : Prostate-specific membrane antigen-based imaging in prostate cancer: impact on clinical decision making process. Prostate 2015; 75: 748. [DOI] [PubMed] [Google Scholar]
- 11.Osborne JR, Akhtar NH, Vallabhajosula S et al. : Prostate-specific membrane antigen-based imaging. Urol Oncol 2013; 31: 144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hijazi S, Meller B, Leitsmann C et al. : Pelvic lymph node dissection for nodal oligometastatic prostate cancer detected by 68Ga-PSMA-positron emission tomography/computerized tomography. Prostate 2015; 75: 1934. [DOI] [PubMed] [Google Scholar]
- 13.Morigi JJ, Stricker PD, van Leeuwen PJ et al. : Prospective comparison of 18F-fluoromethylcholine versus 68Ga-PSMA PET/CT in prostate cancer patients who have rising PSA after curative treatment and are being considered for targeted therapy. J Nucl Med 2015; 56: 1185. [DOI] [PubMed] [Google Scholar]
- 14.Rowe SP, Campbell SP, Mana-Ay M et al. : Prospective evaluation of PSMA-targeted 18F-DCFPyL PET/CT in men with biochemical failure after radical prostatectomy for prostate cancer. J Nucl Med 2020; 61: 58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rowe SP, Gage KL, Faraj SF et al. : 18F-DCFBC PET/CT for PSMA-based detection and characterization of primary prostate cancer. J Nucl Med 2015; 56: 1003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rowe SP, Li X, Trock BJ et al. : Prospective comparison of PET imaging with PSMA-targeted 18F-DCFPyL versus Na18F for bone lesion detection in patients with metastatic prostate cancer. J Nucl Med 2020; 61: 183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rowe SP, Macura KJ, Mena E et al. : PSMA-based [18F]DCFPyL PET/CT is superior to conventional imaging for lesion detection in patients with metastatic prostate cancer. Mol Imaging Biol 2016; 18: 411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rowe SP, Pienta KJ, Pomper MG et al. : PSMA-RADS version 1.0: a step towards standardizing the interpretation and reporting of PSMA-targeted PET imaging studies. Eur Urol 2018; 73: 485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hoffmann MA, Buchholz HG, Wieler HJ et al. : The positivity rate of 68Gallium-PSMA-11 ligand PET/CT depends on the serum PSA-value in patients with biochemical recurrence of prostate cancer. Oncotarget 2019; 10: 6124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ceci F, Bianchi L, Borghesi M et al. : Prediction nomogram for 68Ga-PSMA-11 PET/CT in different clinical settings of PSA failure after radical treatment for prostate cancer. Eur J Nucl Med Mol Imaging 2020; 47: 136. [DOI] [PubMed] [Google Scholar]
- 21.Pereira Mestre R, Treglia G, Ferrari M et al. : Correlation between PSA kinetics and PSMA-PET in prostate cancer restaging: a meta-analysis. Eur J Clin Invest 2019; 49: e13063. [DOI] [PubMed] [Google Scholar]
- 22.Perera M, Papa N, Roberts M et al. : Gallium-68 prostate-specific membrane antigen positron emission tomography in advanced prostate cancer-updated diagnostic utility, sensitivity, specificity, and distribution of prostate-specific membrane antigen-avid lesions: a systematic review and meta-analysis. Eur Urol 2020; 77: 403. [DOI] [PubMed] [Google Scholar]
- 23.Mottet N, Bellmunt J, Bolla M et al. : EAU-ESTRO-SIOG guidelines on prostate cancer. Part 1: screening, diagnosis, and local treatment with curative intent. Eur Urol 2017; 71: 618. [DOI] [PubMed] [Google Scholar]
- 24.Emmett L, van Leeuwen PJ, Nandurkar R et al. : Treatment outcomes from 68Ga-PSMA PET/CT-informed salvage radiation treatment in men with rising PSA after radical prostatectomy: prognostic value of a negative PSMA PET. J Nucl Med 1972; 58: 2017. [DOI] [PubMed] [Google Scholar]
- 25.van Leeuwen PJ, Stricker P, Hruby G et al. : 68Ga-PSMA has a high detection rate of prostate cancer recurrence outside the prostatic fossa in patients being considered for salvage radiation treatment. BJU Int 2016; 117: 732. [DOI] [PubMed] [Google Scholar]
- 26.Markowski MC, Chen Y, Feng Z et al. : PSA doubling time and absolute PSA predict metastasis-free survival in men with biochemically recurrent prostate cancer after radical prostatectomy. Clin Genitourin Cancer 2019; 17: 470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pound CR, Partin AW, Eisenberger MA et al. : Natural history of progression after PSA elevation following radical prostatectomy. JAMA 1999; 281: 1591. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.