Abstract
Assessing for the presence of non–alcoholic steatohepatitis (NASH) and the presence of advanced fibrosis is vital among patients with non–alcoholic fatty liver disease (NAFLD) as each is predictive of disease outcomes. A liver biopsy is the gold standard method for doing so but is impossible to perform among all patients with NAFLD. Reliable methods for noninvasively detecting for the presence of NASH and advanced fibrosis are thus a pressing need. The search for noninvasive tests has been more successful for advanced fibrosis than for NASH. Clinical prediction models and elastography have acceptable accuracy for ruling out advanced fibrosis; when used together, as in a fibrosis prediction algorithm presented in this review, it can avoid the need for liver biopsy among many patients with NAFLD. Several biomarkers for identifying the presence of NASH have been studied but none are sufficiently accurate or validated. Of those studied, the most promising include CK-18 fragments, lipodomic and metabolomics candidates, and magnetic resonance elastography with proton density fat fraction. However, none are ready for clinical use and ultimately large multicenter prospective cohort studies are needed to validate select novel biomarkers.
Keywords: elastography, fibrosis, NASH, biomarkers
Non–alcoholic fatty liver disease (NAFLD) is the most common cause of chronic liver disease, affecting 1 of 4 adults worldwide.1 Although end-stage liver disease and liver–related mortality are among its feared outcomes, NAFLD is also associated with increased risk of several extrahepatic complications. These include risk of cardiovascular disease, cancer, and type 2 diabetes.2,3
NAFLD consists of 2 main subtypes: non–alcoholic fatty liver (NAFL), which is characterized histologically by isolated hepatocyte steatosis, and NASH, which is characterized histologically by steatosis plus hepatocyte ballooning with or without fibrosis. Though both subtypes are associated with increased cardiovascular risk,4 NAFL is associated with low risk of cirrhosis, whereas the NASH subtype may undergo fibrotic progression to cirrhosis in 20% of cases.5 Because of its risk for fibrotic progression to cirrhosis, NASH has been identified as the main target for therapeutic interventions designed to reduce the risk of liver disease progression. In fact, improvement in and/or resolution of NASH is the main outcome measures for pharmacologic agents being tested in clinical trials and required of drugs approved by the Federal Drug Administration for the treatment of NAFLD. The main way NASH activity is measured in clinical trials and in clinical practice right now is on the basis of the finding of hepatocyte ballooning on liver biopsy. Thus, the development of biomarkers that can noninvasively identify the necroinflammatory features of NASH independent of fibrosis is critical.
The presence of advanced fibrosis (Metavir stage 3 or 4) on biopsy is the strongest predictor of liver-related and all-cause mortality among patients with NAFLD. Though advanced fibrosis falls in the spectrum of NASH, the presence of stage 3 or 4 fibrosis—independent of hepatocyte ballooning or any other histologic finding—has been repeatedly shown to the most powerful predictor for adverse outcomes among patients with NAFLD. In a retrospective cohort study consisting of 619 patients with baseline biopsies, advanced fibrosis was associated with a hazard ratio of 85.8 (95% confidence interval, 10.93–673.30) compared with no fibrosis for risk of liver-related mortality and liver transplantation, whereas early fibrosis (Metavir stages 1 and 2) was associated with a hazard ratio of 11.2 (95% confidence interval, 1.33–93.45) over 12 years.6 Increasingly, clinical trials have identified patients with advanced fibrosis as a key target population. Moreover, assessment of fibrosis severity is crucial in managing and counseling patients with NAFLD.
IDENTIFYING ADVANCED FIBROSIS AND NASH
Histologic assessment of liver tissue is the gold standard for diagnosing advanced fibrosis and NASH. However, offering routine liver biopsies to the ~87 million people with NAFLD in the United States alone is an untenable option.7 Thus, reliable noninvasive methods for identifying the presence of advanced fibrosis and the presence of NASH (independent of fibrosis) are a pressing need. Several noninvasive diagnostic methods have been studied for this purpose and in general, have proven more successful for the identification of advanced fibrosis than for NASH.
NONINVASIVE PREDICTION OF ADVANCED FIBROSIS
Clinical prediction scores and elastography are the 2 main tools currently available for the prediction of advanced fibrosis. Of the several studied clinical scores, the most accurate are the FIB4 (AUROC 0.8) and the NAFLD Fibrosis Score (NFS, AUROC 0.78) (Table 1).8 A low cutoff and high cutoff have been derived for each score to maximize sensitivity and specificity for advanced fibrosis; scores that fall in between constitute an indeterminate range. Established diagnostic cutoffs are listed in Table 1 with summary diagnostic statistics. At their low cutoffs, the scores perform very well for ruling out advanced fibrosis and can help avoid liver biopsies in 52% to 62% of patients with a low false–negative rate of 5% to 8%.9 However, they do not perform as well for ruling in advanced fibrosis even at their high cutoffs and a confirmatory liver biopsy is consequently required. A notable drawback to clinical prediction models is that they yield indeterminate results in up to 30% of patients, in which case a second noninvasive assessment through elastography or liver biopsy for risk stratification is necessary. In addition, noninvasive models perform poorly for the detection of advanced fibrosis among patients 35 years and younger and patients younger than 65 years. In general, they tend to underestimate advanced fibrosis among the young (sensitivity 0% for both FIB4 and NFS) and overestimate advanced fibrosis among those 65 years and older (specificity 20% for NFS and 35% for FIB4).10 Although newer age-specific cutoffs have been developed for those 65 years and older they have not yet been widely validated. For those ≤35 years of age it may be prudent to have a low threshold for elastography for fibrosis screening, though this approach has not been formally tested.
TABLE 1.
Noninvasive Diagnostic Methods for Advanced Fibrosis: Summary Statistics
| Noninvasive Test | Sensitivity | Specificity | Positive Predictive Value | Negative Predictive Value | Advantages | Disadvantages |
|---|---|---|---|---|---|---|
| NAFLD Fibrosis Score* | ||||||
| Components: age, IGT/diabetes, AST, ALT, platelets, albumin | ||||||
| Low cutoff: −1.455 | 73% | 74% | 50% | 92% | ||
| Convenient Uses routine clinical data Excellent negative predictive value |
Reduced accuracy in ages <35 y and >65 y Poor positive predictive value Large proportion of indeterminate scores |
|||||
| High cutoff: 0.676 | 43% | 88% | 67% | 89% | ||
| FIB4* | ||||||
| Components: age, AST, ALT, platelets | ||||||
| Low cutoff: 1.3 | 78% | 71% | 40% | 93% | ||
| Convenient Uses routine clinical data Excellent negative predictive value |
Reduced accuracy in patients ≤ 35 y and ≥65 y Poor positive predictive value Large proportion of indeterminate scores |
|||||
| High cutoff: 2.67 | 32% | 96% | 66% | 85% | ||
| Transient elastography | ||||||
| M probe | ||||||
| Cutoff: 6.95–7.25 kPa | 89% | 77% | 43% | 96% | ||
| Point-of-care test Radiology involvement not required High negative predictive value |
Reliability and accuracy reduced by BMI>30 kg/m2, high ALT, cholestasis, congestion, nonfasting state, operator experience | |||||
| Cutoff: 9.6–11.4 kPa | 80% | 90% | 68% | 93% | ||
| Magnetic resonance elastography | ||||||
| Cutoff: 3.6–4.8 kPa | 86% | 91% | 71% | 93% | Most accurate noninvasive testing method Can be used in BMI up to 35 kg/m2 |
Not widely available |
Studies evaluating noninvasive tests have used a wide range of diagnostic cutoffs.
The low and high cutoffs presented here for FIB4 and NFS are ones most widely used in clinical practice. The presented statistics are statistics pooled from studies that used a range of cutoffs clustered around the listed value as presented by the most recent meta-analysis on the topic by Xiao et al.8
ALT, alanine aminotransferase; AST, aspartate transaminase; BMI. body mass index; FIB4, fibrosis 4; IGT, impaired glucose tolerance; NAFLD, non–alcoholic fatty liver disease; NFS, NFS, Non–Alcoholic Fatty Liver Disease Fibrosis Score.
Elastography techniques derive liver stiffness measurements (LSM) from the velocity of liver tissue microdisplacements induced by propagated shear waves. The velocity is directly related to LSM which is in turn directly related to fibrosis. Ultrasound-based vibration controlled transient elastography (VCTE) and magnetic resonance elastography (MRE) are the 2 main methods used among patients with NAFLD.
VCTE, the most extensively studied modality, has reasonably good accuracy (AUROC 0.87)8 and can be used as an outpatient clinic point-of-care test. VCTE values range from 1.5 to 75kPa. Studies have evaluated a wide span of advanced fibrosis diagnostic cutoffs ranging from 6.95 to 12kPa (Table 1). Among these, a cutoff value of 7.9kPa seems to provide the highest negative predictive value for advanced fibrosis and a cutoff value of 9.9kPa, the highest positive predictive value (Table 1).8,11 A recent analysis by Siddiqui et al,12 conducted among 393 biopsy-proven NAFLD subjects in the NASH CRN, found that the best balance between sensitivity and specificity was achieved at a cutoff value of 8.6kPa (sensitivity=0.8; specificity=0.74; negative predictive value=89%; positive predictive value=59%). Like clinical prediction models, VCTE is best used for ruling out rather than ruling in advanced fibrosis.
A disadvantage to VCTE is that there are operator and patient factors that may render its measurements inaccurate or uninterpretable. A VCTE LSM is considered valid if taken as the median of 10 measurements with >60% success rate, an interquartile-to-median ratio of ≤30% in a patient who has been fasting at least for 3 hours.13,14 TE quality is highest when obtained by an operator who has performed >100 exams. Failed or unreliable VCTE measurements occur in up to 27% of patients11 and, ironically, are common because of central obesity.15 Use of the XL probe, designed for use among patients with body mass index (BMI)≥30kg/m2 or a skin-to-liver-capsule distance of ≥2.5cm and up to 3.5cm, has partly, though not completely, solved this problem. Finally, there are several confounding factors that may render even successfully acquired and apparently high-quality VCTE values inaccurate. These include severe necroinflammation (alanine aminotransferase [ALT]>10× upper limit of normal), hepatic congestion, and extrahepatic cholestasis, all of which can lead to overestimation of liver fibrosis by VCTE.16,17
MRE is the most accurate elastography method (AUROC, 0.93)8 and, unlike TE, can discriminate between fibrosis stages (AUROC, 0.86 to 0.91).18 The optimal cutoff for advanced fibrosis is 3.6kPa (diagnostic performance in Table 1), confirmed by 2 meta-analyses.8,18
Additional advantages to MRE are that it can be used in morbidly obese patients and its measurements are not confounded by necroinflammation. Also, although TE only assesses a small region of interest in the liver, MRE assesses the entire liver. Limitations to MRE include its limited availability and cost which prevent its use as a primary screening tool. But it eventually may prove to be a good confirmatory test for the presence of advanced fibrosis and method for longitudinal disease monitoring.
Clinical Strategy for Noninvasive Fibrosis Assessment
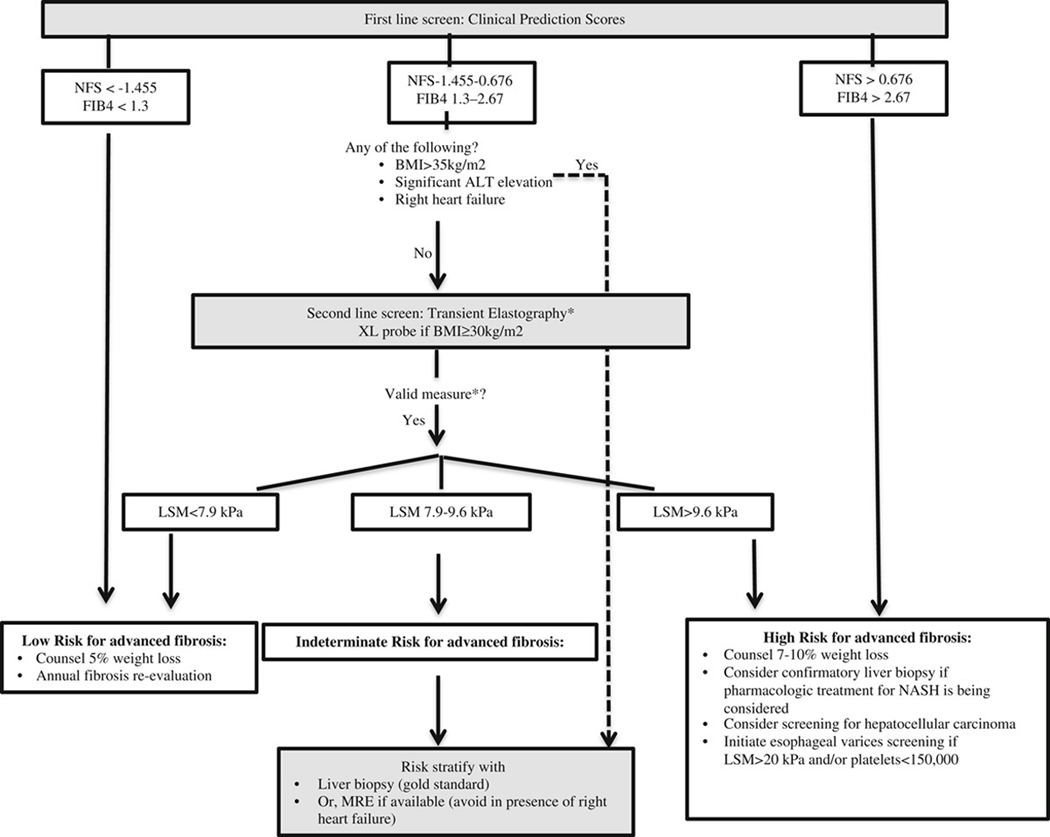
Combining noninvasive tests is a potential way harnessing the advantages of both categories of noninvasive testing and avoiding unnecessary liver biopsies. Petta et al19 tested both a paired and a serial combined approach among patients prospectively evaluated with both clinical prediction scores and VCTE. In the paired testing approach, patients were classified into no/early fibrosis (Metavir stages 0 and 1) and severe fibrosis groups (Metavir stages 2 to 4) on the basis of concordance between clinical prediction scores and LSMs (for example, patients with both a low FIB4 score and low LSM were classified as early fibrosis). This approach yielded improved diagnostic accuracy for severe fibrosis, but over half of patients had discordant findings (for example a low FIB4 but high LSM) and thus could not be classified without a liver biopsy. Next, they tested a serial approach in which only patients with an indeterminate clinical prediction score were evaluated with LSM. This yielded an improved diagnostic accuracy from the use of clinical prediction scores alone (eg, from 52% for NFS alone to 76% for NFS followed by LSM) and reduced the uncertainty rate (39% to 8%). However, the misclassification rate did increase (9% for NFS alone to 16%) but was not significantly different than that observed for LSM alone. Proposed here is a serial method for noninvasive fibrosis prediction in the clinical setting based in part on these data (Fig. 1).
FIGURE 1.
NFS <−1.455 and FIB4< 1.3 are associated with a 92% to 93% negative predictive value for advanced fibrosis. NFS > 0.676 and FIB4> 2.67 are associated with a 66% to 67% positive predictive value for advanced fibrosis.8 Among patients with an indeterminate NFS (−1.455 to 0.676) and FIB4 (1.3 to 2.67), LSM < 7.9 kPa is associated with a 12% to 13% likelihood of advanced fibrosis and LSM > 9.6 is associated with a 72% to 74% likelihood of advanced fibrosis.10 LSM > 20 kPa is associated with 90% accuracy for clinically significant portal hypertension.20 *Transient elastography measure is considered valid if all 3 conditions are met: (a) patient fasting ≥ 3 hours; (b)10 measurements obtained with > 60% success rate; (c) interquartile-to-median ratio ≤ 30%. ALT indicates alanine aminotransferase; BMI, body mass index; FIB4, fibrosis 4; LSM, liver stiffness measurement; MRE, magnetic resonance elastography; NASH, non–alcoholic steatohepatitis; NFS, Non–Alcoholic Fatty Liver Disease Fibrosis Score.
NONINVASIVE PREDICTION OF NASH
The hallmark diagnostic feature of NASH is hepatocyte ballooning on liver biopsy with or without fibrosis. Several studies have tested the diagnostic utility of novel biomarkers to detect histologic NASH independent of fibrosis. Unfortunately, as noted by recently published systematic reviews and meta-analyses, none to date have emerged as sufficiently reliable for clinical use right now.21,22 Here we highlight NASH biomarkers that have been most frequently studied or incorporate novel diagnostic approaches. We provide an overview of each biomarker, propose areas for refinement, and discuss potential future directions.
Aminotransferases
Though serum ALT is the conventional clinical biomarker for detecting and monitoring the chronic liver disease, it is not accurate for diagnosing NASH. Cross-sectional studies have repeatedly shown that a single ALT is poorly predictive for the presence of NASH, regardless of the ALT cutoff used.23–25 A recently published systematic review and meta-analysis confirmed the poor performance of ALT seen in individual studies, estimating a pooled sensitivity and specificity at 64% and 75%, respectively.21
Longitudinal studies suggest that ALT may have value for monitoring disease specifically among patients with histologic NASH and an elevated ALT at baseline. In a secondary analysis of the PIVENS trial, normalization in ALT occurred in most patients who experienced improvement in histologic NASH: 80% of subjects who experienced a 30% reduction in ALT to ≤40U/L had histologic improvement of NASH.26 Similarly, among a cohort of patients with biopsy-proven NASH and elevated ALT at baseline, Vilar-Gomez et al27 observed that the majority of subjects (72%) who attained histologic NASH resolution had normalization of ALT to below gender-specific thresholds (≤19U/L for women and ≤30U/L for men).
Lipidomic, Metabolomic, and Transcriptomic Strategies
“-OMIC” strategies are a novel approach for noninvasive NASH detection. Metabolomics, in particular, have attracted increasing attention within the field of hepatology. Early studies have been small but yielded promising findings. For example, in a proof of concept paper, Loomba et al28 demonstrated that NASH could be accurately diagnosed using a lipidomic panel consisting of either 11,12-diHeTrE alone (AUROC 1) or a consisting of dhk PGD2 plus 20-COOHAA (AUROC 1). Separately, Zhou et al29 found that the NASH ClinLipMet score, which combined lipidomic and metabolomic signatures (glutamate, isoleucine, glycine, lysophosphatidylcholine 16:0 and phosphoethanolamine 40:6) with AST, PNPLA3, and fasting insulin had an AUROC 0.87 for NASH among 318 bariatric patients. In a large multicenter study involving 467 patients with biopsied NAFLD, Mayo et al30 also found a favorable performance of a prediction model that combined 28 selected triglyceride species with BMI (AUROC of 0.95 and 0.79 in the derivation and validation cohorts, respectively).
MicroRNAs (miRNA) are highly conserved noncoding small RNAs that regulate gene expression at translational or posttranslational levels. MiRNAs are appealing clinical biomarkers mainly because they are resistant to ribonucleases and stable in serum, plasma, and urine samples. A handful of proteomic studies have assessed the performance of miRNAs alone or in combination with clinical markers for NASH diagnosis. Evaluated to date are: individual miRNAs (miR122, miR192, and miR375 with AUROCs ranging 0.66 to 0.71)31; a diagnostic panel consisting of mirR122, miR192, miR21 with ALT and CK-18 (AUROC, 0.85); and miRNA combinations miR192/30c and 27b/30c (AUROC, 0.78 and 0.79, respectively).32
Although these early OMIC approaches are promising, they are nowhere close to ready for clinical use. To date, there have been a paucity of studies evaluating their performance. No validation studies have been conducted confirming the use of specific panels. And there is a need for analyses that consider whether and how various coexisting metabolic diseases may impact metabolites that are used for NASH diagnosis.
Markers of Apoptosis: CK-18
CK-18 is the most extensively studied NASH biomarker and has generated a high level of interest on the basis of its significance as a direct molecular byproduct of hepatocyte death. CK-18 is the major intermediate filament protein comprising the cytoskeleton structure of hepatocytes. During hepatocyte apoptosis effector caspases cleave CK-18, generating M30 and M65 fragments into the circulation.
Two types of assays have been used to study CK-18. One mainly detects the M30 fragment, a marker specifically of apoptosis and most frequently studied, and the other detects M65 fragment, a general indicator of cell death. Both have performed moderately well and nearly equivalently for NASH diagnosis (pooled AUROCs: M30=0.82 and M65=0.80).22 The use of separate cutoffs that optimize sensitivity (a low cutoff) and specificity (a high cutoff) seems more useful than the use of a single CK-18 cutoff for NASH detection. For example, the use of a single M30 fragment cutoff had a pooled sensitivity of 66% to 68% and specificity of 75% to 82% in meta-analyses that pooled studies utilizing cutoffs ranging 121.6 to 338.0 U/L.21,33 Diagnostic performance improved considerably with separate low cutoff to optimize sensitivity (pooled estimate, 82% to 83%; cutoffs ranging, 111.6 to 380.0 U/L) and a separate high cutoff to optimize specificity (pooled estimate, 93% to 98%; cutoffs ranging, 261.4 to 670.0 U/L).21
To date, CK-18 has the largest accumulated evidence and has demonstrated the greatest potential among NASH biomarkers. But it, too, has limitations. First, varying CK-18 diagnostic cutoffs and assays (M30 vs M65) have been assessed across studies, making it impossible to hone in on specific useable CK-18 diagnostic value for ruling in or ruling out NASH. Second, the correlation between changes in CK-18 and histologic NASH activity needs further evaluation beyond the handful of small-scale prospective studies that have been completed to date.34,35
Markers of Oxidative Stress and Inflammation
Reactive oxygen species, generated by mitochondrial and peroxisomal fatty acid oxidation, confer liver damage either directly or by depleting antioxidant reserves, rendering hepatocytes more vulnerable to other factors that generate oxidative stress. In keeping with this, higher levels of oxidative stress products have been observed among NASH versus simple steatosis patients.
Markers of oxidative stress have, on the basis of this rationale, been tested for detection of NASH. OxNASH, a panel incorporating 13 hydroxyl octadecadienoic acid/linoleic acid ratio, age, BMI, and AST, is one such example. Although the initial estimated AUROC for oxNASH was promising in the derivation cohort (0.83),36 its accuracy for NASH dropped in the validation group (AUROC 0.73). And, in a subsequent cross-sectional study among 122 patients oxNASH had an AUROC 0.74 for histologic ballooning.37 The drawback to this test is that it has not been externally validated and testing for oxidative markers requires specialized laboratory testing that is not readily available in most centers.
Adipokines
Adipokines are adipose tissue-derived hormones with autocrine, paracrine, and endocrine functions in glucose and fat metabolism. The 2 best-understood adipokines in the context of NAFLD are adiponectin and leptin. Though NASH is associated with lower adiponectin and higher leptin levels than NAFL38 both adipokines have generally performed inadequately for NASH diagnosis with AUROCs<0.8.39–43 Adipokines in combination with inflammatory, clinical, or apoptosis markers have improved their accuracy. For example, although adiponectin alone had an AUROC 0.709 for NASH among 79 patients with biopsied NAFLD patients, its accuracy improved when combined with M65 and IL-6 (AUROC, 0.903).40 Similarly, among 70 patients with biopsied NAFLD, adiponectin alone yielded an AUROC 0.10 for prediction of NASH but improved to 0.90 using a prediction model incorporating adiponectin, visfatin, tumor necrosis factor-alpha, and IL-8.42
A persistent limitation, however, has been the confounding effect of adiposity on adipokines’ ability to predict NASH reliably and the small cross-sectional study designs to date.
Elastography
Neither VCTE nor MRE predict NASH accurately. VCTE had an AUROC of 0.35 for NASH prediction in a cross-sectional analysis among 94 patients with biopsy-proven NAFLD38 and AUROC 0.65 when used with controlled attenuation parameter in a separate analysis among 127 patients. A summary of NASH noninvasive testing and performance is given in (Table 2).44
TABLE 2.
Diagnostic Accuracy of NASH Biomarkers
| Biomarker | AUROC | References |
|---|---|---|
| Routine clinical markers | ||
| ALT | 0.62 | 24 |
| Metabolomic and lipidomic markers | ||
| “NASH ClinLipMet score” (AST, PNPLA3 genotype, fasting insulin, glutamate, isoleucine, glycine, (lysophosphatidylcholine 16:0 and phosphoethanolamine 40:6) | 0.87 | 29 |
| Polyunsaturated fatty acid metabolites (11,12-diHeTrE or dhk PGD2 plus 20-COOHAA) | 1 | 28 |
| Panel of selected triglyceride species+BMI | 0.79 | 30 |
| Cell death markers | ||
| CK-18 M30 fragment | 0.82 (pooled estimate) | 22 |
| CK-18-M65 fragment | 0.80 (pooled estimate) | 22 |
| Oxidative stress and adipokine markers | ||
| OxNASH panel (13 hydroxyl octadecadienoic acid/linoleic acid ratio, age, BMI, and AST) | 0.73–0.74 | 27,36 |
| Adiponectin alone | 0.77–0.71 | 40,41 |
| “NASH diagnostics” adiponectin with CK-18-M65, IL-6 | 0.90 | 41 |
| Elastography | ||
| VCTE | 0.35 | 38 |
| VCTE with CAP | 0.65 | 44 |
| MRE | 0.70–0.94 | 38,44,47 |
| MRE with PDFF | 0.77 | 38 |
ALT, alanine aminotransferase; AST, aspartate transaminase; BMI, body mass index; CAP, controlled attenuation parameter; CK-18, cytokeratin 18; IGT, impaired glucose tolerance; IL-16, interleukin 16; MRE, magnetic resonance elastography; NASH, non–alcoholic steatohepatitis; PDFF, proton density fat fraction; VCTE, vibration controlled transient elastography.
Although MRE performs better than VCTE, its accuracy has varied across studies, with AUROC ranging from 0.70 to 0.9438,45–47 and, when used in combination with proton density fat fraction, AUROC is 0.77.44 These observed disparities in part reflect the NASH definitions used by each study. But they may also reflect the prevalence of advanced fibrosis in each study population. Thus, further clarity is required regarding how MRE performs for detection of NASH independent of fibrosis which may confound its accuracy. Neither VCTE nor MRE can be recommended at this time for the detection of histologic NASH.
CONCLUSIONS
Currently, noninvasive prediction of advanced fibrosis is feasible in the clinical setting and can cut down on the need for biopsies for this purpose. In contrast, although several novel NASH biomarkers have been studied, none are sufficiently accurate for clinical use yet. Consequently, clinicians are left with few tools for diagnosing and monitoring their patients with NASH. Liver biopsy remains the main and only diagnostic method for making an initial diagnosis of NASH.
NASH biomarker analyses to date have been limited by a number of factors including the use of cross-sectional study design and small-sized to moderate-sized study populations from single centers. A few markers have emerged with sound putative basis and intriguing results such as the CK-18 fragments, lipidomic and metabolomic candidates, and MRE with proton density fat fraction. But these are still plagued by a paucity of validation and prospective studies evaluating their performance for NASH diagnosis. Metabolomic and lipidomic markers also require further study addressing how coexisting diseases and exposures may confound their interpretation.
Ultimately what is needed in the field are large multicenter prospective cohort studies to evaluate select novel biomarkers. Alternatively, several phase II and III NASH therapeutic trials that are currently underway are potentially ideal venues for addressing the performance of NASH biomarkers. These trials solve several of the shortcomings of prior studies as, by design, they are prospective and include large study populations of patients with histologically proven NASH derived from multiple centers.
Acknowledgments
R.L. receives funding support from NIEHS (5P42ES010337), NCATS (5UL1TR001442), and NIDDK (R01DK106419).
Footnotes
R.L. serves as a consultant or advisory board member for Arrowhead Pharmaceuticals, AstraZeneca, Bird Rock Bio, Boehringer-Ingelheim, Bristol-Myer Squibb, Celgene, Cirius, CohBar, Conatus, Eli Lilly, Galmed, Gemphire, Gilead, Glympse bio, GNI, GRI Bio, Intercept, Ionis, Janssen Inc., Merck, Metacrine, Inc., NGM Biopharmaceuticals, Novo Nordisk, Pfizer, Prometheus, Sanofi, Siemens, and Viking Therapeutics. In addition, his institution has received grant support from Allergan, Boehringer-Ingelheim, Bristol-Myers Squibb, Cirius, Eli Lilly and Company, Galectin Therapeutics, Galmed Pharmaceuticals, GE, Genfit, Gilead, Intercept, Janssen, Madrigal Pharmaceuticals, Merck, NGM Biopharmaceuticals, NuSirt, Pfizer, Prometheus, and Siemens. He is also cofounder of Liponexus, Inc. The remaining authors declare that they have nothing to disclose.
REFERENCES
- 1.Younossi ZM, Koenig AB, Abdelatif D, et al. Global epidemiology of nonalcoholic fatty liver disease—meta-analytic assessment of prevalence, incidence, and outcomes. Hepatology. 2016;64:73–84. [DOI] [PubMed] [Google Scholar]
- 2.Byrne CD, Targher G. NAFLD: a multisystem disease. J Hepatol. 2015;62(suppl 1):S47–S64. [DOI] [PubMed] [Google Scholar]
- 3.Adams LA, Lymp JF, St Sauver J, et al. The natural history of nonalcoholic fatty liver disease: a population-based cohort study. Gastroenterology. 2005;129:113–121. [DOI] [PubMed] [Google Scholar]
- 4.Mellinger JL, Pencina KM, Massaro JM, et al. Hepatic steatosis and cardiovascular disease outcomes: an analysis of the Framingham Heart Study. J Hepatol. 2015;63:470–476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rinella ME. Nonalcoholic fatty liver disease: a systematic review. JAMA. 2015;313:2263–2273. [DOI] [PubMed] [Google Scholar]
- 6.Angulo P, Kleiner DE, Dam-Larsen S, et al. Liver fibrosis, but no other histologic features, is associated with long-term outcomes of patients with nonalcoholic fatty liver disease. Gastroenterology. 2015;149:389–397.e10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Estes C, Razavi H, Loomba R, et al. Modeling the epidemic of nonalcoholic fatty liver disease demonstrates an exponential increase in burden of disease. Hepatology. 2018;67:123–133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Xiao G, Zhu S, Xiao X, et al. Comparison of laboratory tests, ultrasound, or magnetic resonance elastography to detect fibrosis in patients with nonalcoholic fatty liver disease: a meta-analysis. Hepatology. 2017;66:1486–1501. [DOI] [PubMed] [Google Scholar]
- 9.McPherson S, Stewart SF, Henderson E, et al. Simple non-invasive fibrosis scoring systems can reliably exclude advanced fibrosis in patients with non-alcoholic fatty liver disease. Gut. 2010;59:1265–1269. [DOI] [PubMed] [Google Scholar]
- 10.McPherson S, Hardy T, Dufour JF, et al. Age as a confounding factor for the accurate non-invasive diagnosis of advanced NAFLD fibrosis. Am J Gastroenterol. 2017;112:740–751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tapper EB, Challies T, Nasser I, et al. The performance of vibration controlled transient elastography in a US cohort of patients with nonalcoholic fatty liver disease. Am J Gastroenterol. 2016;111:677–684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Siddiqui MS, Vuppalanchi R, Van Natta ML, et al. Vibration-controlled transient elastography to assess fibrosis and steatosis in patients with nonalcoholic fatty liver disease. Clin Gastroenterol Hepatol. 2019;17:156–163.e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sandrin L, Fourquet B, Hasquenoph JM, et al. Transient elastography: a new noninvasive method for assessment of hepatic fibrosis. Ultrasound Med Biol. 2003;29:1705–1713. [DOI] [PubMed] [Google Scholar]
- 14.Arena U, Lupsor Platon M, Stasi C, et al. Liver stiffness is influenced by a standardized meal in patients with chronic hepatitis C virus at different stages of fibrotic evolution. Hepatology. 2013;58:65–72. [DOI] [PubMed] [Google Scholar]
- 15.Castera L, Foucher J, Bernard PH, et al. Pitfalls of liver stiffness measurement: a 5-year prospective study of 13,369 examinations. Hepatology. 2010;51:828–835. [DOI] [PubMed] [Google Scholar]
- 16.Coco B, Oliveri F, Maina AM, et al. Transient elastography: a new surrogate marker of liver fibrosis influenced by major changes of transaminases. J Viral Hepat. 2007;14:360–369. [DOI] [PubMed] [Google Scholar]
- 17.Tapper EB, Loomba R. Noninvasive imaging biomarker assessment of liver fibrosis by elastography in NAFLD. Nat Rev Gastroenterol Hepatol. 2018;15:274–282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Singh S, Venkatesh SK, Loomba R, et al. Magnetic resonance elastography for staging liver fibrosis in non-alcoholic fatty liver disease: a diagnostic accuracy systematic review and individual participant data pooled analysis. Eur Radiol. 2016;26:1431–1440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Petta S, Wong VW, Camma C, et al. Serial combination of non-invasive tools improves the diagnostic accuracy of severe liver fibrosis in patients with NAFLD. Aliment Pharmacol Ther. 2017;46:617–627. [DOI] [PubMed] [Google Scholar]
- 20.Garcia-Tsao G, Abraldes JG, Berzigotti A, et al. Portal hypertensive bleeding in cirrhosis: risk stratification, diagnosis, and management: 2016 practice guidance by the American Association for the study of liver diseases. Hepatology. 2017;65:310–335. [DOI] [PubMed] [Google Scholar]
- 21.Verhaegh P, Bavalia R, Winkens B, et al. Noninvasive tests do not accurately differentiate nonalcoholic steatohepatitis from simple steatosis: a systematic review and meta-analysis. Clin Gastroenterol Hepatol. 2018;16:837–861. [DOI] [PubMed] [Google Scholar]
- 22.He L, Deng L, Zhang Q, et al. Diagnostic value of CK-18, FGF-21, and related biomarker panel in nonalcoholic fatty liver disease: a systematic review and meta-analysis. BioMed Res Int. 2017;2017:9729107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mofrad P, Contos MJ, Haque M, et al. Clinical and histologic spectrum of nonalcoholic fatty liver disease associated with normal ALT values. Hepatology. 2003;37:1286–1292. [DOI] [PubMed] [Google Scholar]
- 24.Verma S, Jensen D, Hart J, et al. Predictive value of ALT levels for non-alcoholic steatohepatitis (NASH) and advanced fibrosis in non-alcoholic fatty liver disease (NAFLD). Liver Int. 2013; 33:1398–1405. [DOI] [PubMed] [Google Scholar]
- 25.Kunde SS, Lazenby AJ, Clements RH, et al. Spectrum of NAFLD and diagnostic implications of the proposed new normal range for serum ALT in obese women. Hepatology. 2005;42:650–656. [DOI] [PubMed] [Google Scholar]
- 26.Hoofnagle JH, Van Natta ML, Kleiner DE, et al. Vitamin E and changes in serum alanine aminotransferase levels in patients with non-alcoholic steatohepatitis. Aliment Pharmacol Ther. 2013;38:134–143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Vilar-Gomez E, Calzadilla-Bertot L, Friedman SL, et al. Serum biomarkers can predict a change in liver fibrosis 1 year after lifestyle intervention for biopsy-proven NASH. Liver Int. 2017;37:1887–1896. [DOI] [PubMed] [Google Scholar]
- 28.Loomba R, Quehenberger O, Armando A, et al. Polyunsaturated fatty acid metabolites as novel lipidomic biomarkers for noninvasive diagnosis of nonalcoholic steatohepatitis. J Lipid Res. 2015;56:185–192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zhou Y, Oresic M, Leivonen M, et al. Noninvasive detection of nonalcoholic steatohepatitis using clinical markers and circulating levels of lipids and metabolites. Clin Gastroenterol Hepatol. 2016;14:1463–1472.e6. [DOI] [PubMed] [Google Scholar]
- 30.Mayo R, Crespo J, Martinez-Arranz I, et al. Metabolomic-based noninvasive serum test to diagnose nonalcoholic steatohepatitis: results from discovery and validation cohorts. Hepatol Commun. 2018;2:807–820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Pirola CJ, Fernandez Gianotti T, Castano GO, et al. Circulating microRNA signature in non-alcoholic fatty liver disease: from serum non-coding RNAs to liver histology and disease pathogenesis. Gut. 2015;64:800–812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lopez-Riera M, Conde I, Quintas G, et al. Non-invasive prediction of NAFLD severity: a comprehensive, independent validation of previously postulated serum microRNA biomarkers. Sci Rep. 2018;8:10606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kwok R, Tse YK, Wong GL, et al. Systematic review with meta-analysis: non-invasive assessment of non-alcoholic fatty liver disease—the role of transient elastography and plasma cytokeratin-18 fragments. Aliment Pharmacol Ther. 2014;39:254–269. [DOI] [PubMed] [Google Scholar]
- 34.Shen J, Chan HL, Wong GL, et al. Non-invasive diagnosis of non-alcoholic steatohepatitis by combined serum biomarkers. J Hepatol. 2012;56:1363–1370. [DOI] [PubMed] [Google Scholar]
- 35.Vuppalanchi R, Jain AK, Deppe R, et al. Relationship between changes in serum levels of keratin 18 and changes in liver histology in children and adults with nonalcoholic fatty liver disease. Clinl Gastroenterol Hepatol. 2014;12:2121–2130.e1-e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Feldstein AE, Lopez R, Tamimi TA, et al. Mass spectrometric profiling of oxidized lipid products in human nonalcoholic fatty liver disease and nonalcoholic steatohepatitis. J Lipid Res. 2010; 51:3046–3054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Alkhouri N, Berk M, Yerian L, et al. OxNASH score correlates with histologic features and severity of nonalcoholic fatty liver disease. Dig Dis Sci. 2014;59:1617–1624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Park CC, Nguyen P, Hernandez C, et al. Magnetic resonance elastography vs transient elastography in detection of fibrosis and noninvasive measurement of steatosis in patients with biopsy-proven nonalcoholic fatty liver disease. Gastroenterology. 2017; 152:598–607.e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Shimada M, Kawahara H, Ozaki K, et al. Usefulness of a combined evaluation of the serum adiponectin level, HOMA-IR, and serum type IV collagen 7S level to predict the early stage of nonalcoholic steatohepatitis. Am J Gastroenterol. 2007; 102:1931–1938. [DOI] [PubMed] [Google Scholar]
- 40.Grigorescu M, Crisan D, Radu C, et al. A novel pathophysiological-based panel of biomarkers for the diagnosis of nonalcoholic steatohepatitis. J Physiol Pharmacol. 2012;63:347–353. [PubMed] [Google Scholar]
- 41.Machado MV, Coutinho J, Carepa F, et al. How adiponectin, leptin, and ghrelin orchestrate together and correlate with the severity of nonalcoholic fatty liver disease. Eur J Gastroenterol Hepatol. 2012;24:1166–1172. [DOI] [PubMed] [Google Scholar]
- 42.Jamali R, Arj A, Razavizade M, et al. Prediction of non-alcoholic fatty liver disease via a novel panel of serum adipokines. Medicine. 2016;95:e2630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lemoine M, Ratziu V, Kim M, et al. Serum adipokine levels predictive of liver injury in non-alcoholic fatty liver disease. Liver Int. 2009;29:1431–1438. [DOI] [PubMed] [Google Scholar]
- 44.Imajo K, Kessoku T, Honda Y, et al. Magnetic resonance imaging more accurately classifies steatosis and fibrosis in patients with nonalcoholic fatty liver disease than transient elastography. Gastroenterology. 2016;150:626–637.e7. [DOI] [PubMed] [Google Scholar]
- 45.Loomba R, Wolfson T, Ang B, et al. Magnetic resonance elastography predicts advanced fibrosis in patients with non-alcoholic fatty liver disease: a prospective study. Hepatology (Baltimore, Md). 2014;60:1920–1928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Chen J, Talwalkar JA, Yin M, et al. Early detection of nonalcoholic steatohepatitis in patients with nonalcoholic fatty liver disease by using MR elastography. Radiology. 2011;259: 749–756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hsu C, Caussy C, Imajo K, et al. Magnetic resonance vs transient elastography analysis of patients with nonalcoholic fatty liver disease: a systematic review and pooled analysis of individual participants. Clin Gastroenterol Hepatol. 2019;17: 630–637.e8. [DOI] [PMC free article] [PubMed] [Google Scholar]