Abstract
Sleep disruption in older adults living with Alzheimer’s disease and related dementias (ADRD) is debilitating and contributes to increased institutionalization, reduced cognitive function, and accelerated disease progression. Furthermore, sleep disruption is linked to poor health outcomes in caregivers, such as decreased quality of life and increased caregiver burden. Given the potential harmful side effects of pharmacologic treatment, non-pharmacologic approaches, such as music, may provide a safer alternative to reducing sleep disruption in this vulnerable population. A growing body of literature suggests that calming tailored music may improve sleep quality in older adults with memory loss, but its efficacy has not been demonstrated in older adults with ADRD in the community, where most older adults with ADRD live. If shown to be feasible and acceptable, tailored music interventions can then be tested for efficacy in reducing sleep disruption. This protocol details a wait-list randomized controlled trial [NCT04157244], the purpose of which is to test the feasibility, acceptability and examine the preliminary efficacy of a tailored music listening intervention in older adults with ADRD who report sleep disruption. Music selections will be tailored to the music genre preferences of older adults with ADRD and account for known sleep-inducing properties. Feasibility of processes that are key to the success of the subsequent study will be examined. Preliminary efficacy of the intervention will be assessed using objective (actigraphy) and subjective (proxy reported) sleep quality measures. In addition, qualitative data will be solicited examining the acceptability and satisfaction with the intervention by individuals living with dementia.
Keywords: music, dementia, sleep, Alzheimer’s disease
Introduction
Approximately 25–44% of older adults with Alzheimer’s disease and related dementias (ADRD) experience sleep disruption, which includes nighttime sleep fragmentation, decreased sleep efficiency, and increased daytime napping (Deschenes & McCurry, 2009; Vitiello & Borson, 2001). The origin of sleep disruption in ADRD stems from multiple factors, including degeneration of neural pathways responsible for circadian rhythm regulation, presence of psychiatric co-morbidities (Peter-Derex, Yammine, Bastuji, & Croisile, 2015), or nocturnal agitation (Rose et al., 2011). Sleep disruption is pervasive across all three stages of ADRD – mild, moderate, and severe (Rose et al., 2011). The impact of sleep disruption is devastating for both older adults with ADRD and their caregivers (Wulff, Gatti, Wettstein, & Foster, 2010). In older adults with ADRD, untreated sleep disruption is associated with poor quality of life (Hodgson, Gitlin, & Huang, 2014), reduced cognitive function (Jelicic et al., 2002; Yaffe, Falvey, & Hoang, 2014), and accelerated disease progression (Rabins et al., 2013).
Significant age-related changes in circadian regulation contribute to the prevalence of circadian rhythm disorders and sleep disruption in older adults with ADRD (Zee & Vitiello, 2009). Examples of circadian rhythm disorders symptoms include evening agitation, excessive daytime sleepiness, increased sleep latency and frequent nighttime awakenings (Deschenes & McCurry, 2009; Zee & Vitiello, 2009). Interventions aimed at evening hours (between 6 PM and 10 PM) can be particularly helpful to prepare for sleep. The goal of these interventions should be to induce a calm relaxed state and reduce stress hormone production (Safi & Hodgson, 2014). Matching daytime activities with their corresponding optimal windows can be an effective approach to address symptoms of circadian rhythm disorders and improve sleep in older adults with ADRD (Safi & Hodgson, 2014).
Pharmacologic treatment remains the first-line treatment for sleep disruption in ADRD, however, its use is often limited due to potentially harmful side effects (Deschenes & McCurry, 2009; Peter-Derex et al., 2015; Vitiello & Borson, 2001). The absence of effective and safe pharmacologic treatment for sleep disruption warrants closer investigation into alternative approaches. Music has shown promising results in improving sleep quality in adults with insomnia (Jespersen, Koenig, Jennum, & Vuust, 2015; Jespersen, Otto, Kringelbach, Van Someren, & Vuust, 2019), cancer (Lai, Li, & Lee, 2011), and ICU patients (Hu, Jiang, Hegadoren, & Zhang, 2015; Su et al., 2012). In healthy older adults, listening to calming music was associated with shorter wake time after sleep onset (Huang, Chang, & Lai, 2016), shorter sleep-latency onset (Lai et al., 2015), better perceived sleep quality and longer sleep duration (Lai & Good, 2005). The potential therapeutic effects of listening to calming music have been attributed to its anxiolytic properties through its suppressive action on the sympathetic nervous system, contributing to decreased neuromuscular arousal (Chanda & Levitin, 2013).
Music that has personal meaning is appropriate to older adults across all stages of ADRD, from mild to moderate, given that receptive and expressive musical abilities are often preserved long after the diminished ability to process or express verbal language (Basaglia-Pappas et al., 2013; Fornazzari et al., 2006; Johnson et al., 2011; Johnson & Chow, 2015). Therefore, music may be used as a means of communicating even in the advanced stages of dementia when the person is unable to understand verbal language and has a decreased ability to interpret environmental stimuli. Listening to a preferred genre of music may improve adherence to a music intervention. Previous studies have shown that the efficacy of music on sleep is affected by the listener’s enjoyment with the preferred genre having the most beneficial effect (Lai, 2004; Wang, Sun, & Zang, 2014). No empirically validated music protocol exists to address sleep disruption in older adults with ADRD living at home. Such absence indicates a critical barrier to developing widely available non-pharmacologic interventions to improve sleep quality in this vulnerable population.
Purpose and Aims
The purpose of this research project is to examine the feasibility of a tailored music intervention to improve sleep quality in home-dwelling older adults with ADRD. The central hypothesis of this study is that tailored music delivered at bedtime and accounting for known sleep-inducing properties of music can improve sleep quality, if shown to be feasible and acceptable in this population. To test this hypothesis, we propose a wait-list randomized controlled trial of a 30-minute, 4-week nightly tailored home music intervention in a sample of 60 dyads (older adults with ADRD and their caregivers). The aims are to: 1) Examine the feasibility of delivering a tailored music intervention protocol to both family caregivers and older adults with ADRD; 2) Examine the acceptability of the tailored music intervention by both family caregivers and older adults with ADRD using survey and qualitative data; and 3) Obtain preliminary estimates of treatment efficacy on four sleep quality outcomes: wake time after sleep onset, sleep-latency onset, total sleep duration, and perceived sleep quality.
In this study, caregivers are taught to play tailored by genre calming music prior to bedtime which may elicit older adults to relax and fall asleep, shortening time to sleep onset, increasing sleep duration and improving their perceived sleep quality. By providing calming tailored music at bedtime, we aim to change their environment to be more conducive to sleep. Previous research has shown the following music characteristics as efficacious in reducing sleep disruption (Huang et al., 2016; Lai et al., 2015; Lai & Good, 2005) - music selections should be at least 30 minutes in length (Gerdner, 1997, 2018), slow stable tempo (between 60–80 bpm), warm timbre, and absence of lyrics or strong percussion (Lai, 2004; Lai & Good, 2005). Investigator selected music with calming properties, disregarding personal preferences and background, has not been beneficial in promoting sleep (Lai, 2004).
Methods
Design
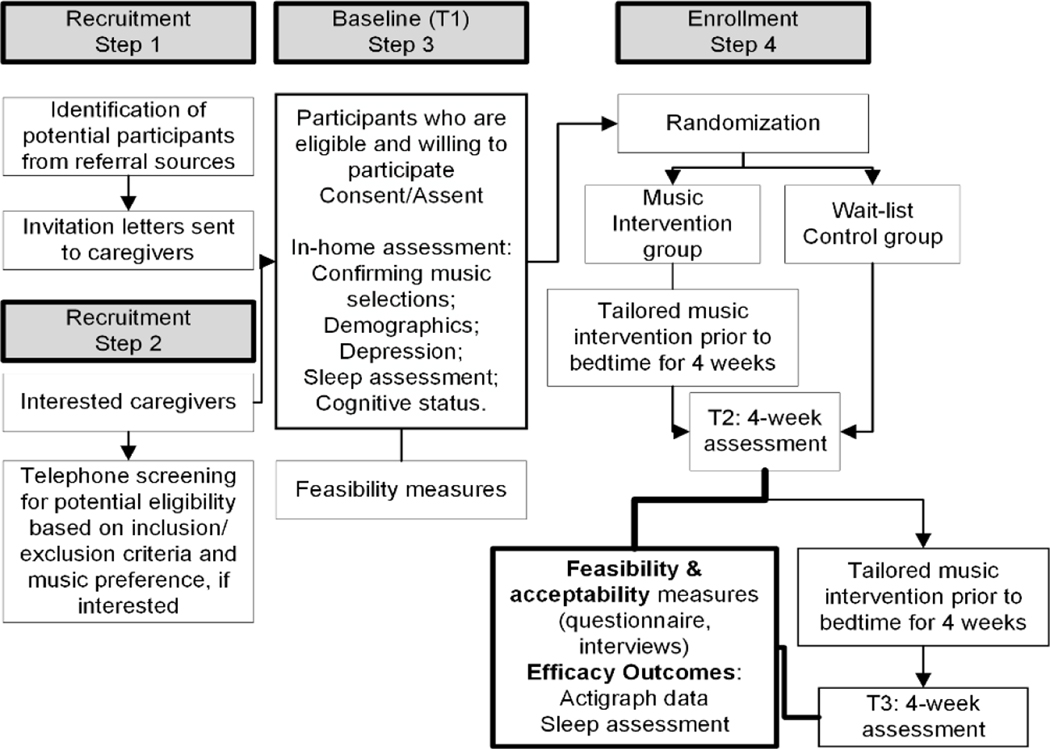
A feasibility randomized wait-list controlled trial will be used. This design was chosen to examine the feasibility and acceptability (Aim 1 and 2) of a tailored 4-week home music intervention for 60 dyads across both the experimental and wait-list control groups. To provide support for a larger randomized controlled trial (RCT), in Aim 3 we will examine preliminary efficacy of the intervention in reducing sleep disruption using actigraphy and subjective sleep measures. Figure 1 outlines the study flow. This study was approved by the University of Pennsylvania Institutional Review Board.
Figure 1: Study Flow.
Participants and Setting
Participation will be offered to 60 dyads from Philadelphia, PA and the surrounding metropolitan area. Table 1 outlines key inclusion and exclusion criteria for older adults with ADRD. To be included in the study participants had to have an existing physician diagnosis of ADRD or self-reported memory impairment and a score of 0.5 or greater on the Clinical Dementia Rating Scale (Morris, 1993) (corresponding to at least mild ADRD severity). This expanded inclusion criteria (beyond an existing diagnosis) is to recognize that most community residing older adults with ADRD are not diagnosed and we wanted to purposely recruit those from underrepresented communities. Caregivers will be included if they provide at least four hours of daily care and live with the older adult, and are able to read and speak in English. All study related visits (except for the brief phone calls) will be done at participants’ homes at late morning or early afternoon hours.
Table 1:
Inclusion and Exclusion Criteria for Older Adults with ADRD
| Inclusion | Exclusion |
|---|---|
| Age 60 and older | Planned transition to another residential or institutional care setting in less than 3 months to decrease attrition rates |
| Existing physician diagnosis of ADRD or self-reported memory impairment and Clinical Dementia Rating (CDR) Scale score of greater than 0.5 (Morris, 1993) | Hearing impairment (defined as inability to hear a normal speaking voice at a distance of 1–1/2 feet) to exclude those who cannot hear the music intervention |
| Presence of sleep problems determined first during phone screening using Neuropsychiatric Inventory sleep disorders item, then using proxy-rated Sleep Disorders Inventory (SDI) (Tractenberg, Singer, Cumming, & Thal, 2003) (presence of at least one sleep disturbance symptom of moderate severity) | Presence of extrapyramidal symptoms affecting non-dominant hand which may include persons with the following diagnoses: schizophrenia, bipolar disorder, Huntington’s disease, Parkinson’s disease, Lewy Body dementia due to REM sleep disorders affecting actigraphy measurement of sleep disruption (Camargos et al., 2013) |
| Stable dose of psychotropic medications, sedatives/hypnotics, anti-dementia or opioids in the past 90 days (typical time frame in clinical trials) prior to enrollment to minimize confounding effects of medications | Currently enrolled in an interventional clinical trial for ADRD aimed to improve sleep as to not to confound the efficacy results |
| Tolerates and agrees to wear wrist actigraph | Acute sleep disruption within 2 weeks of screening as it may indicate sleep disorders not related to ADRD or delirium (Camargos et al., 2013) |
| Responsive to their environment (e.g., able to understand short commands) | End stage disease (i.e. cancer, bed bound) to minimize factors affecting actigraphy measurement |
| Sufficient English to complete questionnaires |
Recruitment and Retention Strategies
This study was designed to overcome three major challenges of behavioral intervention research with community-dwelling, vulnerable older adults: retention of participants, adherence to actigraphy, and the accurate collection of subjective measures. To decrease attrition, we will use several strategies such as beginning intervention within two weeks of recruitment, sending thank you, birthday and holiday cards as well as maintaining strong relationships with community partnerships. A number of participant payments will be offered to incentivize the participants to sustain participation. Based on previous studies in this population and the safeguards we put into place, we predict that most dyads will adhere to the music intervention protocol and complete the study measures. Qualitative data will be sought following the intervention and may help explain the extraneous factors not accounted for in the quantitative data to improve adherence rates in a future trial. Adherence to wear the actigraph might be lower compared to rates of listening to music, since older adults with ADRD might remove the actigraph (Camargos, Louzada, & Nóbrega, 2013). Even though caregivers’ responses on the self-report questionnaires may be influenced by their stress levels and mood, we will use psychometrically sound measures widely employed in this population and behavioral intervention research. In the ongoing meetings with the study team we will review procedures, assignments, and ways to address unexpected challenges.
Procedures
We will send invitation letters to all potential caregivers referred to us detailing the purpose of the study with information on how to contact the research team. Caregivers interested in participating will be screened by telephone for key inclusions/exclusion criteria (Table 1) and music preferences, if interested. After the initial screening and confirmed willingness of caregivers to participate in the research project, we will set a time to meet with the dyad at their home for a consent/baseline assessment. Prior to completing the assessment, we will review study purpose/procedures and obtain written consent. If the older adult with ADRD is unable to provide informed consent based on not being able to verbally identify the study’s purpose and risk/benefits, we will seek proxy consent and the older adult’s assent. We will also collect baseline demographic data (if not available in the medical records), screen for depression, and assess sleep quality. Following the baseline assessment, dyads will be randomized to either the music intervention or wait-list control group. The caregivers will learn about the dyad assignment 72 hours following the randomization. At this point, caregivers may choose to withdraw from the study if they are not satisfied with the assignment, which may lead to higher dropout rates for wait-list control group. In addition, once the investigator becomes aware of the group assignment, their assessment at T1, T2 and T3 may favor the intervention group. To minimize these effects, we will collect all the necessary data at baseline before the dyads learn of their assignment and use a combination of self-report and objective sleep data. It is impossible to blind the research participants to their group assignments at T2 and T3 due to the nature of the intervention. The researcher staff will also be aware of the participants’ group assignments at T2 and T3. This is a limitation of the study. Every effort will be made to minimize unblinding effects to the research staff, including the use and data analysis of actigraphy data. For example, when analyzing actigraphy data the dyad identification will not be linked to their actigraphy data, essentially blinding the research staff from knowing the group assignment of the research participant. Once the actigraphy data analysis is complete, the dyad identification number will be linked again to the participant’s’ actigraphy data. We will collect feasibility information at screening, T1, T2, and T3 as well as acceptability measures (T2 & T3) for all dyads (Figure 1).
Determining Music Preference
We will select calming music selections based on individual genre of music to maximize participant’s enjoyment and music’s effectiveness, using “Assessment of Personal Music Preference” during the telephone screening. The assessment tool is available as part of the evidence-based guidelines on using individualized music and was modified by Gerdner, Hartsock, and Buckwalter in 2000 (Gerdner, 2018). Two versions of the assessment are available, which can be completed by either member of the dyad. Table 2 demonstrates a list of different types of music a person with ADRD may rank in the order of being their favorite.
Table 2:
Genres of Music Available in the “Assessment of Personal Music Preference”
| Country and Western |
| Classical |
| Spiritual/Religious |
| Big Band/Swing |
| Folk |
| Blues |
| Jazz |
| Rock and Roll |
| Easy Listening |
| Cultural or Ethnic Specific (examples: Czech polkas, Ravi Shankar Indian sitar) |
| Other |
Following the assessment and selecting tailored music, we will return to the dyad’s home at baseline (T1) with a tablet to play and discuss selection of seven to eight pieces of music. It is important to note that listening to familiar music selections may be associated with certain emotions connected with the past (Baird & Samson, 2015) and induce a stimulatory, rather than sleep-inducing, effect (Dileo & Bradt, 2007). In addition, specific familiar songs might not have the calming properties to promote sleep in older adults. Therefore, calming music selections will be based on older adults’ preferred genre of music using responses from the “Assessment of Personal Music Preference” tool (Gerdner, 2018) to maximize treatment adherence and enjoyment, but will not contain specific songs/selections familiar to older adults with ADRD. If older adults with ADRD show signs of distress (i.e. grimacing, crying) or excitement we will reassess musical selections and return a week later with new music selections. If at that point during the follow up visit, older adults are not satisfied with the selection or appear to be in distress while listening to music, the dyad will be excluded, and will be considered a drop out, since the dyad has already consented to be randomized. Discontent with the music will be documented as an adverse event. If at halfway enrollment point, more than 10% of the recruited sample has shown dissatisfaction, we will reassess the music preference protocol.
The music intervention consists of listening to tailored calming music at bedtime for 30 minutes every night for four weeks (28 sessions total). We chose four weeks based on previous studies that recommend the duration of the music intervention to be at least three weeks (Wang et al., 2014) and based on a minimum length required to see changes in sleeping patterns (van Someren, 2007). We chose the length of 30 minutes based on previous studies showing that at least 30 minutes of listening to music was associated with improvements in sleep and quality of life (Huang et al., 2016; Jespersen et al., 2019). The dyads may choose to listen to all selections or only listen to one, repeatedly. Caregivers will be instructed to encourage participants to listen to music following bedtime routine, laying down with their eyes closed, at a comfortable volume level, wearing nighttime clothes, and room lights dimmed. We will follow up with the dyads three days after the start of the intervention, ensuring that there are no issues in listening to music. We will provide instructions to the caregivers to watch for any signs of potential overstimulation and/or agitation. The wait-list control group will receive a music intervention four weeks following the baseline assessment (T1). Similar to the music intervention group, we will assess the appropriateness of music selections based on the method described above. We will use a Samsung Galaxy Tablet A to deliver the music by loading all the music selections onto the app. We will work with the app developer from the mHealth Service who will load the music tracks onto the tablet. The tablet will then be delivered to the dyad’s home. This process is then repeated with each dyad, since each dyad receives tailored musical selections. During the home visit, we will show both dyad members how to unlock the tablet and access the music.
To ensure treatment fidelity we will use strategies outlined in the NIH Behavior Change Consortium (Bellg et al., 2004). Specifically, we will create a treatment manual for consistency of delivery of the intervention, have regular contact with the dyads, keep written records of home sessions/phone calls by date and duration. Using the tablet given to the dyads, we will be able to track how often the dyads listen to music, what type of music selections they listened to, and how long they listened to music.
Outcomes
The outcomes in Table 4 are the recommended measures to estimate important feasibility parameters for the subsequent study (Arain, Campbell, Cooper, & Lancaster, 2010; Thabane et al., 2010). To assess acceptability of the study components we will first evaluate caregiver satisfaction with study benefits using a modified survey developed by Gitlin and colleagues (Gitlin, Winter, Dennis, Hodgson, & Hauck, 2010a, 2010b), which examines satisfaction with participation and perceived benefits. Examples of questions include, “Overall, how much do you think you benefitted from participating in the project?”, “How much did participation in the project help improve your person’s with ADRD life?” and “Did the project require too much work or effort?”
Table 4:
Sleep Measures
| Name (Definition) | Role in analysis |
|---|---|
| Sleep latency (Time it takes a person to fall asleep starting from first intention to sleep) | Primary (efficacy) |
| Wake after sleep onset (Time awake during the night, beginning from the time person falls asleep) | Secondary (efficacy) |
| Total sleep duration (Actual time person is asleep) | Secondary (efficacy) |
| Sleep diary (Monk et al., 1994) | Reconciliation with actigraphy data (Efficacy) |
| Neuropsychiatric Inventory sleep item (Cummings et al., 1994) | Screening |
| PROMIS sleep related impairment – SF 8a (Yu et al., 2012) | Efficacy outcome |
| Sleep disorders inventory (SDI) (Tractenberg, Singer, Cumming, & Thal, 2003) | Screening, Efficacy |
Second, we will capture the general level of enjoyment from older adults with ADRD derived from listening to music by asking them a single question – “How enjoyable did you find listening to selected music?” We will record their responses using a 7-point Likert scale (1 - not at all enjoyable …to 7- extremely enjoyable …). We will use this score as a covariate in the analysis, given previous research has shown that general level of enjoyment listening to calming music correlates with its efficacy (Lai, 2004; Shum, Taylor, Thayala, & Chan, 2014; Wang et al., 2014).
Third, we will collect qualitative data to examine the acceptability and satisfaction of the intervention by individuals living with dementia and their caregivers. Gathering qualitative data in addition to quantitative information is helpful in optimizing the results from a feasibility study (Thabane et al., 2010). We will conduct in-person or telephone interviews with all dyads (including the wait-control group) immediately after they complete the intervention. Both persons with ADRD and their caregivers will be asked to participate in the interviews simultaneously in their home or by telephone. Questions will be addressed to both members of the dyad: person with ADRD and their caregiver. In cases where person with ADRD is unable to respond to open-ended questions, caregivers will be asked to comment on person’s with ADRD participation in the trial.
Interviews will take no longer than 20 minutes and include a semi-structured interview guide designed to expand on dyads’ responses in the survey. Example questions will include “What did you think about the music that was selected for you?” and “What else about this experience that you would like to share?” Prompts such as, “Tell me more about that” will be incorporated where needed throughout the interview. The responses of the participants will be audio recorded and then transcribed verbatim using a third-party HIPAA compliant company. Transcriptions will be checked for accuracy by the member of the research team.
Sleep measures in this study include both objective (actigraphy) and subjective measures (Table 4). Older adults with ADRD will be provided instructions on how to wear actigraphs (Philips Respironics Actiwatch Spectrum PRO) on their non-dominant hand to establish their sleep patterns for 28 consecutive 24-hour periods until removal at T2. Wrist actigraphy was chosen because it has been shown to be a sensitive and accurate method for measuring sleep latency, wake after sleep onset (WASO) and total sleep duration in clinical populations, when compared to the gold standard – polysomnography (Marino et al., 2013). Wrist actigraphy monitoring has been shown to be tolerated well by older adults with ADRD (Camargos et al., 2013; Chesson Jr, Coleman, Lee-Chiong, & Pancer, 2007; Figueiro et al., 2015) and provides a reliable way to objectively monitor sleep-wake cycles in this population (Camargos et al., 2013). Caregivers will be asked to complete a sleep diary, documenting the older adults with ADRD’s daily sleep patterns. Using a sleep diary (Monk et al., 1994) in addition to actigraphy provides a more reliable representation of sleep-wake cycles in older adults with ADRD (Figueiro et al., 2015) and is the recommended approach by the American Academy of Sleep Medicine for home-dwelling older adults (Chesson Jr et al., 2007). Sleep diary questions are kept to a minimum to increase compliance and adherence to the protocol. Actigraphy data analysis will be completed using validated scoring algorithms (Camargos et al., 2013). Actigraphs will collect data in 60-second epochs via tri directional accelerometer using a validated algorithm. The primary measure will be sleep latency (Table 4). To analyze the data, we will use Actiware software and the data will be reviewed by Drs. Petrovsky, Hodgson and Gooneratne. Differences between actigraphy data and sleep/wake record data will be reconciled on an individual basis with the dyad. Since the primary aims of this RCT are to examine the feasibility and acceptability of the music listening intervention, we predict a trend towards improving objective and subjective sleep measures, as evidenced by shorter sleep latency, WASO, increased total sleep duration and improved sleep quality.
We will collect the following baseline demographic information (either through the medical records or from the dyad): age, sex, race/ethnicity, relationship status, income, nature of the dyadic relationship, and years of education. We will screen for depression in older adults via proxy caregiver using a validated questionnaire (Patient Health Questionnaire, PHQ-9) (Kroenke, Spitzer, & Williams, 2001). There is evidence of the association between sleep disruption and depression in older adults with AD (Garcia-Alberca et al., 2013). The Clinical Dementia Rating Scale will be used to estimate the level of cognitive impairment at baseline and as a covariate in the analysis (Morris, 1993).
Analyses
The sample size and power calculation for this feasibility trial relies on the estimated confidence intervals width for parameter estimates of interest (feasibility outcome) and is powered sufficiently for these descriptive purposes. By design, this study was not powered to determine the efficacy of music intervention in reducing sleep disruption. We will answer this important question in a future RCT. The primary feasibility outcome is participant adherence to the protocol. We will conclude that this study is feasible if at least 85% of the dyads complete the study components and measures. A value accurate within 10–15 percentage points for adherence mean is sufficient to identify a problem that would justify modification of the intervention (Hertzog, 2008). In the RCT comparing relaxation program and music listening in older adults with mild cognitive impairment, adherence rate exceeded 93% at 12 weeks (Innes, Selfe, Khalsa, & Kandati, 2016). Therefore, with an observed adherence rate of 85% in the proposed study with 50 dyads, we can be 95% confident that the estimate is accurate within 10% points. We will call this study acceptable if the average score is at least 80% (% responding “Yes” or a “Great deal”). With 50 dyads, we can be 95% confident that the estimate is accurate within 12% points. We plan to recruit ten additional dyads for a total of 60 dyads (30 dyads per group) to allow for 16% attrition.
To analyze aims 1 and 2, we will provide descriptive statistics of rates of adherence to the study components, recruitment, participant attrition, reasons for excluding or declining, and acceptability survey results. The study will be considered feasible with 85% protocol adherence and study measures completion. Qualitative data from the dyad interviews will be recorded using field notes with major themes extracted related to the satisfaction and the acceptability of the study components. Data from the interviews will be analyzed after each interview. We will use the analytical technique of conventional content analysis to analyze the focus group data. The goal of conventional content analysis is to subjectively interpret the content of data “through the systematic classification process of coding and identifying themes or patterns” (Hsieh & Shannon, 2005).
To analyze Aim 3, descriptive statistics and univariate comparisons will be used to describe the intervention and wait-list control groups. To explore differences between two groups, we will use Chi-square, Wilcoxon rank-sum tests, and independent t-tests, as appropriate, and we will adjust for the statistically significant variables accordingly in the multivariate analysis. To estimate the effect size, we will use adjusted mean differences in treatment effects on the primary sleep outcome (sleep latency) and secondary outcomes (WASO, total sleep duration and sleep quality). The following data will be used as covariates: baseline cognitive status of the person with ADRD (clinical dementia rating), age, sex, education, general enjoyment of music and depressive symptoms and other background characteristics if large or statistically significant differences between experimental and wait-list control group dyads are found for those variables. Given the purpose of this study is to observe trend and obtain estimates of effect sizes for a future larger study, adjustment for multiple comparisons is not necessary. To examine whether wait-list control group participants experienced the same sleep benefits as the intervention group we will compare four (T2) to eight (T3) week scores of wait-list control group dyads to baseline (T1) to four (T2) week scores of experimental group participants on statistically significant outcomes from the main analyses, using the same methods as described.
Discussion
The long-term impact of this work is significant. First, this research will advance our knowledge of music interventions beyond the nursing home population and into the community, where most older adults with ADRD live. Second, this research will examine the feasibility of an evidence-based music intervention in a vulnerable population. Third, this research will inform our knowledge about the benefits of bedtime music listening in older adults with ADRD and the impact it has in improving sleep quality. Lastly, knowledge gained from this trial related to feasibility and acceptability will guide a larger RCT where the bedtime music protocol is tested for efficacy and later for effectiveness (if shown efficacious). This will increase the number of options for non-pharmacologic approaches to reduce sleep disruption in older adults with ADRD.
Progress to Date
Participant recruitment began in April 2019. To date we have enrolled and randomized thirty-three dyads (16 dyads in the wait-list control group and 17 in the intervention group). Two dyads from the wait-list control group did not complete due to caregiver strain and ongoing health problems for the person with ADRD. One dyad did not complete the intervention group due to a prolonged hospitalization, sudden decline in health and move to a rehabilitation facility of person with ADRD. We anticipate to complete recruitment in Fall 2020 and the study results will be available in Spring 2021.
Lessons Learned
In this project, we have learned several lessons that will help improve future work. First, the data collection method had to be adjusted for caregivers. We had originally planned to leave paper questionnaires for caregivers to fill out on their own and to be picked up a week later. Since most of the questionnaires in this study rely on caregiver report, caregivers expressed being overwhelmed by the amount of paperwork. Therefore, instead of asking caregivers to fill out questionnaires on their own, we would often sit down with both members of the dyad at the baseline visit and go through the questionnaires. This has allowed caregivers to ask clarifying questions, the person with ADRD to participate in the meeting and minimized missing data.
We had also learned that keeping a sleep diary was difficult for some caregivers. In cases of informal caregivers or partners sleeping in separate bedrooms, it was often difficult for caregivers to record accurate wake up and sleep times. In one instance, the spouse caregiver of a person with ADRD reported that she preferred sleeping in a separate room due to person with ADRD experiencing constant sleep disruptions and sometimes she was not aware when her husband went to bed or whether he woke up early.
We also learned the importance of weekly reminders for participants to wear actiwatches. All dyads have been able to complete the actigraphy. However, several dyads have expressed difficulty remembering to put them back on after a shower, only realizing a couple of days later that they needed to wear the actiwatch. In summer months on particularly hot days, one person with ADRD has reported that the watch material has made their skin itch. We have realized from the beginning that it was going to be difficult to ask persons with ADRD to remember to press the event marker buttons. All participants verbalized their understanding of the importance of wearing the actiwatch and filling out the sleep diary. The only person with ADRD who continued to refuse to wear the actiwatch had advanced dementia, ended up being hospitalized and was unable to complete the study. We have learned that it is important to ask the caregiver during the screening process whether person with ADRD is likely to wear an actiwatch, previously unfamiliar to them. With several persons with ADRD wearing an activity watch in the study, such as the Apple Iwatch or a Fitbit, we had to ask the person with ADRD to remove their current wearable device or move it to their dominant hand, so the Actiwatch could be placed on their non-dominant hand.
In this project to date, we had learned the value of working collaboratively with other principal investigators at the university, who are enrolling participants following similar inclusion criteria. For example, participants have been referred for this study from an ongoing R01 study (R01NR015226). The inclusion and exclusion criteria for participants and the targeted population are very similar to this current study; therefore, participants who were deemed eligible and willing to participate in the R01 study would more likely to agree to participate in this study. We had contacted participants from the R01 study four months following their follow up interview as part of the larger R01 study. This referral process has facilitated the enrollment of eligible participants into the current study.
Another lesson that we learned had to do with the tablets that we used for the study. The purpose of using a tablet with music loaded on it (as opposed to the mp3 player) was having the ability to assess the fidelity of the intervention. In order to avoid instances of dyads turning on the music and walking away, the music app required participants to start each track manually. In the qualitative exit interviews, we heard from several participants that it was cumbersome for them to have to start/stop tracks, especially for caregivers since they were simultaneously busy with other tasks. In several dyads this issue has resulted in dyads recording the music provided to them by the team on their smart-phone and playing it back. In response to this feedback, we plan to disable this feature and give participants the opportunity to listen to the whole playlist. In addition, we would have an mp3 available for those dyads who express difficulty using the tablet when it is first introduced to them. Most participants so far have enjoyed the music selections and thought the music has helped them relax.
We expect that some older adults with dementia may not enjoy listening to music when first introduced to music selections. To address this issue, we will determine tailored music using the evidence-based assessment tool (Gerdner, 2018) matching person’s preference and background to maximize enjoyment. We predict that the music intervention will be well received by the dyads. If participants are dissatisfied with the music intervention, we will prompt them for additional details on the cause of their dissatisfaction during qualitative interviews and modify the protocol accordingly. Despite these caveats, the results of this work will inform future efficacy RCTs, potentially adding to the number of palliative care approaches available.
Conclusion
Sleep disruption is debilitating and contributes to increased institutionalization (Pollak, Perlick, Linsner, Wenston, & Hsieh, 1990; Vitiello & Borson, 2001; Wulff et al., 2010) and poor quality of life for more than five million older adults with ADRD living in the United States and their caregivers (Alzheimer’s Association, 2019; Hodgson et al., 2014; Petrovsky et al., 2018). Given the potential harmful side effects of pharmacologic treatment, palliative non-pharmacologic approaches are recommended for this vulnerable population (Deschenes & McCurry, 2009; Gooneratne & Vitiello, 2014; Peter-Derex et al., 2015; Vitiello & Borson, 2001). Despite the prevalence of sleep disruption in older adults with ADRD, there are very few evidence-based, widely available non-pharmacologic interventions to improve sleep quality in this vulnerable population (Livingston et al., 2014). One promising approach is music. Potential therapeutic benefits have been attributed to music’s capacity to reduce stress and modulate arousal levels (Chanda & Levitin, 2013) in older adults with ADRD with a reduced stress threshold (Hall & Buckwalter, 1987). However, such interventions have primarily focused on nursing home residents, included a mix of therapist and nurse-led interventions, and have not examined the impact of listening to music that precedes nighttime sleep (Petrovsky, Cacchione, & George, 2015; Särkämö et al., 2012). The purpose of this research project is to examine the feasibility of a tailored calming music listening intervention to improve sleep quality in home-dwelling older adults with ADRD. This research project will yield preliminary feasibility data needed to conduct a larger RCT powered to assess music’s impact on sleep quality and caregivers’ long-term health outcomes.
Table 3:
Feasibility Measures
| Measures (role in analysis) | Operational definition | Data sources |
|---|---|---|
| Adherence to the study protocol and completion of the study measures (primary) | Number (and %) of subjects following the study protocol (i.e. listening to music and wearing the actigraphs) and completing the study measures. | Field notes, records in the sleep diary, verification by study staff, remote mobile technology applications |
| Recruitment rate (secondary) | Number (and %) of subjects recruited per month | Documentation in the written study records |
| Participant attrition (secondary) | Number (and %) of subjects who dropped out during the study (and reasons for dropping out) | Dyad, documentation in the written study records |
| Reasons for excluding or declining (secondary) | Explanations for why subjects were not recruited or declined to be in the study | Dyad |
Acknowledgement
This work is supported by the National Institute on Aging of the National Institutes of Health under grant numbers F32AG060630 and R01NR015226, the Frank Morgan Jones Fund and the University of Pennsylvania School of Nursing Faculty Grant Award. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Footnotes
Conflict of Interests
None
Contributor Information
Darina V. Petrovsky, University of Pennsylvania School of Nursing.
Nalaka Gooneratne, University of Pennsylvania Perelman School of Medicine.
Joke Bradt, College of Nursing and Health Professions, Drexel University.
Laura N. Gitlin, College of Nursing and Health Professions, Drexel University.
Nancy A. Hodgson, University of Pennsylvania School of Nursing.
References
- Alzheimer’s Association. (2019). 2019 Alzheimer’s disease facts and figures. Alzheimer’s & Dementia, 15(3), 321–387. Retrieved from https://www.alz.org/alzheimers-dementia/facts-figures [DOI] [PubMed] [Google Scholar]
- Arain M, Campbell MJ, Cooper CL, & Lancaster GA (2010). What is a pilot or feasibility study? A review of current practice and editorial policy. BMC Medical Research Methodology, 10(1), 67. doi: 10.1186/1471-2288-10-67 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baird A, & Samson S (2015). Music and dementia. Progress in Brain Research, 217, 207–235. [DOI] [PubMed] [Google Scholar]
- Basaglia-Pappas S, Laterza M, Borg C, Richard-Mornas A, Favre E, & Thomas-Anterion C (2013). Exploration of verbal and non-verbal semantic knowledge and autobiographical memories starting from popular songs in Alzheimer’s disease. International Psychogeriatrics, 25(5), 785–795. doi: 10.1017/S1041610212002359 [DOI] [PubMed] [Google Scholar]
- Bellg AJ, Borrelli B, Resnick B, Hecht J, Minicucci DS, Ory M, . . . Czajkowski S (2004). Enhancing treatment fidelity in health behavior change studies: best practices and recommendations from the NIH Behavior Change Consortium. Health Psychology, 23(5), 443. [DOI] [PubMed] [Google Scholar]
- Camargos EF, Louzada FM, & Nóbrega OT (2013). Wrist actigraphy for measuring sleep in intervention studies with Alzheimer’s disease patients: application, usefulness, and challenges. Sleep Medicine Reviews, 17(6), 475–488. 10.1016/j.smrv.2013.01.006 [DOI] [PubMed] [Google Scholar]
- Chanda ML, & Levitin DJ (2013). The neurochemistry of music. Trends in Cogn Sciences, 17(4), 179–193. doi: 10.1016/j.tics.2013.02.007 [DOI] [PubMed] [Google Scholar]
- Chesson M Jr, Coleman M, Lee-Chiong M, & Pancer D (2007). Practice parameters for the use of actigraphy in the assessment of sleep and sleep disorders: an update for 2007. Sleep, 30(4), 519. [DOI] [PubMed] [Google Scholar]
- Cummings JL, Mega M, Gray K, Rosenberg-Thompson S, Carusi DA, & Gornbein J (1994). The Neuropsychiatric Inventory: comprehensive assessment of psychopathology in dementia. Neurology, 44(12), 2308–2314. [DOI] [PubMed] [Google Scholar]
- Deschenes CL, & McCurry SM (2009). Current Treatments for Sleep Disturbances in Individuals With Dementia. Current Psychiatry Reports, 11(1), 20–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dileo C, & Bradt J (2007). Music therapy: applications to stress management. Principles and Practice of Stress Management, 3rd ed. New York: Guilford. [Google Scholar]
- Figueiro MG, Hunter CM, Higgins PA, Hornick TR, Jones GE, Plitnick B, . . . Rea MS (2015). Tailored lighting intervention for persons with dementia and caregivers living at home. Sleep health, 1(4), 322–330. Retrieved from https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4822719/pdf/nihms758056.pdf [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fornazzari L, Castle T, Nadkarni S, Ambrose M, Miranda D, Apanasiewicz N, & Phillips F (2006). Preservation of episodic musical memory in a pianist with Alzheimer disease. Neurology, 66(4), 610–611. doi: 10.1212/01.WNL.0000198242.13411.FB [DOI] [PubMed] [Google Scholar]
- Garcia-Alberca JM, Lara JP, Cruz B, Garrido V, Gris E, & Barbancho MA (2013). Sleep disturbances in Alzheimer’s disease are associated with neuropsychiatric symptoms and antidementia treatment. Journal of Nervous & Mental Disease, 201(3), 251–257. doi: 10.1097/NMD.0b013e3182848d04 [DOI] [PubMed] [Google Scholar]
- Gerdner LA (1997). An individualized music intervention for agitation. Journal of the American Psychiatric Nurses Association, 3(6), 177–184. [Google Scholar]
- Gerdner LA (2018). Evidence-Based Guideline: Individualized Music for Persons with Dementia (6th Edition). Ann Arbor, Michigan: National Nursing Practice Network; University of Michigan, School of Nursing. [Google Scholar]
- Gitlin LN, Winter L, Dennis MP, Hodgson N, & Hauck WW (2010a). A biobehavioral home-based intervention and the well-being of patients with dementia and their caregivers: the COPE randomized trial. JAMA, 304(9), 983–991. doi: 10.1001/jama.2010.1253 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gitlin LN, Winter L, Dennis MP, Hodgson N, & Hauck WW (2010b). Targeting and managing behavioral symptoms in individuals with dementia: a randomized trial of a nonpharmacological intervention. Journal of American Geriatrics Society, 58(8), 1465–1474. doi: 10.1111/j.1532-5415.2010.02971.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gooneratne NS, & Vitiello MV (2014). Sleep in older adults: normative changes, sleep disorders, and treatment options. Clinics in Geriatric Medicine, 30(3), 591–627. doi: 10.1016/j.cger.2014.04.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall GR, & Buckwalter KC (1987). Progressively lowered stress threshold: A conceptual model for care of adults with Alzheimer’s disease. Archives of Psychiatric Nursing, 1(6), 399–406. [PubMed] [Google Scholar]
- Hertzog MA (2008). Considerations in determining sample size for pilot studies. Research in Nursing & Health, 31(2), 180–191. doi: 10.1002/nur.20247 [DOI] [PubMed] [Google Scholar]
- Hodgson N, Gitlin LN, & Huang J (2014). The influence of sleep disruption and pain perception on indicators of quality of life in individuals living with dementia at home. Geriatric Nursing, 35(5), 394–398. Retrieved from https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4416487/pdf/nihms626717.pdf [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsieh HF, & Shannon SE (2005). Three approaches to qualitative content analysis. Qualitative Health Research, 15(9), 1277–1288. doi: 10.1177/1049732305276687 [DOI] [PubMed] [Google Scholar]
- Hu RF, Jiang XY, Hegadoren KM, & Zhang YH (2015). Effects of earplugs and eye masks combined with relaxing music on sleep, melatonin and cortisol levels in ICU patients: a randomized controlled trial. Critical Care (London, England), 19, 115. Retrieved from http://elinks.library.upenn.edu/?sid=OVID:medline&id=pmid:25881268 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang CY, Chang ET, & Lai HL (2016). Comparing the effects of music and exercise with music for older adults with insomnia. Applied Nursing Research, 32, 104–110. doi: 10.1016/j.apnr.2016.06.009 [DOI] [PubMed] [Google Scholar]
- Innes KE, Selfe TK, Khalsa DS, & Kandati S (2016). Effects of Meditation versus Music Listening on Perceived Stress, Mood, Sleep, and Quality of Life in Adults with Early Memory Loss: A Pilot Randomized Controlled Trial. Journal of Alzheimers Disease, 52(4), 1277–1298. doi: 10.3233/jad-151106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jelicic M, Bosma H, Ponds RW, van Boxtel M, Houx P, & Jolles J (2002). Subjective Sleep Problems in Later Life as Predictors of Cognitive Decline. Report from the Masstricht Ageing Study (MAAS). International Journal of Geriatric Psychiatry, 17, 73–77. 10.1002/gps.529 [DOI] [PubMed] [Google Scholar]
- Jespersen KV, Koenig J, Jennum P, & Vuust P (2015). Music for insomnia in adults. Cochrane Database of Systematic Reviews(8), CD010459. Retrieved from http://elinks.library.upenn.edu/?sid=OVID:medline&id=pmid:26270746 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jespersen KV, Otto M, Kringelbach M, Van Someren E, & Vuust P (2019). A randomized controlled trial of bedtime music for insomnia disorder. Journal of Sleep research, e12817. doi: 10.1111/jsr.12817 [DOI] [PubMed] [Google Scholar]
- Johnson JK, Chang CC, Brambati SM, Migliaccio R, Gorno-Tempini ML, Miller BL, & Janata P (2011). Music recognition in frontotemporal lobar degeneration and Alzheimer disease. Cognitive and Behavioral Neurology, 24(2), 74–84. doi: 10.1097/WNN.0b013e31821de326 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson JK, & Chow ML (2015). Hearing and music in dementia. Handbook of Clinical Neurology, 129, 667–687. doi: 10.1016/B978-0-444-62630-1.00037-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kroenke K, Spitzer RL, & Williams JB (2001). The PHQ-9: Validity of a Brief Depression Severity Measure. Journal of General Internal Medicine, 16(9), 606–613. 10.1046/j.1525-1497.2001.016009606.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lai HL (2004). Music preference and relaxation in Taiwanese elderly people. Geriatric Nursing, 25(5), 286–291. doi: 10.1016/j.gerinurse.2004.08.009 [DOI] [PubMed] [Google Scholar]
- Lai HL, Chang ET, Li YM, Huang CY, Lee LH, & Wang HM (2015). Effects of music videos on sleep quality in middle-aged and older adults with chronic insomnia: a randomized controlled trial. Biological Research for Nursing, 17(3), 340–347. Retrieved from http://elinks.library.upenn.edu/?sid=OVID:medline&id=pmid:25237150 [DOI] [PubMed] [Google Scholar]
- Lai HL, & Good M (2005). Music improves sleep quality in older adults. Journal of Advanced Nursing, 49(3), 234–244. doi: 10.1111/j.1365-2648.2004.03281.x [DOI] [PubMed] [Google Scholar]
- Lai HL, Li YM, & Lee LH (2011). Effects of music intervention with nursing presence and recorded music on psycho-physiological indices of cancer patient caregivers. Journal of Clinical Nursing, 21(5–6), 745–756. Retrieved from http://elinks.library.upenn.edu/?sid=OVID:medline&id=pmid:22098540 [DOI] [PubMed] [Google Scholar]
- Livingston G, Kelly L, Lewis-Holmes E, Baio G, Morris S, Patel N, . . . Cooper C (2014). Non-pharmacological interventions for agitation in dementia: systematic review of randomised controlled trials. British Journal of Psychiatry, 205(6), 436–442. doi: 10.1192/bjp.bp.113.141119 [DOI] [PubMed] [Google Scholar]
- Marino M, Li Y, Rueschman MN, Winkelman JW, Ellenbogen JM, Solet JM, . . . Buxton OM (2013). Measuring sleep: accuracy, sensitivity, and specificity of wrist actigraphy compared to polysomnography. Sleep, 36(11), 1747–1755. doi: 10.5665/sleep.3142 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monk TH, Reynolds CF, Kupfer DJ, Coble PA, Hayes AJ, Machen MA, . . . Ritenour AM (1994). The Pittsburgh Sleep Diary. Journal of Sleep Research, 3, 111–120. [PubMed] [Google Scholar]
- Morris JC (1993). The Clinical Dementia Rating (CDR): Current version and scoring rules. Neurology, 43(11), 2412–2412-a. doi: 10.1212/WNL.43.11.2412-a [DOI] [PubMed] [Google Scholar]
- Peter-Derex L, Yammine P, Bastuji H, & Croisile B (2015). Sleep and Alzheimer’s disease. Sleep Medicine Reviews, 19, 29–38. doi: 10.1016/j.smrv.2014.03.007 [DOI] [PubMed] [Google Scholar]
- Petrovsky D, Cacchione PZ, & George M (2015). Review of the effect of music interventions on symptoms of anxiety and depression in older adults with mild dementia. International Psychogeriatrics, 1–10. doi: 10.1017/S1041610215000393 [DOI] [PubMed] [Google Scholar]
- Petrovsky D, McPhillips MV, Li J, Brody A, Caffeé L, & Hodgson NA (2018). Sleep disruption and quality of life in persons with dementia: A state-of-the-art review. Geriatric Nursing, 39(6), 640–645. doi: 10.1016/j.gerinurse.2018.04.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pollak CP, Perlick D, Linsner JP, Wenston J, & Hsieh F (1990). Sleep problems in the community elderly as predictors of death and nursing home placement. Journal of Community Health, 15(2), 123–135. doi: 10.1007/bf01321316 [DOI] [PubMed] [Google Scholar]
- Rabins PV, Schwartz S, Black BS, Corcoran C, Fauth E, Mielke M, . . . Tschanz J (2013). Predictors of progression to severe Alzheimer’s disease in an incidence sample. Alzheimer’s and Dementia, 9(2), 204–207. doi: 10.1016/j.jalz.2012.01.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rose KM, Beck C, Tsai PF, Liem PH, Davila DG, Kleban M, . . . Richards KC (2011). Sleep disturbances and nocturnal agitation behaviors in older adults with dementia. Sleep, 34(6), 779–786. doi: 10.5665/sleep.1048 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Safi AJ, & Hodgson NA (2014). Timing of Activities and their Effects on Circadian Rhythm in the Elderly with Dementia: A Literature Review. Journal of Sleep Disorders & Therapy, 03(05). doi: 10.4172/2167-0277.1000176 [DOI] [Google Scholar]
- Särkämö T, Laitinen S, Tervaniemi M, Nummien A, Kurki M, & Rantanen P (2012). Music, emotion, and dementia: Insight from neuroscientific and clinical research. Music and Medicine, 4(3), 153–162. https://doi.org/10.1177 [Google Scholar]
- Shum A, Taylor BJ, Thayala J, & Chan MF (2014). The effects of sedative music on sleep quality of older community-dwelling adults in Singapore. Complementary Therapies in Medicine, 22(1), 49–56. doi: 10.1016/j.ctim.2013.11.003 [DOI] [PubMed] [Google Scholar]
- Su CP, Lai HL, Chang ET, Yiin LM, Perng SJ, & Chen PW (2012). A randomized controlled trial of the effects of listening to non-commercial music on quality of nocturnal sleep and relaxation indices in patients in medical intensive care unit. Journal of Advanced Nursing, 69(6), 1377–1389. Retrieved from http://elinks.library.upenn.edu/?sid=OVID:medline&id=pmid:22931483 [DOI] [PubMed] [Google Scholar]
- Thabane L, Ma J, Chu R, Cheng J, Ismaila A, Rios LP, . . . Goldsmith CH (2010). A tutorial on pilot studies: the what, why and how. BMC Medical Research Methodology, 10(1), 1. 10.1186/1471-2288-10-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tractenberg RE, Singer CM, Cumming JL, & Thal LJ (2003). The Sleep Disorders Inventory: an instrument for studies of sleep disturbance in persons with Alzheimer’s disease. Journal of Sleep Research, 12(4), 331–337. 10.1046/j.0962-1105.2003.00374.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Someren EJ (2007). Improving actigraphic sleep estimates in insomnia and dementia: how many nights? Journal of Sleep Research, 16, 269–275. 10.1111/j.1365-2869.2007.00592.x [DOI] [PubMed] [Google Scholar]
- Vitiello MV, & Borson S (2001). Sleep disturbances in patients with Alzheimer’s disease. CNS Drugs, 15(10), 777–796. 10.2165/00023210-200115100-00004 [DOI] [PubMed] [Google Scholar]
- Wang CF, Sun YL, & Zang HX (2014). Music therapy improves sleep quality in acute and chronic sleep disorders: a meta-analysis of 10 randomized studies. International Journal of Nursing Studies, 51(1), 51–62. doi: 10.1016/j.ijnurstu.2013.03.008 [DOI] [PubMed] [Google Scholar]
- Wulff K, Gatti S, Wettstein JG, & Foster RG (2010). Sleep and circadian rhythm disruption in psychiatric and neurodegenerative disease. Nature Reviews Neuroscience, 11(8), 589–599. doi:http://www.nature.com/nrn/journal/v11/n8/suppinfo/nrn2868_S1.html [DOI] [PubMed] [Google Scholar]
- Yaffe K, Falvey CM, & Hoang T (2014). Connections between sleep and cognition in older adults. The Lancet Neurology, 13(10), 1017–1028. doi: 10.1016/s1474-4422(14)70172-3 [DOI] [PubMed] [Google Scholar]
- Yu L, Buysse DJ, Germain A, Moul DE, Stover A, Dodds NE, . . . Pilkonis PA (2012). Development of short forms from the PROMIS™ sleep disturbance and sleep-related impairment item banks. Behavioral Sleep Medicine, 10(1), 6–24. 10.1080/15402002.2012.636266 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zee PC, & Vitiello MV (2009). Circadian Rhythm Sleep Disorder: Irregular Sleep Wake Rhythm Type. Sleep Medicine Clinics, 4(2), 213–218. doi: 10.1016/j.jsmc.2009.01.009 [DOI] [PMC free article] [PubMed] [Google Scholar]