SUMMARY
In this study, we present the photosynthetic oxygen (O2) supply to mammalian cells within a volumetric extracellular matrix-like construct, whereby a three-dimensional (3D)-bioprinted fugitive pattern encapsulating unicellular green algae, Chlamydomonas reinhardtii (C. reinhardtii), served as a natural photosynthetic O2-generator. The presence of bioprinted C. reinhardtii enhanced the viability and functionality of mammalian cells while reducing the hypoxic conditions within the tissues. We were able to subsequently endothelialize the hollow perfusable microchannels formed after enzymatic removal of the bioprinted C. reinhardtii-laden patterns from the matrices following the initial oxygenation period, to obtain biologically relevant vascularized mammalian tissue constructs. The feasibility of co-culture of C. reinhardtii with human cells, the printability and the enzymatic degradability of the fugitive bioink, as well as the exploration of C. reinhardtii as a natural, eco-friendly, cost-effective, and sustainable source of O2 would likely promote the development of engineered tissues, tissue models, and food for various applications.
eTOC blurb
Three-dimensional-bioprinted unicellular green algae, Chlamydomonas reinhardtii was used as a sustainable bionic source of O2 in engineered tissue constructs. O2 photosynthetically produced by bioprinted algae significantly improved the viability and functionality of the human cells within surrounding matrices while reducing their hypoxic conditions. Fugitive patterns encapsulating the algae were enzymatically dissolved by cellulase digestion to create interconnected microchannels, which were subsequently endothelialized, generating vascularized tissues.
Graphical Abstract

INTRODUCTION
Millions of people lose their lives on an annual basis due to health issues associated with organ failures.1 According to the World Health Organization, only about 10% of the global need for organ transplantation is being met as a result of donor organ shortage.2 Thus, there is an increasing demand for artificial tissue substitutes to replace those that are damaged/diseased to restore tissue and organ functions. Tissue engineering represents a promising approach that aims at in vitro production of functional tissues for clinical transplantation to replace and restore the functions of those that are damaged.3 Over the past decade, three-dimensional (3D) bioprinting techniques have been explored extensively for fabricating tissue scaffolds for biomedical and tissue engineering applications.4 3D bioprinting enables in vitro fabrication of volumetric tissue constructs of predefined architectures and shapes, possibly also with embedded vasculature, by precise positioning of bioinks that are typically composed of hydrogels, cells, and/or bioactive agents.4–6 Moreover, 3D-bioprinted tissue models, such as those of the liver, heart, skin, muscle, lung, and tumors, have been used to study the pathophysiology of diseases as well as the pharmacokinetics and pharmacodynamics of drugs in vitro.7–9
Despite advances in the fabrication of 3D tissues and their models in vitro, there are still major limitations such as maintaining high cell viability and functionality throughout a tissue construct, which is largely determined by the availability of oxygen (O2).10 Sufficient and homogeneous distribution of O2 facilitates cell growth,11 and inadequate supply of O2 has been shown to induce cell death within 3D tissue constructs.12 In fact, when the thickness of a tissue construct exceeds a few hundred micrometers to a few millimeters, hypoxic condition occurs especially in the central regions, due to the limited diffusion of O2 into the 3D matrix.13 Efforts have been made to improve O2 supply in engineered tissue constructs such as incorporation of O2-releasing biomaterials, including sodium percarbonate,14 magnesium peroxide,15 calcium peroxide,16 and hydrogen peroxide,17 in the form of scaffolds18 and microspheres,19 among others. Nevertheless, these O2-releasing biomaterials are usually to different levels cytotoxic due to the production of hydrogen peroxide, reactive O2 species, and decomposition of salt byproducts, affecting the cell viability within the tissue constructs, as well as typically fast and rapidly declining release profiles.20 Another approach relies on incorporation of vascular channels, with or without relevant cells, within the tissue constructs.10 3D bioprinting allows rapid fabrication of vascular channels within volumetric tissue constructs using sacrificial (bio)inks, such as Pluronic F127,13 gelatin,21 agarose,22 and hydrocarbons,23 within the tissue scaffolds, which after removal create perfusable microchannels that can be seeded with vascular cells for angiogenic sprouting and/or implanted in vivo to promote the formation of vascular networks.24,25
Very recently, photosynthetic microalgae have been attempted as a source of O2 in engineered tissue constructs. Bloch et al. described the co-encapsulation of pancreatic islets and unicellular alga, Chlorella sorokiniana, in alginate beads and showed that these microalgae could generate sufficient O2 for respiration and function of islets under in vitro anoxic environments. They simply mixed islets and Chlorella sorokiniana (C. sorokiniana) in 1.8% (w/v) sodium alginate and then extruded through a pipette tip into a calcium chloride solution.26 Hopfner et al. established the co-culture of Chlamydomonas reinhardtii (C. reinhardtii) and murine 3T3 fibroblasts in 3D collagen scaffolds. C. reinhardtii and fibroblasts were seeded on commercially available collagen-based scaffolds and demonstrated that the scaffolds harboring C. reinhardtii produced enough O2, thereby decreasing the hypoxic response under low-O2 culture conditions.27 Haraguchi et al. reported the fabrication of 3D tissue scaffolds in which Chlorococcum littorale and C2C12 mouse myoblasts or rat cardiac cells were layered one above the other by the cell sheet engineering approach.28 The study showed the possibility of an in vitro symbiotic relationship between mammalian cells and algae. Moreover, Lode et al. demonstrated the 3D bioprinting of C. reinhardtii and C. sorokiniana encapsulated in alginate/methylcellulose (3:1) blend by means of extrusion bioprinting and demonstrated the growth rate and viability of the bioprinted microalgae.29 The authors also fabricated the patterned human SaOS-2 cells and C. reinhardtii co-cultured 3D scaffolds by alternate plotting of hydrogel laden with SaOS-2 and C. reinhardtii. In this pioneering work, the potential of 3D bioprinting for creation of patterned human cell/algae coculture scaffolds was briefly demonstrated, yet the structures produced were less physiologically relevant as the fully exposed porous patterns did not seem to take advantage of the oxygenation by the algae.
In the present study, we present 3D fabrication of perfusable vascularized tissue constructs via bioprinting of photosynthetic C. reinhardtii encapsulated in a sacrificial cellulose-based blend bioink with predesigned geometries. Cellulose is the water-insoluble natural polymer of glucose and is the main structural component of plants, algae, and fungi, which provides them with the mechanical strength as well as maintains the structural integrity.30,31 Cellulose-based hydrogels are prepared from water-soluble ether-derivatives of cellulose, such as methylcellulose, ethyl cellulose, hydroxyethyl cellulose, hydroxypropyl methylcellulose, and sodium carboxymethylcellulose, through physical or chemical crosslinking.30,32 Carboxymethyl cellulose sodium salt (NaCMC) can also be used as a rheology-modifier, stabilizer, and thickener in a wide range of formulations.33
As a proof-of-principle demonstration, we embedded the bioprinted C. reinhardtii-containing sacrificial structures within gelatin methacryloyl (GelMA)-based hydrogel constructs harboring liver-derived cells, and evaluated the availability of O2 produced by the bioprinted C. reinhardtii as well as their ability to support the viability and functions of human liver-derived (HepG2) cells within the tissue constructs. We subsequently investigated the feasibility of forming hollow microchannels following culture and enzymatic removal of the bioprinted C. reinhardtii patterns, and of seeding endothelial cells in the microchannels to obtain vascularized hepatic tissue-like constructs. Development of such a fugitive bioink that allows initial oxygenation and subsequent vessel-formation within a single tissue construct has not been reported, which however is a critical step towards successful engineering of viable and functional tissues.
RESULTS
3D bioprinting of the NaCMC-based bioink
The bioink was prepared by homogeneously mixing different amounts of NaCMC, poly(vinyl alcohol) (PVA), gelatin, and alginate solutions. NaCMC and gelatin were utilized as the main components, while PVA and alginate as the additives. During bioprinting, a suitable viscosity of the bioink is important to ensure its proper extrusion.34 Deformation and collapse can easily happen when bioprinting low-viscosity materials; on the other hand, nozzles may be frequently jammed when high-viscosity bioinks are used. We therefore examined the printability of bioink formulations with different concentrations of NaCMC (3–10% v/v), PVA (0.01–0.1% v/v), gelatin (2–10% w/v), and alginate (0.5–1% w/v), at different pressures (35–60 psi) as well as at different feeding rates (60–100 mm s−1). The printability studies revealed the optimal bioink formulation as 3% (v/v) NaCMC, 0.03% (v/v) PVA, 4% (v/v) gelatin, and 0.8% (v/v) alginate at 55 psi of pressure and 70 mm s−1 of feeding rate when a 27G extrusion needle was used (Tables S1 and S2, and Figure S1). The appropriate amounts of gelatin and alginate were added to improve the printability of the bioink and to stabilize the shapes of the bioprinted structures. The polymer-polymer interactions for the formation of the hydrogel was mainly due to the intermolecular and/or intramolecular hydrogen bonding among the functional groups35 of the different components (Figure 1A). Indeed, the viscosity of the optimized bioink was found to be higher compared to individual components when measured as a function of shear rate (Figure 1B).
Figure 1. Formulation of the fugitive bioink and 3D bioprinting.
(A) Schematics of the bioink formulation. (B) Viscosity characterizations of the optimized bioink and the different components of the bioink. (C) Schematic illustration of the 3D bioprinting process. (D) Photographs of side and top views of the 3D-bioprinted honeycomb patterns with different layers.
The optimized bioink was then applied to fabricate honeycomb-shaped 3D constructs at room temperature under the optimized pressure and feeding rate. The schematic illustration of the bioprinting process is shown in Figure 1C. The honeycomb construct comprised of seven hexagonal lobules with each lobule having a circumradius of 1.6-mm and the length of each side of the hexagon was 1.6-mm. Immediately after bioprinting, the 3D honeycomb constructs were transferred into a 0.3-M calcium chloride (CaCl2) bath and incubated for 5 min to physically crosslink the alginate component of the constructs. The fabrication of 3-, 4-, 6-, 10- and 20-layered honeycomb constructs were achieved under optimized conditions. Figure 1D illustrates the representative photographs of 3-, 4-, 6-, 10-, and 20-layered honeycomb constructs with the respective side views on the top insets. Further, 3D tubular, grid, and crossed-grid constructs were printed to demonstrate the ability to extrude complex patterns with different aspect ratios and structures using the optimized bioink (Figure S2). Specifically, the 20-layered tubular pattern was 16-mm in diameter while the 20-layered grid and crossed-grid patterns were 20-mm × 20-mm (length × width) and 36-mm × 22-mm (length × width), respectively.
Enzymatic digestion of the bioprinted NaCMC-based sacrificial patterns
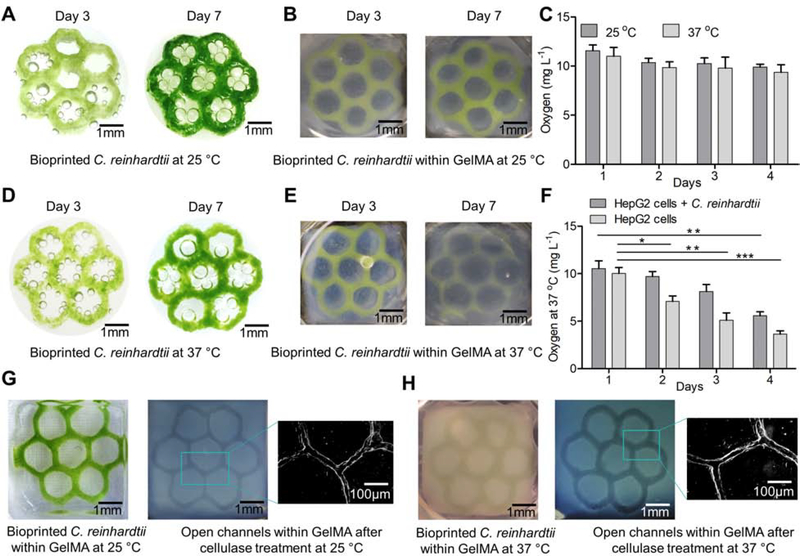
We next demonstrated the unique utility of NaCMC-based hydrogel as a sacrificial structure by cellulase-mediated digestion of NaCMC. Cellulase is an enzyme that hydrolyses cellulose and some related polysaccharides to produce soluble sugars.36 Among the water-soluble cellulose ethers, NaCMC is the only polyelectrolyte that is sensitive to pH.37 Thus, we investigated the digestion of the bioprinted NaCMC-based patterns with cellulase at different concentrations (1–5 mg mL−1) using two different buffers (citrate and acetate buffers), and at different pH values (5, 6, and 7). While the cellulase was found to be inactive in acetate buffer at all concentrations and pH values studied, it was less active in citrate buffer at pH 5 and pH 7. The optimal concentration of cellulase for complete digestion of the bioprinted NaCMC-based patterns was found to be 1 mg mL−1 at pH 6 in citrate buffer at both 25 °C and at 37 °C. However, the cellulase digestion of the NaCMC-based patterns took longer time (~6 h) at 25 °C as compared to that at 37 °C (~2 h). Similarly, we also studied the digestion of the bioprinted NaCMC-based patterns embedded within GelMA to create perfusable microchannels under optimized conditions at both 25 °C and 37 °C. As expected, the digestion of the NaCMC-patterns within GelMA took comparatively longer time when incubated with cellulase (1 mg mL−1 in citrate buffer, pH 6) at both 25 °C and 37 °C, yet both leaving behind the clear open microchannels post-digestion (Figure S3). At 25 °C, it took approximately 12 h while at 37 °C, it was about 4 h. We further confirmed the formation of interconnected lumens within GelMA constructs by the perfusion of colored microbeads as shown in Movie S1.
3D bioprinting of C. reinhardtii-laden patterns
The excellent biocompatibility, bioadhesive properties, and cellulase-mediated degradation38 of NaCMC have prompted the use of NaCMC-based hydrogel as the fugitive matrix for the bioprinting of photosynthetic algae, C. reinhardtii. Different concentrations of freshly pelleted C. reinhardtii (1.0–10×106 cells mL−1) were mixed with the bioink and bioprinted following the same procedure as those without the algae. The density of 5×106 cells mL−1 of C. reinhardtii was found to be optimum for bioprinting. The addition of the C. reinhardtii did not noticeably affect the printability of the bioink as well as the crosslinking, resulting in stable constructs with predefined geometries. As such, 4-, 6-, 10-, and 20-layered C. reinhardtii-laden 3D honeycomb structures were fabricated using 5×106 C. reinhardtii cells mL−1. Due to the transparency of the bioink, the homogenous distribution of green C. reinhardtii was clearly visible within the bioprinted patterns of various heights and thicknesses (Figure 2A). Similarly, 20-layered C. reinhardtii-laden tubular, grid, and crossed-grid constructs were printed demonstrating the faithful extrusion of different patterns of C. reinhardtii-laden structures (Figure 2B). The 20-layered C. reinhardtii-laden tubular pattern was 16-mm in diameter while the 20-layered C. reinhardtii-laden grid and C. reinhardtii-laden crossed-grid patterns were 20-mm × 20-mm (length × width) and 36-mm × 22-mm (length × width), respectively. Immediately after bioprinting, the C. reinhardtii-laden patterns were placed in 0.3-M CaCl2 bath for 5 min and then incubated in the Tris–acetate–phosphate (TAP) medium at 25 °C under continuous light illumination (light intensity: 2,800 Lux). While the C. reinhardtii-laden structures were light green at day 1, they appeared dark green after 7 days of cultivation (Figure 2C). The growth of C. reinhardtii within bioprinted pattern was observed both macroscopically and microscopically (Figure 2C).
Figure 2. 3D bioprinting of C. reinhardtii.
(A) Photographs showing bioprinted C. reinhardtii-laden honeycomb patterns with different layers at day 3. (B) Photographs of bioprinted C. reinhardtii-laden tubular, grid, and cross-grid patterns with different sizes. (C) Optical and fluorescence micrographs of bioprinted C. reinhardtii-laden honeycomb patterns. (D) Viability of C. reinhardtii at different days determined by live/dead assay using Sytox-orange. (E) Viability of the bioprinted C. reinhardtii within 4-layered honeycomb patterns at different days, as represented by OD750. Results are presented as means ± standard deviations.
The viability and growth of C. reinhardtii within the bioprinted patterns were further confirmed by both live/dead assay using Sytox-orange dye and measuring optical density (OD) values at 750 nm. The bioprinted C. reinhardtii was incubated in the TAP medium at 25 °C under continuous light illumination (2,800 Lux), and live/dead assay was performed at day 14. The Sytox-orange dye could penetrate damaged cell membranes and therefore stain dead cells in orange, while viable cells were visualized utilizing the red autofluorescence of chlorophyll (Figure 2D). The viability of the bioprinted C. reinhardtii was about 90% at day 1, which gradually increased with time and reached roughly 98% at day 14 (Figure 2E). Similarly, macroscopic, microscopic, and OD750 measurements using a microplate reader for the bioprinted C. reinhardtii within 4-layered (Figure S4A and S4B), 6-layered (Figure S4C and S4D), and 10-layered (Figure S4E and S4F) honeycomb structures, also revealed a gradual increase of the C. reinhardtii density over the culture period of 14 days. The cell density of C. reinhardtii was proportional to the number of layers in the honeycomb structures demonstrating uniform growth of the algae throughout the bioprinted patterns.
Since we aimed to use the bioprinted C. reinhardtii as the source of O2 for mammalian cells in tissue constructs, we next studied the growth of C. reinhardtii within 3D-bioprinted patterns at 37 °C and compared with that at 25 °C, both under continuous light-illumination conditions (2,800 Lux). C. reinhardtii within the bioprinted structures exhibited similar growth behaviors at 37 °C as observed at 25 °C, over the 14 days of culture period when cultured in TAP medium (Figure S5). A gradual increase of the C. reinhardtii density was observed over the culture period of 14 days at 37 °C within 4-layered (Figure S5A and S5B), 6-layered (Figure S5C and S5D), and 10-layered (Figure S5E and S5F) honeycomb structures. We further investigated the growth of C. reinhardtii in commonly used mammalian cell culture medium, Dulbecco’s modified Eagle medium (DMEM) supplemented with 10% FBS and 1% P/S, for 7 days and compared to that in DMEM:TAP medium (1:1) and TAP media, at both 25 °C and 37 °C under mammalian cell culture conditions and continuous light illumination (2,800 Lux). At both 25 °C and 37 °C, C. reinhardtii showed reduced growth in DMEM when compared to DMEM:TAP and TAP media (Figure S6). When bioprinted C. reinhardtii honeycomb patterns were cultured in DMEM:TAP medium (1:1) at 37 °C under mammalian cell culture conditions, C. reinhardtii within the bioprinted structures exhibited similar growth over the period of 4 days of culture as that observed at 25 °C but the growth was decreased gradually after day 4 due to the presence of P/S in the DMEM (Figures 3A and 3D). Similar growth of C. reinhardtii was observed when the C. reinhardtii-laden patterns were embedded in GelMA matrices at both 25 °C and 37 °C (Figure 3B and 3E). The effect of P/S on the growth of C. reinhardtii was confirmed by observing the growth of bioprinted C. reinhardtii patterns over the period of 14 days in the presence or absence of 1% P/S in TAP media (Figure S7) at both 25 °C and 37 °C. The growth of C. reinhardtii within bioprinted patterns declined gradually over the time, approaching negligible growth at day 14. We then assessed the co-culture of mammalian cells such as HepG2 cells and C2C12 cells with C. reinhardtii in DMEM:TAP medium (1:1) for 4 days at 37 °C and found that these mammalian cells could grow well together with C. reinhardtii when cultured under normal mammalian cell culture conditions (Figure S8). Thus, DMEM:TAP medium (1:1) was chosen as co-culture medium for further cell experiments. We also studied the effect of low light intensity (0 Lux) and high light intensity (11,000 Lux) on the growth of C. reinhardtii at 25 °C as well as 37 °C. C. reinhardtii did not grow at all under both low and high light intensities studied, indicating that absence of light or presence of too much light were not favorable for the growth of C. reinhardtii at both temperatures.
Figure 3. Growth of bioprinted C. reinhardtii and measurements of dissolved O2 levels in medium.
(A) Photographs showing the bioprinted C. reinhardtii grown in TAP medium at 25 °C. (B) Photographs showing the bioprinted C. reinhardtii within a GelMA scaffold grown in TAP medium at 25 °C. (C) Levels of dissolved O2 released by bioprinted C. reinhardtii in TAP medium at 25 °C and 37 °C. Results are presented as means ± standard deviations. (D) Photographs showing the bioprinted C. reinhardtii grown in DMEM:TAP medium (1:1) at 37 °C. (E) Photographs showing the bioprinted C. reinhardtii within a GelMA scaffold grown in DMEM:TAP medium (1:1) at 37 °C. (F) Levels of dissolved O2 in DMEM:TAP medium (1:1) at 37 °C in the presence or absence of bioprinted C. reinhardtii embedded within GelMA/HepG2 matrices. Results are presented as means ± standard deviations. (G) Photographs showing the bioprinted C. reinhardtii pattern within a GelMA matrix at day 7 and the formation of open microchannels within the matrix after digestion of the C. reinhardtii pattern with 1 mg mL−1 of cellulase in citrate buffer (pH 6) at 25 °C for 12 h, followed by microscopic image showing the open microchannel. (H) Photographs showing the bioprinted C. reinhardtii pattern within a GelMA matrix at day 7 and the formation of open microchannels within the matrix after digestion of the C. reinhardtii pattern with 1 mg mL−1 of cellulase in citrate buffer (pH 6) at 37 °C for 4 h, followed by microscopic image showing the open microchannel.
As the bioprinted C. reinhardtii was intended to serve as the source of O2 to boost the growth of mammalian cells encapsulated in the GelMA hydrogel, we also assessed the effect of UV exposure on C. reinhardtii by measuring OD750 and OD665 using a microplate reader for viability and chlorophyll content, respectively. Bioprinted C. reinhardtii patterns were exposed to different UV levels (0, 18, 45, and 72 mW cm−2) for 40 s and incubated for 7 days at 25 °C and 37 °C. At day 7, the growth of C. reinhardtii within bioprinted patterns was measured at OD750 whereas the chlorophyll was further extracted from the C. reinhardtii using 95% ethanol and read at OD665. The OD750 and OD665 evaluations revealed that the range of UV tested had no noticeable adverse effect on the growth and the chlorophyll content of the C. reinhardtii within bioprinted structures at both 25 °C and 37 °C (Figure S9).
O2 release by bioprinted C. reinhardtii
The photosynthetic activity of the bioprinted C. reinhardtii was demonstrated by measuring the O2 released in the medium by photoautotrophic growth of C. reinhardtii within the constructs. Dissolved O2 present in the TAP medium was first removed by flushing with a nitrogen gas for 20 min, followed by incubation with the bioprinted C. reinhardtii patterns at 25 °C and/or 37 °C. The O2 released by bioprinted C. reinhardtii was measured by changes of dissolved O2 concentration in the medium. Under continuous light illumination (2,800 Lux) at 25 °C, the O2 concentration in the medium was found to be 11.5 mg L−1 within the first 24 h. The concentration of dissolved O2 remained almost at the similar level (~10.5 mg L−1) over the period of 4 days at 25 °C (Figure 3C). Similar level of dissolved O2, 10.9 mg L−1, was found over the culture period of 4 days when the bioprinted C. reinhardtii patterns were incubated at 37 °C under continuous light illumination (2,800 Lux), indicating the significant photosynthetic activity of the bioprinted C. reinhardtii at 37 °C (Figure 3C).
It is well-known that the light is an important factor in a photosynthetic process. To gain more insight into how light affects the O2 releasing ability of the bioprinted C. reinhardtii, we further investigated the growth and the subsequent release of O2 by the bioprinted C. reinhardtii patterns under limited light supplies at both 25 °C and 37 °C. To study the effect of the attenuation of light, the cylindrical C. reinhardtii-laden matrices of approximately 6-cm high and 2-cm in diameter were placed in black bioreactors such that C. reinhardtii scaffolds received light only from the top while light exposure to all other directions were prevented. The C. reinhardtii-laden constructs were incubated in TAP medium for 7 days, and the growth of the algae and their O2 production were assessed. The C. reinhardtii-laden constructs that were incubated under normal culture conditions wherein the whole constructs were exposed to normal light source (light intensity: 2,800 Lux), were considered as controls. At both 25 °C and 37 °C, the C. reinhardtii showed similar behaviors of reduced growth corresponding to the attenuation of light with depth. While in the control group C. reinhardtii showed uniform growth throughout the cylindrical constructs at both 25 °C (Figure S10A) and 37 °C (Figure S10C), the growth of the C. reinhardtii was observed only within the top regions of the cylindrical matrices placed in black bioreactors. At both 25 °C and 37 °C, the growth of C. reinhardtii gradually decreased with depth and no growth was observed towards the bottom of the constructs placed inside the black bioreactors. As expected, the O2 production by C. reinhardtii was drastically decreased when light source was blocked at both 25 °C (Figure S10B) and 37 °C (Figure S10D).
Likewise, to evaluate the O2-production behaviors of bioprinted C. reinhardtii under precisely modulated supplies of light source, C. reinhardtii-laden constructs were subjected to the light (2,800 Lux)/dark cycles at 1-h intervals under both 25 °C and 37 °C. When subjected to the 1/1 h light/dark cycles at 25 °C, the level of dissolved O2 in medium was found to increase gradually from 5.7 mg L−1 at 1 h to 6.9 mg L−1 over the culture period of 13 h. After 13 h of culture period, the level of dissolved O2 decreased slightly and remained at 5.9 mg L−1 until the culture period of 20 h. After that, the O2 level decreased rapidly over the time and reached 1.2 mg L−1 at 40 h (Figure S10E). The bioprinted C. reinhardtii followed the similar trend of O2 release under 1/1 h light (2,800 Lux)/dark cycles at 37 °C (Figure S10F). The level of dissolved O2 in medium was found to be 5.5 mg L−1 within 1 h and increased gradually up to 7.1 mg L−1 at 11 h. After 11 h of culture, the level of dissolved O2 decreased slightly and remained at 6.3 mg L−1 until 17 h. Afterwards, the O2 level decreased rapidly over the time and reached 1.0 mg L−1 at 40 h. These results suggested consistency for the growth of bioprinted C. reinhardtii under 1/1 h light/dark cycles at both 25 °C and 37 °C. The growth of C. reinhardtii decreased drastically after 20 h when subjected to the 1/1 h light/dark cycles under both temperature conditions.
We next measured the dissolved O2 in medium, in the presence of bioprinted C. reinhardtii-laden patterns embedded within HepG2 cells-encapsulated GelMA matrices at 37 °C (Figure 3F) and compared to that for the control samples wherein the 3D-bioprinted honeycomb patterns without C. reinhardtii were embedded within HepG2 cell-encapsulated GelMA matrices. The dissolved O2 levels in medium were found to be similar within 24 h in the presence (10.5 mg L−1) and absence (10.0 mg L−1) of C. reinhardtii-laden patterns. Interestingly, while the O2 level was maintained at 9.6 mg L−1 in the presence of the C. reinhardtii-laden patterns within the next 24 h, the O2 level was decreased rapidly to 7.0 mg L−1 in the absence of C. reinhardtii-laden patterns. In the presence of the bioprinted C. reinhardtii-laden patterns, the dissolved O2 was found to decrease slowly and reached up to 5.5 mg L−1 whereas in the absence of C. reinhardtii, the dissolved O2 decreased rapidly to 3.6 mg L−1, over the period of 4 days of culture at 37 °C. These results demonstrated the photosynthetic activity of the bioprinted C. reinhardtii and consumption of O2 by HepG2 cells embedded within GelMA matrices.
Fabrication of vascularized hepatic tissue-like constructs
Our aim was to utilize the bioprinted C. reinhardtii as the source of O2 to boost the initial growth of mammalian cells within the in vitro tissue constructs and to eventually create the vasculature within the tissues by removing the C. reinhardtii-laden construct from tissue scaffolds.
We first examined the digestion of C. reinhardtii, after 3 or 4 days of growth in TAP medium, in the presence of 1 mg mL−1 of cellulase at pH 6. It was found that almost all C. reinhardtii cells died or became inactivated within 2 h of cellulase treatment at 25 °C, as observed by the reduced autofluorescence of chlorophyll as well as ruptured cell walls (Figure S11). We further studied the digestion of the bioprinted C. reinhardtii-laden patterns embedded within GelMA matrices by treating with 1 mg mL−1 of cellulase (pH 6) at both 25 °C and 37 °C. In the control group, the bioprinted honeycomb patterns without C. reinhardtii were embedded within GelMA matrices. The bioprinted C. reinhardtii honeycomb patterns or control patterns embedded within GelMA matrices were cultured in TAP medium at 25 °C or 37 °C for 4 days. At day 4, the constructs were treated with cellulase (1 mg mL−1 in citrate buffer at pH 6) to dissolve the embedded C. reinhardtii patterns or control patterns. While the bioprinted C. reinhardtii or control patterns embedded in GelMA matrices were digested completely within ~12 h at 25 °C (Figure 3G), it took only ~4 h at 37 °C, leaving behind the open channels in the GelMA matrices (Figure 3H). The bioprinted C. reinhardtii patterns embedded within GelMA matrices could be visualized under fluorescence microscopy due to the presence of autofluorescence of chlorophyll. After the enzymatic digestion, the complete removal of the bioprinted C. reinhardtii within HepG2/GelMA matrices was confirmed by observing under fluorescence microscope wherein no autofluorescence of chlorophyll of C. reinhardtii was decerned any longer. The presence of interconnected lumens was further confirmed by the perfusion of the medium through the channels.
We next embedded C. reinhardtii-laden patterns within HepG2 cells-encapsulated GelMA matrices and allowed them to co-culture at 37 °C for up to 3 days. While C. reinhardtii-laden patterns embedded within HepG2/GelMA matrices could be visualized under fluorescence microscopy due to the presence of autofluorescence of chlorophyll, the HepG2 cells were stained with the CellTracker Blue CMF2HC dye to aid observation (Figure 4A). Based on the above results on the growth of the C. reinhardtii and the O2 release, the samples were treated with cellulase (1 mg mL−1 in citrate buffer at pH 6) at day 4 to dissolve the embedded C. reinhardtii-laden patterns. HepG2/GelMA constructs having bioprinted honeycomb patterns without C. reinhardtii embedded served as the control group. The bioprinted C. reinhardtii honeycomb patterns or control patterns embedded within HepG2 cells-encapsulated GelMA matrices were allowed to co-culture in DMEM:TAP medium at 37 °C for 4 days. At day 4, the scaffolds were treated with cellulase (1 mg mL−1 in citrate buffer at pH 6) to dissolve the embedded C. reinhardtii patterns or control patterns. As expected, the C. reinhardtii-laden patterns or control honeycomb patterns embedded in HepG2/GelMA matrices were successfully dissolved within 4 h of cellulase treatment at 37 °C, leaving behind the open microchannels.
Figure 4. Co-culture of bioprinted C. reinhardtii and HepG2 cells.
(A) Fluorescence micrographs showing CellTracker CMF2HC-labeled HepG2 cells (blue) in a GelMA construct and the embedded, bioprinted pattern of C. reinhardtii (red). (B) Fluorescence micrographs showing live (green)/dead (red)-stained HepG2 cells in GelMA constructs at day 4, in the absence (control) or presence of C. reinhardtii. (C) Viability of HepG2 cells in the absence (control) or presence of C. reinhardtii, at day 4 and day 7. Results are presented as means ± standard deviations. (D) Fluorescence micrographs showing the immunostaining of HIF-1α expression by HepG2 cells in the absence (control) or presence of C. reinhardtii at day 7. Nuclei were counter-stained with DAPI (blue). (E) Quantification of HIF-1α expression by HepG2 cells in the absence (control) or presence of C. reinhardtii at day 7. Results are presented as means ± standard deviations.
After ensuring successful removal of the bioprinted fugitive C. reinhardtii-laden patterns or control patterns, the HepG2/GelMA scaffolds with open channels were further incubated in DMEM at 37 °C and 5% CO2 for further functional studies of the HepG2 cells within the GelMA matrices. Viability and proliferation of HepG2 cells within GelMA matrices were evaluated at day 3 and day 7 of the removal of C. reinhardtii-laden patterns or control patterns by Live/Dead assay (Figure 4B) and 3-(4,5-dimethylthiazol-2-yl)-5-(3-carboxymethoxyphenyl)-2-(4-sulfophenyl)-2H-tetrazolium (MTS) assay (Figure 4C), respectively. The viability of HepG2 cells was higher when incubated with the bioprinted C. reinhardtii patterns as compared to the control. We also studied the expression of hypoxia-inducible factor-1α (HIF-1α) by HepG2 cells within the GelMA matrices at day 7 of the removal of C. reinhardtii-laden patterns or control patterns to evaluate if the O2 released by C. reinhardtii-laden patterns in the early stages of co-culture was sufficient for the survival and proliferation of the HepG2 cells within the GelMA matrices. The expression of HIF-1α by HepG2 cells was significantly lower within the GelMA matrices which received O2 released by C. reinhardtii during initial 4 days of co-culture as compared to the control group (Figure 4D and 4E). Thus, the use of the biosynthetic source of O2 within the GelMA constructs during the early stages of culture improved the viability/density of the HepG2 cells in the GelMA matrices.
Further, the morphology of the HepG2 cells within the GelMA matrices were evaluated by staining F-actin, a major component of the cytoskeleton, at day 7 after removal of C. reinhardtii-laden patterns or control patterns (Figure 5A). The cells were counter-stained with 4’,6-diamidino-2-phenylindole (DAPI) for nuclei. The functionality of HepG2 cells within the GelMA matrices was further confirmed by immunostaining for CYP3A4 and CYP1A2. CYP3A4 and CYP1A2 are the major CYP proteins expressed in liver and are important for the metabolism of drugs.39 Fluorescence images of the immunostained HepG2 cells within the GelMA matrices exhibited strong expressions of both CYP3A4 (Figure 5B) and CYP1A2 (Figure 5C) as compared to the controls. Thus, the expressions of these structural and functional markers by HepG2 cells within the GelMA matrices were observed to be improved when incubated with the bioprinted C. reinhardtii patterns during the early stages of culture as compared to those in controls. In addition, we measured the albumin and urea productions by HepG2 cells within the GelMA matrices by ELISA. Both the albumin (Figure 5D) and urea (Figure 5E) secretions were shown to be higher when HepG2 cells were co-cultured with the bioprinted C. reinhardtii-laden patterns during the early stages as compared to the respective controls. Thus, enzymatic digestion of bioprinted C. reinhardtii or control patterns within HepG2/GelMA matrices with 1 mg mL−1 cellulase in citrate buffer (pH 6) for 4 h did not have any negative influence on the HepG2 cells within the GelMA matrices, where the cells maintained higher levels of viability as well as liver-specific functions, including albumin production and urea synthesis, when compared to the controls. Prior to the enzymatic digestion of C. reinhardtii-laden patterns within HepG2/GelMA matrices, the effect of citrate buffer (pH 6) was assessed on the HepG2 cells and C2C12 cells using the same amount of the buffer as used for the enzymatic digestion. These cells were cultured in 2 mL of DMEM complete medium containing 400 μL of citrate buffer (pH 6) and incubated at 37 °C and 5% CO2 in an incubator for 2 days. Any adverse effect of the buffer (pH 6) was not observed on the growth and the viability of the cell types tested.
Figure 5. Functional studies of HepG2 cells.
(A) Fluorescence micrographs showing the expression of F-actin (red) by HepG2 cells within a GelMA construct at day 7 in the absence (control) and presence of C. reinhardtii. Nuclei were counter-stained with DAPI (blue). (B) Fluorescence micrographs showing the expression of CYP3A (green) by HepG2 cells within a GelMA construct at day 7 in the absence (control) and presence of C. reinhardtii. Nuclei were counter-stained with DAPI (blue). (C) Fluorescence micrographs showing the expression of CYP1A (red) by HepG2 cells within a GelMA construct at day 7 in the absence (control) and presence of C. reinhardtii. Nuclei were counter-stained with DAPI (blue). (D) Albumin secretion by HepG2 cells in GelMA constructs as determined by ELISA. Results are presented as means ± standard deviations. (E) Urea production by HepG2 cells in GelMA constructs as determined by ELISA. Results are presented as means ± standard deviations. In all these functional studies, the bioprinted C. reinhardtii-laden patterns within the HepG2/GelMA constructs were dissolve at day 4 by cellulase digestion.
Finally, to generate the vascularized hepatic scaffolds, we seeded the hollow microchannels, formed after enzymatic removal of the C. reinhardtii-laden patterns, with human umbilical vein endothelial cells (HUVECs) at 1.0–1.5×107 cells mL−1 and continued incubation in DMEM:endothelial cell growth medium (1:1) for 7 days under standard cell culture conditions. To facilitate visualization under fluorescence microscope, we initially seeded green fluorescent protein (GFP)-HUVECs (green) in the microchannels while the HepG2 cells were stained with CM-Dil cell tracker (red) before encapsulation in the GelMA matrices (Figure 6A). The GFP-HUVECs were distributed evenly throughout the microchannels over the period of 7 days of culture. HUVECs without GFP was further seeded within the channels to evaluate the viability of HepG2 cells within the matrices and HUVECs within the microchannels by Live/Dead assay (Figure 6B). We assessed the viability of the HepG2 cells within the GelMA matrices after seeding HUVECs in the microchannels and compared that with the control samples in which the microchannels were kept empty (i.e., without HUVECs) after the removal of control bioprinted patterns (i.e., without C. reinhardtii). The viability of HepG2 cells was found higher (~91% at day 3 and ~92% at day 7) in the GelMA matrices wherein HepG2 cells were co-cultured with C. reinhardtii-laden patterns during the early stages of culture and later seeded with HUVECs after removal of the C. reinhardtii-laden patterns. In contrast, the viability of HepG2 cells was approximately 70% at day 3 and 80% at day 7 in controls wherein bioprinted patterns without C. reinhardtii was embedded within HepG2/GelMA matrices and the microchannels formed after the removal of the bioprinted patterns were not seeded with HUVECs (Figure 6C). However, as largely expected, the viability of HepG2 cells within GelMA matrices was similar when compared before and after HUVEC seeding in the channels of the same scaffold. Similarly, the viability values of the HUVECs within the channels were approximately 83% at day 3 and 97% at day 7 (Figure 6D). To further confirm the formation of compact endothelial layer of HUVECs in the channels within hepatic scaffolds, we analyzed the expression of intercellular endothelial junction marker, CD31, by HUVECs. For this, the hollow microchannels were seeded with HUVECs without GFP expression, followed by incubation in DMEM:endothelial cell growth medium (1:1) medium for 7 days. The immunofluorescence staining of HUVEC monolayers revealed strong CD31 expression, indicating the formation of tight junctions among the cells (Figure 6E). Thus, we demonstrated that the O2 supplied by bioprinted C. reinhardtii within the tissue constructs boosted the viability of HepG2 cells during early days of co-culture, which supported the sustained viability and the metabolic activities of HepG2 cells within GelMA matrices that otherwise exhibited suppressed viability and functions even with the perfusion of vascular microchannels.
Figure 6. Vascularization of microchannels.
(A) Fluorescence micrographs showing GFP-HUVECs (green) seeded in the channels surrounded by CM-Dil-stained HepG2 cells (red) in a GelMA construct, at day 5 of GFP-HUVEC seeding in the microchannels after removing the bioprinted C. reinhardtii-laden patterns by cellulase digestion. (B) Fluorescence micrographs showing live (green)/dead (red)-stained HepG2 cells in a GelMA construct and HUVECs in the microchannels at day 7 of HUVEC seeding. (C) Viability of HepG2 cells in the GelMA matrices at day 3 and day 7 of HUVEC seeding in the microchannels. The control group had no HUVECs post-seeded into the microchannels. Results are presented as means ± standard deviations. (D) Viability of HUVECs at day 3 and day 7 of seeding in the microchannels. Results are presented as means ± standard deviations. E) Fluorescence micrographs showing the immunostaining of CD31 expression (green) by the HUVECs at day 7 of HUVEC seeding in the microchannels. Nuclei were counter-stained with DAPI (blue).
DISCUSSIONS
O2 is required for most cell and tissue metabolic processes, and mitochondrial respiration accounts for the majority of O2 consumption in humans,40 where the aerobic metabolism of glucose generates adenosine-5’-triphosphate (ATP). Additionally, the citric acid cycle and β-oxidation of fatty acids are tightly coupled with the process of oxidative phosphorylation and production of ATP. These ATP molecules then provide the required energy for most physiological processes. Thus, O2 bioavailability is vital to all cell functions and asserts its influence in regulating cell fate, morphogenesis, and organogenesis, among others.41 On the other hand, green plants and algae synthesize ATP, reduce nicotinamide adenine dinucleotide phosphate (NADPH), and excrete O2 by using light energy and water (H2O).42 In the carbon-fixation cycle, glyceraldehyde-3-phosphate (G3P) is synthesized from carbon dioxide (CO2) and H2O by utilizing ATP and NADPH. Nutrients including carbohydrates are synthesized from G3P. CO2, produced by respiration, is reused as a core component in photosynthesis.28 This ‘symbiotic recycling system’ has been recently explored in in vitro tissue engineering to supply adequate O2 to mammalian cells, and reuse metabolites and waste products from mammalian cells. For example, C. sorokiniana was co-cultured with pancreatic islets encapsulated in alginate beads, which showed the generation of sufficient O2 by C. sorokiniana for respiration and function of islets under in vitro anoxic environments.26
O2 is a critical nutrient for maintaining the tissue microenvironment.43 The enough supply of O2 and its diffusion into tissues are necessary for survival and physiological functions of tissues and organs. In adult human tissues, O2 concentration varies widely depending on the vasculature, metabolic requirements, and functions of each organ. For example, liver is one of the vital organs that plays major roles in balancing biochemical environments in the human body, including blood protein synthesis, glucose metabolism, fat metabolism, and detoxification of metabolites or other chemicals. The O2 consumption rate of rat hepatocytes was shown to be high, almost 10-fold higher than that of other cell types, and 3-fold more within the first 24 h after the inoculation process, during the attachment, spreading, and reorganizing of the cells into confluent monolayers.44 Similarly, it was shown that O2 is a critical factor for hepatocyte differentiation of human liver-derived cell lines, HepaRG and HepG2-C3A, and that increased levels of O2 lowers expression of HIF1α while increasing the expressions of albumin and hepatic transcription factor CEBPα, thus indicating the importance of pericellular O2 level for in vitro liver cell differentiation and functions.45 Although various studies have been conducted to develop engineered 3D tissue constructs including the hepatic tissues, the lack of efficient O2 supply remains the primary limitation in tissue-engineered constructs. In general, 3D tissue constructs require functional and stable vascular networks to maintain the viability and biological functions of dense cell populations.
Here, we have presented a potent approach that integrates the use of 3D bioprinting of C. reinhardtii as a sustainable source of O2 for HepG2 cells embedded in volumetric hydrogel matrices, aiming the fabrication of in vitro densified hepatic tissue constructs with maintained viability and functions. The unicellular green algae C. reinhardtii has long been used as a model microalgae for photosynthesis research and 3D bioprinting is the process of additively depositing the bioink onto a surface to produce well-defined 3D structures to recapitulate organ- and tissue-level functionality, including the vasculature.46 A bioink is essentially a hydrogel containing one or more types of living cells, biomaterials, and other growth supplements in various amounts, that mimics the ECM of the desired tissue and supports the growth of the embedded cells.47
Considering that algal cell walls contain polysaccharides including cellulose, we selected a structurally relevant polymer, NaCMC, as the main component of the bioink for bioprinting of C. reinhardtii that supported its growth and proliferation. We used the cellulose-based sacrificial bioink formulation consisting of NaCMC, PVA, gelatin, and sodium alginate to facilitate the extrusion bioprinting of well-defined sacrificial patterns and the growth of C. reinhardtii. NaCMC is a water-soluble cellulose ether derivate of natural cellulose, in which sodium carboxymethyl groups (CH2COONa) are introduced into the cellulose molecules to promote water solubility.48 The physical crosslinking of the NaCMC hydrogel occurs mainly through hydrogen bonding due to the presence of a large number of hydroxyl groups on the polymer chains.49,50 Gelatin is a soluble polypeptide produced by partial hydrolysis of collagen, the major component of the natural ECM and is a thermoreversible biopolymer with an upper critical solution temperature of approximately 30–35 °C, i.e., it becomes liquid at above this temperature range, and at the temperatures below roughly 25 °C, it forms a hydrogel.51 Gelatin and gelatin-based hydrogels have been extensively used in developing in vitro tissue models because of their biocompatibility and biodegradability.52 PVA is a water-soluble and biodegradable polyhydroxy polymer with high tensile strength. It is a synthetic polymer and has excellent adhesive properties.53 The presence of hydroxyl groups attached to alternate carbons in PVA favors the formation of hydrogen bonding.54 Alginate is a natural linear polysaccharide polymer that undergoes gelation by addition of divalent cations such as Ca2+, stabilizing the polymer network via physical crosslinking.55,56 These polymers have been used widely in hydrogel-preparations for applications in tissue engineering due to their biodegradability, biocompatibility, lack of toxicity, and cost-effectiveness.53 The aim of our study is to use NaCMC-based bioink as the sacrificial matrix for bioprinting of C. reinhardtii and to apply the bioprinted C. reinhardtii-laden patterns as a living source of O2 in the in vitro tissue constructs. The bioink formulations and their printability studies showed the optimal bioink formulation as 3% (v/v) NaCMC, 0.03% (v/v) PVA, 4% (v/v) gelatin, and 0.8% (v/v) alginate at a 55-psi pressure and 70 mm s−1 of feeding rate when the 27G extrusion nozzle was used (Figure 1).
The temperature and light conditions are the important factors for C. reinhardtii cultivation. Several studies have shown that temperature can significantly influence growth rate,57 carbohydrate and lipid production,58 as well as the induction of pigment-formation.59 While photoautotrophic growth occurs best between 25 °C to 28 °C for C. reinhardtii, 60 in these studies, temperatures ranging from 10 °C to 38 °C were considered optimal based on the desired product. The viability assay of C. reinhardtii within bioprinted C. reinhardtii-laden patterns under continuous light exposure showed that the NaCMC-based hydrogel was compatible for the growth of C. reinhardtii and that these photosynthetic unicellular algae were bioprinting-friendly (Figures 2). Interestingly, the exposure of C. reinhardtii-laden patterns under a range of UV light did not have any adverse effect on the growth and chlorophyll content of the C. reinhardtii-laden patterns at both 25 °C and 37 °C (Figure S9). The effects of the attenuation of light illumination on the growth of C. reinhardtii and the subsequent O2-production were evaluated using tall cylindrical constructs of approximately 6-cm high and 2 cm in diameter at both 25 °C and 37 °C. The attenuation of the growth and a consequent reduction in O2-production by C. reinhardtii within the cylindrical constructs were observed with respect to the attenuation of illuminated light at both 25 °C (Figure S10A and S10B) and 37 °C (Figure S10C and S10D). It should be noted however, the overall size of the GelMA constructs was approximately 1-cm thick with 4 mm on the top and 4 mm at the bottom of the embedded, bioprinted C. reinhardtii. The GelMA layer (4 mm) on the top of the bioprinted C. reinhardtii pattern in each construct was transparent (Figure 3G and 3H) and it was not so thick that it would significantly attenuate the illumination light and affect the growth of bioprinted C. reinhardtii within. Similar growth was observed at 37 °C when the constructs were cultured in TAP medium. However, at 37 °C they showed high cell viability for 4 days and gradually decreased over the period of 7 days in the presence or absence of GelMA matrices when grown in combined media (Figures 3E and S7).
In a related manner, when the C. reinhardtii-laden patterns were subjected to the 1/1 h light (2,800 Lux)/dark cycles, the level of dissolved O2 in medium was found to increase gradually, at both 25 °C and 37 °C, over the culture period of 13 h and then decrease gradually until 20 h, followed by rapid reduction over the 40 h (Figure S10E and S10F). Even though the dissolved O2 levels at 37 °C and 25 °C were comparable when the light was on, the O2 level was relatively low at 37 °C when the light was off. In addition, the bioprinted C. reinhardtii-laden patterns showed high cell viability at 25 °C over the period of 7 days both in the presence and absence of the surrounding GelMA matrices (Figures 3A and 3B). Similarly, these C. reinhardtii-laden patterns exhibited significant photosynthetic activities at both 25 °C and 37 °C over the period of 4 days under continuous light illumination (2,800 Lux), in the presence as well as absence of the surrounding GelMA or HepG2/GelMA matrices (Figures 3C and 3F).
The excellent biocompatibility and the cellulase-mediated degradation38 of NaCMC (Figure S3) has ensured the use of NaCMC-based hydrogel as the fugitive material for the bioprinting of photosynthetic algae, C. reinhardtii. The enzymatic digestion of the bioprinted NaCMC-based hydrogel patterns and C. reinhardtii-laden patterns in the presence or absence of the surrounding GelMA matrices suggested that, the sacrificial NaCMC-based hydrogel, either with or without C. reinhardtii, could be effectively eliminated using cellulase (1 mg mL−1 of cellulase in citrate buffer at pH 6) at both 25 °C and 37 °C, creating the interconnected microchannels within the tissue constructs (Figure 3G and 3H). During the enzymatic digestion process, besides NaCMC, other major components of the bioink were also likely dissociated. The gelatin, PVA, and alginate components were added to improve the printability of the bioink and to stabilize the shapes of the bioprinted structures. The stable bioprinted patterns were formed mainly due to the intermolecular and/or intramolecular hydrogen bonding among the functional groups of the polymers present in the bioink35 (Figure 1). The thermoreversible gelatin hydrogel formed was gradually dissolved out from the bioprinted pattern at temperatures higher than ~30°C,61 leaving the spaces for rapid proliferation of C. reinhardtii within the bioprinted pattern. The PVA is a biodegradable polymer that was present in very small amount in the bioink and alginate is a natural polymer that undergoes gelation by addition of divalent cations such as Ca2+. Therefore, when the NaCMC and/or C. reinhardtii component of a bioprinted structure was digested with cellulase, the entire pattern became easily dissolvable due to the damaged polymer network and significantly reduced hydrogen bonds and thus compromised overall stability. In addition, the treatment of C. reinhardtii-laden patterns embedded in HepG2/GelMA constructs with cellulase in citrate buffer at pH 6 did not have any negative influence on the HepG2 cells, which maintained higher levels of cell viability as well as reduced expression of HIF-1α at day 7 as compared to the control samples (Figure 4). Similarly, HepG2 cells within the constructs exhibited liver-specific functions, including F-actin organization, CYP3A4 and CYP1A2 protein expressions, and albumin production as well as urea synthesis (Figure 5). Since prevascularization of in vitro tissue constructs is critical before implantation for proper O2 and nutrient diffusion and integration with host vasculature,62 we finally seeded HUVECs in the hollow microchannels and proved that the cells were able to form a tight, confluent endothelia lining the inner surfaces of the microchannels within 7 days, thus generating the vascularized hepatic tissue-like constructs (Figure 6).
In summary, to develop vascularized tissue constructs with sufficient supply of O2 in vitro, our present study has presented 3D bioprinting of C. reinhardtii using the NaCMC-based sacrificial bioink. The bioprinted C. reinhardtii-laden patterns served as a natural photosynthetic O2-generator within hepatic tissue constructs and supported the viability and functionality of the HepG2 cells within surrounding GelMA matrices. The enzymatic degradability of the bioprinted NaCMC-based patterns and C. reinhardtii further allowed the formation of hollow perfusable channels within the tissue constructs, which could then be endothelialized with a uniformly distributed layer of HUVECs.
Although the 3D bioprinting of C. reinhardtii and its applicability as a source of oxygen for in vitro tissue constructs as well as formation of channels within the tissue constructs was achieved, more effort is likely needed to optimize the long-term co-culture systems. These include the development of optimized media that facilitate the co-culture of C. reinhardtii and human cells, as well as methods of illumination of light in the standard cell culture incubators, among others, for sustained supply of O2 produced by the photosynthetic algae to the human cells and/or tissues in vitro. Further detailed studies on biosafety, toxicity, and immunocompatibility of algae is important for its application in vivo and possibly in clinical translations in the future. Besides, microalgae represent a rich source of several bioactive compounds such as protein, carbohydrates, polyunsaturated fatty acids, carotenoids, vitamins and essential minerals, which when incorporated can enhance the nutritional value of food products thus providing multiple health benefits.63–65 Such a 3D bioprinting technology can also be explored to develop various healthy innovative food products enriched with both animal and plant proteins, or their subproducts.
EXPERIMENTAL PROCEDURES
Resource Availability
Lead Contact
Further information and requests for resources and reagents should be directed to and will be fulfilled by the Lead Contact, Yu Shrike Zhang (yszhang@research.bwh.harvard.edu)
Materials Availability
This study did not generate new unique reagents. Gelatin from porcine skin (type-A, 300 bloom), sodium alginate (low viscosity), methacrylic anhydride, CaCl2, NaCMC, cellulase from Aspergillus niger, 2-hydroxy-4’-(2-hydroxyethoxy)-2-methylpropiophenone (Irgacure 2959), Triton X-100, bovine serum albumin (BSA), and urea ELISA assay kit were purchased from Sigma-Aldrich (MO, USA). PVA (Mw 78,000 g mol−1) was obtained from Polysciences (PA, USA). Dulbecco’s phosphate-buffered saline (DPBS), fetal bovine serum (FBS), trypsin-ethylenediaminetetraacetic acid (trypsin-EDTA), penicillin/streptomycin (P/S), DAPI, Live/Dead® Viability/Cytotoxicity Kit, PrestoBlue® Cell Viability Reagent, Alexa Fluor® 594-phalloidin, mouse cytochrome P450 3A (CYP3A4/CYP3A5 monoclonal) antibody, Sytox-orange dead cell stain, and dialysis membrane (Mw cutoff 12–14 kDa) were obtained from Thermo Fisher Scientific (MA, USA). Endothelial cell growth medium was obtained from PromoCell (Germany). Mouse anti-hypoxia-inducible factor-1α (HIF-1α) antibody was obtained from Santa Cruz Biotechnology (CA, USA). Mouse anti-CD31 [P2B1] antibody, mouse anti-CYP1A2, Alexa Fluor® 594-conjugated goat anti-mouse, and Alexa Fluor® 488-conjugated goat anti-mouse secondary antibodies were purchased from Abcam (MA, USA). Human albumin ELISA kit was obtained from R&D Systems (MN, USA). Sylgard 184 silicone elastomer kit as was obtained from Dow Corning (MI, USA) for the fabrication of polydimethylsiloxane (PDMS) devices. Syringe filters (0.22 μm in pore size) were purchased from VWR International (MA, USA). The CellTiter 96® AQueous One Solution Cell Proliferation Assay kit was purchased from Promega (WI, USA). All other chemicals used in this study were obtained from Sigma-Aldrich unless otherwise mentioned.
Data and Code Availability
This study did not generate custom code, software, or algorithms. All data needed to evaluate the conclusions in the paper are present in the paper and/or Supplemental Information. Additional datasets that support the findings of this study are available from the corresponding authors upon reasonable request. All requests for raw and analyzed data and materials will be promptly reviewed by the Brigham and Women’s Hospital to verify whether the request is subject to any intellectual property or confidentiality obligations. Any data and materials that can be shared will be released via a Material Transfer Agreement.
Cells
HepG2 cells and murine C2C12 myoblasts were obtained from American Type Culture Collection (VA, USA), whereas HUVECs with or without GFP labeling were purchased from Angio-Proteomie (MA, USA). HepG2 and C2C12 cells were cultured in DMEM supplemented with 10% FBS and 1% P/S while HUVECs were cultured in endothelial cell growth medium supplemented with the supplements. All cells were incubated at 37 °C and 5% CO2 in a humidified incubator until 80–90% confluence. The respective culture medium was replaced every 3 days.
C. reinhardtii (+) was purchased from the Carolina Biological Supply Company (NC, USA) and grown on a standard TAP medium66 in 500-mL Erlenmeyer flasks at 25 °C under continuous light illumination, 2,800 Lux, using a relax LED bulb HD (General Electric Lighting, OH, USA). For all experiments, biological triplicates were simultaneously grown to the mid-log phase (OD750 0.5–0.6) and cells were harvested by centrifuging at 1,000 g for 5 min and discarding the supernatant.
Synthesis of GelMA
GelMA was synthesized as described previously6. Briefly, 10.0 g of type A gelatin from porcine skin was dissolved in 100 mL of DPBS at 60 °C, followed by addition of 8.0 mL of methacrylic anhydride drop-wise with continuous stirring. The reaction was carried out at 50 °C for 3 h and then quenched by a 5-fold dilution with warm DPBS (40 °C). The product was dialyzed with warm distilled water for 7 days using a dialysis membrane (Mw cutoff 12–14 kDa), and finally lyophilized to obtain GelMA in the form of a white porous foam. The GelMA was stored at room temperature until further use.
Preparation of hydrogel-precursor solutions and the bioink
The 10% (w/v) sodium alginate solution was prepared by dissolving sodium alginate powder in distilled water under continuous stirring overnight at 37 °C. Similarly, 9% (w/v) NaCMC, 1.5% (w/v) of PVA, and 10% (w/v) gelatin solutions were prepared in distilled water, separately. All the prepolymer solutions were prepared in glass vials. The NaCMC solution was first mixed with PVA solution and then with gelatin and alginate solutions using a vortex mixer and incubated at 80 °C for 1 h to obtain the homogeneous NaCMC-based hydrogel precursor solution (i.e., the bioink) containing 3% (v/v) NaCMC, 0.03% (v/v) PVA, 4% (v/v) gelatin, and 0.8% (v/v) alginate. This homogenous NaCMC-based hydrogel solution were sterilized by filtration through a sterile 0.22-μm filter. The 0.3-M CaCl2 solution was prepared by dissolving CaCl2 powder in deionized water, autoclaved at 121 °C for 15 min, and stored at 4 °C until use.
The 5% (w/v) GelMA hydrogel precursor solution containing 0.3% (w/v) Irgacure 2959 was prepared by dissolving GelMA and Irgacure 2959 powder in DPBS at 50 °C and sterilized by filtration through a sterile 0.22-μm filter.
Viscosity measurements
The viscosity profiles of the NaCMC-based bioink and its individual components at optimized concentration (3% (v/v) NaCMC, 0.03% (v/v) PVA, 4% (v/v) gelatin, and 0.8% (v/v) alginate) were measured with a hybrid rheometer (HR-3, Waters, VA, USA). All the individual components and the bioink, at the optimized concentrations, were maintained at 25 °C until their viscosity values were measured under a shear ramp mode running from 0.01 to 1,000 s−1 with a 1,000-μm gap size at 25 °C.
3D bioprinting of C. reinhardtii-containing bioinks
Various bioink compositions containing different amount of NaCMC (3–10% v/v), PVA (0.01–0.1% v/v), gelatin (2–10% w/v), and alginate (0.5–1% w/v) were tested for the printability of the predesigned geometry at different pressure (35–60 psi) and at different feeding rate (60–100 mm s−1). The homogeneous mixture of NaCMC, PVA, gelatin, and alginate solutions that gave the best results at a given pressure and feeding rate was used as the matrix for bioprinting of C. reinhardtii. Immediately prior to bioprinting, a suspension of C. reinhardtii was pelleted and different densities of C. reinhardtii (1.0–10×106 cells mL−1) were resuspended in the bioink. The bioink with or without reinhardtii was loaded into a 10-mL syringe (BD Biosciences, MA, USA) fitted with 27G blunt needle and extrusion bioprinting was performed using a bioprinter (Allevi 2, PA, USA) with digitally controlled pneumatic pressures, followed by crosslinking with the 0.3-M CaCl2 solution. Pressure to drive the syringe pistons was supplied by an in-house nitrogen line and maintained at approximately 55 psi by pressure regulators and gauges while the bioink feeding rate was kept constant at 70 mm s−1. The bioink without C. reinhardtii was used for bioprinting of scaffolds to be used as the control groups.
Characterizations of 3D-bioprinted C. reinhardtii patterns
The 3D-bioprinted C. reinhardtii patterns were incubated in the TAP medium at 25 °C under illumination by LED lightbulbs (light intensity: 2,800 Lux). Viability and growth of the algae were evaluated by measuring the optical density at 750 nm at different time points and different temperatures (25 °C and 37 °C). In addition, live/dead assay was performed by using the Sytox-orange fluorescent probe, with excitation and emission peaks of 547 nm and 570 nm, respectively, according to the manufacture’s instruction. Briefly, the bioprinted C. reinhardtii patterns were incubated in the TAP medium containing 5-μM Sytox-orange fluorescent probe for 5 min at 25 °C in the dark. The Sytox-orange dye could penetrate damaged cell membranes and therefore stain dead cells in orange, whereas viable cells would exhibit the red autofluorescence of chlorophyll. The samples were analyzed using a Zeiss Axio Observer inverted fluorescence microscope (Zeiss, NY, USA).
Measurement of chlorophyll
Chlorophyll contents in the bioprinted C. reinhardtii patterns were determined at day 7 using an ethanol extraction method.67,68 1 mL of 95% ethanol was added to each of the bioprinted C. reinhardtii pattern and vortexed to extract green pigments at room temperature. It was then centrifuged at 3,000 × g for 5 min and absorption was measured at 665 nm with a microplate reader (Tecan, Austria).
Measurements of O2 release from the 3D-bioprinted C. reinhardtii patterns
Dissolved O2 present in the TAP medium was removed by flushing with a nitrogen gas for 20 min, followed by incubation with the bioprinted C. reinhardtii patterns at 25 °C and/or 37 °C. Thus, photoautotrophic O2 release by C. reinhardtii was measured by changes of O2 concentrations in the media using a dissolved O2 sensor kit (Atlas Scientific, NY, USA) according to manufacturer’s instructions. Similarly, the O2 levels released by the bioprinted C. reinhardtii patterns embedded within GelMA hydrogel constructs were also measured.
Fabrication of vascularized 3D hepatic tissue-like constructs
The 3D-bioprinted NaCMC-based C. reinhardtii patterns were gently washed with DPBS and embedded in 5% GelMA hydrogels containing HepG2 cells to generate the hepatic tissue constructs. The bioprinted C. reinhardtii-laden constructs were embedded in GelMA hydrogel precursor solution with or without HepG2 cells and crosslinked with ultraviolet (UV, 6.9 mW cm−2) light for 40 s. The overall size of the tissue constructs was approximately 1-cm thick with 4 mm of GelMA on the top and 4 mm at the bottom of the embedded, bioprinted C. reinhardtii. The constructs were incubated in TAP or DMEM:TAP (1:1) media for 3 days at 25 °C or 37 °C under 5% CO2 in a humidified incubator and at day 4, the samples were treated with 1 mg mL−1 of cellulase in citrate buffer (pH 6) for 2–24 h. The enzymatic removal of the sacrificial C. reinhardtii-laden constructs was confirmed by observing under an optical and fluorescent microscope. The 3D-bioprinted patterns without C. reinhardtii were used as the control.
Cell viability and proliferation assays
The viability of HepG2 cells in the constructs was evaluated by live/dead assay using the Live/Dead® Viability/Cytotoxicity Kit (for mammalian cells) according to the manufacturer’s instructions. For the cell viability assay, the constructs were washed three times with DPBS and incubated with 500 μL well−1 of the combined Live/Dead assay reagents (2 μM of calcein AM and 4 μM of ethidium homodimer I (EthD-1)) for 20 min at 37 °C in the dark. The cells were then washed with DPBS and observed under the fluorescence microscope. The MTS assay was performed using the CellTiter 96® AQueous One Solution Cell Proliferation Assay kit. The media were removed, and the constructs were incubated with MTS assay solution for 2 h at 37 °C in the dark. The absorbance was measured at 492 nm with a microplate reader, and the results were expressed as percentages of the control. All the experiments were conducted in triplicates and were repeated for three times.
Measurements of secreted albumin and urea
The concentrations of albumin secreted by the cells in the constructs were measured using ELISA according to the manufacturer’s protocol. The HepG2 constructs with embedded bioprinted patterns, with or without C. reinhardtii, were cultured at 37 °C and 5% CO2 in a humidified incubator for 3 days and then treated with 1 mg mL−1 cellulase in citrate buffer for 2 h to digest the bioink and C. reinhardtii. The samples were further incubated 37 °C until day 7. The culture supernatants were collected at days 3 and 7 and stored at −80 °C until analysis.
For albumin assay, all the reagents were brought to the room temperature before starting the assay. A 50 μL of standard or sample was added to each well of albumin ELISA plate and 50 μL of the antibody cocktail was added to each well and incubated for 1 h at room temperature. Then each well was washed three times with Wash Buffer PT. Next, 100 μL of TMB development solution was added to each well and incubated for 10 min in the dark, followed by three washes. Finally, 100 μL of the stop solution was added to each well, incubated for 1 min and the optical density was recorded at 450 nm with the microplate reader.
Similarly, measurement of urea contents was performed using the Urea Assay Kit, according to the manufacturer’s instructions. Briefly, 0, 1, 2, 3, 4, and 5 nmol well−1 of urea standards were prepared by diluting 100 nmol mL−1 of urea standard solution with urea assay buffer, and 50 μL of each of the standard urea and 50 μL of each sample were placed in separate wells of a flat-bottom 96-well plate. Then, 50 μL of the freshly prepared reaction mix was added to each well and mixed quickly by gently rocking the plate. The reaction was incubated at 37 °C for 1 h in dark and absorbance was measured at 570 nm on the microplate reader. The samples were run in triplicates unless otherwise stated.
F-actin staining of the cells
The cells in the constructs were washed with DPBS, fixed with 4% (v/v) paraformaldehyde for 15 min, and permeabilized with 0.1% (v/v) Triton X-100 in DPBS for 1 h. The samples were then blocked with 5% (w/v) goat serum in DPBS for 1 h, followed by F-actin staining by incubating the samples with Alexa Fluor® 594-phalloidin (1:200 dilution in blocking buffer) overnight at 4 °C. After washing with DPBS, the nuclei were counter-stained with DAPI (1:1000) for 5 min at room temperature. Finally, the cells in the constructs were observed under the fluorescence microscope.
Immunocytochemical analyses
To evaluate the hypoxia conditions and to demonstrate the functions of HepG2 cells within the tissue constructs in the presence or the absence of C. reinhardtii, the cells were immunostained for HIF-1α, CYP3A4, and CYP1A2. At day 7, the cells were washed with DPBS and fixed with 4% (w/v) paraformaldehyde for 30 min at room temperature, followed by incubation with the permeabilization buffer (0.1% (v/v) Triton X-100 in DPBS) for 1 h at room temperature. The cells were then blocked with 5% (w/v) goat serum in DPBS for 2 h at room temperature and incubated with respective primary antibodies (1:200 dilution) overnight at 4 °C. The samples were washed with DPBS and incubated with the corresponding secondary antibody at 1:200 dilution (Alexa Fluor® 594-conjugated goat anti-mouse antibody or Alexa Fluor® 488-conjugated goat anti-mouse antibody) overnight at 4 °C. Finally, the nuclei were counter-stained with DAPI after washing with DPBS and examined under fluorescence microscope. Similarly, HUVECs in the channels were immunostained with primary mouse anti-CD31 antibody and secondary Alexa Fluor® 488-goat anti-mouse antibody.
Statistical analysis
All experiments were performed in triplicates and the results were presented as mean ± standard deviation. Statistical analyses were performed by paired t-test using GraphPad Prism (CA, USA) and p ≤ 0.05 was considered as statistically significant (* p≤0.05, ** p≤0.01, *** p≤0.001).
Supplementary Material
Movie S1. Perfusion of hollow channels within a GelMA construct after enzymatic removal of the bioprinted honeycomb pattern.
Progress and Potential.
Sufficient and homogeneous distribution of oxygen (O2) facilitates cell growth within 3D tissue constructs whereas limited supply of O2 induces cell death. This work reports the unique adoption of three-dimensional (3D)-bioprinted Chlamydomonas reinhardtii (C. reinhardtii) as a natural photosynthetic O2-generator for enhancing functions of engineered tissue constructs in vitro. Interestingly, the cellulase-mediated digestion of the bioprinted C. reinhardtii-laden patterns embedded within mammalian cell-encapsulating GelMA matrices, created perfusable and interconnected microchannels. These microchannels when subsequently endothelialized, made it possible to obtain biologically relevant vascularized tissue constructs. These bioprinted unicellular microalgae represent a bionic and sustainable source of O2 promoting the development of engineered mammalian tissues.
Highlights.
Application of bioprinted algae as O2-generator in tissue (model) engineering.
O2 produced by algae patterns improved functions of surrounding human cells.
Endothelialization of channels post-enzymatic digestion of fugitive algae patterns.
ACKNOWLEDGMENTS
This work was supported by funding from the National Institutes of Health (K99CA201603, R00CA201603, R21EB025270, R21EB026175, R01EB028143, R01GM134036) and National Science Foundation (1936105), the Brigham Research Institute, and the New England Anti-Vivisection Society. to National Science Foundation (1936105), and the Brigham Research Institute.
Footnotes
DECLARATION OF INTERESTS
The authors declare no competing interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Matesanz R, Mahíllo B, Álvarez M, and Carmona M (2009). Global observatory and database on donation and transplantation: world overview on transplantation activities. Transplant Proc. 41, 2297–2301. [DOI] [PubMed] [Google Scholar]
- 2.Giwa S, Lewis JK, Alvarez L, Langer R, Roth AE, Church GM, Markmann JF, Sachs DH, Chandraker A, and Wertheim JA (2017). The promise of organ and tissue preservation to transform medicine. Nat. Biotechnol. 35, 530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Atala A, Kasper FK, and Mikos AG (2012). Engineering complex tissues. Sci. Transl. Med. 4, 160rv12–160rv12. [DOI] [PubMed] [Google Scholar]
- 4.Zhang YS, Yue K, Aleman J, Mollazadeh-Moghaddam K, Bakht SM, Yang J, Jia W, Dell’Erba V, Assawes P, and Shin SR (2017). 3D bioprinting for tissue and organ fabrication. Ann Biomed Eng. 45, 148–163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ying GL, Jiang N, Maharjan S, Yin YX, Chai RR, Cao X, Yang JZ, Miri AK, Hassan S, and Zhang YS (2018). Aqueous Two-Phase Emulsion Bioink-Enabled 3D Bioprinting of Porous Hydrogels. Adv. Mater. 30, 1805460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pi Q, Maharjan S, Yan X, Liu X, Singh B, van Genderen AM, Robledo-Padilla F, Parra-Saldivar R, Hu N, and Jia W (2018). Digitally tunable microfluidic bioprinting of multilayered cannular tissues. Adv. Mater. 30, 1706913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fernandez C, Yen R, Perez S, Bedell H, Povsic T, Reichert W, and Truskey G (2016). Human vascular microphysiological system for in vitro drug screening. Sci. Rep. 6, 21579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ma X, Liu J, Zhu W, Tang M, Lawrence N, Yu C, Gou M, and Chen S (2018). 3D bioprinting of functional tissue models for personalized drug screening and in vitro disease modeling. Adv. Drug Deliv. Rev. 132, 235–251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mazzocchi A, Soker S, and Skardal A (2019). 3D bioprinting for high-throughput screening: Drug screening, disease modeling, and precision medicine applications. Appl. Phys. Rev. 6, 011302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rouwkema J, Koopman BF, Blitterswijk CAV, Dhert WJ, and Malda J (2009). Supply of nutrients to cells in engineered tissues. Biotechnol. Genet. Eng. 26, 163–178. [DOI] [PubMed] [Google Scholar]
- 11.Sarker M, Chen X, and Schreyer D (2015). Experimental approaches to vascularisation within tissue engineering constructs. J. Biomater. Sci. Polym. Ed. 26, 683–734. [DOI] [PubMed] [Google Scholar]
- 12.Lewis MC, MacArthur BD, Malda J, Pettet G, and Please CP (2005). Heterogeneous proliferation within engineered cartilaginous tissue: the role of oxygen tension. Biotechnol. Bioeng. 91, 607–615. [DOI] [PubMed] [Google Scholar]
- 13.Kolesky DB, Homan KA, Skylar-Scott MA, and Lewis JA (2016). Three-dimensional bioprinting of thick vascularized tissues. Proc. Natl. Acad. Sci. USA. 113, 3179–3184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.McQuilling JP, Sittadjody S, Pendergraft S, Farney AC, and Opara EC (2017). Applications of particulate oxygen-generating substances (POGS) in the bioartificial pancreas. Biomater. Sci. 5, 2437–2447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Harrison BS, Eberli D, Lee SJ, Atala A, and Yoo JJ (2007). Oxygen producing biomaterials for tissue regeneration. Biomaterials 28, 4628–4634. [DOI] [PubMed] [Google Scholar]
- 16.Pedraza E, Coronel MM, Fraker CA, Ricordi C, and Stabler CL (2012). Preventing hypoxia-induced cell death in beta cells and islets via hydrolytically activated, oxygen-generating biomaterials. Proc. Natl. Acad. Sci. USA. 109, 4245–4250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Abdi SIH, Ng SM, and Lim JO (2011). An enzyme-modulated oxygen-producing micro-system for regenerative therapeutics. Int. J. Pharm. 409, 203–205. [DOI] [PubMed] [Google Scholar]
- 18.Oh SH, Ward CL, Atala A, Yoo JJ, and Harrison BS (2009). Oxygen generating scaffolds for enhancing engineered tissue survival. Biomaterials 30, 757–762. [DOI] [PubMed] [Google Scholar]
- 19.Li Z, Guo X, and Guan J (2012). An oxygen release system to augment cardiac progenitor cell survival and differentiation under hypoxic condition. Biomaterials 33, 5914–5923. [DOI] [PubMed] [Google Scholar]
- 20.Gholipourmalekabadi M, Zhao S, Harrison BS, Mozafari M, and Seifalian AM (2016). Oxygen-generating biomaterials: a new, viable paradigm for tissue engineering? Trends Biotechnol. 34, 1010–1021. [DOI] [PubMed] [Google Scholar]
- 21.Lee VK, Lanzi AM, Ngo H, Yoo S-S, Vincent PA, and Dai G (2014). Generation of multi-scale vascular network system within 3D hydrogel using 3D bio-printing technology. Cell. Mol. Bioeng. 7, 460–472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zhang YS, Davoudi F, Walch P, Manbachi A, Luo X, Dell’Erba V, Miri AK, Albadawi H, Arneri A, and Li X (2016). Bioprinted thrombosis-on-a-chip. Lab Chip. 16, 4097–4105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cheng F, Cao X, Li H, Liu T, Xie X, Huang D, Maharjan S, Bei HP, Gómez A, and Li J (2019). Generation of Cost-Effective Paper-Based Tissue Models through Matrix-Assisted Sacrificial 3D Printing. Nano Lett. 6, 3603–3611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hegen A, Blois A, Tiron CE, Hellesøy M, Micklem DR, Nör JE, Akslen LA, and Lorens JB (2011). Efficient in vivo vascularization of tissue-engineering scaffolds. J. Tissue. Eng. Regen. Med. 5, e52–e62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wang Z, He Y, Yu X, Fu W, Wang W, and Huang H (2012). Rapid vascularization of tissue-engineered vascular grafts in vivo by endothelial cells in co-culture with smooth muscle cells. J. Mater. Sci. Mater. Med. 23, 1109–1117. [DOI] [PubMed] [Google Scholar]
- 26.Bloch K, Papismedov E, Yavriyants K, Vorobeychik M, Beer S, and Vardi P (2006). Photosynthetic oxygen generator for bioartificial pancreas. Tissue Eng. 12, 337–344. [DOI] [PubMed] [Google Scholar]
- 27.Hopfner U, Schenck T-L, Chávez M-N, Machens H-G, Bohne A-V, Nickelsen J, Giunta R-E, and Egaña J-T (2014). Development of photosynthetic biomaterials for in vitro tissue engineering. Acta Biomater. 10, 2712–2717. [DOI] [PubMed] [Google Scholar]
- 28.Haraguchi Y, Kagawa Y, Sakaguchi K, Matsuura K, Shimizu T, and Okano T (2017). Thicker three-dimensional tissue from a “symbiotic recycling system” combining mammalian cells and algae. Sci. Rep. 7, 41594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lode A, Krujatz F, Brüggemeier S, Quade M, Schütz K, Knaack S, Weber J, Bley T, and Gelinsky M (2015). Green bioprinting: Fabrication of photosynthetic algae-laden hydrogel scaffolds for biotechnological and medical applications. Eng. Life Sci. 15, 177–183. [Google Scholar]
- 30.Hon DN-S (1996). Cellulose and its derivatives: structures, reactions, and medical uses. In Polysaccharides in Medicinal Applications, Dumitriu S, ed. (Taylor & Francis Group; ). [Google Scholar]
- 31.Dutta SD, Patel DK, and Lim K-T (2019). Functional cellulose-based hydrogels as extracellular matrices for tissue engineering. J. Biol. Eng. 13, 55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sannino A, Demitri C, and Madaghiele M (2009). Biodegradable cellulose-based hydrogels: design and applications. Materials. 2, 353–373. [Google Scholar]
- 33.Lopez CG, Colby RH, and Cabral Jo.T. (2018). Electrostatic and hydrophobic interactions in NaCMC aqueous solutions: Effect of degree of substitution. Macromolecules 51, 3165–3175. [Google Scholar]
- 34.Ribeiro A, Blokzijl MM, Levato R, Visser CW, Castilho M, Hennink WE, Vermonden T, and Malda J (2017). Assessing bioink shape fidelity to aid material development in 3D bioprinting. Biofabrication 10, 014102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hu W, Wang Z, Xiao Y, Zhang S, and Wang J (2019). Advances in crosslinking strategies of biomedical hydrogels. Biomater. Sci. 7, 843–855. [DOI] [PubMed] [Google Scholar]
- 36.Eo MY, Fan H, Cho YJ, Kim SM, and Lee SK (2016). Cellulose membrane as a biomaterial: from hydrolysis to depolymerization with electron beam. Biomater. Res. 20, 16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zhivkov AM (2013). Electric properties of carboxymethyl cellulose. In Cellulose - Fundamental Aspects (intechopen; ). [Google Scholar]
- 38.Fei B, Wach RA, Mitomo H, Yoshii F, and Kume T (2000). Hydrogel of biodegradable cellulose derivatives. I. Radiation-induced crosslinking of CMC. J. Appl. Polym. 78, 278–283. [Google Scholar]
- 39.Zanger UM, Schwab M, and therapeutics. (2013). Cytochrome P450 enzymes in drug metabolism: regulation of gene expression, enzyme activities, and impact of genetic variation. Pharmacol. Ther. 138, 103–141. [DOI] [PubMed] [Google Scholar]
- 40.Boveris A, Costa LE, Cadenas E, and Poderoso JJ (1999). Regulation of mitochondrial respiration by adenosine diphosphate, oxygen, and nitric oxide. In Methods in enzymology (Elsevier; ), pp. 188–198. [DOI] [PubMed] [Google Scholar]
- 41.Fathollahipour S, Patil PS, and Leipzig ND (2018). Oxygen Regulation in Development: Lessons from Embryogenesis towards Tissue Engineering. Cells Tissues Organs 205, 350–371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Alberts B, Johnson A, Lewis J, Raff M, Roberts K, and Walter P (2002). Energy conversion: mitochondria and chloroplasts. In Molecular Biology of the Cell (Garland Science; ), pp. 815–878. [Google Scholar]
- 43.Xiao W, Shinohara M, Komori K, Sakai Y, Matsui H, and Osada T (2014). The importance of physiological oxygen concentrations in the sandwich cultures of rat hepatocytes on gas-permeable membranes. Biotechnol. Prog. 30, 1401–1410. [DOI] [PubMed] [Google Scholar]
- 44.Matsui H, Osada T, Moroshita Y, Sekijima M, Fujii T, Takeuchi S, and Sakai Y (2010). Rapid and enhanced repolarization in sandwich-cultured hepatocytes on an oxygen-permeable membrane. Biochem. Eng. J. 52, 255–262. [Google Scholar]
- 45.van Wenum M, Adam AA, van der Mark VA, Chang J-C, Wildenberg ME, Hendriks EJ, Jongejan A, Moerland PD, van Gulik TM, and Elferink RPO (2018). Oxygen drives hepatocyte differentiation and phenotype stability in liver cell lines. Cell Commun. Signal. 12, 575–588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Datta P, Ayan B, and Ozbolat IT (2017). Bioprinting for vascular and vascularized tissue biofabrication. Acta Biomater. 51, 1–20. [DOI] [PubMed] [Google Scholar]
- 47.Chimene D, Lennox KK, Kaunas RR, and Gaharwar AK (2016). Advanced bioinks for 3D printing: a materials science perspective. Ann Biomed Eng. 44, 2090–2102. [DOI] [PubMed] [Google Scholar]
- 48.(2014). Sodium Carboxymethylcellulose. In Cellulose and Cellulose Derivatives in the Food Industry, Wüstenberg T, ed. (Wiley Online Library; ), pp. 387–478. [Google Scholar]
- 49.Edgar KJ, Buchanan CM, Debenham JS, Rundquist PA, Seiler BD, Shelton MC, and Tindall D (2001). Advances in cellulose ester performance and application. Prog. Polym. Sci. 26, 1605–1688. [Google Scholar]
- 50.Shen X, Shamshina JL, Berton P, Gurau G, and Rogers RD (2016). Hydrogels based on cellulose and chitin: fabrication, properties, and applications. Green Chem. 18, 53–75. [Google Scholar]
- 51.Joly-Duhamel C, Hellio D, and Djabourov M (2002). All gelatin networks: 1. Biodiversity and physical chemistry. Langmuir 18, 7208–7217. [Google Scholar]
- 52.Pelling AE, Hickey RJ, and Biotechnology. (2019). Cellulose biomaterials for tissue engineering. Front. Bioeng. Biotechnol. 7, 45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Peng Z, and Shen Y (2011). Study on biological safety of polyvinyl alcohol/collagen hydrogel as tissue substitute (I). Polymer Plast. Technol. Eng. 50, 245–250. [Google Scholar]
- 54.Briscoe B, Luckham P, and Zhu S (2000). The effects of hydrogen bonding upon the viscosity of aqueous poly (vinyl alcohol) solutions. Polym. J. 41, 3851–3860. [Google Scholar]
- 55.Lee KY, and Mooney DJ (2012). Alginate: properties and biomedical applications. Prog. Polym. Sci. 37, 106–126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Augst AD, Kong HJ, and Mooney DJ (2006). Alginate hydrogels as biomaterials. Macromol. Biosci. 6, 623–633. [DOI] [PubMed] [Google Scholar]
- 57.Xin L, Hong-Ying H, and Yu-Ping Z (2011). Growth and lipid accumulation properties of a freshwater microalga Scenedesmus sp. under different cultivation temperature. Bioresour. Technol. 102, 3098–3102. [DOI] [PubMed] [Google Scholar]
- 58.Subhash GV, Rohit M, Devi MP, Swamy Y, and Mohan SV (2014). Temperature induced stress influence on biodiesel productivity during mixotrophic microalgae cultivation with wastewater. Bioresour. Technol. 169, 789–793. [DOI] [PubMed] [Google Scholar]
- 59.Markou G, and Nerantzis E (2013). Microalgae for high-value compounds and biofuels production: a review with focus on cultivation under stress conditions. Biotechnol. Adv. 31, 1532–1542. [DOI] [PubMed] [Google Scholar]
- 60.Adams GMW, Huang B, and Luck DJ (1982). Temperature-sensitive, assembly-defective flagella mutants of Chlamydomonas reinhardtii. Genetics 100, 579–586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Yin J, Yan M, Wang Y, Fu J, and Suo H (2018). 3D Bioprinting of Low-Concentration Cell-Laden Gelatin Methacrylate (GelMA) Bioinks with a Two-Step Cross-linking Strategy. ACS Appl. Mater. Interfaces 10, 6849–6857. [DOI] [PubMed] [Google Scholar]
- 62.Lovett M, Lee K, Edwards A, and Kaplan DL (2009). Vascularization strategies for tissue engineering. Tissue Eng. Part B Rev. 15, 353–370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Chacón-Lee TL, and González-Mariño GE (2010). Microalgae for “Healthy” Foods—Possibilities and Challenges. Compr. Rev. Food Sci. Food Saf. 9, 655–675. [DOI] [PubMed] [Google Scholar]
- 64.Matos à n.P. (2017). The Impact of Microalgae in Food Science and Technology. J. Am. Oil Chem.’ Soc. 94, 1333–1350. [Google Scholar]
- 65.Camacho F, Macedo A, and Malcata F (2019). Potential Industrial Applications and Commercialization of Microalgae in the Functional Food and Feed Industries: A Short Review. Mar. Drugs 17, 312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Harris EH, Stern DB, and Witman GB (2009). The Chlamydomonas Sourcebook, Vol 1 (Academic press; ). [Google Scholar]
- 67.Berberoglu H, Pilon L, and Melis A (2008). Radiation characteristics of Chlamydomonas reinhardtii CC125 and its truncated chlorophyll antenna transformants tla1, tlaX and tla1-CW+. Int. J. Hydrog. Energy 33, 6467–6483. [Google Scholar]
- 68.Wintermans JFGM, and De Mots A (1965). Spectrophotometric characteristics of chlorophylls a and b and their phenophytins in ethanol. Biochim. Biophys. Acta 109, 448–453 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Movie S1. Perfusion of hollow channels within a GelMA construct after enzymatic removal of the bioprinted honeycomb pattern.
Data Availability Statement
This study did not generate custom code, software, or algorithms. All data needed to evaluate the conclusions in the paper are present in the paper and/or Supplemental Information. Additional datasets that support the findings of this study are available from the corresponding authors upon reasonable request. All requests for raw and analyzed data and materials will be promptly reviewed by the Brigham and Women’s Hospital to verify whether the request is subject to any intellectual property or confidentiality obligations. Any data and materials that can be shared will be released via a Material Transfer Agreement.