Abstract
In this review we aim to assess the current state of science in relation to the integration of Patient Generated Health Data (PGHD) and Patient Reported Outcomes (PROs) into routine clinical care with a focus on surgical oncology populations. We will also describe the critical role of artificial intelligence and machine learning methodology in the efficient translation of PGHD, PROs and traditional outcome measures into meaningful patient care models.
Keywords: Telehealth, Biometrics, Patient Reported Outcomes, Machine Learning in Surgery, Artificial Intelligence
INTRODUCTION
Recent events with the COVID-19 pandemic have rapidly evolved the mechanisms where patients engage with their healthcare team. In a very short period of time there has become a normalization of engaging with clinicians via telehealth. Additionally, this is now being reimbursed by insurance. Patients may prefer this method of engagement as it minimizes the inconvenience and cost of time off work, travel, parking and gas.1
In oncology, patient-centered outcomes have been utilized in both the supportive care setting and as an outcome measure in clinical trials.2,3 Further, patient generated health data (PGHD) in the form of patient-reported outcomes (PROs) and biometrics are increasingly being captured. In the field of surgical oncology, a large fraction of care is provided in the outpatient setting and the majority of perioperative recovery occurs at home. With the current climate of telehealth expansion, there is a fundamental need for efficient utilization of PGHD and electronic PROs (ePROs) in oncologic surgery care and recovery.
In this review we aim to address the following questions:
What is the current state of science in relation to the integration of PGHD and PROs into routine clinical care with a focus on surgical oncology populations?
What is the potential role of artificial intelligence and machine learning methodology in the efficient translation of PGHD, PROs and traditional outcome measures into meaningful patient and clinician engagement?
Defining PGHD and PROs in the Context of Telehealth
Telehealth encompasses telemedicine, digital therapeutics and telemonitoring. Telemedicine allows for providers to engage with the patients in assessment, diagnosis and treatment without a face-to-face in person encounter.4 Digital therapeutics can utilize patient data and generate algorithms for care recommendations. Telemonitoring uses digital technology to either frequently or continuously monitor patients. This can be done in the context of patients allowing for passive data collection such as heart rate or steps taken or with the patient engaging in a purposeful manner such as recording their blood sugar or taking their blood pressure. Another form of telemonitoring is in the form of ePROs, where patients fill out prescribed validated surveys either on the internet, via an application or via a portal that connects with the electronic health record. The above are all forms of patient generated health data (PGHD).
Healthy history as a form of PGHD is obtained by the patient prior to a face to face encounter allowing for completion at home or via an electronic device that can then be directly reviewed by the provider or incorporated into the Electronic Health Record (EHR) and then reviewed by the care team. This function of PGHD can expedite clinic visits and allow the patient bidirectional dialogue with their clinician as to the primary issue and options of care. This may eliminate a portion of the encounter that is centered on the practitioner gathering data from the patient to formulate an appropriate plan. As face-to-face time is limited, the value of health surveys lies in enriching the interaction between the provider and patient to improve bidirectional complex decision making. This bidirectional dialogue contributes to patient satisfaction and the sense of autonomy or agency. One limitation is that this requires that the clinician allocates time that is not remunerated separate from the actual patient visit to review the PGHD. Additional limitations will be further addressed in this review.
A second category of PGHD are validated questionnaires and surveys. In oncology these correlate to outcomes of symptomatology, readmission risk, emergency room visits and even survival. In 2017, there was published data demonstrating that ePROs that triggered an email alert to a clinical nurse impacted survival.5 This was a landmark event in oncology and was noted by the American Society of Clinical Oncology in their online Major Milestones Against Cancer Timeline.6 The concept emphasizes the growing recognition that PGHD are meaningful to the patient and can positively inform patient care as outcomes on to their own.7 Utilization of this form of PGHD may take place via a web-based application on the patients home computer, hand held tablet or smart phone. These can be reviewed by the care team and trigger a particular response. The response to the findings of the questionnaires and surveys is an opportunity to intervene to prevent or mitigate symptomatology or impending complications. This form of PGHD in surgery is another method of assessing recovery and expectations. Though these surveys often reflect an individual, the trends can be extrapolated to a larger population and frame expectations for recovery.8,9 Poor responses of mental and physical deterioration have been shown to flag or correlate with complications and readmissions.
The third major category of PGHD are biometrics. These are in the form of digital data that is transferred to the care team. Biometrics can be generated in either an active or passive way and the patient is in charge of releasing this data to their health care provider. Passive generation and release of data can be in the form of measurement of steps via an accelerometer/pedometer, heart rate or continuous pulse oximetry. More active forms of PGHD include weight, blood pressure and glucose or temperature reading. These require the patient to engage by actually obtaining the measurement. The measurement is transferred manually by the patient or via a Bluetooth mechanism that does not require digital input by the patient. An example of such is work done by Peterson et al utilizing home based sensors to identify and intervene in patients with head and neck cancers who developed dehydration while receiving radiation.10 In this study the patients utilized weight, blood pressure and ePROS and 60% of patients developed a dehydration related event that was identified in this manner. Another study evaluated the impact of irinotecan fluorouracil-leucovorin and oxaliplatin (chronoIFL04) delivered at home on the quality of life of patients with cancer in real time using a home-based e-Health multifunction and multiuser platform.11 This involved multidimensional telemonitoring of circadian rest-activity rhythm (CircAct), sleep, patient-reported outcome measures via the MDASI (MD Anderson Symptom Inventory) and body weight changes. This approach was well accepted by patients and was potentially safe for patients at home. Pedometry has also been evaluated as a patient centered outcome in adult patients undergoing hematopoietic cell transplantation (HCT).12 HCT patients wore pedometers and completed PRO assessments during transplant hospitalization (4 weeks) and 4 weeks post-discharge. In this sample, more severe symptoms, impaired physical health and restrictions in the performance of usual daily activities were associated with statistically significant decrements in objectively measured daily steps. This study was an example of associating subjective functional recovery with objectively measured steps. In a pilot to define “normal” postoperative recovery trajectories after elective abdominal, thoracic or inguinal surgery, 68% of patients completed follow up looking at the median number of steps preoperatively compared to the first postoperative day and finally at four weeks.13 Their findings were that in the first postoperative week there was a rapid improvement however, the remaining recovery was at a much slower return to baseline. The recovery trajectories differed based on both admission and operation type. These studies all support the concept that activity recovery is indeed a patient centered outcome that can be used for counseling for recovery expectations.
PROs in Surgical Oncology
This review will further focus on telemonitoring in the form of PGHD and ePROS and its role in telehealth integration for perioperative care in surgical oncology. PGHDs can be categorized as a mechanism of obtaining patients’ health history, surveys and biometrics
Although PROs have gained significant traction in oncology, there is limited data in oncologic surgery and recovery. Recovery has traditionally been measured by conventional forms such as morbidity and length of stay. Surgical recovery in the era of ‘value-based care’ will need to include addressing outcomes such as cognitive decline, persistent pain, reduced functional activity, loss of independence or the inability to return to work.14 PGHD in surgery can be divided into studies that evaluate PROs and those that also included biometrics (either active or passive).
First, we will review work that has been done in PROs. There have been at least 3 insightful studies in gynecology oncology. The first was a single-arm pilot utilizing a Web-based Symptom Tracking and reporting for patients “STAR” questionnaire for patients to fill once preoperatively and then weekly during the 6-week postoperative period.15 When a patient submitted a response that was concerning, an automated email alert was sent to the clinician. The team found that of the 49 patients, most (82%) completed at least 4 sessions and this generated 43 alerts that led to telephone contacts, 2 ER referrals and one new appointment. On exit interviews, 80% of patients found this form of engagement useful and 85% would recommend it to others. Their second study that enrolled 96 patients, had 74% of patient that completed at least 4 sessions.16 Although the patients found it useful and 82% would recommend it to other patients, the clinical personnel found that the STAR system increased their current workload without enhancing patient care. The study speaks to one of the barriers found in the assimilation of PROS as it relates to provider time and will be addressed further in this review. PROs have also been used in perioperative gynecology oncology to assess symptom burden in patients having neoadjuvant chemotherapy with surgery for ovarian cancer.17 A longitudinal study found that in the hospital postoperative setting there were no differences between those who had neoadjuvant chemotherapy and those who did not in the five symptoms with the highest overall burden.
Beyond the hospital, Richards et al published a pilot in the United Kingdom utilizing ePROs post discharge in 40 patients after cancer-related upper gastrointestinal surgery.18 Their symptom-report response rates were in the range of 63–100%. Of the 197 ePROs completed, 39% triggered self-management advice and 36% triggered advice to contact a clinician. Participants found the ePRO system reassuring, providing timely information and advice relevant to supporting their recovery. Clinicians in this study found the system as a useful adjunct to usual care and enhancing their understanding of patients’ experiences during recovery.
Measurement of ePROS in colorectal surgery have also been demonstrated. Dawes et. Al. sought to design and implement a real time surveillance system for postoperative colorectal surgery patients using wireless health technology.19 The patients completed a daily survey regarding their postoperative health status until their first clinic visit. They reported on the first 20 consecutive enrolled patients and found that overall compliance was 63% but varied by patients from 26–100%. Their qualitative data suggested that the experience strengthened patients’ relationship with their surgeon and aided in their recovery. Similarly in Switzerland, 88% of potential users engaged in a mHealth app and up to 83% of post-discharge adverse events were resolved through the app.20 The NIH Patient Reported Outcomes Measurement Information System (PROMIS®) has been used after colorectal surgery as a PRO tool.21 A study of 142 colorectal surgery patients found the majority of patients quickly return to their baseline physical, mental and social function. However, they did find that scores for the interest in the sex domain decreased (worsened) for patients who had oncologic colorectal surgery and that this information can be used preoperatively to counsel patients about the typical impact on quality of life.
As patients are discharged and the majority of recovery occurs at home, questions arise as to the rate and trajectory of recovery post discharge. In a study of 132 patients undergoing short-stay abdominal surgery who were evaluated preoperatively and at 3 weeks and 2 months postoperatively, physical activity and health related quality of life were assessed via validated PROs.22 Their findings were that despite uniformly early discharge, a substantial proportion of patients (33%) had suboptimal recovery after 2 months and that there is variability in trajectories of recovery.
Other groups have developed interventions to accompany ePROS to facilitate recovery. In a prospective feasibility study, patients who had undergone esophagectomy and who had postoperative complications or an increased length of stay were assessed for willingness and adherence to participate in telerehabilitation on functional recovery at home post discharge.23 In the 15/22 patients that completed the intervention, the adherence rate went from 99.8% in the first 6 weeks to 75.6% in the following 6 weeks. This study also demonstrated that initial adherence is good and patients are satisfied, but that adherence decreases with time.
The additional goal of PROs is to complement the care that is being provided. Enhanced recovery pathways have emerged as more efficient and standard ways of managing patients in the postoperative setting. Day et al utilized PROs as a mechanism to accurately measure the enhanced recovery program in liver surgery at MD Anderson Cancer Center.24 They found that in comparing 75 patients in the ERLS (Enhanced Recovery in Liver Surgery) pathway to 43 patients simultaneously treated on a traditional pathway, the ERLS patients reported lower immediate postoperative pain scores and fewer complications and decrease length of stay. They also found that as measured by symptom burden on life interference, ERLS patients were more likely to return to baseline functional status in a shorter time interval. These findings also translated to patients being more likely to return to their intended oncologic therapy.
The role of PROs in surgery can also be used to demonstrated differences in recovery by surgical approach. Patients who had either an open thoracotomy or video assisted thoracoscopic surgery used the MD Anderson Symptom Inventory (MDASI) to report symptom interference with parameters related to functional recovery.25 Repeated measurement of MDASI interference characterized functional recovery and were able to demonstrate that minimally invasive surgery with VATS was associated with an enhanced postoperative recovery.
PGHD in Surgical Oncology
In addition to PROs, PGHD in the form of biometrics are obtained either actively or passively. The form of PGHD that has been most extensively studied is in the context of accelerometers or pedometers as a measure of functional recovery. Our team has previously pilot-tested the feasibility and acceptability of perioperative telemonitoring of ePROs and pedometer-captured daily steps.26 Patients were given a wristband pedometer (Vivofit 2) to monitor daily steps, and completed ePROs (MD Anderson Symptom Inventory - MDASI and EQ-5D-5L). Daily steps were monitored 3–7 days prior to surgery, during hospitalization and up to two weeks after discharge. PROs were completed before surgery (baseline), before hospital discharge and for two weeks post-discharge. Pre-determined outcome thresholds were set as follows: 1) patient reported symptom scores of moderate to severe intensity (4/10 or higher), and 2) QOL score of moderate to extreme problems (2/5 or higher). A real-time alert/feedback system was initiated when an encounter fell within the predetermined thresholds. The triggers prompted a phone call by a RN to the patient for further assessment, triage and management. Over a 4-month period of 29 eligible patients, 20 completed the study. We assessed via an exploratory analysis the correlation between daily steps and risk for postoperative complications as measured by the Comprehensive Complications Index (CCI). The median number of steps at day 7 was 1,689. This strongly correlated with the CCI score (r=−0.64, p<0.05), where patients with fewer daily steps had a higher CCI score (higher score =higher risk for complications. Similar findings were reported in 2020 where post cancer surgery steps as recorded by a cell phone accelerometer correlated inversely to ER visits, readmissions, reoperations and perioperative mortality.27
One of the best studies to date was a randomized 1:1 trial of post liver transplant patients who either had a telemedicine-based home management program (THMP) versus standard of care (SOC).28 Their findings were that 100 of 106 patients completed the study. Participation and adherence with telemedicine was 86% for basic health sessions (vital sign recording), but only 45% for using messaging or FaceTime. Must notably the THMP cohort had a lower 90-day readmission rate compared to SOC (28% vs 58%) The THMP cohort also had improved quality of life in regard to physical function and general health at 90 days. This work is an example of both feasibility and value return for patients, providers and the health system.
In 2019, a group from Wake Forest published their experience of feasibility of compliance with low cost accelerometers in measuring functional recovery after major oncologic surgery.29 In this study compliance varied greatly from 100% preadmission, 19% in-hospital and 82% post discharge. They found that median daily steps decreased from preadmission to post discharge by a median of 77%. In hepato-pancreaticobiliary surgery a recent study demonstrated that preoperative physical activity levels specifically a daily step count of >5,000 steps per day correlated to fewer major postoperative complications.30
This approach extends beyond oncologic surgery into cardiac and lung surgery where functional recovery has been assessed in the context of the trajectory of steps in the perioperative period.31,32 This work has helped lay the foundation for expectations as work done in accelerometer-based physical activity in older cancer patients demonstrates that only 41% returned to baseline levels within 3 months.33 The data presented above encompass the quality of recovery in surgery that must take into account the patients perceptions in PROs alongside the objective conventional data outcomes and cost parameters. The full scope of understanding recovery remains in its infancy and PGHDs and PROs will be necessary tools in establishing norms and expectations for oncologic surgery.
Assessing ePROs is feasible and valuable in surgical oncology however, there have not been any large scale randomized controlled trials evaluating the incorporation of PGHDs, ePROs and triggered intervention on perioperative outcomes. There remains a great opportunity to extend the benefits of telemonitoring to this oncology population during a fixed perioperative time.
The Challenges of Integration of ePROS and PGHD into the Electronic Health Record
Studies in pilot format demonstrate the value of ePROs and PGHD in the perioperative care of cancer patients, however, these concepts have not been assimilated as standard of care due to several challenges (Table I). There has to be recognition of value for patients, clinicians and health systems. There are policy considerations of privacy, and time allocation for clinicians to analyze and interpret data. Last is the operational component to support patients in efficiently generating and sharing their data. Information technology (IT) systems need to be in place to help clinicians and health systems to acquire, package and deliver data for interpretation and action. Ultimately, the goals are to take the data generated and analyze it to serves patients, clinicians and health systems in a meaningful way. Integration of the above data flows into a single descriptive / predictive data analysis and modeling framework, with the overreaching goal of generation of better algorithms of care, is the activity well-suited for the application of machine learning and artificial intelligence methodology, which will be addressed towards the latter portion of this review.
Table I.
Challenges of EHR Integration of PGHD and PROs
| Challenges of Integrating PGHD and PROs into the Electronic Health Record |
|---|
| Data acquisition, Engagement and Perceived Value |
| Web-Based |
| Patient Portal |
| Use of smart phone, tablet |
| Apps |
| Physician encouragement and engagement |
| Large Volume Data Analysis and Interpretation |
| Operational Implementation |
| IT support for patients & care team |
| Real time delivery of data |
| Policy Concepts |
| Adherence to HIPPA |
| Mechanisms to Reimburse Time |
Data Acquisition, Engagement and Perceived Value
In order for progress to be made in an efficient and thoughtful manner, key stakeholders and facilitators must be engaged. Ultimately it is the goal of ePROs and PGHD in cancer surgery to be incorporated in to the EHR. In 2019, Avery et al published their experience in developing a real-time electronic symptom reporting system for patients after discharge following cancer-related surgery.34 They evolved in 2 phases from the development of a web-based ePROS symptom-report from validated European Organization for Research and Treatment of Cancer (EORTC) questionnaires followed by hospital EHR integration. Nearly half (47%) of the patients approached consented and 97% of those completed the self-reports. From this work they developed a hospital EHR-integrated ePRO system that alerts clinicians and provides patients self-management advice to improve the detection and management of postoperative problems and complications after discharge.
EHR integration will require that the patient, care team and health system embrace value in ePROs and PGHD. The collection of ePROs and PGHD renders better engagement in shorter more focused time periods. An example of such was a study that retrospectively reviewed patient participation in submitting blood glucose parameters into the EHR portal in a large multispecialty clinic over 4 years.35 Of nearly 37,000 patients with diabetes, only 53 patients uploaded three or more blood glucose values over any 9 month period. Of these patients, 23 were pregnant women (representing 3% of their portal users with gestational diabetes) and 30 were nonpregnant adults with diabetes (representing <1% of portal users with diabetes). Their findings found that overall adoption is low and that patients in a transient time period may be more inclined to participate actively in a PGHD program.
Most studies that have incorporated PGHD or PROs have an end of study assessment to determine if the process was of value to the patient. The vast majority of patients are in favor of PROs and PGHD.36 The most frequent value adds are an increased sense of empowerment, a greater physician-provider relationship, and a more efficient mode of accessing their care.37
For the value, the role of the clinicians in PGHD can be either that of supporter where they oversee or encourage PGHD use.38 The second role can be that of reviewer that analyzes PGHD to inform counseling and medical decision making in the remote setting. Health systems may identify value in PGHD in contributing to improvements in quality of care, fewer readmissions, less resource utilization and an increase in consumer satisfaction. The work done in transplant surgery was an example of reducing readmissions from 58 to 28% at 90 days.28 This certainly translates to a cost mitigation.
The issues of security and privacy must be taken into consideration when active and passive biometric data is obtained from patients. In general there are 3 tiers of data collection and transmission.39 Tier 1 is at the patient level where patients can generate and transmit PGHD via wireless sensors or with patient input into a portal. This information is sent to Tier 2 as the personal gateway i.e. via tablets or smart phones. The data from Tier 2 is then sent to Tier 3 which is the health care provider or health systems. In a bidirectional dialogue, health systems/providers can then generate responses or services back to the patient or utilize this data in counseling the patient via telemedicine or in person at a future visit. The security and adherence to HIPAA (Healthcare Information Privacy and Portability Act) are important in making these systems functional.
Another consideration of data acquisition is in the context of user characteristics and access. There are differences in digital literacy and access as it pertains to technology. Most apps available in the Apple mobile application market are in English and at reading levels that are too advanced for many patients.40–42 In 2016, 46% of consumers were considered active digital health adopters by having used 3 or more categories of digital health tools.43 These differences can be seen in the ability to engage and consistently participate in follow up using PGHDs.44 Age has become less of a barrier over time as it pertains to digital literacy. In 2016, 38% of users age 65–74 were likely to use their EHR to access their health information compared to only 22% in users age 18–34.45 The proportion of patients that knew what data they have access to grew from 39% in 2014 to 65% in 2016. In 2020, we expect these numbers to grow and result in an even greater proportion of patients engaged with their EHR and as consumers of digital health tools.
Data Analysis and Interpretation
Although intended to bridge a gap to complement clinic visits, PGHD can also have voluminous data that can stymie potential efficiencies. The cognitive demand, increase in labor cost and additional time to assimilate PGHD are all important considerations in data analysis and interpretation.46 In most published studies it is difficult to assess precise data storage formats. Some have integrated with REDCap or similar data collection tools. Web portals are often used.
As for data interpretation, most systems are not feedback triggered. The notable methods of alerts generated as interpretation of PGHD are automated generated emails to providers regarding symptom treatment mismatch, out of range vital signs, symptom severity greater than grade 3, a dashboard that allows the ability to graph findings or change over time, and alerts for symptoms that passed critical thresholds.43,47–50 In most systems, there was a nurse at the interface between the data capture and the patient. As these modes of data interpretation progress, there is need for more sophisticated algorithms to interact with patients in an automated and efficient way and to better predict outcomes. This is a great opportunity for the development of better systems to capture and interpret the PGHD created in the perioperative recovery period for surgery patients.
Operational Implementation
Validated PROs are increasingly used in clinical trials yet their routine adoption in care remains limited and generally separated from the medical record. The 21st Century Cures Act of 2016 includes provisions under Title III to formulate a more robust framework for drug development and product labeling that specifically accounts for “patient experience data,” including PROs to capture not only the disease burden but also treatment burden.51 In oncologic surgery, the patient experience in recovery is no less important a metric to measure in the context of treatment burden. EHRs currently offer patients access to a subset of their health data through “patient portals” and offer secure electronic messaging. There are not any that accept data generated by patients connected devices (e.g., smartphones and wearables).52 There is limited IT support for select PROs and their approach has been non-standardized and rarely incorporated into routine clinical work flows.
Epic Systems Corporation (Verona, WI) is common EHR that has a library of PROs and that allows users to add their own.53 Via the patient portal, PROs can be triggered at particular set time points or in response to particular “events.” In the integration of PGHD and ePROs into the medical record, there needs to be consistent IT support for patients and providers and this will be an investment by health systems. Lastly there is a call for system interoperability where PGHD can be incorporated into a variety of EHRs from wearable devices or mobile phones/tablets.54 The interface needs to have digital flexibility such that the data exchange is convenient and versatile.
Policy Concepts
Policy concepts that will need to be addressed include structure to the interoperability of devices and systems. This will allow PGHD to be compatible with multiple EHR interfaces. There are policy issues around standards for devices used to obtain biometric data. There is debate in the accuracy of low cost commercially available pedometers in recording steps.55 Devices that are deemed medical grade may need Food and Drug Administration approval and rigorously tested to show if they have an impact on health outcomes.43 Tracking modalities and monitoring is an issue of policy in assigning responsibility for the interpretation and analysis of data that can be generated 24 hours/day and 7 days/week. Last, is the issues pertaining to reimbursement. As we are still in the infancy of determining how best to incorporate PGHD in EHRs and work flows, there is a time investment by clinicians in reviewing, analyzing and communicating the relevance of that data to the patient. That extra time needs to be enumerated with innovative payment models and value-based reimbursements.56
Artificial Intelligence and Machine Learning in the Perioperative Care of Surgical Oncology Patients.
PGHD and PROs in the perioperative period for surgical oncology patients can generate large volumes of new heterogeneous data --- something not available prior to the implementation of these perioperative outpatient monitoring protocols. In order to better estimate the utility of this data and to create prediction models of outcomes including complications, readmissions and mortality risk, more complex multivariate data analysis and modeling techniques need to be employed --- specifically, techniques and toolkits (software implementations) explicitly aimed at the secondary analysis of big heterogeneous datasets. These may include predictive analytics and modeling (using various techniques to predict future events based on existing data), descriptive modeling (using various techniques to dissect the patterns and relationships in the observed data) and “traditional” statistical hypothesis testing. Much of the above methodology belongs to the domain of Machine Learning (ML) (itself a subset of Artificial Intelligence (AI) research)56–58
There is growing precedent in utilizing ML / AI techniques and tools to predict surgical outcomes using the available datasets. Monsalve-Torra et. al. focus on patients who underwent surgery for abdominal aortic aneurysm.59 The authors deploy artificial neural networks (ANNs) and Bayesian networks (BNs) to build a system that can predict hospital mortality. The dataset used 57 attributes from 310 cases coming from clinical information systems. The attributes were pre-processed and then analyzed using WEKA (Waikato Environment for Knowledge Analysis) ML workbench ANN/BNs implementations to both generate predictions and gain model insights.
Another recent study utilized a novel ML approach to identify preoperative risk factors associated with super-utilization of Medicare expenditure following surgery.60 They identified patients who underwent abdominal aortic aneurysm repair, coronary artery bypass graft, colectomy, total hip arthroplasty, total knee arthroplasty or lung resection from 2013–2015. They used a Logic Forest ensemble classifier (a classification and variable selection/ranking ML algorithm) to look at the comorbidities and interactions of comorbidities that put patients at an increased chance of becoming a super-utilizer. They found that super-utilizers comprised 4.8% of the overall cohort and that risk factors associated with super-utilization included hemiplegia/paraplegia, weight loss, congestive heart failure with chronic kidney disease. Certain subpopulations were associated with super utilization of health care following surgical intervention despite having lower overall use in the preoperative period. Here, use of a specialized ML tool allows for future work to develop specific evidence-based algorithms aimed at decreasing unnecessary costs.60 Perhaps this population can undergo targeted interventions in the preoperative setting to mitigate the massive burden on the health care system post operatively. A potential application in the realm of PGHD and PROs is that these patients can be monitored and optimized preoperatively to optimize postoperative outcomes and intercept more severe complications.
A second study done by the same group sought to develop a novel easy-to-use surgical complexity score to accurately predict adverse outcomes undergoing elective surgery.61 Comorbid conditions were entered into an AI search/optimization algorithm to assign weights to maximize the correlation with multiple postoperative outcomes including morbidity, readmission, mortality and postoperative super-use. The predictive ability was compared against three of the most commonly used risk adjustment indices: the Charlson Comorbidity index (CCI), Elixhauser Comorbidity Index (ECI) and the Centers for Medicare and Medicaid Service’s Hierarchical Condition Category (CMS-HCC). Stochastic hill-climbing (a standard AI local search/optimization routine) was used to determine the optimal weight for each comorbidity and demographic characteristic. They found that compared to these other indices, the novel Surgical Complexity Score outperformed the other models in predicting postoperative morbidity, 30-day readmission, 90-day readmission and postoperative super-use.
In another recent study, Corey et al used an advanced ML framework, built around the variety of classifiers/variable selection methods (Random Forests, LASSO logistic regression, boosted decision trees) augmented with cross-validation and assorted pre- and post-processing routines, to predict the likelihood of post-surgical complications based on the automatically curated electronic health record data.62 This study exemplifies a comprehensive, adaptive ML approach to the secondary data analysis and predictive modeling that does not rely on just one “off-the-shelf” ML tool.
Doryab et al used step and hearth rate data collected via Fitbit devices to evaluate ML classifiers for predicting readmission for a cohort of 49 patients subjected to pancreatic surgery.63 Classification approach based on boosted logistic regression outperformed baseline clinical approaches to readmission risk calculation such as LACE (which includes data about Length of stay, Acuity of admission, Comorbidity, ER visits in the past 6 months) and HOSPITAL ( which includes data about Hemoglobin at discharge, discharged from Oncology service, Sodium at discharge, Procedure during hospitalization, Index admission Type (emergent or planned), number of Admissions in the past year, and Length of stay).
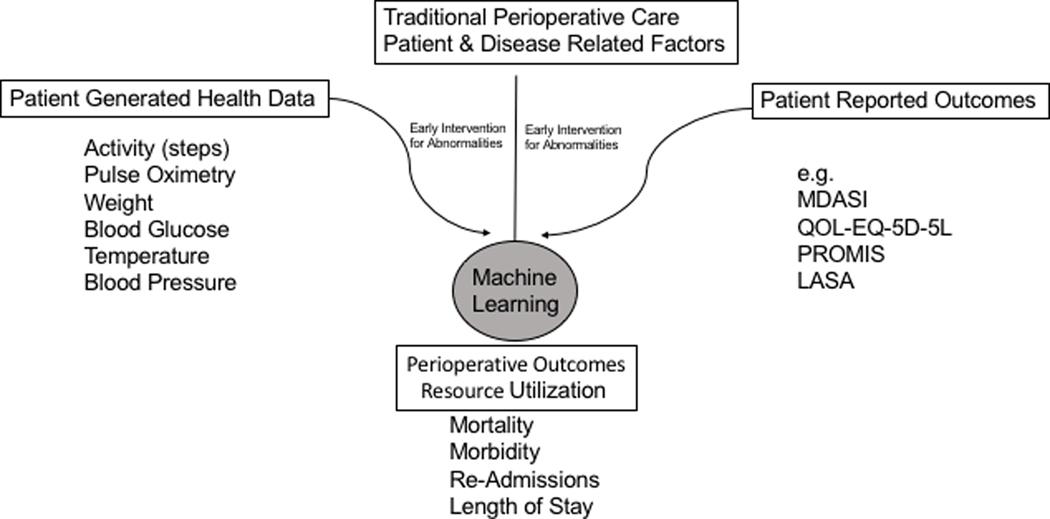
To date we have yet to see in the literature an implementation/application of ML techniques that combines comorbidities and demographics with PGHD and PROs to refine outcome data in oncologic surgery prediction models (Figure 1). The combination of these is what will yield a more robust and comprehensive data analysis framework for generation of improved, comprehensive, prediction models.
Figure 1. Optimizing Models of Care in Surgical Oncology.
Working framework of incorporating PGHD and PROs in the EHR and the utility of predicting outcomes using artificial intelligence. (PROMIS-patient reported outcomes measurement information system)
Limitations
Limitations of this review include the focus on surgical populations as they pertain to PGHD and PROs. Our aims were not to compile the entirety of the literature but rather address a very specific niche in surgical oncology that has yet to be explored.
Conclusions
PGHD and ePROs are being developed in nearly every area of medicine in this era of emerging telehealth. Perioperative optimization is a universal goal in the treatment of surgical oncology patients. Integration of these factors with the aid of ML / AI analytical tools will inform the quality of prediction and interventions for optimizing oncology surgery outcomes.
Synopsis:
Patient Generated Health Data (PGHD) and Patient Reported Outcomes (PROs) are will become more available in the field of surgical oncology. Here we describe the critical role of artificial intelligence and machine learning methodology in the efficient translation of PGHD, PROs and traditional outcome measures into meaningful patient care models.
ACKNOWLEDGEMENTS
ASR is supported by the Susumu Ohno Chair in Theoretical and Computational Biology, NIH NCI Cancer Systems Biology Consortium U01CA232216, and NIH NLM R01LM013138.
Footnotes
Data Availability Statement: This article does not include any shared data.
REFERENCES
- 1.Lee A, Shah K, Chino F. Assessment of Parking Fees at National Cancer Institute-Designated Cancer Treatment Centers. JAMA Oncol. 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Warsame R, D’Souza A. Patient Reported Outcomes Have Arrived: A Practical Overview for Clinicians in Using Patient Reported Outcomes in Oncology. Mayo Clin Proc. 2019;94(11):2291–2301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Basch E, Deal AM, Dueck AC, et al. Overall Survival Results of a Trial Assessing Patient-Reported Outcomes for Symptom Monitoring During Routine Cancer Treatment. JAMA. 2017;318(2):197–198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Aapro M, Bossi P, Dasari A, et al. Digital health for optimal supportive care in oncology: benefits, limits, and future perspectives. Support Care Cancer. 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Basch E, Barbera L, Kerrigan CL, Velikova G. Implementation of Patient-Reported Outcomes in Routine Medical Care. Am Soc Clin Oncol Educ Book. 2018;38:122–134. [DOI] [PubMed] [Google Scholar]
- 6. www.asco.org.
- 7.Petersen C. Patient-generated health data: a pathway to enhanced long-term cancer survivorship. J Am Med Inform Assoc. 2016;23(3):456–461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bowyer A, Royse C. A matter of perspective - Objective versus subjective outcomes in the assessment of quality of recovery. Best Pract Res Clin Anaesthesiol. 2018;32(3–4):287–294. [DOI] [PubMed] [Google Scholar]
- 9.Fagundes CP, Shi Q, Vaporciyan AA, et al. Symptom recovery after thoracic surgery: Measuring patient-reported outcomes with the MD Anderson Symptom Inventory. J Thorac Cardiovasc Surg. 2015;150(3):613–619 e612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Peterson SK, Shinn EH, Basen-Engquist K, et al. Identifying early dehydration risk with home-based sensors during radiation treatment: a feasibility study on patients with head and neck cancer. J Natl Cancer Inst Monogr. 2013;2013(47):162–168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Innominato P, Komarzynski S, Karaboue A, et al. Home-Based e-Health Platform for Multidimensional Telemonitoring of Symptoms, Body Weight, Sleep, and Circadian Activity: Relevance for Chronomodulated Administration of Irinotecan, Fluorouracil-Leucovorin, and Oxaliplatin at Home-Results From a Pilot Study. JCO Clin Cancer Inform. 2018;2:1–15. [DOI] [PubMed] [Google Scholar]
- 12.Bennett AV, Reeve BB, Basch EM, et al. Evaluation of pedometry as a patient-centered outcome in patients undergoing hematopoietic cell transplant (HCT): a comparison of pedometry and patient reports of symptoms, health, and quality of life. Qual Life Res. 2016;25(3):535–546. [DOI] [PubMed] [Google Scholar]
- 13.Carmichael H, Overbey DM, Hosokawa P, et al. Wearable Technology-A Pilot Study to Define “Normal” Postoperative Recovery Trajectories. J Surg Res. 2019;244:368–373. [DOI] [PubMed] [Google Scholar]
- 14.Royse CF. The patient’s surgical journey and consequences of poor recovery. Best Pract Res Clin Anaesthesiol. 2018;32(3–4):253–258. [DOI] [PubMed] [Google Scholar]
- 15.Andikyan V, Rezk Y, Einstein MH, et al. A prospective study of the feasibility and acceptability of a Web-based, electronic patient-reported outcome system in assessing patient recovery after major gynecologic cancer surgery. Gynecol Oncol. 2012;127(2):273–277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cowan RA, Suidan RS, Andikyan V, et al. Electronic patient-reported outcomes from home in patients recovering from major gynecologic cancer surgery: A prospective study measuring symptoms and health-related quality of life. Gynecol Oncol. 2016;143(2):362–366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Meyer LA, Nick AM, Shi Q, et al. Perioperative trajectory of patient reported symptoms: a pilot study in gynecologic oncology patients. Gynecol Oncol. 2015;136(3):440–445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Richards HS, Blazeby JM, Portal A, et al. A real-time electronic symptom monitoring system for patients after discharge following surgery: a pilot study in cancer-related surgery. BMC Cancer. 2020;20(1):543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dawes AJ, Reardon S, Chen VL, et al. Wireless Technology to Track Surgical Patients after Discharge: A Pilot Study. Am Surg. 2015;81(10):1061–1066. [PubMed] [Google Scholar]
- 20.Agri F, Hahnloser D, Demartines N, Hubner M. Gains and limitations of a connected tracking solution in the perioperative follow-up of colorectal surgery patients. Colorectal Dis. 2020;22(8):959–966. [DOI] [PubMed] [Google Scholar]
- 21.Hedrick TL, Harrigan AM, Thiele RH, Friel CM, Kozower BD, Stukenborg GJ. A pilot study of patient-centered outcome assessment using PROMIS for patients undergoing colorectal surgery. Support Care Cancer. 2017;25(10):3103–3112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tran TT, Kaneva P, Mayo NE, Fried GM, Feldman LS. Short-stay surgery: what really happens after discharge? Surgery. 2014;156(1):20–27. [DOI] [PubMed] [Google Scholar]
- 23.van Egmond MA, Engelbert RHH, Klinkenbijl JHG, van Berge Henegouwen MI, van der Schaaf M. Physiotherapy With Telerehabilitation in Patients With Complicated Postoperative Recovery After Esophageal Cancer Surgery: Feasibility Study. J Med Internet Res. 2020;22(6):e16056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Day RW, Cleeland CS, Wang XS, et al. Patient-Reported Outcomes Accurately Measure the Value of an Enhanced Recovery Program in Liver Surgery. J Am Coll Surg. 2015;221(6):1023–1030 e1021–1022. [DOI] [PubMed] [Google Scholar]
- 25.Shi Q, Wang XS, Vaporciyan AA, Rice DC, Popat KU, Cleeland CS. Patient-Reported Symptom Interference as a Measure of Postsurgery Functional Recovery in Lung Cancer. J Pain Symptom Manage. 2016;52(6):822–831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sun V, Dumitra S, Ruel N, et al. Wireless Monitoring Program of Patient-Centered Outcomes and Recovery Before and After Major Abdominal Cancer Surgery. JAMA Surg. 2017;152(9):852–859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Panda N, Solsky I, Huang EJ, et al. Using Smartphones to Capture Novel Recovery Metrics After Cancer Surgery. JAMA Surg. 2019;155(2):1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lee TC, Kaiser TE, Alloway R, Woodle ES, Edwards MJ, Shah SA. Telemedicine Based Remote Home Monitoring After Liver Transplantation: Results of a Randomized Prospective Trial. Ann Surg. 2019;270(3):564–572. [DOI] [PubMed] [Google Scholar]
- 29.Barkley R, Khalil M, Shen P, Levine EA, Votanopoulos K, Clark CJ. Feasibility of low-cost accelerometers in measuring functional recovery after major oncologic surgery. J Surg Oncol. 2019. [DOI] [PubMed] [Google Scholar]
- 30.Nakajima H, Yokoyama Y, Inoue T, et al. How Many Steps Per Day are Necessary to Prevent Postoperative Complications Following Hepato-Pancreato-Biliary Surgeries for Malignancy? Ann Surg Oncol. 2020;27(5):1387–1397. [DOI] [PubMed] [Google Scholar]
- 31.Cook DJ, Thompson JE, Prinsen SK, Dearani JA, Deschamps C. Functional recovery in the elderly after major surgery: assessment of mobility recovery using wireless technology. Ann Thorac Surg. 2013;96(3):1057–1061. [DOI] [PubMed] [Google Scholar]
- 32.Finley DJ, Fay KA, Batsis JA, et al. A feasibility study of an unsupervised, pre-operative exercise program for adults with lung cancer. Eur J Cancer Care (Engl). 2020;29(4):e13254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Jonker LT, Hendriks S, Lahr MM, van Munster BC, de Bock GH, van Leeuwen BL. Postoperative recovery of accelerometer-based physical activity in older cancer patients. Eur J Surg Oncol. 2020. [DOI] [PubMed] [Google Scholar]
- 34.Avery KNL, Richards HS, Portal A, et al. Developing a real-time electronic symptom monitoring system for patients after discharge following cancer-related surgery. BMC Cancer. 2019;19(1):463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ancker JS, Mauer E, Kalish RB, Vest JR, Gossey JT. Early Adopters of Patient-Generated Health Data Upload in an Electronic Patient Portal. Appl Clin Inform. 2019;10(2):254–260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mohanty S, Kinnier CV, Bilimoria KY. Patient satisfaction, outcomes, and the need for cancer-specific quality metrics. J Natl Cancer Inst. 2015;107(3). [DOI] [PubMed] [Google Scholar]
- 37.McCann L, Maguire R, Miller M, Kearney N. Patients’ perceptions and experiences of using a mobile phone-based advanced symptom management system (ASyMS) to monitor and manage chemotherapy related toxicity. Eur J Cancer Care (Engl). 2009;18(2):156–164. [DOI] [PubMed] [Google Scholar]
- 38.Nittas V, Lun P, Ehrler F, Puhan MA, Mutsch M. Electronic Patient-Generated Health Data to Facilitate Disease Prevention and Health Promotion: Scoping Review. J Med Internet Res. 2019;21(10):e13320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Mohsin AH, Zaidan AA, Zaidan BB, et al. Real-Time Medical Systems Based on Human Biometric Steganography: a Systematic Review. J Med Syst. 2018;42(12):245. [DOI] [PubMed] [Google Scholar]
- 40.Davis DW, Logsdon MC, Vogt K, et al. Parent Education is Changing: A Review of Smartphone Apps. MCN Am J Matern Child Nurs. 2017;42(5):248–256. [DOI] [PubMed] [Google Scholar]
- 41.Kim C, Prabhu AV, Hansberry DR, Agarwal N, Heron DE, Beriwal S. Digital Era of Mobile Communications and Smartphones: A Novel Analysis of Patient Comprehension of Cancer-Related Information Available Through Mobile Applications(). Cancer Invest. 2019;37(3):127–133. [DOI] [PubMed] [Google Scholar]
- 42.Purswani JM, Dicker AP, Champ CE, Cantor M, Ohri N. Big Data From Small Devices: The Future of Smartphones in Oncology. Semin Radiat Oncol. 2019;29(4):338–347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Demiris G, Iribarren SJ, Sward K, Lee S, Yang R. Patient generated health data use in clinical practice: A systematic review. Nurs Outlook. 2019;67(4):311–330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kontos E, Blake KD, Chou WY, Prestin A. Predictors of eHealth usage: insights on the digital divide from the Health Information National Trends Survey 2012. J Med Internet Res. 2014;16(7):e172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Psiha MM. Efficient Health Information Management Based on Patient-Generated Digital Data. Adv Exp Med Biol. 2017;989:271–280. [DOI] [PubMed] [Google Scholar]
- 46.Steward DA, Hofler RA, Thaldorf C, Milov DE. A method for understanding some consequences of bringing patient-generated data into health care delivery. Med Decis Making. 2010;30(4):E1–E13. [DOI] [PubMed] [Google Scholar]
- 47.Adams WG, Fuhlbrigge AL, Miller CW, et al. TLC-Asthma: an integrated information system for patient-centered monitoring, case management, and point-of-care decision support. AMIA Annu Symp Proc. 2003:1–5. [PMC free article] [PubMed] [Google Scholar]
- 48.Barrett M, Combs V, Su JG, et al. AIR Louisville: Addressing Asthma With Technology, Crowdsourcing, Cross-Sector Collaboration, And Policy. Health Aff (Millwood). 2018;37(4):525–534. [DOI] [PubMed] [Google Scholar]
- 49.Basch E, Iasonos A, Barz A, et al. Long-term toxicity monitoring via electronic patient-reported outcomes in patients receiving chemotherapy. J Clin Oncol. 2007;25(34):5374–5380. [DOI] [PubMed] [Google Scholar]
- 50.Bauer AM, Iles-Shih M, Ghomi RH, et al. Acceptability of mHealth augmentation of Collaborative Care: A mixed methods pilot study. Gen Hosp Psychiatry. 2018;51:22–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Goble JA. The Potential Effect of the 21st Century Cures Act on Drug Development. J Manag Care Spec Pharm. 2018;24(7):677–681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Sayeed R, Gottlieb D, Mandl KD. SMART Markers: collecting patient-generated health data as a standardized property of health information technology. NPJ Digit Med. 2020;3:9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Jensen RE, Rothrock NE, DeWitt EM, et al. The role of technical advances in the adoption and integration of patient-reported outcomes in clinical care. Med Care. 2015;53(2):153–159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Mandel JC, Kreda DA, Mandl KD, Kohane IS, Ramoni RB. SMART on FHIR: a standards-based, interoperable apps platform for electronic health records. J Am Med Inform Assoc. 2016;23(5):899–908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.West P, Van Kleek M, Giordano R, Weal M, Shadbolt N. Information Quality Challenges of Patient-Generated Data in Clinical Practice. Front Public Health. 2017;5:284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Bhavnani SP, Parakh K, Atreja A, et al. 2017 Roadmap for Innovation-ACC Health Policy Statement on Healthcare Transformation in the Era of Digital Health, Big Data, and Precision Health: A Report of the American College of Cardiology Task Force on Health Policy Statements and Systems of Care. J Am Coll Cardiol. 2017;70(21):2696–2718. [DOI] [PubMed] [Google Scholar]
- 57.Peake JM, Kerr G, Sullivan JP. A Critical Review of Consumer Wearables, Mobile Applications, and Equipment for Providing Biofeedback, Monitoring Stress, and Sleep in Physically Active Populations. Front Physiol. 2018;9:743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Shameer K, Badgeley MA, Miotto R, Glicksberg BS, Morgan JW, Dudley JT. Translational bioinformatics in the era of real-time biomedical, health care and wellness data streams. Brief Bioinform. 2017;18(1):105–124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Monsalve-Torra A, Ruiz-Fernandez D, Marin-Alonso O, Soriano-Paya A, Camacho-Mackenzie J, Carreno-Jaimes M. Using machine learning methods for predicting inhospital mortality in patients undergoing open repair of abdominal aortic aneurysm. J Biomed Inform. 2016;62:195–201. [DOI] [PubMed] [Google Scholar]
- 60.Hyer JM, Ejaz A, Tsilimigras DI, Paredes AZ, Mehta R, Pawlik TM. Novel Machine Learning Approach to Identify Preoperative Risk Factors Associated With Super-Utilization of Medicare Expenditure Following Surgery. JAMA Surg. 2019;154(11):1014–1021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Hyer JM, White S, Cloyd J, et al. Can We Improve Prediction of Adverse Surgical Outcomes? Development of a Surgical Complexity Score Using a Novel Machine Learning Technique. J Am Coll Surg. 2020;230(1):43–52 e41. [DOI] [PubMed] [Google Scholar]
- 62.Corey KM, Kashyap S, Lorenzi E, et al. Development and validation of machine learning models to identify high-risk surgical patients using automatically curated electronic health record data (Pythia): A retrospective, single-site study. PLoS Med. 2018;15(11):e1002701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Doryab A, Dey AK, Kao G, Low C. Modeling Biobehavioral Rhythms with Passive Sensing in the Wild: A Case Study to Predict Readmission Risk after Pancreatic Surgery. Proc ACM Interact Mob Wearable Ubiquitous Technol. 2019;3(1):Article 8. [Google Scholar]