ABSTRACT
Exposure to a cold climate is associated with an increased morbidity and mortality, but the specific mechanisms are largely unknown. People with cardiopulmonary disease and winter endurance athletes are particularly vulnerable. This study aimed to map multiple domains of airway responses to exercise in subzero temperature in healthy individuals.
Thirty-one healthy subjects underwent whole-body exposures for 50 minutes on two occasions in an environmental chamber with intermittent moderate-intensity exercise in +10 °C and -10 °C. Lung function, plasma/urine CC16 , and symptoms were investigated before and after exposures.
Compared to baseline, exercise in -10 °C decreased FEV1 (p=0.002), FEV1/FVC (p<0.001), and increased R20Hz (p=0.016), with no differences between exposures. Reactance increased after +10 °C (p=0.005), which differed (p=0.042) from a blunted response after exercise in -10 °C. Plasma CC16 increased significantly within exposures, without differences between exposures. Exercise in -10 °C elicited more intense symptoms from the upper airways, compared to +10 °C. Symptoms from the lower airways were few and mild.
Short-duration moderate-intensity exercise in -10 °C induces mild symptoms from the lower airways, no lung function decrements or enhanced leakage of biomarkers of airway epithelial injury, and no peripheral bronchodilatation, compared to exercise in +10 °C.
KEYWORDS: Cold temperature, environmental chamber, physical activity, healthy, asthma, respiratory symptoms
Introduction
Cold air is associated with increased morbidity and mortality, but the specific mechanisms are still largely unknown [1]. Particularly vulnerable are the elderly and people with cardiopulmonary disease [2,3]. A Finnish population-based study showed that cold-exposure caused respiratory symptoms in 29% of the population [4]. Similarly, in a study on cold-exposure of Finnish children, almost half of the participants reported respiratory symptoms [5]. To understand the effects of a cold climate in sensitive populations, such as people with cardiopulmonary disease, we must first understand the effects on healthy individuals. Experimental exposure of human subjects to cold air provides one approach to further investigate the epidemiological relationships we have seen in previous studies of cold-related mortality in the population.
Prolonged and repeated exposure to subzero temperatures may induce airway inflammation, bronchoconstriction and bronchial hyperreactivity [6]. In particular, cross-country skiers and other winter endurance athletes who are repeatedly exposed to cold, dry air have a high prevalence of respiratory symptoms, bronchial hyperreactivity and asthma [6]. Nevertheless, the pathogenesis of these airway effects is not fully understood. Even at rest and during low-intensity exercise, cooling of the facial skin may trigger respiratory symptoms [7]. As exercise intensity increases, inhalation of increasing volumes of cold, dry air leads to heat and moisture loss from the airway mucosa, and dehydration of the airways [8]. These cooling and osmotic stressors are thought to trigger release of inflammatory mediators and exercise-induced bronchoconstriction [9]; the severity of which may be determined by minute ventilation and/or air humidity [10]. Cold exposure triggers smooth muscle contraction in the distal airways, while plasma exudation from leaking capillaries can affect the contractile properties of the smooth muscle so that they become hyperresponsive [11]. Exposure to −23°C has also been shown to increase concentrations of neutrophil granulocytes and macrophages in bronchoalveolar lavage fluid in healthy individuals [12], which is indicative of an inflammatory process.
In healthy individuals, experimental exposure to cold air may induce bronchial obstruction [13–16], although not all studies have detected this effect [17,18]. Experimental exposure to subzero temperatures has been shown to induce bronchial obstruction in patients with asthma [19]. Cold air has also been shown to induce and aggravate bronchial hyperreactivity in individuals with asthma [20]. Exposure of asthmatic individuals to −5°C increases sputum neutrophil counts [21].
Club cell protein 16 (CC16) is a protein secreted from club cells in the bronchioles and is thought to protect against oxidative stress and inflammation [22,23]. When the airway epithelium is subjected to stress, there is an increased leakage of CC16 into the bloodstream and urine. CC16 can therefore be used as a marker of damage to airway epithelial integrity [22]. Increased levels of CC16 have been seen after exercise [24], eucapnic hyperventilation [25] and exposure to cold, dry air [26].
While repeated exercise in a cold climate has been shown to cause local signs of airway inflammation [27–29], the systemic immune effects are more uncertain [30]. Generally, short-duration, moderate-intensity exercise at room temperature has not been shown to negatively affect systemic immune function [31,32]. Some studies suggest that exercise in cold temperatures lead to increased stress hormone responses that may in turn interfere with normal immune function [33], although this hypothesis is not well supported by available evidence. While we may expect to see the activation of local inflammatory pathways associated with airway inflammation in response to continuous exercise in cold air, potentially associated systemic immune effects also seem worthy of further investigation.
It is therefore unclear whether the peripheral airways and the airway epithelium, and even systemic immunity are affected by exercise in subzero temperatures in healthy individuals. Deeper knowledge about the physiological responses in healthy human airways can help us to better understand the mechanisms behind the cold-induced morbidity and mortality in the population.
The purpose of this study was to map multiple domains of airway responses to physical exercise in subzero temperature, as well as to compare the responses to the same exercise in a milder temperature. Our primary hypothesis was that moderate-intensity exercise for 35 minutes in −10°C induces more bronchoconstriction than in +10°C. Secondary aims were to investigate the effects of exercise in −10°C on airway resistance and reactance, biochemical signs of epithelial injury, systemic cellular responses and symptoms.
Materials and methods
Study design and subjects
This study was a crossover experimental exposure study. In randomised order, the study subjects were exposed to +10°C or −10°C while exercising in an environmental chamber. Each study subject was exposed on two separate occasions, at least one week apart. The environmental chamber is located at the Swedish Winter Sports Research Centre, Mid Sweden University, Östersund, and has been described elsewhere [34]. The study was carried out during April–June 2017, and the mean (range) outdoor temperature during the exposures was 8.3 (0.2–19.5) °C. The study was conducted in accordance with the Declaration of Helsinki and approved by the Ethical Review Board in Umeå (2016–203-31 M). Written informed consent was obtained from all study subjects.
Thirty-one volunteers were recruited through local advertising. The subjects were 18–65 years of age, healthy, never-smokers, and without allergy or asthma. One subject used oral contraceptives and one used selective serotonin reuptake inhibitors. Before the pre-test and exposures, subjects were asked to refrain from caffeine the same day, avoid strenuous exercise for 24 hours prior to the tests, and to avoid any exhausting means of transportation to the lab. No anti-inflammatory medication during the study period was allowed. Subjects had to be free from lower airway infections for at least 4 weeks prior to their pre-test and exposures. The subjects were told to wear appropriate clothing, and to bring a bag with extra clothing for the exposures. Subject characteristics and baseline lung function are presented in Table 1.
Table 1.
Subject characteristics and baseline data. Lung function measurements in % of predicted. Data are presented as mean (SD) unless noted otherwise
| Characteristic |
Overall n = 31 |
Females n = 16 |
Males n = 15 |
| Age, years mean (range) | 40.4 (18–65) | 37.9 (18–51) | 43.1 (22–65) |
| Height, centimetres | 174.5 (10.8) | 166.7 (6.1) | 182.8 (8.0) |
| Weight, kilograms | 71.7 (12.1) | 64.0 (5.8) | 80.0 (11.8) |
| Body mass index, kg/m2 | 23.4 (1.9) | 23.0 (1.8) | 23.8 (2.0) |
| VO2max, mL/kg/min | 49.1 (6.8) | 46.5 (5.4) | 51.8 (7.4) |
| FEV1 | 104 (10) | 100 (11) | 108 (8) |
| FVC | 104 (9) | 101 (10) | 106 (7) |
| R5Hz | 99 (29) | 102 (33) | 95 (25) |
| R20Hz | 113 (25) | 116 (27) | 109 (24) |
n, number; kg/m2, kilograms/square metres; VO2max, maximum rate of oxygen consumption; mL, millilitres; FEV1, forced expiratory volume in 1 s; FVC, forced vital capacity; R5Hz and R20Hz, resistance at 5 and 20 Hertz.
Pre-test
Each subject performed an integrated submaximal and maximal endurance test on a motorised treadmill (Rodby Innovation, Vänge, Sweden) to determine their maximum rate of oxygen consumption (VO2max), as well as oxygen consumption at four fixed submaximal speeds. Oxygen consumption was measured using AMIS 2001 model C (Innovision AS, Odense, Denmark). These data were used to interpolate the speed that would achieve 70% of the participant’s VO2max during the experimental trials.
Exposures
Each exposure was 50 minutes (min). Subjects conducted a predefined protocol according to Figure 1. The warm-up was at a brisk walking speed; 5.2 kilometers/hour (km/h) for women and 6.4 km/h for men. The running intervals were performed at a speed to elicit 70% of VO2max at 4% inclination. Each running protocol included 2 × 1.5 min running at higher speed (~78% VO2max) followed by a 1.5 min recovery period at decreased speed (~62% VO2max), which was then repeated for 15 min. The running protocol was designed to simulate a range of real-world continuous exercise outdoors in undulating terrain such as skiing, running and cycling. Heart rate was continuously monitored (model s610, Polar Electro Oy, Kempele, Finland).
Figure 1.
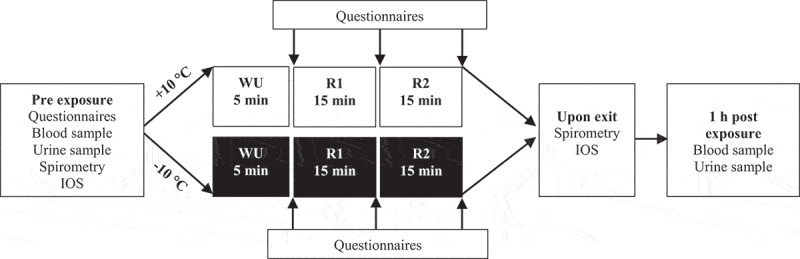
Protocol of exposures
During the +10°C exposures, the chamber mean (SD) temperature was 10.2 (0.1) °C; mean (SD) relative humidity was 22.3 (4.5) %, and mean absolute humidity was 2.0 (0.4) g/m3. During the −10°C exposures, the chamber mean (SD) temperature was −10.7 (0.3) °C, mean (SD) relative humidity was 54.2 (7.2) %, and mean absolute humidity was 1.3 (0.2) g/m3. Absolute humidity (AbsH, grams/cubic metres) was calculated based on the formula
where rh (%) is relative humidity; and T (°C) is temperature [34].
Study variables
Lung function: Before each exposure and immediately after exiting the chamber, participants performed impulse oscillometry (IOS) [35]. Before and after each exposure, dynamic spirometry was performed immediately after IOS (Jaeger Vyntus IOS, CareFusion, Germany) according to the ATS/European Respiratory Society guidelines [36].
Biochemical and cellular markers
Blood and urine samples were taken before and 60 min after each exposure. The blood samples were analysed for CC16, cell counts and creatinine. The urine was analysed for CC16 and corrected for creatinine. To eliminate the postrenal excretion of CC16 from the prostate, the first portion of each male urine sample was discarded [37]. CC16 was analysed with Human Clara Cell Protein ELISA kits (Biovendor, Modrice, Czech Republic). Mean intra-assay coefficients of variation were <5% for all plates.
Symptoms
Two symptom questionnaires were used. One questionnaire had previously been used in experimental exposure studies on air pollutants [38]. The other questionnaire was based on interviews during cold-exposure and simultaneous intermittent easy-moderate exercise for 1 h [34]. The symptoms inquired for in the questionnaires are presented in Table 2 and 4Table 5. For both the questionnaires, the Borg CR10-scale [39] was used to rate the intensity of the symptoms from 0 to 11, where 0 represented “no symptoms” and 11 represented “maximal symptoms”. Questionnaire responses were collected at five time points: (1) outside the chamber immediately before exposure, (2) after warm-up, (3) after the first running interval, (4) after the second running interval, (5) immediately after exiting the chamber. Symptoms within exposures were compared using the scores from before the exposure and after the second running interval. To compare differences in symptom intensity between the exposures, the Borg CR10-scores from each of the five time points were added together to form a single summed score for each exposure.
Table 2.
Comparison of lung function changes within and between exposures. Data presented as mean (SD). Significant p-values in bold
| |
+10°C |
−10°C |
|
||||||
| Measurement |
Pre |
Post |
Percentage change2 |
P-value1 |
Pre |
Post |
Percentage change2 |
P-value1 |
P-value3 |
| FEV1 (L) |
4.078 (0.913) |
4.054 (0.910) |
−0.60 (1.85) |
0.077 | 4.055 (0.892) |
3.987 (0.892) |
−1.67 (2.79) |
0.002 | 0.066 |
| FVC (L) |
5.075 (1.181) |
5.111 (1.171) |
0.82 (1.92) |
0.061 | 5.141 (1.150) |
5.135 (1.145) |
−0.06 (2.02) |
0.739 | 0.114 |
| FEV1/FVC (%) |
79.500 (4.502) |
78.410 (4.794) |
−1.38 (2.23) |
0.002 | 79.070 (4.719) |
77.790 (4.931) |
−1.61 (2.08) |
<0.001 | 0.599 |
| R 5 Hz kPa/(L/s) |
0.313 (0.096) |
0.313 (0.088) |
0.79 (10.39) |
0.953 | 0.319 (0.100) |
0.331 (0.107) |
4.19 (10.08) |
0.117 | 0.243 |
| R 20 Hz kPa/(L/s) |
0.301 (0.075) |
0.307 (0.079) |
2.29 (12.65) |
0.418 | 0.305 (0.082) |
0.317 (0.085) |
4.26 (7.91) |
0.016 | 0.355 |
| X 5 Hz kPa/(L/s) |
−0.072 (0.030) |
−0.062 (0.032) |
18.67 (52.51) |
0.005 | −0.067 (0.030) |
−0.067 (0.037) |
5.12 (35.43) |
0.884 | 0.042 |
| Z 5 Hz kPa/(L/s) |
0.304 (0.089) |
0.302 (0.084) |
0.03 (9.77) |
0.712 | 0.305 (0.087) |
0.316 (0.094) |
3.45 (8.24) |
0.070 | 0.137 |
| Fres Hz |
9.882 (3.112) |
9.636 (2.668) |
−1.76 (7.66) |
0.179 | 9.791 (2.369) |
9.932 (3.122) |
1.01 (12.60) |
0.645 | 0.388 |
| Ax | 0.202 (0.233) |
0.181 (0.169) |
−7.33 (22.68) |
0.181 | 0.204 (0.159) |
0.216 (0.243) |
−1.58 (28.67) |
0.595 | 0.363 |
1Comparison of pre vs. post exposure
2Relative change ((post-pre)/pre *100)
3Comparison between exposures (difference within +10°C vs. difference within −10°C)
4FEV1, forced expiratory volume in 1 s (litres); FVC, forced vital capacity (litres); FEV1/FVC, ratio of FEV1 to FVC; R5Hz, resistance at 5 Hertz (kiloPascal/(litres/second)), R20Hz, resistance at 20 Hertz (kiloPascal/(litres/second)); X5Hz, reactance at 5 Hertz (kiloPascal/(litres/second)); Z5Hz, respiratory impedance at 5 Hertz (kiloPascal/(litres/second)); Fres, resonance frequency Hertz; Ax, reactance area
Table 3.
A description and comparison of CC16 in blood and urine within and between exposures. Data presented as median (IQR). Significant p-values in bold
| |
+10°C |
−10°C |
|
||||
| Measurement |
Pre |
Post |
P-value1 |
Pre |
Post |
P-value1 |
P-value2 |
| P-CC16 (ng/mL) |
5.69 (4.85–8.01) |
7.18 (5.28–8.76) |
<0.001 | 6.61 (4.81–7.91) |
7.14 (5.64–8.78) |
0.005 | 0.903 |
| U-CC16/ creatinine (ng/μmol creatinine) |
0.39 (0.16–0.67) |
0.43 (0.20–0.81) |
0.399 | 0.38 (0.13–0.84) |
0.47 (0.22–0.99) |
0.069 | 0.189 |
1Comparison of pre vs. post exposure
2Comparison between exposures (difference within +10°C vs. difference within −10°C)
P-CC16 (ng/mL), plasma CC16 (nanograms/millilitres); U-CC16, urinary CC16; ng/μmol, nanograms/micromol
Table 4.
Comparison of systemic cellular markers within and between exposures. Data presented as mean (SD). Significant p-values in bold
| |
+10°C |
−10°C |
|
||||
| Measurement |
Pre |
Post |
P-value1 |
Pre |
Post |
P-value1 |
P-value2 |
| Haemoglobin (g/L) |
140.6 (11.9) |
140.2 (12.2) |
0.559 | 140.4 (12.0) |
140.8 (11.7) |
0.564 | 0.439 |
| Thrombocytes (x109/L) |
273.0 (60.3) |
269.2 (59.8) |
0.196 | 269.1 (60.2) |
268.3 (65.4) |
0.775 | 0.176 |
| Leukocytes (x109/L) |
6.7 (1.5) |
8.1 (2.2) |
<0.001 | 6.7 (1.6) |
7.8 (2.3) |
<0.001 | 0.400 |
| Neutrophils (x109/L) |
3.8 (1.3) |
5.4 (2.0) |
<0.001 | 3.6 (1.3) |
5.0 (1.9) |
<0.001 | 0.532 |
| Lymphocytes (x109/L) |
2.13 (0.50) |
1.99 (0.50) |
0.043 | 2.26 (0.53) |
2.01 (0.55) |
0.001 | 0.164 |
| Monocytes (x109/L) |
0.57 (0.16) |
0.59 (0.17) |
0.340 | 0.57 (0.16) |
0.58 (0.18) |
0.689 | 0.687 |
| Eosinophils (x109/L) |
0.14 (0.09) |
0.12 (0.08) |
0.003 | 0.14 (0.09) |
0.12 (0.08) |
0.024 | 0.481 |
| Basophils (x109/L) |
0.050 (0.019) |
0.052 (0.024) |
0.405 | 0.046 (0.021) |
0.053 (0.028) |
0.011 | 0.196 |
1Comparison of pre vs. post exposure
2Comparison between exposures (difference within +10°C vs. difference within −10°C)
2g/L, grams/litres.
Table 5.
Comparison of symptom intensity between the exposures. Data presented as median (IQR). Significant p-values in bold
| Symptom |
+10°C |
−10°C |
P-value |
| Headache |
0 (0–1.6) |
0 (0–0.8) |
0.532 |
| Dizziness | 0 (0–1) | 0 (0–0.5) | 0.858 |
| Nausea | 0 (0–0) | 0 (0–0) | 0.586 |
| Physical fatigue | 6 (3–9) | 6 (1.6–8) | 0.032 |
| Breathlessness | 5 (2.8–8.2) | 3.5 (1.8–6.8) | 0.009 |
| Chest tightness | 0 (0–0) | 0 (0–0) | 0.670 |
| Cough | 0 (0–1) | 0 (0–1.4) | 0.432 |
| Eye irritation | 0.5 (0–3) | 2 (0–6.8) | 0.011 |
| Unpleasant odour | 0 (0–0) | 0 (0–0) | 1.000 |
| Nasal irritation | 0.5 (0–4.8) | 3.5 (1–7.8) | 0.001 |
| Rhinitis | 8 (5.2–10.5) | 12 (9.2–16) | <0.001 |
| Unpleasant taste | 0 (0–0) | 0 (0–0) | 0.311 |
| Irritation in the mouth or pharynx | 5.5 (1.8–8) | 5 (0.5–9.5) | 0.829 |
| Throat irritation | 1.5 (0–4.5) | 1.5 (0–4) | 0.454 |
| Cold face | 1 (0–1.5) | 7 (4–9.2) | <0.001 |
| Cold extremities | 0.5 (0–1.5) | 10 (7–12.2) | <0.001 |
| Physical discomfort | 0 (0–1.8) | 6.5 (4.1–9.4) | <0.001 |
| Feeling warm | 13 (9–17) | 7.5 (3.2–10.2) | <0.001 |
Statistics
A sample size calculation was conducted using ΔFEV1 as the primary outcome variable. We assumed a mean of FEV1 = 4.58 L, standard deviation (SD) = 0.40, and that exercise in −10°C would decrease FEV1 by 6% compared to +10°C [14,16]. We assumed equal variance and a correlation of 0.3 between exposures. With an alpha of 0.05 and a power of 0.80, 20 study subjects were needed.
Analyses were conducted using R [40]. Measurements with a judged normal distribution are presented as mean (SD), and paired t-tests were used for comparison within (post vs. pre) each exposure and between exposures (difference within +10°C vs. difference within −10°C). Measurements that were treated as non-normally distributed are presented as median (interquartile range, IQR) and analysed using Wilcoxon signed-rank test. A p-value <0.05 was considered statistically significant.
Results
A description and comparison of lung function responses within and between exposures are presented in Table 2. Even though physical exercise in −10°C significantly decreased FEV1 (p = 0.002; 95% CI 0.03–0.11 L), the decrease was not larger than that observed in +10°C. Also, the decrease in FEV1/FVC after physical exercise was of similar magnitude in both environments. R20Hz increased after physical exercise in −10°C. Exercise in +10°C increased reactance, indicated by a post-exercise decrease in X5Hz.
Exercise induced an increase in plasma CC16 of similar magnitude in both environments. No significant increases in urinary CC16 were seen following either exposure (Table 3).
No differences in systemic cellular markers were observed between exposures. In both environments, moderate-intensity exercise induced an increase in leukocytes and neutrophils, while the concentration of lymphocytes decreased. After exercise in +10°C eosinophils decreased, while in −10°C basophils increased (Table 4).
Physical exercise induced a wide range of significant increases in symptoms in both environments, as compared to baseline. Symptoms included dizziness, physical fatigue, breathlessness, nasal irritation, rhinitis, irritation in mouth or pharynx, throat irritation, cold face and feeling warm. Significant increases limited to exercise in −10°C were cough (p = 0.014), eye irritation (p = 0.004), cold extremities (p < 0.001) and physical discomfort (p < 0.001).
Exercise in −10°C induced greater summed symptom scores for eye irritation, nasal irritation, rhinitis, cold face, cold extremities and physical discomfort, compared to +10°C (Table 5). Exercise in +10°C gave higher summed symptom scores for physical fatigue, breathlessness and feeling warm, compared to −10°C.
Discussion
Our study shows that moderate-intensity exercise in −10°C induces (1) few and mild symptoms from the lower airways, (2) no lung function decrements or enhanced leakage of biochemical markers of airway epithelial injury, compared to +10°C, and (3) no peripheral bronchodilatation, which significantly increased after exercise in +10°C.
We found that physical exercise in −10°C induces an acute, mostly proximal, airway obstruction in healthy individuals, measured by FEV1, FEV1/FVC and R20Hz. Although in FEV1 the responses were small with a 1.7% decrease and below the clinical threshold of 100 millilitres. Our sample size calculation was based on previous studies [14,16] and an assumption of a 6% decrease in FEV1 in −10°C, compared to +10°C. Thus, our sample size was probably too small to detect diminutive differences between the exposures. However, our findings are in agreement with previous research. Kennedy and Faulhaber [16] detected a reduction in FEV1 in physically active females after intense exercise at different temperatures from 0°C to −20° (40% relative humidity in all environments), but the reductions were not significant when comparing the temperatures. Another study of 12 healthy individuals, who performed moderate-intensity treadmill exercise in +20°C and −11°C, could not either demonstrate any significant differences in FEV1 between temperatures [41]. On the contrary, Therminarias et al. [14] showed that exhaustive exercise in well-trained males in +22°C and −10°C induced a greater decrease in FEV1 in the subzero environment. A possible explanation for the discrepancy between our findings and previous observations could be the exercise intensity. A high minute ventilation is one of the main factors in triggering respiratory symptoms in cold air, and elevated levels of ventilation can lead to bronchoconstriction [7]. Taken together, moderate-intensity exercise in −10°C does not appear to induce significant airway obstruction in healthy individuals as compared to similar exercise in warmer conditions.
Inclusion of impulse oscillometry measurements allowed us to identify a significant increase in reactance after exercise in +10°C, indicating a peripheral bronchodilation. This was significant also when compared to −10°C. Reactance (X) is determined by the elastic properties of the peripheral lung and the inertia of the air to flow through the airways. X at 5 Hz (X5Hz) is a measurement of reactance at a low frequency and can be used to detect changes in the peripheral lung. Circulating adrenaline levels increase during physical activity [42], leading to bronchodilation by binding to β2-adrenoreceptors in the airways [43]. Our finding suggests that cold air inhibits this physiological response to physical exercise.
This study showed an increase of CC16 plasma levels after moderate exercise, with no difference between the warmer and the cold environment. To our knowledge, this is the first study that has measured CC16 after exercise in a subzero temperature. Epithelial stress increases the permeability over the bronchoalveolar-blood barrier, resulting in elevated levels of CC16 in serum [44]. CC16 is thought to play a role as a protective mediator in the airway inflammatory response [22]. It has been suggested that this response after physical exercise is physiological, and not pathogenic, since no difference in plasma CC16 was seen after an exercise challenge test in mild asthmatics and healthy controls [45]. A probable explanation for the increased leakage of CC16 into plasma is the elevated minute ventilation, dehydrating the airway epithelium [45]. Neither the colder nor the drier air in the −10°C environment appeared to induce more epithelial stress than exercise in +10°C; this is in line with our spirometric findings.
Differential cell counts were included in this study to account for systemic signs of inflammation following exercise and cold exposure. A previous exposure-study has shown that cold air causes local inflammation of the airways [12], but it is not known whether this may translate to a difference in exercise-induced systemic immune responses following exercise in cold air. In the present study, as expected, leukocytes and neutrophils significantly increased after moderate-intensity exercise in both environments. This immune response to physical exercise is well known and is probably caused by an increase in plasma catecholamines [33]. Most studies that have set out to investigate immunological effects of cold environments have used temperatures above 0°C [33], which are well above those experienced by winter athletes and the general population in the Nordic countries and similar climatic regions during winter. Our results suggest that exercise-induced increases in circulating leukocytes are not altered by exposure to −10°C compared to +10°C. Had we chosen a larger temperature range between the warm and the cold environments, we may have seen more pronounced differences between conditions, as cold-induced immunological effects could also occur at +10°C [33]. Moreover, future studies designed with the specific purpose of investigating immune responses to exercise in subzero air may wish to incorporate further measures of functional immunity; however, this was not the main purpose of the present study.
The wide range of symptoms, and the increase in symptom-intensity, induced by exercise in −10°C, indicates that our experimental whole-body exposure set-up mimics real-world exposure to cold environments. Moderate-intensity exercise for 35 minutes in −10°C induced few and mild symptoms from the lower respiratory tract, such as breathlessness and cough. The absence of lower airway symptoms in −10°C corresponds well with the absence of a pronounced airway obstruction and the absence of severe epithelial stress. This is also in agreement with previous research, showing that individual predisposition and a high minute ventilation are the two main factors in triggering cold-induced respiratory symptoms [7].
Strengths of this study are the comprehensive range of sensitive measurements, use of whole-body exposure to different temperatures in an environmental chamber with a stable milieu, and exposures that simulate real-world environmental conditions.
The choice of exposing the subjects to +10°C and −10°C had several reasons. Firstly, the exposures should simulate common circumpolar conditions, although occurring temporarily. In the circumpolar regions, +10°C occurs during spring and autumn, and several circumpolar cities, such as Murmansk and Vorkuta (Russia), Fairbanks (USA), Tromsoe (Norway), Kiruna (Sweden) and Rovaniemi (Finland), have mean temperatures around +10°C during summer [46]. Secondly, in order to function as a neutral reference, the “control exposure” should not put excessive strain on the study subjects. Hence, +10°C has been shown to be optimal for preventing thermoregulatory stress, and close to an “optimal” temperature for endurance exercise performance [47]. Most experimental exposure studies evaluating lung function responses to subzero air have used +20°C as control exposure [48]. Pekkarinen et al. did not detect any lung function impairments in healthy males exposed to room temperature and 0°C [17]. We believed that neither room temperature nor +10°C as control exposures would induce significant lung function decrements. However, +10°C is a rather low temperature when considering the conditions in which humans have evolved. Thus, we cannot totally exclude that reactions to cold air might already have started at +10°C, making the difference between “warm” and “cold” exposures less pronounced. Also, relative and absolute humidity differed clearly between the two environments, the air being drier in the cold environment. This makes it difficult to separate whether the etiologic stressor to the responses observed in the present study is cold air, dry air or a combination of both. Low air humidity has an impact on both obstruction and leakage of epithelial injury markers in the airways [10,26].
Experimental whole-body exposure studies simulating real-world exposures incorporate multiple stressors, such as exercise, hyperventilation, inhalation of cold air and hypothermia. Isolating the stressor and outcome does not take into account the complexity of environmental exposures or the extensive responses that may occur. Mapping multiple domains of responses run the risk of providing us with more questions than answers, instead of evidence for safe versus hazardous thresholds for physical exercise in subzero temperatures. Nevertheless, we suggest that short-duration moderate-intensity exercise in −10°C does not induce any acute harm to the lower airways of healthy subjects compared to similar exercise in +10°C, except a lack of bronchodilatation. This novel finding needs to be confirmed in future studies. Before we have developed a deeper knowledge of the meaning of bronchodilatation in this setting, we cannot completely rule out that moderate-intensity exercise in subzero temperatures can be harmful to the airways of vulnerable individuals, or when performed on a regular basis over a season or over many years. The nature of responses occurring among subjects with pre-existing respiratory disease remains to be clarified.
Acknowledgments
The authors would like to thank all the study participants; Anna Eriksson and Agneta Lindberg, research nurses at KFC Östersund, for collection and handling of blood and urine samples; Markus Inderdal, Johan Karlsson and Johanna Oskarsson for their assistance with delivering the exercise tests.
Funding Statement
This study was supported by grants from: Arcum; Gunhild och Assar Karlsson donationsfond; Region Jämtland-Härjedalen; Syskonen Perssons donationsfond; Visare Norr.
Abbreviations
| Abs H | absolute humidity |
| Ax | reactance area |
| CC16 | club cell protein 16 |
| CI | confidence interval |
| FEV1 | forced expiratory volume in one second |
| Fres | resonance frequency |
| FVC | forced vital capacity |
| IOS | impulse oscillometry |
| IQR | interquartile range |
| kPa | kilopascal |
| n | number |
| R | resistance |
| R1 | running interval 1 |
| R2 | running interval 2 |
| rh | relative humidity |
| SD | standard deviation |
| T | temperature |
| VO2max | maximum rate of oxygen consumption |
| vs | versus |
| WU | warm-up |
| X | reactance |
| Z | respiratory impedance |
Disclosure of interest
The authors report no conflict of interest.
Prior abstract publication/presentation
A poster with parts of the results was presented at the European Respiratory Society (ERS) Congress (September 15-19, 2018), Paris, France. A corresponding abstract was published in a supplement of the European Respiratory Journal September 2018 edition:
Eklund LM, Schagatay F, Sjöström R, et al. Symptoms of moderate exercise in subzero temperatures: An experimental exposure study. Eur Respir J. 2018;52: Suppl. 62, PA4514.
References
- [1].Group TE . Cold exposure and winter mortality from ischaemic heart disease, cerebrovascular disease, respiratory disease, and all causes in warm and cold regions of Europe. Lancet. 1997;349(9062):1341–9. [PubMed] [Google Scholar]
- [2].Schwartz J. Who is sensitive to extremes of temperature?: a case-only analysis. Epidemiology. 2005;16(1):67–72. [DOI] [PubMed] [Google Scholar]
- [3].Analitis A, Katsouyanni K, Biggeri A, et al. Effects of cold weather on mortality: results from 15 European cities within the PHEWE project. Am J Epidemiol. 2008;168(12):1397–1408. [DOI] [PubMed] [Google Scholar]
- [4].Nayha S, Hassi J, Jousilahti P, et al. Cold-related symptoms among the healthy and sick of the general population: National FINRISK study data. Public Health. 2011;125(6):380–388. [DOI] [PubMed] [Google Scholar]
- [5].Rasi H, Kuivila H, Polkki T, et al. A descriptive quantitative study of 7- and 8-year-old children’s outdoor recreation, cold exposure and symptoms in winter in Northern Finland. Int J Circumpolar Health. 2017;76(1):1298883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Carlsen KH, Anderson SD, Bjermer L, et al. Exercise-induced asthma, respiratory and allergic disorders in elite athletes: epidemiology, mechanisms and diagnosis: part I of the report from the Joint Task Force of the European Respiratory Society (ERS) and the European Academy of Allergy and Clinical Immunology (EAACI) in cooperation with GA2LEN. Allergy. 2008;63(4):387–403. [DOI] [PubMed] [Google Scholar]
- [7].Koskela HO. Cold air-provoked respiratory symptoms: the mechanisms and management. Int J Circumpolar Health. 2007;66(2):91–100. [DOI] [PubMed] [Google Scholar]
- [8].Carlsen KH. Sports in extreme conditions: the impact of exercise in cold temperatures on asthma and bronchial hyper-responsiveness in athletes. Br J Sports Med. 2012;46(11):796–799. [DOI] [PubMed] [Google Scholar]
- [9].Kippelen P, Anderson SD. Airway injury during high-level exercise. Br J Sports Med. 2012;46(6):385–390. [DOI] [PubMed] [Google Scholar]
- [10].Anderson SD, Daviskas E. The mechanism of exercise-induced asthma is. J Allergy Clin Immunol. 2000;106(3):453–459. [DOI] [PubMed] [Google Scholar]
- [11].Anderson SD, Kippelen P. Exercise-induced bronchoconstriction: pathogenesis. Curr Allergy Asthma Rep. 2005;5(2):116–122. [DOI] [PubMed] [Google Scholar]
- [12].Larsson K, Tornling G, Gavhed D, et al. Inhalation of cold air increases the number of inflammatory cells in the lungs in healthy subjects. Eur Respir J. 1998;12(4):825–830. [DOI] [PubMed] [Google Scholar]
- [13].Koskela H, Tukiainen H. Facial cooling, but not nasal breathing of cold air, induces bronchoconstriction: a study in asthmatic and healthy subjects. Eur Respir J. 1995;8(12):2088–2093. [DOI] [PubMed] [Google Scholar]
- [14].Therminarias A, Oddou MF, Favre-Juvin A, et al. Bronchial obstruction and exhaled nitric oxide response during exercise in cold air. Eur Respir J. 1998;12(5):1040–1045. [DOI] [PubMed] [Google Scholar]
- [15].Mohammadizadeh MA, Ghanbarzadeh M, Habibi A, et al. The effect of high intensity interval exercise in high/low temperatures on exercise-induced bronchoconstriction (EIB) in trained adolescent males. Tanaffos. 2013;12(3):29–43. [PMC free article] [PubMed] [Google Scholar]
- [16].Kennedy MD, Faulhaber M. Respiratory function and symptoms post cold air exercise in female high and low ventilation sport athletes. Allergy Asthma Immunol Res. 2018;10(1):43–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Pekkarinen H, Tukiainen H, Litmanen H, et al. Effect of submaximal exercise at low temperatures on pulmonary function in healthy young men. Eur J Appl Physiol Occup Physiol. 1989;58(8):821–825. [DOI] [PubMed] [Google Scholar]
- [18].Kaminsky DA, Irvin CG, Gurka DA, et al. Peripheral airways responsiveness to cool, dry air in normal and asthmatic individuals. Am J Respir Crit Care Med. 1995;152(6 Pt 1):1784–1790. [DOI] [PubMed] [Google Scholar]
- [19].Koskela H, Tukiainen H, Kononoff A, et al. Effect of whole-body exposure to cold and wind on lung function in asthmatic patients. Chest. 1994;105(6):1728–1731. [DOI] [PubMed] [Google Scholar]
- [20].Ahmed T, Danta I. Effect of cold air exposure and exercise on nonspecific bronchial reactivity. Chest. 1988;93(6):1132–1136. [DOI] [PubMed] [Google Scholar]
- [21].Seys SF, Daenen M, Dilissen E, et al. Effects of high altitude and cold air exposure on airway inflammation in patients with asthma. Thorax. 2013;68(10):906–913. [DOI] [PubMed] [Google Scholar]
- [22].Broeckaert F, Bernard A. Clara cell secretory protein (CC16): characteristics and perspectives as lung peripheral biomarker. Clin Exp Allergy. 2000;30(4):469–475. [DOI] [PubMed] [Google Scholar]
- [23].Lakind JS, Holgate ST, Ownby DR, et al. A critical review of the use of Clara cell secretory protein (CC16) as a biomarker of acute or chronic pulmonary effects. Biomarkers. 2007;12(5):445–467. [DOI] [PubMed] [Google Scholar]
- [24].Chimenti L, Morici G, Paterno A, et al. Bronchial epithelial damage after a half-marathon in nonasthmatic amateur runners. Am J Physiol Lung Cell Mol Physiol. 2010;298(6):L857–862. [DOI] [PubMed] [Google Scholar]
- [25].Bolger C, Tufvesson E, Sue-Chu M, et al. Hyperpnea-induced bronchoconstriction and urinary CC16 levels in athletes. Med Sci Sports Exerc. 2011;43(7):1207–1213. [DOI] [PubMed] [Google Scholar]
- [26].Bolger C, Tufvesson E, Anderson SD, et al. Effect of inspired air conditions on exercise-induced bronchoconstriction and urinary CC16 levels in athletes. J Appl Physiol (1985). 2011;111(4):1059–1065. [DOI] [PubMed] [Google Scholar]
- [27].Sue-Chu M, Larsson L, Moen T, et al. Bronchoscopy and bronchoalveolar lavage findings in crosscountry skiers with and without “ski asthma”. Eur Respir J. 1999;13:626–632. [DOI] [PubMed] [Google Scholar]
- [28].Sue-Chu M, Karjalainen E-M, Altraja A, et al. Lymphoid aggregates in endobronchial biopsies from young elite cross-country skiers. Am J Respir Crit Care Med. 1998;158:597–601. [DOI] [PubMed] [Google Scholar]
- [29].Karjalainen E-M, Laitinen A, Sue-Chu M, et al. Evidence of airway inflammation and remodeling in ski athletes with and without bronchial hyperresponsiveness to methacholine. Am J Respir Crit Care Med. 2000;161:2086–2091. [DOI] [PubMed] [Google Scholar]
- [30].Castellani JW, Brenner IKM, Rhind SG. Cold exposure: human immune responses and intracellular cytokine expression. Med Sci Sports Exerc. 2002;34(12):2013–2020. [DOI] [PubMed] [Google Scholar]
- [31].Diment BC, Fortes MB, Edwards JP, et al. Exercise intensity and duration effects on in vivo immunity. Med Sci Sports Exerc. 2015;47(7):1390–1398. [DOI] [PubMed] [Google Scholar]
- [32].Campbell JP, Turner JE. Debunking the myth of exercise-induced immune suppression: redefining the impact of exercise on immunological health across the lifespan. Front Immunol. 2018;9:648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].LaVoy EC, McFarlin BK, Simpson RJ. Immune responses to exercising in a cold environment. Wilderness Environ Med. 2011;22(4):343–351. [DOI] [PubMed] [Google Scholar]
- [34].Sjostrom R, Soderstrom L, Klockmo C, et al. Qualitative identification and characterisation of self-reported symptoms arising in humans during experimental exposure to cold air. Int J Circumpolar Health. 2019;78(1):1583528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Smith HJ, Reinhold P, Goldman MD. Forced oscillation technique and impulse oscillometry. Eur Respir Mon. 2005;31:72–105. [Google Scholar]
- [36].Miller MR, Hankinson J, Brusasco V, et al. Standardisation of spirometry. Eur Respir J. 2005;26(2):319–338. [DOI] [PubMed] [Google Scholar]
- [37].Andersson L, Lundberg PA, Barregard L. Methodological aspects on measurement of Clara cell protein in urine as a biomarker for airway toxicity, compared with serum levels. J Appl Toxicol. 2007;27(1):60–66. [DOI] [PubMed] [Google Scholar]
- [38].Rudell B, Ledin MC, Hammarström U, et al. Effects on symptoms and lung function in humans experimentally exposed to diesel exhaust. Occup Environ Med. 1996;53(10):658–662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Borg E, Kaijser L. A comparison between three rating scales for perceived exertion and two different work tests. Scand J Med Sci Sports. 2006;16(1):57–69. [DOI] [PubMed] [Google Scholar]
- [40].R: A language and environment for statistical computing [computer program] . Vienna, Austria: R Foundation for Statistical Computing; 2018. [Google Scholar]
- [41].Chapman KR, Allen LJ, Romet TT. Pulmonary function in normal subjects following exercise at cold ambient temperatures. Eur J Appl Physiol Occup Physiol. 1990;60(3):228–232. [DOI] [PubMed] [Google Scholar]
- [42].Warren JB, Dalton N. A comparison of the bronchodilator and vasopressor effects of exercise levels of adrenaline in man. Clin Sci (Lond). 1983;64(5):475–479. [DOI] [PubMed] [Google Scholar]
- [43].Butchers PR, Skidmore IF, Vardey CJ, et al. Characterization of the receptor mediating the antianaphylactic effects of beta-adrenoceptor agonists in human lung tissue in vitro. Br J Pharmacol. 1980;71(2):663–667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Hermans C, Knoops B, Wiedig M, et al. Clara cell protein as a marker of Clara cell damage and bronchoalveolar blood barrier permeability. Eur Respir J. 1999;13(5):1014–1021. [DOI] [PubMed] [Google Scholar]
- [45].Tufvesson E, Svensson H, Ankerst J, et al. Increase of club cell (Clara) protein (CC16) in plasma and urine after exercise challenge in asthmatics and healthy controls, and correlations to exhaled breath temperature and exhaled nitric oxide. Respir Med. 2013;107(11):1675–1681. [DOI] [PubMed] [Google Scholar]
- [46].Hoare R. WorldClimate. 1996-2012; http://www.worldclimate.com.
- [47].Galloway SD, Maughan RJ. Effects of ambient temperature on the capacity to perform prolonged cycle exercise in man. Med Sci Sports Exerc. 1997;29(9):1240–1249. [DOI] [PubMed] [Google Scholar]
- [48].Hanstock HG, Ainegren M, Stenfors N. Exercise in sub-zero temperatures and airway health: implications for athletes with special focus on heat-and-moisture-exchanging breathing devices. Front Sports Act Living. 2020;2:34. [DOI] [PMC free article] [PubMed] [Google Scholar]


