Abstract
Background
Mucosal immunity plays an important role in the pathogenesis of IgA nephropathy (IgAN). This study aimed to investigate if infection of Helicobacter pylori (H. pylori), a common bacteria in the gastrointestinal tract, associated with IgAN.
Methods
This study included 261 patients with IgAN and 46 healthy controls. Clinical information and plasma samples were collected from patients and healthy controls. H. pylori infection was confirmed by western blot. Plasma IgA1 and galactose-deficient IgA1 (Gd-IgA1) levels were detected by specific enzyme-linked immunosorbent assay.
Results
Total H. pylori infection rates showed no statistical differences between IgAN patients and healthy controls, but the infection rates of type I H. pylori in IgAN patients were significantly higher than those in healthy controls (44.4 vs. 28.3%, p = 0.040). Compared with uninfected patients, the systolic blood pressure, 24-h proteinuria, and blood urea nitrogen levels were significantly higher in patients with H. pylori infection (126.0 ± 15.5 vs. 119.6 ± 14.5 mmHg, p = 0.010; 1.8 ± 2.7 vs. 1.2 ± 1.4 g/24h, p = 0.013; 7.9 ± 5.4 vs. 6.7 ± 3.9 μmol/L, p = 0.042), especially in patients with type I infection (126.5 ± 15.4 vs. 119.6 ± 14.5 mmHg, p = 0.002; 1.9 ± 2.9 vs. 1.2 ± 1.4 g/24 h, p = 0.033; 8.1 ± 5.6 vs. 6.7 ± 3.9 μmol/L, p = 0.041). Similarly, patients with IgAN and type I H. pylori infection showed higher plasma Gd-IgA1 levels than uninfected patients (5.5 ± 2.2 vs. 4.5 ± 2.2 μg/mL, p = 0.037).
Conclusions
Virulent type I H. pylori infection is more common in patients with IgAN. Patients with IgAN and type I H. pylori infection showed lower renal function and higher underglycosylation of plasma IgA1.
Keywords: Immunoglobulin A nephropathy, Helicobacter pylori, cytotoxin-associated gene A/vacuolating cytotoxin A, galactose-deficient IgA1
Introduction
Immunoglobulin A nephropathy (IgAN), the most common cause of glomerulonephritis worldwide, is characterized by the predominant or codominant deposition of IgA in the glomerular mesangium [1]. Increasing evidences suggested that mucosal immunity played an important role in the pathogenesis of IgAN [2,3], and Helicobacter pylori (H. pylori) infection might be the most common factor [4,5]; however, the associations between H. pylori infection and clinical manifestations of IgAN and its possible mechanism have not been elucidated yet.
H. pylori, a gram-negative bacterium, colonizes the human gastric mucus layer. Recently, considerable studies changed the paradigm that H. pylori only participates in the pathogenesis of chronic gastritis and peptic ulcer disease, and demonstrated that several extra-intestinal diseases, such as renal-related diseases, were also caused by H. pylori [6–8]. The systemic antibody response to H. pylori was reported to be increased in patients with IgAN [9], suggesting that H. pylori might be involved in the pathogenesis and progression of IgAN. Cytotoxin-associated gene A (CagA) and vacuolating cytotoxin A (VacA) are the main virulence factors of H. pylori [10]. CagA has been observed in the tonsils of most H. pylori infected IgAN patients [11], and it was reported to promote glomerular mesangial cell proliferation and extracellular matrix secretion via suppressing the apoptotic signaling pathway [12]. VacA was found to play some roles in vacuolation, apoptosis, antigen presentation, and multiple cellular activities [13,14]. However, the association of the virulent H. pylori strains and IgAN clinical manifestations and possible mechanism remained to be investigated.
In this study, we investigated the infection rates of different H. pylori types, particularly virulent strains, and the correlation between H. pylori infection and clinical manifestations in patients with IgAN. Moreover, the levels of plasma IgA1 and galactose-deficient IgA1 (Gd-IgA1) in patients with different H. pylori type infection were detected to explore the possible mechanism.
Materials and methods
Study population
A total of 261 patients with IgAN (mean age: 37.7 ± 12.3 years, male ratio: 51.7%) were enrolled in this study from Peking University First Hospital. Forty-six age- and sex-matched healthy individuals (mean age: 38.0 ± 12.2 years, male ratio: 47.8%) were recruited as healthy controls. The patients with IgAN were diagnosed by renal biopsy and confirmed by the deposition of IgA in the glomerular mesangium using immunofluorescence and ultrastructural examination. Patients with secondary causes of IgAN, such as IgA vasculitis, systemic lupus erythematosus, or liver cirrhosis were excluded.
Plasma samples were collected from patients (at renal biopsy) and healthy controls and stored at −80 °C for further use. Baseline demographic and clinical data were collected at the time of renal biopsy, including age, sex, systolic blood pressure (SBP), diastolic blood pressure (DBP), gross/microscopic hematuria, serum creatinine (Scr), serum IgA/IgG/IgM, serum complement 3 (C3), 24-h proteinuria, blood urea nitrogen (BUN), treatment regimes, and histological characteristics. Estimated glomerular filtration rate (eGFR) was calculated using the Chronic Kidney Disease Epidemiology Collaboration equation [15]. Twenty-four hour creatinine clearance (CrCl) was done by the Cockcroft–Gault formula [16]. The histological characteristics were scored according to the Oxford classification [17].
Detection of H. pylori infection
Serum H. pylori-IgG antibodies (CagA, VacA, UreA, and UreB) were detected in all participants using the Typing Detection Kit (Blot Biotech, Shenzhen, China), according to the manufacturer’s protocol. Seropositivity for UreA and/or UreB IgG confirmed H. pylori infection. Type I H. pylori infection was diagnosed if seropositivity for CagA and/or VacA IgG, otherwise, the type II H. pylori infection was diagnosed. The diagnosis of H. pylori infection is summarized in Supplementary Table 1.
Detection of plasma IgA1 and galactose-deficient IgA1
The levels of plasma IgA1 in patients with IgAN were detected by enzyme-linked immunosorbent assay (ELISA), according to a previously described protocol [18]. Briefly, 96-well plates were coated with F(ab’)2 fragments of goat IgG anti-human IgA overnight at 4 °C, followed by blocking with 1% bovine serum albumin for 1 h at 37 °C. Next, plasma samples (1:80,000 dilution) and standard samples were added to the 96-well plates and incubated for 1 h at 37 °C, followed by treatment with horseradish peroxidase-conjugated monoclonal anti-human IgA1 antibodies and tetramethylbenzidine liquid substrate. The absorbance was then measured at 450/570 nm with a microplate reader (Bio-Rad, Japan). Plasma Gd-IgA1 levels were detected using the KM55 ELISA kit (IBL, Japan) [19,20]. Briefly, the ELISA plates were incubated with plasma samples (1:400 dilution in EIA buffer) and standard samples for 1 h at 37 °C, washed four times with wash buffer, incubated with prepared-labeled antibodies, and then treated with 50 µL TMA solution for 30 min in the dark. The absorbance was measured at 450/630 nm by an ELISA reader (Bio-Rad, Japan). The reference range of IgA1 and Gd-IgA1 levels was calculated according to their respective standard curves generated from parallel working standards.
Statistical analysis
Continuous variables with normal distribution were expressed as mean ± standard deviation (SD) and non-normal variables were presented as median and interquartile range (IQR). Categorical variables were reported as absolute frequencies and percentages. For comparison of normally distributed continuous variables, the independent samples t-test was used. For analysis of non-normally distributed data, Mann–Whitney U test or Kruskal–Wallis test was used. The chi-squared test was performed for comparison of categorical variables. Pearson’s correlation and linear regression analyses were used to determine the association between two continuous variables. All results were analyzed by SPSS version 22.0 (SPSS Inc., Chicago, USA) and expressed as hazard ratios with 95% confidence intervals. A two-tailed P value less than 0.05 was considered statistically significant.
Results
The baseline characteristics of patients with IgAN
Two hundred and sixty-one patients with IgAN were enrolled in this study. The baseline characteristics, including age, sex, SBP, DBP, gross/microscopic hematuria, Scr, serum IgA/IgG/IgM, serum C3, 24-h proteinuria, eGFR, CrCl, BUN, treatment regimes, and histological characteristics, were demonstrated in Supplementary Table 2.
H. pylori infection rates
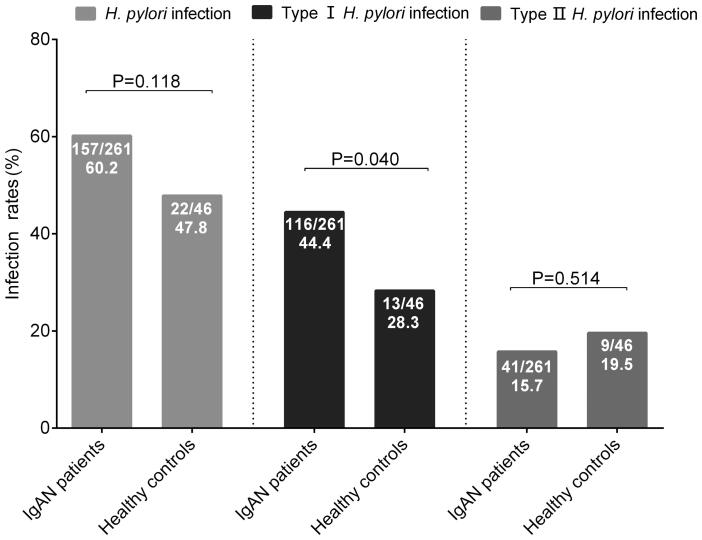
H. pylori infection rates in patients with IgAN tended to be higher than those in healthy controls (157/261, 60.2% vs. 22/46, 47.8%; p = 0.118). Subgroup analyses showed that there were significant higher type I H. pylori infection rates in patients with IgAN, as compared with healthy controls (116/261, 44.4% vs. 13/46, 28.3%; p = 0.040). While, type II H. pylori infection rates were comparable between IgAN patients and healthy controls (41/261, 15.7% vs. 9/46, 19.5%, p = 0.514) (Figure 1).
Figure 1.
The Helicobacter pylori infection rates in IgA nephropathy patients and healthy controls. IgAN: IgA nephropathy; H. pylori: Helicobacter pylori.
Association between H. pylori infection and clinical manifestations
Compared with uninfected IgAN patients, patients with H. pylori infection showed significantly higher SBP (126.0 ± 15.5 vs. 119.6 ± 14.5 mmHg; p = 0.010), 24-h proteinuria (1.8 ± 2.7 vs. 1.2 ± 1.4 g/24h; p = 0.013), and BUN (7.9 ± 5.4 vs. 6.7 ± 3.9 μmol/L, p = 0.042) levels. Further subgroup analyses showed that the above indicators were significantly higher in IgAN patients with type I H. pylori infection (126.5 ± 15.4 vs. 119.6 ± 14.5 mmHg, p = 0.002; 1.9 ± 2.9 vs. 1.2 ± 1.4 g/24h, p = 0.033; 8.1 ± 5.6 vs. 6.7 ± 3.9 μmol/L, p = 0.041), but not in IgAN patients with type II H. pylori infection (124.5 ± 16.0 vs. 119.6 ± 14.5 mmHg, p = 0.081; 1.8 ± 2.0 vs. 1.2 ± 1.4 g/24h, p = 0.088; 7.7 ± 4.7 vs. 6.7 ± 3.9 μmol/L, p = 0.211). And no significant differences were observed in age, sex, SBP, DBP, gross/microscopic hematuria, Scr, serum IgA/IgG/IgM, serum C3, 24-h proteinuria, eGFR, CrCl, BUN, treatment regimes, and histological characteristics between patients with type I infection and patients with type II infection (Table 1).
Table 1.
Association of H. pylori infection and clinical manifestation in patients with IgA nephropathy.
| Uninfected (n = 104) | H. pylori infection (n = 157) | Type I H. pylori infection (n = 116) | Type II H. pylori infection (n = 41) | pa value | pb value | pc value | pd value | |
|---|---|---|---|---|---|---|---|---|
| Age, years | 37.1 ± 11.9 | 38.1 ± 12.5 | 39.0 ± 13.4 | 35.6 ± 9.3 | 0.500 | 0.258 | 0.473 | 0.075 |
| Sex, male, n (%) | 50 (48.1) | 85 (54.1) | 61 (52.6) | 24 (58.5) | 0.337 | 0.504 | 0.257 | 0.511 |
| SBP, mmHg | 119.6 ± 14.5 | 126.0 ± 15.5 | 126.5 ± 15.4 | 124.5 ± 16.0 | 0.010 | 0.002 | 0.081 | 0.101 |
| DBP, mmHg | 75.1 ± 11.3 | 77.3 ± 10.5 | 77.2 ± 9.8 | 77.5 ± 12.4 | 0.109 | 0.138 | 0.257 | 0.593 |
| Hypertension, n (%) | 42 (40.4) | 73 (46.5) | 59 (50.9) | 14 (34.1) | 0.330 | 0.119 | 0.487 | 0.065 |
| Gross hematuria, n (%) | 26 (25.0) | 37 (23.6) | 28 (24.1) | 9 (22.0) | 0.791 | 0.882 | 0.699 | 0.777 |
| Microscopic hematuria, n (%) | 87 (83.7) | 137 (87.3) | 100 (86.2) | 37 (90.2) | 0.246 | 0.399 | 0.221 | 0.505 |
| Serum IgG, g/L | 10.8 ± 3.2 | 10.9 ± 3.1 | 11.0 ± 3.3 | 10.1 ± 2.8 | 0.916 | 0.909 | 0.248 | 0.397 |
| Serum IgA, g/L | 3.3 ± 1.2 | 3.4 ± 1.1 | 3.3 ± 1.1 | 3.4 ± 1.2 | 0.510 | 0.674 | 0.930 | 0.989 |
| Serum IgM, g/L | 1.3 ± 0.7 | 1.4 ± 3.3 | 1.5 ± 3.8 | 1.1 ± 0.6 | 0.671 | 0.464 | 0.231 | 0.550 |
| Serum C3, g/L | 0.9 ± 0.2 | 0.9 ± 0.2 | 0.9 ± 0.2 | 0.9 ± 0.2 | 0.672 | 0.736 | 0.800 | 0.879 |
| Proteinuria, g/24 h | 1.2 ± 1.4 | 1.8 ± 2.7 | 1.9 ± 2.9 | 1.8 ± 2.0 | 0.013 | 0.033 | 0.088 | 0.592 |
| Scr, μmol/L | 126.4 ± 116.0 | 149.8 ± 144.4 | 148.5 ± 128.9 | 153.5 ± 183.0 | 0.171 | 0.187 | 0.292 | 0.851 |
| eGFR, mL/min/1.73m2 | 77.8 ± 36.7 | 72.3 ± 37.0 | 71.8 ± 37.8 | 73.7 ± 34.9 | 0.240 | 0.236 | 0.546 | 0.803 |
| CrCl, mL/min | 82.8 ± 43.4 | 77.1 ± 40.4 | 76.9 ± 42.4 | 77.4 ± 34.9 | 0.284 | 0.322 | 0.477 | 0.952 |
| BUN, μmol/L | 6.7 ± 3.9 | 7.9 ± 5.4 | 8.1 ± 5.6 | 7.7 ± 4.7 | 0.042 | 0.041 | 0.211 | 0.687 |
| Treatment regimes, n (%) | ||||||||
| ACE inhibitors or ARBs | 100 (96.2) | 153 (97.5) | 114 (98.3) | 39 (95.1) | 0.551 | 0.335 | 0.779 | 0.271 |
| Immunosuppressive agents | 34 (32.7) | 48 (30.6) | 36 (31.3) | 12 (29.3) | 0.718 | 0.792 | 0.690 | 0.833 |
| Prednisone | 39 (37.5) | 52 (33.1) | 42 (36.6) | 10 (24.4) | 0.467 | 0.843 | 0.133 | 0.167 |
| Oxford Score, n (%) | ||||||||
| M0/1 | 30/74 (28.8/71.2) | 53/104 (33.8/66.2) | 41/75 (35.3/64.7) | 12/29 (29.3/70.7) | 0.404 | 0.303 | 0.960 | 0.479 |
| E0/1 | 66/38 (63.5/36.5) | 104/53 (66.2/33.8) | 75/41 (64.7/35.3) | 29/12 (70.7/29.3) | 0.644 | 0.854 | 0.407 | 0.479 |
| S0/1 | 31/73 (29.8/70.2) | 49/108 (31.2/68.8) | 40/76 (34.5/65.5) | 9/32 (22.0/78.0) | 0.810 | 0.459 | 0.340 | 0.132 |
| T0/1/2 | 54/41/9 (49.0/39.4/11.6) | 72/72/13 (45.9/45.9/8.2) | 50/55/11 (43.1/47.4/9.5) | 22/17/2 (53.7/41.5/4.8) | 0.581 | 0.418 | 0.741 | 0.418 |
| C0/1/2 | 86/13/5 (82.7/12.5/4.8) | 130/20/7 (82.8/12.7/4.5) | 96/16/7 (80.2/13.8/6.0) | 37/4/0 (90.2/9.8/0.0) | 0.990 | 0.876 | 0.308 | 0.214 |
ACE: angiotensin-converting enzyme; ARB: angiotensin II receptor blocker; BUN: blood urea nitrogen; C: crescents; CrCl: 24-h creatinine clearance; DBP: diastolic blood pressure; E: Endocapillary proliferation; eGFR: estimated glomerular filtration rate; SBP: systolic blood pressure; Scr: serum creatinine; M: mesangial hypercellularity; S: segmental sclerosis; T: interstitial fibrosis and tubular atrophy.
pa value: H. pylori infection group versus uninfected group; pb value: type I H. pylori infection group versus uninfected group; pc value: type II H. pylori infection group versus uninfected group; pd value: type I H. pylori infection group versus type II H. pylori infection group.
Plasma IgA1 and galactose-deficient IgA1 levels
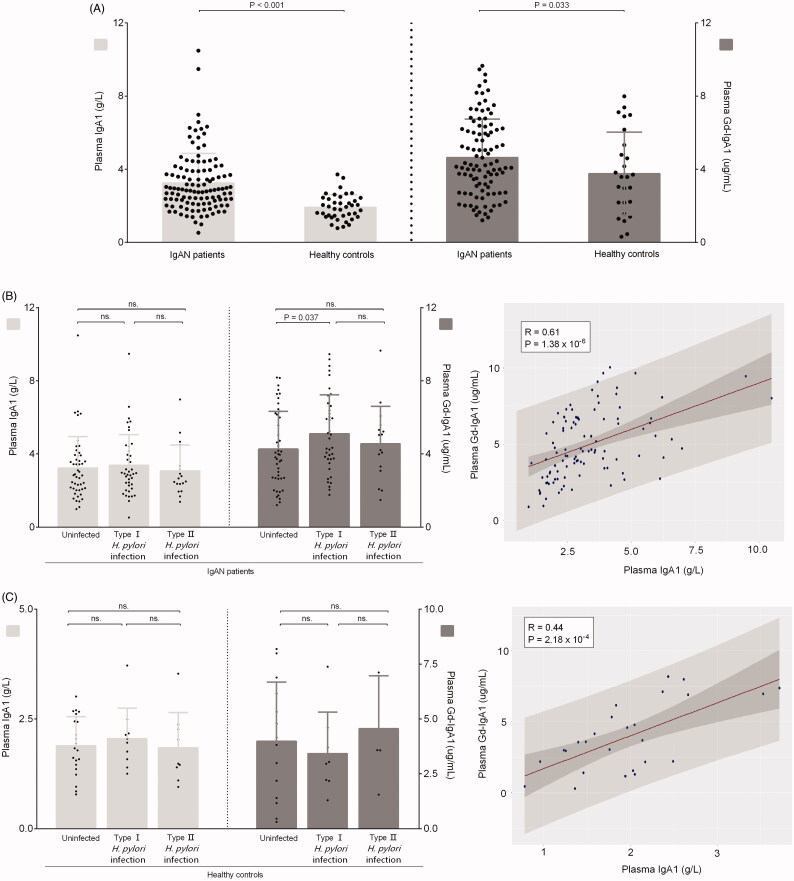
Plasma IgA1 and Gd-IgA1 levels were both significantly higher in patients with IgAN than those in healthy controls (3.3 ± 1.6 vs. 2.1 ± 1.0 g/L; p < 0.001; 5.0 ± 2.2 vs. 3.9 ± 2.4 μg/mL, p = 0.033), which were the common disease characteristics (Figure 2(A)). Plasma Gd-IgA1 levels were significantly higher in patients with type I infection than in uninfected patients (5.5 ± 2.2 vs. 4.5 ± 2.2 μg/mL, p = 0.037). But there was no significance between patients with type I and type II H.pylori infection (5.5 ± 2.2 vs. 4.9 ± 1.9 μg/mL, p = 0.344). Conversely, compared with uninfected patients, plasma IgA1 levels were comparable with patients with type I infection (3.2 ± 1.7 vs. 3.4 ± 1.7 g/L, p = 0.693) and patients with type II H. pylori infection (3.2 ± 1.7 vs. 3.1 ± 1.4 g/L, p = 0.723). Further correlation analyses showed a significantly positive correlation between plasma IgA1 and Gd-IgA1 levels (correlation coefficient = 0.61, p = 1.38 × 10−6) in patients with IgAN (Figure 2(B)). While, in controls, there were no significant differences both in plasma IgA1 and Gd-IgA1 levels whether between uninfected controls and controls with type I infection (1.9 ± 0.7 vs. 2.1 ± 0.7 g/L, p = 0.535; 4.0 ± 2.7 vs. 3.4 ± 1.9 μg/mL, p = 0.608) or between controls and controls with type II infection (1.9 ± 0.7 vs. 1.8 ± 0.8 g/L, p = 0.869; 4.0 ± 2.7 vs. 4.6 ± 2.4 μg/mL, p = 0.682) or between controls with I infection and controls with II infection (2.1 ± 0.7 vs. 1.8 ± 0.8 g/L, p = 0.552; 3.4 ± 1.9 vs. 4.6 ± 2.4 μg/mL, p = 0.363) (Figure 2(C)).
Figure 2.
Levels of plasma IgA1 and galactose-deficient IgA1 in participants. (A) The plasma IgA1 and Gd-IgA1 levels in patients with IgAN and healthy controls. (B) The Plasma IgA1 and Gd-IgA1 levels in IgAN patients with or without H. pylori infection. (C) The Plasma IgA1 and Gd-IgA1 levels in healthy controls with or without H. pylori infection.
Discussion
In this study, patients with IgAN showed higher infection rates of H. pylori, especially type I H. pylori, than healthy controls. And IgAN patients infected with H. pylori, especially type I H. pylori, showed significantly higher SBP, 24-h proteinuria, and BUN levels than uninfected patients. Similarly, higher plasma Gd-IgA1 levels were observed in IgAN patients with type I H. pylori infection.
Mucosal infection was reported to be involved in the development of IgAN through triggering innate and adaptive immune responses and the alternative complement pathway [21,22]. Recently, H. pylori infection and its linkage with extra-gastric diseases has been widely studied [23,24]. However, whether the infection rates of H. pylori are higher in patients with IgAN remains controversial [5,9]. In this study, we recruited 261 patients with IgAN and 46 healthy controls, and results showed that there were slight differences in the infection rates of H. pylori between these two groups, but not reach statistical significance; however, the infection rates of type I H. pylori, the main virulent strain of H. pylori, were significantly higher in patients with IgAN. H. pylori was reported to increase proteinuria and renal vasoconstriction in several renal disease as an infection factor through altering the permeability of the glomerular basement membrane, damaging endothelial dysfunction, and promoting production of inflammatory cytokines [25,26], suggesting that H. pylori was involved in progression of renal disease. Previous study [27] and our clinical practice (data not included) showed that H. pylori eradication reduced proteinuria in patients with IgAN. Decrease trend of eGFR levels were observed in IgAN patients with H. pylori infection [4], but the association between different types of H. pylori infection and clinical manifestations of IgAN remained to be elucidated. In this study, a trend of lower eGFR was showed in patients with type I infection, but it does not reach statistical significance. And significantly higher 24-h proteinuria, SBP, and BUN were showed in IgAN patients with type I infection, but not in patients with type II infection, implying that IgAN patients with virulent H. pylori infection may have lower renal function.
IgA1 with high lectin binding was produced in response to mucosal H. pylori infection [28], and exaggerated systemic antibody response, mainly serum anti-H. pylori IgA1, to mucosal infection was demonstrated in IgAN patients with H. pylori infection [9]. CagA, a crucial virulence factor of type I H. pylori, was reported to promote the production and undergalactosylation of IgA1 in the B cell line, DAKIKI [9,29]. It was reported that aberrant glycosylation of IgA1 was involved in the pathogenesis of IgAN [30]. However, the effects of different H. pylori strains on plasma Gd-IgA1 levels of IgAN patients remain unclear. In present study, patients with IgAN displayed significantly higher plasma IgA1 and Gd-IgA1 levels than controls, which are the common disease characteristics. And the plasma Gd-IgA1 levels were significantly higher in patients with type I H. pylori infection than those in uninfected patients, while the levels in controls and controls with H. pylori infection were comparable, indicating that the higher plasma Gd-IgA1 observed in IgAN patients with virulent H. pylori infection was associated with the disease, and not a general change in healthy controls. This was consistent with previous studies that the levels of serum mucosal-type IgA1 against H. pylori were significantly higher in IgAN patients than healthy controls [9] and the degree of H. pylori antigen and CagA deposition were obviously severe in IgAN patients than patients with non-IgAN primary glomerulonephritis [4]. The immune response to H. pylori infection may be more stronger in IgAN patients than controls.
The main limitations in our study were as followed. We evaluated the serum anti-H. pylori IgG to identify individuals exposed to H. pylori infection rather than testing the current infection status using 13C-UBT. However, the seropositivity of anti-H. pylori IgG could not be converted to seronegativity without H. pylori eradication therapy. To a certain extent, the serum anti-H. pylori IgG represents current infection and has been used for several epidemiological investigations [31, 32]. Although Gd-IgA1 was suggested to play a central role in pathogenesis of IgAN. Some studies reported that Gd-IgA1 was unrelated with proteinuria in IgAN patients [33,34]. While others observed a trend of lower eGFR, higher proteinuria, and increased use of immunosuppressives in IgAN patients with higher Gd-IgA1 levels [18]. In this observation study, we found that the IgAN patients with type I H. pylori infection showed lower renal function and higher plasma Gd-IgA1. Further cohort and H. pylori eradication studies are needed to verify whether H. pylori infection participate in the pathogenesis and progression of IgAN through the elevated plasma Gd-IgA1.
Conclusions
In conclusion, our study revealed that virulent type I H. pylori infection was more common in patients with IgAN. And H. pylori infection, especially type I H. pylori infection, was associated with higher 24-h proteinuria, SBP, and BUN, as well as higher plasma Gd-IgA1 levels in patients with IgAN.
Supplementary Material
Acknowledgements
The authors thank all the patients and healthy controls who participated in this study.
Funding Statement
This work was supported by National Natural Science Foundation of China [81800636], Beijing Natural Science Foundation [7184253], and Peking University Medicine Fund of Fostering Young Scholars’ Scientific & Technological Innovation [BMU2017PY007].
Ethical approval
The study was approved by the Medical Ethics Committee of Peking University, and informed written consent was obtained from all patients.
Disclosure statement
All the authors report no conflicts of interest in this work.
References
- 1.Wyatt RJ, Julian BA.. IgA nephropathy. N Engl J Med. 2013;368(25):2402–2414. [DOI] [PubMed] [Google Scholar]
- 2.Perse M, Veceric-Haler Z.. The role of IgA in the pathogenesis of IgA nephropathy. Int J Mol Sci. 2019;20(24):6199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zhang YM, Zhang H.. Insights into the role of mucosal immunity in IgA nephropathy. Clin J Am Soc Nephrol. 2018;13(10):1584–1586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zhu TT, Wang L, Wang HL, et al. Helicobacter pylori participates in the pathogenesis of IgA nephropathy. Ren Fail. 2016;38(9):1398–1404.27764998 [Google Scholar]
- 5.Kusano K, Inokuchi A, Fujimoto K, et al. Coccoid Helicobacter pylori exists in the palatine tonsils of patients with IgA nephropathy. J Gastroenterol. 2010;45(4):406–412. [DOI] [PubMed] [Google Scholar]
- 6.Niknam R, Barfei M, Mahmoudi L.. Helicobacter pylori, endoscopic, and histologic features among kidney transplant candidates in southern Iran. IDR. 2019;12(1):3687–3693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pan W, Zhang H, Wang L, et al. Association between Helicobacter pylori infection and kidney damage in patients with peptic ulcer. Ren Fail. 2019;41(1):1028–1034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Shin SP, Bang CS, Lee JJ, et al. Helicobacter pylori infection in patients with chronic kidney disease: a systematic review and meta-analysis. Gut Liver. 2019;13(6):628–641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Barratt J, Bailey EM, Buck KS, et al. Exaggerated systemic antibody response to mucosal Helicobacter pylori infection in IgA nephropathy. Am J Kidney Dis. 1999;33(6):1049–1057. [DOI] [PubMed] [Google Scholar]
- 10.Chmiela M, Kupcinskas J.. Review: pathogenesis of Helicobacter pylori infection. Helicobacter. 2019;24(Suppl 1):e12638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kusano K, Tokunaga O, Ando T, et al. Helicobacter pylori in the palatine tonsils of patients with IgA nephropathy compared with those of patients with recurrent pharyngotonsillitis. Hum Pathol. 2007;38(12):1788–1797. [DOI] [PubMed] [Google Scholar]
- 12.Wang L, Tan RZ, Chen Y, et al. CagA promotes proliferation and secretion of extracellular matrix by inhibiting signaling pathway of apoptosis in rat glomerular mesangial cells. Ren Fail. 2016;38(3):458–464. [DOI] [PubMed] [Google Scholar]
- 13.Galmiche A, Rassow J, Doye A, et al. The N-terminal 34 kDa fragment of Helicobacter pylori vacuolating cytotoxin targets mitochondria and induces cytochrome c release. Embo J. 2000;19(23):6361–6370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Molinari M, Salio M, Galli C, et al. Selective inhibition of Ii-dependent antigen presentation by Helicobacter pylori toxin VacA. J Exp Med. 1998;187(1):135–140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Levey AS, Stevens LA, Schmid CH, et al. ; for the CKD-EPI (Chronic Kidney Disease Epidemiology Collaboration) . A new equation to estimate glomerular filtration rate. Ann Intern Med. 2009;150(9):604–612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Doolan PD, Alpen EL, Theil GB.. A clinical appraisal of the plasma concentration and endogenous clearance of creatinine. Am J Med. 1962;32:65–79. [DOI] [PubMed] [Google Scholar]
- 17.Cattran DC, Coppo R, Cook HT, et al. ; Working Group of the International Ig ANN. The Oxford classification of IgA nephropathy: rationale, clinicopathological correlations, and classification. Kidney Int. 2009;76(5):534–545. [DOI] [PubMed] [Google Scholar]
- 18.Zhao N, Hou P, Lv J, et al. The level of galactose-deficient IgA1 in the sera of patients with IgA nephropathy is associated with disease progression. Kidney Int. 2012;82(7):790–796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zhang K, Li Q, Zhang Y, et al. Clinical significance of galactose-deficient IgA1 by KM55 in patients with IgA nephropathy. Kidney Blood Press Res. 2019;44(5):1196–1206. [DOI] [PubMed] [Google Scholar]
- 20.Suzuki H, Yasutake J, Makita Y, et al. IgA nephropathy and IgA vasculitis with nephritis have a shared feature involving galactose-deficient IgA1-oriented pathogenesis. Kidney Int. 2018;93(3):700–705. [DOI] [PubMed] [Google Scholar]
- 21.Kiryluk K, Novak J, Gharavi AG.. Pathogenesis of immunoglobulin A nephropathy: recent insight from genetic studies. Annu Rev Med. 2013;64(1):339–356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Novak J, Moldoveanu Z, Julian BA, et al. Aberrant glycosylation of IgA1 and anti-glycan antibodies in IgA nephropathy: role of mucosal immune system. Adv Otorhinolaryngol. 2011;72(1):60–63. [DOI] [PubMed] [Google Scholar]
- 23.Bravo D, Hoare A, Soto C, et al. Helicobacter pylori in human health and disease: mechanisms for local gastric and systemic effects. World J Gastroenterol. 2018;24(28):3071–3089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Robinson K. Helicobacter pylori-mediated protection against extra-gastric immune and inflammatory disorders: the evidence and controversies. Diseases. 2015;3(2):34–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kanbay M, Kasapoglu B, Akcay A.. An occult risk factor for proteinuria: Helicobacter pylori infection. Med Hypotheses. 2007;69(3):709–710. [DOI] [PubMed] [Google Scholar]
- 26.Migneco A, Ojetti V, Specchia L, et al. Eradication of Helicobacter pylori infection improves blood pressure values in patients affected by hypertension. Helicobacter. 2003;8(6):585–589. [DOI] [PubMed] [Google Scholar]
- 27.Caliskan B, Yazici H, Caliskan Y, et al. The effects of Helicobacter pylori eradication on proteinuria in patients with primary glomerulonephritis. Int J Nephrol. 2014;2014(1):180690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Smith AC, Molyneux K, Feehally J, et al. O-glycosylation of serum IgA1 antibodies against mucosal and systemic antigens in IgA nephropathy. J Am Soc Nephrol. 2006;17(12):3520–3528. [DOI] [PubMed] [Google Scholar]
- 29.Yang M, Li FG, Xie XS, et al. CagA, a major virulence factor of Helicobacter pylori, promotes the production and underglycosylation of IgA1 in DAKIKI cells. Biochem Biophys Res Commun. 2014;444(2):276–281. [DOI] [PubMed] [Google Scholar]
- 30.Novak J, Barratt J, Julian BA, et al. Aberrant glycosylation of the IgA1 molecule in IgA nephropathy. Semin Nephrol. 2018;38(5):461–476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Somily AM, Morshed MG.. An update of laboratory diagnosis of Helicobacter pylori in the Kingdom of Saudi Arabia. J Infect Dev Ctries. 2015;9(8):806–814. [DOI] [PubMed] [Google Scholar]
- 32.Chey WD, Wong BC.. Practice Parameters Committee of the American College of Gastroenterology. American College of Gastroenterology guideline on the management of Helicobacter pylori infection. Am J Gastroenterol. 2007;102(8):1808–1825. [DOI] [PubMed] [Google Scholar]
- 33.Bagchi S, Lingaiah R, Mani K, et al. Significance of serum galactose deficient IgA1 as a potential biomarker for IgA nephropathy: a case control study. PLoS One. 2019;14(3):e0214256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hastings MC, Afshan S, Sanders JT, et al. Serum galactose-deficient IgA1 level is not associated with proteinuria in children with IgA nephropathy. Int J Nephrol. 2012;2012:315467. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.