ABSTRACT
Optic disc drusen (ODD) are a well-recognised cause of an elevated optic disc appearance. When visible with ophthalmoscopy and fundus photography, ODD are readily identified. Yet, in more subtle cases of ODD, ancillary testing may be needed to render the diagnosis. Facilitating the diagnosis of ODD has clinical relevance, because affected individuals may otherwise undergo unnecessary costly and invasive investigations to rule out raised intracranial pressure and other causes of optic disc oedema. In this review, the role of established and emerging optical coherence tomography (OCT) techniques in the diagnosis and management of ODD cases is reviewed. A practical approach is taken to explain how to optimise use of commercially available OCT technology in the clinical setting. Optical coherence tomography provides many advantages over other imaging modalities in the diagnosis of ODD, including the ability to correlate retinal measures of neuroaxonal structure with drusen characteristics. Earlier spectral domain OCT techniques, however, were hindered by poor penetrance. In the modern imaging era, enhanced depth imaging OCT and swept source OCT enable higher resolution of ODD and other optic nerve head structures that might otherwise be mistaken for drusen. Ongoing studies featuring OCT angiography indicate that this technique may provide complementary information about microvascular supply that correlate with structural measures of optic nerve injury. Advances in OCT will continue to improve diagnostic accuracy and inform clinical understanding regarding structure-function correlations germane to the longitudinal follow up of ODD patients.
KEYWORDS: Optic disc drusen (ODD), optical coherence tomography (OCT), enhanced depth imaging OCT (EDI-OCT), swept source OCT (SS-OCT), optical coherence tomography angiography (OCTA), papilloedema, pseudo-papilloedema
Introduction: What is the relevance of optic disc drusen to ophthalmic practice?
Optic disc drusen (ODD) are acellular deposits of calcium, amino acids, nucleic acids, and mucopolysaccharides.1 These lesions are located in front of the lamina cribrosa and typically cause an elevated optic disc appearance. Optic disc drusen are believed to affect 0.3% to 2% of the population.1–3 These reports may underestimate overall prevalence, however, as clinically subtle cases may not be diagnosed. For the vast majority of patients, ODD are bilateral.1 The pathobiology of ODD is still under investigation; yet, impaired axonal metabolism in genetically predisposed individuals, and the presence of narrow scleral canals are factors believed to play a role in drusen development.1
Optic disc drusen are relevant to clinical practice for several reasons. First, ODD can masquerade as papilloedema caused by elevated intracranial pressure (ICP) (Figure 1), posing a diagnostic challenge. This is an important scenario in which a reliable, accessible means of diagnosing ODD could spare patients costly and invasive procedures aimed at excluding causes of raised ICP. Second, ODD are accompanied by visual field defects in up to 87% of adult cases.1,3 Clinicians should therefore consider this diagnosis for patients with cryptogenic perimetric abnormalities and an elevated optic nerve appearance. Finally, ODD may cause sudden-onset painless vision loss through a variety of mechanisms including non-arteritic anterior ischaemic optic neuropathy (NA-AION), central retinal artery occlusion, central retinal vein occlusion, and choroidal neovascularisation.1,3–7 In two recent retrospective studies of young individuals (aged 50 years or less) with NA-AION, 51% to 53% of NA-AION eyes harboured ODD.6,7 Hence, ODD may represent an independent risk factor for NA-AION and should be considered in the differential diagnosis, particularly for younger patients who lack known risk factors for this condition.6,7
Figure 1.
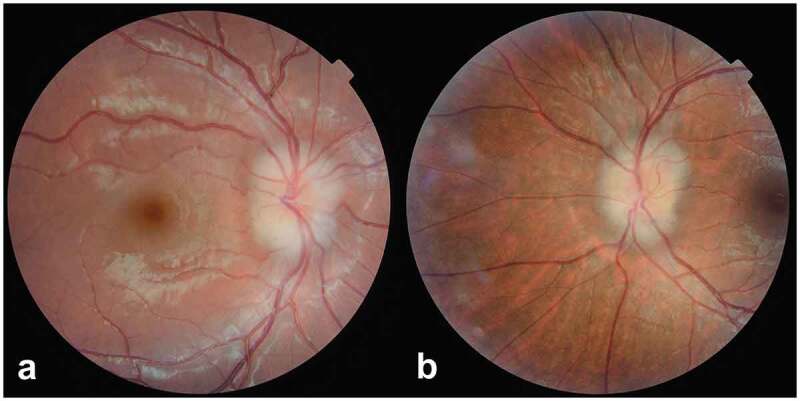
Colour fundus photographs showing the comparable appearance of optic disc elevation in (a) an 8-year-old boy with buried optic disc drusen (right eye) and, (b) a 23-year-old woman with idiopathic intracranial hypertension and associated papilloedema (left eye)
Diagnosing optic disc drusen: A changing paradigm
The diagnosis of ODD is easily rendered in cases of superficial drusen clearly visualised by ophthalmoscopy and fundus photography. Traditionally, B-scan ultrasonography, intravenous fluorescein angiography (FA), colour and autofluorescence fundus photography, computed tomography (CT), and earlier generations of optical coherence tomography (OCT) have all been employed as ancillary tools to confirm diagnosis, when the presence of ODD is less readily apparent (Table 1). Each testing technique has its advantages and disadvantages, the full details of which are beyond the scope of this review. An important limitation of B-scan ultrasonography and CT imaging is that detection requires adequate calcification of the ODD (ergo, less calcified drusen may be missed); whereas, intravenous FA and fundus autofluorescence are insensitive to deeper lying ODD.1,3,8 As an imaging modality, only OCT facilitates ODD detection, and provides the ability to correlate ODD characteristics with surrogate measures of neuroaxonal integrity in the anterior visual pathway, namely peripapillary retinal nerve fibre layer (RNFL) thickness and ganglion cell layer (GCL) thickness. In recent years, OCT has been used more regularly in the evaluation of ODD. Early spectral domain (SD) OCT studies captured superior images of ODD morphology than prior methods, and identified ODD as having a signal-poor core with a hyper-reflective anterior border, likened to a superficial “cap”.1,3,8 With SD-OCT techniques and the time domain OCT techniques that preceded them, posterior borders of ODD and deeper structures in the prelaminar optic nerve remained indistinct, or in some cases invisible1,3,8 due to the technical challenge of poor penetrance (Table 1, Figure 2). This led to erroneous diagnoses of peripapillary hyper-reflective ovoid mass-like structures (PHOMS) as drusen, and prior depictions of the so-called ‘lazy V’ pattern or ‘lumpy bumpy’ internal contour of the subretinal hypo-reflective space as signs of ODD.1,9,10
Table 1.
Ancillary testing modalities used to diagnose optic disc drusen (ODD)
| Imaging Modality | Finding | Advantages | Disadvantages |
|---|---|---|---|
| B-Scan Ultrasonography |
Calcified ODD are hyperechoic, highly reflective round structures identified by their posterior acoustic shadowing | Entire area of the optic disc is scanned, Non-invasive Can be used in children |
Offers relatively poor resolution of ODD Provides no information regarding the neuroaxonal integrity of the optic nerve and retinal structures Requires adequate calcification to detect ODD Is operator dependent; interpretation is subjective |
| Fundus Autofluorescence |
ODD show autofluorescence on scanning laser ophthalmoscopy ODD appear as round or oval hyper-autofluorescent structures with irregular edges |
Cost effective Non-invasive Little subjective interpretation required |
Does not reliably detect deeper lying ODD possibly because of attenuation from overlying tissue |
| Intravenous Fluorescein Angiography |
ODD show nodular optic disc staining and delayed filling of the peripapillary choriocapillaris | Can be useful for differentiating ODD from other causes of optic disc oedema, even in cases of buried ODD, Can detect associated choroidal neovascularisation |
Invasive Allergic reactions Time consuming |
| Computed Tomography |
ODD appear as hyperdense spots in the optic nerve head | Non-invasive Useful for detection of optic nerve sheath meningiomas (which are also calcified) in the diagnostic process |
Costly Radiation exposure Relies on adequate calcification for ODD detection Apt to miss smaller ODD due to slice thickness |
| Spectral domain Optical Coherence Tomography |
ODD have a hyperreflective anterior margin | Non-invasive Can detect associated neuroaxonal injury |
Poor penetrance limits characterisation of ODD, particularly in a swollen optic disc |
Figure 2.

Spectral domain optical coherence tomography (OCT) images of a 25-year-old woman with large optic disc drusen (ODD) (white asterisk). Enhanced depth imaging OCT in the lower image clearly shows the advantage of this technique, by depicting the posterior delineation of the ODD and lack of shadowing of the drusen, as compared with the top image
Subsequent advancements in enhanced depth imaging (EDI) OCT have led to a “changing of the guard” in terms of how ODD are diagnosed and characterised. Compared with ultrasonography and conventional SD-OCT, EDI-OCT better captures the prelaminar optic nerve head, especially the deeper structures below Bruch’s membrane opening. Consequently, EDI-OCT enables sharper spatial resolution and improved identification of structural lesions of the optic nerve head.1,3,8 In the published reports to date, ODD have been consistently identified as rounded, hypo-reflective structures with a full or partial hyper-reflective anterior margin.1,3,8 The hypo-reflective core of a druse results from its uniform refraction index and lack of reflective internal optical interfaces.1,3,8 The hyper–reflective outer rim demarcates the sharp optical interface between a druse and neighbouring tissue. Located anterior to lamina cribrosa, ODD can be solitary, multiple, coalescing or clumped together in conglomerated multilobed structures (Figure 3).1,3,8
Figure 3.
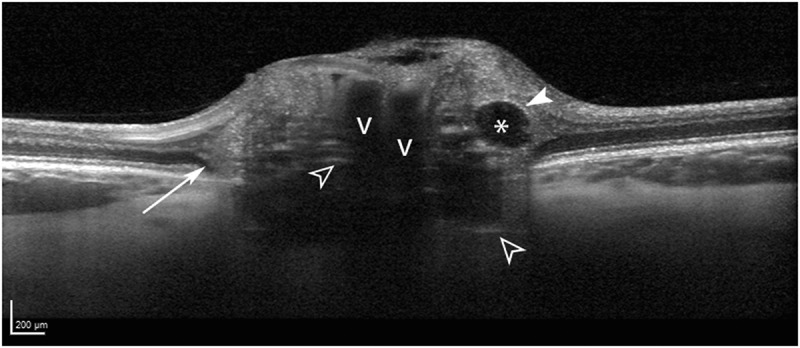
Optic disc drusen morphology in a 22-year-old woman, shown with enhanced depth imaging optical coherence tomography. The drusen are seen as signal-poor structures (asterisk) with a partial hyper-reflective margin (solid white arrowhead). Prelaminar hyper-reflective lines (open white arrowheads) are thought to represent drusen precursors. Peripapillary hyper-reflective ovoid mass-like structures (longer white arrow) circumscribe the disc and likely represent herniated nerve fibres due to crowded optic nerve head conditions. Blood vessel shadows are denoted with v’s
Guidelines put forth by the Optic Disc Drusen Studies (ODDS) Consortium,11 outline how newer iterations of OCT may be used to evaluate optic nerve head morphology, RNFL thickness, macular regions, and autofluorescence properties to diagnose ODD (Table 2). Specifically, EDI-OCT reveals collections of several smaller ODD that coalesce to form larger conglomerates, with a patchy internal reflectivity found within a larger hypo-reflective area.11 Prelaminar, short hyper-reflective lines lying perpendicular to the ganglion cell axons, are often found in eyes with ODD. These may reflect early drusen changes.3,12 Besides the clinical classification of “deep” versus “superficial” drusen, three OCT morphologic types have been described by Traber et al., namely: peripapillary ODD; granular ODD and confluent ODD.13 Yet, there is dispute regarding the true identity of “peripapillary ODD”, which the authors of the present review consider to be PHOMS, not drusen. While the cause of PHOMS is unclear, these lesions are believed to reflect axoplasmic flow stasis. Notably, PHOMS have several imaging features, which are distinct from ODD including their: (1) location in the immediate peripapillary zone; (2) hyper-reflective appearance; (3) lack of clearly defined margins; and, (4) absent hypo-reflective core (Figure 3). Blood vessels, which appear as elongated tube-like structures, may also be mistaken for ODD, but can be distinguished by the absence of a surrounding hyper-reflective signal.3
Table 2.
Enhanced depth imaging (EDI) optical coherence tomography and autofluorescence protocol specifications for identifying optic disc drusen (ODD)
| Prior to Scanning | Optimise scan quality by dilating pupils as needed, measuring corneal curvature and refraction |
| Acquisition | To visualize deeper structures, use EDI mode, then type in corneal curvature and refraction in the operator system |
| Dense optic nerve head (ONH) scan | To identify ODD, select EDI mode and high-resolution acquisition, centre a scan area of 15 × 10 degrees covering the entire optic disc area, scan with 97 sections in that area (30 μm between scans), average at least 30 frames, and perform the volume scan in horizontal (axial) direction only |
| Radial ONH scan | Assess scleral canal size by using EDI mode, select 20-degree 6-line radial scan, and centre scan at optic disc |
| Peripapillary scan | Evaluate RNFL thickness by deselecting EDI mode, select 12-degree peripapillary scan, and centre scan at optic disc |
| Macular scan | To exclude macular pathology, deselect EDI mode, centre scan area of 20 × 20 degrees over macula, scan with at least 25 sections (240 μm between scans), and average at least 9 frames |
| Autofluorescence | To identify autofluorescence, centre scan at optic disc, and average 100 frames |
A “hands on” approach to evaluating optic disc drusen with enhanced depth imaging optical coherence tomography: Optimising image quality
In 2016, the ODDS Consortium convened to develop consensus recommendations for the diagnosis of ODD using EDI-OCT. During this meeting, discussing ODD morphology and creating an optimal OCT scanning protocol were prime objectives. An EDI-OCT protocol (Heidelberg Engineering), which included a dense high-resolution EDI-OCT scan of the optic nerve head was implemented in 38 patients, previously diagnosed with ODD. The scans were assessed and selected by the writing committee for an iterative four-step standardisation process. The EDI-OCT findings were then compared with histopathological specimens from the literature. The process resulted in a protocol, which was later discussed, edited, and approved by consensus, as summarised in Table 2. In addition to characterising EDI-OCT features used to diagnose ODD, the ODDS Consortium identified common artefacts that should be distinguished from ODD.
Practical tips to guide the interpretation of EDI-OCT of the optic nerve head
ODD: Identifying anatomical location
By convention, ODD can be classified on ophthalmoscopy as visible or buried, and on OCT (and histology) as superficial or deep. Yet, the ophthalmic designations of ODD as being “visible” versus “buried” are purely descriptive; in fact, ODD cannot be correlated as being superficial or deep without having matching histology, or alternatively, OCT data from the same patient. With EDI-OCT, ODD can be defined as superficial if the bulk of the lesion(s) is located above a line connecting Bruch’s membrane opening on either side of the optic disc, and deep if the bulk is located below this line (Figure 4).
Figure 4.

Enhanced depth imaging optical coherence tomography from five different patients with optic disc drusen (ODD). Upper panel: a line connecting the Bruch’s membrane openings on either side of the optic disc can be used to categorise ODD by location, namely (a) superficial and (b) deep depending on where the bulk of the drusen material is located. Lower panel: The scale bar in the lower left corner can be used to categorise ODD by size including large (c), referring to drusen measuring greater than 200 μm in at least one direction (arrow), and small (d), referring to drusen that are smaller than 200 μm in all directions (arrow). Conglomerates of ODD (e) are made up of multiple smaller drusen (arrow) that cannot be clearly differentiated from one another
ODD: Determining size
On EDI-OCT, a scale bar measuring 200 µm is present in the lower left corner of the cross-sectional OCT image (Figure 4). Traber et al.13 have categorised ODD as small (<300 µm), medium (300–500 µm), or large (>500 µm) based on the maximum drusen diameter determined with EDI-OCT. Another easy and perhaps more practical way to classify the ODD is to say that large ODD are more than 200 µm in at least one direction, and small ODD measure less than 200 µm in diameter, in both directions. Sometimes small ODD aggregate into conglomerates, in which individual ODD are so small, they cannot be readily visualised.
Calculating ODD volume
The volume of individual ODD can be calculated by manual segmentation of ODD, using 97 EDI-OCT B-scans per optic nerve head with specialised segmentation software. In a study of 37 patients with either visible (seen by ophthalmoscopy) or buried (only visible by EDI-OCT) ODD, lesional volumes determined with this technique directly correlated with optic nerve function.14 In this study, larger ODD volume, in lieu of the anatomic localisation of the ODD within the optic nerve head, was associated with worse visual field defects.14 Notably, the manual ODD segmentation technique is labour intensive and will probably be replaced by artificial intelligence (AI) facilitated automated systems in the future.
Prelaminar hyper-reflective lines
Prelaminar hyper-reflective lines are often seen juxtaposing or completely isolated from ODD (Figure 3). Occasionally, they can be seen in optic nerve heads with no ODD. Hyper-reflective lines on a single OCT section may only seem isolated until their “parent” ODD is detected on adjacent OCT sections.3 When truly isolated hyper-reflective lines are seen, however, they are always situated deep in the optic nerve head, both anterior, and in close proximity, to the lamina cribrosa (Figure 3). Isolated hyper-reflective lines are typically short, oriented perpendicular to the OCT beam, and clustered together in smaller vertical groups. Hyper-reflective lines do not seem to be an imaging artefact, because they persist in their location or develop into ODD over time. In a recent 5-year follow-up study of 17-year-old individuals with ODD, all incident ODD developed at locations where hyper-reflective lines were visualised 5 years prior.12 Furthermore, eight eyes with ODD at baseline had developed new ODD where there were previously hyper-reflective lines.12 The documented progression from prelaminar hyper-reflective lines to ODD support the concept that these lines are ODD precursors. Yet, definitive proof of what the prelaminar hyper-reflective lines truly represent awaits histopathological confirmation.
Peripapillary hyper-reflective ovoid mass-like structures (PHOMS)
Patients with ODD are sometimes observed to have co-existing PHOMS on OCT imaging (Figure 3). In the ODDS Consortium recommendation study, 28 out of the 38 patients with ODD had PHOMS.11 The identity of PHOMS has been a source of confusion and debate in the published literature. These OCT lesions are located in the peripapillary region, and not in the optic disc itself. They characteristically ‘sit’ on top of Bruch’s membrane. Moreover, on the superior aspect of PHOMS there usually is an upward deflection of at least two of the other retinal layers, akin to a “ski slope” (Figures 3 and Figures 5). Unlike ODD, PHOMS do not autofluoresce, are not visible on B-scan orbital ultrasonography, and appear hyper-reflective (instead of hypo-reflective) with OCT. Using these characteristics, the ODDS Consortium found the inter-rater level of agreement for detection of PHOMS to be good (kappa 0.701).15 Notably, PHOMS are not directly visible by ophthalmoscopy but may elevate the optic nerve head at the disc margin, corresponding to the C-shaped halo in myopic obliquely inserted discs. In addition to ODD, PHOMS have also been associated with optic disc oedema from a variety of other causes including: papilloedema, NA-AION, central retinal vein occlusion, papillitis, and tilted or myopic obliquely inserted discs.16 In fact, the first report describing PHOMS was published by Pichi et al. in 2014, in paediatric cases of tilted optic disc syndrome.17 Recently, PHOMS were investigated using OCT angiography (OCTA) in two cases of tilted disc syndrome, and one case of ODD. In this small case series, OCTA identified a vascular complex within the PHOMS consistent with the theory that these structures correspond to herniating axons.18 Finally, histopathological studies of papilloedema and ODD eyes have shown bulging or herniation of distended axons laterally into the regions where PHOMS are typically seen on OCT.19–21
Figure 5.

Enhanced-depth imaging optical coherence tomography imaging shows peripapillary hyper-reflective ovoid mass-like structures (PHOMS) in a 23-year-old woman with idiopathic intracranial hypertension (upper panel), and in a 13-year-old boy with optic disc drusen (lower panel). The two main characteristics of the PHOMS are: the upward deflection of retinal layers on top of the drusen creating the ‘ski slope sign’ (arrowheads), and the visible hyper-reflective Bruch’s membrane (longer white arrows) underneath the PHOMS. The PHOMS (encircled in white) are annotated to the right
Blood vessel artefacts
Normal retinal blood vessels imaged in cross-section can occasionally be mistaken for ODD on OCT, due to the decreased intravascular reflectivity. The vessels appear in the inner superficial layers of the optic disc. The arteriole-venule pair may be recognised by a ‘figure of eight’ configuration.22 Furthermore, a characteristic vessel wall reflection and shadowing of underlying optic nerve head tissue are distinctive imaging features (Figure 3). Vessels imaged in a longitudinal direction demonstrate a tri-layer profile with decreased intravascular reflectivity. In contrast, vessels imaged more obliquely often demonstrate a hyperdense ‘head’ (without a visible ‘figure of eight’ configuration) and show underlying shadowing. As a practical tip, a long tubular blood vessel can be distinguished from an ODD deposit simply by scrolling through adjacent cross-sectional OCT images to delineate the three-dimensional nature of the optic nerve head structures.
Using EDI-OCT to detect ODD: Pearls and pitfalls
For optimum image quality and signal strength, the patient is often dilated prior to OCT. In our experience, pupillary dilation is not essential for diagnosing ODD in the vast majority of cases and is not performed routinely in our clinics. To achieve maximal diagnostic sensitivity, it is very important to scan with 97 sections through the optic nerve head (which equates to 30 µm between each section), averaging at least 30 frames. To reliably exclude ODD using EDI-OCT, this degree of spatial resolution is an absolute prerequisite. In an early ODD histopathology study of 15 cases published by Friedman in 1975, ODD ranged from 50 to 750 µm in diameter.23 A subsequent clinicopathological correlative study of 18 ODD cases showed calcified bodies ranging from 5 to 1000 µm in diameter.21 In this latter study, however, many of the smallest ODD were seen in clusters, equivalent to the conglomerates seen in later OCT reports. In a retrospective multicentre EDI-OCT study, examining the prevalence of ODD in cases of young NA-AION, patients could not be included if their OCT was performed with less than 97 B-scans.7 Notably, OCT imaging can be performed in both horizontal (transverse, or axial) and vertical (sagittal) planes, which increases the chance of detecting ODD smaller than 30 µm in diameter. In our experience this is only necessary to do for research purposes, as the added detection rate is probably low, and the time required to do the scans in both planes increases almost two-fold. In the clinical setting, we only scan in the horizontal plane.
As ODD are located anterior to lamina cribrosa, it is a good idea to visualise this anatomic structure to ensure that the entire prelaminar optic nerve head OCT is available for subsequent analysis. However, in ODD cases with more severe optic nerve head elevation, sub-optimal depth penetration of the imaging laser may be a limitation. Finally, in order to localise and characterise ODD (and detect co-existing PHOMS and hyper-reflective lines) it is important to scroll through all 97 sections of the optic nerve head, using the recommended OCT scanning protocol (Table 2).11 As previously mentioned, this routine also helps to distinguish ODD from potential mimickers, including PHOMS and blood vessels. Ambiguous structures can also be clarified by reformatting the slices from horizontal to vertical, using the OCT viewing software.
Emerging OCT techniques: Swept source OCT versus spectral domain EDI-OCT
Advances in ocular imaging technologies have made the investigation of deeper structures of the posterior pole and optic nerve head possible. The choroid, the sclera and structural details of the optic nerve head, down to the lamina cribrosa can be visualised by OCT. With this non-invasive, in vivo imaging technique, smaller and deeper lying components of ODD can also be detected. Two main approaches are currently commercially available: the method of performing EDI with SD-OCT devices as first described in 2009 by Spaide et al.24; and the more recently introduced swept source OCT (SS-OCT) technology.
State of the art OCT advancements
Low-coherence interferometry is the basic principle of OCT. Analogous to ultrasonography, echoes in backscattered light from ocular tissues and a reference source are compared and analysed. The spectrometer-based SD-OCT uses a broadband light source (around 840 nm wavelength) with good sensitivity and signal-to-noise ratio in retinal tissue, and scan rates up to 85,000 A-scans per second. Inherent to this method is a decrease in signal strength with increasing tissue depth. For visualisation of structures beneath the retinal pigment epithelium, this signal decrement can be countered by positioning the OCT device closer to the eye. This modified EDI technique shifts the maximum sensitivity of the sensor from the conventional position of the vitreoretinal interface, to the level of the choroid and sclera. This adjustment, in turn, enhances visualisation of deep lying structures. Image contrast is further increased by averaging of multiple scans. With real-time eye tracking, multiple scan repetitions per line are acquired (30 times or more as recommended by the ODDS Consortium) and then averaged into one B-scan. This results in significantly reduced speckle-noise, and improved contrast of deeper lying structures.25
Swept source OCT has a different operational system and demonstrates less sensitivity loss with increasing imaging depth. Specifically, SS-OCT uses a 1050 nm light source that allows better tissue penetration, albeit with slightly lower axial resolution. The faster scan rates (100,000 A-scans per second or higher) achieved with SS-OCT yield fewer artefacts caused by eye movement, and provide superior image quality relative to SD-OCT. These properties translate to higher scan-speeds, denser scan-patterns, and improved visualisation of structures beneath the level of the retinal pigment epithelium. A comparative study by Waldstein et al.26 investigated the image penetration depth between EDI-OCT and SD-OCT, when visualising the choroid and choroid-sclera interface. Volume scans obtained with SD-OCT, using the aforementioned EDI technique, were compared with those acquired with SS-OCT imaging (without frame averaging). The authors found a similar signal penetration depth for both devices. They deemed image contrast to be higher for the SD-OCT device, most likely because of the effect of averaging multiple frames. However, SD-OCT imaging was also associated with significantly longer acquisition times, which was a relative disadvantage.26 Unpublished data (personal communication: Rothenbuehler and Hamann) from head-to-head comparisons between SD-OCT and SS-OCT have shown comparable visualisation of ODD lying below the level of Bruch’s membrane opening. Similar to the observations of Waldstein and colleagues, the B-scan image quality was higher with frame average on SD-OCT, whereas SS-OCT allowed faster scan times. In future studies, SS-OCT could be performed with frame averaging to further increase image quality and better delineate deep prelaminar structures. For daily clinical practice, if EDI performed with SD-OCT is not an option, a SS-OCT volume scan performed without frame averaging remains a good imaging alternative to detect superficial and deep ODD (Figure 6, Table 3).
Figure 6.

Comparing spectral domain (SD) optical coherence tomography (OCT) (a) and swept source (SS) OCT (b) images from a volume scan of an eye with deep ODD. The SD-OCT scan was performed with enhanced-depth imaging (EDI), and included frame averaging techniques. Both OCT scans depict comparable images of optic disc drusen, showing a hypo-reflective core (white asterisk) and hyper-reflective margins. The associated prelaminar hyper-reflective lines (white arrow) are slightly more visible with the SD-OCT EDI scan (a)
Table 3.
Alternative swept source optical coherence tomography (OCT) protocol specifications for identifying optic disc drusen
| Prior to Scanning | Optimise scan quality by dilating pupils, and consider lubricating drops for ocular surface disease |
| Dense optic nerve head (ONH) scan | Choose 3D volume scan or OCT angiography scan (scan size of 4.5 mm x 4.5 mm, or 6 mm x 6 mm) with ample coverage of the disc and margin, set resolution to maximum, and set fixation target for disc |
| Radial and circular ONH scan | Chose dedicated disc scan pattern for peripapillary circle scan for retinal nerve fibre layer profile, radial scan pattern for Bruch’s membrane opening assessment, and accurate measurement of scleral canal size |
| Acquisition | Careful instruction of the patient to facilitate good fixation, and ensure scan centration on the disc. If frequent loss of fixation or blinking occurs, consider multiple repetitions of scans to assess and discard later |
Future directions in OCT technology
The longer wavelength and higher scan speeds of SS-OCT permit deeper tissue visualisation, at high scan density. As a further innovation, SS-OCT data can be combined with OCTA data into a single scan. These features, when applied to volume scanning of the optic nerve head, facilitate ODD diagnostics. Specifically, SS-OCT can detect small ODD bodies down to the level of the lamina cribrosa and quantify neuroaxonal injury. Optical coherence tomography angiography and SS-OCT can be used in concert to visualise vascular flow changes associated with ODD, PHOMS and hyper-reflective lines within the optic nerve head. Together, SS-OCT and OCTA can provide complementary information regarding tissue circulation in the peripapillary region. Analogously, volume scans of the macula (including the GCL) provided by SS-OCT and corresponding OCTA measures of vascular density can be correlated with tests of visual function. Hence, SS-OCT alone, or in combination with OCTA provides a wealth of imaging data that can be used to predict vision loss. Ultimately, these emerging OCT techniques will inform our understanding of structure-function relationships in cases of ODD. Future iterations of OCT can be expected to further increase image resolution, penetration depth, and acquisition speed. Currently, identifying the posterior border of ODD proves challenging in many cases, as overlying vascular structures attenuate the OCT signal. Reliable visualisation of the posterior border would allow quantification of ODD volume. Together with the applications of AI, automatic detection and segmentation capabilities will enable detailed 3D-visualisations of ODD and facilitate quantitative measures in longitudinal follow up (Figure 7). In a more immediate time frame, the widespread adoption of OCT devices will continue to expand, so that this technology will become ubiquitous in all eye care settings. Higher numbers of routine screenings with OCT will yield an expanding body of information about deep structures of the optic nerve head. Thus, the rate of incidental detection of ODD will likely increase in the future, along with innovations regarding the optimal strategies to manage ODD patients.
Figure 7.
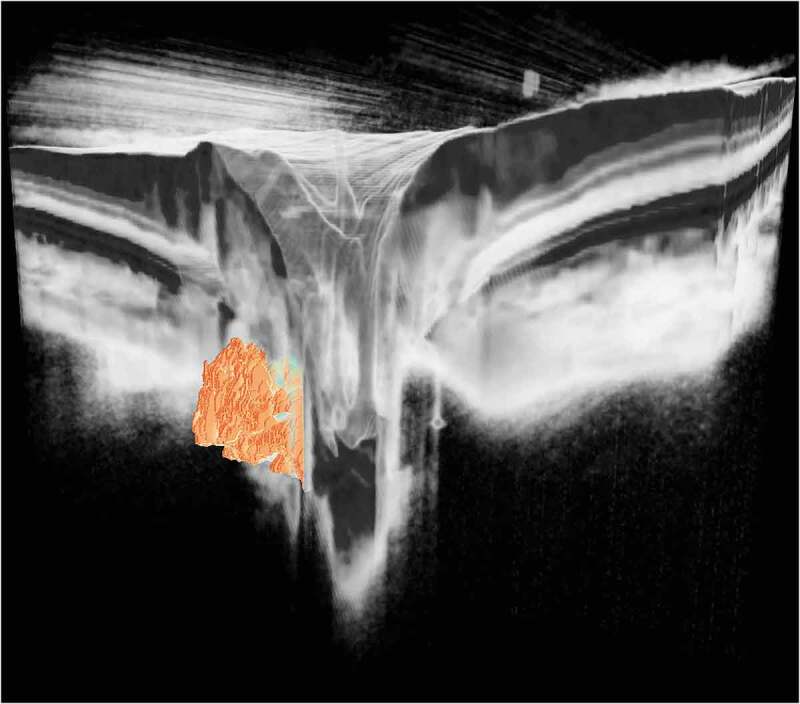
Three-dimensional visualisation of deeply localised optic disc drusen derived from a swept source optical coherence tomography optic disc volume scan. The large, solid drusen are buried within the optic nerve head tissue (which are faded in this artistic rendering, to allow better visibility). (Image processing and visualisation by Peter Maloca, Institute of Molecular and Clinical Ophthalmology Basel, Switzerland)
The role of OCTA in the management of ODD
As mentioned, OCTA is an emerging imaging technique that can be used to indirectly to capture functional changes in macular and peripapillary microvasculature, quantify vessel density, measure vessel length density, evaluate vascular tortuosity, and study vascular flow dynamics.27–31 Previous OCTA studies have shown microvascular attenuation around the optic nerve head in ODD eyes.27–30 Fard et al. used OCTA to demonstrate a significant reduction in peripapillary vessel density in papilloedema and pseudo-papilloedema eyes (including cases of ODD) as compared with control eyes.32 Interestingly, these investigators reported no difference in peripapillary vascular density between eyes with papilloedema versus pseudo-papilloedema. However, when large vessels were excluded from the quantitative analysis, peripapillary capillary density measures in eyes with papilloedema were not different from control eyes. Eyes with pseudo-papilloedema, however showed reduced capillary density, which persisted even after excluding large vessels.32 Thus, OCT measures of whole image capillary density might distinguish cases of papilloedema from pseudo-papilloedema, since the latter have lower peripapillary capillary densities compared with papilloedema eyes.31, 32
Recently, Maloca et al. used OCTA to describe ODD channels through which blood vessels migrate.33 In related work, Cennamo et al.27 evaluated 13 ODD patients with OCTA and reported lower flow indices and peripapillary vessel density measures in ODD eyes relative to normal eyes. Finally, Yan et al.30 compared OCTA and static perimetry findings between 17 patients with ODD (29 eyes) and 35 control subjects (53 eyes). In this study, ODD eyes had significantly worse visual field mean deviation, lower RNFL thickness values, and decreased peripapillary vascular density measures compared with controls.30 These investigators described five key measurements that best correlated with visual field loss including: thinning of the peripapillary RNFL; reduced ganglion cell inner plexiform layer (GCIPL) thickness; decreased peripapillary vessel area density; increased macular vessel diameter; and increased macular flux (defined as the number of blood cells passing through a retinal vessel cross-sectional area over time).30 The relative increase in macular vessel diameter and flux were novel findings, and were noted to occur in ODD cases with mild visual field loss and relative preservation of neuroaxonal integrity.30 The authors suggested that early increases in macular vessel diameter and flux may represent autoregulatory changes in retinal microvasculature. Specifically, these vascular adaptations were proposed to compensate for deleterious effects of ODD on surrounding vasculature and structure by increasing overall retinal blood flow.30 By way of comparison, loss of peripapillary vessel density was suggested to be a later stage phenomenon, manifesting in the setting of progressive retinal ganglion cell axon loss, and worsening visual field function.30 From their findings, the authors suggested that future OCTA studies in patients with ODD should include both macular and peripapillary OCTA measurements in the same eyes, in order to validate increased macular vessel diameter and flux as putative OCT biomarkers of neuroaxonal injury.30 As a technology, OCTA may be used to correlate changes in microvascular supply with structural measures of optic nerve injury. The emerging studies on this topic indicate that the role of OCTA in the study of ODD is an intriguing line of scientific enquiry that warrants further investigation.
Using OCT to distinguish ODD from papilloedema: Putting principles into practice
Optical coherence tomography can be used to distinguish ODD from other causes of optic disc oedema, including papilloedema, even when these diagnoses co-exist in the same patient. In general, OCT is not needed to differentiate relatively severe cases of papilloedema from obvious cases of ODD. In the more challenging clinical setting of mild optic disc elevation, however, EDI-OCT can offer additional information that is not apparent from history or routine ophthalmic assessment, as summarised in Table 4. Previous studies have shown that peripapillary RNFL values may be higher in cases of true papilloedema, as compared with ODD, particularly in the nasal and the inferior sectors.3,34–38 In a SD-OCT study that included 42 eyes with papilloedema, 37 eyes with pseudo-papilloedema, and 34 normal eyes, Fard et al.39 found thicker RNFL values in cases of true papilloedema versus pseudo-papilloedema. An average RNFL thickness of more than 127 μm had 73% sensitivity and specificity for distinction of papilloedema from pseudo-papilloedema.39
Table 4.
A summary of optical coherence tomography (OCT) findings used to distinguish papilloedema and optic disc drusen (ODD)
| OCT finding | Papilloedema | ODD | Key points |
|---|---|---|---|
| Retinal nerve fibre layer (RNFL) thickness | RNFL is thickened in 90% of patients with papilloedema, whereas 10% can have a mean RNFL within the normal range RNFL is decreased in chronic papilloedema |
Thickening of the RNFL is a common finding in children with ODD With time, RNFL gradually thins |
Thickening of the mean RNFL can help distinguish ODD from papilloedema in adults This OCT measure is less useful in children. Coexistent causes of disc oedema should be ruled out |
| Ganglion cell – inner plexiform layer (GCIPL) thickness | The macular GCIPL thickness may show low values in cases of papilloedema, due to OCT software algorithm failures. In chronic papilloedema GCIPL thinning may represent damage to retinal ganglion cells and optic nerve injury |
The macular GCIPL analysis may show thinning earlier than the RNFL thickness in cases of buried ODD due to retinal ganglion cell damage and optic nerve injury | Macular GCIPL measures may be normal or decreased in both ODD and papilloedema, and cannot be relied upon to distinguish these diagnoses |
| Peripapillary Bruch’s membrane layer (BML) shape deformations | Increased intracranial pressure can anteriorly displace the peripapillary BML towards the vitreous in approximately 2/3 of patients with papilloedema and idiopathic intracranial hypertension | Anterior deformation and displacement of BML is rare in ODD | When present, anterior shape deformation and displacement can help distinguish papilloedema from ODD It can also help identify patients with ODD and coexistent disc oedema from papilloedema or anterior ischaemic optic neuropathy (AION). The absence of deformation does not rule out intracranial hypertension |
| Scleral canal | The scleral canal size is enlarged in eyes with mild papilloedema and narrows as the papilloedema resolves | The scleral canal size has been found to be smaller in children with ODD compared to healthy controls | Scleral canal size might differentiate ODD from papilloedema but it has not been validated in studies Use of this OCT metric is challenged by a huge variation of scleral canal size in the general population |
| Retinal and choroidal folds | Optical coherence tomography has been used to detect and characterise retinal and choroidal folds, which accompany papilloedema in a significant proportion of cases | Rare | The presence of retinal and choroidal folds can help distinguish ODD from papilloedema. The presence of retinal and choroidal folds in patients with ODD should prompt a careful evaluation for coexistent papilloedema, AION, and other causes of optic disc oedema |
| Enhanced depth imaging (EDI)-OCT | With improved penetration of EDI-OCT more reliable means of using Bruch’s membrane angling in diagnosing papilloedema, and tracking response to therapy may be identified | ODD can be directly visualised and appear as regions of low reflectivity bordered by hyper-reflective margins | By using EDI-OCT to directly identify ODD, it is possible to distinguish these cases, and also identify cases in which the two diagnoses co-exist |
| Peripapillary hyper-reflective ovoid mass-like structures (PHOMS) | PHOMS represent herniated, distended axons due to optic nerve head congestion and are seen in cases of papilloedema | PHOMS represent herniated, distended axons due to optic nerve head congestion and are seen with ODD | PHOMS are a non-specific marker of axonal congestion and cannot be used to discriminate between papilloedema and ODD |
| Hyper-reflective lines | Hyper-reflective lines are not seen in eyes with papilloedema Using EDI-OCT, the visualisation of lamina cribrosa sometimes mimics hyper-reflective lines found in eyes with ODD However, in this case they are restricted to a very deep anatomical location in the optic nerve head |
Hyper-reflective lines are often seen in eyes with ODD In cases of unilateral ODD the lines will also likely be found in the eye without ODD |
The finding of hyper-reflective lines in different depths of the prelaminar part of the optic nerve head suggests that the eye or fellow eye has ODD However, not all eyes with ODD have these lines, and it is therefore not a reliable tool for the differentiation of papilloedema and ODD |
| OCT angiography (OCTA) | Dilation and tortuosity of large vessels and tangling of capillaries are evident around the optic nerve head, with less vascular density decrements compared with other optic neuropathies like ODD | Decreased peripapillary vascular density and increased macular vessel diameter and flux are seen in ODD eyes | At this time the role for OCTA in diagnosing papilloedema versus ODD is not clear and awaits further study |
As an additional distinguishing OCT feature, Bruch’s membrane layer opening measures have been shown to be smaller in ODD eyes relative to eyes affected by papilloedema. In the Copenhagen Child Cohort Eye Study,40 children with ODD had narrower scleral canal openings, relative to control subjects. Yet, a study of adults revealed larger scleral canal sizes in individuals with ODD relative to control eyes.40 It has therefore been proposed that as ODD grow in size they may cause an outward displacement of Bruch’s membrane, leading to larger scleral canal measurements over time.3 For this reason, scleral canal size may only be useful in differentiation of ODD from optic disc oedema in children, but this metric has yet to be validated (Table 4). Moreover, because of the considerable variance in Bruch’s membrane opening measures between normal individuals, and indeed pathological cases of optic disc oedema, this OCT finding may have limited value in the clinical setting.38
Retinal and choroidal folds commonly occur in patients with papilloedema and other causes of optic disc oedema.41,42 In the recent Idiopathic Intracranial Hypertension (IIH) Treatment Trial retinal folds were detected with OCT in at least one eye, among 73% of patients.41 Sibony et al. have shown that en face and transverse axial OCT are more sensitive in detecting folds than fundus photos alone.41 These investigators have distinguished four types of folds in cases of IIH with papilloedema, including: peripapillary wrinkles; inner retinal folds; outer retinal folds and creases; and choroidal folds.41,42 Notably, the improved sensitivity in detecting folds could help differentiate papilloedema from other causes of optic disc oedema, including ODD (Figure 8). The presence of retinal and choroidal folds in patients with ODD almost always indicates a coexisting cause of optic disc oedema. In a recent study using en face OCT, retinal or choroidal folds were found in 13 of 102 (13%) patients with ODD (six with co-existing papilloedema, four with co-existing NA-AION, and one with co-existing uveitic disc oedema).43 There were, however two patients (2%) with ODD and unexplained peripapillary wrinkles, without associated disc oedema. A prior bout of disc oedema with residual folds could not be entirely excluded in either one of these cases.43
Figure 8.
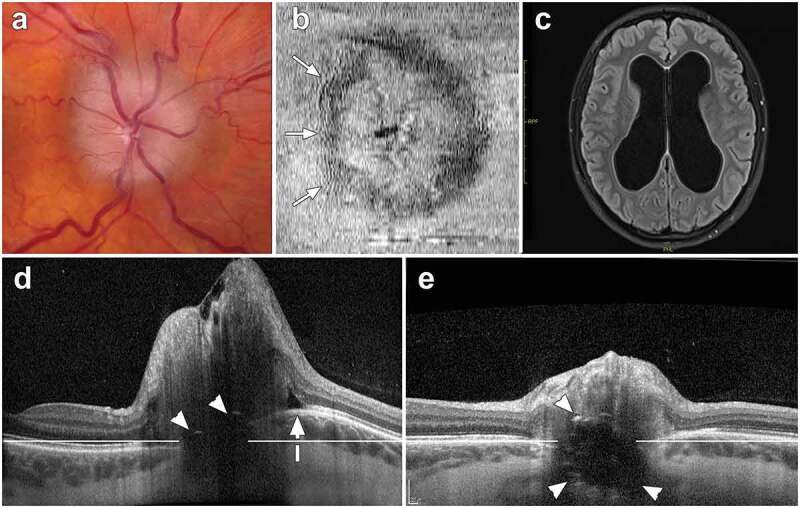
Images from an 18-year-old female with papilloedema, obstructive hydrocephalus and optic disc drusen (ODD). (a) Fundus photograph of an elevated optic nerve head, showing mild vessel tortuosity and obscuration of the entire disc margin. (b) Infrared en face optical coherence tomography (OCT) depicting peripapillary wrinkles on the temporal side of the optic disc (arrows). (c) Magnetic resonance imaging demonstrating ventricular enlargement caused by obstructive hydrocephalus. (d) Transverse axial OCT (30°, vertically scaled 3x, enhanced-depth imaging) at baseline. This image demonstrates ODD with signal-poor region and two small hyper-reflective caps (arrowheads). Bruch’s membrane layer is anteriorly deformed towards the vitreous (white arrow) relative to a horizontal reference line. The mean peripapillary retinal nerve fibre layer thickness measures 316 μm. [e] Follow-up transverse axial OCT performed after a ventriculostomy procedure shows a significant decrease in the disc elevation (mean peripapillary RNFL thickness measures 87 μm). The ODD are more visible. Moreover, the shape of Bruch’s membrane layer is flatter, moving posteriorly away from the vitreous towards the reference line
Notably, longitudinal follow-up with OCT may also offer diagnostic information and help detect cases of subtle papilloedema that might otherwise be missed. Patients presenting with IIH for example tend to have variations in their RNFL measures that exceed the 5–6 μm test-retest variability of conventional OCT techniques. In these IIH cases the broad range of RNFL values observed over serial clinical visits may provide a clue to diagnosis. This observational “pearl” can be especially helpful in identifying cases of mild papilloedema (RNFL thickness measures < 200 µm), particularly for patients with normal vision and a paucity of symptoms related to raised intracranial pressure.
Finally, it bears mentioning that many OCT measures included in Table 4 provide equivocal results. It is therefore imperative to rely on good clinical judgement to avoid misdiagnoses, when trying to identify cases of ODD and/or papilloedema. No one specific OCT measure will reliably exclude papilloedema. Thus, for some patients with an elevated optic nerve appearance, it may be necessary to perform detailed cranial imaging, and a lumbar puncture procedure, to exclude raised intracranial pressure as a potential mechanism. For the specific purpose of determining whether a patient has drusen with or without co-existing papilloedema, we recommend using the ODDS Consortium recommendations to reliably detect ODD (Table 2).
Conclusion
Optic disc drusen are clinically relevant to ophthalmic practice, both as an independent cause of vision loss, and as a potential mimicker of optic disc oedema, including papilloedema. Ongoing advances in OCT will continue to improve diagnostic accuracy and inform clinical understanding regarding structure-function correlations germane to the longitudinal follow up of ODD patients. Recent recommendations put forth by the ODDS Consortium are helpful in distinguishing cases of ODD from other causes of an elevated optic nerve appearance and can be used to identify ODD in patients with more than one reason for optic disc oedema. A comprehensive history and thorough clinical assessment form the bedrock of good clinical care, and these key steps must be taken to avoid misdiagnosis. Importantly, OCT should be viewed not as a substitute for either, but rather an extension of both, representing an ancillary test that complements the clinical assessment. In many cases, OCT may obviate the need for expensive and invasive ancillary testing, if ODD are quickly identified. As research continues to evolve in the area of ocular imaging, so too will our understanding regarding the pathobiology of ODD, and the optimal management of these patients.
Declaration of interest statement
Patrick Sibony received an honorarium from Heidelberg Engineering. The remaining authors declare that there are no conflicts of interest.
Appendix.
Members of the Optic Disc Drusen Studies (ODDS) Consortium are:
Aghsaei Fard, Masoud (Tehran University of Medical Science, Tehran, Iran)
Beres, Shannon (Stanford University Medical Centre, California, USA)
Biousse, Valérie (Emory University School of Medicine, Atlanta, Georgia, USA)
Bursztyn, Lulu (Ivey Eye Institute, Western University, London, Ontario, Canada)
Costello, Fiona (Clinical Neurosciences, Calgary, Canada)
Crum, Alison (University of Utah, Salt Lake City, Utah, USA)
Digre, Kathleen B. (University of Utah, Salt Lake City, Utah, USA)
Fraser, J. Alexander (Ivey Eye Institute, Western University, London, Ontario, Canada)
Fraser, Clare L. (Save Sight Institute, University of Sydney, Australia)
Gale, Jesse (Wellington, New Zealand)
Hamann, Steffen (Rigshospitalet, University of Copenhagen, Glostrup, Denmark)
Huna-Baron, Ruth (Sheba Medical Centre, Tel Hashomer, Israel)
Katz, Bradley (University of Utah, Salt Lake City, Utah, USA)
Lawlor, Mitchell (Sydney Eye Hospital, University of Sydney, Australia)
Liao, Joyce (Stanford University Medical Centre, California, USA)
Malmqvist, Lasse (Rigshospitalet, University of Copenhagen, Glostrup, Denmark)
Maloca, Peter M. (University Hospital Basel, Switzerland)
Moss, Heather (Stanford University Medical Centre, California, USA)
Newman, Nancy J. (Emory University School of Medicine, Atlanta, Georgia, USA)
Peragallo, Jason H. (Emory University School of Medicine, Atlanta, Georgia, USA)
Petzold, Axel (Moorfields Eye Hospital, London, UK)
Rasool, Nailyn (University of California San Francisco, California, USA)
Rodriguez, Amadeo (McMaster University, Hamilton, Ontario, Canada)
Rothenbuehler, Simon P. (University Hospital Basel, Switzerland)
Rougier, Marie-Bénédicte (Bordeaux, France)
Sibony, Patrick A. (Department of Ophthalmology, State University of New York at Stony Brook, New York, USA)
Subramanian, Prem S. (University of Colorado School of Medicine, Denver, Colorado, USA)
Warner, Judith (University of Utah, Salt Lake City, Utah, USA)
Wong, Sui H. (Moorfields Eye Hospital, London, UK)
Correction Statement
This article has been republished with minor changes. These changes do not impact the academic content of the article.
Contributor Information
the Optic Disc Drusen Studies Consortium:
Masoud Aghsaei Fard, Shannon Beres, Valérie Biousse, Lulu Bursztyn, Fiona Costello, Alison Crum, Kathleen B. Digre, J. Alexander Fraser, Clare L. Fraser, Jesse Gale, Steffen Hamann, Jesse Gale, Ruth Huna-Baron, Bradley Katz, Mitchell Lawlor, Joyce Liao, Lasse Malmqvist, Peter M. Maloca, Heather Moss, Nancy Newman, Jason H. Peragallo, Axel Petzold, Nailyn Rasool, Amadeo Rodriguez, Simon Rothenbuehler, Marie-Bénédicte Rougier, Patrick A. Sibony, Prem S. Subramanian, Judith Warner, and Sui H. Wong
References
- 1.Hamann S, Malmqvist L, Costello F.. Optic disc drusen: understanding an old problem from a new perspective. Acta Ophthalmol. 2018;96(7):673–684. doi: 10.1111/aos.13748. [DOI] [PubMed] [Google Scholar]
- 2.Lorentzen SE. Drusen of the optic disk. A clinical and genetic study. Acta Ophthalmol (Copenh). 1966;Suppl 90:91–180. [PubMed] [Google Scholar]
- 3.Fraser JA, Bursztyn LLCD. Optical coherence tomography in optic disc drusen. Annal Eye Sci. 2020;5:5. doi: 10.21037/aes.2019.12.03. [DOI] [Google Scholar]
- 4.Chang MY, Pineles SL. Optic disk drusen in children. Surv Ophthalmol. 2016;61(6):745–758. doi: 10.1016/j.survophthal.2016.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Duncan JE, Freedman SF, El-Dairi MA. The incidence of neovascular membranes and visual field defects from optic nerve head drusen in children. J Aapos. 2016;20(1):44–48. doi: 10.1016/j.jaapos.2015.10.013. [DOI] [PubMed] [Google Scholar]
- 6.Fraser JA, Rueløkke LL, Malmqvist L, Hamann S. Prevalence of optic disc drusen in young patients with nonarteritic anterior ischemic optic neuropathy: A 10-year retrospective study. J Neuroophthalmol. (2020). Advanced online publication. doi: 10.1097/WNO.0000000000000974. [DOI] [PubMed] [Google Scholar]
- 7.Hamann S, Malmqvist L, Wegener M, Biousse V, Bursztyn L, Citirak G, Costello F, Crum AV, Digre K, Fraser JA, Huna-Baron R, Katz B, Lawlor M, Newman NJ, Peragallo JH, Petzold A, Sibony PA, Subramanian PS, Warner JEA, Wong SH, Fraser CL, on behalf of the Optic Dis Drusen Studies Consortium. Young adults with anterior ischemic optic neuropathy: a multicenter optic disc drusen study. Am J Ophthalmol. 2020;217:174–181. doi: 10.1016/j.ajo.2020.03.052. [DOI] [PubMed] [Google Scholar]
- 8.de Carvalho ER, Maloca PM. Overview of optical coherence tomography in neuro-ophthalmology. Annal Eye Sci. 2020;5:14. doi: 10.21037/aes.2019.12.08. [DOI] [Google Scholar]
- 9.Johnson LN, Diehl ML, Hamm CW, Sommerville DN, Petroski GF. Differentiating optic disc edema from optic nerve head drusen on optical coherence tomography. Arch Ophthalmol. 2009;127(45–49):45. doi: 10.1001/archophthalmol.2008.524. [DOI] [PubMed] [Google Scholar]
- 10.Sarac O, Tasci YY, Gurdal C, Can I. Differentiation of optic disc edema from optic nerve head drusen with spectral-domain optical coherence tomography. J Neuroophthalmol. 2012;32(3):207–211. doi: 10.1097/WNO.0b013e318252561b. [DOI] [PubMed] [Google Scholar]
- 11.Malmqvist L, Bursztyn L, Costello F, Digre K, Fraser JA, Fraser C, Katz B, Lawlor MF, Petzold A, Sibony P, Warner J, Wegener M, Wong S, Hamann S.. The optic disc drusen studies consortium recommendations for diagnosis of optic disc drusen using optical coherence tomography. J Neuroophthalmol. 2018;38(3):299–307. doi: 10.1097/WNO.0000000000000585. [DOI] [PubMed] [Google Scholar]
- 12.Malmqvist L, Li XQ, Hansen MH, Thomsen AK, Skovgaard AM, Olsen EM, Larsen M, Munch IC, Hamann S. Progression over 5 years of prelaminar hyperreflective lines to optic disc drusen in the copenhagen child cohort 2000 eye study. J Neuroophthalmol. 2020;40(3):315–321. doi: 10.1097/WNO.0000000000000911. [DOI] [PubMed] [Google Scholar]
- 13.Traber GL, Weber KP, Sabah M, Keane PA, Plant GT. Enhanced depth imaging optical coherence tomography of optic nerve head drusen: a comparison of cases with and without visual field loss. Ophthalmology. 2017;124(1):66–73. doi: 10.1016/j.ophtha.2016.09.022. [DOI] [PubMed] [Google Scholar]
- 14.Malmqvist L, Lindberg AW, Dahl VA, Jorgensen TM, Hamann S. Quantitatively measured anatomic location and volume of optic disc drusen: an enhanced depth imaging optical coherence tomography study. Invest Ophthalmol Vis Sci. 2017;58(5):2491–2497. doi: 10.1167/iovs.17-21608. [DOI] [PubMed] [Google Scholar]
- 15.Petzold A, Biousse V, Bursztyn L, Costello F, Crum A, Digre K, Fraser C, Fraser JA, Katz B, Jurkute N, Newman N, Lautrup-Battistini J, Lawlor M, Liskova P, Lorenz B, Malmqvist L, Perahallo J, Sibony P, Subramanian P, Rejdek R, Nowomiejska K, Touitou V, Warner J, Wegener M, Wong S, Yu-Wai-Man P, Hamann S.. Multirater validation of peripapillary hyperreflective ovoid mass-like structures (PHOMS). Neuro-Ophthalmol. 2020. (advanced online publication):1–2. doi: 10.1080/01658107.2020.1760891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Malmqvist L, Sibony PA, Fraser CL, Wegener M, Heegaard S, Skougaard M, Hamann S for the Optic Disc Drusen Studies Consortium. Peripapillary ovoid hyperreflectivity in optic disc edema and pseudopapilledema. Ophthalmology. 2018;125(10):1662–1664.doi: 10.1016/j.ophtha.2018.04.036. [DOI] [PubMed] [Google Scholar]
- 17.Pichi F, Romano S, Villani E, Lembo A, Gilardoni F, Morara M, Ciardella AP, Ohno-Matsui K, Nucci P. Spectral-domain optical coherence tomography findings in pediatric tilted disc syndrome. Graefes Arch Clin Exp Ophthalmol. 2014;252(10):1661–1667.doi: 10.1007/s00417-014-2701-8. [DOI] [PubMed] [Google Scholar]
- 18.Borrelli E, Barboni P, Battista M, Sacconi R, Querques L, Cascavilla ML, Bandello F, Querques G. Peripapillary hyperreflective ovoid mass-like structures (PHOMS): OCTA may reveal new findings. Eye (Lond). 2020. doi: 10.1038/s41433-020-0890-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Skougaard M, Heegaard S, Malmqvist L, Hamann S. Prevalence and histopathological signatures of optic disc drusen based on microscopy of 1713 enucleated eyes. Acta Ophthalmol. 2020;98(2):195–200. doi: 10.1111/aos.14180. [DOI] [PubMed] [Google Scholar]
- 20.Paton L, Holmes G. The pathology of papilloedema: a histological study of sixty eyes. Brain. 1911;33(4):389–432. doi: 10.1093/brain/33.4.389. [DOI] [Google Scholar]
- 21.Tso MO. Pathology and pathogenesis of drusen of the optic nervehead. Ophthalmology. 1981;88(10):1066–1080. doi: 10.1016/s0161-6420(81)80038-3. [DOI] [PubMed] [Google Scholar]
- 22.Willerslev A, Li XQ, Cordtz P, Munch IC, Larsen M. Retinal and choroidal intravascular spectral-domain optical coherence tomography. Acta Ophthalmol. 2014;92(2):126–132. doi: 10.1111/aos.12048. [DOI] [PubMed] [Google Scholar]
- 23.Friedman AH, Henkind P, Gartner S. Drusen of the optic disc. A histopathological study. Trans Ophthalmol Soc UK. 1975;95:4–9. [PubMed] [Google Scholar]
- 24.Spaide RF. Enhanced depth imaging optical coherence tomography of retinal pigment epithelial detachment in age-related macular degeneration. Am J Ophthalmol. 2009;147(4):644–652. doi: 10.1016/j.ajo.2008.10.005. [DOI] [PubMed] [Google Scholar]
- 25.Sander B, Larsen M, Thrane L, Hougaard JL, Jorgensen TM. Enhanced optical coherence tomography imaging by multiple scan averaging. Br J Ophthalmol. 2005;89(2):207–212. doi: 10.1136/bjo.2004.045989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Waldstein SM, Faatz H, Szimacsek M, Glodan A-M, Podkowinski D, Montuoro A, Simader C, Gerendas BS, Schmidt-Erfurth U Comparison of penetration depth in choroidal imaging using swept source vs spectral domain optical coherence tomography. Eye (Lond). 2015;29(3):409–415.doi: 10.1038/eye.2014.319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cennamo G, Tebaldi S, Amoroso F, Arvanitis D, Breve M, Cennamo G. Optical coherence tomography angiography in optic nerve drusen. Ophthalmic Res. 2018;59(2):76–80.doi: 10.1159/000481889. [DOI] [PubMed] [Google Scholar]
- 28.Engelke H, Shajari M, Riedel J, Mohr N, Priglinger SG, Mackert MJ. OCT angiography in optic disc drusen: comparison with structural and functional parameters. Br J Ophthalmol. 2019. doi: 10.1136/bjophthalmol-2019-314096. [DOI] [PubMed] [Google Scholar]
- 29.Gaier ED, Rizzo JF 3rd, Miller JB, Cestari DM. Focal capillary dropout associated with optic disc drusen using optical coherence tomographic angiography. J Neuroophthalmol. 2017;37(4):405–410. doi: 10.1097/WNO.0000000000000502. [DOI] [PubMed] [Google Scholar]
- 30.Yan Y, Zhou X, Chu Z, Stell L, Shariati MA, Wang RK, Liao YJ Vision loss in optic disc drusen correlates with increased macular vessel diameter and flux and reduced peripapillary vascular density. Am J Ophthalmol. 2020. doi: 10.1016/j.ajo.2020.04.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Fard MA, Jalili J, Sahraiyan A, Khojasteh H, Hejazi M, Ritch R, Subramanian PS. Optical coherence tomography angiography in optic disc swelling. Am J Ophthalmol. 2018;191:116–123. doi: 10.1016/j.ajo.2018.04.017. [DOI] [PubMed] [Google Scholar]
- 32.Fard MA, Sahraiyan A, Jalili J, Hejazi M, Suwan Y, Ritch R, Subramanian PS. Optical coherence tomography angiography in papilledema compared with pseudopapilledema. Invest Ophthalmol Vis Sci. 2019;60(1):168. doi: 10.1167/iovs.18-25453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Maloca PM, Tufail A, Egan C, Zweifel S, Hasler PW, Petzold A, Emanuel Ramos de Carvalho J. Volume rendering of superficial optic disc drusen. Spektrum der Augenheilkunde. 2017;31(6):288–293. doi: 10.1007/s00717-017-0359-4. [DOI] [Google Scholar]
- 34.Sato T, Mrejen S, Spaide RF. Multimodal imaging of optic disc drusen. Am J Ophthalmol. 2013;156(2): 275-282e1. doi: 10.1016/j.ajo.2013.03.039. [DOI] [PubMed] [Google Scholar]
- 35.Flores-Rodriguez P, Gili P, Martin-Rios MD. Sensitivity and specificity of time-domain and spectral-domain optical coherence tomography in differentiating optic nerve head drusen and optic disc oedema. Ophthalmic Physiol Opt. 2012;32(3):213–221. doi: 10.1111/j.1475-1313.2012.00902.x. [DOI] [PubMed] [Google Scholar]
- 36.Carta A, Favilla S, Prato M, Bianchi-Marzoli S, Sadun AA, Mora P. Accuracy of funduscopy to identify true edema versus pseudoedema of the optic disc. Invest Ophthalmol Vis Sci. 2012;53(1–6). doi: 10.1167/iovs.11-8082. [DOI] [PubMed] [Google Scholar]
- 37.OCT Sub-Study Committee for NORDIC Idiopathic Intracranial Hypertension Study Group , Auinger P, Durbin M, Feldon S, Garvin M, Kardon R, Keltner J, Kupersmith M, Sibony P, Plumb K, Wang J-K, Werner JS. Baseline OCT measurements in the idiopathic intracranial hypertension treatment trial, part I: quality control, comparisons, and variability. Invest Ophthalmol Vis Sci. 2014;5(12):8180–8188 doi: 10.1167/iovs.14-14960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Costello F, Malmqvist L, Hamann S . The role of optical coherence tomography in differentiating optic disc drusen from optic disc edema. Asia Pac J Ophthalmol (Phila). 2018;7(271–279). doi: 10.22608/APO.2018124. [DOI] [PubMed] [Google Scholar]
- 39.Fard MA, Fakhree S, Abdi P, Hassanpoor N, Subramanian PS. Quantification of peripapillary total retinal volume in pseudopapilledema and mild papilledema using spectral-domain optical coherence tomography. Am J Ophthalmol. 2014;158(1):136–143. doi: 10.1016/j.ajo.2014.03.008. [DOI] [PubMed] [Google Scholar]
- 40.Malmqvist L, Li XQ, Eckmann CL, Skovgaard AM, Olsen EM, Larsen M, Munch IC, Hamann S. Optic disc drusen in children: the Copenhagen child cohort 2000 eye study. J Neuroophthalmol. 2018;38:140–146. doi: 10.1097/WNO.0000000000000567. [DOI] [PubMed] [Google Scholar]
- 41.Sibony PA, Kupersmith MJ, Feldon SE, Wang J-K, Garvin M, OCT Substudy Group for the NORDIC Idiopathic Intracranial Hypertension Treatment Trial. Retinal and choroidal folds in papilledema. Invest Ophthalmol Vis Sci. 2015;56(10):5670–5680.doi: 10.1167/iovs.15-17459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sibony PA, Kupersmith MJ, OCT Substudy Group of the NORDIC Idiopathic Intracranial Hypertension Treatment Trial. “Paton’s folds” revisited: peripapillary wrinkles, folds, and creases in papilledema. Ophthalmology. 2016;123(6):1397–1399. doi: 10.1016/j.ophtha.2015.12.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Abazari A, Sibony PA. The etiology of retinal and choroidal folds in optic disc drusen. Ophthalmology. 2020. doi: 10.1016/j.ophtha.2020.05.022. [DOI] [PubMed] [Google Scholar]


