Abstract
The microbiome was originally postulated to contribute to the pathogenesis of NAFLD when the first studies of dysbiosis in NAFLD were reported. Since then, a number of studies have investigated this finding further, in order to discern whether the dysbiosis is the result of the metabolic dysregulation seen with NAFLD or a contributor to the pathogenesis of this condition.
Keywords: NASH, dysbiosis, ethanol, intestinal barrier, bacteria
Introduction:
Non-alcoholic fatty liver disease (NAFLD) is the leading cause of liver disease in the world, currently thought to affect approximately one in four adults and one in ten children[1, 2]. The pathophysiology of NAFLD is multifactorial; with genetic, metabolic and environmental factors affecting patients to a variable degree[3–5]. Another contributor to the development and progression of NAFLD is the intestinal microbiome. The role of the microbiome in the pathophysiology of adult and pediatric NAFLD has been previously summarized[6, 7]. The objective of this review is to highlight the most recent discoveries regarding the role of the microbiome in the pathogenesis of, diagnostic approaches for and treatment of NAFLD. Herein we discuss studies on these topics that were published from January 1, 2017 to December 31, 2019.
Updates regarding dysbiosis in adult and pediatric NAFLD:
The differences in the intestinal microbiota composition and function of patients with NAFLD and controls have been a matter of ongoing scientific interest over the past decade. The results of the most recent investigations on the topic are summarized in Table 1. Most studies continue to be characterized by small sample size and infrequent inclusion of obese, non-NAFLD controls, which is necessary to adjust for the known effects of obesity on the microbiome [8–10]. Furthermore, the definition of NAFLD and NASH is variable in these studies, with some using ultrasonography to determine the presence of NAFLD, which is known to have unacceptably low sensitivity and specificity for this purpose[11], and others defining NASH using the histologic NAFLD activity score, which is also not accurate[12]. Considering these limitations, these studies provide mostly explorative associations between dysbiosis, microbial function and disease severity. However, certain results can be hypothesis-generating, predominantly in terms of the pathophysiology of NAFLD, and may also reveal useful disease biomarkers.
Table 1.
Differences in intestinal microbiota composition and function between patients with and without NAFLD.
| Study | Number of patients and controls | Methodology to assess microbiome | Type of dysbiosis | Differences in microbial function |
|---|---|---|---|---|
| Rau et al. 2018[13] | ADULTS, Germany NAFLD, n=32 (NASH, n=18) HC, n=27 |
16S rRNA gene sequencing | NASH had > Fusobacteria and Fusobacteriaceae compared to NAFL and HC | NAFLD patients had > fecal acetate and propionate, associated with lower resting Tregs and higher Th17/rTreg in blood of NASH patients |
| Da Silva et al. 2018[14] | ADULTS, Canada NAFLD, n=39 (NASH, n=24) HC, n=28 |
16S rRNA gene sequencing | NASH not different than NAFL NAFLD have < Ruminococcus, F. prausnitzii and Coprococcus |
NAFLD patients had > fecal propionate, isobutyrate and serum 2-hydroxybutyrate and L-lactic acid |
| Shen et al. 2017[15] | ADULTS, China NAFLD, n=25 (NASH*, n=6) HC, n=22 |
16S rDNA amplicon sequencing | NASH had > Blautia compared to non-NASH Those with ≥F2 had > Escherichia_Shigella vs. those with F0/1 |
N/A |
| Iino et al. 2019[16] | ADULTS, Japan 153 pairs of NAFLD (by u/s) and BMI/sex-matched non-NAFLD |
16S rRNA gene sequencing | NAFLD had lower Ruminococcaceae and Faecalibacterium | N/A |
| Schwimmer et al. 2019[17] | CHILDREN, USA NAFLD, n=87 (NASH, n=48) Obese non-NAFLD, n=37 |
16S rRNA gene sequencing | NASH had lowest α diversity P. copri associated with more severe fibrosis |
N/A |
| Chierico et al. 2017[18] | CHILDREN, Italy NAFLD, n=53 (NASH, n=26) obese, n=8 HC, n=54 |
16S rRNA gene sequencing | No significant differences between NASH, NAFL, obese. Compared to HC, NAFLD had < Oscillospira and NASH had > Ruminococcus, Blautia, Dorea |
Of 292 VOCs, 26 were up- and 2 were down-regulated in NAFLD |
defined as NAS≥5
F: fibrosis; HC: healthy controls; NAFL: non-alcoholic fatty liver; NAFLD: non-alcoholic fatty liver disease; NASH: non-alcoholic steatohepatitis; u/s: ultrasound; VOC: volatile organic compounds
Updates regarding the role of the intestinal microbiome in the pathogenesis of NAFLD (Figure 1):
Figure 1:
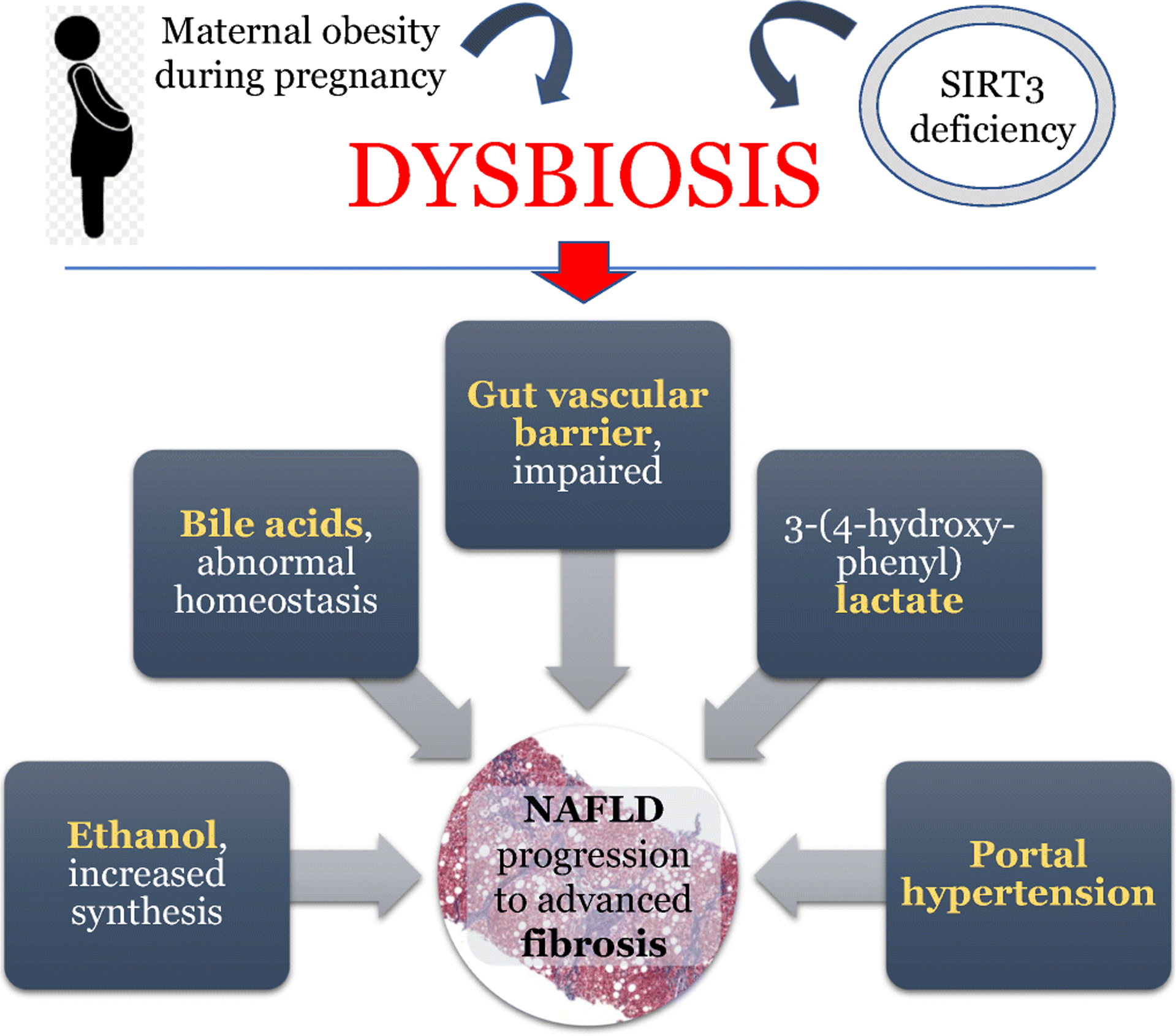
A summary of the recent findings that describe the pathophysiology of NAFLD.
a. Early life exposures
The importance of early metabolic exposures of the individual on the risk of developing hepatic steatosis and inflammation later on in life, and the involvement of the microbiome in this process, have become more apparent recently. Using a murine model, Wankhade et al. showed that maternal obesity during pregnancy causes epigenetic changes that impact on the metabolism of the offspring [19]. Furthermore, they showed that maternal obesity is associated with dysbiosis in the offspring. Soderborg et al. used fecal transplantation studies to discern whether the distinct microbiota composition of infants born to obese mothers can contribute to the risk of NAFLD[20]. When the stool of 2-week old infants was transplanted to germ-free mice there was evidence of impaired bile acid signaling (similar to that previously reported in NAFLD), impaired innate immunity, and portal inflammation in the liver. When these mice were subsequently exposed to a Western-style diet, they gained more weight and fat mass than their counterparts who had received stool from infants born to lean mothers. The diet also led to a phenotype identical to pediatric NAFLD, with hepatic steatosis, portal and lobular inflammation, as well as increased expression of pro-inflammatory cytokines and decreased expression of anti-inflammatory cytokines. This study highlighted that dysbiosis may precede the development of NAFLD and acts synergistically with the diet in the development of the final hepatic phenotype. Importantly, the authors also suggested that this type of microbiome-induced injury could explain the portal pattern of inflammation seen in type 1 (pediatric) NAFLD[21].
b. Ethanol
It has now become apparent that microbiome-driven synthesis of ethanol is an important contributor to the pathogenesis of NAFLD/NASH, in a subset of patients. Aragones et al. recently showed that among 53 Caucasian women with severe obesity and NAFLD, circulating ethanol levels were significantly higher in patients with NASH compared to those with non-alcoholic fatty liver (NAFL)[22]. Studying the intestinal microbiome of an Asian cohort, Yuan et al. revealed that 61% of patients with NASH carried high-ethanol producing Klebsiella pneumonia strains, as opposed to 6% of healthy controls[23]. Using fecal transplantation studies, they showed that these microbes were intimately linked to the pathogenesis of NASH through their ethanol-synthesizing capacity. This effect was both preventable and treatable with the use of bacteriophages and antibiotics, respectively. These findings, should they be validated, suggest that the identification of the high-ethanol producing Klebsiella strain could serve as both a predictor of disease severity and progression, as well as an indication for antimicrobial treatment.
c. Intestinal permeability, including the gut vascular barrier
Recent links between host genotype and dysbiosis in the pathogenesis of NAFLD/NASH were provided by Chen et al.[24]. In a murine model of NASH, they studied the role of Sirtuin 3 (SIRT3), a known regulator of mitochondrial function, on intestinal microbiota composition and gut barrier function. This is important, because single nucleotide polymorphisms of the human SIRT3 gene that lead to low enzyme activity have been previously linked to steatosis and NASH development[25]. Chen et al. showed that SIRT3 deficiency leads to dysbiosis, increased intestinal permeability and endotoxemia, as well as inability to attenuate the intestinal inflammation that is induced by high fat diet feeding. Butyrate supplementation ameliorates this phenotype, as shown by improvements in both intestinal permeability and markers of intestinal steatosis and inflammation. The beneficial impact of butyrate on dysbiosis, gut barrier function and metabolic dysregulation has been shown in other animal models of NASH as well[26].
Beyond the epithelial barrier, an intriguing study by Mouries et al. recently showed the role of the microbiome in disrupting the gut vascular barrier[27]. In a series of elegant in vitro, in vivo and human studies they showed that diet-associated dysbiosis initially leads to the disruption of the intestinal epithelial barrier (tight junctions etc.) and subsequently the disruption of the gut vascular barrier. This study revealed for the first time that the latter is a necessary event for NASH to develop, even in the context of high fat diet feeding and obesity. Importantly, obeticholic acid (a farsenoid X receptor [FXR]) agonist was proven to be efficacious in preventing the disruption of the gut vascular barrier in this context, suggesting that this agent could serve not only as a treatment modality for NASH but also as a preventative approach against progression to end stage liver disease.
d. Bile acids
Using fecal transplantation studies and germ-free mice, Petrov et al. reported on the characteristics of the microbiome and metabolome of mice protected from high fat diet feeding[28]. They showed that beyond specific changes to the microbiome (increased Dusulfovibrio and Oscillospira and decreased Bacteroides and Oribacterium), a “protected phenotype” was associated with lower primary and higher secondary bile acids in both the stool and the plasma, as well as induction of genes involved in hepatic bile acid transport and down regulation of genes involved in de novo lipogenesis and bile acid synthesis.
In contrast, human studies have shown that while serum bile acids are increased in patients with NAFLD, with a predominance of bile acids that antagonize FXR, there is evidence of impaired FXR signaling[29, 30]. Jiao et al. showed that the dysbiosis of patients with NAFLD favors the conversion of primary to secondary bile acids, which may have implications for future treatments[29]. Tan et al. provided another link between the microbiome and the impaired bile acid homeostasis seen in patients with NAFLD. Trimethylamine N-oxide (TMAO) is produced in the liver following the metabolism of the bacterially derived substrate trimethylamine (TMA). Tan et al. demonstrated that serum TMAO levels correlate with serum bile acids in patients with NAFLD. They subsequently performed a series of animal studies showing that TMAO administration to mice, along with a high fat diet, leads to hepatic steatosis associated with a shift in bile acids to an FXR antagonistic profile and a significant increase in the expression of genes involved in de novo lipogenesis[30]. The effect of TMAO supplementation on lipogenesis genes was attenuated with the use of FXR agonists, underscoring the importance of FXR in this process and further supporting the role of FXR agonism for the treatment of NAFLD. Other animal models of NAFLD (e.g. mice fed high fat/high cholesterol/high fructose diet) have failed to show a role for TMAO in the pathogenesis of steatohepatitis[31]. Its exact role in human NAFLD remains to be determined.
e. Portal hypertension
The intestinal microbiome appears to also be involved in the regulation of portal hypertension that develops in the advanced stages of NAFLD/NASH. In a rat model of NASH, fecal transplantation experiments from control animals to those with established (diet-induced) NASH led to significant reduction in portal pressures[32]. Should this be validated in humans and should the key microorganisms driving this process be identified, the future personalized treatment approach for portal hypertension may become targeted antimicrobials.
f. Other
In a proof of concept study, Caussy et al. linked the microbiome to NAFLD severity by demonstrating in both a discovery and a validation cohort, that the microbial metabolite 3-(4-hydroxy-phenyl) lactate had a robust shared gene-effect with steatosis and fibrosis[33]. Such studies are important because they highlight pathways that might serve as treatment targets in the future.
Updates regarding the use of the microbiome as a biomarker of disease severity.
A number of studies have investigated whether microbiome data can be used to predict NAFLD disease presence and severity and the preliminary results have been quite encouraging. Hoyles et al. performed an in-depth metabolomics and transcriptomics study in severely obese, non-diabetic women in Europe and showed that the plasma metabolome could predict the presence of steatosis with 79% accuracy[34]. This was far superior to the widely available clinical variables that had a predictive accuracy of 58%. In another cohort of severely obese women, a combination of serum metabolites including ethanol, betaine, glycocholic acid and deoxycholic acid predicted the presence of NASH with an area under the curve of 0.776[22]. Using whole genome shotgun sequencing, Loomba et al. determined that a combination of 40 variables (37 bacterial species, Shannon diversity, BMI and age) were highly predictive of advanced fibrosis (stage 3 or 4 fibrosis; 94% accuracy) in a cohort of 86 well characterized adults with biopsy-proven NAFLD [35]. In separate cohort of patients with cirrhosis, a model including 30 variables (27 bacterial species, BMI, age and sex) was highly predictive of cirrhosis with an AUC: 0.92[36]. Interestingly, this model performed well in a validation cohort of first-degree relatives of patients with NAFLD cirrhosis, where advanced fibrosis could be predicted with 87% accuracy. Similarly, in children, Schwimmer et al., showed that a combination of serum ALT levels and abundance of gene(s) encoding for either the lipopolysaccharide pathway or flagella biosynthesis had an AUC of 0.92 in predicting NASH and an AUC of 0.87 in predicting moderate to severe fibrosis, respectively[17]. There is currently no other non-invasive biomarker that can accurately predict the presence of NASH or significant fibrosis in pediatric NAFLD[37]. Given how encouraging the results of these studies are, the key next step is to prove the generalizability of the findings. Once that is done, the key microbial biomarkers would have to become clinically available for these tools to become part of daily clinical practice.
Updates regarding treatment options that target the microbiome as an approach to treat NAFLD/NASH:
The past decade has seen an exponential rise in the clinical trials for the treatment of NAFLD[38, 39]. Among the various approaches used, several are aimed at correcting the dysbiosis seen in this context, either directly or indirectly. The most recent data in the field are summarized here.
Dietary changes lead to shifts in intestinal microbial composition, which can be seen rapidly (as early as within 24 hours of the intervention)[40]. Until recently, it was thought that for the treatment of NAFLD, the type of diet used was not as relevant, as long as weight loss was achieved [41]. More recent data however, have revealed that dietary changes can lead to dramatic reductions in hepatic steatosis, without significant weight loss and that the accompanying intestinal microbiome shifts may contribute to the improvements seen. Mardinoglu et al. showed that an isocaloric, carbohydrate-restricted, high protein and high fat diet given to obese adults with NAFLD for 14 days, led to dramatic reductions in hepatic steatosis (~44%), as well as reductions in circulating cytokines (IL-6, TNFa)[42]. The reduction in steatosis was associated with a down-regulation of de novo lipogenesis pathways and an up-regulation of fatty acid oxidation pathways. Accompanying shifts in microbiota composition were seen, such as increases in the abundance of folate-synthesizing bacteria, which were in turn associated with an increase in serum folate levels. Folate-mediated pathways were upregulated in the livers of a separate cohort who was treated with this diet for 7 days, including pathways of glutathione synthesis, an important antioxidant and regulator of fatty acid oxidation. Overall, this important study suggested that diet-induced alterations of the microbiome have direct implications on hepatic metabolism and, ultimately NAFLD treatment, which can be independent of weight loss.
Beyond diet-induced modifications to the dysbiosis seen in patients with NAFLD, studies have addressed the efficacy of pre-, pro- and synbiotics in this context. Loman et al. performed a systematic review and meta-analysis of studies published up to December 2017 the effectiveness of prebiotics and probiotics for patients with NAFLD[43]. The results of the meta-analysis suggested that these therapies lead to statistically, but not clinically significant reductions in serum aminotransferases and lipid levels. Similar results were reported by a subsequent meta-analysis, which included publications up to December 2018[44]. In this study, probiotics/synbiotics were found to improve aminotransferases, liver stiffness and ultrasound-based measures of steatosis. It should be noted, that the major limitation of these meta-analyses is the lack of data regarding histologic outcomes in the assessment of treatment efficacy. Since then, a few more studies have become available, which are summarized in Table 2. In short, the results are encouraging, but still, larger studies are needed to determine the true impact of microbial therapies on key outcomes, such as liver stiffness, inflammation, hepatocellular ballooning and fibrosis.
Table 2:
Recent studies on the role of probiotics and prebiotics for the treatment of NAFLD
| Study | Population/ study design | Intervention | Primary outcomes | Result |
|---|---|---|---|---|
| PROBIOTICS | ||||
| Kobyliak et al. Minerva Med. 2018[46] | 48 adults, RCT x8 weeks | “Symbiter Omega” (probiotic + ω−3 FA) vs. placebo |
|
|
| Duseja et al. BMJ Open Gastro 2019[47] | 39 adults, RCT, 1 year | Multistrain probiotic and lifestyle change or placebo and lifestyle change |
|
|
| Ahn et al. Sci Rep 2o19[48] | 68 adults, RCT, x12 weeks | Probiotic mixtures vs. placebo |
|
|
| PREBIOTICS | ||||
| Chambers et al. Diabetes Obes Metab 2019[49] | 18 adults, RCT, x42 days | Inulin-propionate ester (20 g/day) vs. inulin control |
|
|
IHF: intrahepatic fat; MRI: Magnetic Resonance Elastography; NAS: NAFLD Activity Score; PDFF: Proton Density Fat Fraction; RCT: Randomized Controlled Trial; SWE: Shear Wave Elastography; TC: total cholesterol, TG: triglycerides; VFA: variable flip angle
Antibiotics have also been investigated for the treatment of NAFLD/NASH. Rifaximin, was studied in an open label pilot study by Cobbold et al. for patients with NASH and elevated serum aminotransferase levels[45]. A 6-week treatment of 15 patients with 400 mg of rifaximin twice daily was not associated with improvements in aminotransferases, which in fact, increased further 6 weeks following the cessation of treatment. Glucose tolerance, intrahepatic lipid content and intestinal microbiota abundance remained unchanged with treatment. As mentioned in the ethanol section above, it may be that only a subset of patients would benefit from treatment with antimicrobial agents and these should be carefully selected to target specific microbes.
In terms of fecal microbial transplantation studies, as of January 2020, there are no published studies for the treatment of NAFLD/NASH. However, in clinicaltrials.gov there are four studies on the subject, one of which has completed enrollment. The results of these highly anticipated studies might reveal an additional treatment approach for NASH.
Conclusion and future directions:
In summary, the past three years (2017–2019) have enriched the literature in terms of the role of the microbiome in the pathophysiology of NAFLD, as well as its possible use as both a disease biomarker and treatment target. It is now clear that dysbiosis is a key disease driver for at least a subset of patients with NAFLD. As such, personalized medicine approaches, targeting the offending microorganism (e.g. Klebsiella pneumoniae) or its key metabolites (e.g. ethanol synthesis) may be the ideal approach to treating these patients.
a). Purpose of review:
This review is aimed at highlighting the most important discoveries from the years 2017–2019 that linked the microbiome to the pathogenesis, diagnosis and treatment of NAFLD.
b). Recent findings:
Recent data have revealed further the role of bacterially derived ethanol in the pathogenesis of NAFLD, the crosstalk between microbiome and the gut barrier, as well as the interplay between genetic risk and dysbiosis.
c). Summary:
The microbiome is involved in the pathogenesis of NAFLD in a subset of patients and can be used as a therapeutic target.
Funding Statement
RL receives funding support from NIEHS (5P42ES010337), NCATS (5UL1TR001442), NIDDK (R01DK106419, P30DK120515), and DOD PRCRP (CA170674P2). The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH.
Funding: This work was not funded.
Abbreviations:
- BMI
body mass index
- F
fibrosis
- FXR
Farnesoid X receptor
- HC
healthy controls
- IHF
intrahepatic fat
- MRI-PDFF
Magnetic Resonance Imaging-Proton Density Fat Fraction
- NAFL
non-alcoholic fatty liver
- NAFLD
Non-Alcoholic Fatty Liver Disease
- NAS
NAFLD Activity Score
- NASH
Nonalcoholic Steatohepatitis
- RCT
Randomized Controlled Trial
- SCFA
Short Chain Fatty Acids
- SWE
Shear Wave Elastography
- TC
total cholesterol
- TG
triglycerides
- TMAO
Trimethylamine N-oxide
- u/s
ultrasound
- VFA
variable flip angle
- VOC
volatile organic compounds
Footnotes
Potential conflict of interests: Rohit Loomba:
RL serves as a consultant or advisory board member for Arrowhead Pharmaceuticals, AstraZeneca, Bird Rock Bio, Boehringer Ingelheim, Bristol-Myer Squibb, Celgene, Cirius, CohBar, Conatus, Eli Lilly, Galmed, Gemphire, Gilead, Glympse bio, GNI, GRI Bio, Intercept, Ionis, Janssen Inc., Merck, Metacrine, Inc., NGM Biopharmaceuticals, Novartis, Novo Nordisk, Pfizer, Prometheus, Sanofi, Siemens, and Viking Therapeutics. In addition, his institution has received grant support from Allergan, Boehringer-Ingelheim, Bristol-Myers Squibb, Cirius, Eli Lilly and Company, Galectin Therapeutics, Galmed Pharmaceuticals, GE, Genfit, Gilead, Intercept, Grail, Janssen, Madrigal Pharmaceuticals, Merck, NGM Biopharmaceuticals, NuSirt, Pfizer, pH Pharma, Prometheus, and Siemens. He is also co-founder of Liponexus, Inc.
References:
- 1.Younossi Z, Anstee QM, Marietti M, Hardy T, Henry L, Eslam M, George J, Bugianesi E: Global burden of NAFLD and NASH: trends, predictions, risk factors and prevention. Nature reviews Gastroenterology & hepatology 2018, 15(1):11–20. [DOI] [PubMed] [Google Scholar]
- 2.Anderson EL, Howe LD, Jones HE, Higgins JP, Lawlor DA, Fraser A: The Prevalence of Non-Alcoholic Fatty Liver Disease in Children and Adolescents: A Systematic Review and Meta-Analysis. PloS one 2015, 10(10):e0140908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Eslam M, Valenti L, Romeo S: Genetics and epigenetics of NAFLD and NASH: Clinical impact. J Hepatol 2018, 68(2):268–279. [DOI] [PubMed] [Google Scholar]
- 4.Friedman SL, Neuschwander-Tetri BA, Rinella M, Sanyal AJ: Mechanisms of NAFLD development and therapeutic strategies. Nature medicine 2018, 24(7):908–922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Caussy C, Soni M, Cui J, Bettencourt R, Schork N, Chen CH, Ikhwan MA, Bassirian S, Cepin S, Gonzalez MP et al. : Nonalcoholic fatty liver disease with cirrhosis increases familial risk for advanced fibrosis. The Journal of clinical investigation 2017, 127(7):2697–2704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Leung C, Rivera L, Furness JB, Angus PW: The role of the gut microbiota in NAFLD. Nature reviews Gastroenterology & hepatology 2016, 13(7):412–425. [DOI] [PubMed] [Google Scholar]
- 7.Mouzaki M, Loomba R: Insights into the evolving role of the gut microbiome in nonalcoholic fatty liver disease: rationale and prospects for therapeutic intervention. Therapeutic advances in gastroenterology 2019, 12:1756284819858470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Turnbaugh PJ, Gordon JI: The core gut microbiome, energy balance and obesity. The Journal of physiology 2009, 587(Pt 17):4153–4158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Turnbaugh PJ, Hamady M, Yatsunenko T, Cantarel BL, Duncan A, Ley RE, Sogin ML, Jones WJ, Roe BA, Affourtit JP et al. : A core gut microbiome in obese and lean twins. Nature 2009, 457(7228):480–484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Turnbaugh PJ, Ley RE, Mahowald MA, Magrini V, Mardis ER, Gordon JI: An obesity-associated gut microbiome with increased capacity for energy harvest. Nature 2006, 444(7122):1027–1031. [DOI] [PubMed] [Google Scholar]
- 11.Awai HI, Newton KP, Sirlin CB, Behling C, Schwimmer JB: Evidence and recommendations for imaging liver fat in children, based on systematic review. Clinical gastroenterology and hepatology : the official clinical practice journal of the American Gastroenterological Association 2014, 12(5):765–773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kleiner DE, Brunt EM, Van Natta M, Behling C, Contos MJ, Cummings OW, Ferrell LD, Liu YC, Torbenson MS, Unalp-Arida A et al. : Design and validation of a histological scoring system for nonalcoholic fatty liver disease. Hepatology 2005, 41(6):1313–1321. [DOI] [PubMed] [Google Scholar]
- 13.Rau M, Rehman A, Dittrich M, Groen AK, Hermanns HM, Seyfried F, Beyersdorf N, Dandekar T, Rosenstiel P, Geier A: Fecal SCFAs and SCFA-producing bacteria in gut microbiome of human NAFLD as a putative link to systemic T-cell activation and advanced disease. United European gastroenterology journal 2018, 6(10):1496–1507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Da Silva HE, Teterina A, Comelli EM, Taibi A, Arendt BM, Fischer SE, Lou W, Allard JP: Nonalcoholic fatty liver disease is associated with dysbiosis independent of body mass index and insulin resistance. Scientific reports 2018, 8(1):1466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Shen F, Zheng RD, Sun XQ, Ding WJ, Wang XY, Fan JG: Gut microbiota dysbiosis in patients with non-alcoholic fatty liver disease. Hepatobiliary & pancreatic diseases international : HBPD INT 2017, 16(4):375–381. [DOI] [PubMed] [Google Scholar]
- 16.Iino C, Endo T, Mikami K, Hasegawa T, Kimura M, Sawada N, Nakaji S, Fukuda S: Significant decrease in Faecalibacterium among gut microbiota in nonalcoholic fatty liver disease: a large BMI- and sex-matched population study. Hepatol Int 2019, 13(6):748–756. [DOI] [PubMed] [Google Scholar]
- 17.Schwimmer JB, Johnson JS, Angeles JE, Behling C, Belt PH, Borecki I, Bross C, Durelle J, Goyal NP, Hamilton G et al. : Microbiome Signatures Associated With Steatohepatitis and Moderate to Severe Fibrosis in Children With Nonalcoholic Fatty Liver Disease. Gastroenterology 2019, 157(4):1109–1122. [DOI] [PMC free article] [PubMed] [Google Scholar]; * The largest pediatric study to date (n=87 with NAFLD and n=37 obese controls) studying dysbiosis in NAFLD. While remains to be validated, this study showed that microbiome data are highly accurate (AUC>0.85) in predicting disease phenotypes (e.g. NASH and moderate/severe fibrosis).
- 18.Del Chierico F, Nobili V, Vernocchi P, Russo A, De Stefanis C, Gnani D, Furlanello C, Zandona A, Paci P, Capuani G et al. : Gut microbiota profiling of pediatric nonalcoholic fatty liver disease and obese patients unveiled by an integrated meta-omics-based approach. Hepatology 2017, 65(2):451–464. [DOI] [PubMed] [Google Scholar]
- 19.Wankhade UD, Zhong Y, Kang P, Alfaro M, Chintapalli SV, Thakali KM, Shankar K: Enhanced offspring predisposition to steatohepatitis with maternal high-fat diet is associated with epigenetic and microbiome alterations. PloS one 2017, 12(4):e0175675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Soderborg TK, Clark SE, Mulligan CE, Janssen RC, Babcock L, Ir D, Young B, Krebs N, Lemas DJ, Johnson LK et al. : The gut microbiota in infants of obese mothers increases inflammation and susceptibility to NAFLD. Nature communications 2018, 9(1):4462. [DOI] [PMC free article] [PubMed] [Google Scholar]; **Showing for the first time that, when transplanted to germ-free mice, the microbiome of 2-week-old infants born to obese mothers is able to induce immune dysregulation, gut barrier dysfunction and hepatic inflammation, which when coupled with a Western-style diet leads to a NAFLD phenotype.
- 21.Africa JA, Behling CA, Brunt EM, Zhang N, Luo Y, Wells A, Hou J, Belt PH, Kohil R, Lavine JE et al. : In Children With Nonalcoholic Fatty Liver Disease, Zone 1 Steatosis Is Associated With Advanced Fibrosis. Clinical gastroenterology and hepatology : the official clinical practice journal of the American Gastroenterological Association 2018, 16(3):438–446 e431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Aragones G, Colom-Pellicer M, Aguilar C, Guiu-Jurado E, Martinez S, Sabench F, Antonio Porras J, Riesco D, Del Castillo D, Richart C et al. : Circulating microbiota-derived metabolites: a “liquid biopsy? International journal of obesity 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yuan J, Chen C, Cui J, Lu J, Yan C, Wei X, Zhao X, Li N, Li S, Xue G et al. : Fatty Liver Disease Caused by High-Alcohol-Producing Klebsiella pneumoniae. Cell metabolism 2019, 30(6):1172. [DOI] [PubMed] [Google Scholar]; **** Study showing more definitively the role of bacterially synthesized ethanol on the pathogenesis of NAFLD, in at least a subset of patients. Specifically, high alcohol producing Klebsiella pneumoniae (HiAlc Kpn) was reported to be much more prevalent in adults with NAFLD in China than controls. Animal studies using fecal transplantation showed that HiAlc Kpn was necessary for NAFLD to develop.
- 24.Chen M, Hui S, Lang H, Zhou M, Zhang Y, Kang C, Zeng X, Zhang Q, Yi L, Mi M: SIRT3 Deficiency Promotes High-Fat Diet-Induced Nonalcoholic Fatty Liver Disease in Correlation with Impaired Intestinal Permeability through Gut Microbial Dysbiosis. Molecular nutrition & food research 2019, 63(4):e1800612. [DOI] [PubMed] [Google Scholar]
- 25.Hirschey MD, Shimazu T, Jing E, Grueter CA, Collins AM, Aouizerat B, Stancakova A, Goetzman E, Lam MM, Schwer B et al. : SIRT3 deficiency and mitochondrial protein hyperacetylation accelerate the development of the metabolic syndrome. Molecular cell 2011, 44(2):177–190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zhou D, Pan Q, Xin FZ, Zhang RN, He CX, Chen GY, Liu C, Chen YW, Fan JG: Sodium butyrate attenuates high-fat diet-induced steatohepatitis in mice by improving gut microbiota and gastrointestinal barrier. World journal of gastroenterology 2017, 23(1):60–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mouries J, Brescia P, Silvestri A, Spadoni I, Sorribas M, Wiest R, Mileti E, Galbiati M, Invernizzi P, Adorini L et al. : Microbiota-driven gut vascular barrier disruption is a prerequisite for non-alcoholic steatohepatitis development. J Hepatol 2019, 71(6):1216–1228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Petrov PD, Garcia-Mediavilla MV, Guzman C, Porras D, Nistal E, Martinez-Florez S, Castell JV, Gonzalez-Gallego J, Sanchez-Campos S, Jover R: A Network Involving Gut Microbiota, Circulating Bile Acids, and Hepatic Metabolism Genes That Protects Against Non-Alcoholic Fatty Liver Disease. Molecular nutrition & food research 2019, 63(20):e1900487. [DOI] [PubMed] [Google Scholar]
- 29.Jiao N, Baker SS, Chapa-Rodriguez A, Liu W, Nugent CA, Tsompana M, Mastrandrea L, Buck MJ, Baker RD, Genco RJ et al. : Suppressed hepatic bile acid signalling despite elevated production of primary and secondary bile acids in NAFLD. Gut 2018, 67(10):1881–1891. [DOI] [PubMed] [Google Scholar]
- 30.Tan X, Liu Y, Long J, Chen S, Liao G, Wu S, Li C, Wang L, Ling W, Zhu H: Trimethylamine N-Oxide Aggravates Liver Steatosis through Modulation of Bile Acid Metabolism and Inhibition of Farnesoid X Receptor Signaling in Nonalcoholic Fatty Liver Disease. Molecular nutrition & food research 2019, 63(17):e1900257. [DOI] [PubMed] [Google Scholar]
- 31.Janssen AWF, Houben T, Katiraei S, Dijk W, Boutens L, van der Bolt N, Wang Z, Brown JM, Hazen SL, Mandard S et al. : Modulation of the gut microbiota impacts nonalcoholic fatty liver disease: a potential role for bile acids. Journal of lipid research 2017, 58(7):1399–1416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Garcia-Lezana T, Raurell I, Bravo M, Torres-Arauz M, Salcedo MT, Santiago A, Schoenenberger A, Manichanh C, Genesca J, Martell M et al. : Restoration of a healthy intestinal microbiota normalizes portal hypertension in a rat model of nonalcoholic steatohepatitis. Hepatology 2018, 67(4):1485–1498. [DOI] [PubMed] [Google Scholar]
- 33.Caussy C, Hsu C, Lo MT, Liu A, Bettencourt R, Ajmera VH, Bassirian S, Hooker J, Sy E, Richards L et al. : Link between gut-microbiome derived metabolite and shared gene-effects with hepatic steatosis and fibrosis in NAFLD. Hepatology 2018, 68(3):918–932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hoyles L, Fernandez-Real JM, Federici M, Serino M, Abbott J, Charpentier J, Heymes C, Luque JL, Anthony E, Barton RH et al. : Molecular phenomics and metagenomics of hepatic steatosis in non-diabetic obese women. Nature medicine 2018, 24(7):1070–1080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Loomba R, Seguritan V, Li W, Long T, Klitgord N, Bhatt A, Dulai PS, Caussy C, Bettencourt R, Highlander SK et al. : Gut Microbiome-Based Metagenomic Signature for Non-invasive Detection of Advanced Fibrosis in Human Nonalcoholic Fatty Liver Disease. Cell metabolism 2017, 25(5):1054–1062 e1055. [DOI] [PMC free article] [PubMed] [Google Scholar]; * The first study to study the stool microbiome (using metagenomic sequencing) and the serum metabolome of a well characterized adult cohort with NAFLD to determine microbial biomarkers of advanced fibrosis. The investigators determined that a combination of 37 bacterial species along with the Shannon diversity, age and body mass index had a diagnostic accuracy of 0.936 for the determination of advanced fibrosis
- 36.Caussy C, Tripathi A, Humphrey G, Bassirian S, Singh S, Faulkner C, Bettencourt R, Rizo E, Richards L, Xu ZZ et al. : A gut microbiome signature for cirrhosis due to nonalcoholic fatty liver disease. Nature communications 2019, 10(1):1406. [DOI] [PMC free article] [PubMed] [Google Scholar]; *A large study of patients with NAFLD and their family members that showed that microbiota can serve as biomarkers of cirrhosis. A panel of 30 features, including 27 bacterial features, had an AUC of 0.92 for predicting the presence of cirrhosis in a cohort of probands with NAFLD and an AUC of 0.87 in a validation cohort of relatives of probands with NAFLD
- 37.Jackson JA, Konomi JV, Mendoza MV, Krasinskas A, Jin R, Caltharp S, Mouzaki M, Vos MB: Performance of fibrosis prediction scores in paediatric non-alcoholic fatty liver disease. J Paediatr Child Health 2018, 54(2):172–176. [DOI] [PubMed] [Google Scholar]
- 38.Konerman MA, Jones JC, Harrison SA: Pharmacotherapy for NASH: Current and emerging. J Hepatol 2018, 68(2):362–375. [DOI] [PubMed] [Google Scholar]
- 39.Sumida Y, Yoneda M: Current and future pharmacological therapies for NAFLD/NASH. J Gastroenterol 2018, 53(3):362–376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.David LA, Maurice CF, Carmody RN, Gootenberg DB, Button JE, Wolfe BE, Ling AV, Devlin AS, Varma Y, Fischbach MA et al. : Diet rapidly and reproducibly alters the human gut microbiome. Nature 2014, 505(7484):559–563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Chalasani N, Younossi Z, Lavine JE, Charlton M, Cusi K, Rinella M, Harrison SA, Brunt EM, Sanyal AJ: The diagnosis and management of nonalcoholic fatty liver disease: Practice guidance from the American Association for the Study of Liver Diseases. Hepatology 2018, 67(1):328–357. [DOI] [PubMed] [Google Scholar]
- 42.Mardinoglu A, Wu H, Bjornson E, Zhang C, Hakkarainen A, Rasanen SM, Lee S, Mancina RM, Bergentall M, Pietilainen KH et al. : An Integrated Understanding of the Rapid Metabolic Benefits of a Carbohydrate-Restricted Diet on Hepatic Steatosis in Humans. Cell metabolism 2018, 27(3):559–571 e555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Loman BR, Hernandez-Saavedra D, An R, Rector RS: Prebiotic and probiotic treatment of nonalcoholic fatty liver disease: a systematic review and meta-analysis. Nutrition reviews 2018, 76(11):822–839. [DOI] [PubMed] [Google Scholar]
- 44.Sharpton SR, Maraj B, Harding-Theobald E, Vittinghoff E, Terrault NA: Gut microbiome-targeted therapies in nonalcoholic fatty liver disease: a systematic review, meta-analysis, and meta-regression. The American journal of clinical nutrition 2019, 110(1):139–149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Cobbold JFL, Atkinson S, Marchesi JR, Smith A, Wai SN, Stove J, Shojaee-Moradie F, Jackson N, Umpleby AM, Fitzpatrick J et al. : Rifaximin in non-alcoholic steatohepatitis: An open-label pilot study. Hepatology research : the official journal of the Japan Society of Hepatology 2018, 48(1):69–77. [DOI] [PubMed] [Google Scholar]
- 46.Kobyliak N, Abenavoli L, Falalyeyeva T, Mykhalchyshyn G, Boccuto L, Kononenko L, Kyriienko D, Komisarenko I, Dynnyk O: Beneficial effects of probiotic combination with omega-3 fatty acids in NAFLD: a randomized clinical study. Minerva medica 2018, 109(6):418–428. [DOI] [PubMed] [Google Scholar]
- 47.Duseja A, Acharya SK, Mehta M, Chhabra S, Shalimar, Rana S, Das A, Dattagupta S, Dhiman RK, Chawla YK: High potency multistrain probiotic improves liver histology in non-alcoholic fatty liver disease (NAFLD): a randomised, double-blind, proof of concept study. BMJ open gastroenterology 2019, 6(1):e000315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ahn SB, Jun DW, Kang BK, Lim JH, Lim S, Chung MJ: Randomized, Double-blind, Placebo-controlled Study of a Multispecies Probiotic Mixture in Nonalcoholic Fatty Liver Disease. Scientific reports 2019, 9(1):5688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Chambers ES, Byrne CS, Rugyendo A, Morrison DJ, Preston T, Tedford C, Bell JD, Thomas L, Akbar AN, Riddell NE et al. : The effects of dietary supplementation with inulin and inulin-propionate ester on hepatic steatosis in adults with non-alcoholic fatty liver disease. Diabetes, obesity & metabolism 2019, 21(2):372–376. [DOI] [PMC free article] [PubMed] [Google Scholar]


