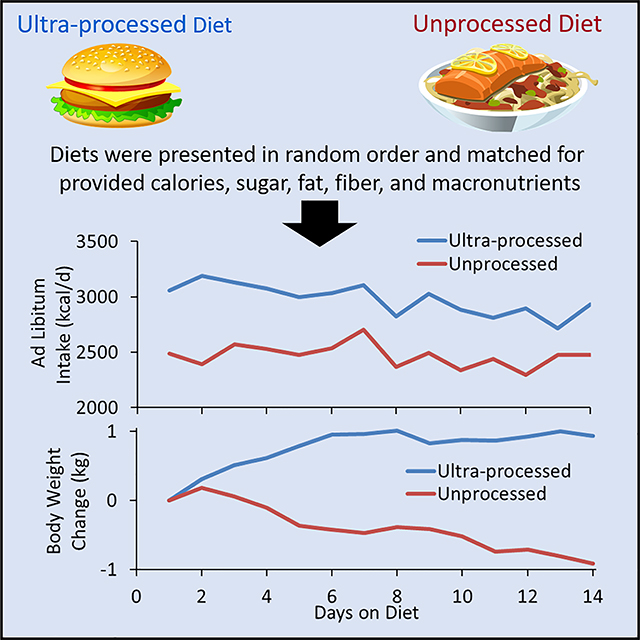
Summary
We investigated whether ultra-processed foods affect energy intake in 20 weight-stable adults, aged (mean±SE) 31.2±1.6 y and BMI=27±1.5 kg/m2. Subjects were admitted to the NIH Clinical Center and randomized to receive either ultra-processed or unprocessed diets for 2 weeks immediately followed by the alternate diet for 2 weeks. Meals were designed to be matched for presented calories, energy density, macronutrients, sugar, sodium, and fiber. Subjects were instructed to consume as much or as little as desired. Energy intake was greater during the ultra-processed diet (508±106 kcal/d; p=0.0001), with increased consumption of carbohydrate (280±54 kcal/d; p<0.0001) and fat (230±53 kcal/d; p=0.0004) but not protein (−2±12 kcal/d; p=0.85). Weight changes were highly correlated with energy intake (r=0.8, p<0.0001) with participants gaining 0.9±0.3 kg (p=0.009) during the ultra-processed diet and losing 0.9±0.3 kg (p=0.007) during the unprocessed diet. Limiting consumption of ultra-processed foods may be an effective strategy for obesity prevention and treatment.
Graphical Abstract
eTOC Blurb
Hall et al. investigated 20 inpatient adults who were exposed to ultra-processed versus unprocessed diets for 14 days each, in random order. The ultra-processed diet caused increased ad libitum energy intake and weight gain despite being matched to the unprocessed diet for presented calories, sugar, fat, sodium, fiber, and macronutrients.
Introduction
The perpetual diet wars between factions promoting low-carbohydrate, keto, paleo, high-protein, low-fat, plant-based, vegan, and a seemingly endless list of other diets has led to substantial public confusion and mistrust in nutrition science. While debate rages about the relative merits and demerits of various so-called “healthy” diets, less attention is paid to the fact that otherwise diverse diet recommendations often share a common piece of advice: avoid ultra-processed foods (Katz and Meller, 2014).
Ultra-processed foods have been described as “formulations mostly of cheap industrial sources of dietary energy and nutrients plus additives, using a series of processes” and containing minimal whole foods (Monteiro et al., 2018). As an alternative to traditional approaches that focus on nutrient composition of the diet, the NOVA (not an acronym) diet classification system considers the nature, extent, and purpose of processing when categorizing foods and beverages into four groups: 1) unprocessed or minimally processed foods; 2) processed culinary ingredients; 3) processed foods; and 4) ultra-processed foods (Monteiro et al., 2018).
While the NOVA system has been criticized as being too imprecise and incomplete to form an adequate basis for making diet recommendations (Gibney, 2019; Gibney et al., 2017; Jones, 2018), Brazil’s national dietary guidelines use the NOVA system and recommend that ultra-processed foods should be avoided (Melo et al., 2015; Moubarac, 2015). However, several attributes of ultra-processed foods make them difficult to replace: they are inexpensive, have long shelf-life, are relatively safe from the microbiological perspective, provide important nutrients, and are highly convenient – often being either ready-to-eat or ready-to heat (Shewfelt, 2017; Weaver et al., 2014).
The rise in obesity and type 2 diabetes prevalence occurred in parallel with an increasingly industrialized food system (Stuckler et al., 2012) characterized by large-scale production of high-yield, inexpensive, agricultural “inputs” (primarily corn, soy, and wheat) that are refined and processed to generate an abundance of “added value” foods (Blatt, 2008; Roberts, 2008). Ultra-processed foods have become more common worldwide (Monteiro et al., 2013; Moubarac, 2015), now constitute the majority of calories consumed in America (Martinez Steele et al., 2016), and have been associated with a variety of poor health outcomes (Fiolet et al., 2018; Mendonca et al., 2017; Mendonca et al., 2016), including death (Schnabel et al., 2019).
Ultra-processed foods may facilitate overeating and the development of obesity (Poti et al., 2017) because they are typically high in calories, salt, sugar, and fat (Poti et al., 2015) and have been suggested to be engineered to have supernormal appetitive properties (Kessler, 2009; Moss, 2013; Moubarac, 2015; Schatzker, 2015) that may result in pathological eating behavior (Schulte et al., 2015; Schulte et al., 2017). Furthermore, ultra-processed foods are theorized to disrupt gut-brain signaling and may influence food reinforcement and overall intake via mechanisms distinct from the palatability or energy density of the food (Small and DiFeliceantonio, 2019).
As compelling as such theories may be, it is important to emphasize that no causal relationship between ultra-processed food consumption and human obesity has yet been established. In fact, there has never been a randomized controlled trial demonstrating any beneficial effects of reducing ultra-processed foods or deleterious effects of increasing ultra-processed foods in the diet. Therefore, to address the causal role of ultra-processed foods on energy intake and body weight change, we conducted a randomized controlled trial examining the effects of ultra-processed versus unprocessed diets on ad libitum energy intake.
Results and Discussion
We admitted 10 male and 10 female weight-stable adults aged (mean±SE) 31.2±1.6 y with BMI=27±1.5 kg/m2 (see Table S1 in the Supplementary Information for more detailed demographics and anthropometrics) as inpatients to the Metabolic Clinical Research Unit (MCRU) at the NIH Clinical Center where they resided for a continuous 28-day period. Subjects were randomly assigned to either the ultra-processed or unprocessed diet for 2 weeks followed immediately by the alternate diet for the final 2 weeks (Figure 1).
Figure 1. Overview of the study design.
Twenty adults were confined to the metabolic ward at the NIH Clinical Center. Every week, subjects spent one day residing in a respiratory chamber to measure energy expenditure, respiratory quotient, and sleeping energy expenditure. Average energy expenditure during each diet period was measured by the doubly labeled water (DLW) method. Body composition was measured by dual-energy X-ray absorptiometry (DXA) and liver fat was measured by magnetic resonance imaging/spectroscopy (MRI/MRS).
During each diet phase, the subjects were presented with three daily meals and were instructed to consume as much or as little as desired. Up to 60 minutes was allotted to consume each meal. Menus rotated on a 7-day schedule and the meals were designed to be well-matched across diets for total calories, energy density, macronutrients, fiber, sugars, and sodium, but widely differing in the percentage of calories derived from ultra-processed versus unprocessed foods (Table 1) as defined according to the NOVA classification scheme (Monteiro et al., 2018). While we attempted to match several nutritional parameters between the diets, the ultra-processed versus unprocessed meals differed substantially in the proportion of added to total sugar (~54% vs 1%, respectively), insoluble to total fiber (~16% versus 77%, respectively), saturated to total fat (~34% vs 19%), and the ratio of omega-6 to omega-3 fatty acids (~11:1 vs. 5:1).
Table 1.
Diet composition of the average 7-day rotating menu presented to the subjects during the Ultra-processed and Unprocessed diet periods.
| Ultra-processed Diet | Unprocessed Diet | |
|---|---|---|
| Three Daily Meals | ||
| Energy (kcal/d) | 3905 | 3871 |
| Carbohydrate (%) | 49.2 | 46.3 |
| Fat (%) | 34.7 | 35.0 |
| Protein (%) | 16.1 | 18.7 |
| Energy Density (kcal/g) | 1.024 | 1.028 |
| Non-beverage Energy Density (kcal/g) | 1.957 | 1.057 |
| Sodium (mg/1000 kcal) | 1997 | 1981 |
| Fiber (g/1000 kcal) | 21.3 | 20.7 |
| Sugars (g/1000 kcal) | 34.6 | 32.7 |
| Saturated Fat (g/1000 kcal) | 13.1 | 7.6 |
| Omega-3 Fatty Acids (g/1000 kcal) | 0.7 | 1.4 |
| Omega-6 Fatty Acids (g/1000 kcal) | 7.6 | 7.2 |
| Energy from Unprocessed (%)1 | 6.4 | 83.3 |
| Energy from Ultra-processed (%)1 | 83.5 | 0 |
| Snacks (available all day) | ||
| Energy (kcal/d) | 1530 | 1565 |
| Carbohydrate (%) | 47.0 | 50.3 |
| Fat (%) | 44.1 | 41.9 |
| Protein (%) | 8.9 | 7.8 |
| Energy Density (kcal/g) | 2.80 | 1.49 |
| Sodium (mg/1000 kcal) | 1454 | 78 |
| Fiber (g/1000 kcal) | 12.1 | 23.3 |
| Sugars (g/1000 kcal) | 24.8 | 95.9 |
| Saturated Fat (g/1000 kcal) | 7.7 | 4.4 |
| Omega-3 Fatty Acids (g/1000 kcal) | 0.3 | 4.0 |
| Omega-6 Fatty Acids (g/1000 kcal) | 9.6 | 21.9 |
| Energy from Unprocessed (%)1 | 0 | 100 |
| Energy from Ultra-processed (%)1 | 75.9 | 0 |
| Daily Meals + Snacks | ||
| Energy (kcal/d) | 5435 | 5436 |
| Carbohydrate (%) | 48.6 | 47.4 |
| Fat (%) | 37.4 | 37.0 |
| Protein (%) | 14.0 | 15.6 |
| Energy Density (kcal/g) | 1.247 | 1.126 |
| Non-beverage Energy Density (kcal/g) | 2.147 | 1.151 |
| Sodium (mg/1000 kcal) | 1843 | 1428 |
| Fiber (g/1000 kcal) | 18.7 | 21.4 |
| Sugars (g/1000 kcal) | 31.9 | 51.0 |
| Saturated Fat (g/1000 kcal) | 11.5 | 6.7 |
| Omega-3 Fatty Acids (g/1000 kcal) | 0.6 | 2.2 |
| Omega-6 Fatty Acids (g/1000 kcal) | 8.1 | 11.5 |
| Energy from Unprocessed (%)1 | 4.6 | 88.1 |
| Energy from Ultra-processed (%)1 | 81.3 | 0 |
The calculated energy percentages refer to the fraction of diet calories contributed from groups 1 and 4 of the NOVA classification system: 1) unprocessed or minimally processed; 2) processed culinary ingredients; 3) processed foods; 4) ultra-processed foods.
The weekly cost for ingredients to prepare 2000 kcal/d of ultra-processed meals was estimated to be $106 versus $151 for the unprocessed meals as calculated using the cost of ingredients obtained from a local branch of a large supermarket chain. Snacks appropriate to the prevailing diet and bottled water were available throughout each day. The meals plus snacks were provided at an amount equivalent to twice each subject’s estimated energy requirements for weight maintenance as calculated by 1.6 × resting energy expenditure measured at screening. Details of the diet menus are provided as Supplemental Information.
Food Intake
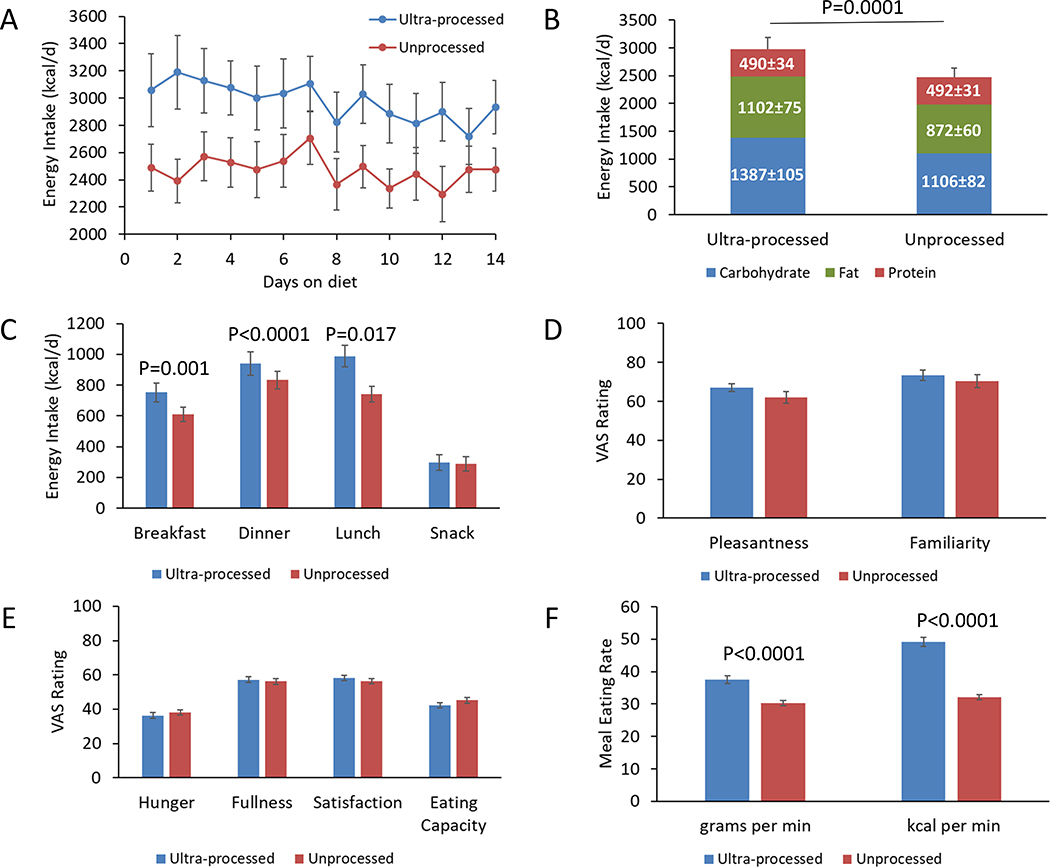
Figures 2A and 2B show that metabolizable energy intake was 508±106 kcal/d greater during the ultra-processed diet (p=0.0001). Neither the order of the diet assignment (p=0.64) nor sex (p=0.28) had significant effects on the energy intake differences between the diets. Baseline BMI was not significantly correlated with the energy intake differences between the diets (r=0.11; p=0.66).
Figure 2. Ad libitum food intake, appetite scores, and eating rate.
A) Energy intake was consistently higher during the ultra-processed diet. B) Average energy intake was increased during the ultra-processed diet because of increased intake of carbohydrate and fat, but not protein. C) Energy consumed at breakfast and lunch was significantly greater during the ultra-processed diet, but energy consumed at dinner and snacks was not significantly different between the diets. D) Both diets were rated similarly on visual analogue scales (VAS) with respect to pleasantness and familiarity. E) Appetitive measures were not significantly different between the diets. F) Meal eating rate was significantly greater during the ultra-processed diet.
During the unprocessed diet, energy intake did not significantly change over time (−7.7±6.4 kcal/d2; p=0.23), whereas there was a significant linear decrease in energy intake during the ultra-processed diet (−25.5±6.4 kcal/d2; p<0.0001) that tended to be different from the unprocessed diet (p=0.051). To partially address the lack of a run-in period before the test diets or a washout period between diets, we compared the final week of each diet period and found that energy intake was 459±105 kcal/d greater during the ultra-processed compared to the unprocessed diet (p=0.0003).
The increased energy intake during the ultra-processed diet resulted from consuming greater quantities of carbohydrate (280±54 kcal/d; p<0.0001) and fat (230±53 kcal/d; p=0.0004), but not protein (−2±12 kcal/d; p=0.85) (Figure 2B). The remarkable stability of absolute protein intake between the diets, along with the slight reduction in overall protein provided in the ultra-processed versus the unprocessed diet (14% versus 15.6% of calories, respectively) (Table 1), suggests that the protein leverage hypothesis could partially explain the increase in energy intake with the ultra-processed diet in an attempt to maintain a constant protein intake (Martinez Steele et al., 2018; Simpson and Raubenheimer, 2005).
Using the mathematical relationship between energy intake changes expected from the observed differences in the protein fraction of the provided diets (Hall, 2019), we calculated that protein leverage could potentially explain at most ~50% of the observed energy intake differences between the diets assuming perfect leverage. However, if protein leveraging was at work in our study, it is unclear why subjects chose to meet their protein targets via compensatory overeating of dietary carbohydrate and fat rather than selecting foods with high protein content. Perhaps within-meal palatability differences between foods or the composite nature of many ultra-processed foods limited the possibility for targeted consumption of higher protein foods without concomitant overeating of carbohydrate and fat during the ultra-processed diet.
Figure 2C illustrates that the ultra-processed diet resulted in increased energy intake at breakfast (144 ± 39 kcal/day; p = 0.0014), lunch (248 ± 39 kcal/day; p < 0.0001), and dinner (108 ± 41 kcal/day; p = 0.017) as compared to the unprocessed diet. Carbohydrate intake was significantly increased during the ultra-processed diet at breakfast (76 ± 22 kcal/day; p = 0.002), lunch (139 ± 21 kcal/day; p < 0.0001), and dinner (73 ± 25 kcal/day; p = 0.009). Fat intake was significantly increased during the ultra-processed diet at breakfast (69 ± 14 kcal/day; p < 0.0001) and lunch (130 ± 17 kcal/day; p < 0.0001), and tended to be increased at dinner (26 ± 13 kcal/day; p = 0.06). Protein intake was significantly lower during the ultra-processed diet at lunch (−22 ± 6 kcal/day; p = 0.0013) but was not significantly different from other meals (p > 0.17).
Whereas sodium intake was significantly increased during the ultra-processed versus the unprocessed diet (5.8±0.2 g/d vs. 4.6±0.2 g/d; p<0.0001), there were no significant differences in consumption of total fiber (48.5±2.3 g/d vs. 45.8±2.3 g/d; p=0.41) or total sugars (93.3±4.0 g/d vs. 96.6±4.0 g/d; p=0.57).
The foods and beverages consumed during the ultra-processed diet had greater energy density than the unprocessed diet (1.36±0.02 kcal/g vs. 1.09±0.02 kcal/g; p<0.0001). While the presented ultra-processed and unprocessed meals had similar energy densities (Table 1), this was due to inclusion of beverages as vehicles for the dissolved fiber supplements in the ultra-processed meals that were otherwise low in fiber. However, because beverages have limited ability to affect satiety (DellaValle et al., 2005) the ~85% higher energy density of the non-beverage foods in the ultra-processed versus unprocessed diets (Table 1) likely contributed to the observed excess energy intake (Rolls, 2009).
Appetitive measurements and eating rate
Participants did not report significant differences in the pleasantness (4.8±3.1; p=0.13) or familiarity (2.7±4.6; p=0.57) of the meals between the ultra-processed and unprocessed diets as measured using 100-point visual analogue scales (Figure 2D). This suggests that the observed energy intake differences were not due to greater palatability or familiarity of the ultra-processed diet. Furthermore, differences in the energy intake-adjusted scores for hunger (−1.7±2.5; p=0.5), fullness (1.1±2.5; p=0.67), satisfaction (1.9±2.4; p=0.42), and capacity to eat (−2.9±2.5; p=0.25) (Figures 2E) were not significant between the diets suggesting that they did not differ in their subjective appetitive properties.
Interestingly, Figure 2F illustrates that meal eating rate was significantly greater during the ultra-processed diet whether expressed as kcal/min (17±1 kcal/min; p<0.0001) or g/min (7.4±0.9 g/min; p<0.0001). Individual differences in average eating rate in kcal/min between the ultra-processed and unprocessed diets were moderately correlated with overall energy intake differences (r= 0.45; p=0.047).
Previous studies have demonstrated that higher eating rates can result in increased overall energy intake (de Graaf and Kok, 2010; Forde et al., 2013; McCrickerd et al., 2017; Robinson et al., 2014) such that a 20% change in eating rate can impact energy intake by between 10–13% (Forde, 2018). Perhaps the oro-sensory properties of the ultra-processed foods (e.g., softer food that was easier to chew and swallow) led to the observed increased eating rate and delayed satiety signaling thereby resulting in greater overall intake (de Graaf and Kok, 2010). Future studies should examine whether the observed energy intake differences persist when ultra-processed and unprocessed diets are more closely matched for dietary protein and non-beverage energy density while at the same time including ultra-processed foods that are typically eaten slowly.
Body weight and composition
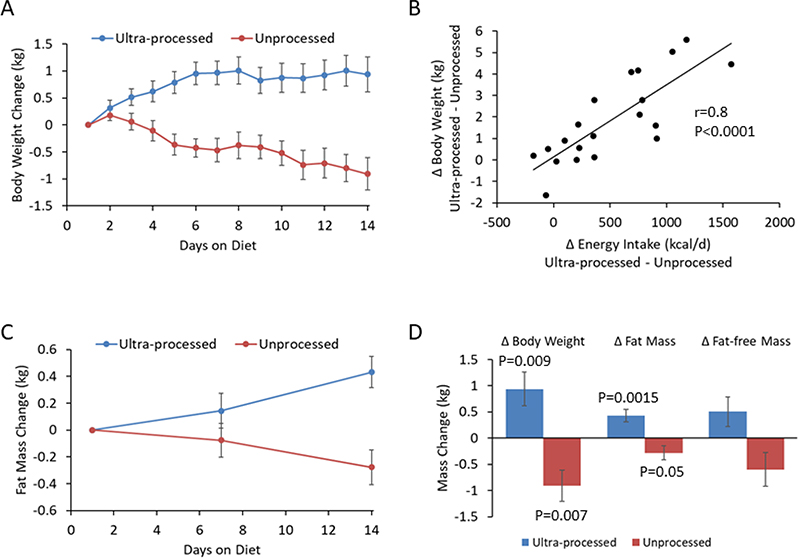
Figure 3A illustrates that participants gained 0.9±0.3 kg (p=0.009) during the ultra-processed diet and lost 0.9±0.3 kg (p=0.007) during the unprocessed diet. The individual differences in weight change between the diets were not significantly correlated with baseline BMI (r=0.01; p=0.97) but Figure 3B shows that they were highly correlated with energy intake differences between the diets (r=0.8, p<0.0001).
Figure 3. Body weight and composition changes.
A) The ultra-processed diet led to increased body weight over time whereas the unprocessed diet led to progressive weight loss. B) Differences in body weight change between the ultra-processed and unprocessed diets were highly correlated with the corresponding energy intake differences. C) Body fat mass increased over time with the ultra-processed diet and decreased with the unprocessed diet. D) Body weight, body fat, and fat-free mass changes between the beginning and end of each diet period..
Body fat mass increased by 0.4±0.1 kg (p=0.0015) during the ultra-processed diet and decreased by 0.3±0.1 kg during the unprocessed diet (p=0.05) (Figure 3C) whereas fat-free mass tended to increase during the ultra-processed diet (0.5±0.3 kg; p=0.09) and decrease during the unprocessed diet (0.6±0.3 kg; p=0.08) (Figure 3D). While the dual energy X-ray absorptiometry (DXA) methodology used to measure body composition in our study tends to underestimate body fat changes (Pourhassan et al., 2013), the relatively large fat-free mass changes may be due to extracellular fluid shifts associated with differences in sodium intake between the diets. Indeed, individual differences in sodium intake between the diets were significantly correlated with changes in fat-free mass (r=0.63; p=0.004) and body weight (r=0.64; p=0.002). Such fluid shifts may also affect the accuracy and precision of the measured body fat changes (Lohman et al., 2000; Muller et al., 2012).
Thirteen subjects completed measurements of liver fat content by magnetic resonance spectroscopy at baseline and the end of each diet period (Ouwerkerk et al., 2012). Baseline liver fat was 1.2±0.1% and liver fat was not significantly changed after the unprocessed diet (0.95±0.1%; p=0.24) or the ultra-processed diet (1.1±0.2%; p=0.74).
Energy expenditure, physical activity, and energy balance
Subjects spent one day each week residing in respiratory chambers to measure the components of 24hr energy expenditure. On the chamber days subjects were presented with identical meals within each diet period and those meals were not offered on non-chamber days. Table 2 shows that there was no significant difference in energy intake between the diets on the chamber days, but the food quotient differences indicated that subjects consumed relatively more carbohydrate versus fat during the chamber days on the ultra-processed diet. While subjects tended to have greater 24hr energy expenditure during the ultra-processed diet (51±27 kcal/d; p=0.06), there were no significant differences in sleeping energy expenditure, sedentary energy expenditure, or physical activity. These results contrast with a previous study suggesting that energy expenditure was ~60 kcal lower for 6 hours following consumption of processed versus unprocessed sandwiches (Barr and Wright, 2010).
Table 2.
Energy expenditure and food intake during the respiratory chamber and doubly labeled water periods.
| Ultra-processed Diet (week 1) | Ultra-processed Diet (week 2) | Ultra-processed Diet (2-week average) | Unprocessed Diet (week 1) | Unprocessed Diet (week 2) | Unprocessed Diet (2-week average) | P-value1 | |
|---|---|---|---|---|---|---|---|
| Respiratory Chamber Days | |||||||
| Energy Intake (kcal/d) | 2715±86 | 2588±66 | 2651±53 | 2657±86 | 2597±66 | 2627±53 | 0.75 |
| Food Quotient | 0.850±0.002 | 0.856±0.003* | 0.853±0.002 | 0.846±0.002 | 0.843±0.003 | 0.845±0.002 | 0.002 |
| Energy Expenditure (kcal/d) | 2328±28 | 2344±29 | 2336±19 | 2320±28 | 2248±29* | 2284±19 | 0.056 |
| 24hr Respiratory Quotient | 0.907±0.005 | 0.899±0.005 | 0.903±0.003 | 0.875±0.005 | 0.869±0.005 | 0.872±0.003 | <0.0001 |
| Sleeping Energy Expenditure (kcal/d) | 1515±28 | 1550±33 | 1532±19 | 1516±27 | 1535±33 | 1525±19 | 0.81 |
| Sedentary Energy Expenditure (kcal/d) | 1590±21 | 1573±30 | 1581±17 | 1550±21 | 1530±30 | 1540±17 | 0.084 |
| Physical Activity Expenditure (kcal/d) | 738±29 | 771±21 | 755±18 | 771±29 | 717±21 | 744±18 | 0.67 |
| Doubly Labeled Water Period2 | |||||||
| Energy Intake (kcal/d) | 3099±87 | 2865±64* | 2963±74 | 2555±82 | 2486±64 | 2491±74 | 0.0003 |
| Food Quotient | 0.851±0.002 | 0.854±0.002* | 0.854±0.002 | 0.852±0.002 | 0.856±0.002* | 0.855±0.002 | 0.93 |
| Adjusted Respiratory Quotient | 0.903±0.01 | 0.902±0.009 | 0.901±0.007 | 0.847±0.01 | 0.836±0.009 | 0.842±0.007 | <0.0001 |
| Daily CO2 production (L/d) | 468±13 | 505±19 | 473±7.5 | 444±13 | 388±19 | 422±7.5 | 0.0001 |
| Daily Energy Expenditure (kcal/d) | 2496±83 | 2693±80 | 2526±43 | 2497±79 | 2309±85 | 2385±43 | 0.033 |
| Daily physical activity METs (via accelerometry) | 1.502±0.002 | 1.509±0.003 | 1.5055±0.002 | 1.507±0.002 | 1.506±0.003 | 1.5065±0.002 | 0.71 |
P-value refers to the comparison between the 2-week average values for ultra-processed versus unprocessed diets.
N=19 because one subject’s doubly-labeled water data failed quality control for the calculated deuterium dilution space.
p<0.05 comparing means for week 2 with week 1 within each diet period. Mean ± SE.
The significantly higher 24hr respiratory quotient observed during the ultra-processed diet indicates that fat oxidation was decreased compared to the unprocessed diet. This was likely due to differences in food quotient between ultra-processed and unprocessed diet periods during the chamber days along with differences in energy intake and energy balance on the days prior to the chamber stays.
During the chamber days on the ultra-processed diet, both insulin secretion measured by 24-hour urinary C-peptide excretion (38.9±2.8 nmol/d vs. 30.9±2.8 nmol/d; p=0.052) and average daily glucose levels measured by continuous glucose monitoring (CGM) (99.1±1.3 mg/dl vs. 96.0±1.3 mg/dl; p=0.10) tended to be slightly higher compared to the unprocessed diet.
Table 2 reports the average daily energy expenditure as measured by the doubly labeled water (DLW) method during each diet period. The respiratory chamber measurements of energy expenditure were 191±73 kcal/d lower than the DLW measurements during the ultra-processed diet (p=0.02) and not significantly different during the unprocessed diet (−70±75 kcal/d; p=0.36). The ultra-processed diet led to slightly higher energy expenditure by DLW compared to the unprocessed diet (171±56 kcal/d; p=0.006). Since overall physical activity quantified by accelerometry did not detect significant differences between the diet periods (Table 2), the DLW energy expenditure differences were likely due to the differing states of energy balance between the diets.
Energy intake was calculated from the measured foods and beverages consumed using their estimated nutrient composition and metabolizable energy densities. Table 2 shows that energy intake was 417±121 kcal/d (p=0.003) more than energy expenditure by DLW during the ultra-processed diet in accordance with the observed gain in body weight and fat. However, despite significant body weight and fat loss during the unprocessed diet, energy intake was nominally higher than energy expenditure by DLW by 116±111 kcal/d, but this difference was not statistically significant (p=0.31).
Changes in body energy stores were calculated using the repeated body composition measurements and were found to be increasing by 307±85 kcal/d (p=0.002) during the ultra-processed diet and decreasing by 220±88 kcal/d (p=0.02) during the unprocessed diet. Energy balance calculated as energy intake minus expenditure by DLW was not significantly different from the calculated rate of change of body energy stores during the ultra-processed diet (111±111 kcal/d; p=0.33) but was 382±92 kcal/d (p=0.0007) greater during the unprocessed diet.
The limited precision of the DLW method, with an intrasubject coefficient of variation of ~8–15% (Black and Cole, 2000), along with the limited precision and accuracy of measured body composition changes (Lohman et al., 2000; Muller et al., 2012; Pourhassan et al., 2013) may have led to the discrepant energy balance calculations during the unprocessed diet simply by chance (type-1 error). However, another possibility is that the metabolizable energy content of the unprocessed diet may have been substantially overestimated.
Metabolizable energy content of mixed diets has been shown to decrease at a rate of ~7.2 kcal per gram of total or insoluble fiber intake, whereas intake of soluble fiber (as supplemented during the ultra-processed diet) does not consistently affect metabolizable energy (Baer et al., 1997). Given that subjects consumed ~46 g/d of total fiber during the unprocessed diet, the vast majority of which was insoluble (~77%), the expected decrease in metabolizable energy amounts to ~330 kcal/d thereby bringing the energy balance calculations into approximate alignment with the measured changes in body energy stores. Of course, this implies that the metabolizable energy intake difference between the ultra-processed and unprocessed diets was even larger than the ~500 kcal/d difference calculated from the nutrient estimates in the measured foods consumed. Future studies should include fecal collections to directly assess digestibility and metabolizable energy intake.
Fasting blood measurements
Table 3 presents the fasting blood measurements obtained at baseline and on the final days of the ultra-processed and unprocessed diet periods. Overall, compared to the unprocessed diet, the measurements obtained after the ultra-processed diet were largely unchanged from baseline suggesting that these subjects likely consumed a habitual diet high in ultra-processed foods which might be expected given the high prevalence of ultra-processed food consumption in America (Martinez Steele et al., 2016).
Table 3.
Fasting blood measurements at baseline and at the end of the ultra-processed and unprocessed diet periods.
| Baseline | Ultra-processed Diet | P-value Ultra-processed vs. Baseline Diet | Unprocessed Diet | P-value Unprocessed vs. Baseline Diet | |
|---|---|---|---|---|---|
| Leptin (ng/ml) | 44.3±1.7 | 45.1±1.7 | 0.75 | 40.4±1.7 | 0.11 |
| Active Ghrelin (pg/ml) | 61.4±3.5 | 54.1±3.5 | 0.15 | 48.3±3.5 | 0.01 |
| PYY (pg/ml) | 28.9±1.9 | 25.1±1.9 | 0.15 | 34.3±1.9 | 0.047 |
| FGF-21 (pg/ml) | 397±59 | 289±59 | 0.21 | 362±59 | 0.67 |
| Adiponectin (mg/L) | 7.3±0.7 | 8.0±0.7 | 0.43 | 4.6±0.7 | 0.007 |
| Resistin (ng/ml) | 13.5±0.4 | 12.4±0.4 | 0.05 | 12.1±0.4 | 0.01 |
| Active GLP-1 (pg/ml) | 1.88±0.19 | 1.25±0.19 | 0.027 | 1.57±0.19 | 0.26 |
| Total GIP (pg/ml) | 79.7±5.4 | 67.9±5.4 | 0.13 | 64.3±5.4 | 0.052 |
| Active GIP (pg/ml) | 27.4±2.8 | 20.0±2.8 | 0.07 | 18.2±2.8 | 0.025 |
| Glucagon (pmol/L) | 12.0±0.8 | 11.0±0.8 | 0.42 | 9.8±0.8 | 0.07 |
| Hgb A1C (%) | 4.98±0.03 | 5.02±0.03 | 0.28 | 5.00±0.03 | 0.55 |
| Glucose (mg/dl) | 90.5±0.9 | 88.6±0.9 | 0.16 | 88.0±0.9 | 0.06 |
| Insulin (μU/ml) | 11.9±0.9 | 11.3±0.9 | 0.64 | 8.9±0.9 | 0.03 |
| C-Peptide (ng/ml) | 2.19±0.06 | 2.14±0.06 | 0.62 | 1.94±0.06 | 0.01 |
| HOMA-IR | 2.8±0.3 | 2.5±0.3 | 0.50 | 1.9±0.3 | 0.03 |
| HOMA-Beta | 152±10 | 159±11 | 0.63 | 129±10 | 0.13 |
| Total Cholesterol (mg/dl) | 155±3 | 152±3 | 0.54 | 137±3 | 0.0002 |
| HDL Cholesterol (mg/dl) | 58.2±0.8 | 55.0±0.9 | 0.01 | 48.3±0.8 | <0.0001 |
| LDL Cholesterol (mg/dl) | 82±3 | 84±3 | 0.61 | 77±3 | 0.21 |
| Triglycerides (mg/dl) | 72±3 | 62±3 | 0.02 | 59±3 | 0.003 |
| Free Fatty Acids (μmol/L) | 409±40 | 384±40 | 0.67 | 556±40 | 0.013 |
| Uric Acid (mg/dl) | 4.9±0.3 | 4.5±0.3 | 0.0007 | 4.9±0.3 | 0.55 |
| TSH (μIU/ml) | 2.2±0.1 | 2.6±0.1 | 0.054 | 2.5±0.1 | 0.24 |
| Free T3 (pg/ml) | 3.17±0.06 | 3.20±0.06 | 0.72 | 3.03±0.06 | 0.11 |
| Free T4 (ng/dl) | 1.19±0.02 | 1.22±0.02 | 0.36 | 1.27±0.02 | 0.019 |
| T3 (ng/dl) | 113±2 | 112±2 | 0.80 | 104±2 | 0.011 |
| T4 (μg/dl) | 6.8±0.1 | 6.9±0.1 | 0.70 | 6.8±0.1 | 0.91 |
| PAI-1 (ng/ml) | 4.0±0.5 | 4.6±0.5 | 0.42 | 4.7±0.5 | 0.34 |
| hsCRP (mg/L) | 2.7±0.3 | 2.5±0.3 | 0.48 | 1.5±0.3 | 0.014 |
Mean ± SE.
Interestingly, the appetite-suppressing hormone PYY increased during the unprocessed diet as compared with both the ultra-processed diet and baseline. Also, the hunger hormone ghrelin was decreased during the unprocessed diet compared to baseline. The unprocessed diet led to reduced adiponectin, total cholesterol, hsCRP, and total T3, whereas free T4 and free fatty acids were increased compared to baseline. Uric acid decreased after the ultra-processed diet compared with baseline. Triglycerides and HDL cholesterol were significantly decreased compared to baseline after both diets. After the unprocessed diet, fasting glucose and insulin levels tended to decrease compared to baseline and the homeostasis model assessment of insulin resistance (HOMA-IR) (Matthews et al., 1985) was significantly decreased compared to baseline. There were no significant differences in HOMA-IR after the ultra-processed diet as compared to either baseline or the unprocessed diet.
Glucose Tolerance
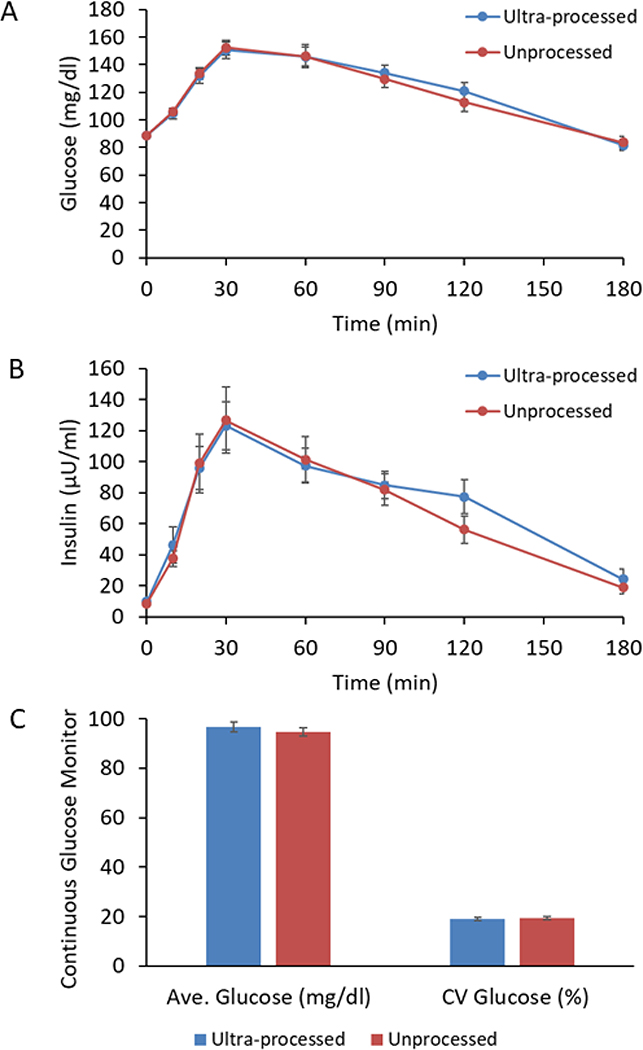
Despite substantial differences in energy intake and body weight between the ultra-processed and unprocessed diets, oral glucose tolerance tests performed at the end of each diet period indicated no significant differences in glucose tolerance (Figure 4A and B). Therefore, insulin sensitivity as measured by the Matsuda index (Matsuda and DeFronzo, 1999) was not significantly different between the ultra-processed and unprocessed diets (3.9±0.2 versus 4.5±0.2, respectively; p=0.1). Furthermore, there were no significant differences in either average daily glucose concentrations or glycemic variability between the diets as measured by daily CGM (Figure 4C).
Figure 4. Glucose tolerance and continuous glucose monitoring.
A) Glucose concentrations following a 75g oral glucose tolerance test (OGTT) was not significantly different between the diets. B) Insulin concentrations following the OGTT were not significantly different between the diets. C) Continuous glucose monitoring throughout the study did not detect significant differences in average glucose concentrations or glycemic variability as measured by the coefficient of variation (CV) of glucose.
It is possible that differences in glucose tolerance and insulin sensitivity would have emerged after longer periods on each diet. However, shorter durations of overfeeding have previously been demonstrated to result in rapid impairments in glucose tolerance and insulin sensitivity (Lagerpusch et al., 2012; Walhin et al., 2013), albeit with greater differences in energy intake than the present study.
Another possible explanation is that exercise can prevent changes in insulin sensitivity and glucose tolerance during overfeeding (Walhin et al., 2013). Our subjects performed daily cycle ergometry exercise in three 20-minute bouts at a constant intensity corresponding to 30–40% of each subjects’ estimated heart rate reserve. This relatively low intensity exercise was mandated to avoid the sedentary behavior and de-training that often occurs during inpatient metabolic ward studies. Indeed, the average physical activity level (defined by total energy expenditure by DLW divided by resting energy expenditure) during the inpatient stay was 1.59±0.06 which is representative of free-living adults (SACN, 2011). It is intriguing to speculate that perhaps even this modest dose of exercise prevented any differences in glucose tolerance or insulin sensitivity between the ultra-processed and unprocessed diets.
Limitations of Study
Ultra-processed foods are less expensive and more convenient than preparing meals using unprocessed whole foods and culinary ingredients. Because the meals were prepared and presented at no cost to our subjects, and they could not choose their meals or their mode of presentation, our study did not address how consumer choices between ultra-processed versus unprocessed meals may be influenced by cost and convenience.
Our study was not designed to identify the cause of the observed differences in energy intake. Many of the potential negative effects of ultra-processed foods have been hypothesized to relate to their elevated sugar, fat, and sodium content while being low in protein, and fiber (Poti et al., 2017). However, we attempted to match these nutritional variables in the presented meals to investigate whether other aspects of ultra-processed diets contribute to excess energy intake. Had the experimental diets used in our study allowed for greater differences in sugar, fat, sodium content more typical of differences between ultra-processed versus unprocessed diets, we may have observed larger differences in energy intake.
Our study did not include a weight-maintenance run-in period or a washout period between test diets. These design choices were made to lessen the burden to the subjects and reduce the likelihood of dropouts, which was successful because all 20 subjects who successfully screened for the study also completed. To partially address the lack of run-in or washout periods, we compared ad libitum energy intake during the final week of each test diet period and the substantial diet differences persisted. The lack of a run-in period complicates the interpretation of the baseline blood measures in comparison to those obtained at the end of each test diet and all such diet comparisons were potentially confounded by the substantial differences in energy intake and corresponding weight changes.
Finally, the inpatient environment of the metabolic ward makes it difficult to generalize our results to free-living conditions. However, current dietary assessment methods are insufficient to accurately or precisely measure energy intake outside the laboratory (Schoeller, 1990; Schoeller et al., 2013) and adherence to study diets cannot be guaranteed in free-living subjects. While the 28-day duration of our study was relatively modest, most laboratory-based studies of food intake are typically much shorter in duration, often occurring within a single day of testing with one or two meals (Gibbons et al., 2014).
In conclusion, our data suggest that eliminating ultra-processed foods from the diet decreases energy intake and results in weight loss whereas a diet with a large proportion of ultra-processed food increases energy intake and leads to weight gain. Whether reformulation of ultra-processed foods could eliminate their deleterious effects while retaining their palatability and convenience is unclear. Until such reformulated products are widespread, limiting consumption of ultra-processed foods may be an effective strategy for obesity prevention and treatment. Such a recommendation could potentially be embraced across a wide variety of healthy dietary approaches including low-carb, low-fat, plant-based, or animal-based diets. However, policies that discourage consumption of ultra-processed foods should be sensitive to the time, skill, expense, and effort required to prepare meals from minimally processed foods – resources that are often in short supply for those who are not members of the upper socioeconomic classes.
STAR★Methods
CONTACT FOR REAGENT AND RESOURCE SHARING
Further information and requests for resources and reagents should be directed to and will be fulfilled by the Lead Contact, Kevin Hall (kevinh@nih.gov).
EXPERIMENTAL MODEL AND SUBJECT DETAILS
The study protocol was approved by the Institutional Review Board of the National Institute of Diabetes & Digestive & Kidney Diseases (ClinicalTrials.gov Identifier NCT03407053). Eligible subjects were between 18–50 years old with a body mass index (BMI) > 18.5 kg/m2 and were weight-stable (< ± 5% over the past 6 months). Volunteers were excluded if they had anemia, diabetes, cancer, thyroid disease, eating disorders or other psychiatric conditions such as clinical depression or bipolar disorder. Volunteers with strict dietary concerns, including food allergies or adherence to particular diets (e.g., vegetarian, vegan, kosher, etc.) were also excluded.
Subjects were told that the purpose of the study was to learn about how a processed versus unprocessed diet affects the amount of food they eat, glucose tolerance, hormone levels, markers of inflammation, body weight and composition, energy expenditure, and liver fat. The subjects were told that this was not a weight loss study. They wore loose fitting clothing throughout the study and were blinded to daily weight and continuous glucose measurements.
METHOD DETAILS
Diets
The diets were designed and analyzed using ProNutra software (version 3.4, Viocare, Inc., Princeton, NJ) with nutrient values derived from the USDA National Nutrient Database for Standard Reference, Release 26 and the USDA Food and Nutrient Database for Dietary Studies, 4.0. The ultra-processed and unprocessed meals were provided on 7-day rotating menus (see the Supplemental Information for detailed menu information). Foods and beverages were categorized according to the NOVA system (Monteiro et al., 2018).
Bottled water and snacks representative of the prevailing diet were provided ad libitum throughout the day in snack boxes located in the subjects’ inpatient rooms. Meals were presented to the subjects plated approximately as shown in the photographs included in the Supplemental Information with instructions to eat as much or as little as desired. Subjects were given up to 60 minutes to eat and when they finished each meal a nurse removed the meal and documented the meal duration. Remaining food and beverages were identified and weighed by nutrition staff to calculate the amount of each food consumed and the nutrient and metabolizable energy intake were calculated using the nutrition software described above. Meal eating rate was calculated by dividing the measured food intake by the meal duration.
Subjective assessment of appetite, sensory, and palatability
During each diet period, subjects were asked to complete appetitive surveys over the course of three separate days implemented using REDCap (Research Electronic Data Capture) electronic data capture tools (Harris et al., 2009). The surveys comprised visual analog scales (VAS) in response to four questions: 1) “How hungry do you feel right now?” 2) “How full do you feel right now?” 3) “How much do you want to eat right now?” and 4) “How much do you think you can eat right now?”. Subjects answered the questions using 100-point VAS line scale anchored at 0 and 100 by descriptors such as “not at all” and “extremely”. The questions were answered immediately prior to each meal and at least every 30 to 60 minutes over the 2–3 hours following the consumption of each meal. We calculated the mean values of the responses adjusted for the energy consumed using multiple linear regression.
On the last two days of the first diet period and the first two days of the second diet period, subjects were asked to complete another survey to assess the palatability and familiarity of the meals provided. The questions were embedded amongst distracter “mood” ratings (e.g., alert, happy, and clear-headed). Survey items were completed after the first bite of the meal.
Body weight and composition
Daily body weight measurements were performed at 6am each morning after the first void (Welch Allyn Scale-Tronix 5702; Skaneateles Falls, NY, USA). Subjects wore hospital-issued top and bottom pajamas which were pre-weighed and deducted from scale weight. To minimize the influence of fluctuations in body fluids, weight changes during each 14-day diet period were calculated by linear regression. Body composition measurements were performed at baseline and weekly using dual-energy X-ray absorptiometry (General Electric Lunar iDXA; Milwaukee, WI, USA). Changes in body energy stores were calculated using the measured changes in body fat and fat-free mass along with the corresponding energy densities of 9300 kcal/kg and 1100 kcal/kg, respectively. Liver fat measurements were performed using T1 and T2 corrected proton magnetic resonance spectroscopy with a breath-holding technique in a 3T scanner (MAGNETOM Verio; Siemens, Tarrytown, NY) (Ouwerkerk et al., 2012).
Physical Activity Monitoring
Overall physical activity was quantified by calculating average daily metabolic equivalents (MET) using small, portable, pager-type accelerometers (Actigraph, Pensacola, FL) sampled at 80 Hz and worn on the hip (Freedson et al., 1998).
Energy expenditure via respiratory chamber
All chamber measurement periods were >23 hours and we extrapolated the data to represent 24hr periods by assuming that the mean of the measured periods was representative of the 24hr period. Energy expenditure was calculated as follows:
where VO2 and VCO2 were the volumes of oxygen consumed and carbon dioxide produced, respectively, and N was the 24hr urinary nitrogen excretion measured by chemiluminescence (Antek MultiTek Analyzer, PAC, Houston, TX).
Sleeping energy expenditure was determined by the lowest energy expenditure over a continuous 180 minute period between the hours of 00:00–06:00 (Schoffelen and Westerterp, 2008). Sedentary energy expenditure and physical activity expenditure were defined as previously described (Hall et al., 2016).
Energy expenditure via doubly labeled water
Subjects drank from a stock solution of 2H2O and H218O water where 1 g of 2H2O (99.99% enrichment) was mixed with 19 g of H218O (10% enrichment). An aliquot of the stock solution was saved for dilution to be analyzed along with each set of urine samples. The water was weighed to the nearest 0.1 g into the dosing container. The prescribed dose was 1.0 g per kg body weight and the actual dose amounts were entered in a dose log. Spot urine samples were collected daily. Isotopic enrichments of urine samples were measured by isotope ratio mass spectrometry. The average CO2 production rate (rCO2) were estimated from the rate constants describing the exponential disappearance of the labeled 18O and D water isotopes (kO and kD) in repeated spot urine samples collected over several days and were corrected for previous isotope doses (Bhutani et al., 2015). We used the parameters of Racette et al. (Racette et al., 1994) with the weighted dilution space calculation, Rdil, proposed by Speakman (Speakman, 1997):
where (ND / NO)ave is the mean of the ratio of the body water pool sizes ND / NO from the n subjects. In cases where the individual values for the total body water, N, differed by > 5% from that calculated as 73% of the fat-free mass determined by DXA within a few days of the dose, N was adjusted to agree with the DXA data.
The average total energy expenditure (EEDLW) from the DLW measurement of rCO2 was calculated as:
where RQ was calculated by adjusting the respiratory chamber RQ measurements for the overall degree of energy imbalance of each subject as determined by body composition changes during the DLW period as previously described (Hall et al., 2019).
Continuous glucose monitoring
Subjects wore the Dexcom G4 Platinum (Dexcom Inc, San Diego, CA, USA) continuous glucose monitor (CGM) daily during the inpatient stay. The device consisted of a small sensor, a transmitter, and a hand-held receiver. The sensor was inserted subcutaneously in the lower abdomen to measure interstitial glucose concentrations every 5 minutes which were transmitted to the receiver. Finger stick calibrations were required at insertion as well as each morning and night. The sensor was changed every 7 days. Subjects were blinded to their glucose readings. The CGM was removed during MRI/MRS procedures and DXA scans. All the data was downloaded at the end of the inpatient stay.
QUANTIFICATION AND STATISTICAL ANALYSIS
This study was powered to detect a difference in mean ad libitum energy intake over each 14-day test diet period (the primary endpoint) of 125–150 kcal/d in 20 subjects with probability (power) of 0.8 with a Type I error probability of 0.05. This sample size calculation was informed by previous studies measuring day to day variability of ad libitum energy intake having a standard deviation of about 500–600 kcal/d (Bray et al., 2008; Edholm et al., 1970; Tarasuk and Beaton, 1992). Using the conservative assumption that within-subject energy intake correlations were zero, over the 14-day diet period each subject was expected to have a mean energy intake with a standard error of about 130–160 kcal/d and the mean energy intake difference between the study diets was therefore estimated to have a standard error of about 190–230 kcal/d.
Statistical analyses were performed using SAS (version 9.4; SAS Institute Inc, Cary, NC, USA). The baseline data are presented as mean ± SE. Data were analyzed by analysis of variance (PROC GLM, SAS). The data tables and figures present least squares mean ± SE and two-sided t-tests were used to compare the diet groups. Significance was declared at p < 0.05.
Supplementary Material
KEY RESOURCES TABLE.
Highlights.
20 inpatient adults received ultra-processed and unprocessed diets for 14 days each
Diets were matched for presented calories, sugar, fat, fiber, and macronutrients
Ad libitum intake was ~500 kcal/d more on the ultra-processed vs unprocessed diet
Body weight changes were highly correlated with diet differences in energy intake
Context and Significance.
Increased availability, marketing, and consumption of ultra-processed foods has been associated with rising obesity prevalence, but scientists have not yet demonstrated that ultra-processed food causes obesity or adverse health outcomes. Researchers at the NIH investigated whether people ate more calories when exposed to a diet composed of ultra-processed foods compared with a diet composed of unprocessed foods. Despite the ultra-processed and unprocessed diets being matched for daily presented calories, sugar, fat, fiber, and macronutrients, people consumed more calories when exposed to the ultra-processed diet as compared to the unprocessed diet. Furthermore, people gained weight on the ultra-processed diet and lost weight on the unprocessed diet. Limiting consumption of ultra-processed food may be an effective strategy for obesity prevention and treatment.
Acknowledgements
This work was supported by the Intramural Research Program of the National Institutes of Health, National Institute of Diabetes & Digestive & Kidney Diseases. We thank the nursing and nutrition staff at the NIH MCRU for their invaluable assistance with this study. We also thank Ms. Shavonne Pocock for taking the photos of the study diets. We are most thankful to the study subjects who volunteered to participate in this demanding protocol.
Footnotes
Declaration of Interests
CG Forde has received reimbursement for speaking at conferences sponsored by companies selling nutritional products, serves on the scientific advisory council for Kerry Taste and Nutrition, and is part of an academic consortium that has received research funding from Abbott Nutrition, Nestec, and Danone. The other authors have no conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Baer DJ, Rumpler WV, Miles CW, and Fahey GC Jr. (1997). Dietary fiber decreases the metabolizable energy content and nutrient digestibility of mixed diets fed to humans. J Nutr 127, 579–586. [DOI] [PubMed] [Google Scholar]
- Barr SB, and Wright JC (2010). Postprandial energy expenditure in whole-food and processed-food meals: implications for daily energy expenditure. Food & nutrition research 54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhutani S, Racine N, Shriver T, and Schoeller DA (2015). Special Considerations for Measuring Energy Expenditure with Doubly Labeled Water under Atypical Conditions. Journal of obesity & weight loss therapy 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Black AE, and Cole TJ (2000). Within- and between-subject variation in energy expenditure measured by the doubly-labelled water technique: implications for validating reported dietary energy intake. Eur J Clin Nutr 54, 386–394. [DOI] [PubMed] [Google Scholar]
- Blatt H (2008). America’s food: What you don’t know about what you eat. (Cambridge: The MIT press; ). [Google Scholar]
- Bray GA, Flatt JP, Volaufova J, Delany JP, and Champagne CM (2008). Corrective responses in human food intake identified from an analysis of 7-d food-intake records. Am J Clin Nutr 88, 1504–1510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Graaf C, and Kok FJ (2010). Slow food, fast food and the control of food intake. Nature reviews. Endocrinology 6, 290–293. [DOI] [PubMed] [Google Scholar]
- DellaValle DM, Roe LS, and Rolls BJ (2005). Does the consumption of caloric and non-caloric beverages with a meal affect energy intake? Appetite 44, 187–193. [DOI] [PubMed] [Google Scholar]
- Edholm OG, Adam JM, Healy MJ, Wolff HS, Goldsmith R, and Best TW (1970). Food intake and energy expenditure of army recruits. Br J Nutr 24, 1091–1107. [DOI] [PubMed] [Google Scholar]
- Fiolet T, Srour B, Sellem L, Kesse-Guyot E, Alles B, Mejean C, Deschasaux M, Fassier P, Latino-Martel P, Beslay M, et al. (2018). Consumption of ultra-processed foods and cancer risk: results from NutriNet-Sante prospective cohort. Bmj 360, k322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forde CG (2018). From perception to ingestion; The role of sensory properties in energy selection, eating behaviour and food intake. Food Quality and Preference 66, 171–177. [Google Scholar]
- Forde CG, van Kuijk N, Thaler T, de Graaf C, and Martin N (2013). Texture and savoury taste influences on food intake in a realistic hot lunch time meal. Appetite 60, 180–186. [DOI] [PubMed] [Google Scholar]
- Freedson PS, Melanson E, and Sirard J (1998). Calibration of the Computer Science and Applications, Inc. accelerometer. Med Sci Sports Exerc 30, 777–781. [DOI] [PubMed] [Google Scholar]
- Gibbons C, Finlayson G, Dalton M, Caudwell P, and Blundell JE (2014). Metabolic Phenotyping Guidelines: studying eating behaviour in humans. The Journal of endocrinology 222, G1–12. [DOI] [PubMed] [Google Scholar]
- Gibney MJ (2019). Ultra-Processed Foods: Definitions and Policy Issues. Current developments in nutrition 3, nzy077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibney MJ, Forde CG, Mullally D, and Gibney ER (2017). Ultra-processed foods in human health: a critical appraisal. Am J Clin Nutr 106, 717–724. [DOI] [PubMed] [Google Scholar]
- Hall KD (2019). The potential role of protein leverage in the US obesity epidemic. Obesity (Silver Spring), In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall KD, Chen KY, Guo J, Lam YY, Leibel RL, Mayer LE, Reitman ML, Rosenbaum M, Smith SR, Walsh BT, et al. (2016). Energy expenditure and body composition changes after an isocaloric ketogenic diet in overweight and obese men. Am J Clin Nutr 104, 324–333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall KD, Guo J, Chen KY, Leibel RL, Reitman ML, Rosenbaum M, Smith SR, and Ravussin E (2019). Methodologic considerations for measuring energy expenditure differences between diets varying in carbohydrate using the doubly labeled water method. Am J Clin Nutr. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris PA, Taylor R, Thielke R, Payne J, Gonzalez N, and Conde JG (2009). Research electronic data capture (REDCap)--a metadata-driven methodology and workflow process for providing translational research informatics support. Journal of biomedical informatics 42, 377–381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones JM (2018). Food processing: criteria for dietary guidance and public health? Proc Nutr Soc, 1–15. [DOI] [PubMed] [Google Scholar]
- Katz DL, and Meller S (2014). Can we say what diet is best for health? Annual review of public health 35, 83–103. [DOI] [PubMed] [Google Scholar]
- Kessler DA (2009). The end of overeating: controling the insatiable American appetite. (New York: Rodale Inc.). [Google Scholar]
- Lagerpusch M, Bosy-Westphal A, Kehden B, Peters A, and Muller MJ (2012). Effects of brief perturbations in energy balance on indices of glucose homeostasis in healthy lean men. Int J Obes (Lond) 36, 1094–1101. [DOI] [PubMed] [Google Scholar]
- Lohman TG, Harris M, Teixeira PJ, and Weiss L (2000). Assessing body composition and changes in body composition. Another look at dual-energy X-ray absorptiometry. Annals of the New York Academy of Sciences 904, 45–54. [DOI] [PubMed] [Google Scholar]
- Martinez Steele E, Baraldi LG, Louzada ML, Moubarac JC, Mozaffarian D, and Monteiro CA (2016). Ultra-processed foods and added sugars in the US diet: evidence from a nationally representative cross-sectional study. BMJ open 6, e009892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinez Steele E, Raubenheimer D, Simpson SJ, Baraldi LG, and Monteiro CA (2018). Ultra-processed foods, protein leverage and energy intake in the USA. Public Health Nutr 21, 114–124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuda M, and DeFronzo RA (1999). Insulin sensitivity indices obtained from oral glucose tolerance testing: comparison with the euglycemic insulin clamp. Diabetes Care 22, 1462–1470. [DOI] [PubMed] [Google Scholar]
- Matthews DR, Hosker JP, Rudenski AS, Naylor BA, Treacher DF, and Turner RC (1985). Homeostasis model assessment: insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia 28, 412–419. [DOI] [PubMed] [Google Scholar]
- McCrickerd K, Lim CM, Leong C, Chia EM, and Forde CG (2017). Texture-Based Differences in Eating Rate Reduce the Impact of Increased Energy Density and Large Portions on Meal Size in Adults. J Nutr 147, 1208–1217. [DOI] [PubMed] [Google Scholar]
- Melo EA, Jaime PC, Monteiro CA, and Nutrition GCo.F.a. (2015). Dietary Guidelines for the Brazilian population. (Brasília: Ministry of Health of Brazil), p. 152. [Google Scholar]
- Mendonca RD, Lopes AC, Pimenta AM, Gea A, Martinez-Gonzalez MA, and Bes-Rastrollo M (2017). Ultra-Processed Food Consumption and the Incidence of Hypertension in a Mediterranean Cohort: The Seguimiento Universidad de Navarra Project. American journal of hypertension 30, 358–366. [DOI] [PubMed] [Google Scholar]
- Mendonca RD, Pimenta AM, Gea A, de la Fuente-Arrillaga C, Martinez-Gonzalez MA, Lopes AC, and Bes-Rastrollo M (2016). Ultraprocessed food consumption and risk of overweight and obesity: the University of Navarra Follow-Up (SUN) cohort study. Am J Clin Nutr 104, 1433–1440. [DOI] [PubMed] [Google Scholar]
- Monteiro CA, Cannon G, Moubarac JC, Levy RB, Louzada MLC, and Jaime PC (2018). The UN Decade of Nutrition, the NOVA food classification and the trouble with ultra-processing. Public Health Nutr 21, 5–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monteiro CA, Moubarac JC, Cannon G, Ng SW, and Popkin B (2013). Ultra-processed products are becoming dominant in the global food system. Obes Rev 14 Suppl 2, 21–28. [DOI] [PubMed] [Google Scholar]
- Moss M (2013). Salt, sugar, fat: how the food giants hooked us. (New York: Random House; ). [Google Scholar]
- Moubarac JC (2015). Ultra-processed food and drink products in Latin America: Trends, impact on obesity, policy implications. (Washington D.C.: Pan American Health Organization; ), p. 60. [Google Scholar]
- Muller MJ, Bosy-Westphal A, Lagerpusch M, and Heymsfield SB (2012). Use of Balance Methods for Assessment of Short-Term Changes in Body Composition. Obesity (Silver Spring) 20, 701–707. [DOI] [PubMed] [Google Scholar]
- Ouwerkerk R, Pettigrew RI, and Gharib AM (2012). Liver metabolite concentrations measured with 1H MR spectroscopy. Radiology 265, 565–575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poti JM, Braga B, and Qin B (2017). Ultra-processed Food Intake and Obesity: What Really Matters for Health-Processing or Nutrient Content? Current obesity reports 6, 420–431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poti JM, Mendez MA, Ng SW, and Popkin BM (2015). Is the degree of food processing and convenience linked with the nutritional quality of foods purchased by US households? Am J Clin Nutr 101, 1251–1262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pourhassan M, Schautz B, Braun W, Gluer CC, Bosy-Westphal A, and Muller MJ (2013). Impact of body-composition methodology on the composition of weight loss and weight gain. Eur J Clin Nutr 67, 446–454. [DOI] [PubMed] [Google Scholar]
- Racette SB, Schoeller DA, Luke AH, Shay K, Hnilicka J, and Kushner RF (1994). Relative dilution spaces of 2H- and 18O-labeled water in humans. Am J Physiol 267, E585–590. [DOI] [PubMed] [Google Scholar]
- Roberts P (2008). The end of food. (New York: Houghton Mifflin Harcourt Publishing Company; ). [Google Scholar]
- Robinson E, Almiron-Roig E, Rutters F, de Graaf C, Forde CG, Tudur Smith C, Nolan SJ, and Jebb SA (2014). A systematic review and meta-analysis examining the effect of eating rate on energy intake and hunger. Am J Clin Nutr 100, 123–151. [DOI] [PubMed] [Google Scholar]
- Rolls BJ (2009). The relationship between dietary energy density and energy intake. Physiol Behav 97, 609–615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SACN SACo.N. (2011). Dietary Reference Values for Energy. (London), p. 225. [Google Scholar]
- Schatzker M (2015). The dorito effect: The surprising new truth about food and flavor. (New York, NY: Simon & Schuster; ). [Google Scholar]
- Schnabel L, Kesse-Guyot E, Alles B, Touvier M, Srour B, Hercberg S, Buscail C, and Julia C (2019). Association Between Ultraprocessed Food Consumption and Risk of Mortality Among Middle-aged Adults in France. JAMA internal medicine. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schoeller DA (1990). How accurate is self-reported dietary energy intake? Nutr Rev 48, 373–379. [DOI] [PubMed] [Google Scholar]
- Schoeller DA, Thomas D, Archer E, Heymsfield SB, Blair SN, Goran MI, Hill JO, Atkinson RL, Corkey BE, Foreyt J, et al. (2013). Self-report-based estimates of energy intake offer an inadequate basis for scientific conclusions. Am J Clin Nutr 97, 1413–1415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schoffelen PF, and Westerterp KR (2008). Intra-individual variability and adaptation of overnight- and sleeping metabolic rate. Physiol Behav 94, 158–163. [DOI] [PubMed] [Google Scholar]
- Schulte EM, Avena NM, and Gearhardt AN (2015). Which foods may be addictive? The roles of processing, fat content, and glycemic load. PLoS One 10, e0117959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schulte EM, Smeal JK, and Gearhardt AN (2017). Foods are differentially associated with subjective effect report questions of abuse liability. PLoS One 12, e0184220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shewfelt RL (2017). In defense of processed food: It’s not nearly as bad as you think. (Springer International Publishing; ). [Google Scholar]
- Simpson SJ, and Raubenheimer D (2005). Obesity: the protein leverage hypothesis. Obes Rev 6, 133–142. [DOI] [PubMed] [Google Scholar]
- Small DM, and DiFeliceantonio AG (2019). Processed foods and food reward. Science 363, 346–347. [DOI] [PubMed] [Google Scholar]
- Speakman JR (1997). Doubly labelled water: Theory and practice. (London: Chapman & Hall; ). [Google Scholar]
- Stuckler D, McKee M, Ebrahim S, and Basu S (2012). Manufacturing epidemics: the role of global producers in increased consumption of unhealthy commodities including processed foods, alcohol, and tobacco. PLoS medicine 9, e1001235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tarasuk V, and Beaton GH (1992). Day-to-day variation in energy and nutrient intake: evidence of individuality in eating behaviour? Appetite 18, 43–54. [DOI] [PubMed] [Google Scholar]
- Walhin JP, Richardson JD, Betts JA, and Thompson D (2013). Exercise counteracts the effects of short-term overfeeding and reduced physical activity independent of energy imbalance in healthy young men. J Physiol 591, 6231–6243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weaver CM, Dwyer J, Fulgoni VL 3rd, King JC, Leveille GA, MacDonald RS, Ordovas J, and Schnakenberg D (2014). Processed foods: contributions to nutrition. Am J Clin Nutr 99, 1525–1542. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.