Abstract
Enhancing the solubility of active drug ingredients is a major challenge faced by scientists and researchers. Different approaches have been explored for the enhancement of solubility and physicochemical properties of drugs, without affecting their stability or pharmacological activity. Among the various strategies available, pharmaceutical co-crystals, co-amorphous systems, and pharmaceutical salts as multicomponent systems (MCS) have gained interest to improve physicochemical properties of drugs. Development of MCS by conventional methods involves the utilization of excess amount of solvents, thus, making the product prone to instability, and may also cause harmful side effects in patients. Scale up is critical and involves the investment of huge capital and time. Lately, hot-melt extrusion has been utilized in the development of MCS to enhance solubility, bioavailability, stability, and physicochemical properties of the drugs. In this review, the authors discussed the development of different MCS produced via hot-melt extrusion technology. Specifically, approaches for screening of co-formers and co-crystals, selection of excipients for co-amorphous systems, pharmaceutical salts, and significance of MCS and process parameters affecting product quality are discussed.
Keywords: Hot-melt extrusion, Multicomponent systems, Co-crystals, Co-amorphous systems, Pharmaceutical salts, Physicochemical properties
Graphical Abstract
1. Introduction
With increasing patient population, the requirement of drug formulations has also increased tremendously. Approximately 80% of new chemical entities (NCE) within the pipeline have been claimed to be poorly water-soluble, which in turn affects their bioavailability and efficacy. Solubility is a critical prerequisite for developing a dosage form. While enhancing the solubility of an NCE, it is important to preserve its chemical structure and maintain its stability [1–3]. Till date, various formulation approaches, such as the use of amorphous solid dispersions, liposomes, pro-liposomes, self-emulsifying drug delivery systems, co-crystals, nanosuspensions, cyclodextrin complexes, co-amorphous systems, and salts, have been examined. Different strategies that have been employed to improve the solubility of drugs include hot-melt extrusion (HME), spray drying, solvent evaporation, supercritical fluid process, high-pressure homogenization, ultrasonic precipitation, and freeze drying. The selection of an appropriate strategy to develop a formulation with improved solubility and bioavailability depends on the nature of materials to be utilized or processed [4–8].
Synthesis of these multicomponent systems (MCS) by conventional process involves multiple steps, is time-consuming, and requires excessive solvent. In addition, scale up of these systems remains a significant issue for the pharmaceutical industries. Nevertheless, the applicability of HME in pharmaceutical industry is well-documented. HME provides the flexibility of single-step continuous extrusion or continuous granulation, with the ease of scale-up/scale-down process. Several process analytical technology tools (PAT) are available for inline monitoring of critical process parameters and their effect on product quality [9,10]. Furthermore, the viability of HME technology for developing various formulations has drawn the attention of researchers, industries, and the FDA.
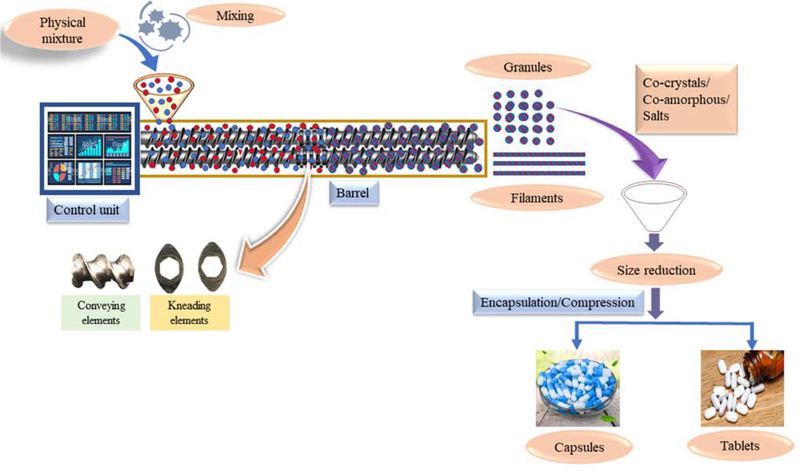
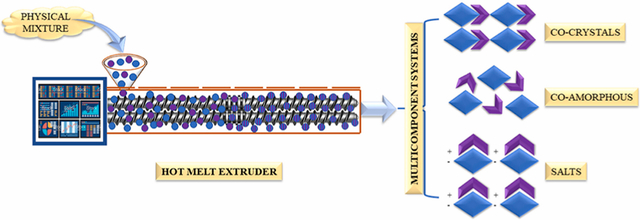
Recently, HME has been explored in the development of MCS [11–14]. HME involves the feeding of a physical mixture (formulation blend), which is then conveyed along the barrel length using co-rotating twin screws by applied thermal and mechanical energy. The conveyed material is extruded through a die and collected as extrudates (powder, granules, filaments) [14–16]. The collected filaments can be subjected to downstream processing, such as milling, followed by compression/encapsulation, or the obtained filaments can be directly cut into pellets, using pelletizer, and filled into capsules [17–19]. The process parameters involved in HME are barrel temperature, feed rate, screw configuration, screw speed, and torque [20–23]. The entire barrel is divided into zones, and the temperature of each zone can be controlled, depending on the thermal properties of the processing materials. This provides an added advantage for heat-sensitive drugs [24]. Among the various screw elements, conveying and mixing elements are most commonly used for developing MCS, and the screw configuration can be customized, as required. Conveying elements are intended to convey the processing material from one zone to another and have zero mixing property. Mixing elements can be configured at 0°, 30°, 60°, and 90° offset angles. Increasing the offset angle results in decreased conveying property and increased shear [14,25]. The processed material is collected as granules by removing the die at the discharge point and can be directly encapsulated or compressed, without any further downstream processing.
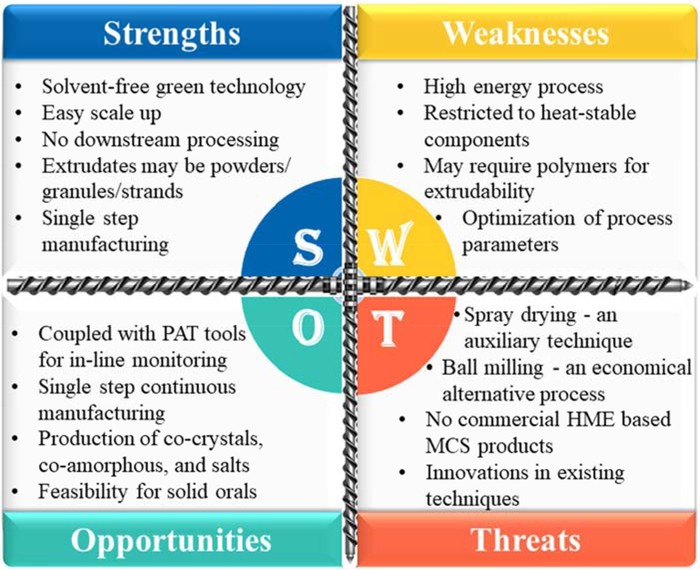
An overview of pharmaceutical co-crystals, co-amorphous systems, and pharmaceutical salts, prepared by various conventional methods such as solvent evaporation, spray drying, ball milling, melt quenching, solvent-assisted grinding, and quench cooling techniques, is available in the literature. However, the current literature lacks the review of HME-based MCS and its significance in formulation development. This review focused on studies related to the development of MCS (co-crystals, co-amorphous systems, and pharmaceutical salts) and HME technology used to enhance the physicochemical properties, such as solubility, bioavailability, and stability of drugs. A SWOT (Strengths, Weaknesses, Opportunities, and Threats) analysis of HME-based MCS is presented in Figure 1. In addition, different approaches for screening of co-formers and co-crystals, selection of excipients for co-amorphous systems, pharmaceutical salts, and significance of MCS and process parameters that affect product quality are discussed. Schematic representation of synthesizing MCS by HME is presented in Figure 2.
Figure 1:
SWOT analysis of hot-melt extrusion-based multicomponent systems (SWOT: Strengths, Weaknesses, Opportunities, and Threats; PAT: Process Analytical Technology; HME: Hot-Melt Extrusion; MCS: Multicomponent Systems).
Figure 2:
Process flow of manufacturing multicomponent systems by hot-melt extrusion.
2. Pharmaceutical co-crystals and hot-melt extrusion
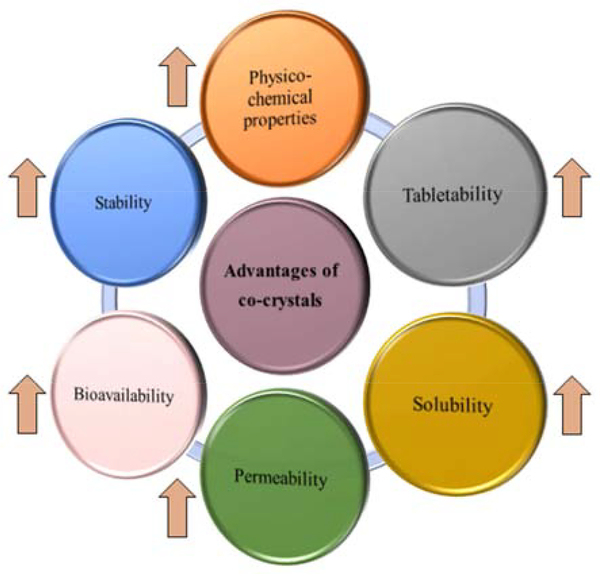
The use of co-crystals is considered as an emerging strategy for enhancing the physicochemical properties, stability [26], taste masking [27], and bioavailability [28] of drugs, without affecting their pharmacological properties [29]. The various advantages of formulating a co-crystal dosage form are listed in Figure 3.
Figure 3:
Various advantages of co-crystal formulations.
Co-crystals are defined as “solids that are crystalline single-phase materials of two or more different molecular and/or ionic compounds, generally in a stoichiometric ratio, which are neither solvates nor simple salts” [30]. Co-crystals are classified as either molecular or ionic, depending on the co-former used. In the molecular system, either neutral or unionized co-formers are used, whereas in ionic systems, ionized co-formers are used. Screening of suitable co-former plays a vital role in the successful formation of co-crystal. Conventionally, a hit and trial approach is mostly used for the selection of co-formers; however, it is expensive and time-consuming. Some of the other approaches that are employed in screening of co-formers and co-crystals are described below. The various USFDA approved materials can be utilized as co-formers. Use of any new excipient as a co-former must be added to the list, EAFUS (Everything added to food in the United States). The co-formers employed in synthesis of co-crystals can be either ionized/unionized/neutral, should be inert, with no adverse effects, and approved as Generally Regarded as Safe (GRAS).
2.1. Methods for screening of co-formers and co-crystals
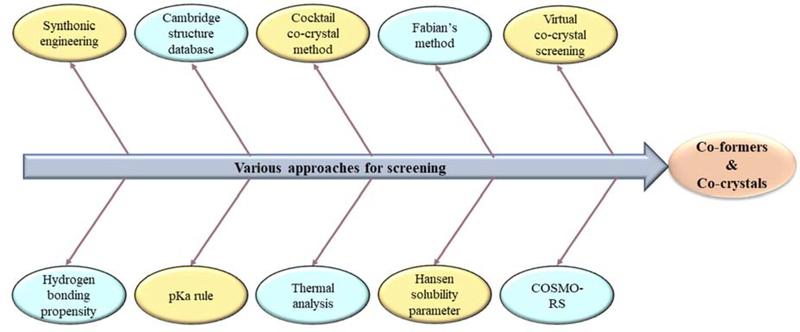
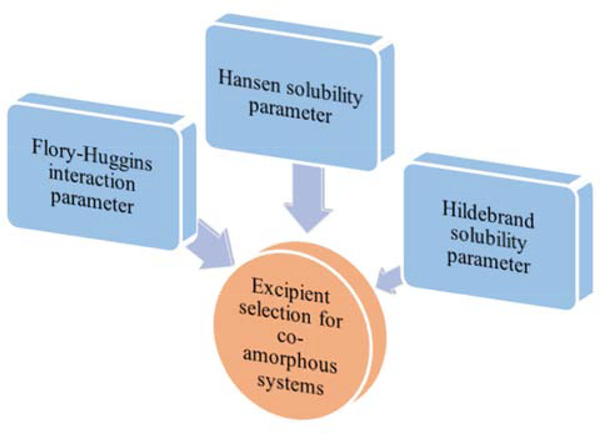
To establish a robust screening technique and save costs and development time, researchers have investigated the suitability of various approaches for the screening of co-formers and co-crystals (Figure 4). Among them, the synthonic engineering approach is most widely used to describe molecular interactions between functional groups of active pharmaceutical ingredient (API) and co-formers. Synthons are basic structural units present in super molecules and are of two types: homosynthons and heterosynthons. Homosynthons involve interaction between the same functional groups of API and co-former (such as amide-amide), whereas heterosynthons involve interaction between different functional groups of API and co-former (such as acid-pyridine). However, this approach is complicated for molecules with multiple H-bond acceptors or donor capacity [31]. The simple rule of pKa can also be employed, where a pKa difference of < 0 results in co-crystal formation [32]. Cambridge structure data base (CSD) is a computer-based approach that provides a list of co-formers based on the functional groups of API, and is associated with a reduction in research time and costs [33]. Fábián’s method involves extraction of co-former structures from CSD, followed by calculation of various molecular properties, which results in the formation of a suitable co-former for co-crystal. Among the estimated molecular properties, shape, and polarity of the co-former have been identified as potential factors [34]. COSMO-RS software is used to predict miscibility of co-former in molten phase. The probability of co-crystallization was predicted based on the excess enthalpy (key factor of H-bonding) of drug-co-former mixture when compared with that of pure components [35]. Hansen solubility parameter (HSP) has also been employed for predicting co-crystal formation, depending on the miscibility of two components. A difference of HSP < 7 MPa0.5 between the components results in successful co-crystal formation [36]. In the virtual screening co-crystal approach, energy difference (ΔE) is estimated for two pure solid components and co-crystal, and a value of > 11 kJ/mol results in 50% more chances of co-crystal formation [37]. Screening of co-formers using cocktail co-crystal approach involves simultaneous ball milling of API with co-formers, which results in the formation of synthons (homosynthons or heterosynthons) between the functional groups of the components [38]. Thermal analysis using differential scanning calorimetry (DSC) was also employed for the screening of co-formers. When a physical mixture (1:1) is subjected to heating, the generation of either three endotherms with two exotherms or two endotherms with one exotherm results in co-crystal formation, and the obtained results can be either confirmed using hot stage microscopy or any of the other screening tools [39,40]. However, this approach is not suitable for heat-sensitive and volatile materials [41,42].
Figure 4:
Various approaches for screening of co-formers and co-crystals.
Co-crystals are conventionally synthesized either by the solid-state technique or solvent-based technique. The former method utilizes little or no solvent, while the latter technology utilizes an excess amount of solvent, which is subsequently separated or evaporated. The solid-state technique is a green technique and advantageous over solvent-based technique [43]. Conventional methods, such as solid-state grinding [44], solvent-assisted grinding [45], and ball milling [46], have been widely used for synthesizing co-crystals. However, co-crystal synthesis using a conventional process has limitations for large scale application. In addition, it requires careful monitoring of crystallization conditions, such as super saturation, temperature, and concentration of compounds. Recently, HME was investigated as a versatile continuous process for the synthesis of co-crystals, and various researchers have focused and reported co-crystals using HME [43,47–53].
HME involves a continuous single-step synthesis of co-crystals by applying mechanical and thermal energy, without the use of any organic solvent. Physical mixture containing co-crystal components (API:co-former) are processed at suitable temperature, using optimized screw configuration. At the processing temperature, the materials being processed might exhibit high melt viscosity, thus resulting in increased torque values to process HME. Thus, incorporation of polymer into the physical mixture, which results in smooth extrusion owing to reduction in melt viscosity, has been investigated. Various studies that have investigated the development of co-crystals by HME, with or without incorporation of polymers, have been detailed in the sections below.
2.2. Plain pharmaceutical co-crystals
The manufacture of plain co-crystals by HME involves extrusion of API and its suitable co-former through an extruder to obtain a co-crystal extrudate. The complete co-crystallization during extrusion depends on the screw configuration, screw speed, and processing temperature employed. Various reports indicating the suitability and development of co-crystals by HME are presented here.
Fernandes et al. [54] examined the suitability of HME for synthesizing carvedilol co-crystals, using different ratios of nicotinamide (NIC) (1:1, 1:2, 2:1, 1:3, and 3:1). HME process parameters, such as temperature, screw speed, and feed rate, were controlled to obtain the desired output of co-crystals. HME was demonstrated to be an appropriate technique to obtain a high practical yield (86.26%) of co-crystals, at a 1:2 ratio of carvedilol:nicotinamide. In addition, solubility of the synthesized co-crystals increased by 15-fold compared to that of pure carvedilol. This study suggested the importance of the selection of appropriate stoichiometric ratio of API and co-former in the formation of co-crystals for improved solubility and dissolution rate of poorly soluble carvedilol.
Moradiya et al. [51] investigated melt extrusion as a continuous co-crystallization process for the formation of carbamazepine (CBZ) and trans-cinnamic acid (TCA) co-crystals, by employing both single-screw extrusion (SSE) and twin-screw extrusion (TSE) processing. The spiral screw design of SSE produced low quality co-crystals because of the lack of sufficient shear during material mixing within the single-screw design. The extrusion process exhibited high torque values at low barrel temperature. By contrast, excellent mixing was achieved in TSE by modifying the screw configuration in mixing zones. According to this study, TSE resulted in high-quality CBZ–TCA co-crystals, when compared with those produced by SSE and solvent crystallization technique. Furthermore, dissolution studies revealed faster dissolution rates for co-crystals produced by TSE. This report indicated the suitability of an appropriate extruder for the production of high-quality co-crystals within the processing temperatures. Additionally, Daurio et al. [48] investigated the application of TSE and the effect of processing parameters on the co-crystallization process, using different co-crystal systems: caffeine–oxalic acid, nicotinamide–TCA, and CBZ–saccharin, in a twin-screw extruder. In this study, caffeine–oxalic acid (2:1) was not transformed into co-crystal at different processing temperatures (25 °C, 75 °C, and 90 °C), with only conveying elements in a screw configuration. However, using the mixing elements at processing temperatures of 25 °C and 75 °C resulted in co-crystal formation, thus, indicating the significance of mixing in co-crystallization. Similarly, nicotinamide–TCA mixture was studied in a 1:1 ratio at different processing temperatures (80 °C, 90 °C, 100 °C, 110 °C, and 120 °C) and 75 rpm screw speed. There was a complete co-crystal conversion at 110 °C and 120 °C, indicating that the co-crystal formation is temperature dependent. In another combination of 1:1 CBZ–saccharin co-crystal preparation, 95% of the mixture was converted to co-crystal at an elevated temperature of 190 °C, which was close to the melting point of CBZ. The authors also investigated liquid-assisted extrusion by incorporating water to anhydrous CBZ–saccharin mixture. The presence of small amount of solvent helped in lowering the extrusion temperature for co-crystal formation. These observations highlight the importance of selection of the appropriate combinations of API and co-formers, extrusion temperature, and shear due to mixing in the formation of co-crystals.
Dhumal et al. [43] developed co-crystals of ibuprofen (IBU) and NIC using HME and investigated the effect of process temperatures (70 °C, 80 °C, and 90 °C), screw speed (20, 30, and 40 rpm) and screw configurations. Only partial co-crystallization was observed when extrusion was performed below the eutectic temperature (74 °C), indicating the significant role of processing temperature in mass transfer, an essential feature in co-crystal formation. In contrast, high purity IBU–NIC co-crystals were produced at high processing temperature (above eutectic temperature), high shear, and low screw speed (increased residence time). This study demonstrated the impact of process parameters in the extent of co-crystallization and agglomeration in a single processing step. Additionally, the extrudates produced by HME were directly compressible and demonstrated enhanced dissolution rate. Similarly, Li et al. [55] investigated the influence of screw geometry and temperature on co-crystal yield, and assessed the solubility of co-crystal components in a given matrix, using the concepts of HSP and Flory–Huggins theory. The authors prepared IBU and isonicotinamide (INA) co-crystal in a 1:1 ratio, with 50% w/w xylitol as the matrix former. The authors reported that the drug co-former miscibility was essential for co-crystallization and the incorporation of solubility parameters was coherent for the screening of co-former candidates. Extrusion trials performed with only conveying elements resulted in a low co-crystal yield. However, increasing the mixing intensity led to an improved co-crystal yield. Selection of optimum processing temperature and a careful choice of screw design enhanced the co-crystal yield. Based on the above literature, twin-screw HME was considered as a suitable single-step manufacturing process for the development of pharmaceutical co-crystals. However, selection and optimization of appropriate extrusion parameters is of prime importance in the production of high-quality co-crystals. In addition, the utilization of appropriate techniques for the screening of co-formers could save significant development time and cost.
2.3. Polymer-assisted co-crystals
Most of the materials (API and co-formers) exhibit thixotropic behavior (decrease in melt viscosity with increasing temperature) when exposed to high temperatures. However, employing high processing temperatures may result in degradation of the processing materials. Thus, the use of polymeric carriers with low glass transition temperature (Tg) and melting temperature, along with co-crystal components, will aid the execution of the extrusion process at lower temperatures, thereby protecting the stability of the formulation ingredients. Various studies that have shown the development of polymer-assisted co-crystals are presented below.
Butreddy et al. [56] prepared aripiprazole (ARP) and adipic acid (ADP) (1:1) co-crystals, with Soluplus® (SOL) (5%) as the polymeric matrix, using HME and investigated the effect of process parameters on co-crystallization. The presence of SOL in the ARP-ADP mixture decreased the torque and enhanced processability during extrusion. This study emphasized the role of processing temperature in producing high-quality co-crystals. The authors observed complete conversion of the physical blend into co-crystals, regardless of screw speed. In addition, the prepared co-crystals showed enhanced solubility (eight-fold) and dissolution rate (seven-fold) when compared with those of pure ARP. Similarly, Karimi-Jafari et al. [57] examined the effect of SOL on co-crystallization of IBU and NIC, using HME, and evaluated the suitability of co-crystals for tableting. IBU–NIC formulations were prepared in a 1:1 M ratio, with and without SOL. Regardless of the screw speed, samples extruded at 90 °C were completely transformed into co-crystals, as confirmed by DSC and Raman spectra. However, powder X-ray diffraction (PXRD) analysis showed highest intensity of the characteristic peak for IBU–NIC co-crystals processed at 40 rpm. The authors identified that the addition of SOL decreased the co-crystallization temperature. Among the different proportions of SOL (10, 20, & 30%) investigated, the physical mixture with 10% SOL resulted in better co-crystal yield, along with reduced processing temperature and improved tabletability, compactibility, and compressibility of the extruded blends. Physical mixtures and co-crystals showed enhanced dissolution when compared with that by pure IBU. The physical nature, viscosity, and glass-forming ability of the polymer dictated the physical appearance of the HME extrudates. These reports corroborate the applicability of SOL in the manufacture of co-crystals at relatively low processing temperatures, when compared with plain co-crystals. Furthermore, it confirms the feasibility of SOL in the development of suitable solid dosage form.
Ross et al. [58] successfully improved the physicochemical stability of indomethacin–saccharin co-crystals by coprocessing with an amorphous hydrophilic polymer (hydroxypropyl methylcellulose [HPMC] grade pharmacoat 603 (3cP)) and aluminometasilicate inorganic (Neusilin® US2), using HME. Addition of HPMC and Neusilin® to the extruder conveying zones, after co-crystallization, allowed the co-crystals to disperse in the dense matrix formed by the molten excipients. This prevented any interactions between the excipient and the parent co-crystal, enhanced dissolution of the co-former, and improved the stability. In contrast, co-crystal extruded with crystalline hydrophilic polymer (PEG 6000) was chemically unstable because of either thermal degradation of the polymer and/or interaction of the polymer with indomethacin. Gajda et al. [59] investigated the role of amorphous and semicrystalline polymers in co-crystallization of flufenamic acid (FFA) and NIC, using HME. Polymers with different structural features and physicochemical properties (poloxamer P407 [PXM], polyethylene glycol-polyvinyl alcohol [PEG-PVA] copolymer, SOL, vinylpyrrolidone and vinyl acetate copolymer [PVPVA64], and hydroxypropyl methylcellulose acetate succinate [HPMCAS]) were examined as functional matrices for FFA–NIC co-crystal synthesis. Semicrystalline polymers (PXM and PEG-PVA) resulted in complete transformation of the physical mixture into co-crystals, whereas partial co-crystallization was observed with amorphous polymers (SOL, PVPVA64, HPMCAS). Co-crystal formulations with semicrystalline polymers (PXM and PEG-PVA) enhanced the dissolution of FFA due to increased wetting of the co-crystal. Thus, the type of polymer played a vital role in the formation of quality co-crystals.
Gajda et al. [60] investigated the effect of semicrystalline (poloxamer P407 and PXM) and amorphous (SOL) polymers on the co-crystallization of theophylline (THP) and NIC, using HME. Incorporation of polymers with low Tg (SOL) or low melting point (PXM), as carriers, significantly reduced the torque values during the process, and thereby increased the efficiency of co-crystal formation when compared with extrusion of pure components. PXRD data revealed that the extent of THP-NIC co-crystals, formed in the presence of PXM, was high for all the investigated API-co-former/polymer ratios. SOL assisted co-crystallization resulted in reduced yield, which could be attributed to the interaction of co-crystal components with amorphous polymer. In THP–NIC/SOL formulations, crystallinity increased when stored at 25 ± 2 °C/60 ± 5% relative humidity (RH) for 12 months because of the interaction of amorphous co-former with the unreacted THP. In contrast, co-crystals of THP–NIC/PXM did not show any further changes, thus, indicating the physicochemical stability of the obtained formulations on storage. This suggests the importance of the selection of an appropriate polymer in the production of stable co-crystals.
Shaikh et al. [61] successfully used twin-screw melt granulation for co-crystallization of THP and 4-aminobenzoic acid (4ABA) in a 1:1 ratio, with different concentrations of polyethylene glycol (PEG) (PEG 1500 and PEG 8000) as hydrophilic binder. Extrusion at processing temperature (125°C) reduced the melt viscosity and thus promoted the molecular collision between drug and co-former resulting in successful co-crystallization and granulation. Co-crystals synthesized at all the proportions of PEG 1500 resulted in co-crystals with poor crystallinity, which could be attributed to the solubility of co-crystal in polymeric carrier. In contrast, 5% PEG 8000 produced co-crystals with good crystallinity. Increasing PEG concentrations resulted in decrease in purity of the co-crystal because of molecular collisions between API and co-former or interaction of polymer with THP. This case study demonstrated the importance of variables such as processing temperature, molecular weight, and concentration of polymer selection in the production of co-crystals.
Walsh et al. [62] compared co-crystals of IBU-INA formed by spray drying and HME techniques. IBU and INA (in 1:1 molar ratio) were synthesized in the presence of various excipients: mannitol, xylitol, SOL, and PVP K15. The use of both spray drying and HME techniques resulted in successful co-crystallization of the API and co-former. Differences in HSP between the API, co-former, and carrier played major role in formation of co-crystals. Besides, this study also highlighted the importance of parameters such as the excipient:co-crystal ratio and the miscibility of the API, co-former, and carrier, in co-crystal formation. In HME, intense mixing for prolonged duration eventually promoted the interactions between co-crystal ingredients and excipients. This study highlighted the importance of solubility parameters in co-crystal formation.
Li et al. [52] reported the use of HME for the mechanochemical synthesis of IBU-INA co-crystal suspensions in a 1:1 stoichiometric ratio, in the presence of xylitol (10, 30, 50% w/w) or 10% w/w eudragit EPO and 10% w/w SOL, as matrix carriers. Co-crystal yield was limited in formulations extruded with polymeric matrix carrier owing to entrapment of the drug in highly viscous polymeric (SOL) network, which prevented the interaction of the drug with co-former. The presence of xylitol significantly increased the dissolution rate, when compared with the formulations consisting of polymeric excipients. In general, for successful co-crystal synthesis, the matrix carrier should be chemically inert and should have low melt viscosity and the processing temperature should be sufficiently lower than the onset of co-crystal melting point. This study substantiated the effect of melting point and concentration of the polymer utilized in the development of polymer-assisted co-crystals. Fernandes and Rathnanand [63] designed a gastroretentive drug delivery system of carvedilol-nicotinamide co-crystals (in a 1:2 ratio) by HME, and optimized it using the Box Behnken design. Co-crystals were formed when the extrusion was carried above the eutectic temperature, indicating the significance of the processing parameters. The prepared co-crystals were compressed into tablets, along with HPMC E50, HPMC K4M, carbopol 934P, sodium bicarbonate, methyl crystalline cellulose, talc, and magnesium stearate, by direct compression. In this study, the desired gastroretention of tablets was achieved only when the formulation consisted of both HPMC E50 and carbopol 934P. Results from this study confirmed that HME, in conjunction with the design of experiment approach, is a suitable method for developing gastroretentive floating tablet.
Liu et al. [64] developed a stable co-crystal solid dispersion of CBZ and NIC, using polymeric carriers such as PVP/VA, SOL, and HPMC E5, by melting method and HME. Extrusion was carried at a processing temperature of 160 °C (which was 30 °C lower than the melting point of the drug) and a screw speed of 30 rpm. DSC data revealed a single melting peak at 160 °C for CBZ–NIC co-crystal, in the presence of PVP/VA and SOL. The hot stage polarized optical microscopy micro photograms revealed that CBZ–NIC–HPMC system melted at 190 °C, which may be attributed to the hydrogen bonding interaction of HPMC with NIC, resulting in the formation of free CBZ. CBZ–NIC co-crystal showed faster dissolution rate in the presence of polymer, which could be because of the co-crystal itself or could be attributed to the hydrophilic nature of the polymers employed. Similarly, Boksa et al. [49] utilized SOL matrix-assisted co-crystallization to develop CBZ and NIC co-crystals (1:1) using HME. Compared to the CBZ anhydrous form, CBZ–NIC co-crystal tended to dissociate in aqueous media to form CBZ dihydrate, thus, resulting in reduced dissolution. The authors found that the incorporation of CBZ–NIC co-crystal into SOL matrix significantly increased the rate and extent of in vitro dissolution. These studies present the need of an appropriate analytical tool to understand whether the improved solubility of polymer-assisted co-crystals is due to co-crystallization, the type of polymer used, or a synergistic effect of both. Polymer-assisted co-crystallization is a suitable approach to produce quality co-crystals for high melting point APIs, with reduced torque and processing temperatures.
Overall, the preparation of pharmaceutical co-crystals is feasible using a single step, solvent-free HME technology, and may be used as an alternative approach to improve solubility, stability, and physicochemical properties of APIs. However, the selection of appropriate processing conditions and polymers is crucial in the manufacture of pharmaceutical co-crystals. The major limitations of this technique in synthesizing co-crystals is non-suitability for thermolabile materials and in some cases the polymers may be required to improve processing conditions.
3. Co-amorphous systems and hot-melt extrusion
The use of a co-amorphous system is an alternative approach to the use of polymeric amorphous solid dispersions. Within the co-amorphous system, the drug is stabilized in its amorphous form using low-molecular-weight material, such as the drug [65–69] or excipient [70–74]. Strong intermolecular interactions exist between the two components of the co-amorphous system [75]. It has been observed that formulations with 1:1 molar ratio are more stable than all other reported ratios, which could be attributed to intermolecular interactions, such as hydrogen bonding [65,66,76–78]. In such cases, recrystallization would be complex as it involves the breaking of intermolecular bonds. In some cases, the anti-plasticizing property of drugs enhances physical stability by increasing Tg [67]. The co-amorphous system can be categorized as either drug-excipient or drug–drug systems. Löbmann et al. examined the suitability of amino acids for the preparation of co-amorphous systems using vibrational ball milling. Along with amino acids, other low-molecular-weight excipients, such as citric acid, succinic acid, tartaric acid, meglumine, quercetin, saccharin, and nicotinamide have also been studied. All these excipients resulted in the formation of co-amorphous systems with enhanced stability, either by the formation of intermolecular interactions between drug and excipient or by enhanced Tg. Similar to the approach adopted for co-crystals, a hit and the trial method was followed for excipient selection. Figure 5 showed some of the prominent approaches used for excipient selection. Drug–drug systems are suitable for drugs which have to be administered together to achieve the desired therapeutic action [79–81]. Conventionally, co-amorphous systems are prepared by milling, solvent evaporation, and melt quenching methods. The major drawback of the milling process is incomplete transition of the crystalline drug to the amorphous state. The leftover partial crystalline drug initiates nucleation and recrystallization of the amorphous drug. The solvent evaporation technique is not suitable for scale up and large scales. The limitations of the conventional processes has encouraged researchers to focus on evaluating the suitability of HME for manufacturing co-amorphous systems [82–84]. In ternary systems, the use of polymers other than co-amorphous components resulted in enhanced stability, owing to the inhibition of phase separation. Similar to co-crystals, extrusion of binary mixtures would result in increased torque, which could be attributed to the thixotropic behavior of materials when exposed to the processing temperatures. Incorporation of additional polymer (ternary system) would result in decreased melt viscosity and increased processability. Some of the studies that were carried for developing co-amorphous systems using HME are described below.
Figure 5:
Prominent approaches employed for the selection of excipients for co-amorphous systems.
Lenz et al. [85], for the first time, examined the feasibility of HME for continuous manufacturing of co-amorphous drug-amino acid formulations, and compared the results with spray drying and solvent evaporation methods. In this study, indomethacin (IND) and arginine co-amorphous system was prepared, with or without copovidone. Thermal analysis data of solvent evaporation and HME samples showed a second Tg at approximately 116 °C, with or without copovidone, because of phase separation of IND and arginine, when compared to those of spray dried samples (which resulted in only single Tg at 55 °C). The phase separation in HME may be due to the slow evaporation of water, whereas in samples prepared by solvent evaporation, a relatively faster evaporation of the solvent (acetone/water) was observed. In contrast, solvent evaporation is rapid in the spray drying technique. Irrespective of the preparation methods, all the formulations exhibited similar dissolution patterns.
Arnfast et al. [86] investigated the feasibility of HME for the development of co-amorphous system of IND–cimetidine (CIM) as a model drug–drug combination, with and without polymer (5% w/w polyethylene oxide [PEO]). Results from the DSC analysis confirmed the eutectic behavior of the IND–CIM physical mixture, with a reduction in the melting temperature of individual components of approximately 30–50 °C. Rheological analysis suggested that high melt viscosities were observed with drug–drug mixtures. In this study, PEO showed a plasticizing effect on the IND–CIM system by decreasing melt viscosity, and thus, favored the extrusion process. Furthermore, the presence of PEO prevented phase separation in co-amorphous extrudates during storage. The above findings demonstrate the suitability of HME for developing stable co-amorphous systems with improved processability and lowered extrusion temperature, thus, preventing degradation of materials and making the process suitable for heat-sensitive components. In the future, a thorough investigation of process parameters and formulation parameters is warranted for the development of quality co-amorphous systems.
4. Pharmaceutical salts and hot-melt extrusion
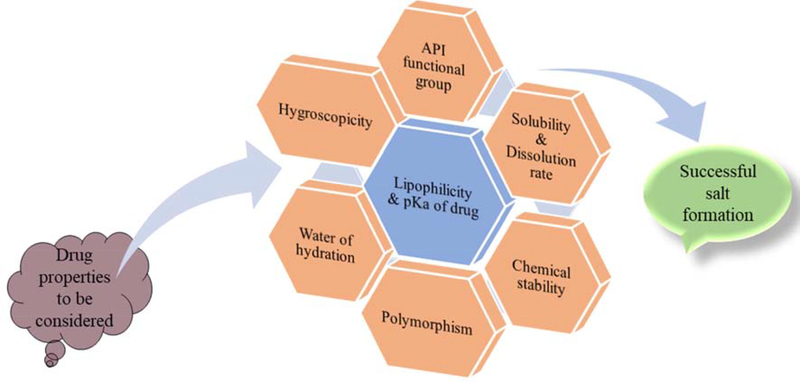
Pharmaceutical salts are used for enhancing the solubility and bioavailability of poorly aqueous soluble drugs. Most of the drugs are either weak acids or weak bases, which assists in salt formation efficiently by using suitable counterions. More than 50% of the drug formulations on the market exist in salt form, making this the most effective technique for enhancing solubility and bioavailability of poorly soluble drugs [87,88]. Salt formation is an acid–base reaction that involves the neutralization reaction or transfer of a proton from the acidic moiety to the basic group and is suitable for all molecules of acidic or basic nature. The strength of the acid and base determines salt formation. Complete transfer of proton takes place in salts, whereas no proton transfer takes place in co-crystals [89]. The formulation of pharmaceutical salt begins with the identification of the acidic or basic functional groups, followed by the selection of suitable counterion. Mostly, low-molecular-weight APIs possess a low melting point, and thus, converting such APIs into the salt form increases the melting point, leading to enhanced stability [89]. A difference in pKa of 2–3 units between drug and counterion enhances the probability of salt formation [90,91]. For basic drugs, pKa should be at least 2 units greater than that of the counterion. For acidic drugs, pKa should be 2 units less than that of the counterion. The difference in pKa ensures formation of stronger interactions that would not dissociate easily [90]. A drug must possess hydrophilic and lipophilic properties. Hydrophilic property helps in aqueous solubility, whereas lipophilic property enhances membrane permeability of drugs. The formation of lipophilic salt is also of utmost importance for hydrophilic drugs. Unlike hydrophilic salts, lipophilic salts do not take up moisture and remain stable even at high humidity [92–94]. Hygroscopicity of API should also considered, as hygroscopic salt form could enhance hydrolysis, which in turn would affect the performance of the drug [95]. Salts with bound water are known as hydrated salts, and those with unbound water are known as anhydrous salts. The bound water is incorporated in the crystalline structure. Exposure to dry environment results in the loss of water molecules from the hydrated form of the salt, leading to the formation of the anhydrate form, which in turn affects the solubility and mechanical properties of the drug [96,97]. Besides pKa, lipophilicity, hygroscopicity, and hydration, other properties such as polymorphism, chemical stability, solubility, and dissolution rate of the drug must be considered in product development. [98–102]. Synthesis of pharmaceutical salts using conventional batch process requires an excess amount of solvent, which has to be subsequently removed. Disposal of the excess solvents requires special environmentally safe procedures, leading to increase in manufacturing cost [103,104]. HME is documented as a single-step, solvent-free manufacturing process, which has prompted researchers to investigate the suitability of HME for synthesizing salts. Depending on the stability of the resultant product, HME provides the flexibility of manufacturing both amorphous and crystalline salts. As per the new FDA guidance document, the salt form of an active ingredient is considered as a new API, which opens the possibilities of patent extensions; however, this scenario is not applicable for API co-crystals, which are regarded as intermediate product or in-process material [105]. Some of the essential properties of the drug that have to be considered for the formulation of salts are shown in Figure 6.
Figure 6:
Properties of drug to be considered for successful formulation of pharmaceutical salts.
Bookwala et al. [106] used HME to improve the solubility, and thereby the dissolution rate of IND, by converting it to the crystalline salt form, using tromethamine as the counter ion. The physical mixture of drug and counter ion was extruded through 11 mm co-rotating twin-screw extruder, at a screw speed of 150 rpm and a processing temperature of 135 °C. The pH-solubility profiles revealed that IND and tromethamine salt had improved solubility (more than 1000 times) compared to that of pure indomethacin. PXRD and scanning electron microscopy confirmed the crystalline nature of the prepared salt. In addition, solution-phase NMR and FTIR results indicated strong ionic interaction, accompanied by proton transfer from IND to tromethamine. The dissolution rate of IND salt was increased by 4.6 times in the aqueous medium. Both the solvent evaporation and HME methods produced crystalline salts with similar physicochemical properties. The processing temperature and screw configuration of TSE were identified as the critical processing parameters in salt formation. This study shows the suitability of HME as an alternative approach in developing pharmaceutical salts.
Similarly, Lee et al. [107] synthesized pharmaceutical salts of haloperidol and maleic acid using TSME, and investigated the effect of temperature and screw configuration on salt formation. High operating temperature and a small ΔT (difference between the operating temperature and melting temperature) resulted in decreased crystallinity of the salt. In TSME, screw configuration with more mixing and shear aided in salt formation. The solubility of resulting haloperidol-maleic acid salt was improved (4.7 mg/mL) and was found to be stable for 90 days at 25 °C and 75% RH.
These studies conducted validated the suitability of HME process for developing pharmaceutical salts. However, very few studies regarding pharmaceutical salts and HME are available in literature. Substantial research has to be conducted for the evaluation of various counterions and confirm the applicability of HME in the preparation of pharmaceutical salts.
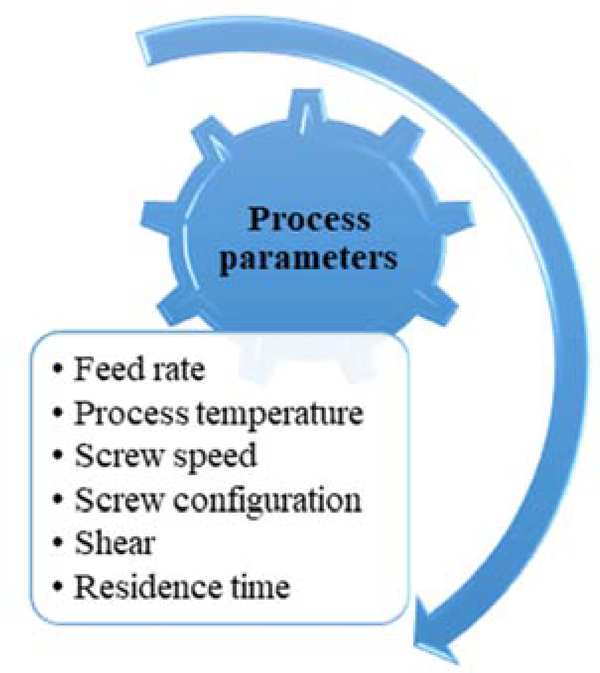
Overall, HME was found to be a suitable technique for the manufacturing of MCS, which would be an added advantage to the pharmaceutical industry as most of the drugs are available in the salt form. The HME technique would enable the production of MCS as a continuous manufacturing line, without compromising the quality of the final product. Some of the critical process parameters that would affect the output are captured in Figure 7. However, before transitioning completely from conventional methods to HME, a thorough understanding of the formulation parameters (concentration and suitability of polymeric carriers, viscosity, molecular weight), process parameters (feed rate, screw speed, screw configuration, process temperature, and effect of shear), and evaluation of various PAT tools is required. There is a potential scope for research in development of MCS. Different MCS formulations developed using the HME technique are listed in Table 1. Among the various co-formers investigated for the preparation of co-crystals, nicotinamide is the most widely utilized co-former (Table 1).
Figure 7:
Process parameters affecting the quality of hot-melt extrusion (HME)-based multicomponent system (MCS).
Table 1:
Various multicomponent systems (MCS) developed using hot-melt extrusion (HME) technology
| Composition of Multicomponent system (MP* °C) | Matrix | Screw configuration elements | Process Temperature (°C) | Comments | Reference |
|---|---|---|---|---|---|
| Co-crystals | |||||
| Theophylline (270–274) – Nicotinamide (128) | HPMCAS-MG/PEO N80/Kollidon VA64® | Conveying, Kneading elements | 140–170 | Addition of polymers improved extrudability. Kollidon VA64® resulted in successful co-crystal with no eutectic formation. | [108] |
| Ibuprofen(75 to 78)-Nicotinamide (128) | Soluplus® | Conveying elements | 70–90 | Addition of Soluplus® enhanced tableting properties and decreased processing temperature. | [57] |
| Flufenamic acid (125) – Nicotinamide (128) | Poloxamer P407, PEG-PVA, Soluplus®, PVPVA64, HPMCAS | Conveying, Kneading elements | 70–135 | Polymers resulted in reduced torque. Poloxamer and PEG-PVA resulted in successful matrix assisted cocrystal formation. | [59] |
| Theophylline (270–274) – 4 Aminobenzoic acid (187) | PEG | Conveying, Kneading elements | 40–125 | Melt granulation technique was employed. PEG was used as a binder for enhancing tableting properties | [61] |
| Ibuprofen (75–78) – Isonicotinamide (155–157) | Xylitol, Soluplus®, PVP K15 | Conveying elements | 70–90 | Hansen solubility parameter was used for estimating co-crystal formation, Soluplus® and PVP resulted in single components of cocrystal components along with co-crystal. | [62] |
| Carvedilol (114) – Nicotinamide (128) | NA | Conveying, Kneading elements | 85–92 | Process temperature above the eutectic melting point resulted in successful cocrystal formation. | [54] |
| Theophylline (270–274) – Nicotinamide (128) | Poloxamer P407, Soluplus® | Conveying, Kneading elements | 70–135 | Semi-crystalline poloxamer resulted in successful cocrystal formation. | [60] |
| Ibuprofen (75–78) – Isonicotinamide (155–157) | Xylitol, Soluplus®, Eudragit EPO | Conveying elements | 85–92 | Carrier (Xylitol) should have limited interaction with the pure drug and co-former. | [52] |
| Carbamazepine (193) – Trans cinnamic acid (133) | NA | Conveying, Kneading elements | 110–135 | Process temperature and screw type affected co-crystal quality. In-line NIR probe was mounted to monitor co-crystal formation during the process. | [51] |
| Ibuprofen (75–78) – Nicotinamide (128) | NA | Conveying, Kneading elements | 70–90 | Process temperature above the eutectic melting point resulted in successful cocrystal formation. | [43] |
| a. Caffeine (238)-Oxalic acid (189–191); b. Nicotinami de (128) – Trans cinnamic acid (133); c. Carbamaze pine (193) – Saccharin (228); d. Theophylli ne (270274) – Citric acid (153) |
NA | Conveying, Kneading elements | a. 25, 75, 90°C b. 80, 90,100, 110, 120°C c. 50, 100, 190°C d. 20, 50, 153°C |
Process temperature, residence time, mixing efficiency are key factors affecting formation of co-crystals. | [48] |
| Carvedilol (114) – Nicotinamide (128) | HPMC E50, HPMC K4M, Carbopol 934P | Conveying, Kneading elements | 85–90 | Co-crystals of Carvedilol were directly compressed into gastroretentive tablets using suitable excipients. | [63] |
| Carbamazepine (193) – Nicotinamide (128) | PVP/VA, Soluplus®, HPMC | NA | 160 | Carrier assisted cocrystal formation resulted in enhanced stability. | [64] |
| Carbamazepine (193) – Nicotinamide (128) | Soluplus® | NA | 115 | Addition of polymer improves in vitro dissolution. | [49] |
| Co-amorphous systems | |||||
| Indomethacin (150–160) – Arginine (226- 230) |
Copovidone | Conveying, Kneading elements | 120–200 | Stable co-amorphous system was prepared with or without copovidone. Presence of copovidone resulted in enhanced dissolution when compared with formulations with only Indomethacin – Arginine |
[85] |
| Indomethacin (150–160) – Cimetidine (142) | PEO | NA | 120 | Addition of PEO reduced melt viscosity and also inhibited drug-drug phase separation | [86] |
| Pharmaceutical Salts | |||||
| Indomethacin (162) – Tromethamine (142) | NA | Conveying, Kneading elements | 135 | Crystalline salt of Indomethacin was prepared | [106] |
| Haloperidol (151) – Maleic acid (140) | NA | Conveying, Kneading elements | 30, 80°C | Process temperature and screw configuration are identified as key parameters | [107] |
MP: Melting Point; NA: Not available
5. Conclusion
Traditionally, HME and twin-screw granulation have been used successfully for the development of amorphous solid dispersions. Recently, HME has been explored for the manufacture of different MCS with improved solubility and bioavailability. HME involves a single continuous step for the synthesis of MCS with no requirement of solvent. The use of polymeric carriers in MCS facilitates the extrusion process by reducing the melt viscosity and results in lowered processing temperatures and inhibition of phase separations. Process temperatures and screw configurations are the crucial parameters affecting the quality of MCS. Mixing zones with high offset angles were found to be successful in the execution of HME, without leaving any unreacted formulation components. The process of HME can be scaled up quickly, thus, saving colossal capital and time for the pharmaceutical industries. Implementation of PAT tools will make HME technology suitable as a single continuous manufacturing line for manufacturing MCS by inline monitoring critical process parameters, without affecting product quality. Future studies should extensively evaluate various polymers, effect of various process parameters (feed rate, screw speed, screw configuration, screw elements, and process temperatures) on MCS quality, and suitability of various PAT tools.
Highlights.
SWOT analysis of hot-melt extrusion (HME) based multi component systems (MCS).
Processing temperature and screw configuration critically affect quality of MCS.
Polymer assisted systems enhanced HME processability.
Both crystalline and amorphous salts can be synthesized using HME technique.
Nicotinamide was reported as a common co-former in pharmaceutical co-crystals.
Acknowledgements
This project was also partially supported by Grant Number P30GM122733-01A1, funded by the National Institute of General Medical Sciences (NIGMS) a component of the National Institutes of Health (NIH) as one of its Centers of Biomedical Research Excellence (COBRE).
Abbreviations
- MCS
multicomponent systems
- NCE
new chemical entities
- HME
hot-melt extrusion
- PAT
process analytical technology tools
- EAFUS
everything added to food in the United States
- API
active pharmaceutical ingredient
- CSD
Cambridge structure data base
- HSP
Hansen solubility parameters
- DSC
differential scanning calorimetry
- NIC
nicotinamide
- CBZ
carbamazepine
- TCA
trans-cinnamic acid
- TSE
twin-screw extrusion
- IBU
ibuprofen
- INA
isonicotinamide
- Tg
glass transition temperature
- ARP
aripiprazole
- ADP
adipic acid
- SOL
soluplus®
- PXRD
powder X-ray diffraction
- HPMC
hydroxypropyl methylcellulose
- PEG
polyethylene glycol
- FFA
flufenamic acid
- PXM
poloxamer P407
- PEG-PVA
polyethylene glycol-polyvinyl alcohol copolymer
- PVPVA 64
vinylpyrrolidone and vinyl acetate copolymer
- HPMCAS
hydroxypropyl methylcellulose acetate succinate
- THP
theophylline
- RH
relative humidity
- 4ABA
4-aminobenzoic acid
- IND
indomethacin
- CIM
cimetidine
- PEO
polyethylene oxide
- NMR
nuclear magnetic resonance spectroscopy
- FTIR
Fourier-transform infrared spectroscopy
- MP
melting point
Footnotes
Declaration of interests
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- [1].Hauss DJ, Oral Lipid-based Formulations, Adv. Drug Deliv. Rev. 59 (2007) 667–676. [DOI] [PubMed] [Google Scholar]
- [2].Williams H, Strategies to address low drug solubility in discovery and development, ASPET. 65 (2013) 315–499. 10.1124/pr.112.005660. [DOI] [PubMed] [Google Scholar]
- [3].Bauer F, Challenges in today’s pharmaceutical formulation, Eur. Pharm. Rev. 25 (2020) 64–64. https://www.europeanpharmaceuticalreview.com/article/113038/challenges-in-todays-pharmaceutical-formulation/ (accessed September 14, 2020). [Google Scholar]
- [4].Savjani K, Gajjar A, Savjani J, Drug solubility: importance and enhancement techniques, Int. Sch. Res. Not. 2012 (2012) 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Sareen S, Mathew G, Joseph L, Improvement in solubility of poor water-soluble drugs by solid dispersion, Int J Pharm Investig. 2 (2012) 12–17. 10.4103/2230-973X.96921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Singh D, Bedi N, Tiwary AK, Enhancing solubility of poorly aqueous soluble drugs: critical appraisal of techniques, J. Pharm. Investig. 48 (2018) 509–526. 10.1007/s40005-017-0357-1. [DOI] [Google Scholar]
- [7].Bandari S, Gangishetty S, Eedara BB, Jukanti R, Veerareddy PR, Proliposomes of lisinopril dihydrate for transdermal delivery: Formulation aspects and evaluation, Korean J. Chem. Eng. 30 (2013) 1659–1666. 10.1007/s11814-013-0110-z. [DOI] [Google Scholar]
- [8].Eedara BB, Kallakunta VR, Bandari S, Self-nanoemulsifying powders for improved oral delivery of poorly water-soluble drugs, Ther. Deliv. 6 (2015) 899–901. 10.4155/tde.15.43. [DOI] [PubMed] [Google Scholar]
- [9].Fonteyne M, Vercruysse J, De Leersnyder F, Van Snick B, Vervaet C, Remon JP, De Beer T, Process analytical technology for continuous manufacturing of solid-dosage forms, TrAC Trends Anal. Chem. 67 (2015) 159–166. [Google Scholar]
- [10].Islam MT, Maniruzzaman M, Halsey SA, Chowdhry BZ, Douroumis D, Development of sustained-release formulations processed by hot-melt extrusion by using a quality-by-design approach, Drug Deliv. Transl. Res. 4 (2014) 377–387. 10.1007/s13346-014-0197-8. [DOI] [PubMed] [Google Scholar]
- [11].Allison G, Cain YT, Cooney C, Garcia T, Bizjak TG, Holte O, Jagota N, Komas B, Korakianiti E, Kourti D, Madurawe R, Morefield E, Montgomery F, Nasr M, Randolph W, Robert JL, Rudd D, Zezza D, Regulatory and quality considerations for continuous manufacturing May 20–21, 2014 continuous manufacturing symposium, J. Pharm. Sci. 104 (2015) 803–812. 10.1002/jps.24324. [DOI] [PubMed] [Google Scholar]
- [12].Byrn S, Futran M, Thomas H, Jayjock E, Maron N, Meyer RF, Myerson AS, Thien MP, Trout BL, Achieving Continuous Manufacturing for Final Dosage Formation: Challenges and How to Meet Them. May 20–21, 2014 Continuous Symposium, J. Pharm. Sci. 104 (2015) 792–802. [DOI] [PubMed] [Google Scholar]
- [13].Nepveux K, Sherlock JP, Futran M, Thien M, Krumme M, How Development and Manufacturing Will Need to Be Structured-Heads of Development/Manufacturing. May 20–21, 2014 Continuous Symposium, J. Pharm. Sci. 104 (2015) 850–864. 10.1002/jps.24286. [DOI] [PubMed] [Google Scholar]
- [14].Bandari S, Nyavanandi D, Kallakunta VR, Janga KY, Sarabu S, Butreddy A, Repka MA, Continuous twin screw granulation – An advanced alternative granulation technology for use in the pharmaceutical industry, Int. J. Pharm. 580 (2020) 119215. 10.1016/j.ijpharm.2020.119215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Censi R, Rosa M, Id G, Casadidio C, Di Martino P, Hot Melt Extrusion: Highlighting Physicochemical Factors to Be Investigated While Designing and Optimizing a Hot Melt Extrusion Process, Pharmaceutics. 10 (2018) 89. 10.3390/pharmaceutics10030089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Repka MA, Bandari S, Kallakunta VR, Vo AQ, McFall H, Pimparade MB, Bhagurkar AM, Melt extrusion with poorly soluble drugs – An integrated review, Int. J. Pharm. 535 (2018) 68–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Saerens L, Vervaet C, Remon JP, De Beer T, Process monitoring and visualization solutions for hot-melt extrusion: A review, J. Pharm. Pharmacol. 66 (2014) 180–203. 10.1111/jphp.12123. [DOI] [PubMed] [Google Scholar]
- [18].Vervaet C, Vercruysse J, Remon JP, De Beer T, Continuous Processing of Pharmaceuticals, Encycl. Pharm. Sci. Technol. (2013) 37–41. 10.1081/E-EPT4-120050224. [DOI] [Google Scholar]
- [19].Vo AQ, Kutz G, He H, Narala S, Bandari S, Repka MA, Continuous Manufacturing of Ketoprofen Delayed Release Pellets using Melt Extrusion Technology: Application of QbD Design Space, Inline Near Infrared, and Inline Pellet Size Analysis, J. Pharm. Sci. (2020). 10.1016/j.xphs.2020.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Dhenge R, Washino K, Cartwright J, M.H.-P. Technology, U. 2013, Twin screw granulation using conveying screws: Effects of viscosity of granulation liquids and flow of powders, Powder Technol. 238 (2013) 77–90. [Google Scholar]
- [21].El Hagrasy A, Hennenkamp J, M.B.-P. Technology, U. 2013, Twin screw wet granulation: influence of formulation parameters on granule properties and growth behavior, Powder Technol. 238 (2013) 108–115. [Google Scholar]
- [22].Lee K, Ingram A, N.R.-E. journal of pharmaceutics And, U. 2012, Twin screw wet granulation: the study of a continuous twin screw granulator using Positron Emission Particle Tracking (PEPT) technique, Eur. J. Pharm. Biopharm. 81 (2012) 666–673. [DOI] [PubMed] [Google Scholar]
- [23].Tu W, Ingram A, J.S.-C. engineering Science, U. 2013, Regime map development for continuous twin screw granulation, Chem. Eng. Sci. 87 (2013) 315–326. [Google Scholar]
- [24].Vervaet C, Remon JP, Continuous granulation in the pharmaceutical industry, Chem. Eng. Sci. 60 (2005) 3949–3957. 10.1016/j.ces.2005.02.028. [DOI] [Google Scholar]
- [25].Thompson M, Sun J, Wet granulation in a twin-screw extruder: Implications of screw design, J. Pharm. Sci. 99 (2010) 2090–2103. [DOI] [PubMed] [Google Scholar]
- [26].V Trask A, Sam Motherwell WD, Jones W, Physical stability enhancement of theophylline via cocrystallization, Int. J. Pharm. 320 (2006) 114–123. 10.1016/j.ijpharm.2006.04.018. [DOI] [PubMed] [Google Scholar]
- [27].Maeno Y, Fukami T, Kawahata M, Yamaguchi K, Novel pharmaceutical cocrystal consisting of paracetamol and trimethylglycine, a new promising cocrystal former, Int. J. Pharm. 473 (2014) 179–186. [DOI] [PubMed] [Google Scholar]
- [28].Serrano DR, Walsh D, O’Connell P, Mugheirbi NA, Worku ZA, Bolas-Fernandez F, Galiana C, Dea-Ayuelae MA, Healy AM, Optimising the in vitro and in vivo performance of oral cocrystal formulations via spray coating, Eur. J. Pharm. Biopharm. 124 (2018) 13–27. 10.1016/j.ejpb.2017.11.015. [DOI] [PubMed] [Google Scholar]
- [29].Luo C, Liang W, Chen X, Wang J, Deng Z, Zhang H, Pharmaceutical cocrystals of naringenin with improved dissolution performance, CrystEngComm. 20 (2018) 3025–3033. [Google Scholar]
- [30].Aitipamula S, Banerjee R, Bansal AK, Biradha K, Cheney ML, Choudhury AR, Desiraju GR, Dikundwar AG, Dubey R, Duggirala N, Ghogale PP, Ghosh S, Goswami PK, Goud NR, Jetti RKR, Karpinski P, Kaushik P, Kumar D, Kumar V, Moulton B, Mukherjee A, Mukherjee G, Myerson AS, Puri V, Ramanan A, Rajamannar T, Reddy CM, Rodriguez-Hornedo N, Rogers RD, Row TNG, Sanphui P, Shan N, Shete G, Singh A, Sun CC, Swift JA, Thaimattam R, Thakur TS, Kumar Thaper R, Thomas SP, Tothadi S, Vangala VR, Vishweshwar P, Weyna DR, Zaworotko MJ, Polymorphs, salts, and cocrystals: What’s in a name?, Cryst. Growth Des. 12 (2012) 2147–2152. 10.1021/cg3002948. [DOI] [Google Scholar]
- [31].Desiraju GR, Supramolecular Synthons in Crystal Engineering—A New Organic Synthesis, Angew. Chemie Int. Ed. English. 34 (1995) 2311–2327. 10.1002/anie.199523111. [DOI] [Google Scholar]
- [32].Bhogala BR, Basavojua S, Nangia A, Tape and layer structures in cocrystals of some di-and tricarboxylic acids with 4, 4’-bipyridines and isonicotinamide. From binary to ternary cocrystals, CrystEngComm. 7 (2005) 551–562. [Google Scholar]
- [33].Qiao N, Li M, Schlindwein W, Malek Nazneen, Davies A, Trappitt G, Pharmaceutical cocrystals: an overview, Int. J. Pharm. 419 (2011) 1–11. [DOI] [PubMed] [Google Scholar]
- [34].Fábián L, Cambridge structural database analysis of molecular complementarity in cocrystals, Cryst. Growth Des. 9 (2009) 1436–1443. 10.1021/cg800861m. [DOI] [Google Scholar]
- [35].Abramov YA, Loschen C, Klamt A, Rational coformer or solvent selection for pharmaceutical cocrystallization or desolvation, J. Pharm. Sci. 101 (2012) 3687–3697. [DOI] [PubMed] [Google Scholar]
- [36].Mohammad MA, Alhalaweh A, Velaga SP, Hansen solubility parameter as a tool to predict cocrystal formation, Int. J. Pharm. 407 (2011) 63–71. [DOI] [PubMed] [Google Scholar]
- [37].Musumeci D, Hunter C, Prohens R, Scuderi S, Virtual cocrystal screening, Chem. Sci. 2 (2011) 883–890. [Google Scholar]
- [38].Yamamoto K, Tsutsumi S, Ikeda Y, Establishment of cocrystal cocktail grinding method for rational screening of pharmaceutical cocrystals, Int. J. Pharm. 437 (2012) 162–171. [DOI] [PubMed] [Google Scholar]
- [39].Lu E, Rodríguez-Hornedo N, Suryanarayanan R, A rapid thermal method for cocrystal screening, CrystEngComm. 10 (2008) 665–668. [Google Scholar]
- [40].Zhou Z, Chan HM, Sung HHY, Tong HHY, Zheng Y, Identification of New Cocrystal Systems with Stoichiometric Diversity of Salicylic Acid Using Thermal Methods, Pharm. Res. 33 (2016) 1030–1039. 10.1007/s11095-015-1849-1. [DOI] [PubMed] [Google Scholar]
- [41].Duggirala NK, Perry ML, Almarsson Ö, Zaworotko MJ, Pharmaceutical cocrystals: along the path to improved medicines, Chem. Commun. 52 (2016) 640–655. 10.1039/c5cc08216a. [DOI] [PubMed] [Google Scholar]
- [42].Thipparaboina R, Kumar D, Chavan RB, Shastri NR, Multidrug co-crystals: towards the development of effective therapeutic hybrids, Drug Discov. Today. 21 (2016) 481–490. 10.1016/j.drudis.2016.02.001. [DOI] [PubMed] [Google Scholar]
- [43].Dhumal RS, Kelly AL, York P, Coates PD, Paradkar A, Cocrystalization and simultaneous agglomeration using hot melt extrusion, Pharm. Res. 27 (2010) 2725–2733. 10.1007/s11095-010-0273-9. [DOI] [PubMed] [Google Scholar]
- [44].Zhang G, Lin H, Lin S, Thermal analysis and FTIR spectral curve-fitting investigation of formation mechanism and stability of indomethacin-saccharin cocrystals via solid-state grinding Process, J. Pharm. Biomed. Anal. 66 (2012) 162–169. [DOI] [PubMed] [Google Scholar]
- [45].Shan N, Toda F, Jones W, Mechanochemistry and co-crystal formation: effect of solvent on reaction kinetics, Chem. Commun. (2002) 2372–2373. [DOI] [PubMed] [Google Scholar]
- [46].Friščic T, Friščić1 F, Halasz I, Beldon PJ, Belenguer AM, Adams F, Kimber SAJ, Honkimäki V, Dinnebier RE, Real-time and in situ monitoring of mechanochemical milling reactions, Nat. Chem. 5 (2013) 66–73. 10.1038/NCHEM.1505. [DOI] [PubMed] [Google Scholar]
- [47].Medina C, Daurio D, Nagapudi K, Alvarez-Nunez F, Manufacture of pharmaceutical co-crystals using twin screw extrusion: A solvent-less and scalable process, J. Pharm. Sci. 99 (2010) 1693–1696. [DOI] [PubMed] [Google Scholar]
- [48].Daurio D, Medina C, Saw R, Nagapudi K, Alvarez-Núñez F, Application of Twin Screw Extrusion in the Manufacture of Cocrystals, Part I: Four Case Studies, Pharmaceutics. 3 (2011) 582–600. 10.3390/pharmaceutics3030582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Boksa K, Otte A, Pinal R, Matrix-assisted cocrystallization (MAC) simultaneous production and formulation of pharmaceutical cocrystals by hot-melt extrusion, J. Pharm. Sci. 103 (2014) 2904–2910. 10.1002/jps.23983. [DOI] [PubMed] [Google Scholar]
- [50].Daurio D, Nagapudi K, Li L, Quan P, Nunez F-A, Application of Twin Screw Extrusion to the Manufacture of Cocrystals: Scale up of AMG 517-Sorbic acid cocrystal production, Faraday Discuss. 170 (2014) 235–249. [DOI] [PubMed] [Google Scholar]
- [51].Moradiya HG, Islam MT, Halsey S, Maniruzzaman M, Chowdry B, Snowden MJ, Douroumis D, Continuous cocrystallisation of Carbamazepine and trans-Cinnamic acid via melt extrusion processing, CrystEngComm. 16 (2014) 3573–3583. [Google Scholar]
- [52].Li S, Yu T, Tian Y, McCoy CP, Jones DS, Andrews GP, Mechanochemical synthesis of pharmaceutical cocrystal suspensions via hot melt extrusion: Feasibility studies and physicochemical characterization, Mol. Pharm. 13 (2016) 3054–3068. 10.1021/acs.molpharmaceut.6b00134. [DOI] [PubMed] [Google Scholar]
- [53].Moradiya HG, Islam MT, Scoutaris N, Halsey SA, Chowdhry BZ, Douroumis D, Continuous Manufacturing of High Quality Pharmaceutical Cocrystals Integrated with Process Analytical Tools for In-Line Process Control, Cryst. Growth Des. 16 (2016) 3425–3434. 10.1021/acs.cgd.6b00402. [DOI] [Google Scholar]
- [54].Fernandes GJ, Rathnanand M, Kulkarni V, Mechanochemical Synthesis of Carvedilol Cocrystals Utilizing Hot Melt Extrusion Technology, J. Pharm. Innov. 14 (2019) 373–381. 10.1007/s12247-018-9360-y. [DOI] [Google Scholar]
- [55].Li S, Yu T, Tian Y, Lagan C, Jones DS, Andrews GP, Mechanochemical Synthesis of Pharmaceutical Cocrystal Suspensions via Hot Melt Extrusion: Enhancing Cocrystal Yield, Mol. Pharm. 15 (2018) 3741–3754. 10.1021/acs.molpharmaceut.7b00979. [DOI] [PubMed] [Google Scholar]
- [56].Butreddy A, Sarabu S, Bandari S, Dumpa N, Zhang F, Repka MA, Polymer-Assisted Aripiprazole-Adipic Acid Cocrystals Produced by Hot Melt Extrusion Techniques, Cryst. Growth Des. 20 (2020) 4335–4345. 10.1021/acs.cgd.0c00020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [57].Karimi-Jafari M, Ziaee A, Iqbal J, O’Reilly E, Croker D, G. W, Impact of polymeric excipient on cocrystal formation via hot-melt extrusion and subsequent downstream processing, Int. J. Pharm. 566 (2019) 745–755. [DOI] [PubMed] [Google Scholar]
- [58].Ross SA, Ward A, Basford P, McAllister M, Douroumis D, Coprocessing of Pharmaceutical Cocrystals for High Quality and Enhanced Physicochemical Stability, Cryst. Growth Des. 19 (2019) 876–888. 10.1021/acs.cgd.8b01440. [DOI] [Google Scholar]
- [59].Gajda M, Nartowski KP, Pluta J, Karolewicz B, The role of the polymer matrix in solvent-free hot melt extrusion continuous process for mechanochemical synthesis of pharmaceutical cocrystal, Eur. J. Pharm. Biopharm. 131 (2018) 48–59. 10.1016/j.ejpb.2018.07.002. [DOI] [PubMed] [Google Scholar]
- [60].Gajda M, Nartowski KP, Pluta J, Karolewicz B, Tuning the cocrystal yield in matrix-assisted cocrystallisation via hot melt extrusion: A case of theophylline-nicotinamide cocrystal, Int. J. Pharm. 569 (2019) 118579. 10.1016/j.ijpharm.2019.118579. [DOI] [PubMed] [Google Scholar]
- [61].Shaikh R, Walker G, Croker D, Continuous, simultaneous cocrystallization and formulation of Theophylline and 4-Aminobenzoic acid pharmaceutical cocrystals using twin screw melt granulation, Eur. J. Pharm. Sci. 137 (2019) 104981. [DOI] [PubMed] [Google Scholar]
- [62].Walsh D, Serrano DR, Worku ZA, Madi AM, O’Connell P, Twamley B, Healy AM, Engineering of pharmaceutical cocrystals in an excipient matrix: Spray drying versus hot melt extrusion, Int. J. Pharm. 551 (2018) 241–256. 10.1016/j.ijpharm.2018.09.029. [DOI] [PubMed] [Google Scholar]
- [63].Fernandes GJ, Rathnanand M, Formulation Optimization for Gastroretentive Drug Delivery System of Carvedilol Cocrystals Using Design of Experiment, J. Pharm. Innov. 15 (2020) 455–465. 10.1007/s12247-019-09393-5. [DOI] [Google Scholar]
- [64].Liu X, Lu M, Guo Z, Huang L, Feng X, Wu C, Improving the chemical stability of amorphous solid dispersion with cocrystal technique by hot melt extrusion, Pharm. Res. 29 (2012) 806–817. 10.1007/s11095-011-0605-4. [DOI] [PubMed] [Google Scholar]
- [65].Allesø M, Chieng N, Rehder S, Rantanen J, Rades T, Aaltonen J, Enhanced dissolution rate and synchronized release of drugs in binary systems through formulation: Amorphous naproxen–cimetidine mixtures prepared by mechanical activation, J. Control. Release. 136 (2009) 45–53. [DOI] [PubMed] [Google Scholar]
- [66].Chieng N, Aaltonen J, Saville D, Rades T, Physical characterization and stability of amorphous indomethacin and ranitidine hydrochloride binary systems prepared by mechanical activation, Eur. J. Pharm. Biopharm. 71 (2009) 47–54. [DOI] [PubMed] [Google Scholar]
- [67].Löbmann K, Strachan C, Grohganz H, Rades T, Korhonen O, Laitinena R, Co-amorphous simvastatin and glipizide combinations show improved physical stability without evidence of intermolecular interactions, Eur. J. Pharm. Biopharm. 81 (2012) 159–169. [DOI] [PubMed] [Google Scholar]
- [68].Shayanfar A, Jouyban A, Drug-Drug Coamorphous Systems: Characterization and Physicochemical Properties of Coamorphous Atorvastatin with Carvedilol and Glibenclamide, J. Pharm. Innov. 8 (2013) 218–228. 10.1007/s12247-013-9162-1. [DOI] [Google Scholar]
- [69].Dengale S, Ranjan O, Hussen S, Krishna B, Musmade B, Shenoy G, Bhat K, Preparation and characterization of co-amorphous Ritonavir–Indomethacin systems by solvent evaporation technique: Improved dissolution behavior and physical stability without evidence of intermolecular interactions, Eur. J. Pharm. Sci. 62 (2014) 57–64. [DOI] [PubMed] [Google Scholar]
- [70].Lu Q, Zografi G, Phase behavior of binary and ternary amorphous mixtures containing indomethacin, citric acid, and PVP, Pharm. Res. 15 (1998) 1202–1206. 10.1023/A:1011983606606. [DOI] [PubMed] [Google Scholar]
- [71].Masuda T, Yoshihashi Y, Yonemochi E, Fujii K, Uekusa H, Terada K, Cocrystallization and amorphization induced by drug–excipient interaction improves the physical properties of acyclovir, Int. J. Pharm. 422 (2012) 160–169. [DOI] [PubMed] [Google Scholar]
- [72].Gao Y, Liao J, Qi X, Zhang J, Coamorphous repaglinide–saccharin with enhanced dissolution, Int. J. Pharm. 450 (2013) 290–295. [DOI] [PubMed] [Google Scholar]
- [73].Shayanfar A, Ghavimi H, Hamishehkar H, Jouyban A, Coamorphous Atorvastatin Calcium to Improve its Physicochemical and Pharmacokinetic Properties, J. Pharm. Pharm. Sci. 16 (2013) 577–587. [DOI] [PubMed] [Google Scholar]
- [74].Han Y, Pan Y, Lv J, Guo W, Wang J, Powder grinding preparation of co-amorphous β-azelnidipine and maleic acid combination: molecular interactions and physicochemical properties, Powder Technol. 291 (2016) 110–120. [Google Scholar]
- [75].Zhao Y, Inbar P, Chokshi H, Malick A, Prediction of the thermal phase diagram of amorphous solid dispersions by Flory–Huggins theory, J. Pharm. Sci. 100 (2011) 3196–3207. [DOI] [PubMed] [Google Scholar]
- [76].Löbmann K, Laitinen R, Grohganz H, Gordon KC, Strachan C, Rades T, Coamorphous drug systems: Enhanced physical stability and dissolution rate of indomethacin and naproxen, Mol. Pharm. 8 (2011) 1919–1928. 10.1021/mp2002973. [DOI] [PubMed] [Google Scholar]
- [77].Ueda H, Muranushi N, Sakuma S, Ida Y, Endoh T, Kadota K, Tozuka Y, A Strategy for Co-former Selection to Design Stable Co-amorphous Formations Based on Physicochemical Properties of Non-steroidal Inflammatory Drugs, Pharm. Res. 33 (2016) 1018–1029. 10.1007/s11095-015-1848-2. [DOI] [PubMed] [Google Scholar]
- [78].Tantishaiyakul V, Songkro S, Suknuntha K, Permkum P, Pipatwarakul P, Crystal structure transformations and dissolution studies of cimetidine-piroxicam coprecipitates and physical mixtures, AAPS PharmSciTech. 10 (2009) 789–795. 10.1208/s12249-009-9263-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [79].Löbmann K, Grohganz H, Laitinen R, Strachan C, Rades T, Amino acids as co-amorphous stabilizers for poorly water soluble drugs–Part 1: Preparation, stability and dissolution enhancement, Eur. J. Pharm. Biopharm. 85 (2013) 873–881. [DOI] [PubMed] [Google Scholar]
- [80].Löbmann K, Laitinen R, Strachan C, Rades T, Grohganz H, Amino acids as co-amorphous stabilizers for poorly water-soluble drugs–Part 2: Molecular interactions, Eur. J. Pharm. Biopharm. 85 (2013) 882–888. [DOI] [PubMed] [Google Scholar]
- [81].Korhonen O, Pajula K, Laitinen R, Rational excipient selection for co-amorphous formulations, Expert Opin. Drug Deliv. 14 (2017) 551–569. 10.1080/17425247.2016.1198770. [DOI] [PubMed] [Google Scholar]
- [82].Wojnarowska Z, Grzybowska K, Adrjanowicz K, Kaminski K, Paluch M, Hawelek L, Wrzalik R, Dulski M, Sawicki W, Mazgalski J, Tukalska A, Bieg T, Study of the amorphous glibenclamide drug: Analysis of the molecular dynamics of quenched and cryomilled material, Mol. Pharm. 7 (2010) 1692–1707. 10.1021/mp100077c. [DOI] [PubMed] [Google Scholar]
- [83].Zhang F, Aaltonen J, Tian F, Saville D, Influence of particle size and preparation methods on the physical and chemical stability of amorphous simvastatin, Eur. J. Pharm. Biopharm. 71 (2009) 64–70. [DOI] [PubMed] [Google Scholar]
- [84].Wanapun D, Kestur US, Taylor LS, Simpson GJ, Single particle nonlinear optical imaging of trace crystallinity in an organic powder, Anal. Chem. 83 (2011) 4745–4751. 10.1021/ac1031397. [DOI] [PubMed] [Google Scholar]
- [85].Lenz E, Löbmann K, Rades T, Knop K, Kleinebudde P, Hot melt extrusion and spray drying of co-amorphous indomethacin-arginine with polymers, J. Pharm. Sci. 106 (2017) 302–312. [DOI] [PubMed] [Google Scholar]
- [86].Arnfast L, Kamruzzaman M, Löbmann K, Aho J, Baldursdottir S, Rades T, Rantanen J, Melt Extrusion of High-Dose Co-Amorphous Drug-Drug Combinations: Theme: Formulation and Manufacturing of Solid Dosage Forms Guest Editors: Tony Zhou and Tonglei Li, Pharm. Res. 34 (2017) 2689–2697. 10.1007/s11095-017-2254-8. [DOI] [PubMed] [Google Scholar]
- [87].Serajuddin ATM, Salt formation to improve drug solubility, Adv. Drug Deliv. Rev. 59 (2007) 603–616. 10.1016/j.addr.2007.05.010. [DOI] [PubMed] [Google Scholar]
- [88].Stephenson G, Aburub A, Woods T, Physical stability of salts of weak bases in the solid-state, J. Pharm. Sci. 100 (2011) 1607–1617. [DOI] [PubMed] [Google Scholar]
- [89].Gross TD, Schaab K, Ouellette M, Zook S, Reddy JP, Shurtleff A, Sacaan AI, Alebic-Kolbah T, Bozigian H, An approach to early-phase salt selection: Application to NBI-75043, Org. Process Res. Dev. 11 (2007) 365–377. 10.1021/op060221a. [DOI] [Google Scholar]
- [90].Cruz-Cabeza A, Acid–base crystalline complexes and the pKa rule, CrystEngComm. 14 (2012) 6362–6365. [Google Scholar]
- [91].Kramer SF, Flynn GL, Solubility of organic hydrochlorides, J. Pharm. Sci. 61 (1972) 1896–1904. 10.1002/jps.2600611203. [DOI] [PubMed] [Google Scholar]
- [92].Miller J, Dahan A, Gupta D, Varghese S, Amidon GL, Quasi-equilibrium analysis of the ion-pair mediated membrane transport of low-permeability drugs, J. Control. Release. 137 (2009) 31–37. [DOI] [PubMed] [Google Scholar]
- [93].Miller JM, Dahan A, Gupta D, Varghese S, Amidon GL, Enabling the intestinal absorption of highly polar antiviral agents: Ion-pair facilitated membrane permeation of zanamivir heptyl ester and guanidino oseltamivir, Mol. Pharm. 7 (2010) 1223–1234. 10.1021/mp100050d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [94].Sarveiya V, Templeton JF, Benson HAE, Ion-pairs of ibuprofen: increased membrane diffusion, J. Pharm. Pharmacol. 56 (2004) 717–724. 10.1211/0022357023448. [DOI] [PubMed] [Google Scholar]
- [95].Visalakshi NA, Mariappan TT, Bhutani H, Singh S, Behavior of moisture gain and equilibrium moisture contents (EMC) of various drug substances and correlation with compendial information on hygroscopicity and loss on drying, Pharm. Dev. Technol. 10 (2005) 489–497. 10.1080/10837450500299883. [DOI] [PubMed] [Google Scholar]
- [96].Suzuki T, Araki T, Kitaoka H, Terada K, Characterization of Non-stoichiometric Hydration and the Dehydration Behavior of Sitafloxacin Hydrate, Chem. Pharm. Bull. (Tokyo). 60 (2012) 45–55. 10.1248/cpb.60.45. [DOI] [PubMed] [Google Scholar]
- [97].Li Y, Chow PS, Tan RBH, Black SN, Effect of water activity on the transformation between hydrate and anhydrate of carbamazepine, Org. Process Res. Dev. 12 (2008) 264–270. 10.1021/op7001497. [DOI] [Google Scholar]
- [98].Singhal D, Curatolo W, Drug polymorphism and dosage form design: a practical perspective, Adv. Drug Deliv. Rev. 56 (2004) 335–347. [DOI] [PubMed] [Google Scholar]
- [99].Bučar DK, Lancaster RW, Bernstein J, Disappearing Polymorphs Revisited, Angew. Chemie - Int. Ed. 54 (2015) 6972–6993. 10.1002/anie.201410356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [100].Narang AS, Desai D, Badawy S, Impact of excipient interactions on solid dosage form stability, in: Excip. Appl. Formul. Des. Drug Deliv., Springer International Publishing, 2015: pp. 93–137. 10.1007/978-3-319-20206-8_5. [DOI] [Google Scholar]
- [101].Elder D, Delaney E, Teasdale A, Eyley S, Reif VD, Jacq K, Facchine KL, Schulte Oestrich R, Sandra P, David F, The utility of sulfonate salts in drug development, J. Pharm. Sci. 99 (2010) 2948–2961. [DOI] [PubMed] [Google Scholar]
- [102].Bastin RJ, Bowker MJ, Slater BJ, Salt selection and optimisation procedures for pharmaceutical new chemical entities, Org. Process Res. Dev. 4 (2000) 427–435. 10.1021/op000018u. [DOI] [Google Scholar]
- [103].Vasconcelos T, Sarmento B, Today PC, Solid dispersions as strategy to improve oral bioavailability of poor water soluble drugs, Drug Discov. Today. 12 (2007) 1068–1075. [DOI] [PubMed] [Google Scholar]
- [104].Berge SM, Bighley LD, Monkhouse DC, Pharmaceutical salts, J. Pharm. Sci. 66 (1977) 1–19. 10.1002/jps.2600660104. [DOI] [PubMed] [Google Scholar]
- [105].Regulatory Classification of Pharmaceutical Co-Crystals Guidance for Industry, (2018). http://www.fda.gov/Drugs/GuidanceComplianceRegulatoryInformation/Guidances/default.htm (accessed April 6, 2020).
- [106].Bookwala M, Thipsay P, Ross S, Zhang F, Bandaria S, Repka MA, Preparation of a crystalline salt of indomethacin and tromethamine by hot melt extrusion technology, Eur. J. Pharm. Biopharm. 131 (2018) 109–119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [107].Lee HL, Vasoya JM, De Lima Cirqueira M, Yeh KL, Lee T, Serajuddin ATM, Continuous preparation of 1:1 haloperidol-maleic acid salt by a novel solvent-free method using a twin screw melt extruder, Mol. Pharm. 14 (2017) 1278–1291. 10.1021/acs.molpharmaceut.7b00003. [DOI] [PubMed] [Google Scholar]
- [108].Srinivasan P, Almutairi M, Dumpa N, Sarabu S, Bandari S, Zhang F, Ashour E, Repka MA, Theophylline-nicotinamide pharmaceutical co-crystals generated using hot melt extrusion technology: Impact of polymeric carriers on processability, J. Drug Deliv. Sci. Technol. (2020) 102128. 10.1016/j.jddst.2020.102128. [DOI] [PMC free article] [PubMed] [Google Scholar]