Abstract
The development of non-fouling and antimicrobial materials have shown great promise for reducing thrombosis and infection associated with medical devices with aims of improving device safety and decreasing the frequency of antibiotic administration. Here, the design of a antimicrobial, anti-inflammatory and antithrombotic vascular catheter is assessed in vivo over 7-d in a rabbit model. Antimicrobial and antithrombotic activity is achieved through the integration of a nitric oxide donor, while the non-fouling surface is achieved using a covalently-bound phosphorylcholine-based polyzwitterionic copolymer top coat. Effect of sterilization on the non-fouling nature and nitric oxide release are presented. Catheters significantly reduced viability of S. aureus in long term (7 d CDC bioreactor) studies and inflammation in the 7-d rabbit model. Overall, this approach provides a robust method for decreasing thrombosis, inflammation, and infections associated with vascular catheters.
Keywords: nitric oxide, zwitterion, CLABSI, antimicrobial, catheter, antifouling, in vivo
Graphical Abstract

Introduction
Interaction of bodily fluids with implanted devices such as intravascular catheters, urinary catheters, vascular catheters, and extracorporeal life support circuits is a critical process to the success of the devices.1 This involves favorable and/or inert interactions with blood along with prevention of fouling by biomacromolecules such as proteins and bacteria.2 Even for short-term catheterization, any unfavorable phenomena such as extraluminal microbial colonization and thrombosis (leading to embolism) can cause a huge increase in healthcare associated costs and fatality within a week.3 Within seconds of biomaterials coming in contact with tissues, proteins from blood and interstitial fluids adsorb to the surface.4–5 Following the adsorption of proteins on the surface, activation of the coagulation cascade, complement system, platelets and immune cells takes place. Adsorption of blood proteins, like fibrinogen, have also been highly linked to an increase in the adhesion of microbes.6–8 Once the surface of the catheter has been “fouled”, it can lead to various cascading effects like thrombus formation and microbial infection which can ultimately lead to device failure (due to occlusion) and can even result in patient death if not detected early or if the patient has a compromised immune system and/or is undergoing mechanical ventilation.3 Fouling of central venous catheters can be particularly problematic as even a small number of bacteria infiltrating the vascular system can cause central line-associated bloodstream infections (CLABSIs), the most costly HAI (Healthcare-associated infection) on a per-case basis at about $46,000.4 According to CDC, HAIs are defined as “complications of healthcare and linked with high morbidity and mortality”. Additionally, according to the Centers for Disease Control and Prevention, 30,100 CLABSI cases are reported every year in the intensive care units and acute care facilities of the US.9 As such, the development of a thromboresistant and antimicrobial catheter coating material is of utmost importance.10–13 However, another consideration for the development of a catheter coating materials is to design a material that would form a ‘biocompatible system’ when placed in the major vein.14–16 The term biocompatible has been defined as ‘the ability of a material to perform with an appropriate host response in a specific situation’.17 To explain in more simpler terms, this means that a material is considered biocompatible if it does not elicit any adverse reactions in the living system. Therefore, in case of central venous catheters, it is important to also characterize the interaction between the coating materials and the endothelium environment.18
Several strategies have been introduced in the past decade to reduce protein attachment through antifouling, antithrombogenic and antimicrobial methods.19–29 However, even though much progress has been made, very few studies have proven to combine all of the three properties to prevent protein and bacterial attachment and blood clots with vascular catheter implantations.30–33 While all of these three properties can be combined to make a material almost biocompatible, a highly efficient biocompatible polymer system would also exude the least inflammatory response from the hosts’ cells. This is where most researchers struggle to succeed since achieving some of the other medical device sustainability goals like antifouling, antithrombogenic, or antimicrobial properties can elicit an immune response. Therefore, a very careful interplay of characteristics is required to achieve a pristine coating that has all the above-mentioned properties.
In this work we have built on our lab’s previous work to combine the antifouling, antithrombogenic and antimicrobial properties of a medical grade polymer that has a nitric oxide (NO) releasing donor (S-nitroso-N-acetylpenicillamine, SNAP) and a phosphorylcholine-based polyzwitterionic copolymer (2-methacryloyloxyethyl phosphorylcholine-co-butyl methacrylate co-benzophenone, BPMPC) topcoat.30 It was the first study to be able to design a facile treatment of phosphorylcholine-based polyzwitterion to covalently attach it to any hydrophobic polymer. Superhydrophilic properties of zwitterion materials are a challenge in forming uniform coating on hydrophobic materials and can involve several difficult steps to achieve robust attachment. However, we were able to achieve covalent attachment with BPMPC that utilizes the UV-crosslinking properties of the vinyl benzophenone. Zwitterionic polymers having a phosphorylcholine group have been shown to prevent blood cell adhesion even when the polymers contact human whole blood without an anticoagulant.34 Besides being a potent antimicrobial agent that is physiologically produced by macrophages and the sinus cavities to prevent infection, NO has also been found to reduce inflammatory response by the reduction of inflammatory cell recruitment.35–36 In the past it has been found to have sustained effects on reduction of inflammation as NO-release from biomaterials induce the release of more NO from macrophages.5 Therefore, the two polymers were combined to synergistically act as a biocompatible system. In brief, 10 wt.% of the NO donor (SNAP) was blended within a medical polymer (CarboSil 2080A from DSM Biomedical) and allowed to form disks of films with the solvent evaporation method. Following this, the samples were spray coated with the BPMPC/ethanol solution and dried in air. Then the samples were irradiated with UV light (UVP, 254 nm, 6.5 mW cm−2) for 1 min to covalently bond the BPMPC to the NO-releasing polymer. The fabricated samples were able to achieve antifouling (~84–93% reduction for 14 days) and antimicrobial properties (99.91 ± 0.06% reduction in Staphylococcus aureus adhesion for 24 hours).
To further advance the studies and design of the biocompatible system, this paper looks into the in vivo characteristics of the biocompatible system previously designed and studied for proof-of-concept. Vascular catheters were first fabricated to test for wettability and presence of zwitterion coatings on the catheters, followed by NO-release measurements over a 7 d period. The catheters were then tested in vitro for their antimicrobial efficacy against S. aureus. To demonstrate the catheter’s robustness in retaining its properties after sterilization, contact angle and NO-release measurements were done pre- and post- hydrogen peroxide vapor sterilization. Finally, sterilized catheters were implanted into rabbits for 7 d and evaluated for clot formation and inflammation. The rigorous tests performed through the study were able to confirm the Z-NO material’s superior quality in retaining it’s antimicrobial and hemocompatibility properties for the preservation of the biocompatible system.
MATERIALS AND METHODS
Materials.
4-vinylbenzophenone (BP) was synthesized as previously published.30 Sodium chloride (NaCl), potassium chloride (KCl), methacryloyloxyethyl phosphorylcholine (MPC), N-acetyl-D-penicillamine (NAP), ethylenediamine tetraacetic acid (EDTA), sodium phosphate dibasic (Na2HPO4), sodium phosphate monobasic (NaH2PO4), concentrated sulfuric acid (conc. H2SO4), tetrahydrofuran (THF), and sodium nitrite (NaNO2) were purchased from Sigma Aldrich (St. Louis, MO). 2–2’-azobis(2-methyl propionitrile (AIBN) and n-butyl methacrylate (BMA) were bought from Alfa-Aesar (Haverhill, MA). Concentrated hydrochloric acid (conc. HCl), methanol (CH3OH) and sodium hydroxide (NaOH) were obtained from Fisher Scientific (Hampton, NH). Potassium phosphate monobasic (KH2PO4) was purchased from BDH Chemicals-VWR International (West Chester, PA). CarboSil™ 20 80A UR STPU (referred to as CarboSil hereon) was obtained from DSM Biomedical Inc. (Berkeley, CA). De-ionized water (DI H2O) was obtained from in-house Milli-Q filtration system. The bacteria strains for the antimicrobial studies were obtained from American Type Culture Collection. Nitrogen and oxygen gas cylinders were from Air gas (Kennesaw, GA).
Synthesis of S-nitroso-N-acetylpenicillamine (SNAP, NO donor).
The NO donor, SNAP, was synthesized using a previously reported method.37 Briefly, 1M H2SO4 and 1M HCl were added to an equimolar solution of NAP dissolved in methanol. This was followed by addition of DI H2O. The reaction mixture was allowed to cool to room temperature before adding an equimolar concentration of NaNO2. This reaction was allowed to take place for 8 hours in an ice bath. After obtaining the greenish red SNAP crystals through vacuum filtration, the crystals were allowed to dry overnight before storing it in the freezer for further experiments.
Synthesis of 2-methacryloyloxyethyl phosphoryl-choline-co-butyl methacrylate-co-benzophenone (ZI, zwitterionic terpolymer).
The zwitterionic copolymer was synthesized using a previously reported free radical polymerization method.30 Briefly, MPC (1.85 mmol), n-BMA (0.66 mmol), and BP (0.132 mmol) were reacted in the presence of ethanol as the solvent, and AIBN as the initiator (60°C, 16h). Precipitation of the polymer was done by pouring the reaction mixture into ethyl ether at the end of the 16h. The polymer was collected by vacuum filtration. Finally, the polymer (white solid) was dried under vacuum for 12h. 1H NMR (dH2O) was done to confirm the copolymer’s composition.
Fabrication of Catheters.
A dip coating method was employed to fabricate catheters: single layered with plain CarboSil (no NO or ZI), double layered with a CarboSil and a ZI layer (ZI catheters), trilayered with CarboSil, ZI and NO layers (NO-releasing catheters) (Figure 1a). A more detailed design of the Z-NO catheter is given in Figure 1b. In this method, solutions with the following polymer concentrations were first made: 10 mg ml−1 of BPMPC in ethanol (zwitterion coat), 40 mg ml−1 of CarboSil in THF (CarboSil coat), 10 wt.% SNAP in 70 mg ml−1 of CarboSil in THF (SNAP solution). Steel rods (outer dia.=1.6 mm) were used to first dip coat with CarboSil coat solution (5x). The coatings were left to dry for 2–3 hours and then coated with the SNAP solution (15x). This was then coated with another 5x of CarboSil coat after overnight drying. The NO-releasing catheters were then additionally dip coated with a zwitterion topcoat. The ZI coated catheters were treated with UV light for 2 mins followed by ethanol wash to remove any uncrosslinked zwitterionic polymer. NO-releasing catheters were stored in dark, dry, low temperature conditions to preserve the NO-donor quantity. After all the layers were dip-coated and dried, the steel rods were removed from the catheters. For convenience, the samples will be called Control, ZI, NO, Z-NO from hereon. The details are in Table 1.
Figure 1.

Overall design of catheters. a) A representative diagram of the four types of catheters tested in this in vivo study. b) Cross-sectional view of Z-NO catheter.
Table 1.
Names of different samples and their compositions.
| Catheter Tested | Composition |
|---|---|
| Control | 25 coats of CarboSil only |
| ZI | Only ZI, no SNAP (25 coats of CarboSil, 1 coat of 10% ZI) |
| NO | only SNAP, no ZI (5 coats of CarboSil, 15 coats of 10 wt.% SNAP, 5 coats of CarboSil) |
| Z-NO | SNAP and ZI (5 coats of CarboSil, 15 coats of 10 wt.% SNAP, 5 coats of CarboSil, topcoated with 10% BPMPC). |
Surface Analysis of Catheters.
To ensure coverage of the catheters with the BPMPC coat, catheters were cut after coating and static water contact angle measurements were carried out. A DSA 100 drop shape analysis system (Krüss) with a computer controlled dispensing system was used for this purpose. The contact angles were obtained through quick photographic analysis of 1 μl droplets on the substrates.
NO Release Kinetics of Catheters.
Once the catheters were fabricated, NO release was measured using a Sievers chemiluminescence nitric oxide analyzer (NOA 280i, GE Analytical, Boulder, CO, USA).38 It is the gold standard instrument used to measure nitric oxide release with high sensitivity unlike the Griess assay that might falsely detect nitrates and nitrites as nitric oxide release whilst also having the advantage of real-time measurement. All samples were characterized for NO release at 37°C in PBS containing EDTA as the metal ion chelator. Each sample was shielded from light and soaked in 3.5 mL of PBS during the measurement stored in the PBS solution inside a 37°C incubator between measurements. Nitric oxide released by the sample was swept into the analyzer with the help of a constant flow of nitrogen and vacuum supplied by the analyzer. The voltage signal produced was converted to concentration and displayed on the analyzer’s screen. Using the raw data in ppb form and NOA constant (mol ppb−1 s−1), the data in ppb was normalized and converted to NO flux units (×10−10 mol cm−2 min−1) according to the surface area of the sample used for analysis.
In vitro analysis
Bacterial Culture and 7-d exposure to Staphylococcus aureus in a “Centers for Disease Control and Prevention” Bioreactor Model.
For in vitro bacterial adhesion study, a Centers for Disease Control and Prevention (CDC) biofilm bioreactor (model CR 90) from Biosurface Technologies (Bozeman, MT) was used to measure antimicrobial efficacy of the test samples versus control samples over a period of 7 d (Figure 2) using a modified version of the ASTM International E2562 – 17 protocol. S. aureus was subcultured and grown to a mid-log phase (12–14 hours). This mid-log culture was then centrifuged at 4400 rpm for 7.5 min to extract the bacterial pellet from the growth medium and waste. The bacterial pellet was then suspended in PBS and washed at 4400 rpm for 7 min. Following this, the optical density of bacterial solution in LB broth was adjusted to 0.1 at 600 nm and used for incubation of the samples in the bioreactor for 2 h. During the 2 h incubation period, the bioreactor was stirred at 100 rpm and not supplied with additional nutrient medium. After 2 h of incubation, nutrient medium (2 g L−1 of LB broth) was allowed to flow through the bioreactor at 1.67 ml min−1 and the waste allowed to flow into a waste bottle from the outlet of the CDC bioreactor. During the 7 d period of bioreactor study, nutrient was continuously supplied and waste continuously discharged from the system.
Figure 2.
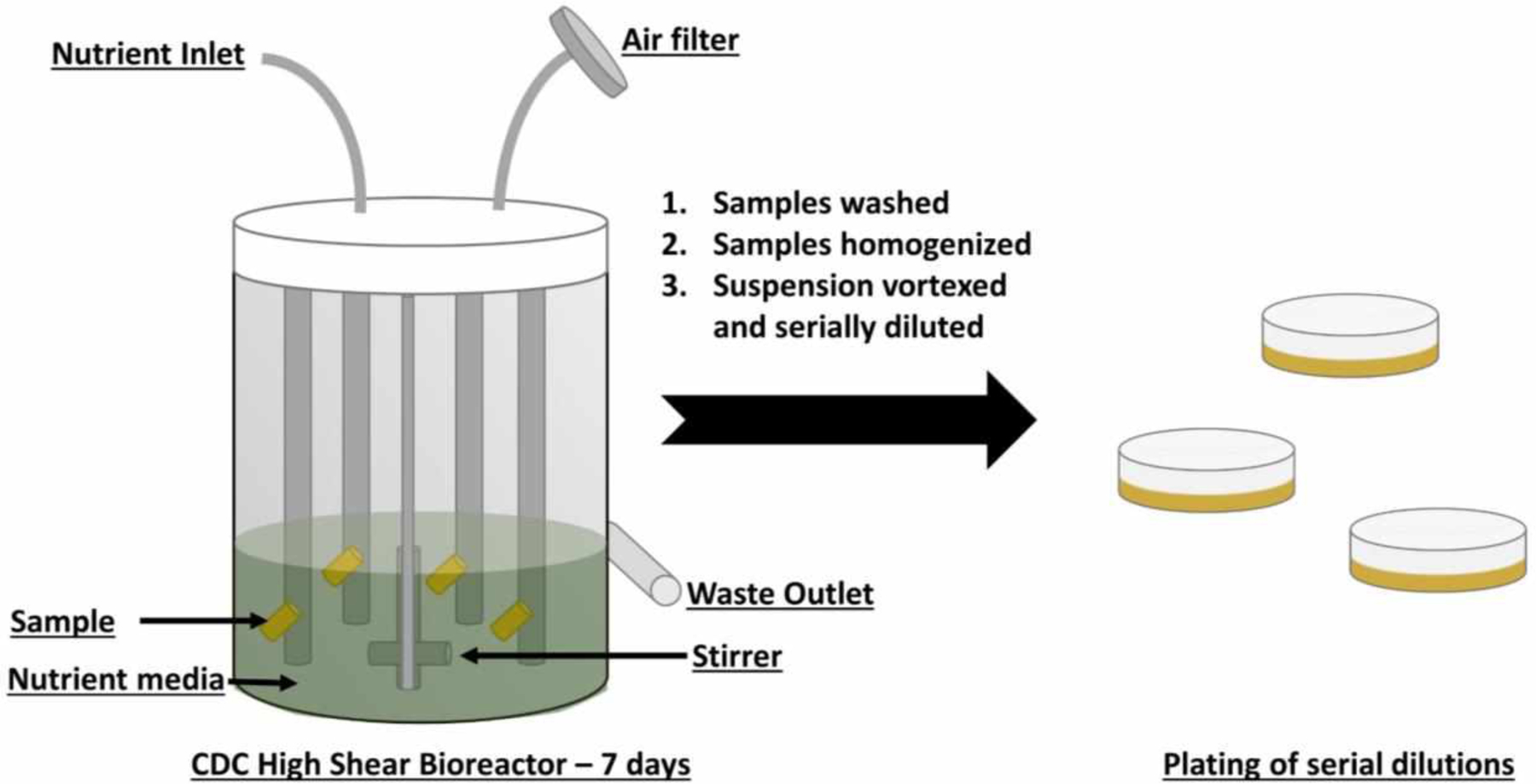
A schematic of the 7-d in vitro antimicrobial test carried out in a CDC high shear bioreactor.
At the end of the 7 d test period, samples were removed from the bioreactor for colony counting analysis and SEM imaging analysis. For colony counting analysis, samples were gently rinsed with sterile PBS to remove any unattached bacteria and dropped into equal amounts of PBS. The samples were then homogenized at 25000 rpm to remove the attached bacteria and mix them into the PBS solution. Finally, the solutions were vortexed and serially diluted to be plated on LB agar plates. The colonies were counted post 24 h incubation in 37°C.
The surface characterization of materials after 7-d bioreactor study was performed by a field emission scanning electron microscope (FESEM, FEI Teneo). Before analysis by SEM, materials were washed with PBS to remove any unadhered bacteria. The attached bacteria were then fixed with 3 vol.% glutaraldehyde in PBS, pH 7.4. The fixed samples were dehydrated by gradual water replacement using a series of increasing concentration of ethanol solutions (50%, 60%, 70%,80%, and 90%) in DI water and a final dehydration step with 100% ethanol. The dehydrated materials were then dried using increasing amounts of hexamethyldisilazane and left overnight to dry completely. Once dried, samples were mounted on stubs and gold-palladium coated in vacuum using a sputter coater (Leica sputter coater)
Effect of Hydrogen Peroxide (H2O2) Vapor Sterilization.
To ensure that H2O2 vapor sterilization did not have any significant effects on catheters to be used for in vivo study, they were first characterized for their NO-releasing and surface wetting properties pre- and post- sterilization. Sterilization was done using a Sterix VHP MD140X for 28 min, where the chamber is placed under vacuum followed by the injection and vaporization of H2O2 to a chamber concentration of 6 mg L−1. Post sterilization, catheters were analyzed for their NO release before and after sterilization. This was done using the chemiluminescence NO analyzer as described previously. Additionally, to ensure the presence of zwitterion coating on the zwitterion and Z-NO samples, static water contact angle was measured before and after sterilization using the drop shape analyzer.
In vivo analysis
Rabbit Catheter Implantation.
All animals were cared for by the standards of the University Committee on Use and Care of Animals (UCUCA) at the University of University of Georgia. The surgical area was sanitized and dedicated to the purpose of performing surgery. All surgical instruments were sterilized using steam sterilization and sterile drapes were used to create a sterile field around the dorsal and ventral sides of rabbit neck. Catheters were sterilized using hydrogen peroxide vapor sterilization at the University of Georgia College of Veterinary Medicine. A total of 16 New Zealand white rabbits (Charles River Laboratories, Wilmington, MA) were used in this study. All rabbits (2.5–3.5 kg) were initially anesthetized with intramuscular injections of 5 mg kg−1 xylazine injectable (AnaSed Lloyd Laboratories Shenandoah, Iowa) and 30 mg kg−1 ketamine hydrochloride (Hospira, Inc. Lake Forest, IL). Maintenance anesthesia was administered via isoflurane gas inhalation at a rate of 1.5–3% via mechanical ventilation which was done through an A.D.S. 2000 Ventilator (Engler Engineering Corp. Hialeah, FL). Each rabbit’s neck region was cleaned with povidone iodine and ethanol prior to incision. Under sterile conditions, a small skin incision (2 cm) was made over the right external jugular vein and the facial vein branch isolated for the catheter insertion. Briefly, the facial vein is ligated proximally and under distal occlusion, a small venotomy is made through which the catheter is introduced into the jugular vein and then advanced into the cranial vena cava (Figure 3). About 6 cm of a catheter length is inserted and then fixed to the vein at its entrance by two sterile silk sutures (4–0, Ethicon).39 By using the facial vein, the external jugular vein blood flow was maintained over the catheter which provided thrombosis assessments. The incision was then closed using uninterrupted stitches (4–0 absorbable suture, Ethicon).
Figure 3.

A schematic of the in vivo rabbit model for 7-day implantation of venous catheter.
Post-operative recovery.
After removal from anesthesia, animals were placed in an oxygenated and 37 °C incubator for post-operative recovery. Animals were continuously checked during the 1–2 h recovery period until they were able to maintain sternal recumbency before being transferred to the animal facility. The rabbits that recovered from anesthesia after the catheter placements were housed individually with a respective cage card identifying the animal in the animal facility. Routine daily check-ups were carried out for the duration of the study to ensure that the animals were in good health and the implantation and skin incision sites were free of inflammation (redness). 4 mg kg−1 Rimadyl (analgesic) was given for 2 days after surgery and 5 mg kg−1 Baytril (antibiotic) was given for 4 days post-surgery. The catheter was flushed with 2 mL of sterile saline every day. After 9 d, rabbits were given 400 IU kg−1 sodium heparin just prior to euthanasia to prevent necrotic thrombosis. The animals were euthanized using a dose of Fatal Plus (130 mg kg−1 sodium pentobarbital) (Vortech Pharmaceuticals, Dearborn, MI).
Ex vivo analysis
Catheter Evaluation and Vein Histological Evaluation.
The jugular and facial vein were cut proximal to the catheter, as well as the jugular vein distal to the catheter tip. The vein was then cut longitudinally, and the catheter was carefully removed. Veins were stored immediately in formalin. The left jugular vein (2 cm) was excised to be used as controls for histological imaging. After explanting, the catheters were rinsed in PBS. Pictures were taken of the exterior of the whole catheter. Starting at the distal tip of the catheter, 1 cm sections were cut for SEM, and bacterial adhesion. To quantify the viable bacteria, a 1 cm piece was cut longitudinally and was placed in 1 mL PBS buffer. Explanted veins were flushed with PBS and immediately placed in 10% buffered formalin for 48 h. After immersion fixation, veins were rinsed in cold water for 5 min and stored in PBS at 4°C. The fixed veins were then dehydrated in ethanol and cleared in xylenes before finally being cut into 3–4 mm segments, placed within tissue cassettes, and embedded in paraffin wax. The veins were then cut into 10 μm sections and stained with hematoxylin and eosin (H&E) per facility SOPs. Images were taken using an EVOS FL inverted microscope.
RESULTS AND DISCUSSION
Surface analysis of catheters.
Once the catheters were fabricated, surface analysis was done to confirm the deposition and durability of the ZI coating. The NO releasing polymer, CarboSil, is a hydrophobic polymer with a contact angle of approx. 100° and hence a drop in the contact angle was expected on the ZI-coated catheters. The wetting properties of the ZI coated catheters were analyzed using a drop shape analyzer which is seen in Figure 4. Further characterization of the surfaces and kinetics of the zwitterion’s polymerization have been previously demonstrated in a published study by our group.30 As seen from the wetting characteristics, the ZI was well-coated on the insides and the outsides of the NO-releasing catheters.
Figure 4.

Contact angle measured before and after coating with zwitterionic polymer.
In vitro NO-Release Kinetics from catheters.
As we have previously stated, one of the desirable properties of the Z-NO materials was to have sustained NO release for the application period of the catheters. This would indicate that the catheters are suitable for antithrombotic and antimicrobial applications. Nitric oxide is released from S-nitrosothiols upon exposure to heat, moisture, metal ions (e.g. copper and zinc) or light irradiation (340 and/or 590 nm, S-nitroso adsorption bands).40–41 For the catheters fabricated in this study, NO release is catalyzed by heat (physiological temperature of 37 °C) and moisture upon contact with bodily fluids (blood). To minimize burst release of NO, which was seen in our previously published study,30 the catheters were fabricated via a dip coating method in which several layers of NO-releasing CarboSil were topcoated with five layers of CarboSil and the final topcoat of zwitterion (as shown in Figure 1). This eliminated the possibility of a loss of SNAP, the NO donor. There is also little apprehension regarding the low possibility of leaching of SNAP, NAP (the parent thiol and used to clinically treat heavy metal poisoning), or NAP disulfide from the catheters as none of them would result in any toxicity issues. S-nitrosothiols, like SNAP, release NO through their spontaneous decomposition which yields a disulfide (RSSR) product and NO.38 This NO release from the catheters was measured and monitored using chemiluminescence NO analyzers (NOA, Sievers, Boulder, CO), the gold standard for measuring nitric oxide release, over a period of 7 d. The catheters were incubated in PBS with EDTA at 37°C for the entirety of the study. Unlike the previously published study wherein the samples exhibited a high NO release on the first day because of initial water uptake and leaching of SNAP,30 no such burst release of NO was observed from these catheters (NO: 1.393 ±.612 and Z-NO: 1.289 ±.625 (×10−10 mol cm−2 min−1)). This is desirable as it means that SNAP storage is optimal, and the NO release is gradual and can be expected to remain around physiological levels for a long period of time. However, it is also important to note here that a higher NO release would not be a problem as besides NO’s short half-life due to its swift scavenging by hemoglobin, the catheter surface area in blood vessels is significantly lesser than the surface area of blood vessels which are continuously releasing NO at endogenous levels.1 The results indicated a general trend of comparable NO release from both NO and Z-NO catheters as seen on Figure 5. It’s also important to note here that despite the presence of a hydrophilic topcoat like zwitterion, NO release was not significantly higher for Z-NO samples. This demonstrates the robustness of layering NO-releasing polymers to have a gradual NO release instead of burst release. Just as seen on day 1 of the study, day 3, 5, and 7 show comparable NO-release profile from both NO and Z-NO samples (Table 2). This trend of comparative NO-release is desirable as it means that even adding a hydrophilic zwitterion topcoat to promote antifouling characteristics does not result in losing the beneficial properties of sustained NO release.
Figure 5.

NO release kinetics over a 7-day period. (n=3)
Table 2.
NO flux for NO and Z-NO catheters over a 7-day period. (n=3)
| Day of Measurement | NO Flux (× 10−10 mol cm−2 min−1) | |
|---|---|---|
| NO | Z-NO | |
| 1 | 1.4 ±0.6 | 1.3 ±0.6 |
| 3 | 0.5 ±0.2 | 0.6 ±0.1 |
| 5 | 0.5 ±0.2 | 0.5 ±0.1 |
| 7 | 0.5 ±0.1 | 0.3 ±0.1 |
Antimicrobial efficacy in a continuous flow “Centers for Disease Control and Prevention” biofilm bioreactor over 7 d.
Antimicrobial materials can be developed through passive and active strategies. While a passive method (e.g. PEG, zwitterion) involves the use of strategies that do not release any biocidal agents into the environment, active strategies (e.g. silver nanoparticles, quaternary ammonium ions, nitric oxide) involve the use of agents that actively kill microbes.42 The Z-NO catheters combine both passive and active strategies through the incorporation of a zwitterion topcoat on a NO-releasing polymer. While the zwitterion topcoat provides protection from the contamination of proteins (as demonstrated in the previous publication), the NO-releasing polymer acts as a biocidal agent releasing polymer that does not have the risk of antibiotic resistance and acts locally. Nitric oxide’s antimicrobial mechanisms include lipid oxidation, deamination of DNA, and denaturation of enzymes so the possibility of bacteria developing resistance is minimal.36 Catheters fabricated via dip coating were tested in a Centers for Disease Control and Prevention (CDC) bioreactor (model CBR 90) over 7 d with S. aureus culture (initial inoculum of ~108 CFU mL−1) to demonstrate their antimicrobial efficacy. S. aureus is a Gram-positive bacterium that is a leading pathogen known for causing bacteremia and endocarditis.43
Figure 6 a represents the surface of the catheter samples after a 7 d exposure to S. aureus in the CDC bioreactor. As expected, a high amount of growth and a well-formed biofilm is seen on the control catheters (A and E. This growth of biofilm is dense and shows a well-connected layer of exopolysaccharide that provides nutrition and protection to the bacterial cells present within the network. In contrast, NO catheters represented on image B and F show little biofilm formation. However, a layer of protein is seen on the catheters as NO cannot prevent the adsorption of protein produced by the bacteria that are able to encounter the surface and use proteins to attach themselves. The reduction of intact bacteria present on the surface is clearly visible through the SEM images. The ZI catheters represented on image C and G show the structure of the coating on the catheter along with the presence of few bacteria on the surface. The pores seen on the surface could be due to the quick evaporation of the ethanol solvent that is used to coat the zwitterion polymer. The surface of the ZI catheters clearly show a reduction in the protein coverage unlike the NO catheters. Z-NO catheters on the other hand demonstrate both improved surfaces and highest reduction in protein and bacteria attachment (image D and H). A more detailed discussion of the bacterial results will be discussed in the next few paragraphs with the quantitative results.
Figure 6.

Quantitative and qualitative results from 7-day exposure to infection level CFU mL−1 of Gram-positive bacteria, S. aureus. a) Scanning electron micrographs of materials exposed to S. aureus for 7 d. b) Magnified section of SEM image from Figure 6a(H) showing disrupted cells with red arrows c) Log10 CFU cm−2 counts of bacteria present on each material after 7 d exposure to S. aureus in a high shear CDC bioreactor. (n=4)
Figure 6 c and Table 3 represent the results from the 7 d antimicrobial study carried out in the continuous flow CDC bioreactor. The continuous flow CDC bioreactor is an ideal design to study vascular catheters materials as the shear rate is high and it provides an ideal platform to continuously supply nutrients and remove waste without exposing the materials to an unsterile environment (as expected in implantation). Several studies have been carried out to monitor biofilm growth and efficacy of antimicrobials with the continuous flow CDC bioreactor.44–47
Table 3.
Complementary table for Figure 6c: Reduction of bacteria/cm2 on ZI, NO, and Z-NO catheters compared to the controls.
| Control | ZI | NO | Z-NO | |
|---|---|---|---|---|
| Avg CFU of S. aureus cm−2 | 1.3 × 109 | 1.9 × 108 | 1.5 × 108 | 3.5 × 107 |
| Reduction % compared to Control | 85.0 ±7.4 | 88.2 ±4.3 | 97.3 ±1.3 | |
| p value vs. Control | - | 0.015 | 0.013 | 0.009 |
| p value vs Z-NO | 0.009 | 0.031 | 0.010 | - |
In this study, Z-NO was expected to have a higher reduction of bacteria compared to the other materials (ZI and NO). This was due to the additive effects expected from the antifouling nature of the zwitterion topcoat along with the bactericidal effects of NO. As observed, the presence of a zwitterion alone reduces the adhesion of S. aureus by 85.0 ±7.4% (p=0.015) after the 7 d exposure in the bioreactor. This reduction is attributed to the zwitterion’s hydrophilic nature which in turn prevents the attachment of proteins and other biomacromolecules produced by bacteria for the formation of biofilms. Thus, the repulsion property of zwitterion itself is able to provide significant protection from biofilm formation. Similarly, a reduction of 88.2 ±4.3% (p=0.013) is seen on NO catheters which is attributed to the bactericidal property of NO. As seen from NO release measurements in the range of 0.5–1.4 (×10−10 mol cm−2 min−1) flux, the consistent NO release at physiological levels helps in this reduction. An even more significant reduction is seen with Z-NO catheters. At 97.3 ±1.3% (p=0.009) reduction compared to the control catheters for a 7-d bioreactor, Z-NO catheters establish the additive antimicrobial action of the zwitterion and NO components of the catheters. This is a greater reduction compared to the single modifications on the ZI and NO catheters and hence increases the chances of prevention against microbial infections. It is important to note here that though these are promising results; further studies need to be carried out with longer term in vitro and in vivo bacterial infection models.
Effects of hydrogen peroxide vapor sterilization on fabricated materials.
Sterilization or biodecontamination is a necessary step required before any medical implants are introduced into living beings for in vivo testing. It is also imperative that medical devices withstand sterilization process to be used clinically. The University of Georgia’s College of Veterinary Medicine provides the facilities for both sterilization and in vivo tests. The sterilization instrument uses low temperature for biodecontamination of temperature sensitive devices and hence is ideal for sterilizing heat-sensitive NO-releasing materials. Another advantage of this sterilization is that it uses hydrogen peroxide in its vapor state and has no condensation of active ingredient onto surfaces, therefore making sure that the only antimicrobial present post-sterilization is NO itself. Hydrogen peroxide vapor sterilization is slowly replacing ethylene oxide as the main low temperature sterilization technique due to its turnaround time and device compatibility.
The NO flux for pre-sterilized materials was 1.4 and 1.3 (×10−10 mol cm−2 min−1) flux for NO and Z-NO samples, respectively. For post-sterilized materials, the NO flux was 1.7 and 1.0 (× 10−10 mol cm−2 min−1) for NO and Z-NO samples, respectively. From NO release analysis of the samples, the NO release for Day 1 is only minutely different for both NO and Z-NO pre- and post-sterilized samples. This is a desirable result since it indicates that the NO donor in the catheter is not affected by the sterilization process and H2O2 vapor sterilization is a safe method. It is also an expected result since we know from previous studies that only heat affects the NO content.38 From table 4, it is seen that contact angle remains the same and does not change pre- and post-sterilization. This demonstrates that the zwitterion coating is not affected by the sterilization method. Hence, both the analyses validate the H2O2 vapor sterilization technique as an ideal biodecontamination method for the samples used in this study.
Table 4.
Pre- and post-sterilization wetting properties of fabricated coatings.
| Material | Pre-sterilization Contact Angle (°) | Post-sterilization Contact Angle (°) |
|---|---|---|
| Control | 102.8 ±1.7 | 100.6 ±0.5 |
| ZI | 49.8 ±2.4 | 58.7 ±0.1 |
| NO | 97.8 ±3.0 | 97.2 ±1.3 |
| Z-NO | 61.8 ±3.67 | 61.4 ±0.6 |
Antithrombogenicity efficacy in an in vivo model over 1 week.
Central venous catheters were prepared via dip coating and implanted for 7 d in rabbit cranial vena cava (1 catheter per rabbit) without systemic anticoagulation. After the 7-d period, the catheter and vein were explanted to evaluate the various materials to reduce thrombosis via imaging and scanning electron microscopy (Figure 7 a and b). The control CarboSil catheters showed the highest levels of thrombus formation, with large amounts of both fibrin formation and platelet and red blood cell attachment.
Figure 7.

Images of catheters post-explantation from 7-day implantation in rabbit model. a) Digital images comparing blood clots on the surface of catheters implanted within the vena cava of rabbits for 7 d. b) Scanning Electron micrograph Imaging of thrombus formation in catheters implanted in in vivo rabbit model for 7 d.
The NO-releasing surfaces showed a dense layer of protein on the surface, with minimal cell adhesion. These results coincide with previous findings of NO releasing materials in central venous catheters after 9 d in a sheep model.48 While the NO release levels of the previous work are on the higher range of physiological levels, similar reductions in thrombus formation and degree of protein adhesion are observed when compared to the respective controls. While it is estimated the physiological range is between 0.5 – 4 × 10−10 mol min−1 cm−2, the correlation of NO release levels to the degree of thrombus formation have yet to be determined, including the differences in flow conditions for venous and arterial thrombi.
The zwitterionic coating was also able to significantly reduce thrombus formation when compared to the CarboSil control. However, small localized regions of thrombus formed. This may be the result of the adhesion and activation of platelets on the catheter surface, which then can provide a substrate for fibrinogen adhesion and fibrin formation. This small location can then grow over time despite the non-fouling nature of the material surface.
The combination of the zwitterionic coating along with NO release showed the lowest level of thrombus formation, with minimal protein and cell attachment when viewed on SEM. With no significant difference in the NO release rates from NO and Z-NO, this demonstrates the importance of both the platelet and protein contributions to thrombus formation. While similar results have been reported on the effect of non-fouling coatings with NO release in vitro, this is the first study to investigate their ability to prevent thrombus formation in a long term in vivo application.49–50 Overall, the combination of the non-fouling zwitterionic coating coupled with the active release of nitric oxide demonstrate a strong potential for limiting thrombosis associated with central venous catheters for long term applications.
In addition to the surface analysis of the catheters by SEM for the presence of any thrombus formed, the catheters were also tested to check for any bacterial adhesion due to possible contamination via the surgical site. While all the catheters were found to have no viable bacterial growth, some amount of non-viable bacteria is seen on all catheters as indicated by the disrupted cells on the SEM images. This is desirable because it indicates a good sterile environment in which the surgery was performed, and surgical site was maintained with no contamination through the course of the 7-d implantation of the catheters.
Histology Assessment of Foreign body response in an in vivo model over 1 week
Implantation of a CVC can cause a variety of anatomical changes to the venous wall that lead to further complications that can jeopardize the health of the patient. Direct vascular wall damage immediately occurring from the surgical procedure can be unavoidable, often leading to smooth muscle cell (SMC) proliferation. After implantation, any further disturbance to the inner lumen of the vein from the CVC will only accelerate this problem51. Blood moving around the catheter also has a high chance of clotting onto the endothelium. There are several types of thrombotic occlusions that occur from implanted CVCs, which originate from the fibrin sheath that initially forms on the catheter surface. The two specific types that typically occur are mural thrombosis, where a blood clot adheres to the catheter and vein wall but does not completely prevent blood flow through the vein, and complete venous thrombosis, where the clot that forms stops all blood flow52.
While SMC proliferation is primarily an issue for long term implanted CVCs, early stages of SMC migration to the pericatheter fibrin sheath that forms have been shown to be present within one week53. Explanted veins were stained with H&E to observe the foreign body response to the implanted catheters and are shown in Figure 8. Specifically, the formation of mural thrombosis on the vein adjacent to the catheter implantation site was investigated. Catheters without zwitterionic coatings or NO release demonstrated high thrombus formation along with SMC proliferation at the catheter-vein interface. This was anticipated as the previously shown clotting on the surface of the untreated catheters was much more significant compared to ZI or NO functionalized catheters. The combination of ZI-NO showed little clotting as well as a decreased amount of SMC proliferation on the vein endothelium. While the NO was able to inhibit a majority of the clotting on the catheter surface, there was still the presence of clotting near the venous surface. This is most likely due to the catheters reaching the lower threshold of NO release by the end of the 7 d implantation in combination with increased activity of platelets flowing around the catheter itself. Since the ZI coating prevents or slows the formation of the fibrin sheath that intensifies the thrombotic complications of CVCs, it was expected that its combination with NO shows even less occurrences of thrombus formation around the adjacent vessel wall.
Figure 8.

Histological imaging with hematoxylin and eosin stains post-explantation after 7 d of implantation in rabbit model. A) Control Catheter – Endothelium and SMC proliferation is seen along with detached thrombus formation B) Zwitterion catheter – endothelium and SC proliferation is seen along with detached thrombus formation C) NO catheter – Little to no SMC proliferation present and thrombus formation is seen D) Z-NO catheter – rare occurrences of clotting and little to no SMC proliferation is seen. [* indicates lumen location, Red Arrow = endothelium and SMC proliferation, Green Arrow = detached thrombus formation]
Conclusion
Vascular catheters with non-fouling, antimicrobial, and antithrombotic activity were developed using phosphorylcholine-based polyzwitterionic copolymer (2 methacryloyloxyethyl phosphorylcholine-co-butyl methacrylate co-benzophenone, BPMPC) topcoat coupled with NO release. Both NO and Z-NO formulations released physiological levels of NO over 1 week. Initial functionalization of the zwitterionic top coat was confirmed via decreases in the contact angle from ca. 100° (control, NO) to 60° (ZI, Z-NO). The presence of the ZI topcoat did not significantly affect the release kinetics of NO, and the Z-NO combination demonstrates a 97.2 ± 1.3% reduction in viable S. aureus after 7 d in a CDC bioreactor environment. The Z-NO combination withstood hydrogen peroxide vapor sterilization, and showed decreases in thrombosis and smooth muscle proliferation in vivo over 7 d rabbit model.
Acknowledgements
The authors declare that this work was supported by the National Institutes of Health, Grant R01HL134899 and Centers for Disease Control and Prevention contract 200-2016-91933. The authors would like to thank the Melissa Martin and Tara Denley (Veterinary College of Medicine, University of Georgia) for assistance with animal handling and in vivo experiments performed at the University of Georgia Veterinary College of Medicine, Athens.
References
- (1).Brisbois EJ; Davis RP; Jones AM; Major TC; Bartlett RH; Meyerhoff ME; Handa H Reduction in Thrombosis and Bacterial Adhesion With 7 Day Implantation of S-nitroso-N-acetylpenicillamine (SNAP)-Doped Elast-eon E2As Catheters in Sheep. Journal of Materials Chemistry B 2015, 3 (8), 1639–1645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (2).Xu L-C; Bauer JW; Siedlecki CA Proteins, Platelets, and Blood Coagulation at Biomaterial Interfaces. Colloids and Surfaces B: Biointerfaces 2014, 124, 49–68, DOI: 10.1016/j.colsurfb.2014.09.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (3).Parienti J-J; Mongardon N; Mégarbane B; Mira J-P; Kalfon P; Gros A; Marqué S; Thuong M; Pottier V; Ramakers M; Savary B; Seguin A; Valette X; Terzi N; Sauneuf B; Cattoir V; Mermel LA; du Cheyron D Intravascular Complications of Central Venous Catheterization by Insertion Site. New England Journal of Medicine 2015, 373 (13), 1220–1229, DOI: 10.1056/NEJMoa1500964. [DOI] [PubMed] [Google Scholar]
- (4).Neoh KG; Li M; Kang E-T; Chiong E; Tambyah PA Surface Modification Strategies for Combating Catheter-Related Complications: Recent Advances and Challenges. Journal of Materials Chemistry B 2017, DOI: 10.1039/C6TB03280J. [DOI] [PubMed] [Google Scholar]
- (5).Franz S; Rammelt S; Scharnweber D; Simon JC Immune responses to implants – A review of the Implications for the Design of Immunomodulatory Biomaterials. Biomaterials 2011, 32 (28), 6692–6709, DOI: 10.1016/j.biomaterials.2011.05.078. [DOI] [PubMed] [Google Scholar]
- (6).Zhang Z; Chen S; Chang Y; Jiang S Surface Grafted Sulfobetaine Polymers via Atom Transfer Radical Polymerization as Superlow Fouling Coatings. The Journal of Physical Chemistry B 2006, 110 (22), 10799–10804, DOI: 10.1021/jp057266i. [DOI] [PubMed] [Google Scholar]
- (7).Messersmith PB; Textor M Nanomaterials: Enzymes on Nanotubes Thwart Fouling. Nature nanotechnology 2007, 2 (3), 138–139. [DOI] [PubMed] [Google Scholar]
- (8).Zhang H; Chiao M Anti-fouling Coatings of Poly(dimethylsiloxane) Devices for Biological and Biomedical Applications. Journal of Medical and Biological Engineering 2015, 35 (2), 143–155, DOI: 10.1007/s40846-015-0029-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (9).Control, C. f. D.; Prevention. Bloodstream Infection Event (Central Line-Associated Bloodstream Infection and Non-Central Line-Associated Bloodstream Infection). Device-associated Module BSI 2017, 1–38. [Google Scholar]
- (10).Obiweluozor FO; Tiwari AP; Lee JH; Batgerel T; Kim JY; Lee D; Park CH; Kim CS Thromboresistant semi-IPN hydrogel coating: Towards Improvement of the Hemocompatibility/Biocompatibility of Metallic Stent Implants. Materials Science and Engineering: C 2019, 99, 1274–1288. [DOI] [PubMed] [Google Scholar]
- (11).Wilson AC; Chou S-F; Lozano R; Chen JY; Neuenschwander PF Thermal and Physico-Mechanical Characterizations of Thromboresistant Polyurethane Films. Bioengineering 2019, 6 (3), 69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (12).Yang T; Du Z; Qiu H; Gao P; Zhao X; Wang H; Tu Q; Xiong K; Huang N; Yang Z From Surface to Bulk Modification: Plasma Polymerization of Amine-Bearing Coating by Synergic Strategy of Biomolecule Grafting andNitric Oxide Loading. Bioactive Materials 2020, 5 (1), 17–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (13).Shitole AA; Giram PS; Raut PW; Rade PP; Khandwekar AP; Sharma N; Garnaik B Clopidogrel Eluting Electrospun Polyurethane/Polyethylene Glycol Thromboresistant, Hemocompatible Nanofibrous Scaffolds. Journal of biomaterials applications 2019, 33 (10), 1327–1347. [DOI] [PubMed] [Google Scholar]
- (14).Williams DF There is no such thing as a biocompatible material. Biomaterials 2014, 35 (38), 10009–10014, DOI: 10.1016/j.biomaterials.2014.08.035. [DOI] [PubMed] [Google Scholar]
- (15).Sun M; Qiu H; Su C; Shi X; Wang Z; Ye Y; Zhu Y Solvent-Free Graft-From Polymerization of Polyvinylpyrrolidone Imparting Ultralow Bacterial Fouling and Improved Biocompatibility. ACS Applied Bio Materials 2019, 2 (9), 3983–3991. [DOI] [PubMed] [Google Scholar]
- (16).Wang L; Zhang S; Keatch R; Corner G; Nabi G; Murdoch S; Davidson F; Zhao Q In-Vitro Antibacterial and Anti-Encrustation Performance of Silver-Polytetrafluoroethylene Nanocomposite Coated Urinary Catheters. Journal of Hospital Infection 2019, 103 (1), 55–63. [DOI] [PubMed] [Google Scholar]
- (17).Black J Biological Performance of Materials: Fundamentals of Biocompatibility, Crc Press: 2005. [Google Scholar]
- (18).Mathew E; Domínguez-Robles J; Larrañeta E; Lamprou DA Fused Deposition Modelling as a Potential Tool for Antimicrobial Dialysis Catheters Manufacturing: New Trends vs. Conventional Approaches. Coatings 2019, 9 (8), 515. [Google Scholar]
- (19).Kingshott P; Wei J; Bagge-Ravn D; Gadegaard N; Gram L Covalent Attachment of Poly(ethylene glycol) to Surfaces, Critical for Reducing Bacterial Adhesion. Langmuir 2003, 19 (17), 6912–6921, DOI: 10.1021/la034032m. [DOI] [Google Scholar]
- (20).Chen S; Zheng J; Li L; Jiang S Strong Resistance of Phosphorylcholine Self-Assembled Monolayers to Protein Adsorption: Insights into Nonfouling Properties of Zwitterionic Materials. Journal of the American Chemical Society 2005, 127 (41), 14473–14478. [DOI] [PubMed] [Google Scholar]
- (21).Li L; Chen S; Zheng J; Ratner BD; Jiang S Protein Adsorption on Oligo (ethylene glycol)-Terminated Alkanethiolate Self-Assembled Monolayers: the Molecular Basis for Nonfouling Behavior. The Journal of Physical Chemistry B 2005, 109 (7), 2934–2941. [DOI] [PubMed] [Google Scholar]
- (22).Cheng G; Zhang Z; Chen S; Bryers JD; Jiang S Inhibition ofBacterial Adhesion and Biofilm Formation on Zwitterionic Surfaces. Biomaterials 2007, 28 (29), 4192–4199, DOI: 10.1016/j.biomaterials.2007.05.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (23).Sundaram HS; Han X; Nowinski AK; Ella-Menye JR; Wimbish C; Marek P; Senecal K; Jiang S One-step Dip Coating of Zwitterionic Sulfobetaine Polymers on Hydrophobic and Hydrophilic Surfaces. ACS Appl Mater Interfaces 2014, 6 (9), 6664–71, DOI: 10.1021/am500362k. [DOI] [PubMed] [Google Scholar]
- (24).Sundaram HS; Han X; Nowinski AK; Brault ND; Li Y; Ella-Menye J-R; Amoaka KA; Cook KE; Marek P; Senecal K; Jiang S Achieving One-Step Surface Coating of Highly Hydrophilic Poly(Carboxybetaine Methacrylate) Polymers on Hydrophobic and Hydrophilic Surfaces. Advanced Materials Interfaces 2014, 1 (6), n/a–n/a, DOI: 10.1002/admi.201400071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (25).Padmanabhan NT; Jayaraj MK; John H Graphene Hybridized High Energy Faceted Titanium Dioxide for Transparent Self-Cleaning Coatings. Catalysis Today 2019. [Google Scholar]
- (26).Valenzuela L; Iglesias A; Faraldos M; Bahamonde A; Rosal R Antimicrobial Surfaces with Self-Cleaning Properties Functionalized by Photocatalytic ZnO Electrosprayed Coatings. Journal of hazardous materials 2019, 369, 665–673. [DOI] [PubMed] [Google Scholar]
- (27).Su Q; Wen F; Huang Y; Wang B Abrasion Resistant Semitransparent Self-Cleaning Coatings Based on Porous Silica Microspheres and Polydimethylsiloxane. Ceramics International 2019, 45 (1), 401–408. [Google Scholar]
- (28).Narayana JL; Mishra B; Lushnikova T; Golla RM; Wang G Modulation of Antimicrobial Potency of Human Cathelicidin Peptides Against the ESKAPE Pathogens and in vivo Efficacy in a Murine Catheter-Associated Biofilm Model. Biochimica et Biophysica Acta (BBA)-Biomembranes 2019, 1861 (9), 1592–1602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (29).Kagan E; Salgado CD; Banks AL; Marculescu CE; Cantey JR Peripherally Inserted Central Catheter–Associated Bloodstream Infection: Risk Factors and the Role of Antibiotic-Impregnated Catheters for Prevention. American journal of infection control 2019, 47 (2), 191–195. [DOI] [PubMed] [Google Scholar]
- (30).Liu Q; Singha P; Handa H; Locklin J Covalent Grafting of Antifouling Phosphorylcholine-Based Copolymers with Antimicrobial Nitric Oxide Releasing Polymers to Enhance Infection-Resistant Properties of Medical Device Coatings. Langmuir 2017, DOI: 10.1021/acs.langmuir.7b02970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (31).Werner BG; Wu JY; Goddard JM Antimicrobial and Antifouling Polymeric Coating Mitigates Persistence of Pseudomonas Aeruginosa Biofilm. Biofouling 2019, 35 (7), 785–795. [DOI] [PubMed] [Google Scholar]
- (32).Fan X; Liu Y; Wang X; Quan X; Chen S Improvement of Antifouling and Antimicrobial Abilities on Silver–Carbon Nanotube Based Membranes under Electrochemical Assistance. Environmental science & technology 2019, 53 (9), 5292–5300. [DOI] [PubMed] [Google Scholar]
- (33).Zheng H-T; Bui HL; Chakroborty S; Wang Y; Huang C-J PEGylated Metal-Phenolic Networks for Antimicrobial and Antifouling Properties. Langmuir 2019, 35 (26), 8829–8839. [DOI] [PubMed] [Google Scholar]
- (34).Ishihara K; Nomura H; Mihara T; Kurita K; Iwasaki Y; Nakabayashi N Why do Phospholipid Polymers Reduce Protein Adsorption? Journal of biomedical materials research 1998, 39 (2), 323–330. [DOI] [PubMed] [Google Scholar]
- (35).Fang FC Antimicrobial Actions of Nitric Oxide. Nitric oxide : biology and chemistry / official journal of the Nitric Oxide Society 2012, 27, Supplement, S10, DOI: 10.1016/j.niox.2012.04.036. [DOI] [Google Scholar]
- (36).Carpenter AW; Schoenfisch MH Nitric Oxide Release: Part II. Therapeutic Applications. Chem Soc Rev 2012, 41 (10), 3742–52, DOI: 10.1039/c2cs15273h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (37).Brisbois EJ; Handa H; Major TC; Bartlett RH; Meyerhoff ME Long-Term Nitric Oxide Release and Elevated Temperature Stability with S-nitroso-N-acetylpenicillamine (SNAP)-Doped Elast-eon E2As Polymer. Biomaterials 2013, 34 (28), 6957–66, DOI: 10.1016/j.biomaterials.2013.05.063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (38).Goudie MJ; Brisbois EJ; Pant J; Thompson A; Potkay JA; Handa H Characterization of an S-nitroso-N-acetylpenicillamine–Based Nitric Oxide Releasing Polymer From a Translational Perspective. International Journal of Polymeric Materials and Polymeric Biomaterials 2016, 65 (15), 769–778, DOI: 10.1080/00914037.2016.1163570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (39).Klement P; Du YJ; Berry LR; Tressel P; Chan AK Chronic Performance of Polyurethane Catheters Covalently Coated with ATH Complex: a Rabbit Jugular Vein Model. Biomaterials 2006, 27 (29), 5107–5117. [DOI] [PubMed] [Google Scholar]
- (40).Frost MC; Meyerhoff ME Controlled Photoinitiated Release of Nitric Oxide from Polymer Films Containing S-Nitroso-N-acetyl-dl-penicillamine Derivatized Fumed Silica Filler. Journal of the American Chemical Society 2004, 126 (5), 1348–1349, DOI: 10.1021/ja039466i. [DOI] [PubMed] [Google Scholar]
- (41).Frost MC; Meyerhoff ME Synthesis, Characterization, and Controlled Nitric Oxide Release from S-nitrosothiol-derivatized Fumed Silica Polymer Filler Particles. Journal of Biomedical Materials Research Part A 2005, 72 (4), 409–419. [DOI] [PubMed] [Google Scholar]
- (42).Singha P; Locklin J; Handa H A Review of the Recent Advances in Antimicrobial Coatings for Urinary Catheters. Acta biomaterialia 2017, 50, 20–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (43).Corey GR Staphylococcus Aureus Bloodstream Infections: Definitions and Treatment. Clinical Infectious Diseases 2009, 48 (s4), S254–S259, DOI: 10.1086/598186. [DOI] [PubMed] [Google Scholar]
- (44).Ren H; Wu J; Colletta A; Meyerhoff ME; Xi C Efficient Eradication of Mature Pseudomonas aeruginosa Biofilm via Controlled Delivery of Nitric Oxide Combined with Antimicrobial Peptide and Antibiotics. Frontiers in Microbiology 2016, 7, DOI: 10.3389/fmicb.2016.01260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (45).Valquier-Flynn H; L Wilson C; E Holmes A; D Wentworth C Growth Rate of Pseudomonas aeruginosa Biofilms on Slippery Butyl Methacrylate-Co-Ethylene Dimethacrylate (BMA-EDMA), Glass and Polycarbonate Surfaces. Journal of Biotechnology & Biomaterials 2017, 07 (04), DOI: 10.4172/2155-952x.1000274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (46).Percival SL; Mayer D; Salisbury A-M Efficacy of a Surfactant-Based Wound Dressing on Biofilm Control. Wound Repair and Regeneration 2017, 25 (5), 767–773, DOI: 10.1111/wrr.12581. [DOI] [PubMed] [Google Scholar]
- (47).Kandimalla KK; Borden E; Omtri RS; Boyapati SP; Smith M; Lebby K; Mulpuru M; Gadde M Ability of Chitosan Gels to Disrupt Bacterial Biofilms and Their Applications in the Treatment of Bacterial Vaginosis. Journal of Pharmaceutical Sciences 2013, 102 (7), 2096–2101, DOI: 10.1002/jps.23571. [DOI] [PubMed] [Google Scholar]
- (48).Brisbois EJ; Major TC; Goudie MJ; Meyerhoff ME; Bartlett RH; Handa H Attenuation of Thrombosis and Bacterial Infection Using Dual Function Nitric Oxide Releasing Central Venous Catheters in a 9 Day Rabbit Model. Acta Biomaterialia 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (49).Goudie MJ; Pant J; Handa H Liquid-InfusedNitric Oxide-Releasing (LINORel) Silicone for Decreased Fouling, Thrombosis, and Infection of Medical Devices. Scientific Reports 2017, 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (50).Amoako KA; Sundaram HS; Suhaib A; Jiang S; Cook KE Multimodal, Biomaterial-Focused Anticoagulation via Superlow Fouling Zwitterionic Functional Groups Coupled with Anti-Platelet Nitric Oxide Release. Advanced Materials Interfaces 2016. [Google Scholar]
- (51).Xiang DZ; Verbeken E; Van Lommel A; Stas M; De Wever I Intimal Hyperplasia After Long-Term Venous Catheterization. European surgical research 2000, 32 (4), 236–245. [DOI] [PubMed] [Google Scholar]
- (52).Baskin JL; Pui C-H; Reiss U; Wilimas JA; Metzger ML; Ribeiro RC; Howard SC Management of Occlusion and Thrombosis Associated with Long-Term Indwelling Central Venous Catheters. The Lancet 2009, 374 (9684), 159–169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (53).Kim S; Kim Y; Moon S-B Histological Changes of the Unligated Vein wall Adjacent to the central Venous Catheter After Open Cutdown in Rats. Journal of pediatric surgery 2015, 50 (11), 1928–1932. [DOI] [PubMed] [Google Scholar]


