Abstract
Background:
People who use drugs (PWUD) continue to experience a disproportionate HIV burden due to drug- and sex-related risk behaviors. Pre-exposure prophylaxis (PrEP) is highly effective at preventing HIV infection, but very little is known about PrEP use among PWUD and their willingness to initiate PrEP.
Methods:
We conducted a cross-sectional survey among 234 HIV-negative, opioid-dependent individuals recruited from an urban methadone clinic. Participants were assessed using an audio-computer assisted self-interview technique. Bivariate and multiple logistic regressions were used to explore independent correlates of actual PrEP use and willingness to initiate PrEP.
Results:
One-fourth (25.6%) of participants had previously used PrEP. Over two-thirds (67.1%) of participants had previously heard of PrEP, and 65.0% were willing to take it. In multivariable logistic regression analyses, the number of times participants engaged in HIV testing (aOR=1.66, p<0.01) and whether they visited a healthcare provider (aOR=20.81, p=0.02) were associated with a higher likelihood of PrEP use, while perceived HIV risk (aOR=2.71, p<0.01) and previous use of PrEP (aOR=3.57, p<0.01) were significantly associated with willingness to initiate PrEP.
Conclusion:
PrEP use was low among PWUD, but their willingness to initiate PrEP was moderate, which indicated a significant discrepancy between actual PrEP use and willingness to use it. Our findings highlight the importance of healthcare providers engaging opioid-dependent individuals in discussions about PrEP and the need for innovative strategies to increase their awareness of PrEP and modify their perceptions of HIV risk.
Keywords: People who use drugs (PWUD), HIV prevention, pre-exposure prophylaxis (PrEP), substance use, sexual risk behavior, opioid-dependent, opioid use disorder
1. Introduction
People who use drugs (PWUD) are one of the most vulnerable groups to HIV transmissions in the United States.1,2 Their vulnerability is elevated by injection behaviors.3 For example, HIV outbreaks in rural Indiana and other rural parts of the United States serve as a cautionary tale for HIV to rapidly disseminate within the networks of people who inject drugs (PWID).4,5 In 2017, 9% of all newly diagnosed HIV cases in the United States were among PWID, and this percentage in Connecticut State was 5.6%.6,7 By 2019, there were 10,719 people living with HIV in Connecticut, and 25.5% of them were PWID.7 According to the Centers for Disease Control and Prevention, over 200 counties and 26 states and jurisdictions in the United States are experiencing or are at risk of HIV outbreaks due to injection drug use.8 Evidence-based harm reduction programs such as opioid agonist therapy (OAT) and syringe services programs (SSP) can help reduce the risk of HIV acquisition in PWUD.9,10 Those programs, however, do not eliminate risk. PWUD on OAT remain at elevated risk by continued injection of opioids (suboptimal dosing) or stimulants or through sexual risk.11,12 Previous studies show that PWUD are more likely to involve in transactional sex, experience sexual violence, have multiple sexual partners, and engage in condomless sex.13-15 Given the potential for future HIV outbreaks amidst a burgeoning opioid epidemic, innovative strategies are urgently needed to curtail HIV transmission among PWUD.
Pre-exposure prophylaxis (PrEP) is an effective tool to prevent HIV and is recommended for high-risk PWUD.16-18 PrEP, however, remains underutilized in PWUD with the minimal programmatic rollout.19 A systematic review examined the PrEP care cascade in PWID and found that PrEP use among PWID was very low, ranging from 0~3%, indicating the urgency to promote PrEP knowledge and improve linkage to care among PWID.6 Evidence about factors that influence PrEP use (e.g., willingness to initiate, perceived risk of acquiring HIV) has primarily focused on MSM.20-22 Although PWUD are ideal candidates to use PrEP, interventions and strategies to improve PrEP use among this population are inadequate. The known barriers to PrEP use among PWUD include individual-level, interpersonal-level, clinical and structural barriers.23 Individual-level barriers include limited HIV risk perception, concerns about PrEP side effects, and competing health priorities and needs.23 Interpersonal-level barriers include HIV- and PrEP-related stigma and discrimination within the social network, and negative experiences interacting with healthcare providers.19,23,24 Clinical and structural barriers include poor infrastructure for PrEP delivery, healthcare providers' capacity or willingness to prescribe PrEP to PWID.19,23,24
With the evolving landscape in PrEP-related research and implementation, more research is warranted on factors that can influence PrEP use in order to inform future interventions. In this study, we therefore explored prior use and willingness to initiate PrEP among opioid-dependent individuals enrolled in medication for opioid use disorder (MOUD) program. We also investigated factors related to these outcomes. Such findings are necessary to guide future implementation of PrEP among high-risk PWUD in the context of common drug treatment settings.
2. Methods
2.1. Study design and participants
We conducted an anonymous, cross-sectional survey of 234 opioid-dependent individuals enrolled in a larger HIV prevention study between July 2018 and October 2019. Inclusion criteria included: a) ≥18 years of age; b) self-reported HIV-negative or HIV status unknown; c) engagement in drug-related (sharing of injection equipment) or sex-related (condomless sex, multiple sexual partners) risk behaviors in the past 6 months; d) met the criteria for OUD based on the American Psychiatric Association's Diagnostic and Statistical Manual of Mental Disorders (DSM-V); e) enrolled in MOUD program (methadone); and f) able to read and speak English.
Participants were recruited using clinic-based advertisements and flyers, word-of-mouth, and direct referral from substance use counselors at Connecticut's largest addiction treatment center, which provides OAT for >7000 patients. All screening, enrollment, and interview activities were conducted in a private room by trained research assistants. Following informed consent, participants completed a 45-minute survey using audio computer-assisted self-interview (ACASI). All participants were reimbursed $25 for their time. The study protocol was approved by the Institutional Review Board at the University of Connecticut and received board approval from the partnering methadone clinic.
2.2. Measures
Explanatory variables were based on prior research. Sociodemographic characteristics included sex, age, sexual orientation, race and ethnicity, marital status, educational status, employment status, income, homelessness, and methadone dose. Healthcare access was measured by health insurance status (yes/no), whether participants had visited healthcare providers (in the past 12 months), and whether participants had taken HIV testing (in the past 12 months). A standardized scale was used to assess depression (scores ≥16 indicative of moderate to severe depression using the 20-item Center for Epidemiological Studies Depression Scale (CES-D)).25
Participants reported their age of first drug injection, length of drug use (in years), whether they had injected drugs in the last 30 days, and whether they experienced an overdose of Fentanyl in the last month. The Alcohol Use Disorders Identification Test for Consumption (AUDIT-C) was used to measure the presence and degree of an alcohol use disorder (AUD). The standardized score of ≥ 4 for males and ≥ 3 for females indicating the presence of an AUD.26
Sex-related risk behaviors included participants' age of sexual debut, number of sexual partners in the last month, and condomless sex. Condomless sex was assessed by the question "In the past month, how much of the time did you use a condom or other latex protection when you had oral, anal, or vaginal sex?" Perceived risk of acquiring HIV was measured by a single question, "What do you think is your current risk of getting HIV (yes/no)? Participants' satisfaction with previous HIV prevention methods was assessed using the question, "Are you satisfied with your current method of HIV protection (e.g., condom use, clean needle use, daily oral PrEP use)?" with a dichotomized response of "Yes" and "No."
A single-item question measured participants' awareness of PrEP prior to the interview (yes/no); "Before participating in this survey, have you ever heard of oral PrEP for protection against HIV?".
Outcome variables are i) participants' willingness to initiate PrEP and ii) participants' PrEP use. Participants’ willingness to initiate PrEP was assessed after providing a brief description of PrEP — "Would you be interested in taking PrEP to reduce the risk of HIV infection (yes/no)?". The latter was assessed by the question, "Have you ever used PrEP (yes/no)?"
2.3. Statistical analyses
All data analyses were performed using SAS 9.4 (SAS Institute, Cary, North Carolina, United States). We used frequencies and percentages to describe categorical variables and means and standard deviations to describe continuous variables. Logistic regression models were used to examine the association between explanatory and outcome variables. The statistical significance level was set as 0.10 in bivariate logistic regression models. Variables with a p value of less than 0.10 were entered into the multivariable logistic regression model, in which the statistical significance level was set as 0.05. Odds ratios and corresponding 95% confidence intervals were used to indicate significant variables.
3. Results
3.1. Participant characteristics
Most participants were non-Hispanic Whites (63.3%), high school graduates (72.2%), and unemployed (87.6%) with an annual income of less than $10,000 (70.9%). Participants were mostly in their early 40s, with most (74.4%) meeting criteria for depression and 31.2% meeting criteria for AUD. Participants' average age of sexual debut was 14.8 years, with an average of three sexual partners in the last month, and most (65.4%) reported condomless sex in the last month. Among all participants, 4.7% were MSM. In terms of drug use behaviors, 44.9% reported injecting illicit drugs in the past 30 days, and, of those, 41.0% reported sharing injection equipment. Almost two-thirds (62%) of participants reported being satisfied with their current method of HIV prevention (e.g., condom use, clean needle use, PrEP use), and 64.1% perceived that they were at risk of acquiring HIV (Table 1).
Table 1.
Characteristics of participants
| Variables | Entire sample (N=234) | |
|---|---|---|
| Frequency | % | |
| Patients’ characteristics | ||
| Sex | ||
| Female | 115 | 49.1 |
| Male | 119 | 50.9 |
| Age (years), mean (SD) | 234 | 42.7 (10.2) |
| Race | ||
| Non-Hispanic White | 148 | 63.3 |
| African American or Black | 46 | 19.6 |
| Hispanic or Latino | 35 | 15.0 |
| Asian or Pacific Islander | 5 | 2.1 |
| Currently married or living with partner | ||
| Yes | 51 | 21.8 |
| No | 183 | 78.2 |
| High school graduate | ||
| Yes | 169 | 72.2 |
| No | 65 | 27.8 |
| Currently employed | ||
| Yes | 29 | 12.4 |
| No | 205 | 87.6 |
| Yearly income | ||
| < $10,000 | 166 | 70.9 |
| ≥ $10,000 | 68 | 29.1 |
| Health insurance | ||
| Yes | 230 | 98.3 |
| No | 4 | 1.7 |
| Homeless in the past 12 months | ||
| Yes | 131 | 56.0 |
| No | 103 | 44.0 |
| Heterosexual orientation | ||
| Yes | 185 | 79.1 |
| No | 49 | 20.9 |
| Visited healthcare provider in the past 12 months | ||
| Yes | 207 | 88.5 |
| No | 27 | 11.5 |
| Current methadone dose (mg), mean (SD) | 234 | 81.9 (30.6) |
| Ever tested for HIV | ||
| Yes | 228 | 97.4 |
| No | 6 | 2.6 |
| Moderate to severe depression | ||
| Yes | 174 | 74.4 |
| No | 60 | 25.6 |
| Number of times HIV tested in the past 12 months, mean (SD) | 234 | 1.64 (1.35) |
| Perceived risk of getting HIV | ||
| Yes | 150 | 64.1 |
| No | 84 | 35.9 |
| Sex-related behaviors | ||
| Number of sexual partners in the past 30 days, mean (SD) | 234 | 3.0 (4.3) |
| Condomless sex in the past 30 days | ||
| Yes | 153 | 65.4 |
| No | 81 | 34.6 |
| Satisfied with current method of HIV protection | ||
| Yes | 145 | 62.0 |
| No | 89 | 38.0 |
| Enaged in sexual activitiy while using alchol | ||
| Yes | 73 | 31.2 |
| No | 161 | 68.8 |
| Drug-related behaviors | ||
| Age of first drug injection (years), mean (SD) | 234 | 25.3 (9.2) |
| Length of using drugs (years), mean (SD) | 234 | 19.1 (12.6) |
| Drug injection in the past 30 days | ||
| Yes | 105 | 44.9 |
| No | 129 | 55.1 |
| Daily injection of drugs | ||
| Yes | 214 | 91.5 |
| No | 20 | 8.5 |
| Shared drug injection equipment | ||
| Yes | 43 | 18.4 |
| No | 191 | 81.6 |
| Used Cocaine in the past 30 days | ||
| Yes | 127 | 54.3 |
| No | 107 | 45.7 |
| Current alcohol use disorder a | ||
| Yes | 73 | 31.2 |
| No | 161 | 68.8 |
| Experienced an overdose of Fentanyl in the past 30 days | ||
| Yes | 21 | 9.0 |
| No | 213 | 91.0 |
| Outcome variables | ||
| PrEP use (current or ever) | ||
| Yes | 60 | 25.6 |
| No | 174 | 74.4 |
| Willingness to initiate PrEP | ||
| Yes | 152 | 65.0 |
| No | 82 | 34.6 |
The number of female and male participants who didn’t have alcohol use disorder was 85 and 76, respectively.
Over two-thirds (67.1%) had ever heard about PrEP, and only 25.6% had previously used it as a method to prevent HIV. Conversations with healthcare providers (49.7%), friends (44.6%), and HIV prevention counselors (42.7%) were noted as the top sources of PrEP knowledge. Nearly two-thirds of participants (65.0%) reported that they would be willing to initiate PrEP to reduce their risk of HIV infection (Figure 1).
Figure 1.
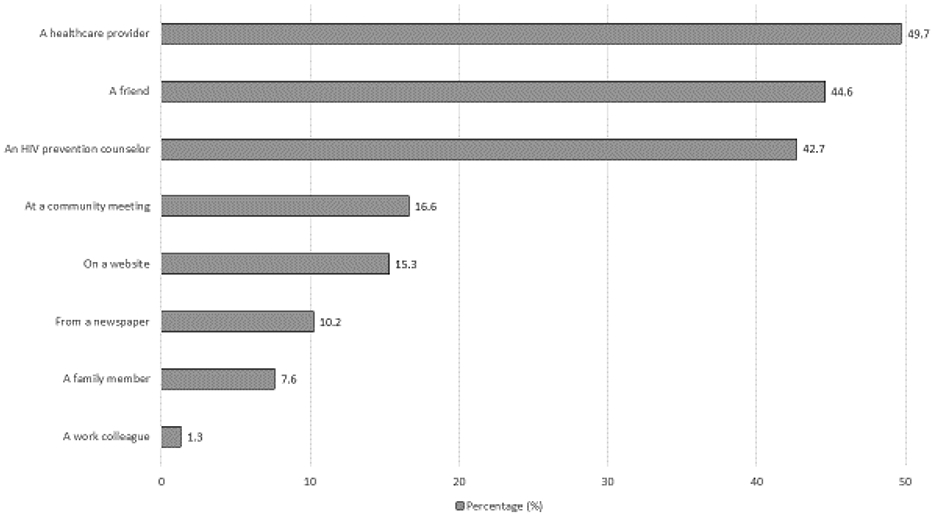
Where participants heard about PrEP (n=157)
3.2. Correlates of prior PrEP use
At the bivariate level, length of using drugs (OR=1.03, p=0.03) and condomless sex in the past 30 days (OR=2.00, p=0.02) were positively related to PrEP use. Additionally, we examined the independent correlates associated with PrEP use (Table 2). Specifically, visiting a healthcare provider in the past 12 months was significantly related to participants' PrEP use (aOR=20.81, p=0.02). Also, a higher frequency of HIV testing in the past 12 months was significantly associated with PrEP use (aOR=1.66, p<0.01).
Table 2.
Bivariate and Independent Correlates of Having Ever Used PrEP (N=234)
| Variable | Bivariate Associations | Multivariate Analysis | ||||
|---|---|---|---|---|---|---|
| OR | 95% CI | P-value | aOR | 95% CI | P-value | |
| Sex | ||||||
| Female | 1.50 | 0.83, 2.71 | 0.18 | |||
| Male (ref) | ||||||
| Age (years; continuous) | 1.01 | 0.98, 1.04 | 0.63 | |||
| Race | ||||||
| White | 0.76 | 0.42, 1.38 | 0.36 | |||
| Non-white (ref) | ||||||
| Currently married or living with partner | 0.99 | 0.49, 2.02 | 0.98 | |||
| High school graduate | 0.57 | 0.30, 1.06 | 0.08* | 0.76 | 0.38, 1.53 | 0.45 |
| Currently employed | 0.57 | 0.21, 1.56 | 0.27 | |||
| Yearly income | ||||||
| < $10,000 | 0.94 | 0.50, 1.79 | 0.85 | |||
| ≥ $10,000 (ref) | ||||||
| Having health insurance | 0.34 | 0.05, 2.45 | 0.28 | |||
| Homeless in the past 12 months | 0.79 | 0.44, 1.43 | 0.44 | |||
| Heterosexual orientation | 0.73 | 0.36, 1.46 | 0.37 | |||
| Visited healthcare provider in the past 12 months | 10.37 | 1.38, 78.13 | 0.02* | 20.81 | 1.67, 258.82‡ | 0.02** |
| Current methadone dose (continuous per mg) | 1.00 | 0.99, 1.01 | 0.49 | |||
| Moderate to severe depression | 1.05 | 0.53, 2.06 | 0.90 | |||
| Number of times HIV tested in the past 12 months (continuous) | 1.54 | 1.20, 1.97 | <0.001* | 1.66 | 1.27, 2.18 | <0.001** |
| Perceived at risk of getting HIV | 0.96 | 0.52, 1.76 | 0.89 | |||
| Number of sexual partners in the past 30 days (continuous) | 0.99 | 0.92, 1.08 | 0.86 | |||
| Condomless sex in the past 30 days | 2.00 | 1.10, 3.64 | 0.02* | 1.79 | 0.93, 3.45 | 0.08 |
| Satisfied with current method of HIV protection | 1.99 | 1.04, 3.79 | 0.04* | 1.76 | 0.88, 3.53 | 0.11 |
| Enaged in sexual activitiy while using alchol | 1.03 | 0.55, 1.94 | 0.93 | |||
| Age of first drug injection (years; continuous) | 0.99 | 0.98, 1.00 | 0.17 | |||
| Length of using drugs (years; continuous) | 1.03 | 1.00, 1.05 | 0.03* | 1.02 | 1.00, 1.05 | 0.07 |
| Drug injection in the past 30 days | 0.93 | 0.45, 1.92 | 0.84 | |||
| Daily injection of drugs | 0.74 | 0.40, 1.34 | 0.32 | |||
| Shared drug injection equipment | 0.61 | 0.27, 1.40 | 0.25 | |||
| Used Cocaine in the past 30 days | 0.95 | 0.88, 1.03 | 0.19 | |||
| Current alcohol use disorder | 0.59 | 0.30, 1.17 | 0.13 | |||
| Experienced an overdose of Fentanyl in the past 30 days | 1.18 | 0.44, 3.12 | 0.75 | |||
OR odds ratio, aOR adjusted odds ratio CI confidence interval, ref referent
In bivariate logistic regression models, those variables whose P-value is less than 0.1 was included in the multiple logistic regression
Variables that have been significant at 0.05 level in multiple logistic regression model
Given the large confidence intervals, we also conducted a multivariate analysis that didn’t include the variable – visited healthcare provider in the past 12 months. This change didn’t alter the results
3.3. Correlates of willingness to initiate PrEP
As shown in Table 3, participants' willingness to initiate PrEP was significantly related to their perceived risk of getting HIV and previous PrEP use. Compared to those who did not perceive themselves to be at risk for HIV, those with perceived risk were over two times more willing to initiate PrEP (aOR=2.71, p<0.01). Similarly, those who had prior experience of using PrEP were over three times more willing to initiate PrEP (aOR=3.57, p<0.01) compared to those who had no such experience.
Table 3.
Bivariate and Independent Correlates of Willingness to Initiate PrEP (N=234)
| Variable | Bivariate Associations | Multivariate Analysis | ||||
|---|---|---|---|---|---|---|
| OR | 95% CI | P-value | aOR | 95% CI | P-value | |
| Sex | ||||||
| Female | 1.19 | 0.64, 2.04 | 0.53 | |||
| Male (ref) | ||||||
| Age (years; continuous) | 1.00 | 0.98, 1.03 | 0.80 | |||
| Race | ||||||
| White | 0.99 | 0.57, 1.73 | 0.97 | |||
| Non-white (ref) | ||||||
| Currently married or living with partner | 1.39 | 0.71, 2.72 | 0.34 | |||
| High school graduate | 1.12 | 0.62, 2.03 | 0.71 | |||
| Currently employed | 0.74 | 0.33, 1.62 | 0.45 | |||
| Yearly income | ||||||
| < $10,000 | 0.77 | 0.42, 1.41 | 0.39 | |||
| > $10,000 (ref) | ||||||
| Having health insurance | 5.73 | 0.59, 56.00 | 0.13 | |||
| Homeless in the last year | 0.73 | 0.42, 1.26 | 0.26 | |||
| Heterosexual orientation | 0.87 | 0.45, 1.71 | 0.69 | |||
| Visited healthcare provider in the past 12 months | 1.57 | 0.70, 3.53 | 0.28 | |||
| Current methadone dose (continuous per mg) | 1.00 | 0.99, 1.01 | 0.42 | |||
| Moderate to severe depression | 0.74 | 0.39, 1.39 | 0.34 | |||
| Number of times HIV tested in the past 12 months (continuous) | 0.98 | 0.81, 1.20 | 0.86 | |||
| Perceived at risk of getting HIV | 2.99 | 1.70, 5.24 | <0.001* | 2.71 | 1.47, 5.00 | 0.002** |
| Number of sexual partners in the past 30 days (continuous) | 1.11 | 0.99, 1.25 | 0.08* | 1.05 | 0.95, 1.17 | 0.34 |
| Condomless sex in the past 30 days | 0.74 | 0.43, 1.30 | 0.30 | |||
| Satisfied with current method of HIV protection | 0.60 | 0.34, 1.07 | 0.08* | 0.69 | 0.37, 1.29 | 0.24 |
| Enaged in sexual activitiy while using alchol | 1.38 | 0.76, 2.49 | 0.29 | |||
| Age of first drug injection (years; continuous) | 1.00 | 0.99, 1.01 | 0.98 | |||
| Length of using drugs (years; continuous) | 0.99 | 0.97, 1.02 | 0.56 | |||
| Drug injection in the past 30 days | 0.97 | 0.91, 1.04 | 0.40 | |||
| Daily injection of drugs | 0.73 | 0.42, 1.29 | 0.28 | |||
| Shared drug injection equipment | 0.97 | 0.91, 1.04 | 0.39 | |||
| Used Cocaine in the past 30 days | 0.97 | 0.91, 1.04 | 0.41 | |||
| Current alcohol use disorder | 0.96 | 0.54, 1.72 | 0.90 | |||
| Experienced an overdose of Fentanyl in the past 30 days | 0.97 | 0.91, 1.03 | 0.32 | |||
| PrEP use (ever) | 3.07 | 0.49, 6.31 | 0.002* | 3.57 | 1.68, 7.61 | 0.001** |
OR odds ratio, aOR adjusted odds ratio CI confidence interval, ref referent
In bivariate logistic regression models, those variables whose P-value is less than 0.1 was included in the multiple logistic regression
Variables that have been significant at 0.05 level in multiple logistic regression model
4. Discussion
In this study, we investigated the willingness to initiate PrEP and its use among opioid-dependent individuals in a community-based MOUD program. We found that the percentage of opioid-dependent individuals who were aware of PrEP was moderate (67.1%), and the percentage who had ever used PrEP was low (25.6%). Comparing the findings with that previously reported by our team within the same MOUD program,27 there was an encouraging increase in the percentage of participants aware of PrEP (67.1% vs. 18%) and taking it (25.6% vs. 1.8%) as HIV prevention, respectively. These improvements may reflect effects of the expansion of PrEP services from MSM to include PWUD among many OAT clinics in the Northeast. Also, this could be attributed to many local initiatives implemented in Connecticut aimed to increase awareness about HIV and reduce stigma from communities and medical professionals, such as Connecticut Getting to Zero initiative,28 and programs providing medical and treatment services, including Connecticut PrEP Local Medical Services,29 and the Community Health Care Van (CHCV) program, which provides education and medical services for marginalized populations in impoverished neighborhoods.30 Although these improvements are encouraging and might also reflect improvements in other regions, more tailored interventions are needed to increase PrEP awareness and use among PWUD. This urgent need stems from the fact that: 1) PrEP is an effective HIV prevention method, and 2) sex-related (condomless sex, multiple sexual partners) and drug-related (sharing of injection equipment) high-risk behaviors are disproportionately high among PWUD, as reported in this study.31 These results are consistent with the broader literature that PWUD are among the most vulnerable groups to the rapid spread of HIV,32 echoing the argument that PrEP would be ideal for PWUD and actions to reduce high-risk behaviors among PWUD were emergently needed.19,27,33
In this study, although participants’ willingness to initiate PrEP was moderate (65%), their actual PrEP use remained relatively low (25.6%). The gap between the two indicates that the actual uptake of PrEP among PWUD, as a high-risk group, has been grossly inadequate in fully incorporating PrEP as a primary HIV prevention strategy. Our findings echo a critical analysis of a report of a community consultation led by the International Network of PWUD, pointing out fundamental gaps between enthusiasm about PrEP among PWUD and actual use.34 Multiple factors may have contributed to the gap, including PWUD’s concerns of side effects, PrEP-related stigma and discrimination, and variation in physicians’ prescription practices.19,24 Although approved PrEP medications have no serious side effects,35 consideration of potential side effects of PrEP medications are still major barriers to PrEP use among PWUD.33,36,37 PrEP-related stigma can also discourage PWUD from taking PrEP. For example, concerns exist among PWUD that they would be regarded as an HIV-infected person if they are known to take PrEP.33 Moreover, some studies reported that some physicians, as a treatment gatekeeper, were reluctant to prescribe PrEP to PWID, believing that PWID were less likely to adhere to it or that prescribing PrEP could increase the potential for drug resistance. As an injectable form of PrEP has become available, it is plausible that more PWID will be initiated on PrEP since this could directly improve a number of concerns regarding adherence.38 Future research is needed to explore the various facilitators and barriers for translating willingness to actual PrEP use behavior, as well as the development of more innovative strategies to enhance PrEP adherence over time.
In this study, we found that participants who visited a healthcare provider in the past 12 months and were HIV tested were more likely to use PrEP to prevent HIV transmission. It could be that participants on PrEP need to visit their healthcare providers for the lab work required for continued PrEP maintenance, as well as regular HIV testing to reduce the risk of antiretroviral medication resistance, which may occur when an individual seroconverts.39 It might also be because participants who engaged in more HIV testing tended to be more aware of their health and potential health risks.40 HIV testing is a prerequisite for the initiation and ongoing uptake of PrEP.39 Integrating PrEP counseling and prescription within HIV testing services in MOUD clinics might improve PrEP use among PWUD. For example, when PWUD come to MOUD clinics to test for HIV, clinical providers could initiate discussions about HIV prevention, such as PrEP-related information including effectiveness, affordability, and behavioral skills needed to properly adhere to PrEP, such as taking PrEP together with daily methadone. This engagement might greatly improve PrEP use among PWUD enrolled in common addiction treatment settings.31 Additionally, compared to clinic-based HIV testing promotion, the application of HIV self-testing can also increase PrEP use. For example, recent studies in Keyna,41 Zambia, and Uganda42 have shown that HIV self-testing could support PrEP implementation and scale-up. This is largely because promoting HIV self-testing can increase first-time and repeat testing for HIV,39,43 thus increasing individual’s awareness of their HIV status and frequency of PrEP counseling. Our study indicates that future research to improve PrEP use with HIV testing outreach and pre/post-test counseling is warranted. Future work should also examine PWUD who have previously taken PrEP and thoroughly explore why some have discontinued vs. those who have been able to adhere over time.
Interestingly, we found that PWUD's overall perception of acquiring HIV was high, and the perceived risk of acquiring HIV infection was positively correlated with PWUD’s willingness to initiate PrEP. This finding is consistent with findings in the broader literature about PWUD27 and MSM44-46 that self-assessed the risk of HIV infection related to higher adherence to PrEP.33 Given that there were still 32.9% of participants who had never heard about PrEP in our study, it appears necessary to improve PWUD's understanding of their sex- and drug-related risk behaviors. Integrating this education with PrEP services might be an effective way to improve willingness to initiate and use PrEP. Our study found that healthcare providers and friends were the top two sources for participants to hear about PrEP. This result is similar to a prior study conducted among 400 PWUD in New Haven, Connecticut.27 Therefore, it would seem helpful to create an environment for PWUD to easily spread information about PrEP and encourage their peers to seek PrEP-related information in OAT clinics.
Although this study contributed important knowledge to the field, we also acknowledge several limitations. First, participants in this study were recruited from the largest community-based addiction treatment center in Connecticut, which provides MOUD. This center has also been a site for previous PrEP clinical trails among PWUD. Therefore, healthcare providers might have been more willing to endorse taking PrEP than providers from other outside centers. This limits the generalizability of the study findings to other MOUD programs in Connecticut and elsewhere, such as programs in rural regions of the US. Second, this was a cross-sectional study in which we investigated the association between variables obtained during a larger HIV prevention study. No causal relationships were studied, and we predicted the causal relationships and relevance among variables using odds ratios and corresponding 95% confidence intervals.
5. Conclusion
The perceived risk of acquiring HIV and awareness of PrEP were high in our sample, but PrEP uptake was found to be relatively low. Our study underscores the important role of healthcare providers in engaging PWUD in discussions about PrEP and the need for innovative strategies to increase PrEP uptake among PWUD. As discussed, possible solutions include integrating PrEP education/prescription alongside HIV-focused services within common OAT programs. Future research is greatly needed in order to enhance PrEP uptake and adherence among very high-risk groups, including PWUD.
Highlights.
Over two-thirds of PWUD had heard about PrEP, but only 25.6% had previously used it.
PWUD’s willingness to initiate PrEP was related to their perceived risk of getting HIV.
A higher frequency of HIV testing was positively associated with PrEP use among PWUD.
Acknowledgments
Source of Funding:_This work was supported by grants from the National Institute on Drug Abuse for research (R01 DA032290 to MMC) and for career development (K01DA051346 to RS; K24 DA017072 to FLA; K24DA051344 to MMC; K01 DA038529 for JAW).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest: The authors have no conflicts of interest to disclose.
Ethical approval: The study protocol was approved by the Investigational Review Board (IRB) at the University of Connecticut and received board approval from APT Foundation Inc. All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Informed consent
Informed consent was obtained from all individual participants included in the study.
References
- 1.Alpren C, Dawson EL, John B, et al. Opioid Use Fueling HIV Transmission in an Urban Setting: An Outbreak of HIV Infection Among People Who Inject Drugs-Massachusetts, 2015-2018. Am J Public Health. 2020;110(1):37–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Centers for Disease Control and Prevention (CDC). HIV and People Who Inject Drugs, https://www.cdc.gov/hiv/group/hiv-idu.html. Published 2020. Updated February 6, 2020. Accessed June 19, 2020. [Google Scholar]
- 3.Scholl L, Seth P, Kariisa M, Wilson N, Baldwin G. Drug and Opioid-Involved Overdose Deaths - United States, 2013-2017. MMWR Morb Mortal Wkly Rep. 2018;67(5152):1419–1427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ungar Laura. 5 Years After Indiana's Historic HIV Outbreak, Many Rural Places Remain At Risk. https://www.npr.org/sections/health-shots/2020/02/16/801720966/5-years-after-indianas-historic-hiv-outbreak-many-rural-places-remain-at-risk. Published 2020. Accessed February 23, 2020. [Google Scholar]
- 5.Varney Sarah. Rural Indiana Struggles With Drug-Fueled HIV Epidemic. Kasier Health News. https://khn.org/news/rural-indiana-struggles-with-drug-fueled-hiv-epidemic/. Published 2015. Accessed February 23, 2020. [Google Scholar]
- 6.Mistler CB, Copenhaver MM, Shrestha R. The Pre-exposure Prophylaxis (PrEP) Care Cascade in People Who Inject Drugs: A Systematic Review. AIDS and Behavior. 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Health CSDoP. HIV Surveillance. https://portal.ct.gov/DPH/AIDS--Chronic-Diseases/Surveillance/Connecticut-HIV-Statistics. Published 2020. Accessed. [Google Scholar]
- 8.Centers for Disease Control and Prevention (CDC). Vulnerable Counties and Jurisdictions Experiencing or At-Risk of Outbreaks, https://www.cdc.gov/pwid/vulnerable-counties-data.html. Published 2018. Accessed February 24, 2020.
- 9.Centers for Disease Control and Prevention (CDC). Summary of Information on The Safety and Effectiveness of Syringe Services Programs (SSPs). https://www.cdc.gov/ssp/syringe-services-programs-summary.html. Published 2019. Accessed February 07, 2020.
- 10.Wiessing L, Ferri M, Běláčková V, et al. Monitoring quality and coverage of harm reduction services for people who use drugs: a consensus study. Harm Reduct J. 2017;14(1):19–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Centers for Disease Control and Prevention (CDC). Persons Who Inject Drugs (PWID). https://www.cdc.gov/pwid/index.html. Published 2020. Updated July 19, 2018. Accessed June 19, 2020. [Google Scholar]
- 12.Tetrault JM, Kozal MJ, Chiarella J, Sullivan LE, Dinh AT, Fiellin DA. Association between risk behaviors and antiretroviral resistance in HIV-infected patients receiving opioid agonist treatment. J Addict Med. 2013;7(2): 102–107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sopheab H, Chhea C, Tuot S, Muir JA. HIV prevalence, related risk behaviors, and correlates of HIV infection among people who use drugs in Cambodia. BMC Infect DIs. 2018;18(1):562–562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Williams JE, Dangerfield DT 2nd, Krai AH, Wenger LD, Bluthenthal RN. Correlates of Sexual Coercion among People Who Inject Drugs (PWID) in Los Angeles and San Francisco, CA. J Urban Health. 2019;96(3):469–476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Braitstein P, Li K, Tyndall M, et al. Sexual violence among a cohort of injection drug users. Soc Scl Med. 2003;57(3):561–569. [DOI] [PubMed] [Google Scholar]
- 16.Baeten JM, Donnell D, Ndase P, et al. Antiretroviral prophylaxis for HIV prevention in heterosexual men and women. N Engl J Med. 2012;367(5):399–410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Choopanya K, Martin M, Suntharasamai P, et al. Antiretroviral prophylaxis for HIV infection in injecting drug users in Bangkok, Thailand (the Bangkok Tenofovir Study): a randomised, double-blind, placebo-controlled phase 3 trial. Lancet. 2013;381(9883):2083–2090. [DOI] [PubMed] [Google Scholar]
- 18.Thigpen MC, Kebaabetswe PM, Paxton LA, et al. Antiretroviral preexposure prophylaxis for heterosexual HIV transmission in Botswana. N Engl J Med. 2012;367(5):423–434. [DOI] [PubMed] [Google Scholar]
- 19.Shrestha R, Karki P, Altice FL, et al. Measuring Acceptability and Preferences for Implementation of Pre-Exposure Prophylaxis (PrEP) Using Conjoint Analysis: An Application to Primary HIV Prevention Among High Risk Drug Users. AIDS Behav. 2018;22(4):1228–1238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hoornenborg E, Krakower DS, Prins M, Mayer KH. Pre-exposure prophylaxis for MSM and transgender persons in early adopting countries. AIDS. 2017;31(16):2179–2191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wong NS, Kwan TH, Tsang OTY, et al. Pre-exposure prophylaxis (PrEP) for MSM in low HIV incidence places: should high risk individuals be targeted? Sci Rep. 2018;8(1):11641–11641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Stein M, Thurmond P, Bailey G. Willingness to use HIV pre-exposure prophylaxis among opiate users. AIDS Behav. 2014;18(9):1694–1700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Biello KB, Bazzi AR, Mimiaga MJ, et al. Perspectives on HIV pre-exposure prophylaxis (PrEP) utilization and related intervention needs among people who inject drugs. Harm reduction journal. 2018;15(1):55–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Warren MJ, Bass ES. From efficacy to impact: an advocate's agenda for HIV pre-exposure prophylaxis implementation. Am J Prev Med. 2013;44(1 Suppl 2):S167–170. [DOI] [PubMed] [Google Scholar]
- 25.Radloff LS. The CES-D Scale: A Self-Report Depression Scale for Research in the General Population. Applied Psychological Measurement. 1977;1(3):385–401. [Google Scholar]
- 26.Bush K, Kivlahan DR, McDonell MB, Fihn SD, Bradley KA. The AUDIT alcohol consumption questions (AUDIT-C): an effective brief screening test for problem drinking. Ambulatory Care Quality Improvement Project (ACQUIP). Alcohol Use Disorders Identification Test. Arch Intern Med. 1998;158(16):1789–1795. [DOI] [PubMed] [Google Scholar]
- 27.Shrestha R, Karki P, Altice FL, et al. Correlates of willingness to initiate pre-exposure prophylaxis and anticipation of practicing safer drug- and sex-related behaviors among high-risk drug users on methadone treatment. Drug Alcohol Depend. 2017;173:107–116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.The Connecticut Getting to Zero Commission. A comprehensive report on ending the HIV epidemic in Connecticut. 2018. [Google Scholar]
- 29.Connecticut Department of Public Health. CT Pre-exposure Prophylaxis (PrEP) Local Medical Services. https://portal.ct.gov/-/media/Departments-and-Agencies/DPH/AIDS--Chronic-Diseases/Prevention/PrEP_Services.pdf?la=en. Published 2019. Accessed October 20,, 2020. [Google Scholar]
- 30.Gibson BA, Ghosh D, Morano JP, Altice FL. Accessibility and utilization patterns of a mobile medical clinic among vulnerable populations. Health Place. 2014;28:153–166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Shrestha R, Altice F, Karki P, Copenhaver M. Developing an Integrated, Brief Biobehavioral HIV Prevention Intervention for High-Risk Drug Users in Treatment: The Process and Outcome of Formative Research. Front Immunol. 2017;8:561–561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Avert. People who inject drugs-HIV and AIDS. https://www.avert.org/professionals/hiv-social-issues/key-affected-populations/people-inject-drugs#footnote1_t9ojaej. Published 2019. Accessed February 09, 2020. [Google Scholar]
- 33.Shrestha R, Copenhaver M. Exploring the Use of Pre-exposure Prophylaxis (PrEP) for HIV Prevention Among High-Risk People Who Use Drugs in Treatment. Frontiers in public health. 2018;6:195–195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Guise A, Albers ER, Strathdee SA. 'PrEP is not ready for our community, and our community is not ready for PrEP': pre-exposure prophylaxis for HIV for people who inject drugs and limits to the HIV prevention response. Addiction. 2017;112(4):572–578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Centers for Disease Control and Prevention. PrEP. https://www.cdc.gov/hiv/basics/prep.html. Published 2020. Updated June 4, 2020. Accessed July 22, 2020. [Google Scholar]
- 36.Galea JT, Kinsler JJ, Salazar X, et al. Acceptability of pre-exposure prophylaxis as an HIV prevention strategy: barriers and facilitators to pre-exposure prophylaxis uptake among at-risk Peruvian populations. Int J STD AIDS. 2011;22(5):256–262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Mustanski B, Johnson AK, Garofalo R, Ryan D, Birkett M. Perceived likelihood of using HIV pre-exposure prophylaxis medications among young men who have sex with men. AIDS Behav. 2013;17(6):2173–2179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Shrestha R, DiDomizio EE, Kim RS, Altice FL, Wickersham JA, Copenhaver MM. Awareness about and willingness to use long-acting injectable pre-exposure prophylaxis (LAI-PrEP) among people who use drugs. Journal of Substance Abuse Treatment. 2020;117:108058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ngure K, Heffron R, Mugo N, et al. Feasibility and acceptability of HIV self-testing among pre-exposure prophylaxis users in Kenya. J Int AIDS Soc. 2017;20(1):21234–21234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sherman SG, Schneider KE, Park JN, et al. PrEP awareness, eligibility, and interest among people who inject drugs in Baltimore, Maryland. Drug Alcohol Depend. 2019;195:148–155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ortblad KF, Kearney JE, Mugwanya K, et al. HIV-1 self-testing to improve the efficiency of pre-exposure prophylaxis delivery: a randomized trial in Kenya. Trials. 2019;20(1):396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ortblad KF, Chanda MM, Musoke DK, et al. Acceptability of HIV self-testing to support pre-exposure prophylaxis among female sex workers in Uganda and Zambia: results from two randomized controlled trials. BMC Infect Dis. 2018;18(1):503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.World Health Organization. World Health Organization Guidelines on HIV self-testing and partner notification: supplement to consolidated guidelines on HIV testing services. 2016. [PubMed] [Google Scholar]
- 44.Eisingerich AB, Wheelock A, Gomez GB, Garnett GP, Dybul MR, Piot PK. Attitudes and acceptance of oral and parenteral HIV preexposure prophylaxis among potential user groups: a multinational study. PloS one. 2012;7(1):e28238–e28238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Golub SA, Gamarel KE, Rendina HJ, Surace A, Lelutiu-Weinberger CL. From Efficacy to Effectiveness: Facilitators and Barriers to PrEP Acceptability and Motivations for Adherence Among MSM and Transgender Women in New York City. AIDS Patient Care and STDs. 2013;27(4):248–254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wheelock A, Eisingerich AB, Ananworanich J, et al. Are Thai MSM willing to take PrEP for HIV prevention? An analysis of attitudes, preferences and acceptance. PloS one. 2013;8(1):e54288–e54288. [DOI] [PMC free article] [PubMed] [Google Scholar]


