Summary
The mechanism of systolic annular expansion in mitral valve prolapse (MVP) is not clarified. Since annular expansion is systolic outward shift of MV leaflet/chorda tissue complex at superior and outer ends, annular expansion could be related to inward (superior) shift of the complex at another inferior and inner end of the papillary muscle (PM) tip and/or systolic lengthening of the tissue complex, especially MV leaflets.
MV annulus systolic expansion, PMs’ systolic superior shift, and MV leaflets’ systolic lengthening were evaluated by echocardiography with a speckle tracking analysis in 25 normal subjects, 25 subjects with holo-systolic MVP and 20 subjects with late-systolic MVP.
PMs’ superior shift, MV leaflets’ lengthening, MV annular area at the onset of systole and subsequent MV annulus expansion were significantly greater in late-systolic MVP than in holo-systolic MVP (4.6 ± 1.6 versus 1.5 ± 0.7 mm/m2, 2.5 ± 1.4 versus 0.6 ± 2.0 mm/m2, 6.8 ± 2.5 versus 5.7 ± 1.0 cm2/m2 and 1.6 ± 0.8 versus 0.1 ± 0.5 cm2/m2, P < 0.001, respectively). Multivariate analysis identified MV leaflets’ lengthening and PMs’ superior shift as independent factors associated with MV annular expansion.
Conclusions:
These results suggest that systolic MV annular expansion in MVP is related to abnormal MV leaflets’ lengthening and PMs’ superior shift.
Keywords: Valvular heart disease, Sudden death
Systolic augmented mitral valve (MV) annular expansion is a unique echocardiographic finding in patients with global or late-systolic MV prolapse (MVP).1) Although the MV annular area remains approximately constant during systole in patients with segmental or holo-systolic MVP and in normal subjects, the MV annular area considerably expands during systole in those with late-systolic MVP.1) Systolic annular expansion is considered to be of potential and critical importance, despite the lack of proof, with its possible influences to cause myocardial fibrosis in the left ventricular (LV) base2,3) influencing malignant ventricular arrhythmia.4) However, the mechanism of systolic annular expansion in patients with MVP is not yet fully clarified.
Systolic annular expansion in MVP can be expressed as “systolic outward shift of MV leaflet/chorda tissue complex at superior and outer ends” (Figure 1, red arrows). This can be related to 1) systolic inward (superior) shift of MV leaflet/chorda tissue complex at another inferior and inner end of papillary muscle (PM) tips (Figure 1, lower blue arrow) and/or 2) systolic lengthening of the tissue complex. Systolic superior shift of PMs has been confirmed.5,6) Systolic lengthening of MV leaflets has also been observed in patients with late-systolic MVP.1) However, significant or clear tissue elongation of chordae is not expected because of its highly limited tissue distensibility.7) Therefore, we hypothesized that systolic annular expansion in MVP can be related to a systolic superior shift of PM tips (Figure 1, lower blue arrow) and/or systolic lengthening of MV leaflet tissue (Figure 1, upper small black arrows). Consequently, the purpose of this study was to investigate the relation among the systolic expansion of MV annulus, superior shift of the PM tip, systolic lengthening of MV leaflet, and other cardiac structural measurements in patients with late-systolic MVP.
Figure 1.
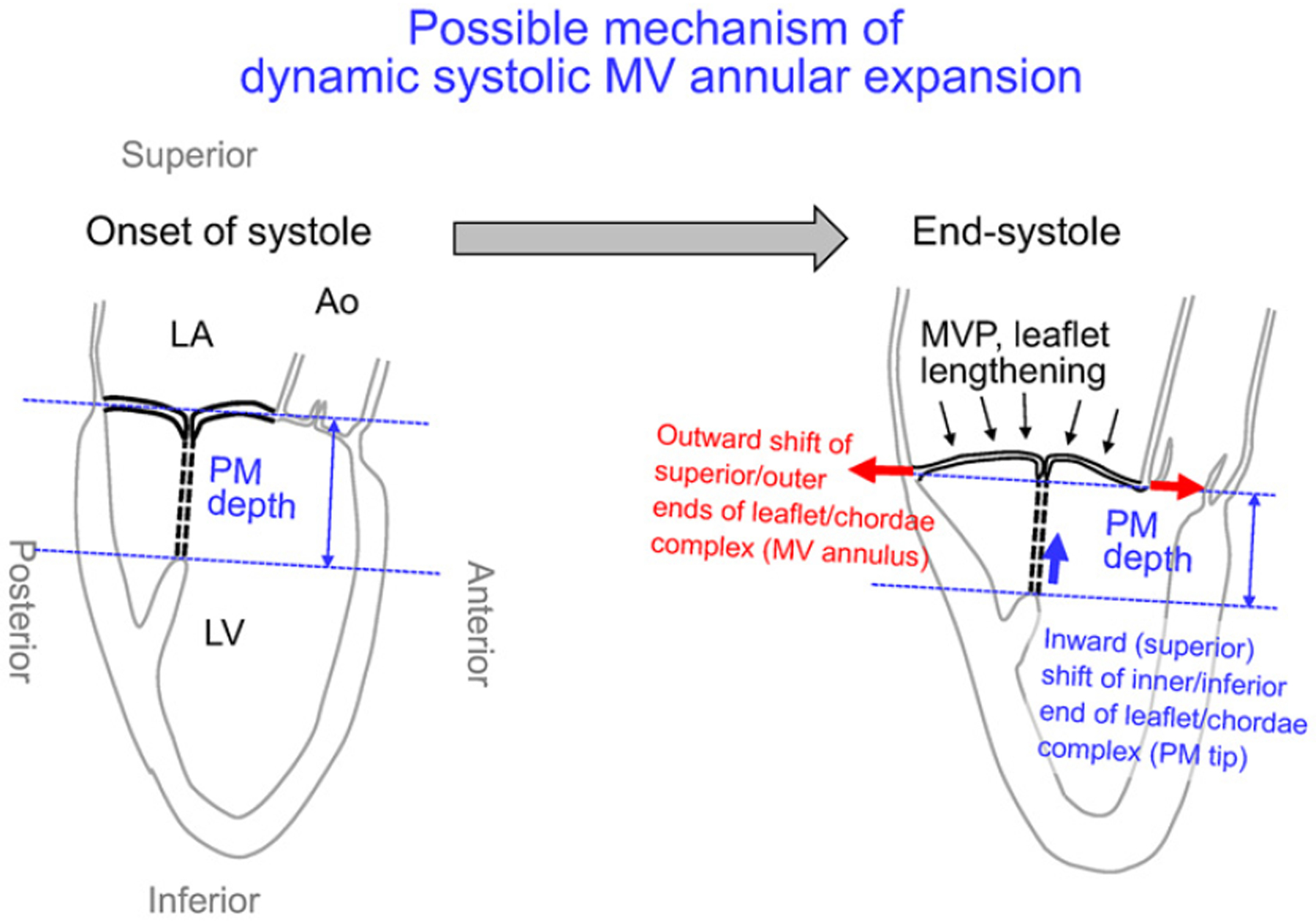
Potential mechanism of systolic mitral valve (MV) annular expansion in patients with late-systolic MV prolapse (MVP) expected from the structural characteristics of the MV complex. Detailed explanation is in the main text. Ao indicates aorta; LA, left atrium; LV, left ventricle; and PM, papillary muscle.
Methods
Study population:
Consecutive 30 patients with holo-systolic MVP and 23 with late-systolic MVP who underwent conventional and three-dimensional transthoracic echocardiographic examination were retrospectively enrolled in this study, and those with only conventional echocardiography without a three-dimensional study were not included. Definition of MVP will be provided later. Age-matched 30 controls consisted of normal volunteers and those who had undergone echocardiography for clinical reasons but were finally judged to have no significant cardiac disease. Among these enrolled subjects, five controls, five patients with holo-systolic MVP, and three with late-systolic MVP were excluded because of inadequate images for the speckle tracking analysis. The remaining 25 controls, 25 patients with holo-systolic MVP, and 20 with late-systolic MVP were study subjects.
The institutional ethics committee approved this study (19–051), and the requirement to obtain informed consent was waived because of the non-invasive and retrospective nature of the study. The information of this study including anonymity, risks, and benefits were provided to the public on the homepage of the institution, and all subjects were given the opportunity to decline their enrollment in this study.
General echocardiographic measurements:
Standard two-dimensional Doppler echocardiography was performed using an iE33 ultrasound system (Philips Healthcare, Andover, MA, USA) equipped with a 2.5 MHz transducer. In apical four- and two-chamber views, LV end-diastolic volume (LVEDV), LV end-systolic volume (LVESV), and left atrial (LA) end-systolic volume were measured by the biplane disk summation method.8) Stroke volume (SV) was measured by LV outflow tract dimensions and velocity profile. Mitral regurgitation (MR) vena contracta width (narrowest jet width by color Doppler echocardiography) was measured in the parasternal or apical view with the best MR jet visualization at the onset of systole and at end-systole.9,10) Onset of systole was defined as the echocardiographic initial frame of systolic MV closure, and end-systole was defined as end-ejection by pulsed Doppler aortic flow. Frame rates were 50–90 (mean ± SD = 67 ± 13)/second. Increase in MR vena contracta width from onset to end-systole was calculated. MR volume was quantified as the difference between LV ejection volume (LVEDV - LVESV) and forward aortic SV.
Measurement of the dynamic MV structure:
Systolic superior shift of MV coaptation toward the LA was measured as follows. In two-dimensional echocardiographic apical views with the best visualization of the MV leaflet and its prolapse, the distance from the MV annulus level to leaflet coaptation (from the orange line to the white broken one in Figure 2A) was measured and defined as “coaptation depth.” MVP was defined as systolic superior displacement (> 2.0 mm) of MV leaflet coaptation into the LA beyond the annular level.11) This measurement was repeated in the onset of systole and in end-systole, and the difference was defined as systolic superior shift of MV coaptation. Systolic superior shift of MV coaptation in normal subjects was 1.4 ± 0.5 mm/m2, and excessive superior shift beyond the normal range (> 2.4 mm/m2 = mean + 2 SD) was defined as late-systolic MVP. Widths of anterior and posterior MV leaflets (AML and PML) were measured in late-diastole. The presence of both thickened MV leaflets beyond normal range (> 2.3 mm/m2) obtained from the data of normal controls in this study and bileaflet prolapse was defined as Barlow type MVP in this study.
Figure 2.
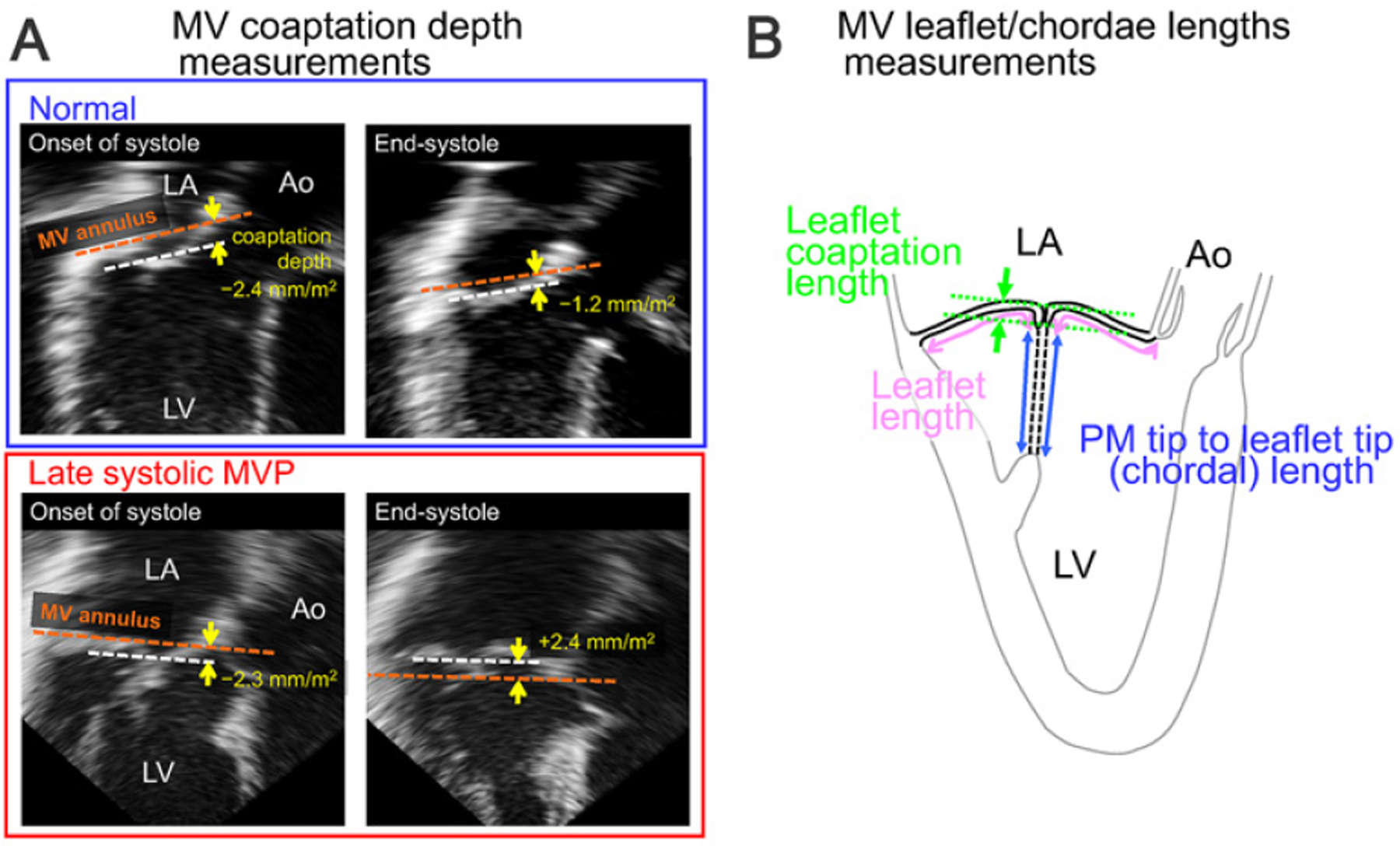
A: Measurement of the systolic superior shift of mitral valve (MV) coaptation. Depths of MV coaptation (the white broken line) in early and late systole were measured as its distance (yellow arrows) from the line connecting the MV annulus (the orange broken line) in apical echocardiographic views with the best visualization of the MV and/or mitral valve prolapse (MVP). In this normal subject (upper), MV coaptation depth slightly increases in systole (−2.4 to −1.2 mm/m2). By contrast, MV coaptation depth considerably increases in this patient with late systolic MV prolapse (MVP) (−2.3 to + 2.4 mm/m2) (lower). B: Measurements of MV leaflet length (pink arrows), papillary muscle (PM) tip to MV leaflet tip length (blue arrows) as an alternative to chordal tissue length, and MV coaptation length (green arrows). Detailed explanation is in the main text. Ao indicates aorta; LA, left atrium; and LV, left ventricle.
The MV annular area was measured by the annular dimension in apical four- and two-chamber views with an elliptical assumption at the onset of systole and end-systole. Systolic annular expansion was defined as the increase in the MV annular area from the onset of systole to end-systole.
Systolic lengthening of MV leaflets and chordae was evaluated by three-dimensional echocardiography-oriented standardized two-dimensional transthoracic echocardiography. From the full volume datasets from an apical approach, two apical log axis views with aorta and anterolateral or posteromedial PM visualization were obtained. In both views, AML and PML lengths along the curvilinear tissue line from its tip to anterior and posterior MV annulus were measured and averaged (Figure 2B, pink arrows). This measurement was repeated in the onset of systole and in end-systole, and systolic lengthening of AML and PML tissue was obtained. Length from PM tip to AML or PML tip was also measured as an alternative to chordal tissue length (Figure 2B, blue arrows). This length was also measured in two apical views and repeated in the onset of systole and in end-systole. MV leaflet coaptation length (Figure 2B, green arrows) was measured in two apical views through averaging and repeated in the onset of systole and in end-systole. Systolic reduction in the coaptation length was calculated.
Systolic superior shift of PM tips was evaluated using the echocardiographic speckle tracking analysis, and details were described previously.6) Briefly, in apical four-and two-chamber views with PM visualization, distances 1) between the PM tip and the fixed external reference point (ERP) around the cardiac apex (Figure 3A, red points) and 2) between the MV annulus and ERP (Figure 3A, blue points) were dynamically monitored using a two-dimensional echocardiographic speckle tracking analysis in a commercially available software (free strain, Philips) (Figure 3A–C). Measurements of medial and lateral PMs were averaged. Details are described in the figure legends.
Figure 3.
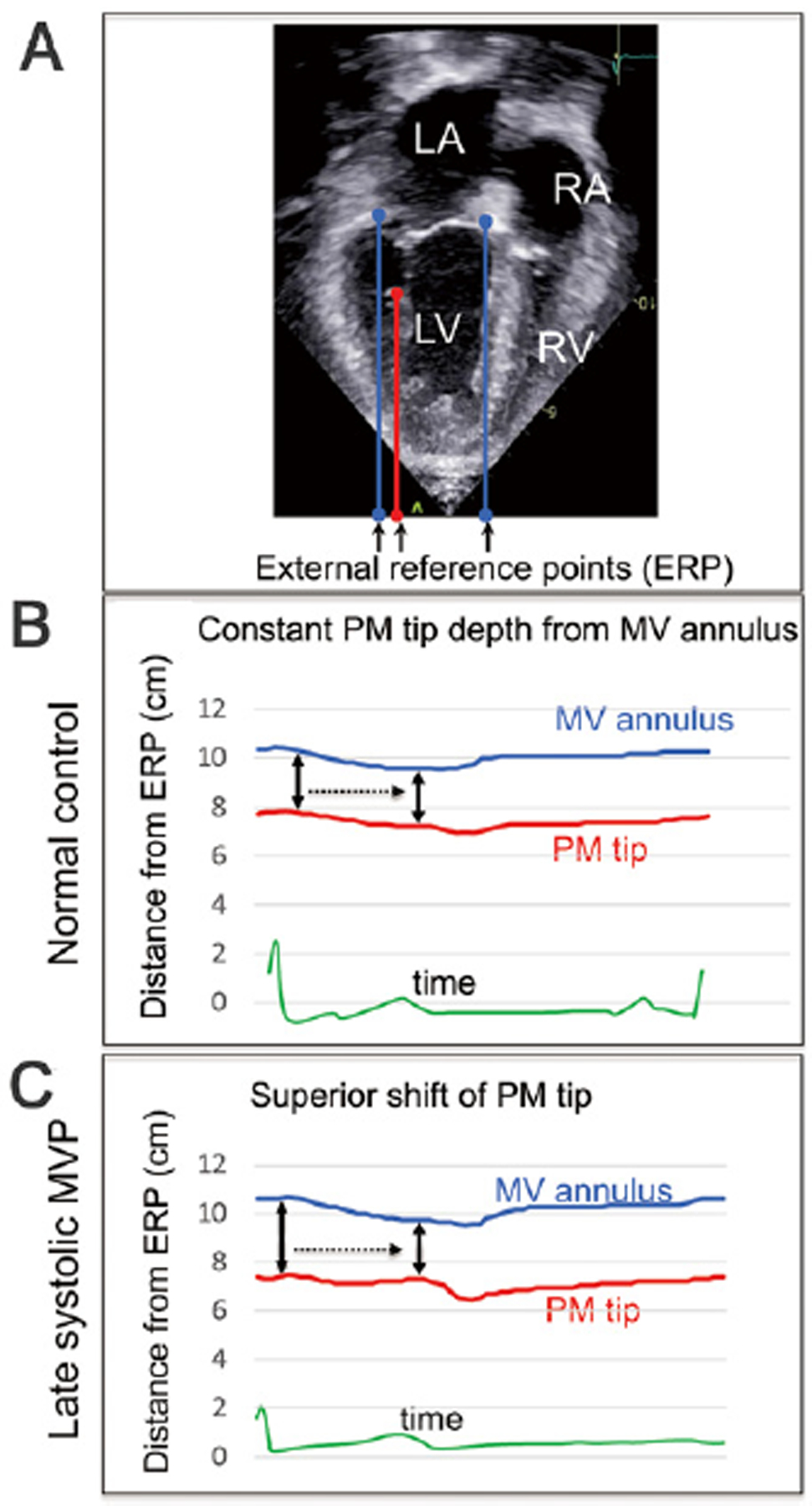
Dynamic evaluation of systolic superior shift of papillary muscles (PMs) tip by any two-point speckle tracking analysis (free strain by Philips) in a normal subject and in a patient with late-systolic mitral valve prolapse (MVP). In apical four- and two-chamber views with PM visualization, distances between 1) the PM tip and fixed external reference point (ERP) around the apex (red points) and 2) the MV annulus and the ERP (blue points) were dynamically tracked and monitored. Of note is that supero-inferior direction in this echocardiographic image is reversed and consistent with the actual direction in the body (A). In a normal subject, the upper blue and lower red lines indicate the distances from the ERP to the MV annulus or PM tip, respectively. Consequently, the distance between the blue and red lines indicates the PM tip depth from the MV annulus. Since the blue and red lines are approximately parallel in systole, the PM tip depth is approximately constant during whole systole in this normal subject (black arrows) (B). A patient with late-systolic MVP, compared to panel B of a normal subject: The PM tip depth from the MV annulus is considerably reduced from the onset of systole toward end-systole (black arrows), demonstrating an abnormal systolic superior shift of the PM tip (C).
Statistics:
Measurements of properties such as lengths, areas and volumes were normalized by body surface area for statistical analysis. Continuous variables were compared among three groups using ANOVA. When statistical significance was detected by ANOVA, the Wilcoxon test was used to test the difference in continuous variables between two groups. In patients with late-systolic MVP, the univariate Spearman correlation analysis was performed to investigate the relationship between the systolic expansion of MV annulus and other echocardiographic measurements, including superior shift of the PM tip toward the LA, systolic lengthening of MV leaflets, the MV annular area at onset of systole, LVEDV, and EF. A multivariate analysis was further performed for variables with significant correlations as determined by the univariate analysis. Because the superior shift of PMs and the superior shift of MV coaptation showed an extremely strong correlation (these two variables are theoretically identical but measured using different methods, given the constant chordal length during systole), superior shift of MV coaptation was not included in the analysis to avoid errors from col-linearity.
Results
Profiles of patients:
The profiles of patients are summarized in Table I. No patient with MVP had the diagnosis of specific connective tissue disease such as Marfan syndrome and Loeys-Dietz syndrome. In the 20 patients with late-systolic MVP, 17 had Barlow type MVP, and two had thickened and large single anterior or posterior leaflet prolapse, and one had bileaflet prolapse without leaflet thickening. The LVEDV, LVESV, and EF were not significantly different between patients with late-systolic MVP and those with holo-systolic MVP. MR vena contracta width at the onset of systole was significantly reduced in patients with late-systolic MVP compared to those with holo-systolic MVP, but it was not different at end-systole between the two groups, indicating pronounced increase in the width during systole in late-systolic MVP. Superior shift of MV coaptation during systole was significantly increased in patients with late-systolic MVP compared to those with holo-systolic MVP by definition. MV annular area at the onset of systole tended to be larger and MR volume was significantly less in late-systolic MVP, compared to holo-systolic MVP, reconfirming structurally large MV annulus beyond MR severity in this disease.1) In addition, the increase in the MV annulus area during systole (systolic MV annular expansion) was significantly greater in patients with late-systolic MVP compared to that in patients with holo-systolic MVP (P < 0.01), demonstrating the presence of abnormal systolic MV annular expansion in this group. Superior shift of the PM tip relative to the MV annulus was also significantly increased in late-systolic MVP (P < 0.01). Since the systolic inferior shift of the MV annulus relative to fixed ERP around the apex was not different between the two groups, these indicate an augmented superior shift of PM rather than an augmented inferior shift of MV annulus in patients with late-systolic MVP. AML and PML lengths, and the sum at the onset of systole tended to be longer in late-systolic MVP compared to holo-systolic MVP but without statistical significance. Both AML and PML lengths significantly lengthened at end-systole in patients with late-systolic MVP (P < 0.01), but these lengths only slightly increased in holo-systolic MVP. The MV coaptation length at the onset of systole was significantly longer in patients with late-systolic MVP; however, this length at end-systole was not different between the two groups. Lengths from the PM tip to the MV leaflet tip at the onset of systole were not different between the two groups and remained constant at end-systole in both groups.
Table I.
Clinical Characteristics and Basic Echocardiographic Measurements
| Control (n = 25) | Holosystolic MVP (n = 25) | Late systolic MVP (n = 20) | P (ANOVA or chi-square test) | |
|---|---|---|---|---|
| Age (years) | 58 ± 9 | 65 ± 9 | 61 ± 10 | 0.07 |
| Height (cm) | 163 ± 9 | 165 ± 11 | 167 ± 9 | 0.22 |
| Weight (kg) | 60 ± 11 | 61 ± 12 | 58 ± 11 | 0.61 |
| BSA (m2) | 1.6 ± 0.2 | 1.7 ± 0.2 | 1.7 ± 0.2 | 0.80 |
| Barlow type MVP | 0 / 25 | 0 / 25 | 17 / 20*† | < 0.001 |
| Gender (male/female) | 17 / 8 | 14 / 11 | 12 / 8 | 0.42 |
| Heart Rate (bpm) | 65 ± 9 | 71 ± 13 | 64 ± 12 | 0.11 |
| Rhythm (Sinus/AF) | 25 / 0 | 25 / 0 | 19 / 1 | 0.36 |
| Systolic BP (mmHg) | 127 ± 17 | 139 ± 21 | 136 ± 18 | 0.11 |
| Diastolic BP (mmHg) | 74 ± 13 | 78 ± 13 | 77 ± 13 | 0.58 |
| LVEDV (mL/m2) | 62 ± 10 | 103 ± 25† | 97 ± 30† | < 0.001 |
| LVESV (mL/m2) | 23 ± 5 | 43 ± 11† | 42 ± 14† | < 0.001 |
| EF (%) | 63 ± 6 | 58 ± 4† | 57 ± 5† | < 0.001 |
| SV (ml/m2) | 40 ± 7 | 37 ± 5 | 39 ± 6 | 0.32 |
| LA volume (mL/m2) | 24 ± 8 | 60 ± 28† | 49 ± 16*† | < 0.001 |
| VC width (OS) (mm/m2) | 0.3 ± 0.5 | 3.8 ± 1.5† | 0.9 ± 0.9* | < 0.001 |
| VC width (ES) (mm/m2) | 0.2 ± 0.4 | 3.9 ± 1.3† | 3.2 ± 1.1† | < 0.001 |
| ΔVC width (mm/m2) | −0.1 ± 0.5 | 0.2 ± 0.6 | 2.4 ± 0.9†* | < 0.001 |
| MR volume (mL/m2) | 1 ± 1 | 23 ± 7† | 16 ± 6*† | < 0.001 |
| MV superior shift (mm/m2) | 1.4 ± 0.5 | 1.4 ± 0.6 | 4.6 ± 1.5†* | < 0.001 |
| MV annulus area (OS) (cm2/m2) | 4.5 ± 0.9 | 5.7 ± 1.0† | 6.8 ± 2.5† | < 0.001 |
| MV annulus area (ES) (cm2/m2) | 4.3 ± 0.8 | 5.7 ± 0.9† | 8.4 ± 3.1†* | < 0.001 |
| ΔMV annulus area (cm2/m2) | −0.2 ± 0.3 | 0.1 ± 0.5 | 1.6 ± 0.8†* | < 0.001 |
| Superior PM shift (mm/m2) | 1.3 ± 0.4 | 1.5 ± 0.7 | 4.6 ± 1.6†* | < 0.001 |
| MV annulus inferior shift (mm/m2) | 6.1 ± 1.0 | 6.7 ± 1.1 | 6.9 ± 1.3 | 0.10 |
| MV leaflet length (OS) (mm/m2) | ||||
| AML + PML | 19.9 ± 2.8 | 23.7 ± 4.6† | 27.2 ± 5.2† | < 0.001 |
| AML | 12.8 ± 2.7 | 12.9 ± 4.7 | 14.2 ± 4.8 | 0.56 |
| PML | 7.1 ± 1.6 | 10.8 ± 2.5† | 12.9 ± 3.9† | < 0.001 |
| MV leaflet length (ES) (mm/m2) | ||||
| AML + PML | 19.6 ± 2.8 | 24.3 ± 4.4† | 29.6 ± 5.1†* | < 0.001 |
| AML | 12.4 ± 2.4 | 13.3 ± 4.5 | 15.5 ± 4.7 | 0.07 |
| PML | 7.2 ± 1.7 | 11.1 ± 2.7† | 14.2 ± 3.7†* | < 0.001 |
| ΔAML + PML length (mm/m2) | −0.3 ± 1.2 | 0.6 ± 2.0† | 2.5 ± 1.4†* | < 0.001 |
| MV coaptation (OS) (mm/m2) | 2.1 ± 0.5 | 1.0 ± 1.2† | 4.0 ± 1.2†* | < 0.001 |
| MV coaptation (ES) (mm/m2) | 1.6 ± 0.6 | 1.0 ± 1.1† | 1.4 ± 1.0 | 0.05 |
| ΔMV coaptation (mm/m2) | −0.5 ± 0.4 | −0.1 ± 0.6 | −2.6 ± 0.9†* | < 0.001 |
| PM tip to MV tip length (OS) (mm/m2) | 12.6 ± 2.1 | 14.0 ± 2.6 | 14.4 ± 2.7 | 0.07 |
| PM tip to MV tip length (ES) (mm/m2) | 12.6 ± 2.1 | 13.9 ± 2.5 | 14.6 ± 2.6 | 0.05 |
| ΔPM tip to MV tip length (mm/m2) | 0.1 ± 0.5 | −0.1 ± 0.4 | 0.2 ± 0.3 | 0.08 |
Values are the mean ± SD. AF indicates atrial fibrillation; AML, anterior mitral leaflet; BP, blood pressure; bpm, beat per minute; EF, ejection fraction; ES, end systole; LA, left atrium; LVEDV, left ventricular end-diastolic volume; LVESV, left ventricular end-systolic volume; MR, mitral regurgitation; MV, mitral valve; MVP, mitral valve prolapse; OS, onset of systole; PM, papillary muscle; PML, posterior mitral leaflet; SV, stroke volume; and VC, vena contracta.
P < 0.05 versus holo-systolic MVP and
P < 0.05 versus control.
Factors associated with dynamic MV abnormalities:
Table II shows factors associated with systolic expansion of the MV annulus. Using univariate analysis, systolic lengthening of MV leaflets, PM superior shift, the structurally large MV annular area at the onset of systole and younger age were associated with systolic expansion of the MV annulus. The multivariate analysis showed systolic lengthening of MV leaflets and superior PM shift as independent factors associated with systolic MV annular expansion. Of note is that the MR volume itself was not related to systolic MV annulus expansion. Figure 4 shows these relations through scatter graphs. Further multivariate analysis identified a structurally large MV annulus at the onset of systole and PM superior shift as independent factors of systolic lengthening of MV leaflets.
Table II.
Factors Associated with Dynamic Changes of MV
| Univariate | Multivariate | |||
|---|---|---|---|---|
| r | P value | Standardized β | P value | |
| Systolic expansion of MV annulus | ||||
| Age | −0.528 | 0.01 | −0.113 | 0.17 |
| LVEDV | 0.326 | 0.08 | ||
| EF | −0.209 | 0.19 | ||
| LA volume | 0.196 | 0.20 | ||
| MR volume | −0.258 | 0.09 | ||
| PM superior shift | 0.840 | < 0.001 | 0.456 | 0.01 |
| MV leaflet systolic lengthening | 0.852 | < 0.001 | 0.506 | < 0.001 |
| MV annular area (OS) | 0.725 | < 0.001 | 0.011 | 0.15 |
| MV leaflet systolic lengthening | ||||
| Age | −0.496 | 0.01 | −0.166 | 0.30 |
| LVEDV | 0.372 | 0.05 | ||
| EF | −0.207 | 0.19 | ||
| LA volume | 0.213 | 0.18 | ||
| MR volume | −0.253 | 0.09 | ||
| PM superior shift | 0.759 | < 0.001 | 0.426 | 0.04 |
| MV annular area (OS) | 0.767 | < 0.001 | 0.456 | 0.02 |
EF indicats ejection fraction; LA, left atrium; LVEDV, left ventricular end-diastolic volume; MV, mitral valve; OS, onset of systole and PM, papillary muscle. All parameters related with physical size normalized by body surface area.
Figure 4.
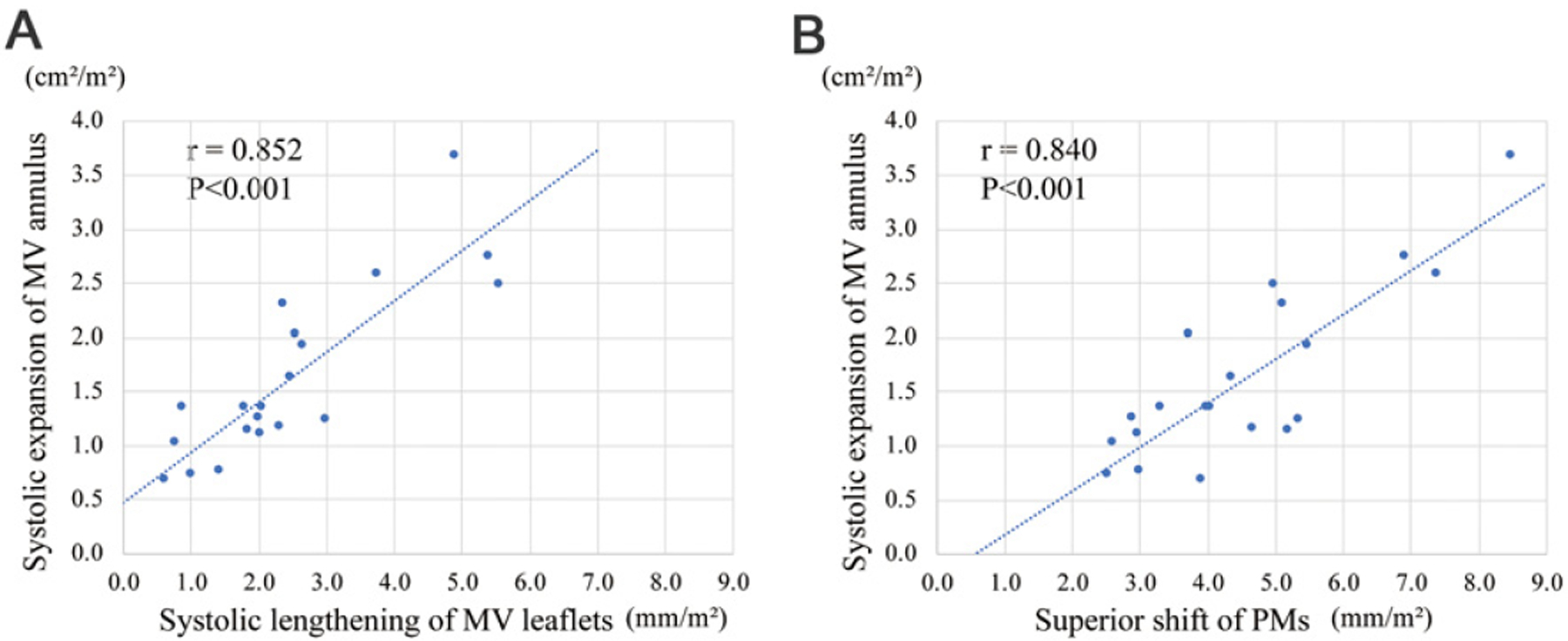
Relations between systolic expansion of mitral valve (MV) annulus and 1) systolic lengthening of MV leaflets (A) and 2) systolic superior shift of papillary muscles (PMs) (B). MV annulus expansion is related to both MV leaflet lengthening and PM superior shift.
Discussion
This study has demonstrated that systolic lengthening of MV leaflets and systolic superior shift of PMs are closely associated with systolic expansion of the MV annulus in patients with MVP. Systolic lengthening of MV leaflets was further related to dilated MV annulus at the onset of systole. These associations are consistent with the study hypothesis. This can be interpreted as follows. Possibility #1 is that systolic lengthening of MV leaflets, superior shift of PMs and structurally large MV annulus cause abnormal expansion of MV annulus. A previous investigation on late-systolic MVP has demonstrated that a systolic LV-LA pressure gradient on larger MV leaflets/annulus can cause greater MV superiorly pushing force (Figure 5), shifting MV leaflets superiorly, which secondarily tract PMs to shift superiorly.6) Secondary PM traction has been confirmed by the disappearance of PM superior shift following surgical MV annuloplasty to reduce the MV annular area and MV superiorly pushing force.6) Such abnormal pathophysiology does not develop in patients with holo-systolic MVP and only modest MV annular dilatation. Results of the present study along with those of the previous studies suggest that augmented leaflet tissue distensibility, in addition to structurally large MV annulus, suggesting primary tissue fragility and leaflet tissue elongation, may further cause abnormal superior shift of PMs and contribute to systolic MV annular expansion in patients with MVP. This leaflet tissue elongation (structurally large MV annulus) and augmented distensibility may be the central and primary pathophysiology of late-systolic MVP, and prolapse itself can be a secondary consequence of this disease, which requires further studies. Possibility #2 is that primary systolic expansion of the MV annulus elongates MV leaflet tissue and tracts PMs to shift superiorly. Because MV annulus tissue lacks muscular cells to develop active motions, its motion is passive and is influenced by forces from LV pressure, LV wall, LA wall and MV leaflets. The mechanism and probability of primary MV annular expansion is not clear, but this possibility is not denied by the present study. Other interpretations may also be possible. Notwithstanding the lack of causal proof, this study in patients with MVP has demonstrated a novel finding of the close association of augmented systolic expansion of MV annulus versus systolic lengthening of MV leaflets and structurally large MV annulus beyond MR severity, suggesting primarily affected leaflet tissue in this disease, and possibly a secondary superior shift of PMs.
Figure 5.
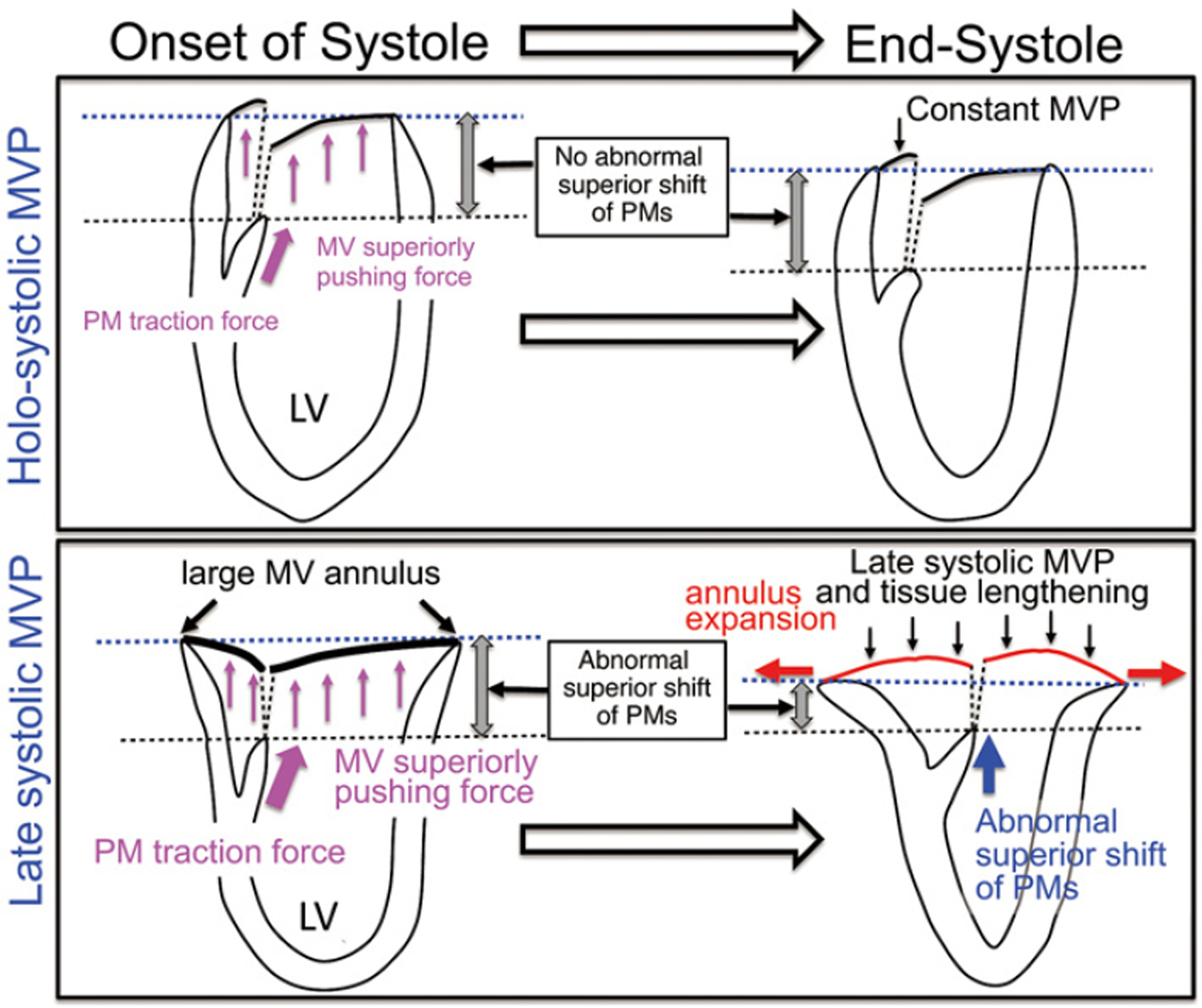
Suggested mechanism of systolic expansion of mitral valve (MV) annulus from this study. The lower panel is comparison with the upper panel: It is suggested that a structurally large MV annulus at the onset of systole causes greater MV superiorly pushing force (pink arrows) by the systolic left ventricle (LV) to the left atrium pressure gradient, shifting MV leaflets superiorly toward end-systole (small black arrows), which tracts subvalvular papillary muscles (PMs) to shift superiorly (blue arrow). The PM superior shift along with systolic lengthening of MV leaflets may in turn contribute to augmented systolic expansion of the MV annulus (red arrows) in patients with late-systolic MV prolapse (MVP). Causal relations are not yet proved, and further studies are thus required.
Relation with Previous Studies:
Clavel, et al. have observed systolic lengthening of MV leaflets, especially in patients with late-systolic MVP,1) whereas such abnormalities were not clear in those with holo-systolic MVP and normal subjects. He, et al. performed ex vivo experiments on the relation among MV annulus size, PM position, and forces acting on the MV annulus. They reported that force to dilate MV annulus increases with a larger MV annulus and slack PM position (superiorly located PM).12) Topilsky, et al. performed an echocardiographic analysis and found that patients with late-systolic MVP have larger MV annulus at the onset of systole and its greater expansion during subsequent systole compared to those with holo-systolic MVP.13) Sanfilippo, et al. found an abnormal superior shift of PMs during systole in patients with late-systolic MVP, whereas such abnormalities were not observed in normal subjects.5) The present study is consistent with these preceding studies and further demonstrated the close relation among systolic MV annular expansion versus systolic MV leaflets lengthening, an abnormal systolic superior shift of PMs and structurally large MV annulus at the onset of systole.
MV and LV develop mutual interactions. Primary LV dilatation causes MV leaflet tethering and secondary MR.14) Primary MR can cause secondary LV dilatation and dysfunction, which further causes secondary MV tethering.15) A large MV annulus with regional dilatation of the LV base leads to regionally reduced contraction in the LV base.16) A large MV annulus causes systolic superior shift of PMs and reduction in PM contraction.6) This study has demonstrated a novel MV-LV interaction of the close association of MV annulus systolic expansion, systolic lengthening of MV leaflets, structurally large MV annulus, and abnormal systolic superior shift of PMs.
Clinical Implications:
Systolic expansion of the MV annulus is considered important as a potential factor in the pathogenesis of myocardial fibrosis in the LV base to cause malignant ventricular arrhythmia.2,4) Systolic lengthening of MV leaflets and structurally large MV annulus beyond MR severity, constituting characteristics of late-systolic MVP, seem to cause PM superior shift and contribute to the MV annular expansion. Therefore, interventions to address abnormal leaflet tissue distensibility and a structurally large MV annulus are required. For this direction, basic and molecular investigations are on their way.17) In addition, potential and practical therapeutic interventions to address this fundamental pathology seems to be surgical or transcatheter MV annuloplasty. Beneficial effects of early surgery for MVP have been reported.18) In addition to eliminating MR, the beneficial effects may partially derive from stabilization of MV annular expansion and dynamic superior shift of PMs. Beneficial effects of surgical MV plasty for MVP on associated ventricular tachycardia have been reported.19) Current study may promote early surgery, especially in patients with systolic MV annular expansion and severe MR. Beneficial effects of transcatheter MV annuloplasty on MR have been reported.20) The present study may suggest further beneficial influences of transcatheter annuloplasty on the MV annulus and PMs’ dynamic instabilities.
When MR is eliminated by surgical MV leaflet repair, it is controversial whether ring implantation is still necessary or not.21) The present study may suggest the need of annuloplasty even in such cases with controlled MR to stabilize MV annulus and PMs’ dynamic abnormalities. Although MitraClip has important beneficial effects on MR,22) this study suggests that MitraClip without direct effects on MV annulus stabilization may not be ideal for patients with late-systolic MVP and systolic annular expansion.
Limitations:
Lack of proof for the causal relation among systolic superior shift of PMs, systolic lengthening of MV leaflets, structurally large MV annulus at the onset of systole and systolic MV annular expansion is the main limitation of this study. Therefore, the present study may warrant redoing Gornick’s experiment.23) Gornick, et al. mechanically tracted PMs superiorly during systole and introduced ventricular arrhythmia in intact canine hearts. This is still an innovative and important animal model of ventricular arrhythmia in MVP. If MV annulus systolic expansion is introduced by mechanical PM traction toward MV or LA, it will at least partially prove that superior PM shift in systole contributes to MV annulus systolic expansion.
Abnormal myocardial fibrosis develops, especially in LV base and PMs, in patients with MVP.2,3) The present study suggests the possibility of augmented leaflet tissue distensibility, and a structurally dilated MV annulus may promote systolic dynamic MV annular expansion with a PM superior shift. However, relation between these PMs and MV annulus dynamic instabilities and myocardial fibrosis was not investigated. In general patients with MVP, those with bileaflet prolapse and of a younger age and female gender are at high risk of sudden death.24) Identification of additional echocardiographic characteristics with higher risk will benefit patients. Clinical studies to compare dynamic MV annulus systolic expansion and PMs’ superior shift versus LV/PM myocardial fibrosis and ventricular arrhythmia are required. The number of studied subjects is small, and studies with larger sample sizes are required. Consecutive patients with MVP and conventional and three-dimensional echocardiographic examination were enrolled; however, those with only conventional echocardiography were not included with a potential selection bias. Although all systolic annular expansion, leaflet lengthening and PMs’ superior shift are three-dimensional phenomena, measurements in this study were two-dimensional.
Conclusions
Systolic MV annular expansion in MVP is related to systolic abnormal lengthening of MV leaflets, a systolic superior shift of PMs and a structurally large MV annulus. These may promote further studies to investigate 1) causal relations among MV annulus expansion, PM traction, leaflet distensibility, and a structurally large MV annulus; 2) relations between MV annulus and PMs dynamic instabilities versus myocardial fibrosis and ventricular arrhythmia; and 3) beneficial influences of surgical or transcatheter MV annuloplasty to stabilize dynamic MV annulus and PMs’ abnormalities in patients with MVP.
Acknowledgments
This study was supported by Grants-in-Aid for Scientific Research from the Japan Society of the Promotion of Science (17K09538, 19K20721, 19K18199, 19K11405, and 19K17541 for Y.O., M.I., S.H., M.A., and T.O, respectively).
Footnotes
Conflicts of interest: The authors report no relationships that could be construed as a conflict of interest.
References
- 1.Clavel MA, Mantovani F, Malouf J, et al. Dynamic phenotypes of degenerative myxomatous mitral valve disease: quantitative 3-dimensional echocardiographic study. Circ Cardiovasc Imaging 2015; 8: e002989. [DOI] [PubMed] [Google Scholar]
- 2.Basso C, Perazzolo Marra M, Rizzo S, et al. Arrhythmic mitral valve prolapse and sudden cardiac death. Circulation 2015; 132: 556–66. [DOI] [PubMed] [Google Scholar]
- 3.Han Y, Peters DC, Salton CJ, et al. Cardiovascular magnetic resonance characterization of mitral valve prolapse. JACC Cardiovasc Imaging 2008; 1: 294–303. [DOI] [PubMed] [Google Scholar]
- 4.Chesler E, King RA, Edwards JE. The myxomatous mitral valve and sudden death. Circulation 1983; 67: 632–9. [DOI] [PubMed] [Google Scholar]
- 5.Sanfilippo AJ, Harrigan P, Popovic AD, Weyman AE, Levine RA. Papillary muscle traction in mitral valve prolapse: quantitation by two-dimensional echocardiography. J Am Coll Cardiol 1992; 19: 564–71. [DOI] [PubMed] [Google Scholar]
- 6.Hei S, Iwataki M, Jang JY, et al. Possible mechanism of late-systolic mitral valve prolapse: systolic superior shift of leaflets secondary to annular dilatation that causes papillary muscle traction. Am J Physiol Heart Circ Physiol 2019; 316: H629–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Clark RE. Stress-strain characteristics of fresh and frozen human aortic and mitral leaflets and chordae tendineae. Implications for clinical use. J Thorac Cardiovasc Surg 1973; 66: 202–8. [PubMed] [Google Scholar]
- 8.Shi J, Xing Y, Qian J, et al. Early assessment of left ventricular function by layer-specific strain and its relationship to pulsatile arterial load in patients with coronary slow flow. Int Heart J 2019; 60: 586–92. [DOI] [PubMed] [Google Scholar]
- 9.Noack T, Kiefer P, Mallon L, et al. Changes in dynamic mitral valve geometry during percutaneous edge-edge mitral valve repair with the MitraClip system. J Echocardiogr 2019; 17: 84–94. [DOI] [PubMed] [Google Scholar]
- 10.Ji Q, Zhao Y, Shen JQ, et al. Risk factors for moderate or more residual regurgitation in patients with moderate chronic ischemic mitral regurgitation undergoing surgical revascularization alone. Int Heart J 2019; 60: 1268–75. [DOI] [PubMed] [Google Scholar]
- 11.Levine RA, Handschumacher MD, Sanfilippo AJ, et al. Three-dimensional echocardiographic reconstruction of the mitral valve, with implications for the diagnosis of mitral valve prolapse. Circulation 1989; 80: 589–98. [DOI] [PubMed] [Google Scholar]
- 12.He Z, Bhattacharya S. Papillary muscle and annulus size effect on anterior and posterior annulus tension of the mitral valve: an insight into annulus dilatation. J Biomech 2008; 41: 2524–32. [DOI] [PubMed] [Google Scholar]
- 13.Topilsky Y, Michelena H, Bichara V, Maalouf J, Mahoney DW, Enriquez-Sarano M. Mitral valve prolapse with mid-late systolic mitral regurgitation: pitfalls of evaluation and clinical outcome compared with holosystolic regurgitation. Circulation 2012; 125: 1643–51. [DOI] [PubMed] [Google Scholar]
- 14.Otsuji Y, Handschumacher MD, Schwammenthal E, et al. Insights from three-dimensional echocardiography into the mechanism of functional mitral regurgitation: direct in vivo demonstration of altered leaflet tethering geometry. Circulation 1997; 96: 1999–2008. [DOI] [PubMed] [Google Scholar]
- 15.Otani K, Takeuchi M, Kaku K, et al. Evidence of a vicious cycle in mitral regurgitation with prolapse: secondary tethering attributed to primary prolapse demonstrated by three-dimensional echocardiography exacerbates regurgitation. Circulation 2012; 126(Suppl 1): S214–21. [DOI] [PubMed] [Google Scholar]
- 16.Fukuda S, Song JK, Mahara K, et al. Basal left ventricular dilatation and reduced contraction in patients with mitral valve prolapse can be secondary to annular dilatation: preoperative and postoperative speckle-tracking echocardiographic study on left ventricle and mitral valve annulus interaction. Circ Cardiovasc Imaging 2016; 9: e005113. [DOI] [PubMed] [Google Scholar]
- 17.Levine RA, Hagége AA, Judge DP, et al. Mitral valve disease: morphology and mechanisms. Nat Rev Cardiol 2015; 12: 689–710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ling LH, Enriquez-Sarano M, Seward JB, et al. Early surgery in patients with mitral regurgitation due to flail leaflets: a longterm outcome study. Circulation 1997; 96: 1819–25. [DOI] [PubMed] [Google Scholar]
- 19.Vaidya VR, DeSimone CV, Damle N, et al. Reduction in malignant ventricular arrhythmia and appropriate shocks following surgical correction of bileaflet mitral valve prolapse. J Interv Card Electrophysiol 2016; 46: 137–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Daimon M, Shiota T, Gillinov AM, et al. Percutaneous mitral valve repair for chronic ischemic mitral regurgitation: a real-time three-dimensional echocardiographic study in an ovine model. Circulation 2005; 111: 2183–9. [DOI] [PubMed] [Google Scholar]
- 21.De Bonis M, Lapenna E, Maisano F, et al. Long-term results (≤ 18 years) of the edge-to-edge mitral valve repair without annuloplasty in degenerative mitral regurgitation: implications for the percutaneous approach. Circulation 2014; 130(Suppl 1): S19–24. [DOI] [PubMed] [Google Scholar]
- 22.Feldman T, Kar S, Elmariah S, et al. Randomized comparison of percutaneous repair and surgery for mitral regurgitation: 5-year results of EVEREST II. J Am Coll Cardiol 2015; 66: 2844–54. [DOI] [PubMed] [Google Scholar]
- 23.Gornick CC, Tobler HG, Pritzker MC, Tuna IC, Almquist A, Benditt DG. Electrophysiologic effects of papillary muscle traction in the intact heart. Circulation 1986; 73: 1013–21. [DOI] [PubMed] [Google Scholar]
- 24.Sriram CS, Syed FF, Ferguson ME, et al. Malignant bileaflet mitral valve prolapse syndrome in patients with otherwise idiopathic out-of-hospital cardiac arrest. J Am Coll Cardiol 2013; 62: 222–30. [DOI] [PubMed] [Google Scholar]


