Abstract
Background:
The proportion of patients with kidney failure at time of liver transplantation is at an historic high in the United States. The optimal timing of kidney transplantation with respect to the liver transplant is unknown.
Methods:
We used a modified cost-effectiveness analysis to compare four strategies: the old system (“pre-OPTN”), the new Organ Procurement Transplant Network (OPTN) system since August 10, 2017 (“OPTN”), and two strategies which restrict simultaneous liver-kidney transplants (“safety net” and “stringent”). We measured “cost” by deployment of deceased donor kidneys (DDKs) to liver transplant recipients and effectiveness by life years (LYs) and quality-adjusted life years (QALYs) in liver transplant recipients. We validated our model against Scientific Registry for Transplant Recipients data.
Results:
The OPTN, safety net and stringent strategies were on the efficient frontier. By rank order, OPTN > safety net > stringent strategy in terms of LY, QALY and DDK deployment. The pre-OPTN system was dominated, or outperformed, by all alternative strategies. The incremental LY per DDK between the strategies ranged from 1.30 to 1.85. The incremental QALY per DDK ranged from 1.11 to 2.03.
Conclusion:
These estimates quantify the “organ”-effectiveness of various kidney allocation strategies for liver transplant candidates. The OPTN system will likely deliver better liver transplant outcomes at the expense of more frequent deployment of DDKs to liver transplant recipients.
Introduction
In 2002, the United States (US) Organ Procurement and Transplant Network (OPTN) adopted the Model for End-stage Liver Disease (MELD) score for assigning priority in liver transplantation1. The presence of kidney failure increases the MELD score and thus liver transplant priority. Due to broader acceptance criteria for liver transplant candidates and universal application of the MELD score, the proportion of patients with kidney failure at time of liver transplant has risen, leading to a surge in simultaneous liver-kidney (SLK) transplants. OPTN data show that 531, or 5%, of all deceased donor kidneys (DDKs) transplanted in 2014 were allocated as SLK. Of these kidneys, approximately half were allocated to recipients with conventional indications for kidney transplantation, i.e. metabolic disease (e.g. primary hyperoxaluria) or prolonged dialysis-dependence (Table 1). However, the rest were allocated without meeting any formal criteria2. Given the ongoing scarcity of organs, every organ allocated to one patient is necessarily withheld from another3. The practice of SLK transplantation has come under considerable scrutiny in recent years and was reformed on August 10, 2017 by the OPTN.
Table 1.
Indications for first-time simultaneous liver-kidney transplantation (SLK) in adults, March 1 2002 – December 31 2013.
| Indication | Number |
|---|---|
| Dialysis duration ≥6 weeks | 1640 (44%) |
| Metabolic disorder, e.g. primary hyperoxaluria | 96 (3%) |
| Triple-organ transplant (heart-liver-kidney or liver-intestine-kidney) | 10 (<1%) |
| Other | 1943 (53%) |
| Meeting OPTN AKI criteria for SLK | 433 (11%) |
| Meeting OPTN CKD criteria for SLK | 267 (7%) |
| Not meeting OPTN criteria for SLK | 351 (10%) |
| Unknown if meeting OPTN criteria for SLK | 892 (24%) |
| Total | 3689 |
OPTN = Organ Procurement and Transplant Network. AKI = acute kidney injury. CKD = chronic kidney disease.
For those liver transplant candidates without a conventional indication for kidney transplantation, four strategies for liver-kidney transplantation are possible (Figure 1a). In the old US system (“pre-OPTN”), they undergo SLK or liver transplantation alone (LTA) at the transplant center’s discretion. Common indications include varying dialysis duration, varying degrees of chronic kidney disease, and varying duration of acute kidney injury, although regional differences abound4. Since August 10, 2017, the OPTN has implemented a new system (“OPTN”), by which5:
Patients will be accepted for SLK transplantation only by meeting certain medical eligibility criteria based on the duration and extent of acute and chronic kidney disease. In theory, these criteria identify patients at a high risk of irreversible kidney failure after LTA.
An express kidney transplant waitlist (“safety net”) will be in place: any LTA recipient whose kidneys fail within one year of transplant will receive priority on the kidney transplant waitlist.
These eligibility criteria were arrived at via consensus, and many patients who met them have received LTA with acceptable outcomes6. These criteria are to be interpreted as minimal acceptability criteria, rather than designating hard indications for SLK. Two scenarios are thus possible under the new system: 1) only patients who absolutely need SLK are listed for SLK and the remainder receive LTA (“low-utilization scenario”); 2) all patients who meet medical eligibility criteria for SLK are listed for SLK (“high-utilization scenario”) by their transplant centers.
Figure 1.
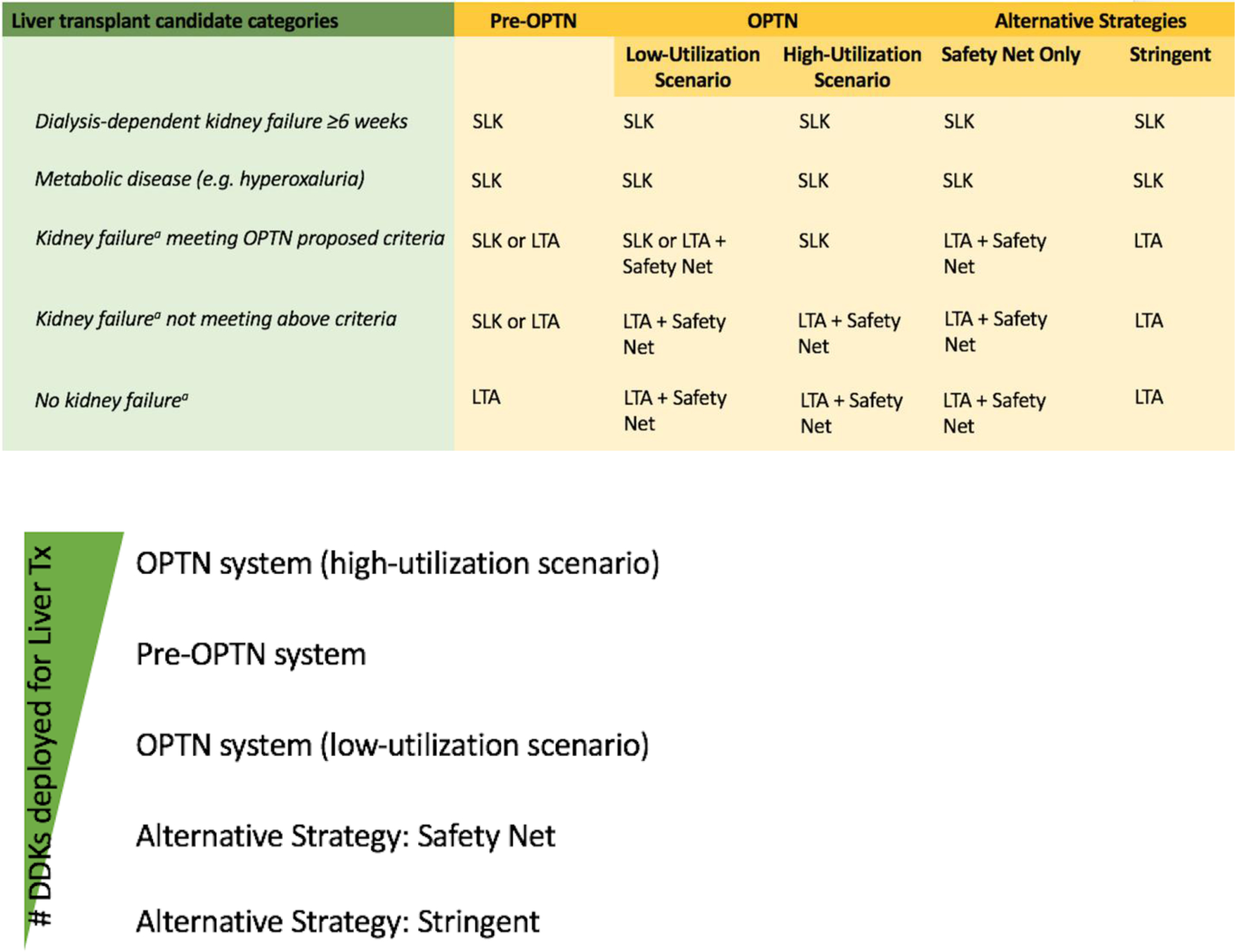
Kidney transplant strategies for liver transplant candidates, in detail (panel A) and ranked by number of deceased donor kidneys needed (panel B).
Members in the nephrology and transplant community have proposed a third strategy (“safety net”)2,7. This strategy reserves SLK only to liver transplant candidates with conventional kidney transplant indication. All other candidates will undergo LTA backed by “safety net” allocation, as per the second half of OPTN’s new system. A fourth strategy (“stringent”) is similar to “safety net” strategy, but eliminates “safety net” allocation altogether. These two strategies form the most conservative kidney transplant strategies for liver transplant candidates.
Controversy exists regarding whether OPTN’s criteria for SLK are too broad and will utilize too many DDKs, leading to diminished access for patients with end-stage kidney disease (ESKD) without liver disease who are awaiting kidney transplantation, a far larger pool, already strained by wait times that typically exceed life expectancy6,8. We therefore set out to compare four transplantation strategies—pre-OPTN, OPTN, safety net, and stringent—that span the full range of strategies for liver transplant recipients, and included both scenarios within the new OPTN system in the evaluation. Since a prospective randomized controlled trial comparing allocation strategies is not feasible, we turned to decision modeling to fulfill our objective.
Methods
This study consisted of two parts: 1) development and validation of a Markov model for liver transplant candidates, using inputs from a cohort assembled from Scientific Registry of Transplant Recipients (SRTR) 2002–2013 data to recreate the post-SLK and liver transplant trajectories over the same time period; and 2) an assessment of the patient outcomes (life years [LYs] and quality-adjusted life years [QALYs]) and organ costs of the strategies outlined above using this model. We used a simulated study cohort consisting of 55-year-old first-time liver transplant candidates without a conventional indication for kidney transplant (primary diagnosis of metabolic disorder or dialysis-dependent for ≥6 weeks). Age 55 was selected, as it is the mean and median age for first-time liver transplant recipients in 2002–2013.
Cohort Definition
Characteristics and outcomes of the simulated cohort were obtained from first-time liver and SLK transplant recipients assembled from SRTR, March 1 2002 through December 31, 2013. March 2002 marks the beginning of the MELD era. The cut-off at December 2013 ensured that we had at least 1-year follow-up for all patients. The SRTR contains de-identified data on all solid organ transplants in the US, including status history (clinical information from the pre-transplant period), recipient information collected at the time of transplant, and dates of graft failure and death. Liver status history forms record pre-transplant serum creatinine and dialysis-dependence at set time intervals, which are required submissions from transplant centers to maintain waitlist priority. We used this information to divide patients into three strata based on their kidney function at time of the liver transplant: little or no kidney disease (estimated glomerular filtrate rate ≥60 mL/min), kidney disease that meets OPTN’s criteria for SLK5 (OPTN+), and kidney disease that does not meet OPTN’s criteria (OPTN-). We sequentially excluded 454 recipients of multi-organ transplants other than SLK transplant and 2,036 recipients with a conventional indication for kidney transplant, as noted above. We further excluded 6,365 (11%) patients whose status history forms did not contain sufficient information to place them into the OPTN− versus OPTN+ group. We included these patients as separate stratum in a secondary analysis. Meanwhile, the primary analysis cohort consisted of three strata (Table 2) with outcomes modeled separately by stratum.
Table 2.
A theoretical cohort is assembled based on adult, first-time liver transplant recipients from Scientific Registry of Transplant Recipients (SRTR), 2013 data.
| Stratum | SRTR Cohort (N=4649) | Proportion in Theoretical Cohort | |
|---|---|---|---|
| Received LTA | Received SLK | ||
| 1: No kidney failure | 2826 | 0 | 0.6079 |
| 2: Kidney failure (OPTN−) | 1510 | 34 | 0.3321 |
| 3: Kidney failure (OPTN+) | 188 | 91 | 0.0600 |
OPTN+ and OPTN− refer to whether the patient’s degree of kidney disease qualifies them for simultaneous liver-kidney (SLK) or liver transplant alone (LTA) based on Organ Procurement and Transplant Network’s proposed SLK criteria.
Model Structure
We followed patients in each stratum for a lifetime in a discrete-time Markov model. We calculated each outcome in each stratum, and the overall population mean as a weighted average across the three strata. The outcomes included post-transplant life years (LYs), quality-adjusted life years (QALYs), and number of DDKs utilized. A 3% annual discount rate was applied to outcomes and costs.
We modeled four strategies: pre-OPTN, OPTN (both scenarios), safety net, and stringent (Figure 2a). In the high-utilization scenario of OPTN system, all patients who met the SLK eligibility criteria received a SLK transplant. In the low-utilization scenario, only the patients who received SLK transplant in the pre-OPTN system and met the SLK eligibility criteria received a SLK transplant. The probability of receiving a SLK transplant pre-OPTN was derived from the stratum-specific proportions in 2013 (Table 2).
Figure 2.
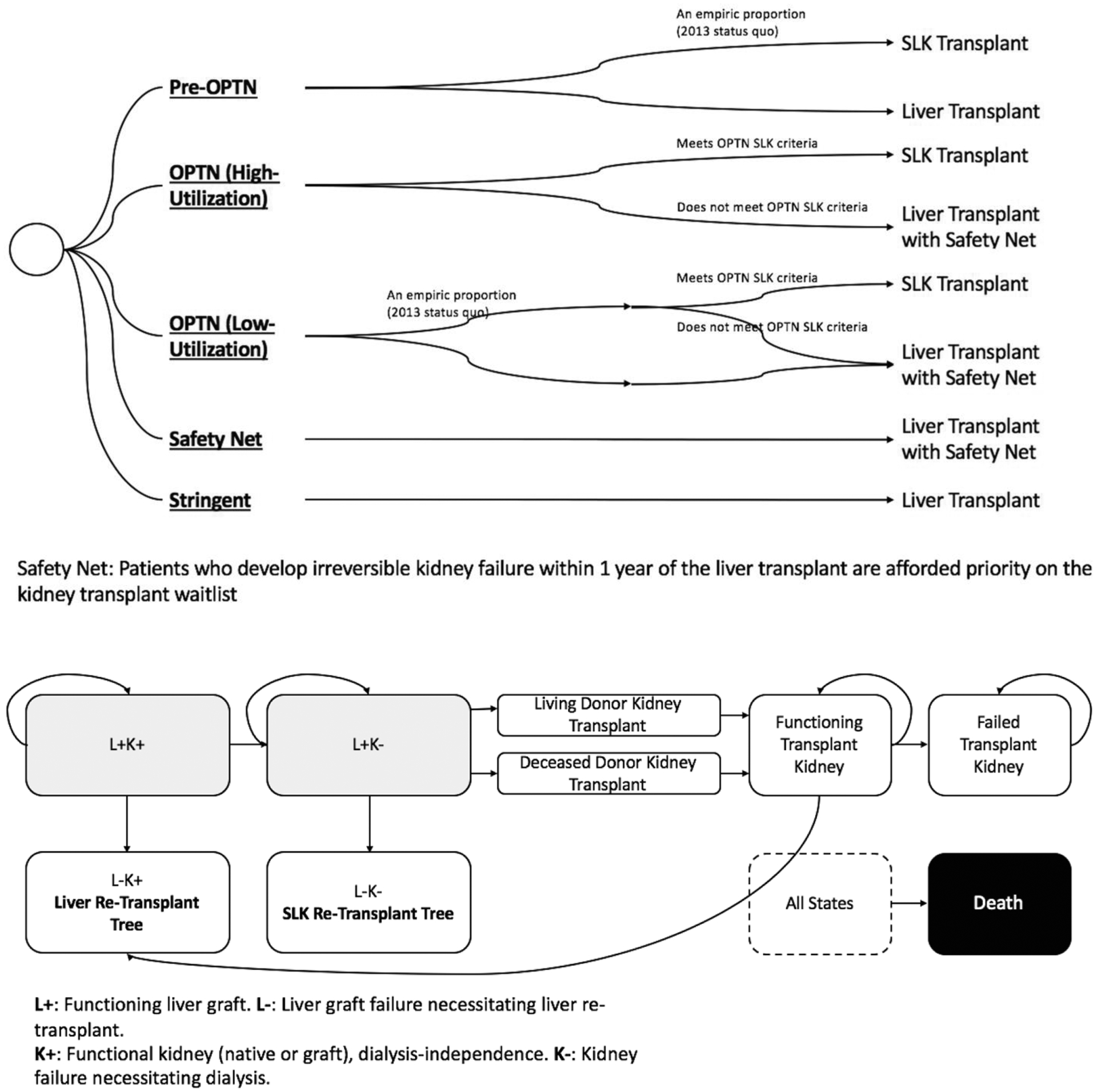
Decision model layout. Panel A: Decision tree schematic depicting the four strategies. Panel B: Schematic diagram depicting the health states and their flow post-transplant in the Markov model. Death could happen in any health state.
The model was run in monthly cycles for 30 years, at which point <10% of the cohort remained alive. In each cycle, a patient could transition from one health state to another based on a probability placed into the model. As can be seen in Figure 2b, at any point after the index transplant (LTA or SLK), a patient could die or undergo liver graft failure or kidney (native or graft) failure. Liver graft failure led to liver re-transplantation. Kidney failure led to dialysis and/or entering the kidney transplant waitlist. Kidney failure followed by liver allograft failure led to SLK re-transplantation. To approximate real-life scenarios, we allowed no more than one liver and one kidney transplant after the index transplant. DDK transplant occurred when time-on-waitlist equaled time-to-transplant and the subject had not experienced a competing event (death or liver graft failure). Living donor kidney transplant occurred at 3 months after onset of kidney failure, if a donor were available.
Model Inputs
We derived values for model parameters, especially transition probabilities, from two data sources:
Post-LTA and post-SLK transplant outcomes, analyzed based on the SRTR dataset.
Published literature, which consisted of registry-based analyses (similar to SRTR) and single-center reports.
Table 3 details parameters used in the model. A detailed discussion of each parameter is included Supplemental S0: Technical Appendix. To reflect uncertainty in average patient risks and costs, each parameter was represented by a continuous probability distribution. We determined the distribution with non-parametric assumptions from simulation (as detailed in Supplemental S0: Technical Appendix), or with back-calculation, using parametric assumptions from the expected value (base model) and 95% confidence interval (range). Some assumptions were necessary; we arrived at them through consensus among the clinician co-authors and stated them explicitly in Supplemental S0: Technical Appendix. In general, we tried to select assumptions such that they biased the model in one direction: favoring strategies utilizing SLK transplantation more liberally. We selected this direction of bias to counteract the bias in observational literature: the benefit of SLK versus LTA may be underestimated in observational studies, because SLK recipients may be sicker4. Biasing the model in one direction, toward less stringent use of SLK, also enhances the interpretability of our results.
Table 3.
Parameter inputs for decision tree and Markov model. L+/−: functioning or non-functioning liver allograft. K +/−: functioning or non-functioning kidney, allograft (if initial transplant is SLK) or native (if initial transplant is liver). KD: Kidney disease. OPTN-: Kidney disease not meeting OPTN criteria for SLK. OPTN+: Kidney disease meeting OPTN criteria for SLK.
| Parameter | Stratum | Base Model | Range | Distribution | Source | Technical Appendix |
|---|---|---|---|---|---|---|
| Probability of outcome at month 1 post-index transplant | ||||||
| Post-SLK transplant: | ||||||
| L+K− | OPTN− | 0.012 | 0.003–0.026 | beta (4,334) | SRTR cohort | A |
| L−K+ | OPTN− | 0.003 | 0.000–0.011 | beta (1,337) | SRTR cohort | A |
| Death | OPTN− | 0.024 | 0.010–0.042 | beta (8,330) | SRTR cohort | A |
| Post-liver transplant: | ||||||
| L+K− | No KD | 0.003 | 0.001–0.005 | beta (11,3289) | 9 | A |
| L−K+ | No KD | 0.022 | 0.020–0.023 | beta (722,32933) | SRTR cohort | A |
| Death | No KD | 0.023 | 0.021–0.024 | beta (759,32896) | SRTR cohort | A |
| Transition probabilities per cycle, month 1 and beyond post-index transplant | ||||||
| Post-SLK transplant: | ||||||
| Death (L+K+ → death) | time-dependent, see Table A5 | non-parametric | SRTR cohort | A | ||
| Liver graft fails (L+K+ → L−K+L+K− → L−K−) | time-dependent, see Table A5 | non-parametric | SRTR cohort | A | ||
| Kidney graft fails (L+K+ → L+K−) | time-dependent, see Table A5 | non-parametric | SRTR cohort | A | ||
| Post-liver transplant: | ||||||
| Death (L+K+ → death) | time-dependent, see Table B5 | non-parametric | SRTR cohort | A | ||
| Liver graft fails (L+K+ → L−K+L+K− → L−K−) | time-dependent, see Table A5 | non-parametric | SRTR cohort | A | ||
| Native kidneys fail (L+K+ → L+K−) | time-dependent, see Table A5 | non-parametric | 9 | A | ||
| Post-both transplants: | ||||||
| Death on dialysis (L+K− → death) | all | death rate without dialysis × hazard ratio (3.32) | hazard ratio: 2.96–3.71 | hazard ratio: log-normal (1.200,0.058) | 10 | A |
| Liver graft failure on dialysis (L+K− → L−K−) | all | liver failure rate without dialysis × hazard ratio (1.49) | hazard ratio: 1.10–2.04 | hazard ratio: log-normal (0.3988,0.1573) | 11 | A |
| Death after second transplant (kidney) | all | death rate without second transplant × time-dependent hazard ratio | time-dependent, see Figure B2 | non-parametric | 12 | B |
| Kidney graft failure after second transplant (kidney) | time-dependent, see Table B2 | non-parametric | 12 | B | ||
| Kidney wait-list information | ||||||
| Proportion of patients who have matching living donors | all | 0.059 | 0.049–0.069 | beta (132,2105) | 12 | B |
| Adjustment for liver / SLK re-transplantation | ||||||
| Hazard ratio for death (compared to first transplant) | all | 1.7 | 1.56–1.84 | log-normal (0.531,0.042) | 13 | A |
| Probability of receiving SLK pre-OPTN (based on 2013 data) | ||||||
| No KD | 0.000 | 0 | none | 2013 SRTR cohort | ||
| Utility weights | ||||||
| Post-liver transplant: | ||||||
| No kidney failure | all | 0.747 | 0.720–0.774 | beta (742,251) | 14 | D |
| With kidney failure | all | 0.573 | 0.523–0.624 | beta (210,156) | 14,15 | D |
Gamma distribution is described here by a shape parameter, α, and an inverse scale parameter, β.
We tested a series of assumptions in sensitivity analyses (Supplemental S1). Kidney waitlist length and therefore wait-time to transplant vary regionally, but transplant policies are national. We therefore assessed the strategies across assumptions regarding wait-time to transplant and effects of safety net allocation on wait-time and living donation rate. Because all available studies detailing the mortality risk after SLK versus LTA are observational, there is uncertainty surrounding the probability of death after LTA when the patient might have otherwise received SLK. We therefore assessed the direction of strategies across a range of assumptions regarding this death risk.
Some parameters were correlated with others in the model. For instance, in a world where patients experience a higher probability of death after LTA, we would expect that the probability of death is also higher after SLK transplant, i.e. the two rates are correlated. We induced a modest correlation (ρ=0.5) using a published rank order approach to create joint distributions for correlated parameters16, and then built the model based on these joint distributions.
Model Validation
To augment model credibility, we validated the model17 by plotting the actual versus modelled patient outcomes (survival, liver re-transplant-free survival, and death-censored ESKD-free survival) using the Kaplan-Meier method. We used three related SRTR cohorts for actual clinical outcomes:
Derivation cohort: First-time adult LTA and SLK transplant recipients, March 1 2002 through December 31 2013, meeting the exclusion criteria as defined in “Cohort Definition”, with follow-up until December 31 2014 (N=53,648);
Validation cohort 1: Same cohort as the derivation dataset, with extended follow-up until December 31 2016 (N=53,648);
Validation dataset 2: first-time adult LTA and SLK transplant recipients, January 1 2014 through December 31 2015, meeting the exclusion criteria as defined in “Cohort Definition”, with follow-up until December 31 2016 (N=10,147).
Comparison of model output to the derivation cohort tests internal validity, i.e. whether the model is able to reproduce the same outcomes from its derivation dataset. The two validation cohorts enable a test external validity, i.e. whether the model is able to predict outcomes in a different dataset.
Outcomes and Analysis
Main outcomes are LYs, QALYs, and number of DDKs deployed to liver transplant recipients. To capture a key aspect of the SLK controversy, i.e., whether the increase in DDK allocated to SLK provides sufficient benefit to justify the practice, we calculated an incremental LY/QALY gained per DDK deployed when comparing one strategy to another. This is a modified form of the incremental cost-effectiveness ratio commonly used in economic analyses: instead of monetary cost, we focus on the organ “cost”. For comparison purposes, a metric developed for kidney and kidney-pancreas transplants is the life year from transplant (LYFT)18. This may be seen also as an incremental LY gained per DDK when we compared two strategies for treating ESKD: transplantation and dialysis.
We calculated the outcomes as follows. For each patient stratum, we performed 1,000,000 simulations (1,000 microsimulations, nested within 1,000 probability sensitivity analyses [PSAs]). Each microsimulation represented an individual patient’s post-transplant course. Each PSA represented a “parallel universe,” in which a set of parameters, e.g. probability of death after liver transplant, were sampled independently from distributions defined in Table 3. The 1,000,000 simulations therefore represent 1,000 “parallel universes,” each of which contains 1,000 individual patients and their unique trajectories. Thus we propagated the uncertainty in the parameters to uncertainty in outcomes modelled. For each study outcome, we constructed the population average within each PSA. We then bootstrapped these population averages to arrive at a pooled result that represented the weighted-average of the three strata: little or no kidney disease (estimated glomerular filtrate rate ≥60 mL/min), kidney disease that meets OPTN’s criteria for SLK5 (OPTN+), and kidney disease that does not meet OPTN’s criteria (OPTN-)
Simulations and calibrations to generate model inputs were conducted in SAS 9.4 (Cary, NC). Model validations and simulations were performed in TreeAge Pro 2016 (Williamstown, MA). Stanford University’s Institutional Review Board approved this study in accordance with the Declaration of Helsinki (protocol number IRB-40876). The data reported here have been supplied by the Minneapolis Medical Research Foundation (MMRF) as the contractor for the Scientific Registry of Transplant Recipients (SRTR). The interpretation and reporting of these data are the responsibility of the authors and in no way should be seen as an official policy of or interpretation by the SRTR or the US government. Findings in this manuscript were partly reported in abstract form at the National Kidney Foundation Young Investigator Forum in April, 2017 and American Transplant Congress in May, 2017.
Results
Model Validation
As internal validation, actual versus modelled Kaplan-Meier curves for post-transplant outcomes are virtually superimposable (Figure S3). As external validation, our model slightly overestimates death and liver re-transplant, but closely approximates kidney-specific outcomes (Figure 3). The overestimation is slightly more pronounced in LTA than in SLK transplants.
Figure 3.
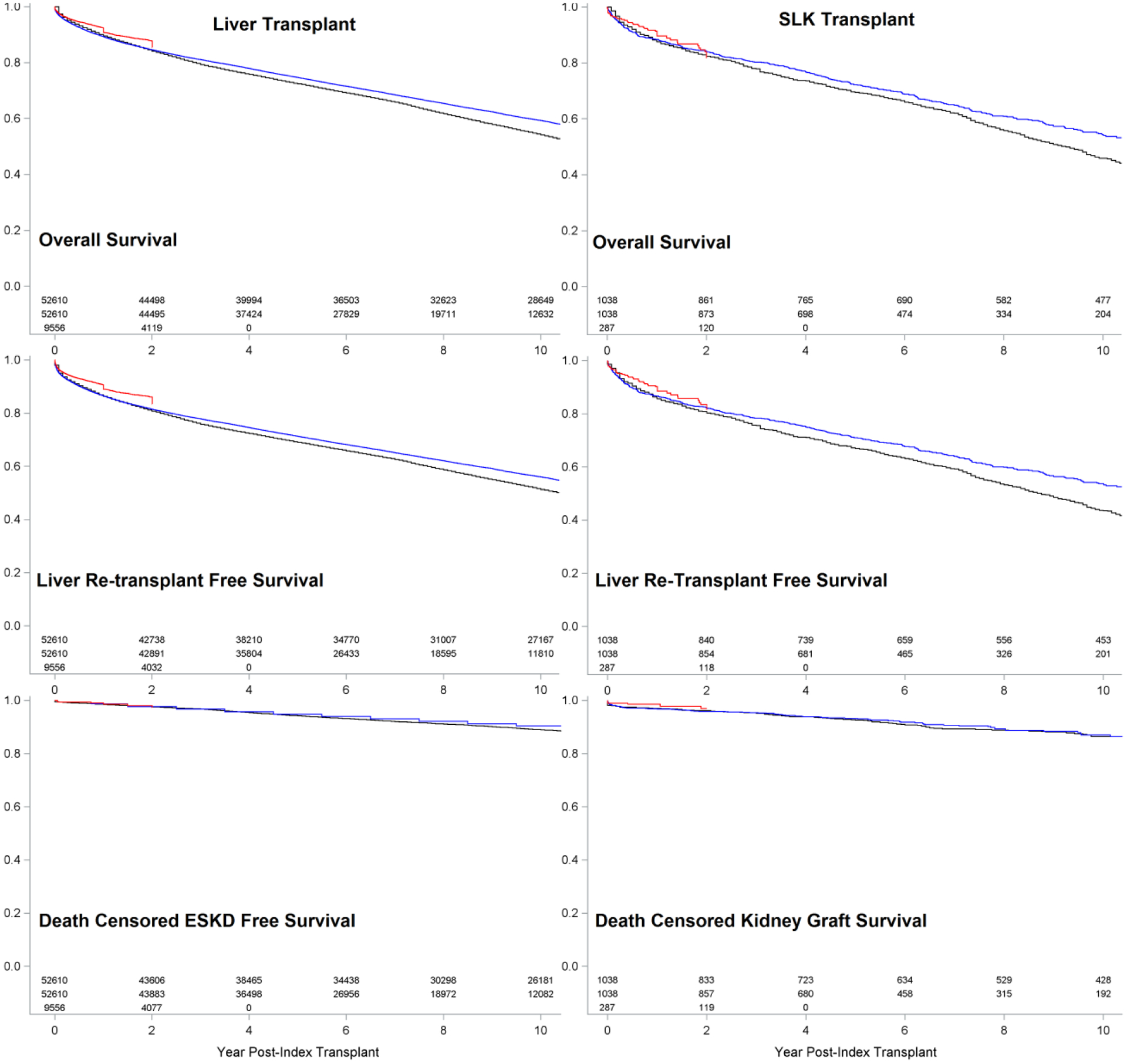
External validation: Actual SRTR (blue: validation set #1; red: validation set #2) versus modelled (black) outcomes after LTA or SLK transplantation.
Analysis
Table 4 summarizes our primary outcomes. The stringent strategy results in the fewest DDKs deployed to liver transplant recipients (3.6 per-100-persons) and the lowest LYs and QALYs per person (9.56 and 7.09, respectively). Moving from the stringent strategy to the safety net strategy results in more LYs and QALYs per person at the expense of more DDKs, with an incremental effectiveness of 1.85 LYs or 2.03 QALYs per DDK deployed. The OPTN and pre-OPTN strategies deploy yet more DDKs. In the low-utilization scenario, wherein an expansion in SLK transplantation does not occur as a result of the new rule, the OPTN strategy generates more LYs/QALYs per person using fewer DDKs, compared to the pre-OPTN strategy. In the high-utilization scenario, where an expansion in SLK transplantation occurs, and every liver transplant candidate eligible for SLK receives one, the pre-OPTN strategy is also not favored. Assuming a starting point with a slightly worse clinical outcome (i.e., safety net), we can choose to move to the pre-OPTN or OPTN strategy by deploying more DDKs to liver transplant recipients. With every additional DDK deployed, we gain 1.15 (1.08–1.22) LY by moving to the OPTN strategy, compared to only 0.89 (0.81–0.98) LY by moving to the pre-OPTN strategy. Thus under no circumstance would we prefer the pre-OPTN over the other strategies, given the set of trade-offs. In sensitivity analysis, these results are robust to the discount rate used, adjustment for the liver transplant counterfactual, wait time to DDK transplant, varying effectiveness of safety net allocation and live kidney donation rate for safety net-eligible patients (Supplemental S1).
Table 4.
“Cost”-effectiveness of each kidney allocation strategy, with 3% annual discounting. LY: life year. QALY: quality-adjusted life year. DDK: deceased donor kidney. Dom: Dominated completely (i.e. there is another strategy present which delivers more LY or QALY using fewer DDKs). DomEx: Dominated by extension (i.e. other strategies present provide more LY or QALY at the same incremental increase in DDK). Highlighted cells indicate the dominated strategy.
| Low-Utilization Scenario for OPTN Implementation | |||||
|---|---|---|---|---|---|
| Strategy | LY (year) | QALY (year) | # DDK | Incremental LY per DDK* | Incremental QALY per DDK* |
| Stringent | 9.56 (9.55–9.57) | 7.09 (7.08–7.10) | 0.036 (0.036–0.037) | - | - |
| Safety Net | 9.57 (9.56–9.59) | 7.10 (7.09–7.11) | 0.042 (0.041–0.042) | 1.85 (1.77–1.94) | 2.03 (1.97–2.09) |
| OPTN | 9.61 (9.59–9.61) | 7.12 (7.11–7.13) | 0.061 (0.060–0.061) | 1.59 (1.52–1.66) | 1.22 (1.17–1.27) |
| Pre-OPTN | 9.59 (9.57–9.60) | 7.11 (7.10–7.12) | 0.062 (0.062–0.063) | Dom | Dom |
| High-Utilization Scenario for OPTN Implementation | |||||
| Strategy | LY (year) | QALY (year) | # DDK | Incremental LY per DDK* | Incremental QALY per DDK* |
| Stringent | 9.56 (9.55–9.57) | 7.09 (7.08–7.10) | 0.036 (0.036–0.037) | - | - |
| Safety Net | 9.57 (9.56–9.59) | 7.10 (7.09–7.11) | 0.042 (0.041–0.042) | 1.85 (1.77–1.94) | 2.03 (1.97–2.09) |
| Pre-OPTN | 9.59 (9.57–9.60) | 7.11 (7.10–7.12) | 0.062 (0.062–0.063) | DomEx: 0.89 (0.81–0.98) | DomEx: 0.54 (0.48–0.60) |
| OPTN | 9.64 (9.62–9.65) | 7.15 (7.14–7.16) | 0.098 (0.098–0.099) | 1.30 (1.21–1.38) | 1.11 (1.05–1.17) |
Current strategy compared to the immediately preceding strategy.
Table 5 summarizes detailed organ utilization per strategy. The OPTN strategy has the potential to increase the number of SLKs significantly (high-utilization scenario), but may also decrease the number of SLKs somewhat compared to the pre-OPTN strategy (low-utilization scenario). DDK utilization tracks SLK numbers. Live kidney donation rates are comparable under all the strategies. Activation on safety net is more common in strategies that are more conservative with SLKs. The proportion of patients activated on safety net who ultimately receive DDK transplantation is 70–72%. Average liver graft lifespan ranges from 11.92 (11.90–11.94) years under stringent strategy to 12.02 (11.99–12.04) years under OPTN strategy (high-utilization scenario).
Table 5.
Expected organ usage (per liver transplant recipient) under each kidney transplant strategy. SLK: simultaneous liver-kidney. For Safety Net, activation refers activation under the Safety Net system, and transplant refers to receiving a deceased donor kidney transplant under the Safety Net system.
| SLK | Liver | Kidney | Safety Net | |||
|---|---|---|---|---|---|---|
| Deceased Donor | Living Donor | Activation | Transplant | |||
| Stringent | - | 1.07 | 0.05 | 0.006 | - | - |
| Safety Net | - | 1.07 | 0.06 | 0.006 | 0.02 | 0.01 |
| OPTN: | ||||||
| Low-utilization | 0.02 | 1.07 | 0.07 | 0.006 | 0.02 | 0.01 |
| High-utilization | 0.06 | 1.07 | 0.11 | 0.006 | 0.01 | 0.01 |
| Pre-OPTN | 0.03 | 1.07 | 0.08 | 0.006 | - | - |
Discussion
In the past few decades, health policy experts have used decision analysis with Markov models to approach problem where an intervention modifies an ongoing risk. In the present study, we compared kidney allocation strategies, which modify the ongoing risks of death and kidney failure after the initial liver transplant. Because organ scarcity lies at the heart of this problem, we used a modified cost-effectiveness analysis approach to evaluate the expected clinical benefit (life year and quality-adjusted life years) as well as the organ “cost,” herein expressed in terms of DDKs deployed per strategy, rather than conventional currencies. We hypothesized a trade-off between maximizing clinical benefit and minimizing DDK deployment1,19,20, and our objective was to quantify this trade-off.
Our first important finding is that the new OPTN system has the potential to substantially increase the number of DDKs deployed to liver transplant recipients, both in the form of SLK transplants and subsequent kidney transplants (3.6 [3.5–3.6] per 100-persons, or 167 kidneys in 2013 terms). Our estimates likely underestimate the true number of patients who will qualify for SLK transplants for two reasons. First, we excluded from the analysis patients for whom we could not establish SLK eligibility based on liver status form data, but a proportion of whom likely will meet criteria for SLK transplantation by additional data to which transplant centers would have access. Furthermore, we used the 4-variable Modification of Diet in Renal Disease (MDRD4) equation, which has been shown to overestimate kidney function in cirrhosis21–23—and therefore underqualify patients for SLK. These two factors increase the theoretical upper bound of proportion of patients who will qualify for SLK transplants under OPTN’s eligibility criteria. The extent to which transplant centers will pursue SLK listing for these eligible patients is unknown. We modelled the OPTN strategy under two scenarios: one in which transplant centers do not expand their indication for SLK transplants, and one in which they do, assuming that the truth will lie somewhere in between. Nonetheless, given that all metrics for transplant centers focus on post-transplant outcomes, we hypothesize that the truth will lean slightly toward the high-utilization scenario in which centers expand their indication for SLK transplants in the hope of improving survival after liver transplant in their sickest patients. As the supply of DDKs has remained rather constant over the past decade, we project that the increase in DDK deployment to liver transplant candidates may diminish access to DDKs for kidney transplant candidates without liver disease, an opportunity cost we have formerly quantified at 5.92 years (1st-3rd quartile: 5.50–6.39 years) per DDK24, the LYFT18 that would be expected if we used each DDK deployed to SLK to transplant kidney transplant candidates without liver disease.
Our second important finding is that in both scenarios of OPTN system, all strategies, except the pre-OPTN system, are efficient uses of DDKs with a specific trade-off between liver transplant outcomes and DDK deployment. In the low-utilization scenario, the OPTN system increases LY/QALY per person and decreases DDK deployment compared to pre-OPTN system, so in no scenario would we choose the pre-OPTN system. In the high-utilization scenario, the four strategies are ranked as OPTN > pre-OPTN > safety net > stringent strategy in both liver transplant outcomes and DDK deployment. For any level of DDK use, the OPTN, safety net and stringent strategies are all deploy DDKs more efficiently than the pre-OPTN system. We have additional confidence in our results because our model is biased, as previously discussed, to favor strategies more liberal with SLK transplants: that more restrictive strategies still perform well is therefore all the more striking. Our decision analysis therefore fully supports changing the pre-OPTN system to the new system, even as controversy over the specifics exist6,8. Future modifications of the OPTN system may consider setting stricter eligibility criteria for SLK, and our analysis provides the quantification of trade-offs to inform these policy modifications.
Two possible mechanisms account for the relative efficiency of the OPTN system and safety net strategy: 1) in patients who do not meet OPTN’s SLK medical eligibility criteria, SLK transplant may yield little appreciable benefit over LTA5, and thus eliminating these “low-value” SLK transplants reduces DDK deployment without too much adverse effect on patient outcomes; 2) certain patients develop severe kidney failure after LTA due to stochastic peri- and post-operative events, and these patients benefit greatly from the earlier kidney transplants that the OPTN system and safety Net strategy afford.
Our model performed well in internal and external validation. Rates of death and liver re-transplantation are slightly overestimated, especially for LTA recipients. We anticipate that this would bias our results toward policies more liberal with SLK transplants and lead us to understate the effectiveness of the OPTN system (restricted use) and the safety net strategy. This bias thus does not alter our conclusion.
To our knowledge, this is the first comprehensive evaluation of the OPTN’s new liver-kidney transplantation strategy, and the first comparison of the new system to the pre-OPTN system and alternative strategies. Compared to prior decision analyses7,20,25, advantages of this study include the inclusion of all liver transplant candidates who might be affected by a change in allocation policy, comparison of clearly defined allocation strategies, and incorporation of SRTR data to provide the highest degree of assurance that the model faithfully mimics reality.
Our study has several important limitations. We relied on observational data, where any comparison of SLK versus LTA suffers from confounding by indication, and had to exclude many patients whose kidney status are not as well-defined. We considered some of these factors in sensitivity analyses, which did not alter our qualitative results. Factors we did not consider included different rates of native kidney failure or alternative criteria to define SLK eligibility. Conceivably, there may be biases in the opposite direction. In one study, a subset of liver transplant recipients was listed for, but too unstable to undergo, SLK transplant26. The desire to avoid futile kidney transplants in extremely sick liver transplant recipients27 may also bias the studies by enriching the liver transplant pool with sicker patients, although this is unlikely to overcome the powerful incentive that the SRTR program-specific reporting—which excludes SLK from liver transplant report cards—provides. These biases may lead us to overestimate the effectiveness of the OPTN system and underestimate the effectiveness of the safety net and stringent strategies. Nonetheless, they are unlikely to alter our conclusion that all of these strategies are preferable to the pre-OPTN system. The use of past data to “predict” future outcomes in a complex system is, at best, an imperfect endeavor. For instance, we did not account for changes in future patient mix, future organ supply, technologic changes that may substantially change the field of transplantation, or other policy or structural changes in the transplant field (including liver allocation re-districting). Discounting both benefits and costs, as we have done, is a partial safeguard against weighing the unpredictable future too heavily. Finally, we made the overly simplistic assumption that all liver transplant recipients with kidney failure will move to the kidney transplant waitlist. The scarcity of data on the proportion of such patients who are eligible for kidney transplant versus not and their differential rates of survival drove this decision. This bias may lead us to overestimate the effectiveness of the OPTN and the safety set strategy.
In summary, both the new liver-kidney transplantation system put forth by OPTN and competing strategies far more parsimonious in SLK allocation are preferable to the pre-OPTN system of SLK allocation. The net effect on patient outcomes and organ utilization will require further study. We raise concerns over aspects of the current proposal, including a potential increase in the number of DDKs required. Our modelling provides a quantification of the trade-off between liver transplant outcomes and DDK deployment which may inform future modifications to the OPTN SLK allocation system.
Supplementary Material
Acknowledgments
Research reported here was supported by the John M. Sobrato Gift Fund (J.C.T.) and National Institute of Diabetes and Digestive and Kidney Diseases of the National Institutes of Health under award number K24DK092336 (W.R.K.) and K24 DK085446 (G.M.C.).
Abbreviations:
- AKI
Acute kidney injury.
- CKD
Chronic kidney disease.
- DDK
Deceased donor kidney.
- ESKD
End-stage kidney disease.
- LTA
Liver transplant alone.
- LY
Life year.
- LYFT
Life year from transplant.
- MELD
Model for End-stage Liver Disease.
- MMRF
Minneapolis Medical Research Foundation.
- OPTN
Organ Procurement and Transplant Network.
- PSA
Probability sensitivity analysis.
- QALY
Quality-adjusted life year.
- SLK
Simultaneous liver-kidney.
- SRTR
Scientific Registry of Transplant Recipients.
- US
United States.
Footnotes
The authors declare no conflict of interest.
References
- 1.Gonwa TA, McBride MA, Anderson K, Mai ML, Wadei H, Ahsan N. Continued influence of preoperative renal function on outcome of orthotopic liver transplant (OLTX) in the US: where will MELD lead us? Am J Transplant. 2006;6(11):2651–2659. [DOI] [PubMed] [Google Scholar]
- 2.Nadim MK, Sung RS, Davis CL, et al. Simultaneous liver-kidney transplantation summit: current state and future directions. Am J Transplant. 2012;12(11):2901–2908. [DOI] [PubMed] [Google Scholar]
- 3.Reese PP, Veatch RM, Abt PL, Amaral S. Revisiting multi-organ transplantation in the setting of scarcity. Am J Transplant. 2014;14(1):21–26. [DOI] [PubMed] [Google Scholar]
- 4.Nadim MK, Davis CL, Sung R, Kellum JA, Genyk YS. Simultaneous liver-kidney transplantation: a survey of US transplant centers. Am J Transplant. 2012;12(11):3119–3127. [DOI] [PubMed] [Google Scholar]
- 5.Formica RN, Aeder M, Boyle G, et al. Simultaneous Liver-Kidney Allocation Policy: A Proposal to Optimize Appropriate Utilization of Scarce Resources. Am J Transplant. 2016;16(3):758–766. [DOI] [PubMed] [Google Scholar]
- 6.Wadei HM, Gonwa TA, Taner CB. Simultaneous Liver Kidney Transplant (SLK) Allocation Policy Change Proposal: Is It Really a Smart Move? Am J Transplant. 2016;16(9):2763–2764. [DOI] [PubMed] [Google Scholar]
- 7.Chang Y, Gallon L, Jay C, et al. Comparative effectiveness of liver transplant strategies for end-stage liver disease patients on renal replacement therapy. Liver Transplant. 2014;20(9):1034–1044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Asch WS, Bia MJ. New Organ Allocation System for Combined Liver-Kidney Transplants and the Availability of Kidneys for Transplant to Patients with Stage 4–5 CKD. Clin J Am Soc Nephrol. 2017;12(5):848–852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sharma P, Goodrich NP, Schaubel DE, Guidinger MK, Merion RM. Patient-specific prediction of ESRD after liver transplantation. J Am Soc Nephrol. 2013;24(12):2045–2052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sharma P, Schaubel DE, Guidinger MK, Goodrich NP, Ojo AO, Merion RM. Impact of MELD-based allocation on end-stage renal disease after liver transplantation. Am J Transplant. 2011;11(11):2372–2378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mindikoglu AL, Raufman JP, Seliger SL, Howell CD, Magder LS. Simultaneous liver-kidney versus liver transplantation alone in patients with end-stage liver disease and kidney dysfunction not on dialysis. Transplant Proc. 2011;43(7):2669–2677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cassuto JR, Reese PP, Sonnad S, et al. Wait list death and survival benefit of kidney transplantation among nonrenal transplant recipients. Am J Transplant. 2010;10(11):2502–2511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Montenovo MI, Hansen RN, Dick AAS. Outcomes of adult liver re-transplant patients in the model for end-stage liver disease era: is it time to reconsider its indications? Clin Transplant. 2014;28(10):1099–1104. [DOI] [PubMed] [Google Scholar]
- 14.Ratcliffe J, Longworth L, Young T, et al. Assessing health-related quality of life pre- and post-liver transplantation: a prospective multicenter study. Liver Transplant. 2002;8(3):263–270. [DOI] [PubMed] [Google Scholar]
- 15.Wyld M, Morton RL, Hayen A, Howard K, Webster AC. A systematic review and meta-analysis of utility-based quality of life in chronic kidney disease treatments. PLoS Med. 2012;9(9):e1001307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Goldhaber-Fiebert JD, Jalal HJ. Some Health States Are Better Than Others: Using Health State Rank Order to Improve Probabilistic Analyses. Med Decis Mak. 2016;36(8):927–940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Goldhaber-Fiebert JD, Stout NK, Goldie SJ. Empirically evaluating decision-analytic models. Value Health. 2010;13(5):667–674. [DOI] [PubMed] [Google Scholar]
- 18.Wolfe RA, McCullough KP, Schaubel DE, et al. Calculating life years from transplant (LYFT): methods for kidney and kidney-pancreas candidates. Am J Transplant. 2008;8(4 Pt 2):997–1011. [DOI] [PubMed] [Google Scholar]
- 19.Fong T-L, Bunnapradist S, Jordan SC, Selby RR, Cho YW. Analysis of the United Network for Organ Sharing database comparing renal allografts and patient survival in combined liver-kidney transplantation with the contralateral allografts in kidney alone or kidney-pancreas transplantation. Transplantation. 2003;76(2):348–353. [DOI] [PubMed] [Google Scholar]
- 20.Chang Y, Gallon L, Shetty K, et al. Simulation modeling of the impact of proposed new simultaneous liver and kidney transplantation policies. Transplantation. 2015;99(2):424–430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Francoz C, Nadim MK, Baron A, et al. Glomerular filtration rate equations for liver-kidney transplantation in patients with cirrhosis: validation of current recommendations. Hepatol. 2014;59(4):1514–1521. [DOI] [PubMed] [Google Scholar]
- 22.De Souza V, Hadj-Aissa A, Dolomanova O, et al. Creatinine- versus cystatine C-based equations in assessing the renal function of candidates for liver transplantation with cirrhosis. Hepatol. 2014;59(4):1522–1531. [DOI] [PubMed] [Google Scholar]
- 23.Mindikoglu AL, Dowling TC, Weir MR, Seliger SL, Christenson RH, Magder LS. Performance of chronic kidney disease epidemiology collaboration creatinine-cystatin C equation for estimating kidney function in cirrhosis. Hepatold. 2014;59(4):1532–1542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cheng XS, Stedman MR, Chertow GM, Kim WR, Tan JC. Utility in Treating Kidney Failure in End-Stage Liver Disease With Simultaneous Liver-Kidney Transplantation. Transplantation. 2017;101(5):1111–1119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kiberd B, Skedgel C, Alwayn I, Peltekian K. Simultaneous liver kidney transplantation: a medical decision analysis. Transplantation. 2011;91(1):121–127. [DOI] [PubMed] [Google Scholar]
- 26.Hmoud B, Kuo Y-F, Wiesner RH, Singal AK. Outcomes of liver transplantation alone after listing for simultaneous kidney: comparison to simultaneous liver kidney transplantation. Transplantation. 2015;99(4):823–828. [DOI] [PubMed] [Google Scholar]
- 27.Lunsford KE, Bodzin AS, Markovic D, et al. Avoiding Futility in Simultaneous Liver-kidney Transplantation: Analysis of 331 Consecutive Patients Listed for Dual Organ Replacement. Ann Surg. 2017;265(5):1016–1024. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


