Abstract
The coronavirus disease 2019 (COVID-19) pandemic required transplant nephrologists, surgeons, and care teams to make decisions about the full spectrum of transplant program operations and clinical practices in the absence of experience or data. Initially, across the country, there was a reduction in kidney transplant procedures and a striking pause in the conduct of living donation and living-donor transplant surgeries. Aspects of candidate evaluation and follow-up rapidly converted to telehealth. Months into the pandemic, much has been learned from experiences worldwide, yet many questions remain. In this Perspective, we reflect on some of the practice decisions made by the transplant community in the initial response to the pandemic and consider lessons learned, including those related to the risks, benefits, and logistical considerations of proceeding with versus delaying deceased-donor transplantation, living donation, and living-donor transplantation during the pandemic. We review the evolution of therapeutic strategies for severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection and their use in transplant recipients, current consensus related to immunosuppression management in infected transplant recipients, and emerging information on vaccination against SARS-CoV-2. We share our thoughts on research priorities, discuss the areas in which we are still practicing with uncertainty, and look ahead to the next phase of the pandemic response.
Index Words: Coronavirus disease 2019 (COVID-19), severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), pandemic, evaluation, follow-up, kidney transplantation, living kidney donation, screening, telehealth, therapeutics, vaccines, immunosuppression management, allograft rejection
Introduction
Transplant and nontransplant nephrologists’ practices were greatly and differentially affected by the coronavirus disease 2019 (COVID-19) pandemic. Unlike dialysis, which cannot be stopped or even paused, transplant evaluations and surgery were paused as an early response to the pandemic. While transplantation generally resumed, emerging reports of increased mortality raised questions for the response to future surges.1, 2, 3 In this Perspective, we describe some of the key practice decisions made by the transplant community in response to the pandemic. Focused on US and emerging international experiences, we consider lessons learned and look ahead to the next phase of the pandemic response.
Living Donor Program Management
Suspension of Practice: When Is it Necessary?
The COVID-19 pandemic substantially impacted the practice of living-donor kidney transplantation (LDKT). During the first week after the pandemic declaration on March 19, 2020, 80% of US deceased-donor kidney transplantation (DDKT) programs were operating with restrictions, whereas 72% of US LDKT programs reported that they had fully suspended living donation and LDKT.4 Similar suspensions were reported globally.5 , 6 The rationale for the dramatic early disruption of practices included the categorization of LDKT as “elective” (ie, distinct from some “essential” DDKT) and possible to safely delay, especially in locations with strained resources, including the availability of ventilators (Fig 1 ).7 In addition, LDKT has additional complexity related to safety concerns for recipients (given early reports suggesting that they faced substantially increased mortality risks following severe acute respiratory syndrome coronavirus 2 [SARS-CoV-2] infection)8 , 9 and also for living donors. A by-proxy effort to synthesize consensus based on 19 professional society bulletins published in March 2020 offered strong agreement (14 of 19) for reduction to a minimum, if not complete postponement, of elective transplantation, in particular LDKT, during the pandemic.10 Comments and experiences described on the American Society of Transplantation (AST) electronic community discussion board reached similar conclusions.11
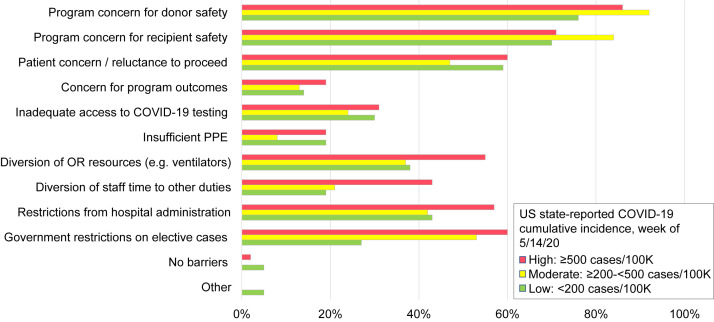
Figure 1.
Barriers to LDKT surgery during the COVID-19 pandemic. Original graphic ©2020 International Society of Nephrology; adapted from Lentine et al7 with permission of the copyright holder.
We recommend that transplant programs provide up-to-date communication regarding the operational status of their transplant programs and current practices and policies for ensuring donor and recipient safety. A survey of US programs in May 2020 identified multiple barriers to living donation and LDKT surgery during the early phase of the pandemic, including program concerns for donor (85%) and recipient (75%) safety, patient concerns (56%), elective case restrictions (47%), and hospital administrative restrictions (48%).7 Programs in which COVID-19 cumulative incidence was higher locally reported more barriers related to staff and resource diversion. By June 28, 2020, monthly US LDKT rates returned to prepandemic levels, even though the volume decrement from deferred procedures did not recover (Fig 2 A), in contrast to DDKT, which recovered to exceed 2019 levels (Fig 2B).12 Similar volume-change patterns occurred across programs of all sizes (Fig 2C and D). Kidney paired donation, which incurs additional complexities due to organ or patient travel, decreased by more than 50% in the pandemic period (249 procedures from March 1, 2020, to September 1, 2020).13 Key aspects of living donor care that warrant attention in transplant program policies and guidelines during the pandemic include presurgical screening and self-quarantine, processes for conducting routine evaluation and follow-up, and living donor counseling.
Figure 2.
Impact of the COVID-19 pandemic on US kidney transplant volumes. Cumulative LDKT (A) and DDKT (B) activity based on Organ Procurement and Transplantation Network and Scientific Registry of Transplant Recipients data as of September 2020 (green line) with cumulative volume for 2019 shown for comparison (blue line).12 Changes in volume of LDKT (C) and DDKT (D) in each US transplant program during the first 6 months of the pandemic (March 1, 2020, through August 31, 2020) compared with the preceding 6 months (September 1, 2019, through February 29, 2020) grouped by prepandemic program volume. For LDKT, the vast majority of US programs had a decrease in volume, which was particularly noticable in high-volume programs. For DDKT activity, even though volume was lower than at baseline at many programs, a substantial number of programs (mostly large and medium-sized, along with few small programs) performed more DDKT procedures than during the prepandemic period.
Prevention Strategies: COVID-19–Related Screening and Self-Quarantine
Early reports of increased morbidity and mortality among asymptomatic patients with SARS-CoV-2 infection undergoing surgical procedures in Wuhan, China,14 highlighted the need to ensure that living donors are not infected at the time of surgery. The American Society of Transplant Surgeons, the AST, and The Transplantation Society developed recommendations to promote donor and recipient safety.15, 16, 17 These societies endorse proceeding with living donation for asymptomatic individuals with a negative nasopharyngeal swab nucleic acid test result close to the time of donation surgery, and surveys of living-donor programs suggest strong agreement with this recommendation.6 , 7 However, the duration of predonation quarantine is more controversial. The American Society of Transplant Surgeons recommends a period of at least 7 days and preferably 2 weeks before donation for donors and recipients of LDKTs.15 The Transplantation Society recommends a 2-week quarantine for donors.17 Recent surveys show wide variation in adoption of these recommendations.6 , 7 In July 2020, the AST modified recommendations to remove the mandate for self-quarantine, but it was still recommended as a preventive strategy.16
Given the widespread nature of the pandemic, individuals who have recovered from COVID-19 may present for living donor candidate evaluation. The highly variable manifestations of COVID-19 render symptomatic screening alone insufficient. The protective effects of immunoglobulin G antibody—and the clinical implications of serologic findings in general—remain undefined.18 The potential for viral shedding in the urine19 may also hold implications for disease transmission via kidney donation.20, 21, 22 For living donors with a history of COVID-19, AST recommends considering donation only if the donor is at least 28 days from symptom resolution with a negative nucleic acid test result.16 This recommendation is opinion-based, and some have opened the conversation about considering when donation may be safe in the context of “mild” active SARS-CoV-2 infection.23
In April 2020, the Organ Procurement and Transplantation Network/United Network for Organ Sharing (OPTN/UNOS) temporarily paused data collection and submission requirements for follow-up of living donors (as well as recipient follow-up and recipient malignancy forms), retroactive to March 17, 2020.24 , 25 The purpose was to reduce potential testing-related patient exposure to SARS-CoV-2 and to decrease administrative burden on centers. Surveys of US and international centers identified barriers to living donor follow-up during the pandemic.6 , 7 The waiver was initially heavily used, with 40% of living donor follow-up in amnesty status by late June 2020, including as many as 60% of these forms in some regions.26 However, as of December 2020, more than 70% of forms have been completed. OPTN/UNOS is now considering how to handle the amnesty forms and when to resume follow-up,26 but, as of December 2020, amnesty remains in place. As discussed below, clinical follow-up during the pandemic can be facilitated by telehealth.
Living Donor Counseling Related to COVID-19
A recent survey of US programs found that less than half and less than a third, respectively, counsel donors that donation does not affect the risk of contracting COVID-19 or the risk of COVID-19 complications, whereas 57% educate donors about associations of COVID-19 and acute kidney injury (AKI).7 Although being a donor should not increase the risk of contracting COVID-19 in the community or lead to an immunosuppressed state, reduced renal reserve from surgical nephrectomy could heighten the risk of severe AKI in the context of severe infection. There have been no reports thus far of a recent living donor contracting COVID-19 and facing AKI, but this should continue to be monitored. Recent emergency use authorization of vaccines by the US Food and Drug Administration (FDA) in December 2020 raises the option of presurgical immunization of living donors and their transplant recipients 3-4 weeks before surgery as vaccine becomes available. The risk of vaccine-induced allosensitization is unknown, and impact on the final crossmatch should be monitored.
Changing Practices: Telehealth
Before the pandemic, and, in part, because of reimbursement limitations, telehealth was not widely used in transplant practice in the United States.27, 28, 29, 30 Experience in Germany indicated that telehealth can reduce acute hospitalizations and be cost-saving in caring for LKDT recipients.31 Several programs within the US Department of Veterans Affairs system had experience with the use of telehealth in evaluation of transplant candidates. Unlike most programs that draw the majority of patients from a local catchment, Veterans Affairs programs often evaluate patients who reside a long distance from the center and have significant comorbidities, making travel challenging. Notably, the Veterans Affairs experience involved patients who used local Veterans Affairs staff and facilities to perform physical examinations and provide internet equipment. Forbes et al demonstrated that telehealth costs less than in-person visits when used in this manner.32
The transplant community responded to the pandemic with rapid adoption of telehealth, facilitated by national reimbursement and policy changes. The US Centers for Medicare & Medicaid Services and insurance companies were responsive in permitting telehealth use in posttransplant care, recipient evaluations, and donor evaluations.33 In the past, Medicare paid for telehealth only for routine care in a limited set of circumstances, including those in which beneficiaries lived in remote areas, and the expectation was that the patient would receive telehealth services at a local facility, not from home. Despite the liberalization of telehealth restrictions, transplant programs have not embraced the technology for all aspects of transplant evaluations. This reluctance was illustrated in a recent survey that found that, even though programs successfully incorporated telehealth into their practices for education purposes as well as medical and social-work evaluations, most do not allow use of telehealth for the surgical evaluation of living donors and mandate at least 1 in-person visit before donation.7 Telehealth visits can decrease the need for patients to visit facilities, but do not directly address the critical issues of monitoring by laboratory testing. Some programs have adopted the use of home phlebotomy services, whereas others have advised patients to reduce testing frequency and/or time their visits to minimize waiting times. Home blood-pressure monitoring and other remote monitoring tools may increase options for adjunctive testing and monitoring outside of the clinic setting.34
During and after the pandemic, telehealth can provide programs and patients with flexibility and convenience for some aspects of the evaluation and follow-up care, as well as facilitating communication. Data are needed to ascertain whether telehealth-based communication with donors and recipients affects quality of care (eg, the possibility of information being missed without in-person evaluation by various practitioners) or access to care (eg, among patients or potential donors lacking internet access).26 Reimbursement policies that allow for telehealth are critical to sustained use. An August 2020 Executive Order endorsed development of regulations to support access to telehealth services after the public health emergency, including investments in physical and communications infrastructure.35 Given the large lapse in living donor and longer-term recipient follow-up during the pandemic,26 we believe continuation of telehealth waivers and reimbursement are vital to allowing programs to maintain long-term patient engagement.
Challenges to Kidney Transplant Patient Care
Information and guidelines on COVID-19 management are published online by the National Institutes of Health (NIH), including information on solid organ transplant (SOT) recipients.36 These guidelines support standard clinical practice for treatment of COVID-19 in transplant recipients based on expert opinion, as data are lacking and unlikely to be generated as a result of relatively smaller numbers of transplant patients compared with the general population. In addition, even though most infected individuals are outpatients, most published data focus on hospitalized patients.
Management of Immunosuppression
Currently, there are no evidence-based recommendations for managing immunosuppression in the context of the pandemic, either for prevention or as part of the management of patients with confirmed COVID-19. A recent US registry study of experience through July 2020 found a decrease in the use of lymphocyte-depleting induction agents in favor of basiliximab and no induction during the pandemic (adjusted odds ratio, 0.53), associated with trends toward increased rejection, but no change in use of maintenance steroids.37 The AST noted that the decision to reduce immunosuppression (generally antimetabolites or calcineurin inhibitors) should consider the balance between disease severity and risk of rejection.38 Whether to modify induction immunosuppression is also unclear.39
Treatment Agents for COVID-19
Remdesivir and Dexamethasone
A limitation to the treatment of COVID-19 is the lack of study in immunosuppressed populations. Remdesivir has received emergency use authorization from the FDA for use in severe disease, with a modest but statistically significant reduction in hospital stay length.40 Although only limited transplant patients were included in the initial study, the drug may be used in SOT recipients; however, use is not recommended when the estimated glomerular filtration rate is <30 mL/min/1.73 m2.41 Results from a dexamethasone trial (RECOVERY) indicate a small but statistically significant improvement in patient mortality at 28 days in those who required mechanical ventilation or supplemental oxygen, but not in those who already required ventilation at the time of randomization.42
Antibody Treatments and Convalescent Plasma
Infusions of immune globulin have been used in other viral diseases such as Ebola virus43 and in some diseases in SOT recipients such as metapneumovirus in the absence of approved, effective antiviral agents. Although the initial reports of treated patients were promising,44 subsequently, several thousand infected patients have received plasma infusions, with inconsistent benefit. Problematic here may be the lack of standards for selection of immune plasma and variability in the antibody response to the virus based on infection severity.45 Moreover, there is no standard for the frequency or volume of infusions. The National COVID-19 Convalescent Plasma Project is a collaborative effort of multiple academic centers and research entities to maintain a data registry for treated patients.46 Regardless of limited findings, the FDA issued an emergency use authorization for the use of convalescent plasma within 3 days of onset of symptoms. However, NIH treatment guidelines note insufficient data to recommend for or against its use.47
Emergency use authorizations have also been recently granted for COVID-19–specific antibodies, namely bamlanivimab and the antibody cocktail of casirivimab and imdevimab. The former is a monoclonal antibody targeting the receptor-binding domain of the spike protein of SARS-CoV-2 (disrupting viral entry into the host’s cells),48 and the latter consists of a combination of recombinant monoclonal antibodies that also bind the same domain.49 Both therapies are approved for the treatment of nonhospitalized patients with mild to moderate COVID-19 who are at high risk for progression to severe disease and/or hospitalization. Neither agent is recommended by the NIH expert guidelines as a result of lack of sufficient data, although academic centers have pivoted the treatment of many infected patients to outpatient infusion centers. Use in transplant recipients has not yet been assessed, but some use is being implemented.
Other Ineffective Agents
There are other agents that were used empirically or studied as antiviral agents based on in vitro studies that used relatively high doses.50 A number of these that were used commonly early in the pandemic—including azithromycin, hydroxychloroquine, chloroquine, HIV reverse transcriptase inhibitors, and ribavirin—are now avoided because of associated complications.36 Tocilizumab, an anti–interleukin 6 receptor monoclonal antibody, was used on an off-label basis to mitigate the inflammatory cytokine storm associated with viral infection.51 The phase 3 COVACTA trial for severe COVID-19 pneumonia demonstrated nonimprovement in 452 randomized patients, 294 of whom received tocilizumab (none of whom had transplants), in terms of clinical disease acuity and ventilator dependence compared with placebo controls.52 Another randomized trial of 243 hospitalized patients not receiving mechanical ventilation at the time of treatment found no effect on prevention of intubation or death.53 Despite its initial empirical use for management of patients with COVID-19, including transplant recipients,8 , 54 and limited adverse-event profile, this agent is not recommended as a result of its lack of efficacy.
Vaccine Development
Successful vaccine development against coronaviruses had previously been elusive. Animal models for efficacy have not been shown to prevent human disease and may not be effective on a long-term basis.55 , 56 The most recently published studies demonstrate marked efficacy of 2 mRNA vaccines, neither of which has been tested in SOT recipients57, 58, 59; however, there are efforts to develop registries of transplant recipients who receive these vaccines.60 At issue is vaccine safety and avoidance of syndromes associated with vaccine-enhanced disease, be it antibody-dependent enhancement or vaccine-associated enhanced respiratory disease, but these serious adverse events have not been reported in phase 3 trials.61 , 62 The Centers for Disease Control and Prevention (CDC)’s advisory committee on immunization practices has added transplant recipients to their phased delivery to the public, including them in the third group of phase 1 (1c), following the other phase 1 groups: health care workers and residents of long-term care facilities (1a) and front-line workers (1b).63 These administration priorities may be further adapted by state governments and local health authorities. Based on previous vaccination guidelines for SOT recipients, the AST recommends that all transplant candidates and their household members receive vaccination when it becomes available, ideally more than 2 weeks before transplantation, or starting 1-6 months after transplantation.64 As complex as mass delivery will be, patient acceptance may prove even more challenging.
Allograft Rejection
An ongoing debate is whether rejection is more frequent with COVID-19 and whether maintenance immunosuppression has some beneficial impact on outcomes. This is complicated by reports of relatively higher recipient mortality rates than in the general population, primarily due to the multiple comorbidities as opposed to immunosuppressed condition.8 , 9 , 65 There are limited data, as the ability to perform biopsies in actively infected patients with graft dysfunction was eliminated by the critical nature of their illness. Hence, it is unclear if the reported level of AKI in this population represents rejection or AKI associated with volume depletion, hypoperfusion, or systemic infection.8 , 65 , 66 The long-term consequences of immunosuppressive reduction in the context of COVID-19 are unknown but may include precipitating de novo donor-specific antibodies and/or subclinical rejection. Some groups have reported performing immune monitoring by using commercially available tools such as donor-derived cell-free DNA and peripheral blood mononuclear cell transcriptomics, but there are no data supporting this approach and insufficient data to assess the impact of these tests in context of a systemic illness with intrinsic graft dysfunction that may not be related to alloimmunity. For now, we recommend consulting the patient’s transplant team before making any changes to maintenance immunosuppression.
Research Priorities
As outlined above, ongoing research priorities related to COVID-19 in transplantation include developing a better understanding of the risks of waiting for a transplant versus undergoing transplantation with requisite immunosuppression. The safety and efficacy of COVID-19 treatments have not been defined in this population. Further information is needed to identify best practices in immunosuppression management, long-term immunologic outcomes, and the role of novel monitoring tools. There is also a need for ongoing research to define optimal living donation screening strategy and timing, as well as the use of presurgical vaccination. Resource considerations aside, when the risk-benefit ratio favors transplantation versus delay is not yet defined. A study of UK registries demonstrated that kidney transplant candidates on the waitlist were more likely to be positive for SARS-CoV-2 but less likely to die than transplant recipients with SARS-CoV-2 infection.67 Patients who received transplants in 2020 were more likely to become infected, as well as more likely to die, than those who received a transplant earlier.67 However, a simulation model suggests that, in most cases, patients benefit from receiving a kidney transplant compared with waiting until the pandemic subsides.68 Continued assessment of outcomes information and translation to accessible tools69 are needed to guide decisions about when to perform the transplant and subsequent patient management.
Conclusion
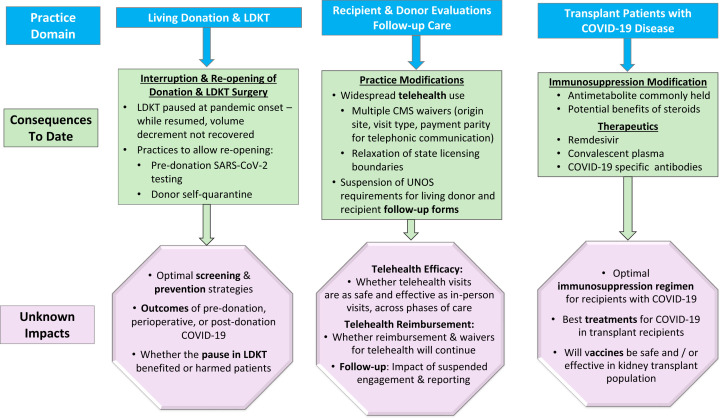
The COVID-19 pandemic has presented transplant clinicians with unprecedented uncertainties across the spectrum of practice (Fig 3 ). LKDT procedures paused at the onset of the pandemic and then resumed, even though the overall number of LDKT procedures performed in 2020 remains lower than in the previous year. By comparison, DDKT volume has surpassed even the record 2019 levels (Fig 2B). Strategies that enabled transplant practice to resume include widespread use of telehealth and presurgical screening. Although grounded in science, the field of transplantation has always incorporated elements of art, experience, theory, and preference. Decisions regarding how to manage and treat transplant patients with COVID-19, and whether to undertake the risk of vaccination, will continue to require judgment, art, and theory as the evidence for best practices evolves. In the face of these uncertainties, we are learning from case series, registry reporting, shared experiences, and consensus recommendations. In the absence of trial-based evidence, we should include transplant patients and living donors in prospective studies and registries to help guide optimal care.
Figure 3.
Currently known and uncertain future impacts of the COVID-19 pandemic across the spectrum of kidney transplantation practice.
Article Information
Authors’ Full Names and Academic Degrees
Krista L. Lentine, MD, PhD, Roslyn B. Mannon, MD, and Michelle A. Josephson, MD.
Support
Dr Lentine receives support for living donation research from National Institute of Diabetes and Digestive and Kidney Diseases grant R01DK120551 and the Mid-America Transplant/Jane A. Beckman Endowed Chair in Transplantation.
Financial Disclosure
The authors declare that they have no relevant financial interests.
Other Disclosures
Drs Lentine, Mannon, and Josephson are members of the American Society of Nephrology (ASN) COVID-19 Response Team. Dr Lentine is a member of the ASN Quality Committee, and Drs Mannon and Josephson are members of the ASN Policy and Advocacy Committee.
Disclaimer
The views expressed are those of the authors and in no way should be seen as an official policy of any committee or funding agency. Data on US kidney transplant procedures reported here have been supplied by the Hennepin Healthcare Research Institute as the contractor for the Scientific Registry of Transplant Recipients (SRTR). The interpretation and reporting of these data are the responsibility of the authors and in no way should be seen as an official policy of or interpretation by the SRTR or the US Government.
Peer Review
Received September 30, 2020, in response to an invitation from the journal. Evaluated by 2 external peer reviewers, with direct editorial input from an Associate Editor and a Deputy Editor. Accepted in revised form December 19, 2020.
Footnotes
Complete author and article information provided before references.
References
- 1.Jager K.J., Kramer A., Chesnaye N.C. Results from the ERA-EDTA Registry indicate a high mortality due to COVID-19 in dialysis patients and kidney transplant recipients across Europe. Kidney Int. 2020;98(6):1540–1548. doi: 10.1016/j.kint.2020.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Thaunat O., Legeai C., Anglicheau D. IMPact of the COVID-19 epidemic on the moRTAlity of kidney transplant recipients and candidates in a French Nationwide registry sTudy (IMPORTANT) Kidney Int. 2020;98(6):1568–1577. doi: 10.1016/j.kint.2020.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Caillard S., Anglicheau D., Matignon M. An initial report from the French SOT COVID Registry suggests high mortality due to COVID-19 in recipients of kidney transplants. Kidney Int. 2020;98(6):1549–1558. doi: 10.1016/j.kint.2020.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Boyarsky B.J., Po-Yu Chiang T., Werbel W.A. Early impact of COVID-19 on transplant center practices and policies in the United States. Am J Transplant. 2020;20(7):1809–1818. doi: 10.1111/ajt.15915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ahn C., Amer H., Anglicheau D. Global Transplantation COVID Report March 2020. Transplantation. 2020;104(10):1974–1983. doi: 10.1097/TP.0000000000003258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Salvalaggio P.R., Ferreira G.F., Caliskan Y. An international survey on living kidney donation and transplant practices during the COVID-19 Pandemic. Transpl Infect Dis. 2020:e13526. doi: 10.1111/tid.13526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lentine K.L., Vest L.S., Schnitzler M.A. Survey of US living kidney donation and transplantation practices in the COVID-19 era. Kidney Int Rep. 2020;5(11):1894–1905. doi: 10.1016/j.ekir.2020.08.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Akalin E., Azzi Y., Bartash R. Covid-19 and kidney transplantation. N Engl J Med. 2020;382(25):2475–2477. doi: 10.1056/NEJMc2011117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pereira M.R., Mohan S., Cohen D.J. COVID-19 in solid organ transplant recipients: initial report from the US epicenter. Am J Transplant. 2020;20(7):1800–1808. doi: 10.1111/ajt.15941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ritschl P.V., Nevermann N., Wiering L. Solid organ transplantation programs facing lack of empiric evidence in the COVID-19 pandemic: a by-proxy society recommendation consensus approach. Am J Transplant. 2020;20(7):1826–1836. doi: 10.1111/ajt.15933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cravedi P., Schold J.D., Safa K. The COVID-19 pandemic: a community approach. Clin Transplant. 2020;34(11) doi: 10.1111/ctr.14059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.United Network for Organ Sharing (UNOS) COVID-19 and solid organ transplant. https://unos.org/covid/ Accessed September 30, 2020.
- 13.Scientific Registry of Transplant Recipients (SRTR). Kidney Pancreas Transplant Standard Analytic File (SAF). Data through September 2020. Accessed December 10, 2020. SRTR data are publicly available at: https://www.srtr.org/requesting-srtr-data/data-requests/
- 14.Lei S., Jiang F., Su W. Clinical characteristics and outcomes of patients undergoing surgeries during the incubation period of COVID-19 infection. EClinicalMedicine. 2020;21:100331. doi: 10.1016/j.eclinm.2020.100331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.American Society of Transplant Surgeons (ASTS) re-engaging organ transplantation in the COVID-19 era. Posted June 5, 2020. https://asts.org/advocacy/covid-19-resources/asts-covid-19-strike-force/re-engaging-organ-transplantation-in-the-covid-19-era#.XwnX7ROSmUk Accessed December 10, 2020.
- 16.American Society of Transplantation (AST) 2019-nCoV (coronavirus): recommendations and guidance for organ donor testing. https://www.myast.org/covid-19-information Updated October 5, 2020. Accessed December 10, 2020.
- 17.The Transplantation Society (TTS) Guidance on coronavirus disease 2019 (covid-19) for transplant clinicians. https://tts.org/tid-about/tid-presidents-message/23-tid/tid-news/657-tid-update-and-guidance-on-2019-novel-coronavirus-2019-ncov-for-transplant-id-clinicians Updated June 8, 2020. Accessed December 10, 2020.
- 18.Sethuraman N., Jeremiah S.S., Ryo A. Interpreting diagnostic tests for SARS-CoV-2. JAMA. 2020;323(22):2249–2251. doi: 10.1001/jama.2020.8259. [DOI] [PubMed] [Google Scholar]
- 19.Abrishami A., Samavat S., Behnam B., Arab-Ahmadi M., Nafar M., Sanei Taheri M. Clinical course, imaging features, and outcomes of COVID-19 in kidney transplant recipients. Eur Urol. 2020;78(2):281–286. doi: 10.1016/j.eururo.2020.04.064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Walsh K.A., Jordan K., Clyne B. SARS-CoV-2 detection, viral load and infectivity over the course of an infection. J Infect. 2020;81(3):357–371. doi: 10.1016/j.jinf.2020.06.067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Widders A., Broom A., Broom J. SARS-CoV-2: the viral shedding vs infectivity dilemma. Infect Dis Health. 2020;25(3):210–215. doi: 10.1016/j.idh.2020.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.He X., Lau E.H.Y., Wu P. Temporal dynamics in viral shedding and transmissibility of COVID-19. Nat Med. 2020;26(5):672–675. doi: 10.1038/s41591-020-0869-5. [DOI] [PubMed] [Google Scholar]
- 23.Kates O.S., Fisher C.E., Rakita R.M., Reyes J.D., Limaye A.P. Emerging evidence to support not always “just saying no” to SARS-CoV-2 positive donors. Am J Transplant. 2020;20(11):3261–3262. doi: 10.1111/ajt.16119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Organ Procurement and Transplantation Network (OPTN) COVID-19: temporary changes to data requirements. https://optn.transplant.hrsa.gov/covid-19/ Accessed December 10, 2020.
- 25.Organ Procurement and Transplantation Network (OPTN) COVID-19 Policy Actions Implemented. https://optn.transplant.hrsa.gov/governance/policy-notices/ Accessed December 10, 2020.
- 26.Organ Procurement and Transplantation Network (OPTN) COVID-19 emergency policies and data collection. https://optn.transplant.hrsa.gov/governance/public-comment/ Accessed December 10, 2020.
- 27.Lee T.C., Kaiser T.E., Alloway R., Woodle E.S., Edwards M.J., Shah S.A. Telemedicine based remote home monitoring after liver transplantation: results of a randomized prospective trial. Ann Surg. 2019;270(3):564–572. doi: 10.1097/SLA.0000000000003425. [DOI] [PubMed] [Google Scholar]
- 28.Pape L., de Zwaan M., Tegtbur U. The KTx360 degrees -study: a multicenter, multisectoral, multimodal, telemedicine-based follow-up care model to improve care and reduce health-care costs after kidney transplantation in children and adults. BMC Health Serv Res. 2017;17(1):587. doi: 10.1186/s12913-017-2545-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Murgia F., Corona B., Bianciardi F., Romano P., Tagliente I., Bella S. The application of telemedicine in the follow-up of lung transplantation in a patient with cystic fibrosis. Clin Ter. 2014;165(5):e382–e383. doi: 10.7417/T.2014.1769. [DOI] [PubMed] [Google Scholar]
- 30.Mammas C.S., Geropoulos S., Kavantzas N. Telemedicine systems in organ transplantation: a feasibility and reliability study of the integrated teleradiological and tele-pathological evaluation of the cardiac graft. Stud Health Technol Inform. 2014;202:303–306. [PubMed] [Google Scholar]
- 31.Schmid A., Hils S., Kramer-Zucker A. Telemedically supported case management of living-donor renal transplant recipients to optimize routine evidence-based aftercare: a single-center randomized controlled trial. Am J Transplant. 2017;17(6):1594–1605. doi: 10.1111/ajt.14138. [DOI] [PubMed] [Google Scholar]
- 32.Forbes R.C., Rybacki D.B., Johnson T.B., Hannah-Gillis A., Shaffer D., Hale D.A. A cost comparison for telehealth utilization in the kidney transplant waitlist evaluation process. Transplantation. 2018;102(2):279–283. doi: 10.1097/TP.0000000000001903. [DOI] [PubMed] [Google Scholar]
- 33.Centers for Medicare & Medicaid Services (CMS) President Trump expands telehealth benefits for medicare beneficiaries during COVID-19 outbreak. March 17, 2020. https://www.cms.gov/newsroom/press-releases/president-trump-expands-telehealth-benefits-medicare-beneficiaries-during-covid-19-outbreak Accessed December 10, 2020.
- 34.Manta C., Jain S.S., Coravos A., Mendelsohn D., Izmailova E.S. An evaluation of biometric monitoring technologies for vital signs in the era of COVID-19. Clin Transl Sci. 2020;13(6):1034–1044. doi: 10.1111/cts.12874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.The White House Executive Order on Improving Rural Health and Telehealth Access. Posted August 3, 2020. www.whitehouse.gov/presidential-actions/executive-order-improving-rural-health-telehealth-access/ Accessed December 10, 2020.
- 36.National Institutes of Health (NIH) COVID-19 Treatment Guidelines. Special considerations in solid organ transplant, hematopoietic stem cell transplant, and cellular therapy candidates, donors, and recipients. https://www.covid19treatmentguidelines.nih.gov/special-populations/transplant/ Accessed December 10, 2020.
- 37.Bae S., McAdams-DeMarco M.A., Massie A.B. Early changes in kidney transplant immunosuppression regimens during the covid-19 pandemic. Transplantation. 2021;105(1):170–176. doi: 10.1097/TP.0000000000003502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.American Society of Transplantation (AST) 2019-nCoV (Coronavirus): 2019-nCoV (Coronavirus): FAQs for organ transplantation. Updated October 13, 2020. https://www.myast.org/covid-19-information Accessed December 10, 2020.
- 39.Imam A., Tzukert K., Merhav H. Practical recommendations for kidney transplantation in the COVID-19 pandemic. World J Transplant. 2020;10(9):223–229. doi: 10.5500/wjt.v10.i9.223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Beigel J.H., Tomashek K.M., Dodd L.E. Remdesivir for the treatment of Covid-19 - final report. N Engl J Med. 2020;383(19):1813–1826. doi: 10.1056/NEJMoa2007764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Adamsick M.L., Gandhi R.G., Bidell M.R. Remdesivir in patients with acute or chronic kidney disease and COVID-19. J Am Soc Nephrol. 2020;31(7):1384–1386. doi: 10.1681/ASN.2020050589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.RECOVERY Collaborative Group. Horby P., Lim W.S., Emberson J.R. Dexamethasone in hospitalized patients with covid-19. N Engl J Med. 2021;384:693–704. doi: 10.1056/NEJMoa2021436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Marano G., Vaglio S., Pupella S. Convalescent plasma: new evidence for an old therapeutic tool? Blood Transfus. 2016;14(2):152–157. doi: 10.2450/2015.0131-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Shen C., Wang Z., Zhao F. Treatment of 5 critically ill patients with COVID-19 with convalescent plasma. JAMA. 2020;323(16):1582–1589. doi: 10.1001/jama.2020.4783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wang Y., Zhang L., Sang L. Kinetics of viral load and antibody response in relation to COVID-19 severity. J Clin Invest. 2020;130(10):5235–5244. doi: 10.1172/JCI138759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.National COVID-19 Convalescent Plasma Project “Who are we?”. https://ccpp19.org/about/index.html Accessed September 23, 2020.
- 47.National Institutes of Health (NIH) COVID-19 Treatment Guidelines. Convalescent plasma. https://www.covid19treatmentguidelines.nih.gov/immune-based-therapy/blood-derived-products/convalescent-plasma/ Last updated October 9, 2020. Accessed December 10, 2020. [PubMed]
- 48.Chen P., Nirula A., Heller B., BLAZE-1 Investigators SARS-CoV-2 neutralizing antibody LY-CoV555 in outpatients with covid-19. N Engl J Med. 2021;384(3):229–237. doi: 10.1056/NEJMoa2029849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Food and Drug Administration (FDA) Fact sheet for health care providers Emergency Use Authorization (EAU) of casirivimab and imdevimab. https://www.fda.gov/media/143892/download Accessed December 10, 2020.
- 50.Dong L., Hu S., Gao J. Discovering drugs to treat coronavirus disease 2019 (COVID-19) Drug Discov Ther. 2020;14(1):58–60. doi: 10.5582/ddt.2020.01012. [DOI] [PubMed] [Google Scholar]
- 51.Tay M.Z., Poh C.M., Renia L., MacAry P.A., Ng L.F.P. The trinity of COVID-19: immunity, inflammation and intervention. Nat Rev Immunol. 2020;20(6):363–374. doi: 10.1038/s41577-020-0311-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Rosas I, Bräu N, Waters M, et al. Tocilizumab in hospitalized patients with COVID-19 pneumonia [published online ahead of print September 12, 2020]. medRxiv. https://doi.org/10.1101/2020.08.27.20183442
- 53.Stone J.H., Frigault M.J., Serling-Boyd N.J. Efficacy of tocilizumab in patients hospitalized with covid-19. N Engl J Med. 2020;383(24):2333–2344. doi: 10.1056/NEJMoa2028836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Alberici F., Delbarba E., Manenti C. A single center observational study of the clinical characteristics and short-term outcome of 20 kidney transplant patients admitted for SARS-CoV2 pneumonia. Kidney Int. 2020;97(6):1083–1088. doi: 10.1016/j.kint.2020.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Edridge A.W.D., Kaczorowska J., Hoste A.C.R. Seasonal coronavirus protective immunity is short-lasting. Nat Med. 2020;26(11):1691–1693. doi: 10.1038/s41591-020-1083-1. [DOI] [PubMed] [Google Scholar]
- 56.Roper R.L., Rehm K.E. SARS vaccines: where are we? Expert Rev Vaccines. 2009;8(7):887–898. doi: 10.1586/erv.09.43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Lurie N., Saville M., Hatchett R., Halton J. Developing covid-19 vaccines at pandemic speed. N Engl J Med. 2020;382(21):1969–1973. doi: 10.1056/NEJMp2005630. [DOI] [PubMed] [Google Scholar]
- 58.Thanh Le T., Andreadakis Z., Kumar A. The COVID-19 vaccine development landscape. Nat Rev Drug Discov. 2020;19(5):305–306. doi: 10.1038/d41573-020-00073-5. [DOI] [PubMed] [Google Scholar]
- 59.Graham B.S. Rapid COVID-19 vaccine development. Science. 2020;368(6494):945–946. doi: 10.1126/science.abb8923. [DOI] [PubMed] [Google Scholar]
- 60.Epidemiology Research Group in Organ Transplantation (ERGOT) COVID-19 antibody testing of recipients of solid organ transplants. https://transplantvaccine.org/ Accessed December 10, 2020.
- 61.Voysey M., Clemens S.A.C., Madhi S.A., Oxford COVID Vaccine Trial Group Safety and efficacy of the ChAdOx1 nCoV-19 vaccine (AZD1222) against SARS-CoV-2: an interim analysis of four randomised controlled trials in Brazil, South Africa, and the UK. Lancet. 2021;397(10269):99–111. doi: 10.1016/S0140-6736(20)32661-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Doshi P. Covid-19 vaccine trial protocols released. BMJ. 2020;371:m4058. doi: 10.1136/bmj.m4058. [DOI] [PubMed] [Google Scholar]
- 63.Dooling K. ACIP COVID-19 Vaccines Work Group. Phased allocation of COVID-19 vaccines. ACIP meeting 11/23/2020. https://www.cdc.gov/vaccines/acip/meetings/downloads/slides-2020-11/COVID-04-Dooling.pdf Accessed December 10, 2020.
- 64.American Society of Transplantation (AST) COVID-19 vaccine FAQ sheet. Posted 12/10/2020. https://www.myast.org/covid-19-vaccine-faq-sheet Accessed December 10, 2020.
- 65.Kates OS, Haydel BM, Florman SS, et al; UW COVID-19 SOT Study Team. COVID-19 in solid organ transplant: a multi-center cohort study [published online ahead of print August 7, 2020]. Clin Infect Dis. https://doi.org/10.1093/cid/ciaa1097
- 66.Hoek R.A.S., Manintveld O.C., Betjes M.G.H. COVID-19 in solid organ transplant recipients: a single-center experience. Transpl Int. 2020;33(9):1099–1105. doi: 10.1111/tri.13662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Ravanan R., Callaghan C.J., Mumford L. SARS-CoV-2 infection and early mortality of waitlisted and solid organ transplant recipients in England: a national cohort study. Am J Transplant. 2020;20(11):3008–3018. doi: 10.1111/ajt.16247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Massie A.B., Boyarsky B.J., Werbel W.A. Identifying scenarios of benefit or harm from kidney transplantation during the COVID-19 pandemic: a stochastic simulation and machine learning study. Am J Transplant. 2020;20(11):2997–3007. doi: 10.1111/ajt.16117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Scientific Registry of Transplant Recipients (SRTR) Effect of COVID-19 on the transplant system. https://www.srtr.org/reports-tools/covid-19-evaluation/ Accessed December 10, 2020.