Abstract
Background:
There is currently no consensus about standardized gait bout definitions when passively monitoring walking during normal daily life activities. It is also not known how different definitions of a gait bout in daily life monitoring affects the ability to distinguish pathological gait quality. Specifically, how many seconds of a pause with no walking indicates an end to one gait bout and the start of another bout? In this study, we investigated the effect of 3 gait bout definitions on the discriminative ability to distinguish quality of walking in people with multiple sclerosis (MS) from healthy control subjects (HC) during a week of daily living.
Methods:
15 subjects with MS and 16 HC wore instrumented socks on each foot and one Opal sensor over the lower lumbar area for a week of daily activities for at least 8 h/day. Three gait bout definitions were based on the length of the pause between the end of one gait bout and start of another bout (1.25 s, 2.50 s, and 5.0 s pause). Area under the curve (AUC) was used to compare gait quality measures in MS versus HC.
Results:
Total number of gait bouts over the week were statistically significantly different across bout definitions, as expected. However, AUCs of gait quality measures (such as gait speed, stride length, stride time) discriminating people with MS from HC were not different despite the 3 bout definitions.
Significance:
Quality of gait measures that discriminate MS from HC during daily life are not influenced by the length of a gait bout, despite large differences in quantity of gait across bout definitions. Thus, gait quality measures in people with MS versus controls can be compared across studies using different gait bout definitions with pause lengths ≤5 s.
Keywords: Gait, Free-living, Multiple sclerosis, Bout, Wearable sensors
1. Introduction
Gait impairments are very common in patients with multiple sclerosis (MS), leading to an elevated risk of falls and reduced quality of life [1,2]. Although the contributing factors for gait disability are complex in MS [3]; sensory deficits, imbalance, weakness, spasticity, and/or ataxia impairments are thought to contribute to most of the gait impairments in MS [3]. Gait analysis can often determine the problem(s) underlying the gait disability and then can be useful to test the efficacy of new interventions. Quantitative analysis of gait may be particularly helpful for clinical trials that investigate the effectiveness of novel pharmacologic and non-pharmacologic interventions to ameliorate gait impairments in MS.
Recently, the use of wearable sensors has made it possible to quantify the quality of gait outside the clinic during real-life situations [4,5, 6–25]. Wearable sensors can augment the standard clinical assessment of gait by active monitoring, such as performing predefined walking tests [1,17–20]. Additionally, wearable inertial sensors can be used for passive monitoring to increase the ecological validity of gait assessment by measuring walking quality during daily life in the community (for details, see [6,7,14]).
However, a key challenge in using wearable sensors to passively monitor walking in daily life is no agreement on how to define the start and end of each walking bout to investigate potential digital biomarkers of gait quality [6,26]. Specifically, it is not clear how long a subject can pause walking within a gait bout before identifying it as a separate bout. Allowing variable pause lengths of 1–5 s within walking bouts is likely to alter the number and duration of bouts included in analyses. Since recent studies have shown that bout duration may affect gait quality measures such as naturally faster walking velocity for longer than shorter walking bouts [9,27], it is important to define what constitutes a single walking bout. Daily and weekly average gait quality measures are derived after each walking bout is identified, and the gait quality measures are then used to determine the most discriminative gait measures to use as a digital endpoints for clinical trials [11,24].
In this study, we investigated the effects of different walking bout definitions based on 3 different pause lengths bouts on the discriminative ability of gait quantity and quality measures to distinguish MS from age-matched healthy control subjects (HC). All gait measures (e.g., gait speed, stride length), except activity measures, were referred to as “quality” whereas gait measures reflecting the activity (e.g., number of bouts/hour) were referred to as “quantity” of gait. We hypothesized that quantity and quality of gait will differ across the 3 bout definitions and hence, will affect the ability to discriminate gait quality between MS and HC groups.
2. Methods
2.1. Participants
Fifteen MS (49 ± 10.25 years), and 16 age-matched HC (44.75 ± 10.77 years) participated in this study. Inclusion criteria for MS were a confirmed diagnosis of relapsing-remitting MS [28], a mild-to-moderate MS-associated disability (EDSS score ≤ 6.0) confirmed by a neurology specialist, and complaints about mobility. Exclusion criteria for all subjects included the inability to follow protocol instructions, other factors affecting gait such as hip replacement, musculoskeletal disorder, uncorrected vision or vestibular problem. All participants provided informed consent approved by the Oregon Health & Science University Institutional Review Board.
2.2. Daily life gait data collection
Subjects were asked to wear instrumented socks (prototype developed by APDM Wearable Technologies, Portland, Oregon, USA) on each foot, and one Opal sensor over the lower lumbar area with an elastic belt for a week of daily activities for at least 8 h/day, details in Shah et al. [29]. Briefly, the inertial sensor within the sock is located on the dorsum of the foot like the Opal sensors worn in the laboratory. The main unit containing the battery is located in a second pocket just above the lateral malleolus. To maximize fit, the socks come in different sizes, and the Velcro attachment around the foot and ankle is adjustable to ensure that a snug fit and that the sensor does not move on the foot while being worn. The instrumented socks are synchronized with the Opal (sampling rate =128 Hz) worn on the lumbar area. The subjects removed the socks and the belt at night to recharge the batteries. Data were stored in the internal memory (8 GB) of the Opals. Subjects mailed back the sensors using a pre-paid box after completion of a week of data collection. Data were uploaded to a secure cloud-based database upon return of the devices and downloaded to a local computer for further processing.
2.3. Gait measures
The algorithms used for extracting spatial and temporal measures of gait during daily life gait have been detailed previously [30,31]. The algorithm begins by using a fast step detection algorithm that searches for periods when one foot is stationary while the other foot is moving. The algorithm then groups the detected steps into potential bouts, that are periods of continuous walking with sequences of at least 3 steps detected. A sequence of steps only qualifies as a potential bout if the duration between steps is no more than some threshold (depending upon a bout definition), the duration of the sequence of steps is at least 3 s, and the potential bout includes at least 3 steps. When possible, the start of the potential bout is defined as 5 s prior to the first detected step and the end of the potential bout is defined as 5 s after the last detected steps (referred to as potential bouts). This allows the next stage of processing to detect other steps that may not have been detected by the fast step detection algorithm.
Each potential bout is then processed with the commercial gait analysis algorithms included in Mobility Lab (APDM Wearable Technologies, Portland, Oregon, USA) [32] which have been validated previously [33,34]. These algorithms use the Unscented Kalman Filter (UKF) and zero-velocity updates to estimate the orientation of the sensors on the feet from the accelerometers and gyroscopes [35,36]. These algorithms also estimate the orientation and position trajectory of each foot between stance periods. These algorithms also perform another, independent step-detection algorithm with many validation criteria to ensure the final detection is very accurate. After this stage of processing, only bouts that contained validated steps with at least two complete strides during normal forward-progression walking are retained as the final bouts for further processing (referred to as valid bouts). The data processing flow is summarized in Fig. 1.
Fig. 1.
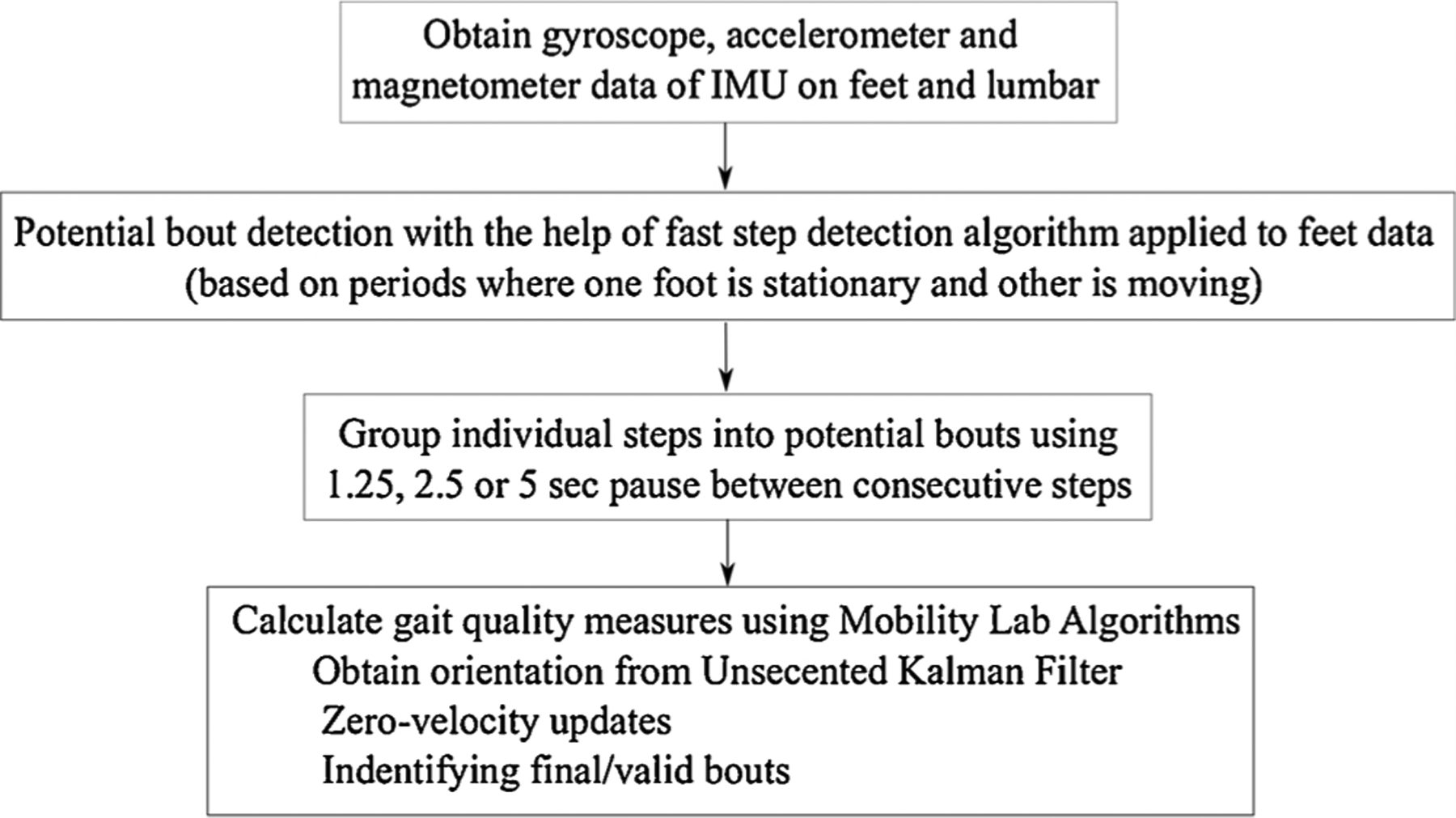
Flowchart of data processing to calculate gait measures.
A set of gait quality measures are calculated for each of the valid bouts. We calculated the average value of each gait quality measure for each bout. Finally, we calculated the mean and coefficient of variation (CV) across all of the measures’ bout averages. The list of all gait quality measures that we extracted from the data is given in Table 1.
Table 1.
List of the 24 gait quality measures.
| Gait Speed (m/s) | Elevation at Mid Swing (cm) |
|---|---|
| Stride Duration (s) | Stride Length (m) |
| Double Support (%) | Cadences (strides/min) |
| Swing Time (%) | Pitch at Initial Contact (°) |
| Pitch at Toe Off (°) | Transverse Range of Motion (rad) |
| Coronal Range of Motion (rad) | Sagittal Range of Motion (rad) |
| Gait Speed CV (-) | Elevation at Mid Swing CV (-) |
| Stride Duration CV (-) | Stride Length CV (-) |
| Double Support CV (-) | Cadence CV (-) |
| Swing Time CV (-) | Pitch at Initial Contact CV (-) |
| Pitch at Toe Off CV (-) | Transverse Range of Motion CV (-) |
| Coronal Range of Motion CV (-) | Sagittal Range of Motion CV (-) |
CV = Coefficient of Variation.
The thresholds for defining 3 bout definitions for potential bouts were as follows:
Bout Definition 1 (1.25 s pause): Individual steps are combined into potential bouts of walking, as long as the duration from one step to the next step is no longer than 1.25 seconds.
Bout Definition 2 (2.50 s pause): Individual steps are combined into potential bouts of walking, as long as the duration from one step to the next step is no longer than 2.50 seconds [8].
Bout Definition 3 (5.00 s pause): Individual steps are combined into potential bouts of walking, as long as the duration from one step to the next step is no longer than 5.00 seconds.
2.4. Statistical analysis
To compare a total number of potential and valid bouts/hour across 3 bout definitions, we used the Kruskal–Wallis rank-sum test, and for the post-hoc pairwise analysis, we used the Wilcoxon rank-sum test. To investigate which specific gait quality measures best discriminated mobility characteristics between the MS from the control group, we calculated ROC curves [37] and computed the AUC [38]. All the statistical analyses were performed using R Software (version 3.6.1). The same procedure was repeated for all 3 bout definitions to investigate the effects of bout definitions on AUC between MS and controls for each gait quality measures.
3. Results
Total wear time was similar in both MS and HC groups with a minimum of 5 days of recordings. Specifically, the grouped average of total wear time was 60.20 ± 11.41 (mean ± SD) hours (range: [44–78] h) for MS and 64.15 ± 9.91 h (range: [44–76] h) for HC (p = 0.319).
Both the total number of weekly average potential bouts/hour (p < 0.001) and valid bouts/hour (p = 0.001) (combining both groups) were statistically significantly different among 3 bout definitions (see Fig. 2). Specifically, post-hoc analysis for potential bouts/hour showed that total number of potential bouts/hour were significantly different between bout definition 1 (1.25 s pause) and definition 3 (5.00 s pause) (p < 0.001), and between bout definitions 2 (2.50 s pause) and 3 (p = 0.007). Similarly, post-hoc analysis for valid bouts/hour showed that total number of valid bouts/hour were significantly different between definitions 1 and 3 (p < 0.001), and between bout definitions 2 and 3 (p = 0.034). The 3 bout definitions also resulted in different length gait bouts (see Fig. 3). The longest pause between the consecutive steps (5 s pause) resulted in a smaller number of shorter bouts. The detailed group-wise analysis showed that the difference across the 3 bout definitions for weekly average potential and valid bouts/hour was due to the HC group and not from the MS group (see Fig. 4).
Fig. 2.
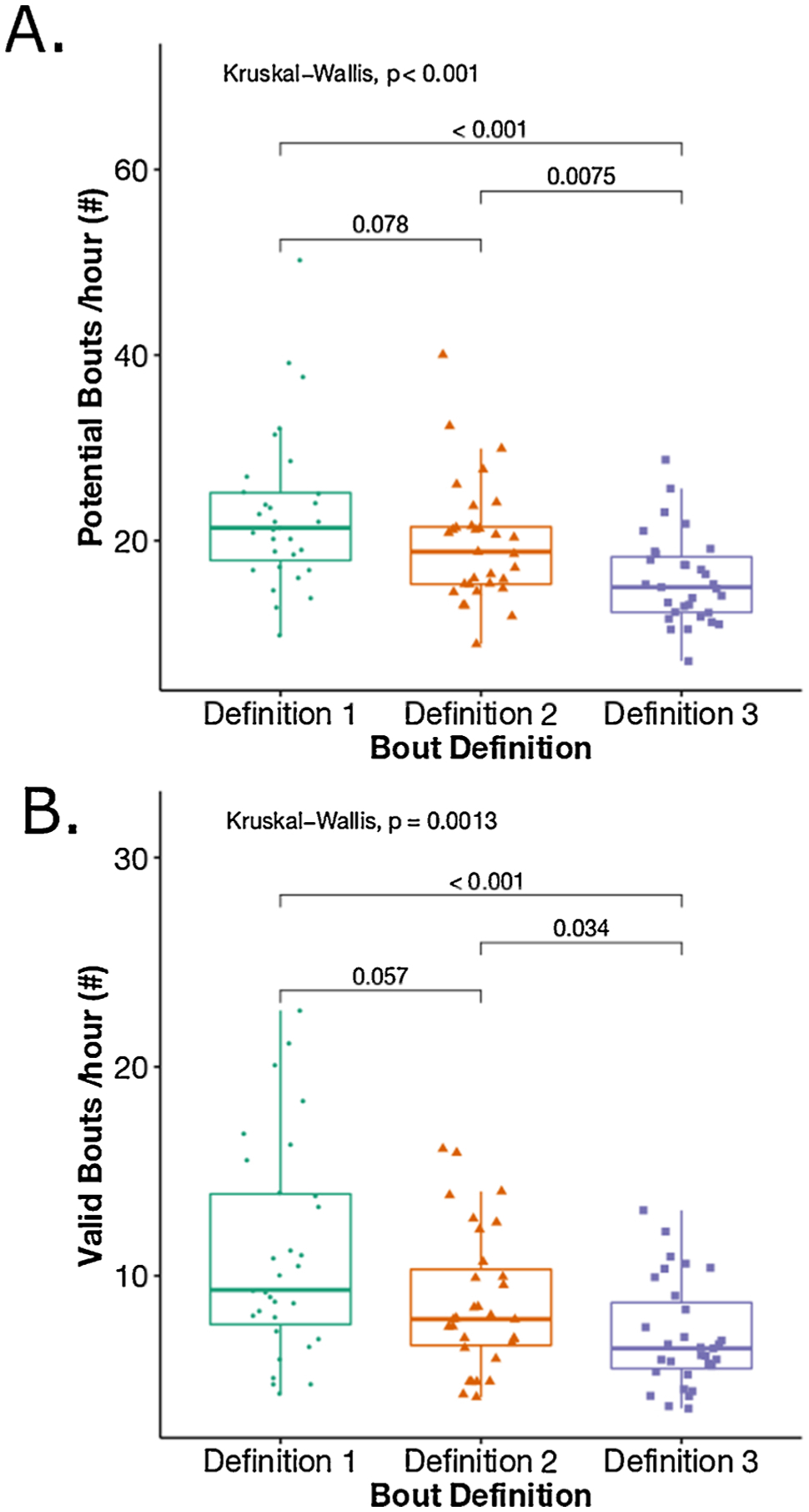
Boxplot representation of a total number of A) potential bouts/hour, and B) valid bouts/hour across the three definitions combining both MS and HC.
Fig. 3.
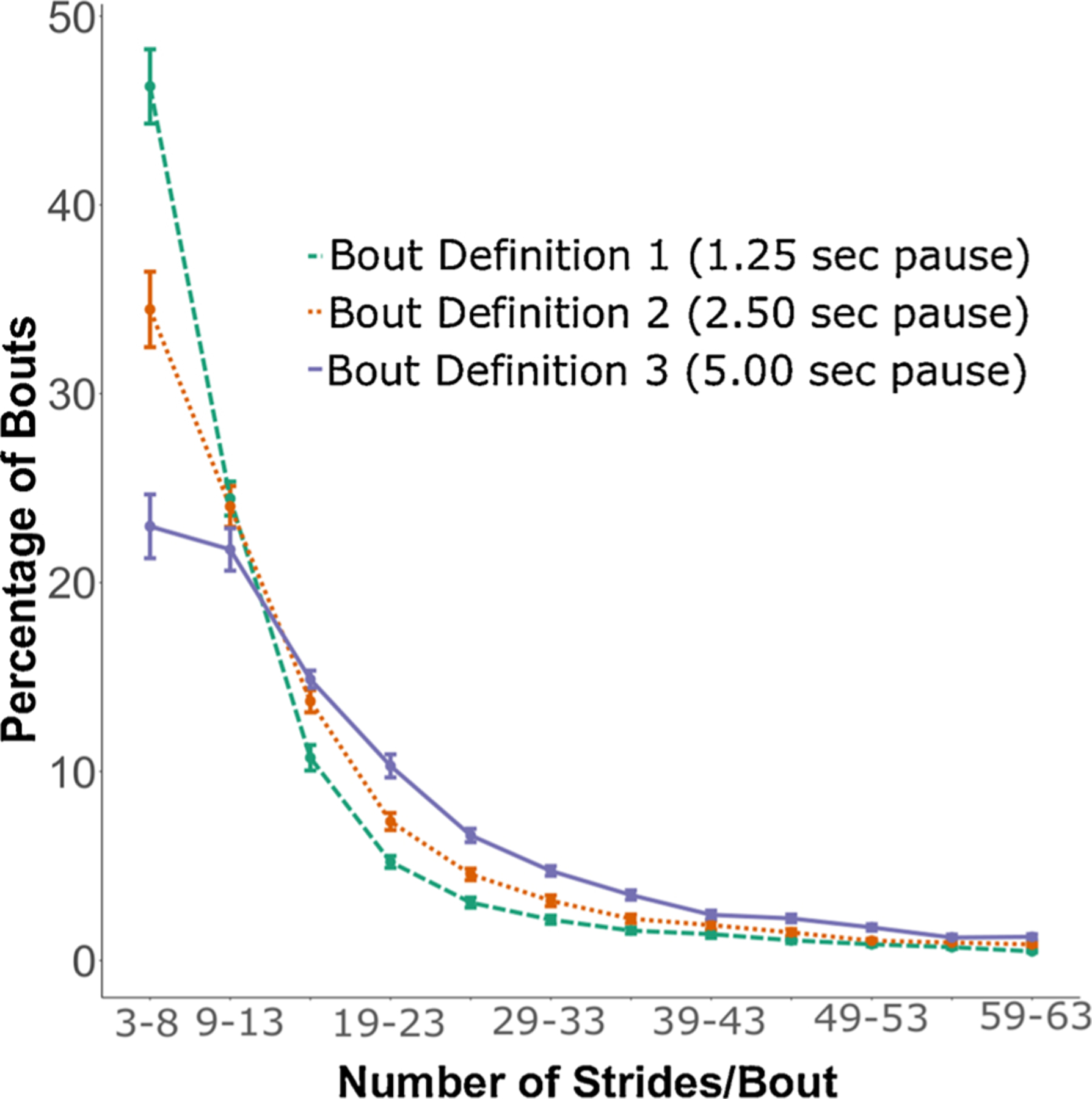
Percentage of bouts (mean and SE) vs bout length (# strides/bout) across 3 bout definitions (combining HC and MS groups).
Fig. 4.
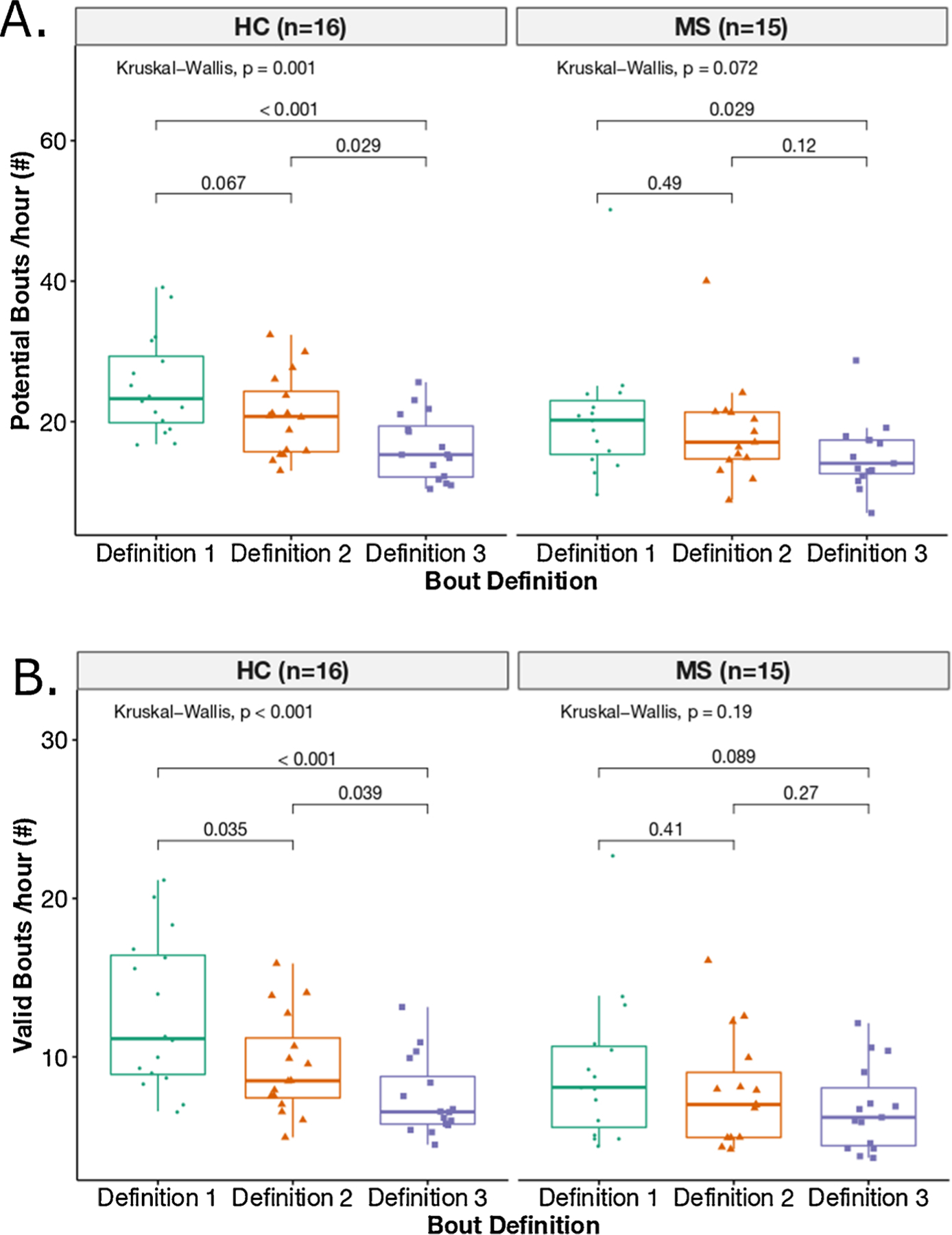
Boxplot representation of a total number of potential bouts/hour A) and valid bouts/hour B) across the three definitions per each group (HC and MS).
AUC for quantity of gait (i.e., number of valid bouts/hour) to distinguish MS from HC changed from 0.71 to 0.60 to 0.56, across the 1.25 s, 2.5 s and 5 s pause bout definitions, respectively (see Table 2A).
Table 2A.
AUC for gait quantity and quality measures discriminating MS from HC (AUC > 0.8) for different bout definitions.
| Gait measures | AUC using bout def. 1 (1.25 s pause) | AUC using bout def. 2 (2.5 s pause) | AUC using bout def. 3 (5 s pause) |
|---|---|---|---|
| Quantity | |||
| Valid bouts/hour (#) | 0.71 | 0.60 | 0.56 |
| Quality | |||
| Gait Speed (m/s) | 0.89 | 0.89 | 0.88 |
| Double Support (%) | 0.84 | 0.84 | 0.83 |
| Stride Length (m) | 0.84 | 0.81 | 0.80 |
| Swing Time (%) | 0.83 | 0.83 | 0.83 |
| Cadence (strides/minutes) | 0.81 | 0.80 | 0.80 |
| Stride Duration (s) | 0.80 | 0.80 | 0.79 |
AUC representing quality of gait measures (such as gait speed, stride length, stride duration) were almost unaltered and the most discriminative measures remained the same across the 3 bout definitions (see Table 2A). Table 2B shows the most discriminative gait quality measures for the MS and HC groups, calculated using three bout definitions.
Table 2B.
Mean and SD of gait quality measures with AUC > 0.8 for different bout definitions.
| Gait quality measures | Boutdef. 1 (1.25 s pause) | Bout def. 2 (2.5 s pause) | Bout def. 3 (5 s pause) |
|---|---|---|---|
| Gait Speed (m/s) | |||
| HC | 1.1 ± 0.16 | 1.07 ± 0.16 | 1.05 ± 0.15 |
| MS | 0.85 ± 0.13 | 0.82 ± 0.12 | 0.82 ± 0.13 |
| Double Support (%) | |||
| HC | 21.49 ± 3.15 | 21.96 ± 3.19 | 22.14 ± 3.12 |
| MS | 26.04 ± 3.33 | 26.48 ± 3.4 | 26.57 ± 3.46 |
| Stride Length (m) | |||
| HC | 1.17 ± 0.15 | 1.15 ± 0.15 | 1.14 ± 0.15 |
| MS | 0.99 ± 0.15 | 0.97 ± 0.15 | 0.97 ± 0.15 |
| Swing Time (%) | |||
| HC | 39.33 ± 1.62 | 39.12 ± 1.66 | 39.05 ± 1.63 |
| MS | 36.97 ± 1.73 | 36.77 ± 1.76 | 36.71 ± 1.77 |
| Cadence (strides/minutes) | |||
| HC | 54.99 ± 3.96 | 54.48 ± 3.79 | 54.22 ± 3.75 |
| MS | 50.66 ± 3.26 | 50.24 ± 3.49 | 50.08 ± 3.58 |
| Stride Duration (s) | |||
| HC | 1.13 ± 0.09 | 1.14 ± 0.08 | 1.15 ± 0.08 |
| MS | 1.22 ± 0.08 | 1.23 ± 0.09 | 1.24 ± 0.09 |
4. Discussion
The ability to discriminate quantity of gait (number of valid bouts/hour) between people with MS and heatlhy control subjects decreased from short to long gait-pause definitions. That is, when pauses of ≤1.25 s defined the end of one bout and start of another, people with MS showed statistically fewer gait bouts than HC but when pauses of up to ≤ 5 s defined the end of one bout and start of another, differences in number of gait bouts per week were no longer significant between groups. The detailed group-wise analysis showed that differences in gait quantity (bouts/hour) across the 3 bout definitions was only observed in the HC group, and not in the MS group suggesting that people with MS might stop for longer periods of time between walking bouts compared to HC and likely longer than our longest bout definition of 5 s pause between steps.
Unlike quantity of gait bouts, the ability to discriminate quality of gait measures (such as gait speed, stride length, and stride duration) were almost unaltered by the different gait bout definitions contrary to our hypothesis. The lack of effect of bout definitions on AUCs for gait quality measures might be due to the fact that one average gait measure per bout was used to characterize gait quality so long bouts were given just as much weight as short bouts. When a shorter pause duration was used, longer bouts were separated into multiple shorter bouts, giving the quality measures in these shorter bouts more weight statistically. However, this also occurred with shorter bouts, so the effect of dividing bouts into shorter bouts was almost insignificant on the weekly average value of the gait quality measures.
Our gait quality measures are slightly lower than the previously published literature. Specifically, Shema-Shiratzky et al. [21] reported the mean gait speed of 1.15 m/s for HC and 0.94 m/s for MS (calculated from only bouts >30 s), whereas we found the mean gait speed of 1.0 m/s for HC and 0.85 m/s for MS. Similarly, Storm et al. [22] reported the mean stride duration of 1.35 s (<50 steps), whereas we found the mean stride duration of 1.22 s. This discrepancy may be explained by the following possibilities: (1) definition of a bout was different, (2) various bouts durations were considered to calculate gait quality measures, (3) different algorithms used to estimate the gait measures.
Since the difference between groups by gait quality measures have been shown to influence by the bout length [6,27], and merging the shorter periods of no walking resulting in longer bout length, we expected differences in the ability to discriminate gait quality between groups with different bout definitions. However, our comparison of bout lengths was based on the same data just processed in a different way. Unlike comparing the effects of bout length in daily life on gait quality, comparing the effects of bout length based on definitions of pauses within bouts does not affect subjects’ intention to walk short or long distances, which may be responsible for altering gait speed and other gait measures [9].
It is also important to note that during normal daily activities, there is a much broader range of gait speeds than observed in a laboratory. The average value of quality measures should be interpreted with due caution since this is sensitive to the behavior of subjects and their daily habits as well as their ability and impairments due to disease or injury.
There are several limitations to the current study. First, we had a modest sample size of 15–16 subjects in each group and three different thresholds to define a bout with a short to long gait-pauses. Future work is needed to validate these findings with a larger number of subjects and with a broader range of maximum resting pauses between bouts [37]. Second, other gait quantity measures related to the activity such as alpha, mean bout length, and their variability, might be interesting to explore. Last, we performed all the analysis by taking the mean of each gait measure for all the bouts over a week for each subject and thus gave equal weight to each bout. But in reality, gait speed and other measures vary for gait bouts of different lengths [9,27]. Hence, future work will focus on analyzing the effect of bout length on each gait measure and how gait bout length affects the discriminatory power of each gait measure.
5. Conclusion
We investigated the effect of different walking bout definitions on the ability to discriminate gait quantity and quality measures between people with multiple sclerosis from healthy control subjects during a week of continuous monitoring of daily living. Results showed that the bout definition did not change the AUC for the most discriminative gait quality measures, although it did affect the difference in quantity of gait bouts between MS and HC.
Supplementary Material
Acknowledgment
We thank our participants for generously donating their time to participate in this study. This study was supported by grants from the National Multiple Sclerosis Society Mentor Fellowship (MB0027) and National Institutes of Health (National Institute on Aging; #R44AG055388).
Footnotes
Declaration of Competing Interest
Drs. McNames, El-Gohary and Horak have significant financial interests in APDM Wearable Technologies, a company that may have a commercial interest in the results of this research. Dr. Horak has also received honoraria from: British Columbia PT Association, Neuropore, Sanofi, Takeda, Adamas, Penn State University, University of Michigan, Johns Hopkins University. This potential conflict of interest has been reviewed and managed by OHSU. Dr. Spain receives honoraria from TG therapeutics.
Appendix A. Supplementary data
Supplementary material related to this article can be found, in the online version, at doi:https://doi.org/10.1016/j.gaitpost.2020.11.024.
References
- [1].Comber L, Galvin R, Coote S, Gait & Posture Gait deficits in people with multiple sclerosis: a systematic review and meta-analysis, Gait Posture 51 (2017) 25–35, 10.1016/j.gaitpost.2016.09.026. [DOI] [PubMed] [Google Scholar]
- [2].Allali G, Laidet M, Herrmann FR, Armand S, Elsworth-Edelsten C, Assal F, Lalive PH, Gait variability in multiple sclerosis: a better falls predictor than EDSS in patients with low disability, J. Neural. Transm 123 (2016) 447–450, 10.1007/s00702-016-1511-z. [DOI] [PubMed] [Google Scholar]
- [3].Cameron MH, Wagner JM, Gait abnormalities in multiple sclerosis: pathogenesis, evaluation, and advances in treatment, Curr. Neurol. Neurosci. Rep 11 (2011) 507–515, 10.1007/s11910-011-0214-y. [DOI] [PubMed] [Google Scholar]
- [4].Patel S, Lorincz K, Hughes R, Huggins N, Growdon J, Standaert D, Akay M, Dy J, Welsh M, Bonato P, Member S, Monitoring motor fluctuations in patients with Parkinson’s disease using wearable sensors, IEEE Trans. Inf. Technol. Biomed 13 (2009) 864–873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Chen BR, Patel S, Buckley T, Rednic R, McClure DJ, Shih L, Tarsy D, Welsh M, Bonato P, A web-based system for home monitoring of patients with Parkinson’s disease using wearable sensors, IEEE Trans, Biomed. Eng 58 (2011) 831–836, 10.1109/TBME.2010.2090044. [DOI] [PubMed] [Google Scholar]
- [6].Del Din S, Godfrey A, Mazzà C, Lord S, Rochester L, Free-living monitoring of Parkinson’s disease: lessons from the field, Mov. Disord 31 (2016) 1293–1313, 10.1002/mds.26718. [DOI] [PubMed] [Google Scholar]
- [7].Block VAJ, Pitsch E, Tahir P, Cree BAC, Allen DD, Gelfand JM, Remote physical activity monitoring in neurological disease: a systematic review, PLoS One 11 (2016) e0154335, 10.1371/journal.pone.0154335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Hickey A, Del Din S, Rochester L, Godfrey A, Detecting free-living steps and walking bouts: validating an algorithm for macro gait analysis, Physiol. Meas 38 (2017) N1–N15, 10.1088/1361-6579/38/1/N1. [DOI] [PubMed] [Google Scholar]
- [9].Del Din S, Godfrey A, Galna B, Lord S, Rochester L, Free-living gait characteristics in ageing and Parkinson’s disease: impact of environment and ambulatory bout length, J. Neuroengineering Rehabil 13 (2016) 1–12, 10.1186/s12984-016-0154-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Arora S, Baig F, Lo C, Barber TR, Lawton MA, Zhan A, Rolinski M, Ruffmann C, Klein JC, Rumbold J, Louvel A, Zaiwalla Z, Lennox G, Quinnell T, Dennis G, Wade-Martins R, Ben-Shlomo Y, Little MA, Hu MT, Smartphone motor testing to distinguish idiopathic REM sleep behavior disorder, controls, and PD, Neurology 91 (2018) e1528–e1538, 10.1212/WNL.0000000000006366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Lipsmeier F, Taylor KI, Kilchenmann T, Wolf D, Scotland A, Schjodt-Eriksen J, Cheng WY, Fernandez-Garcia I, Siebourg-Polster J, Jin L, Soto J, Verselis L, Boess F, Koller M, Grundman M, Monsch AU, Postuma RB, Ghosh A, Kremer T, Czech C, Gossens C, Lindemann M, Evaluation of smartphone-based testing to generate exploratory outcome measures in a phase 1 Parkinson’s disease clinical trial, Mov. Disord 33 (2018) 1287–1297, 10.1002/mds.27376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Salarian A, Russmann H, Vingerhoets FJG, Dehollain C, Blanc Y, Burkhard PR, Aminian K, Gait assessment in Parkinson’s disease: toward an ambulatory system for long-term monitoring, IEEE Trans. Biomed. Eng 51 (2004) 1434–1443, 10.1109/TBME.2004.827933. [DOI] [PubMed] [Google Scholar]
- [13].Weiss A, Brozgol M, Dorfman M, Herman T, Shema S, Giladi N, Hausdorff JM, Does the evaluation of gait quality during daily life provide insight into fall risk? A novel approach using 3-Day accelerometer recordings, Neurorehabil. Neural Repair 27 (2013) 742–752, 10.1177/1545968313491004. [DOI] [PubMed] [Google Scholar]
- [14].Giggins OM, Clay I, Walsh L, Physical activity monitoring in patients with neurological disorders: a review of novel body-worn devices, Digit. Biomark 4 (2017) 14–42, 10.1159/000477384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].El-Gohary M, Pearson S, McNames J, Mancini M, Horak F, Mellone S, Chiari L, Continuous monitoring of turning in patients with movement disability, Sensors (Switzerland) 14 (2014) 356–369, 10.3390/s140100356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Mancini M, Schlueter H, El-Gohary M, Mattek N, Duncan C, Kaye J, Horak FB, Continuous monitoring of turning mobility and its association to falls and cognitive function: a pilot study, J. Gerontol. - Ser. A Biol. Sci. Med. Sci 71 (2016) 1102–1108, 10.1093/gerona/glw019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Casey B, Coote S, Galvin R, Donnelly A, Objective physical activity levels in people with multiple sclerosis : meta- - analysis, Scand. J. Med. Sci. Sport (2018) 1960–1969, 10.1111/sms.13214. [DOI] [PubMed] [Google Scholar]
- [18].Motl RW, Pilutti L, Sandroff BM, Dlugonski D, Sosnoff JJ, Pula JH, Accelerometry as a measure of walking behavior in multiple sclerosis, Acta Neurol. Scand 127 (6) (2013) 384–390, 10.1111/ane.12036. [DOI] [PubMed] [Google Scholar]
- [19].Bradshaw MJ, Farrow S, Motl RW, Chitnis T, Wearable biosensors to monitor disability in multiple sclerosis, Neurol. Clin. Pract (2017) 354–362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Midaglia L, Mulero P, Montalban X, Graves J, Scotland A, Lipsmeier F, Van Beek J, Bernasconi C, Adherence and satisfaction of smartphone- and smartwatch-based remote active testing and passive monitoring in people with multiple sclerosis : nonrandomized interventional feasibility study corresponding author : related article, J. Med. Internet Res 21 (2019) 1–15, 10.2196/14863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Shema-Shiratzky S, Hillel I, Mirelman A, Regev K, Hsieh KL, Karni A, Devos H, Sosnoff JJ, Hausdorff JM, A wearable sensor identifies alterations in community ambulation in multiple sclerosis: contributors to real-world gait quality and physical activity, J. Neurol 267 (2020) 1912–1921, 10.1007/s00415-020-09759-7. [DOI] [PubMed] [Google Scholar]
- [22].Storm FA, Nair KPS, Clarke AJ, Van Der Meulen JM, Mazz C, Free-living and laboratory gait characteristics in patients with multiple sclerosis, PLoS One 13 (2018) 1–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Neven A, Vanderstraeten A, Janssens D, Wets G, Feys P, Understanding walking activity in multiple sclerosis: step count, walking intensity and uninterrupted walking activity duration related to degree of disability, Neurol. Sci 37 (2016) 1483–1490, 10.1007/s10072-016-2609-7. [DOI] [PubMed] [Google Scholar]
- [24].Mueller A, Hoefling HA, Muaremi A, Praestgaard J, Walsh LC, Schieker M, Wolfgang B, Roubenoff R, Continuous digital monitoring of walking speed in frail elderly patients : noninterventional validation study and longitudinal clinical trial, JMIR Mhealth Uhealth 7 (2019) 1–12, 10.2196/15191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Hillel I, Gazit E, Nieuwboer A, Avanzino L, Rochester L, Cereatti A, Della Croce U, Rikkert MO, Bloem BR, Pelosin E, Del Din S, Ginis P, Is every-day walking in older adults more analogous to dual-task walking or to usual walking? Elucidating the gaps between gait performance in the lab and during 24 / 7 monitoring, Eur. Rev. Aging Phys. Act 16 (2019) 1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Espay AJ, Hausdorff JM, Sanchez-Ferro A, Klucken J, Merola A, Bonato P, Paul SS, Horak FB, Vizcarra JA, Mestre TA, Reilmann R, Nieuwboer A, Dorsey RE, Rochester L, Bloem BR, Maetzler W, On behalf of the MDS technology task force, a roadmap for implementation of patient-centered digital outcome measures in parkinson’s disease obtained using mobile health technologies, Mov. Disord 34 (5) (2019) 657–663, 10.1002/mds.27671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Shah VV, McNames J, Harker G, Mancini M, Carlson-Kuhta P, Nutt JG, El-Gohary M, Curtze C, Horak FB, Effect of bout length on gait measures in people with and without Parkinson’s disease during daily life, Sensors (Basel) 20 (5769) (2020) 1–16, 10.3390/s20205769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Thompson AJ, Banwell BL, Barkhof F, Carroll WM, Coetzee T, Comi G, Correale J, Fazekas F, Filippi M, Freedman MS, Fujihara K, Galetta SL, Hartung HP, Kappos L, Lublin FD, Marrie RA, Miller AE, Miller DH, Montalban X, Mowry EM, Sorensen PS, Tintoŕe M, Traboulsee AL, Trojano M, Uitdehaag BMJ, Vukusic S, Waubant E, Weinshenker BG, Reingold SC, Cohen JA, Diagnosis of multiple sclerosis: 2017 revisions of the McDonald criteria, Lancet Neurol. 17 (2018) 162–173, 10.1016/S1474-4422(17)30470-2. Position paper. [DOI] [PubMed] [Google Scholar]
- [29].Shah VV, McNames J, Mancini M, Carlson-Kuhta P, Spain RI, Nutt JG, El-Gohary M, Curtze C, Horak FB, Laboratory versus daily life gait characteristics in patients with multiple sclerosis, Parkinson’s disease, and matched controls, J NeuroEngineering and Rehab 17 (2020) 159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Shah VV, McNames J, Mancini M, Carlson-Kuhta P, Nutt JG, El-Gohary M, Lapidus JA, Horak FB, Curtze C, Digital biomarkers of mobility in Parkinson’s disease during daily living, J. Parkinson’s. Dis 10 (2020) 1099–1111, 10.3233/JPD-201914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Shah VV, McNames J, Mancini M, Carlson-Kuhta P, Spain RI, Nutt JG, El-Gohary M, Curtze C, Horak FB, Quantity and quality of gait and turning in people with multiple sclerosis, Parkinson’s disease and matched controls during daily living, J. Neurol 267 (4) (2020) 1188–1196, 10.1007/s00415-20-09696-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Mancini M, King L, Salarian A, Holmstrom L, James M, Horak FB, Mobility lab to assess balance and gait with synchronized body-worn sensors, J. Bioeng. Biomed. Sci (2013) 1–5, 10.4172/2155-9538.s1-007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Morris R, Stuart S, McBarron G, Fino PC, Mancini M, Curtze C, Validity of mobility lab (version 2) for gait assessment in young adults, older adults and Parkinson’s disease, Physiol. Meas 40 (2019) 0–8, 10.1088/1361-6579/ab4023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Washabaugh EP, Kalyanaraman T, Adamczyk PG, Claflin ES, Krishnan C, Validity and repeatability of inertial measurement units for measuring gait parameters, Gait Posture 55 (2017) 87–93, 10.1016/j.gaitpost.2017.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Wan EA, Van Der Merwe R, Rd NWW, The unscented Kalman filter for nonlinear estimation, Proc. IEEE 2000 Adapt. Syst. Signal Process. Commun. Control Symp. (Cat. No. 00EX373) (2000) 153–158. [Google Scholar]
- [36].Van Der Merwe R, Wan E, Sigma-Point Kalman Filters for Probabilistic Inference in Dynamic State-Space Models, Oregon Health and Science University, 2004. [Google Scholar]
- [37].Barry G, Galna B, Lord S, Rochester L, Godfrey A, Defining ambulatory bouts in free-living activity: impact of brief stationary periods on bout metrics, Gait Posture 42 (2015) 594–597, 10.1016/j.gaitpost.2015.07.062. [DOI] [PubMed] [Google Scholar]
- [38].Turck N, et al. , pROC: an open-source package for R and S+ to analyze and compare ROC curves, BMC Bioinf. 8 (2011) 12–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


