Abstract
Ameloblastoma is benign odontogenic tumours that mainly occur in the jawbone. This tumour induces aggressive invasion into the surrounding bone and has a high recurrence rate after surgery. Therefore, mandibular resection is performed in many patients with this tumour, causing aesthetic and functional problems. It is necessary to develop a novel treatment strategy for ameloblastoma, but there are currently no innovative treatments. Although our understanding of the molecular biological mechanisms of ameloblastoma is still insufficient, there have been many recent reports of new molecular biological findings on ameloblastoma. Therefore, bioactive factors that have potential for novel therapeutic methods, such as molecular targeted therapy, have been discovered in ameloblastoma. In this review, we summarize the molecular biological findings of ameloblastoma reported over several decades, focusing on factors involved in invasion into surrounding tissues and disease-specific gene mutations. We also mention the effect of the interaction between tumour cells and stromal components in ameloblastoma on tumour development.
Scientific field of dental Science: Oral surgery, Odontogenic tumor, Ameloblastoma.
Keywords: Ameloblastoma, Biology, Review
1. Introduction
Ameloblastoma is representative benign odontogenic tumour that commonly occurs in the jawbone. This tumour is thought to arise from the remaining cells of the dental lamina, the epithelial cell rests of Malassez, or the basal cell layer of the epithelial surface [1]. Most patients with ameloblastoma are between the ages of 30 and 50 years, and this tumour occurs with equal frequency in men and women, with 80% occurring in the mandible [2,3]. Ameloblastoma develops invasively [4], and recurrence rates are higher if they are not adequately resected during surgery [5,6]. Extended resection of the jaw is effective in controlling recurrence, but it presents significant aesthetic and functional problems [7].
Over the past few decades, many research groups have carried out molecular biological studies on ameloblastoma, and our understandings of this tumour has been dramatically improved. However, many points of the molecular mechanisms of the invasive development of ameloblastoma have not been clarified, and surgeons usually empirically decide on the treatment plan. Ameloblastoma is a rare disease, and the primary culture of ameloblastoma cells is difficult. Previously, there were few studies using immortalized ameloblastoma cell lines, and many studies focused on histological verification. However, recently, many studies using primary cell cultures and immortalized ameloblastoma cell lines have been reported. Harada et al. [8] established AM-1 cells, which are an immortalized cell line derived from the plexiform type of human ameloblastoma using papillomavirus. Kibe et al. [9] also established AM-3 cells, which are an immortalized cell line derived from the follicular type of human ameloblastoma, using a lentivirus. In addition, several research groups performed in vitro experiments using novel immortalized cell lines of ameloblastoma and reported various molecular biological findings [[10], [11], [12]]. Regarding ameloblastoma, a stable animal experimental model is necessary for preliminary experiments of novel molecular targeted therapy. However, there are few reports of animal experimental models of ameloblastoma. Possible reasons are the difficulty of primary culture, the immortalization of ameloblastoma cells, and the rarity of this tumour. Zhang et al. [13,14] established an experimental model of ameloblastoma by transplanting ameloblastoma tissue and primary culture cells into immunodeficient mice. In addition, our research group transplanted immortalized ameloblastoma cells derived from follicular and plexiform types subcutaneously into the heads of immunodeficient mice and established stable animal experimental models of two different types of ameloblastoma [15]. By using such experimental animal models, we expect progress in the development of a novel therapeutic method for ameloblastoma. Recently, gene mutations of BRAF and SMO specific to ameloblastoma have been reported [[16], [17], [18]]. We consider that research on the factors involved in the control of tumour development, including these mutant genes and factors related to invasion, will progress in the near future.
2. Effects on bone remodelling in ameloblastoma
Ameloblastoma invades aggressively into the surrounding bone. Therefore, it is considered that the role of osteoclasts in bone resorption of ameloblastoma is important. Receptor activator nuclear factor kappa B (RANK), receptor activator nuclear factor kappa B ligand (RANKL), and osteoprotegerin (OPG) in osteoclast formation are crucial for bone remodelling [19,20]. RANK is a central activator of NF-kB that regulates DNA transcription and is a signal transduction receptor of RANKL. RANKL is normally membrane-bound to osteoblasts, binds to RANK on the surface of pre-osteoclasts, and stimulates osteoclast differentiation and activation. OPG is a soluble decoy receptor for RANKL that inhibits the pre-osteoclastic interaction between RANKL and RANK, thereby inhibiting bone resorption [21]. The balance of these factors is important in controlling bone remodelling [22]. The RANKL, RANK, and OPG systems have also been shown to be aberrantly regulated in several osteolytic lesions, including neoplastic lesions [[23], [24], [25], [26]].
Several reports have pointed out the expression of RANKL, RANK, and OPG in odontogenic diseases, including ameloblastoma, and pointed out the role of osteoclast regulators in the progression of odontogenic lesions [[27], [28], [29], [30]]. da Silva et al. [27] showed that both RANK and RANKL were more highly expressed in ameloblastoma than in odontogenic keratocysts. This finding supports the phenomenon that ameloblastoma have a higher degree of bone invasiveness and a higher recurrence rate than odontogenic keratocysts [27]. In contrast, Tekkesin et al. [28] reported that the expression of RANKL in radicular cysts and odontogenic keratocysts and ameloblastoma is the same in all three diseases, and the variable factors that determine osteoclast formation depend on RANK expression. da Silva et al. [27] found that stromal cells in solid/multicystic ameloblastoma have higher levels of RANK expression than unicystic ameloblastoma, and the rate of positive cells for RANKL and OPG was high in solid/multicystic and unicystic ameloblastoma. The balance of OPG, RANKL, and RANK expression regulates osteoclast activation, leading to tumour-induced bone and tooth resorption, and may be involved in the clinical behaviour of ameloblastoma.
Sandra et al. [31] suggested that ameloblastoma can induce osteoclastogenesis by secreting soluble RANKL and tumour necrosis factor α (TNF-α). They also found that RANKL was contained in the culture supernatant of AM-1 cells, a human ameloblastoma cell line, and induced osteoclast differentiation [31]. However, Yoshimoto et al. [32] reported that RANKL expression sufficient for osteoclast differentiation was not detected in the ameloblastoma cell line AM-1. Kumamoto et al. [33] reported that RANKL expression was scarcely observed in both plexiform and follicular ameloblastoma. Previous studies have reported that RANKL-positive cells are more distributed throughout the stroma than ameloblastoma tumour cells [27,32]. These indicate that both tumour cells and stromal components may act as a source of osteoclastogenic factors in ameloblastoma. Liu et al. [34] revealed that the interaction between AM cells and bone marrow stromal cells (BMSCs) induces osteoclastogenesis by upregulating interleukin (IL)-8 and activin A. In our study, the interaction between ameloblastoma cells and stromal fibroblasts promoted the production of cytokines such as IL-6 and IL-8 from stromal fibroblasts and promoted tumour growth and osteoclast differentiation [35]. The overexpression of IL-6 and IL-8 is known not only to correlate with tumour growth and metastasis and angiogenesis but also to play an important role in osteoclast formation [[36], [37], [38]]. These findings suggest that the interaction between ameloblastoma cells and the surrounding stromal cells may influence the bone invasion of ameloblastoma.
Although there are many studies on the effects of ameloblastoma on bone remodelling, most of them focus on osteoclast differentiation and activation, and there are few studies investigating the suppression of osteogenesis. Sathi et al. [39] reported that sFRP-2 is strongly expressed in ameloblastoma tissue and AM-1 cells, and sFRP-2 derived from ameloblastoma cells can lead to decreased osteogenesis. The same group also suggested that ameloblastoma cells secrete sFRP-2, RANKL, and IL-6 to create a favourable environment for the inhibition of bone formation and bone resorption [40].
From the above, it was suggested that ameloblastoma realize aggressive invasion by promoting bone resorption and suppressing bone formation. Factors involved in these mechanisms may be targets for the regulation of tumour development in ameloblastoma.
3. Digestive proteinase: matrix metalloproteinase
Matrix metalloproteinases (MMPs) are a family of zinc-dependent proteinases that mediate the degradation of extracellular matrix (ECM) and basement membrane and are classified into gelatinases, matrilysin, collagenase, stromelysin, and membrane-bound MMPs (MB-MMPs) [41]. The degradation of extracellular matrix and basement membrane components promotes the migration of tumour cells and the entry into adjacent tissue compartments. There are numerous reports on the role of MMPs in the invasion of ameloblastoma. Among them, there are many reports that MMP-2 and MMP-9 are expressed in ameloblastoma [[42], [43], [44], [45], [46], [47], [48]]. MMP-2 and MMP-9 are expressed in various cancers and are involved in angiogenesis and tumour growth, and many reports suggested a relationship with tumour aggressiveness and clinical course [[49], [50], [51]]. MMP-2 degrades type IV collagen, one of the major components of the basement membrane, and promotes tumour invasion, and its importance is well known [52]. High expression and activity of MMP-2 have been documented in many tumours, and many studies of ameloblastoma indicate similar findings [53]. In particular, high expression and activity of MMP-2 have been shown to be associated with aggressive invasive behaviour in ameloblastoma [13,53,54]. Wang et al. [55] reported that the suppression of MMP-2 expression in ameloblastoma cells by RNA interference inhibited MMP-2 activity and the invasion of ameloblastoma cells. MMP-9 is considered to be involved in the process of tumour invasion by mediating basement membrane degradation and ECM remodelling [56]. Several studies have proposed that MMP-9 is associated with the local invasiveness of ameloblastoma, and they found its expression in ameloblastoma epithelial and stromal components [54,57]. Qian et al. [58] found a large discrepancy in MMP-9 expression levels in many patients with ameloblastoma. They further argued that there was a correlation between the levels of MMP-9 expression and the histological type and biological behaviour of ameloblastoma [58]. Kibe et al. [9] evaluated the activity of MMP-2 and MMP-9 in AM-1 cells derived from the plexiform type and AM-3 cells derived from the follicular type of ameloblastoma. As a result, the activities of MMP-2 and MMP-9 from AM-3 cells were more active than those from AM-1 cells [9]. Kumamoto et al. [59] evaluated the correlation of the expression of MMP-9 and tumour growth in ameloblastoma and concluded that MMP-9 plays a role in regulating tumour progression. However, it was reported that tissue inhibitor of metalloproteinase (TIMP)-1, which is a selective inhibitor of MMP-9, did not show a significant inhibitory effect on bone resorption of osteoclasts, and the results suggest that MMP-9 may not be involved in the degradation of bone matrix [58]. Similar to other findings, the expression of other MMP types, such as MMP-1, MMP-13, and MMP-14, has also been reported in ameloblastoma [[60], [61], [62]]. Statistically, there is a difference in recurrence rate and prognosis among the histological types, and it has been suggested that the difference in invasion ability is the factor [57,63]. The difference in MMP expression may be the cause of the difference in aggressiveness in various ameloblastoma.
TIMPs are key regulatory molecules that inhibit MMP expression and activity [41]. Several reports have shown the expression of TIMPs in ameloblastoma, such as TIMP-1 and TIMP-2 [45,59]. Zhang et al. [64] found that the relative expression levels of TIMP-2 and MMP-14 were significantly higher in recurrent ameloblastoma than in primary ameloblastoma. Furthermore, Nunia et al. [57] suggested that the expression of TIMP-2 in stromal components plays a role in the regulation of tumour progression by its inhibitory effect on MMP-9 in ameloblastoma. TIMPs counteract the effects of MMPs, and the relationship between the expression of TIMPs and the behaviour of ameloblastoma is unclear. The imbalance of the expression of MMPs and TIMPs may influence the progression of ameloblastoma.
There are several reports on factors that promote MMP expression in ameloblastoma. Yang et al. [12] suggested that hydrostatic pressure on ameloblastoma cells causes the upregulation of MMP-2, MMP-9, and RANKL via the Wnt/β-catenin pathway in ameloblastoma. Kibe et al. [9] found that ameloblastoma cells enhanced MMP-9 expression in response to Wnt3a. Additionally, Ohta et al. [65] reported that TNF-α promotes the expression of IL-6 and MMP-9 in ameloblastoma cells. da Rosa et al. [10] reported that epidermal growth factor (EGF) activated tumour-cell-derived MMP-2 and MMP-9 to enhance the invasive ability of ameloblastoma. They showed that epidermal growth factor receptor (EGFR) signalling downstream of EGFR regulates the migration, invasion, and MMP secretion of ameloblastoma-derived cells.
Controlling MMPs may lead to effective control of invasion into surrounding tissues of different types of ameloblastoma. However, MMPs were originally proteolytic enzymes with an important role in biological functions, including tissue remodelling, and the inhibition of MMP activity using an MMP inhibitor causes many side effects. Therefore, further studies are needed for disease control by MMP inhibition in ameloblastoma.
4. Gene mutations in ameloblastoma: BRAF and SMO
Kurppa et al. [16] found frequent mutations in the BRAF gene in ameloblastoma, and they indicated V600E mutations in all cases. BRAF is a component of the mitogen-activated protein kinase (MAPK) pathway, which is the intracellular signal transduction pathway, and other studies have also reported high-frequency MAPK pathway mutations in ameloblastoma [17,18]. The BRAF V600E mutation was found in many neoplasms, and the mutation can promote cell growth and neoplastic transformation via the activation of BRAF, MEK, and ERK signalling in cancer [66]. Brown et al. [18] found that the BRAF V600E mutation was detected in 62% (31/50) of ameloblastoma and that the mutation was not only in the BRAF gene but also in the RAS gene (KRAS, 8%, NRAS, 6%, HRAS, 6%) and FGFR2 (6%). Brown et al. [18] indicated that BRAF V600E was more common in mandibular ameloblastoma, whereas ameloblastoma containing wild-type BRAF occurred more frequently in the maxilla and showed earlier recurrence. Other reports also suggested a low recurrence rate in cases of ameloblastoma with the BRAF V600E mutation [[16], [17], [18]]. This result suggests a role for the BRAF V600E mutation as a prognostic marker for ameloblastoma, and maxillary ameloblastoma are typically considered to be more aggressive. Regarding histological types, Sweeney et al. [17] suggested that mutations in SMO and BRAF tend to be mutually exclusive and that these changes may define two independent genetic aetiologies of ameloblastoma. They also showed that most cases of follicular type ameloblastoma carried either SMO or BRAF mutations, whereas almost all plexiform cases of ameloblastoma had SMO mutations [17]. do Canto et al. [67] suggested that the BRAF V600E mutation is common in mandibular ameloblastoma, especially in tumours larger than 4 cm and in the posterior region of the mandible, in which mutations may occur regardless of histological type, age, sex, and radiographic profile. Thus, although many studies have suggested the superiority of BRAF mutations in mandibular ameloblastoma, there are also reports that BRAF V600E activating mutations occur regardless of the site or type of ameloblastoma [68,69].
It has also been reported that SMO is the most commonly mutated gene other than BRAF in ameloblastoma and is involved in the aetiology of these tumours [17,18]. Sweeney et al. [17] indicated that SMO mutations were more frequently found in maxillary ameloblastoma than in mandibular ameloblastoma, whereas BRAF mutations were more frequently found in mandibular ameloblastoma. The SMO gene encodes the hedgehog pathway protein smoothened (SMO). Hedgehog pathway activation was independent and suggested to be synergistic with MAPK pathway activation [18]. The expression of Sonic Hedgehog (SHH), PTCH1 and SMO in ameloblastoma has been reported [70,71]. Commonly, the membrane protein PTCH1 suppresses SMO activity in the absence of SHH proteins. Kanda et al. [72] investigated the association between SHH signalling and cell proliferation of ameloblastoma cells using AM-1 cells. As a result, they found that AM-1 cells express SHH and PTCH, and their growth was suppressed by SHH antibody or cyclopamine. This result indicates that SHH plays an anti-apoptotic role in the growth of ameloblastoma cells [72]. The relationship between the aforementioned SMO gene mutation and the overexpression of a protein involved in the SHH signalling pathway is unknown, but both of them may affect the characteristics of ameloblastoma.
Vemurafenib and dabrafenib, which are selective inhibitors of mutant BRAF, and trametinib, which is a MEK inhibitor, are approved for the treatment of BRAF mutation-positive metastatic melanoma [73]. Ameloblastoma cells carrying the BRAF V600E mutation have been shown to be sensitive to vemurafenib treatment in vitro, suggesting that mutant BRAF inhibition may be effective for ameloblastoma [17,18]. Furthermore, several groups have already reported the use of mutant BRAF inhibitors in patients with ameloblastoma [[74], [75], [76]]. Tan et al. [74] reported that preoperative therapy with dabrafenib was given to patients with recurrent BRAF-mutant mandibular ameloblastoma and that these pretreated ameloblastoma lesions showed a >90% reduction in tumour volume. Kaye et al. [75] also reported that patients with ameloblastoma with the BRAF V600E mutation were treated with a combination of dabrafenib and trametinib and showed a dramatic response after eight weeks of treatment. These reports suggest that MAPK pathway inhibitors should be evaluated as a novel treatment for ameloblastoma. However, the reports of the therapeutic effect of BRAF inhibitors on ameloblastoma remains scarce, and no large scale studies have yet reported. Vismodegib, which is a specific inhibitor of SMO, has been approved for the treatment of basal cell carcinoma [77]. In addition, there is a report that itraconazole and arsenic trioxide (ATO), which are hedgehog pathway inhibitors, inhibit the growth of medulloblastoma and basal cell carcinoma in vivo and in Phase II clinical trials [78]. However, there is a report that vismodegib and itraconazole have been shown to be ineffective at inhibiting the activity of SMO mutants in ameloblastoma [17]. Thus, it is necessary to verify the utility of these agents for ameloblastoma. The number of cases of ameloblastoma is generally small. In addition, the tumor size and the general condition of the patient should be taken into account in the treatment with molecular targeted agents. Therefore, the number of patients who can participate in clinical trials may be smaller and proving the therapeutic efficacy of these agents may require a longer study period.
The elucidation of gene mutations in ameloblastoma may help to develop novel non-invasive treatments for ameloblastoma. We expect that the gene mutations in ameloblastoma will be better understood and that an excellent therapeutic method for this tumour will be developed in the future.
5. The role of stromal components in ameloblastoma
Ameloblastoma is composed of odontogenic tissue and abundant tumour stroma. Recently, it has been reported that stromal cells play an essential role in various diseases. For example, various types of cancer cells and stromal fibroblasts secrete soluble factors such as cytokines and growth factors into the microenvironment, and these factors upregulate the growth and invasion of cancer [79,80]. These studies indicate that the interaction between ameloblastoma cells and surrounding stromal cells, such as fibroblasts, may contribute to the pathogenesis of ameloblastoma.
The authors' research group hypothesized that ameloblastoma cells and stromal fibroblasts might be reciprocally activated via cytokines and co-ordinately create a microenvironment that promotes the growth of ameloblastoma. We indicated that the IL-1α-mediated interaction between ameloblastoma cells and stromal fibroblasts increased the production of IL-6 and IL-8 from fibroblasts [35]. Furthermore, IL-6 and IL-8 from fibroblasts promoted cell motility and the proliferation of ameloblastoma cells [35]. It has been reported that IL-6 and IL-8 are associated with invasive growth and metastasis by activating tumour cell proliferation and cell motility in various malignant tumours [[81], [82], [83], [84]]. Goh et al. [85] suggested that the overexpression of IL-1α in ameloblastoma may play a role in the promotion of bone resorption and local infiltration. They also indicated that tumour parenchymal and stromal components of ameloblastoma interact through IL-1α and IL-6 to create a microenvironment that promotes tumour progression [85]. Furthermore, as described above, Liu et al. [34] showed that ameloblastoma cells and stromal cells interacted with each other and induced the expression of IL-8 and activin A from stromal cells. They suggested that the expression of these factors may play an essential role in osteoclastogenesis in ameloblastoma [34]. Additionally, Jiang et al. [86] indicated that IL-6 secreted from stromal cells may promote the EMT of ameloblastoma cells, promote tumour-like epithelial stem cell formation and play an essential role in the progression of ameloblastoma. These reports suggest that ameloblastoma cells and stromal components interact with each other to form a specific microenvironment, which may significantly influence tumour characteristics.
Syamala et al. [87] reported the presence of α-SMA-positive myofibroblasts in the stromal components of odontogenic cysts and tumour. They also found that ameloblastoma showed the highest number of myofibroblasts compared with dentigerous cysts and odontogenic keratocysts [87]. Mudaliar et al. [88] also found that solid/multicystic ameloblastoma contain more myofibroblasts in the stroma than unicystic ameloblastoma. Myofibroblasts are variants of fibroblasts that express α-SMA; are found in both fibroblasts and smooth muscle cells; and secrete cytokines, growth factors, and abundant extracellular matrix [89]. Therefore, the association of myofibroblasts with invasiveness has been pointed out in various malignant tumours [89]. There are still many unclear points about the role of myofibroblasts in ameloblastoma, but they may influence the progression of these tumour-like stromal fibroblasts described above.
As mentioned above, ameloblastoma is characterized by different histological types, such as the follicular type and the plexiform type. However, the factors that affect invasive growth patterns are not clear in ameloblastoma. Our research group focused on the role of stromal components as described above and considered that the interaction between ameloblastoma cells and stromal cells might influence the invasion behaviour of ameloblastoma cells. We evaluated the behaviour of two different ameloblastoma cell lines (AM-1 cells derived from the plexiform type and AM-3 cells derived from the follicular type) in collagen gel and the role of fibroblasts in this process using a double-layered collagen gel hemisphere (DL-CGH) system, which is a three-dimensional (3D) culture method [90]. As a result, AM-1 and AM-3 cells had different invasive patterns, and the presence of fibroblasts changed the invasive form of ameloblastoma cells, promoting more aggressive, collective cell invasion [90]. These results suggested that ameloblastoma cells from different types indicate different collective invasion patterns and that the existence of fibroblasts influences the behaviour of ameloblastoma cells [90]. Moreover, the difference in the properties of the stromal components may be responsible for the various invasive forms in ameloblastoma.
These reports suggest that the stromal components of ameloblastoma are involved in tumour proliferation, invasion, histological pattern (growth pattern), and osteoclast differentiation. In the future, it will be possible to establish effective treatments for various types of ameloblastoma through the clarification of the effect of stromal components on the behaviour of ameloblastoma.
6. Conclusion
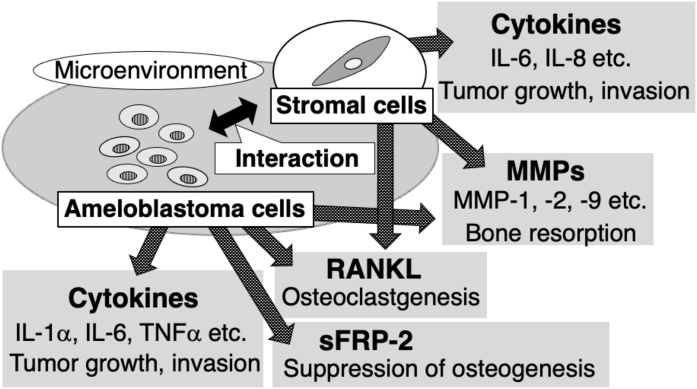
We summarized the molecular biological findings that may be related to the aggressive invasion of ameloblastoma in this review. In recent years, the detailed molecular mechanisms for the character of this tumour have been investigated, and the biological factors that cause invasive development have become more apparent. These findings indicate that tumour cells secrete factors related to bone resorption and invasion, such as MMPs and RANKL, and interact with surrounding stromal components to construct a microenvironment favourable for tumour development in ameloblastoma (Fig. 1). Even so, there are many unclear points about this tumour, and the generating mechanisms of ameloblastoma and the factors that cause the difference in histological type are still unknown. Currently, invasive surgery, such as jaw resection including surrounding tissues, is often performed to reduce the risk of recurrence, causing many problems in patients. Therefore, it is desirable to develop a novel non-invasive treatment including molecular targeted therapy with less postoperative QOL reduction in patients. There are several reports that molecular targeted therapy with inhibitors against mutant BRAF is significant, as mentioned in this review. In addition to BRAF, candidates for therapeutic target factors have been reported, and we expect further research results in the future.
Fig. 1.
Microenvironment constructed by tumour cells and stromal components in ameloblastoma.
Funding
No funding involved in this review.
Conflict of interest
None.
References
- 1.You Z., Liu S.P., Du J., Wu Y.H., Zhang S.Z. Advancements in MAPK signaling pathways and MAPK-targeted therapies for ameloblastoma: a review. J Oral Pathol Med. 2019;48:201–205. doi: 10.1111/jop.12807. [DOI] [PubMed] [Google Scholar]
- 2.Sham E., Leong J., Maher R., Schenberg M., Leung M., Mansour A.K. Mandibular ameloblastoma: clinical experience and literature review. ANZ J Surg. 2009;79:739–744. doi: 10.1111/j.1445-2197.2009.05061.x. [DOI] [PubMed] [Google Scholar]
- 3.Adebayo E.T., Fomete B., Adekeye E.O. Delayed soft tissue recurrence after treatment of ameloblastoma in a black African: case report and review of the literature. J Craniomaxillofac Surg. 2011;39:615–618. doi: 10.1016/j.jcms.2010.05.010. [DOI] [PubMed] [Google Scholar]
- 4.Cawson R.A. 5th ed. Churchill Livingstone; London: 1998. Lucas’s pathology of tumors of the oral tissues. [Google Scholar]
- 5.Fregnani E.R., da Cruz Perez D.E., de Almeida O.P., Kowalski L.P., Soares F.A., de Abreu Alves F. Clinicopathological study and treatment outcomes of 121 cases of ameloblastomas. Int J Oral Maxillofac Surg. 2010;39:145–149. doi: 10.1016/j.ijom.2009.11.022. [DOI] [PubMed] [Google Scholar]
- 6.Reichart P.A., Philipsen H.P., Sonner S. Ameloblastoma: biological profile of 3677 cases. Eur J Cancer B Oral Oncol. 1995;31b:86–99. doi: 10.1016/0964-1955(94)00037-5. [DOI] [PubMed] [Google Scholar]
- 7.Nakamura N., Higuchi Y., Mitsuyasu T., Sandra F., Ohishi M. Comparison of long-term results between different approaches to ameloblastoma. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2002;93:13–20. doi: 10.1067/moe.2002.119517. [DOI] [PubMed] [Google Scholar]
- 8.Harada H., Mitsuyasu T., Nakamura N., Higuchi Y., Toyoshima K., Taniguchi A. Establishment of ameloblastoma cell line, AM-1. J Oral Pathol Med. 1998;27:207–212. doi: 10.1111/j.1600-0714.1998.tb01943.x. [DOI] [PubMed] [Google Scholar]
- 9.Kibe T., Fuchigami T., Kishida M., Iijima M., Ishihata K., Hijioka H. A novel ameloblastoma cell line (AM-3) secretes MMP-9 in response to Wnt-3a and induces osteoclastogenesis. Oral Surg Oral Med Oral Pathol Oral Radiol. 2013;115:780–788. doi: 10.1016/j.oooo.2013.03.005. [DOI] [PubMed] [Google Scholar]
- 10.da Rosa M.R., Falcao A.S., Fuzii H.T., da Silva Kataoka M.S., Ribeiro A.L., Boccardo E. EGFR signaling downstream of EGF regulates migration, invasion, and MMP secretion of immortalized cells derived from human ameloblastoma. Tumour Biol. 2014;35:11107–11120. doi: 10.1007/s13277-014-2401-3. [DOI] [PubMed] [Google Scholar]
- 11.Liang Q.X., Liang Y.C., Xu Z.Y., Chen W.L., Xie H.L., Zhang B. RECK overexpression reduces invasive ability in ameloblastoma cells. J Oral Pathol Med. 2014;43:613–618. doi: 10.1111/jop.12179. [DOI] [PubMed] [Google Scholar]
- 12.Yang Z., Li K., Liang Q., Zheng G., Zhang S., Lao X. Elevated hydrostatic pressure promotes ameloblastoma cell invasion through upregulation of MMP-2 and MMP-9 expression via Wnt/beta-catenin signalling. J Oral Pathol Med. 2018;47:836–846. doi: 10.1111/jop.12761. [DOI] [PubMed] [Google Scholar]
- 13.Zhang B., Zhang J., Huang H.Z., Chen W.L., Tao Q., Zeng D.L. Inhibition of ameloblastoma invasion in vitro and in vivo by inhibitor of metalloproteinase-2 activity. J Oral Pathol Med. 2009;38:731–736. doi: 10.1111/j.1600-0714.2009.00771.x. [DOI] [PubMed] [Google Scholar]
- 14.Zhang L., Zeng D., Huang H., Wang J., Tao Q., Pan C. Tissue inhibitor of metalloproteinase-2 inhibits ameloblastoma growth in a new mouse xenograft disease model. J Oral Pathol Med. 2010;39:94–102. doi: 10.1111/j.1600-0714.2009.00812.x. [DOI] [PubMed] [Google Scholar]
- 15.Fuchigami T., Suzuki H., Yoshimura T., Kibe T., Chairani E., Kiyono T. Ameloblastoma cell lines derived from different subtypes demonstrate distinct developmental patterns in a novel animal experimental model. J Appl Oral Sci. 2020;28 doi: 10.1590/1678-7757-2019-0558. e20190558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kurppa K.J., Caton J., Morgan P.R., Ristimaki A., Ruhin B., Kellokoski J. High frequency of BRAF V600E mutations in ameloblastoma. J Pathol. 2014;232:492–498. doi: 10.1002/path.4317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sweeney R.T., McClary A.C., Myers B.R., Biscocho J., Neahring L., Kwei K.A. Identification of recurrent SMO and BRAF mutations in ameloblastomas. Nat Genet. 2014;46:722–725. doi: 10.1038/ng.2986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Brown N.A., Rolland D., McHugh J.B., Weigelin H.C., Zhao L., Lim M.S. Activating FGFR2-RAS-BRAF mutations in ameloblastoma. Clin Cancer Res. 2014;20:5517–5526. doi: 10.1158/1078-0432.CCR-14-1069. [DOI] [PubMed] [Google Scholar]
- 19.Boyce B.F., Xing L. Biology of RANK, RANKL, and osteoprotegerin. Arthritis Res Ther. 2007;9(Suppl. 1):S1. doi: 10.1186/ar2165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Boyce B.F., Xing L. The RANKL/RANK/OPG pathway. Curr Osteoporos Rep. 2007;5:98–104. doi: 10.1007/s11914-007-0024-y. [DOI] [PubMed] [Google Scholar]
- 21.Kong Y.Y., Boyle W.J., Penninger J.M. Osteoprotegerin ligand: a regulator of immune responses and bone physiology. Immunol Today. 2000;21:495–502. doi: 10.1016/s0167-5699(00)01718-7. [DOI] [PubMed] [Google Scholar]
- 22.Boyce B.F., Xing L. Functions of RANKL/RANK/OPG in bone modeling and remodeling. Arch Biochem Biophys. 2008;473:139–146. doi: 10.1016/j.abb.2008.03.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Liu W., Zhang X. Receptor activator of nuclear factor-kB ligand (RANKL)/RANK/osteoprotegerin system in bone and other tissues (review) Mol Med Rep. 2015;11:3212–3218. doi: 10.3892/mmr.2015.3152. [DOI] [PubMed] [Google Scholar]
- 24.Wright H.L., McCarthy H.S., Middleton J., Marshall M.J. RANK, RANKL and osteoprotegerin in bone biology and disease. Curr Rev Musculoskelet Med. 2009;2:56–64. doi: 10.1007/s12178-009-9046-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wada T., Nakashima T., Hiroshi N., Penninger J.M. RANKL-RANK signaling in osteoclastogenesis and bone disease. Trends Mol Med. 2006;12:17–25. doi: 10.1016/j.molmed.2005.11.007. [DOI] [PubMed] [Google Scholar]
- 26.Renema N., Navet B., Heymann M.F., Lezot F., Heymann D. RANK-RANKL signalling in cancer. Biosci Rep. 2016;36 doi: 10.1042/BSR20160150. e00366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.da Silva T.A., Batista A.C., Mendonca E.F., Leles C.R., Fukada S., Cunha F.Q. Comparative expression of RANK, RANKL, and OPG in keratocystic odontogenic tumors, ameloblastomas, and dentigerous cysts. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2008;105:333–341. doi: 10.1016/j.tripleo.2007.06.009. [DOI] [PubMed] [Google Scholar]
- 28.Tekkesin M.S., Mutlu S., Olgac V. The role of RANK/RANKL/OPG signalling pathways in osteoclastogenesis in odontogenic keratocysts, radicular cysts, and ameloblastomas. Head Neck Pathol. 2011;5:248–253. doi: 10.1007/s12105-011-0271-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.de Matos F.R., de Moraes M., das Neves Silva E.B., Galvao H.C., de Almeida Freitas R. Immunohistochemical detection of receptor activator nuclear kB ligand and osteoprotegerin in odontogenic cysts and tumors. J Oral Maxillofac Surg. 2013;71:1886–1892. doi: 10.1016/j.joms.2013.05.023. [DOI] [PubMed] [Google Scholar]
- 30.Andrade F.R., Sousa D.P., Mendonca E.F., Silva T.A., Lara V.S., Batista A.C. Expression of bone resorption regulators (RANK, RANKL, and OPG) in odontogenic tumors. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2008;106:548–555. doi: 10.1016/j.tripleo.2008.05.042. [DOI] [PubMed] [Google Scholar]
- 31.Sandra F., Hendarmin L., Kukita T., Nakao Y., Nakamura N., Nakamura S. Ameloblastoma induces osteoclastogenesis: a possible role of ameloblastoma in expanding in the bone. Oral Oncol. 2005;41:637–644. doi: 10.1016/j.oraloncology.2005.02.008. [DOI] [PubMed] [Google Scholar]
- 32.Yoshimoto S., Morita H., Matsubara R., Mitsuyasu T., Imai Y., Kajioka S. Surface vacuolar ATPase in ameloblastoma contributes to tumor invasion of the jaw bone. Int J Oncol. 2016;48:1258–1270. doi: 10.3892/ijo.2016.3350. [DOI] [PubMed] [Google Scholar]
- 33.Kumamoto H., Ooya K. Expression of parathyroid hormone-related protein (PTHrP), osteoclast differentiation factor (ODF)/receptor activator of nuclear factor-kappaB ligand (RANKL) and osteoclastogenesis inhibitory factor (OCIF)/osteoprotegerin (OPG) in ameloblastomas. J Oral Pathol Med. 2004;33:46–52. doi: 10.1111/j.1600-0714.2004.00204.x. [DOI] [PubMed] [Google Scholar]
- 34.Liu X., Chen Z., Lan T., Liang P., Tao Q. Upregulation of interleukin-8 and activin a induces osteoclastogenesis in ameloblastoma. Int J Mol Med. 2019;43:2329–2340. doi: 10.3892/ijmm.2019.4171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Fuchigami T., Kibe T., Koyama H., Kishida S., Iijima M., Nishizawa Y. Regulation of IL-6 and IL-8 production by reciprocal cell-to-cell interactions between tumor cells and stromal fibroblasts through IL-1alpha in ameloblastoma. Biochem Biophys Res Commun. 2014;451:491–496. doi: 10.1016/j.bbrc.2014.07.137. [DOI] [PubMed] [Google Scholar]
- 36.Bendre M.S., Margulies A.G., Walser B., Akel N.S., Bhattacharrya S., Skinner R.A. Tumor-derived interleukin-8 stimulates osteolysis independent of the receptor activator of nuclear factor-kappaB ligand pathway. Cancer Res. 2005;65:11001–11009. doi: 10.1158/0008-5472.CAN-05-2630. [DOI] [PubMed] [Google Scholar]
- 37.Bendre M.S., Montague D.C., Peery T., Akel N.S., Gaddy D., Suva L.J. Interleukin-8 stimulation of osteoclastogenesis and bone resorption is a mechanism for the increased osteolysis of metastatic bone disease. Bone. 2003;33:28–37. doi: 10.1016/s8756-3282(03)00086-3. [DOI] [PubMed] [Google Scholar]
- 38.Huynh P.T., Beswick E.J., Coronado Y.A., Johnson P., O’Connell M.R., Watts T. CD90+ stromal cells are the major source of IL-6, which supports cancer stem-like cells and inflammation in colorectal cancer. Int J Cancer. 2016;138:1971–1981. doi: 10.1002/ijc.29939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sathi G.A., Inoue M., Harada H., Rodriguez A.P., Tamamura R., Tsujigiwa H. Secreted frizzled related protein (sFRP)-2 inhibits bone formation and promotes cell proliferation in ameloblastoma. Oral Oncol. 2009;45:856–860. doi: 10.1016/j.oraloncology.2009.02.001. [DOI] [PubMed] [Google Scholar]
- 40.Sathi G.S., Nagatsuka H., Tamamura R., Fujii M., Gunduz M., Inoue M. Stromal cells promote bone invasion by suppressing bone formation in ameloblastoma. Histopathology. 2008;53:458–467. doi: 10.1111/j.1365-2559.2008.03127.x. [DOI] [PubMed] [Google Scholar]
- 41.Nagase H., Visse R., Murphy G. Structure and function of matrix metalloproteinases and TIMPs. Cardiovasc Res. 2006;69:562–573. doi: 10.1016/j.cardiores.2005.12.002. [DOI] [PubMed] [Google Scholar]
- 42.Bibichenko I.I., Semkin V.A., Katushkina A.A. Ki-67 and matrix metalloproteinase-9 expression in the follicular cyst, keratocystic odontogenic tumor, and ameloblastoma. Arkh Patol. 2013;75:10–14. [PubMed] [Google Scholar]
- 43.Singh T., Chandu A., Clement J., Angel C. Immunohistochemistry of five molecular markers for typing and management of ameloblastomas: a retrospective analysis of 40 cases. J Maxillofac Oral Surg. 2017;16:65–70. doi: 10.1007/s12663-016-0923-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Farias L.C., Gomes C.C., Rodrigues M.C., de Castro W.H., Lacerda J.C., Ferreira E.F.E. Epigenetic regulation of matrix metalloproteinase expression in ameloblastoma. BMC Clin Pathol. 2012;12:11. doi: 10.1186/1472-6890-12-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Siqueira A.S., Carvalho M.R., Monteiro A.C., Freitas V.M., Jaeger R.G., Pinheiro J.J. Matrix metalloproteinases, TIMPs and growth factors regulating ameloblastoma behaviour. Histopathology. 2010;57:128–137. doi: 10.1111/j.1365-2559.2010.03596.x. [DOI] [PubMed] [Google Scholar]
- 46.da Silva A.D., Nobrega T.G., Saudades A.W., Otero M.I., Danilevicz C.K., Magnusson A.S. Ameloblastic neoplasia spectrum: a cross-sectional study of MMPS expression and proliferative activity. Oral Surg Oral Med Oral Pathol Oral Radiol. 2016;121:396–401.e1. doi: 10.1016/j.oooo.2015.11.011. [DOI] [PubMed] [Google Scholar]
- 47.Nadalin M.R., Fregnani E.R., Silva-Sousa Y.T., da Cruz Perez D.E. Presence of myofibroblasts and matrix metalloproteinase 2 in radicular cysts, dentigerous cysts, and keratocystic odontogenic tumors: a comparative immunohistochemical study. J Endod. 2012;38:1363–1367. doi: 10.1016/j.joen.2012.05.020. [DOI] [PubMed] [Google Scholar]
- 48.Aloka D., Padmakumar S.K., Sathyan S., Sebastian M., Banerjee M., Beena V.T. Association of matrix metalloproteinase 2 and matrix metalloproteinase 9 gene polymorphism in aggressive and nonaggressive odontogenic lesions: a pilot study. J Oral Maxillofac Pathol. 2019;23:158. doi: 10.4103/jomfp.JOMFP_2_17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.de Vicente J.C., Fresno M.F., Villalain L., Vega J.A., Vallejo G.H. Expression and clinical significance of matrix metalloproteinase-2 and matrix metalloproteinase-9 in oral squamous cell carcinoma. Oral Oncol. 2005;41:283–293. doi: 10.1016/j.oraloncology.2004.08.013. [DOI] [PubMed] [Google Scholar]
- 50.Stankovic S., Konjevic G., Gopcevic K., Jovic V., Inic M., Jurisic V. Activity of MMP-2 and MMP-9 in sera of breast cancer patients. Pathol Res Pract. 2010;206:241–247. doi: 10.1016/j.prp.2009.12.003. [DOI] [PubMed] [Google Scholar]
- 51.Fouad H., Salem H., Ellakwa D.E., Abdel-Hamid M. MMP-2 and MMP-9 as prognostic markers for the early detection of urinary bladder cancer. J Biochem Mol Toxicol. 2019;33 doi: 10.1002/jbt.22275. e22275. [DOI] [PubMed] [Google Scholar]
- 52.Baum O., Hlushchuk R., Forster A., Greiner R., Clezardin P., Zhao Y. Increased invasive potential and up-regulation of MMP-2 in MDA-MB-231 breast cancer cells expressing the beta3 integrin subunit. Int J Oncol. 2007;30:325–332. [PubMed] [Google Scholar]
- 53.Pinheiro J.J., Freitas V.M., Moretti A.I., Jorge A.G., Jaeger R.G. Local invasiveness of ameloblastoma. Role played by matrix metalloproteinases and proliferative activity. Histopathology. 2004;45:65–72. doi: 10.1111/j.1365-2559.2004.01902.x. [DOI] [PubMed] [Google Scholar]
- 54.Florescu A., Margaritescu C., Simionescu C.E., Stepan A. Immunohistochemical expression of MMP-9, TIMP-2, E-cadherin and vimentin in ameloblastomas and their implication in the local aggressive behavior of these tumors. Rom J Morphol Embryol. 2012;53:975–984. [PubMed] [Google Scholar]
- 55.Wang A., Zhang B., Huang H., Zhang L., Zeng D., Tao Q. Suppression of local invasion of ameloblastoma by inhibition of matrix metalloproteinase-2 in vitro. BMC Cancer. 2008;8:182. doi: 10.1186/1471-2407-8-182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Huang H. Matrix metalloproteinase-9 (MMP-9) as a cancer biomarker and MMP-9 biosensors: recent advances. Sensors (Basel) 2018;18:E3249. doi: 10.3390/s18103249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Nunia K., Urs A.B., Kumar P. Interplay between MMP-9 and TIMP-2 regulates ameloblastoma behavior and tooth morphogenesis. Appl Immunohistochem Mol Morphol. 2016;24:364–372. doi: 10.1097/PAI.0000000000000196. [DOI] [PubMed] [Google Scholar]
- 58.Qian Y., Huang H.Z. The role of RANKL and MMP-9 in the bone resorption caused by ameloblastoma. J Oral Pathol Med. 2010;39:592–598. doi: 10.1111/j.1600-0714.2009.00882.x. [DOI] [PubMed] [Google Scholar]
- 59.Kumamoto H., Yamauchi K., Yoshida M., Ooya K. Immunohistochemical detection of matrix metalloproteinases (MMPs) and tissue inhibitors of metalloproteinases (TIMPs) in ameloblastomas. J Oral Pathol Med. 2003;32:114–120. doi: 10.1034/j.1600-0714.2003.00086.x. [DOI] [PubMed] [Google Scholar]
- 60.Ribeiro B.F., Iglesias D.P.P., Nascimento G.J.F., Galvão H.C., Medeiros A.M.C., Freitas R.A. Immunoexpression of MMPs-1, -2, and -9 in ameloblastoma and odontogenic adenomatoid tumor. Oral Dis. 2009;15:472–477. doi: 10.1111/j.1601-0825.2009.01575.x. [DOI] [PubMed] [Google Scholar]
- 61.de Andrade Santos P.P., Nonaka C.F.W., Barboza C.A.G., Pereira Pinto L., de Souza L.B. Immunohistochemical analysis of MMP-13 and EMMPRIN in epithelial odontogenic lesions. Eur Arch Otorhinolaryngol. 2019;276:3203–3211. doi: 10.1007/s00405-019-05589-0. [DOI] [PubMed] [Google Scholar]
- 62.Freitas V.S., de Araujo C.R.F., Alves P.M., de Souza L.B., Galvao H.C., de Almeida Freitas R. Immunohistochemical expression of matrilysins (MMP-7 and MMP-26) in ameloblastomas and adenomatoid odontogenic tumors. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2009;108:417–424. doi: 10.1016/j.tripleo.2009.03.035. [DOI] [PubMed] [Google Scholar]
- 63.Anne R., Krisnuhoni E., Chotimah C., Latief B.S. Matrix metalloproteinase-9 (mmp-9) expression in different subtypes of ameloblastoma. J Maxillofac Oral Surg. 2014;13:281–285. doi: 10.1007/s12663-013-0538-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Zhang B., Zhang J., Huang H.Z., Xu Z.Y., Xie H.L. Expression and role of metalloproteinase-2 and endogenous tissue regulator in ameloblastoma. J Oral Pathol Med. 2010;39:219–222. doi: 10.1111/j.1600-0714.2009.00827.x. [DOI] [PubMed] [Google Scholar]
- 65.Ohta K., Naruse T., Ishida Y., Shigeishi H., Nakagawa T., Fukui A. TNF-alpha-induced IL-6 and MMP-9 expression in immortalized ameloblastoma cell line established by hTERT. Oral Dis. 2017;23:199–209. doi: 10.1111/odi.12594. [DOI] [PubMed] [Google Scholar]
- 66.Niault T.S., Baccarini M. Targets of Raf in tumorigenesis. Carcinogenesis. 2010;31:1165–1174. doi: 10.1093/carcin/bgp337. [DOI] [PubMed] [Google Scholar]
- 67.do Canto A.M., da Silva Marcelino B.M.R., Schussel J.L., Wastner B.F., Sassi L.M., Correa L. Immunohistochemical analysis of BRAF V600E mutation in ameloblastomas. Clin Oral Investig. 2019;23:779–784. doi: 10.1007/s00784-018-2494-y. [DOI] [PubMed] [Google Scholar]
- 68.Diniz M.G., Gomes C.C., Guimaraes B.V., Castro W.H., Lacerda J.C., Cardoso S.V. Assessment of BRAFV600E and SMOF412E mutations in epithelial odontogenic tumours. Tumour Biol. 2015;36:5649–5653. doi: 10.1007/s13277-015-3238-0. [DOI] [PubMed] [Google Scholar]
- 69.Fregnani E.R., Perez D.E., de Almeida O.P., Fonseca F.P., Soares F.A., Castro-Junior G. BRAF-V600E expression correlates with ameloblastoma aggressiveness. Histopathology. 2017;70:473–484. doi: 10.1111/his.13095. [DOI] [PubMed] [Google Scholar]
- 70.Kumamoto H., Ohki K., Ooya K. Expression of sonic hedgehog (SHH) signaling molecules in ameloblastomas. J Oral Pathol Med. 2004;33:185–190. doi: 10.1111/j.0904-2512.2004.00070.x. [DOI] [PubMed] [Google Scholar]
- 71.Zhang L., Chen X.M., Sun Z.J., Bian Z., Fan M.W., Chen Z. Epithelial expression of SHH signaling pathway in odontogenic tumors. Oral Oncol. 2006;42:398–408. doi: 10.1016/j.oraloncology.2005.09.008. [DOI] [PubMed] [Google Scholar]
- 72.Kanda S., Mitsuyasu T., Nakao Y., Kawano S., Goto Y., Matsubara R. Anti-apoptotic role of the sonic hedgehog signaling pathway in the proliferation of ameloblastoma. Int J Oncol. 2013;43:695–702. doi: 10.3892/ijo.2013.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Menzies A.M., Long G.V. Systemic treatment for BRAF-mutant melanoma: where do we go next? Lancet Oncol. 2014;15:e371–e381. doi: 10.1016/S1470-2045(14)70072-5. [DOI] [PubMed] [Google Scholar]
- 74.Tan S., Pollack J.R., Kaplan M.J., Colevas A.D., West R.B. BRAF inhibitor treatment of primary BRAF-mutant ameloblastoma with pathologic assessment of response. Oral Surg Oral Med Oral Pathol Oral Radiol. 2016;122:e5–e7. doi: 10.1016/j.oooo.2015.12.016. [DOI] [PubMed] [Google Scholar]
- 75.Kaye F.J., Ivey A.M., Drane W.E., Mendenhall W.M., Allan R.W. Clinical and radiographic response with combined BRAF-targeted therapy in stage 4 ameloblastoma. J Natl Cancer Inst. 2015;107:378. doi: 10.1093/jnci/dju378. [DOI] [PubMed] [Google Scholar]
- 76.Abe M., Zong L., Abe T., Takeshima H., Ji J., Ushijima T. BRAF inhibitor: a novel therapy for ameloblastoma in mandible. Chin J Cancer Res. 2018;30:677–678. doi: 10.21147/j.issn.1000-9604.2018.06.12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Meiss F., Andrlová H., Zeiser R. Vismodegib. In: Krämer A., Lu J.J., editors. Recent results in cancer research. Springer; New York, NY: 2018. pp. 125–139. [Google Scholar]
- 78.Kim J., Aftab B.T., Tang J.Y., Kim D., Lee A.H., Rezaee M. Itraconazole and arsenic trioxide inhibit hedgehog pathway activation and tumor growth associated with acquired resistance to smoothened antagonists. Cancer Cell. 2013;23:23–34. doi: 10.1016/j.ccr.2012.11.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.von Ahrens D., Bhagat T.D., Nagrath D., Maitra A., Verma A. The role of stromal cancer-associated fibroblasts in pancreatic cancer. J Hematol Oncol. 2017;10:76. doi: 10.1186/s13045-017-0448-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Affo S., Yu L.X., Schwabe R.F. The role of cancer-associated fibroblasts and fibrosis in liver cancer. Annu Rev Pathol. 2017;12:153–186. doi: 10.1146/annurev-pathol-052016-100322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Kumari N., Dwarakanath B.S., Das A., Bhatt A.N. Role of interleukin-6 in cancer progression and therapeutic resistance. Tumour Biol. 2016;37:11553–11572. doi: 10.1007/s13277-016-5098-7. [DOI] [PubMed] [Google Scholar]
- 82.Alfaro C., Sanmamed M.F., Rodriguez-Ruiz M.E., Teijeira A., Onate C., Gonzalez A. Interleukin-8 in cancer pathogenesis, treatment and follow-up. Cancer Treat Rev. 2017;60:24–31. doi: 10.1016/j.ctrv.2017.08.004. [DOI] [PubMed] [Google Scholar]
- 83.Li W., Lin S., Li W., Wang W., Li X., Xu D. IL-8 interacts with metadherin promoting proliferation and migration in gastric cancer. Biochem Biophys Res Commun. 2016;478:1330–1337. doi: 10.1016/j.bbrc.2016.08.123. [DOI] [PubMed] [Google Scholar]
- 84.Nguyen D.P., Li J., Tewari A.K. Inflammation and prostate cancer: the role of interleukin 6 (IL-6) BJU Int. 2014;113:986–992. doi: 10.1111/bju.12452. [DOI] [PubMed] [Google Scholar]
- 85.Goh Y.C., Chan S.W., Siar C.H. Parenchyma-stromal interleukin-1 alpha and interleukin-6 overexpressions in ameloblastoma correlate with the aggressive phenotype. Malays J Pathol. 2019;41:303–311. [PubMed] [Google Scholar]
- 86.Jiang C., Zhang Q., Shanti R.M., Shi S., Chang T.H., Carrasco L. Mesenchymal stromal cell-derived interleukin-6 promotes epithelial-mesenchymal transition and acquisition of epithelial stem-like cell properties in ameloblastoma epithelial cells. Stem Cells. 2017;35:2083–2094. doi: 10.1002/stem.2666. [DOI] [PubMed] [Google Scholar]
- 87.Syamala D., Suresh R., Janardhanan M., Savithri V., Anand P.P., Jose A. Immunohistochemical evaluation of myofibroblasts in odontogenic cysts and tumors: a comparative study. J Oral Maxillofac Pathol. 2016;20:208–213. doi: 10.4103/0973-029X.185898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Mudaliar U., Tamgadge A., Tamgadge S., Pereira T., Dhouskar S., Rajhans S. Immunohistochemical expression of myofibroblasts using alpha-smooth muscle actin (SMA) to assess the aggressive potential of various clinical subtypes of ameloblastoma. J Microsc Ultrastruct. 2019;7:130–135. doi: 10.4103/JMAU.JMAU_10_19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Carthy J.M. TGFbeta signaling and the control of myofibroblast differentiation: implications for chronic inflammatory disorders. J Cell Physiol. 2018;233:98–106. doi: 10.1002/jcp.25879. [DOI] [PubMed] [Google Scholar]
- 90.Fuchigami T., Koyama H., Kishida M., Nishizawa Y., Iijima M., Kibe T. Fibroblasts promote the collective invasion of ameloblastoma tumor cells in a 3D coculture model. FEBS Open Biol. 2017;7:2000–2007. doi: 10.1002/2211-5463.12313. [DOI] [PMC free article] [PubMed] [Google Scholar]