Abstract
There is a complex interaction between titanium dental implants, bone, and the immune system. Among them, specific immune cells, macrophages play a crucial role in the osseointegration dynamics. Infiltrating macrophages and resident macrophages (osteomacs) contribute to achieving an early pro-regenerative peri-implant environment. Also, multinucleated giant cells (MNGCs) in the bone-implant interface and their polarization ability, maintain a peri-implant immunological balance to preserve osseointegration integrity. However, dental implants can display cumulative levels of antigens (ions, nano and microparticles and bacterial antigens) at the implant–tissue interface activating an immune-inflammatory response. If the inflammation is not resolved or reactivated due to the stress signals and the immunogenicity of elements present, this could lead implants to aseptic loosening, infections, and subsequent bone loss. Therefore, to maintain osseointegration and prevent bone loss of implants, a better understanding of the osteoimmunology of the peri-implant environment would lead to the development of new therapeutic approaches. In this line, depicting osteoimmunological mechanisms, we discuss immunomodulatory strategies to improve and preserve a long-term functional integration between dental implants and the human body.
Scientific field of dental science: implant dentistry.
Keywords: Osteoimmunology, Osseointegration, Dental implant, Bone loss, Immunomodulation
1. Introduction
Branemark in the 60 s was the first researcher to report bone growing in direct contact with a titanium implant naming it osseointegration. However, osseointegration was more a concept rather than a biological term. Currently, a new paradigm for osseointegration has been proposed, suggesting it as an immune-driven process that leads to new bone formation surrounding the implant surface rather than a pure bone response [1]. Upon this new concept, titanium would activate a tolerogenic balance with peri-implant tissues leading to a foreign body equilibrium (FBE) response [[2], [3], [4]]. Following this, evidence shows that immune response regulates wound/tissue healing and regenerative mechanisms [5]. Findings support the idea that osseointegration could be activated by the same processes during the early stage of peri-implant tissues heal [2,[5], [6], [7]].
Traditionally, osseointegration has been described according to the mechanical stability of titanium implants within bone tissue [8]. On the other hand, myelointegration describes the tolerance of the bone marrow microenvironment after the insertion of a titanium implant [9]. Today, novel insights represent the complexity of interactions between both the peri-implant bone niche and bone marrow niche. These interactions include heterogeneous cell populations (immune cells, bone, and vascular cells) [10,11]. The specific interaction between immune and bone cells is called the “osteoimmune system” [[12], [13], [14], [15]]. In turn, osteoimmunology has become a fundamental discipline for the study of several inflammatory diseases, the crosstalk between bone and the immune system, and the influence of the immune system on bone regeneration and consumption [7,16,17].
Briefly, upon titanium dental implant insertion, bone and immune cells interact with a specific protein adsorption pattern that is formed instantly onto the titanium surface, which is critical for modulating the immune response, cell survival, growth, and differentiation [18]. In a recent quantitative polymerase chain reaction (qPCR) and histological study, it was observed that many immune and inflammation related RNA markers adjacent to titanium were up or down regulated at 1–4 weeks of observation, confirming the relationship between immune responses and the formation of osseointegration [3]. These facts lead us to the assumption that the osseointegration of intra-osseous titanium dental implants would depend on an osteoimmunological balance. The concept of osteoimmune balance implies a comprehensive view of the complex harmony of this triad of elements, i.e., bone cells, immune cells, and implants, which finally determines the fate of FBE [3,11,18]. Thus, to improve osseointegration and prevent bone loss of implants, a better understanding of the osteoimmunology of the peri-implant environment would lead to the development of new therapeutic approaches. In this line, several efforts are being developed in the field of immunomodulation to maintain the integrity of FBE in the long term [3,11,19], which will also probably improve the functional integration of the artificial implant-based prosthetic [20].
Growing evidence supports the critical role of the immune system in the modulation of bone homeostasis, healing and repair. However, its role at the peri-implant tissue healing after dental implant insertion remains poorly described. In this narrative review, we summarize new insights in the understanding of how the immune system leads to new bone formation in the peri-implant tissues. We also propose immune-centered therapeutic approaches to improve osseointegration.
2. Immunity and regenerative dynamics in the early peri-implant environment
While fish and amphibians exhibit extensive tissue regeneration, mammals only replicate this property in some specific tissues (e.g., skin and bone) [6,7,21]. Particularly, bone undergoes a genuinely regenerative process after injury, recapitulating embryonic mechanisms [5,22]; however, this inherent capacity of regeneration has a biological limit in humans known as “critical size” [23]. After the implantation procedure, it seems this capacity of bone regeneration is strongly activated, which seems to indicate that titanium implants complement the bone healing process [1,2]. Interestingly, in these biological events both innate and acquired immune mechanisms are required [2,[5], [6], [7]].
Inflammation is the main mechanism of innate immunity and in turn, an initial and tightly controlled inflammatory immune response is critical for bone formation, osseointegration and successful regenerative capacity [5,11,24]. In this initial inflammatory phase, macrophages are widely involved [25], even if the injury is chronic and there is persistent inflammation [26]. Hence today macrophages are considered important to guide the tissue microenvironment at the site of the wound [27]. Macrophages polarize into two phenotypes: the antimicrobial and proinflammatory M1-macrophages, and the anti-inflammatory and pro-regenerative M2-macrophages [28]. An unbalanced M1/M2 ratio with a dominant M1 environment may lead to chronic low-grade inflammation, osteolysis, and loosening of implants [29]. On the other hand, modulation of the M1/M2 balance of macrophages is important in wound healing, regeneration and osseointegration [2,5]. In fact, a balanced M1/M2 macrophage ratio-correlates with M2-associated bone growth at the peri-implant niche at post-implant day (PID) 10 [30,31]. During fracture healing, M2 macrophages participate in both the resolution phase of inflammation and in the homing of mesenchymal stem cells (MSCs) [32,33]. Also, M2 contributes to the ossification phase of fracture repair [34,35]. Surprisingly, for epimorphic regeneration of axolotl limb and zebrafish tail fin regeneration, macrophages also gradually shift to an M2 phenotype during the tissue remodeling stage [6,36]. In addition, it is important to note that a pro-regenerative M2 phenotype can produce trophic molecules, such as Wnt ligands [37,38]. In turn, higher Wnt signaling has been correlated with faster bone healing, faster implant osseointegration [39], a range of functions during embryonic development and organ development [40,41].
On the other hand, the role of the adaptative immune system also seems to be relevant for tissue healing and regeneration [24]. Recent research shows that lower levels of pro-inflammatory cytokines secreted by CD8+ T stimulate the new bone formation by MSC and bone healing [42]. In addition, it is known that CD4+CD25+FOXP3+ regulatory (Treg) cells are essential modulators of the immune response, being able to suppress the inflammatory response and allow reparative processes to stop some forms of autoimmune diseases [5,30]. Interestingly, recent results on bone immune response to titanium implant showed the activation of CD4+ T cells, while the phenotype of CD8+ T cells is suppressed. Therefore, these findings also indicate an adaptive immune response around Titanium [30,31]. Nevertheless, it unknown whether lymphocytes make an acquired immunity participation in the process or if it remains within the innate immunity limits [18].
The role of M macrophages, Tregs, on the activation of osteogenic pathways (i.e.: Wnt/B cathenin pathway); strongly suggest that immunomodulatory signals occurred in the early stage of osseointegration recapitulate regenerative mechanisms [2,6,5,7]. However, local immune cell infiltration and macrophage polarization can regulate both bone dynamics [43] and the progression of bone resorption [44], ectopic bone calcification [45] and solid tumors development [36]. Therefore, intermediate stages of M macrophage polarization causing multiples biological scenarios, making the understanding of its role still challenging (Table 1).
Table 1.
Contribution of macrophage phenotypes to biological events: macrophages are considered important to guide the tissue microenvironment and probably are widely involved in several biological events. The interplay between M1- and M2-dominated microenvironments (M1/M2relationship) and the temporal modulation of the transition M1 to M2, determinates de fate of the tissue response through the release of anti-inflammatory/ proinflammatory cytokines, respectively. (M1: pro-inflammatory macrophage phenotype; M2: anti-inflammatory macrophage phenotype; [+]: predominance; and [-]: low-level).
| Biological event | M1/M2 relationship | Predominant macrophage phenotype | Proinflammatory environment | Ref. |
|---|---|---|---|---|
| Osseointegration | Balance | M2 | (−) | [2,30,31,57,111] |
| Loosening of implants | Unbalanced | M1 | (+) | [29] |
| Epimorphic regeneration | Balance | M2 | (−) | [5,5,36] |
| Fracture repair | Balance | M2 | (−) | [34,35] |
| Bone resorption | Unbalanced | M1 | (+) | [44] |
3. Immunity: a pivotal player to achieve and maintain osseointegration throughout the implant-bone interface
For an implant become osseointegrated and myelointegrated, it needs to trigger an osteoformative response around the titanium surface at both cortical and cancellous bone levels, respectively [9,46,47]. Cancellous bone is organized in trabeculae surrounded by bone marrow spaces. In turn, the bone marrow is the natural reservoir of MSCs. These cells maintain a stable bone mass and then bone homeostasis through life by differentiating to the osteoblastic lineage, able to secrete bone matrix [13,33,40]. Beside it, MSCs can differentiate into the chondrogenic and adipogenic lineages [3]. Multifactorial inputs, including mechanical stability, aging, and metabolic diseases, will define the fate of these progenitor/stem cells, leading to establishing either an optimal or adverse environment for osseointegration at the bone-implant interface [31,39,[48], [49], [50], [51], [52]].
Besides the stromal component, bone marrow host the hematopoietic cell lineage. Between it, both myeloid and lymphoid progenitor cells give rise to the monocyte-macrophage-osteoclast cell lineage and T cells, respectively [9]. Specifically, bone marrow myelomonocytic cells will support the population of circulant monocytes, which will differentiate into macrophages upon defensive environmental demands [10]. Interestingly, a number of these myelomonocytic cells will give rise to a population of resident or osteal macrophages, named osteomacs, constituting approximately one-sixth of total cell type residing in the bone marrow [53]. Osteomacs participate in bone homeostasis, supporting osteoblast differentiation, function, and bone matrix mineralization [54]. Also, they contribute to bone repair after fracture [43,55].
Regarding the tolerance of biomaterials, osteomacs has been proposed as immune surveillance cells by being in contact with implanted biomaterials [56]. It has been postulated that monocytic-derived cells, possibly osteomacs, arrive at the titanium implant surface differentiating into pro-regenerative M2 and then recruiting osteoprogenitor cells to build the peri-implant new bone [57]. In fact, a macrophage depleted model showed both a reduced number of osteoblasts and less bone formation around implants [58].
Multinucleated giant cells (MNGCs) are fused macrophages contributing to the removal of cell debris after tissue aggressions [59]. Also, MNGCs is associated with foreign body reaction (FBR) and fibrotic encapsulation around biomedical implants [[60], [61], [62]]. Growing evidence from experimental models of oral osseointegration, reported MNGCs at the peri-implant tissues [9,63,64]. This could be related with the adsorbed protein layer on the implant surfaces and macrophages interaction through cell surface receptors called integrins [11,18,65]. In addition, macrophages can bind to a group of endogenous molecules acting as local “danger signals” called damage-associated molecular patterns (DAMPs), which are important for regulate healing outcome [66,67]. In fact, it has been demonstrated that the inhibition of a prototypic DAMPs impaired the osseointegration, causing a higher expression M1 markers and resulting in FBR, with persistence of MNGCs [68]. Interestingly, precursor cells of MNGCs are thought to be derived from osteomacs [56], and in turn osteomacs and osteoclasts (OCs) have a common RANKL-induced macrophage-derived cell line [57,69]. Furthermore, important findings have shown that if the antigen accumulation occurs in surrounding tissues and in the intercellular spaces close to implant surfaces, “it is likely that MNGCs will polarize toward M1-MNGCs, creating an inflammatory environment and probably, their interaction with other cells such as osteoclasts (direct interaction vs. indirect interaction through e.g., T-cells)”. This opens up a whole new field of research, because it has been postulated that they can act as key regulators during peri-implant bone loss [45]
Dental implants are transmucosal devices exposed to oral antigens (i.e., food, bacteria, viruses, fungi, and by their products) during its lifespan [45]. This anatomical feature is associated with the strong immunoreactivity of periodontal tissues [70]. In addition, could be related to the higher T cell co-stimulating capacity of oral Langerhans cells (LCs) phenotype [71]. LCs are dendritic cell (DC) [72], the most potent antigen-presenting cells (APCs) [73,74]. In turn, DCs would participate in the host response against biomaterials raising the inflammatory response [75]. In fact, DCs are critically situated at the osteo–immune interface [73]; however, their roles in implantable devices has been poorly investigated [76], despite there is growing evidence that DCs promoting bone loss by RANKL-activated osteoclasts [77,78]. The primary function of DCs is to present antigens to lymphocytes by the major histocompatibility complex MHC [79,80], influencing their development and differentiation, to initiate an antigen-specific immune response [81,82]. For the lymphocyte activation, it requires engagement of T cell receptor (TCRs) with MHC/peptide complexes expressed on the surface of DCs, and interestingly, metal ions can interact with these MHC/peptide complexes, like bacterial derived antigens [83].
Free Titanium metal ions can bind to serum proteins and form haptens or hapten-like complexes activating the adaptive immune system driven by a Th1-type response [[84], [85], [86]]. Also, TiO2 nanoparticles can in multiple cell types disrupt epigenetic integrity, through DNA methylation [87]. Furthermore, cobalt alloy particles induce activation of NF-κB, the master inflammatory transcription factor, leading to the release of TNF-α and IL-8 through TLR4-dependent signaling [83]. Cobalt can also modulate the vital and functional parameters of human macrophages, like titania (TiO2), silica (SiO2) and zirconia (ZrO2) [88]. This is an important issue because the cobalt-chromium alloy is widely used for dental implant supra structures [89], and there are several potential sources of titanium ions and particles in implant dentistry [90,91]. Besides, organic and inorganic contaminants have been reported on dental implant surfaces [92]. On the other hand, increased levels of metal ions and pro-inflammatory cytokines (i.e.: IL-1β, IL-2, IL-8, IFN-γ, and TNF-α) have been reported in retrieved tissues from aseptically loosened metallic orthopaedic implants [93]. These findings suggest that both the innate and acquired immune response is associated with aseptic metallic implant failure [86].
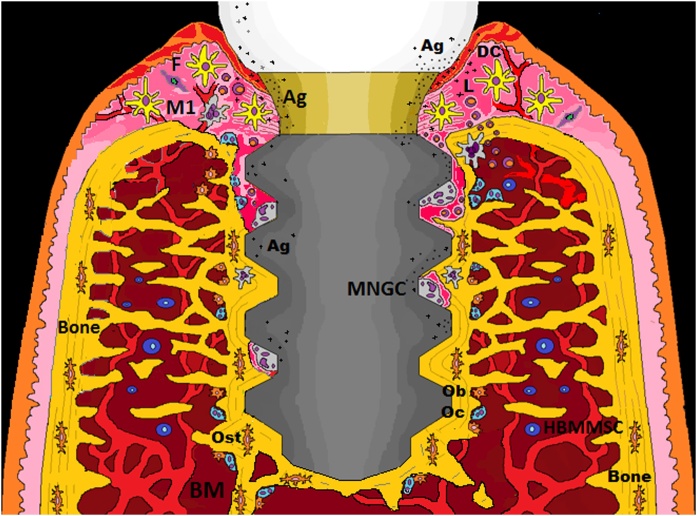
The above reveals that DCs and macrophages are immunological sentinels present in the peri-implant environment that could determine the lifespan of dental implants [18,45,94]. Moreover, these findings lead us to think that the bone loss around an implant device can occur through aseptic and/or septic osteolysis with similar underlying immune reactions [95]. Therefore, we must be aware of their immune cell capacity when we are planning a dental implant treatment (Fig. 1).
Fig. 1.
Hypothetical scenario: Functional dental implant and eventual accumulation of antigens (ions, nano and microparticles and bacterial antigens) with the presence of activated immunological sentinels (DCs and macrophages) throughout the implant-tissue interface. If the inflammation is not resolved or reactive due to the stress signals and the immunogenicity of the elements present, there is a risk that initially relatively harmless peri-implant bone loss progresses to a more damaging and vicious stage, due to the polarization capacity of the MNGC. Ag = antigen; DC = dendritic cell; F = fibroblast; HBMMSC = human mesenchymal stem cells derived from bone marrow; L = lymphocyte; M1 = macrophage; MNGCs = multinucleated giant cells; Ob = osteoblast; Oc = osteoclast; Ost = osteocyte.
4. Strategies to modulate the peri-implant immune component to achieve and maintain dental implant osseointegration
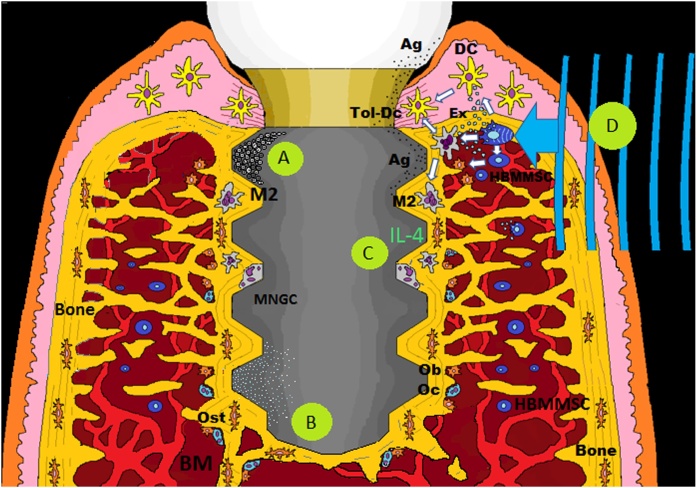
Functional dental implants are in contact with both hard and soft tissues, through an intraosseous part that reacts with host bone to achieve osseointegration and a transmucosal part, the implant–abutment connection structure that supports prosthesis [[96], [97]]. This special anatomical arrangement predisposes dental implants to several factors from the local oral environment: implant overloading, periodontal infection, cement, and metallic debris or ions released from the implant components [98]. In addition, in the presence of metallic ions, such as impurities or alloys like Co and Ni, the anatase–rutile phase transformation takes place even at normal conditions, that would alter the biological characteristics of a TiO2 surface [99]. Thus, oral implants can be exposed to various clinical factors, however, also many factors associated with the patient. Certain genetic polymorphisms of cytokines such as interleukin (IL)-1b, habits such as smoking and alcohol consumption, the intake of medication for certain diseases, are thought to influence the host response [18]. But even more importantly, these factors (clinical and patients) together may trigger the immune system to a different reaction possibly resulting in implant rejection [100]. In general, when a device is implanted, within either soft or hard tissue, the material surface adsorbs proteins from blood initiating the inflammation cascade mediated by the innate immune system [101]. However, if the inflammation is not resolved or reactivated due to the stress signals and the immunogenicity of elements present, there is a risk of implant failure [100,102,103]. Fortunately, there is evidence that immune response during biomaterial-mediated osteogenesis can be manipulated through beneficial “osteoimmunomodulation” [82] (Fig. 2).
Fig. 2.
Immunomodulation strategies to improve, maintain and eventually recover osseointegration: A) Modification of implant surfaces properties may improve osseointegration, probably by switching the phenotype of peri-implant macrophages from the pro-inflammatory (M1) subset to a pro-regenerative one (M2). B) Osseointegration needs to be maintained, this could be possible by reducing an eventual secretion of pro-inflammatory cytokines through ionic-treated implant surfaces with LiCl or Mg. C) Another approach is centered on the modulation of macrophage phenotype using polarizing cytokines such IL-4. D) Recently, it has been proposed that an external mechanical stimulus directed to the peri-implant tissue could promote the innate immunomodulatory capacities of BMMSCs, influencing CDs and macrophages, this through mechano-signal transduction and the release of exosomes (ex). Ag = antigen; Ex = exosome; HBMMSC = human mesenchymal stem cells derived from bone marrow; M2 = macrophage; MNGCs = multinucleated giant cells; Ob = osteoblast; Oc = osteoclast; Ost = osteocyte; Tol-DC = tolerogenic dendritic cell.
Modifying material properties may decrease the immune response to implanted biomaterial [102]. In this sense, it is known that the modification of implant surfaces with titanium oxide (TiO2) nanotubes positively affect the osseointegration, probably by switching the phenotype of peri-implant macrophages from the pro-inflammatory (M1) subset to a pro-regenerative one (M2) [[104], [105], [106], [107], [108], [109]] (Fig. 2A). The immunomodulatory capability to promote pro-regenerative macrophage polarization, through additive manufacturing (AM) porous titanium, has also been studied [110]. Furthermore, the use of hydrophilic surfaces appears to be able to influence macrophages to produce an anti-inflammatory microenvironment [111]. These surface modifications are a critical approach to induce osteogenesis for the next-generation of intraosseous implants [104]. However, the main studies analyzing the osteoimmunological response around dental implants consider c.p. titanium implants [2,30,31]. Therefore, there is still a lack of knowledge about the osteoimmunological response upon implant surface modifications such sandblasted/acid-etched (SAE) [111].
Attaining the FBE-mediated osseointegration, it needs to be maintained during the lifespan of a loaded implant. Biologically, the interplay between bone cells, immune cells, and implant surface would determine the fate of FBE [11,112]. Thus, ionic-treated implant surfaces would modulate a pro-regenerative immune response and then optimizing osseointegration. For example, high concentrations of magnesium (Mg) in the implant surface reduce the secretion of pro-inflammatory cytokines, such as TNF-α, IL-1b, IL-6, and PEG2 [113]. A recent study highlights the immunomodulatory effect of lithium chloride (LiCl) by mitigating the macrophage-driven periprosthetic inflammation in particle-induced osteolysis models [114] (Fig. 2B). Another approach is centered on the modulation of macrophage phenotype using polarizing cytokines [112]. Indeed, macrophages can be activated to the anti-inflammatory M2 phenotype by the exogenous addition of polarizing cytokines IL-4, IL-13, or IL-10 [115]. Therefore, adding IL-4, the macrophage phenotype polarizes from the pro-inflammatory (M1) to the tissue regenerative (M2), leading to a pro-osteogenic response. This transition from M1 to M2 has been associated with an increase in bone anabolic factors CCL2/MCP-1, CCL5/RANTES, and IGF-1 in vitro [116] (Fig. 2C).
On the other hand, MSCs represent one of the most promising tools in regenerative medicine, thanks to their potential for proliferation, differentiation, and their innate immunomodulatory capabilities [112,117]. In this line, MSCs influence not only T cells but also other cells of the immune system, such as DCs and macrophages [118]. Interestingly, bone marrow-derived MSCs (BMMSCs) modulate the immune response through a series of mechanisms, among these the generation of tolerogenic DCs (Tol-DCs) [119]. Current evidence indicates that BMMSCs can modulate the immune response by inhibiting polarization induced to M1 macrophages and promoting polarization to M2 macrophages through the release of paracrine factors [120]. Hence, local BMMSCs could immunomodulate the local response in favor of osseointegration [121]. Furthermore, studies about the mechanobiology of stem cells, have shown that mechanical stimuli induced signaling pathways that play essential roles in cellular differentiation and the determination of stem cells' fate (mechanotransduction) [[122], [123], [124]]. Interestingly, there are sensory organelles called primary cilia that play a critical role in mechanotransduction and BMMSCs do indeed possess these organelles [125]. A recent study has confirmed the importance of this organelles in MSC biology. Thus, stem cell mechanotransduction could be targeted therapeutically [126]. Mechanosignaling also affect the phenotype of immune cells, such as macrophage and dendritic cells [127,128] and is a potent pathway to counteract inflammation activated by the NF-κB signaling cascade [129]. In this line, osseointegration was associated with mechanotransduction to maintain FBE in the long term [11,121] (Fig. 2D)
5. Functional integration of dental implants after achieving the immune equilibrium
Despite the inherent complexity of the peri-implant environment, oral implants have developed rapidly, and their development has shifted mainly toward esthetics and simplified use [130]. However, the importance of the peri-implant osteoimmunology [68] and the neurophysiological integration of dental implants remain only partially understood [131]. In relation to the latter, new evidence relates tooth loss with the increased risk of diminished cognitive function [132] and in turn it is thought that dental implants may play a role in maintaining cognitive function [133]. Moreover, it has been demonstrated that dental implants activate cortical somatosensory areas [134], and osseoperception increases over time [135].
It has been suggested that the extent of bone apposition to the implant might be of importance in the grade of osseoperception. In fact, it has been demonstrated that implants with a SAE surface have high bone to implant contact and are more sensitive than machined implants [136,137]. Interestingly, it is known that nerves in bone can interact with bone cells [138] and regenerative paradigms exhibit nerve dependency [139]. It is for this reason that the use of neurotrophins (e.g., nerve growth factor (NGF)) has been proposed in dental implants, because NGF is related to the survival of peripheral sensory neurons [140] and bone formation after bone fracture [141]. Hence, the use of suitable methods to induce peri-implant nerve regeneration could be effective for improving proprioception of dental implants [142]. In addition, recent findings have related the polarization of M2 macrophages with the restoration of axonal regeneration [143], proving the functional links between the immune system and the nervous system [144]. Surprisingly, there is an M2 regulation in the peri-implant environment [2,31,111] and both neurofilament-positive fibers and nerve bundles have been observed near the titanium implant surface [63,145]. Furthermore, it has been demonstrated that nerves retain a degree of physiological function suitable for creating an osseointegrated neural interface [146]. Nevertheless, the role of this peri-implant innervation remains only partially understood [131].
Evidence of osseoperception shows that an appropriate peripheral feedback pathway can be restored with the use of osseointegrated implants [147]. Thereby, the restoration of the somatosensorial control loops would allow the patient to live a normal life with a prosthesis and reduce the risk of overloading artificial prosthetic and/or implants [136]. Hence, from the psychoprosthetic point of view, a better understanding of the complex human interaction with implantable devices is necessary [148]. But at the same time, it is necessary a reliable and long-term stable host–implant relationship [149] which would not be possible if the FBE were to be compromised [4,18]. Therein lies the importance of understanding host osteoimmunology and the development of peri-implant immunomodulation strategies [11].
6. Conclusion
-
1
It is suggested that titanium dental implant induces a critical pro-regenerative environment in the early stage of osseointegration.
-
2
It seems that osteomacs and dendritic cells are immunological sentinel highlights present in the implant–tissue interface, being able to activate a T cell-mediated response.
-
3
If the antigen accumulation occurs in surrounding tissues close to the implant surface, and the inflammation is not resolved or reactivated, there could be a peri-implant bone loss through M1-MNGC polarization.
-
4
Metal ions released can activate the adaptive immune system like bacterial derived antigens, hence bone loss around an implant device could occur through aseptic and/or septic osteolysis.
-
5
FBE needs to be maintained in the long-term, therefore immunomodulatory strategies focused on the osteoimmunology of the host are a promising approach.
-
6
The osteoimmunology field will permit a greater understanding and integration of osseointegration, myelointegration and osseoperception concepts.
Funding acknowledgement
No funding to declare
Conflicts of interest
The authors declare no conflict of interest.
Contributor Information
Luis Amengual-Peñafiel, Email: luisamengualp@gmail.com.
Luis A. Córdova, Email: lcordova@uchile.cl.
M. Constanza Jara-Sepúlveda, Email: mconstanzajara@gmail.com.
Manuel Brañes-Aroca, Email: drmanuelbranesa@gmail.com.
Francisco Marchesani-Carrasco, Email: francisco@marchesani.cl.
Ricardo Cartes-Velásquez, Email: cartesvelasquez@gmail.com.
References
- 1.Albrektsson T., Chrcanovic B., Jacobsson M., Wennerberg A. Osseointegration of implants- a biological and clinical overview. JSM Dent Surg. 2017;2(3):1022–1028. [Google Scholar]
- 2.Trindade R., Albrektsson T., Galli S., Prgomet Z., Tengvall P., Wennerberg A. Osseointegration and foreign body reaction: titanium implants activate the immune system and suppress bone resorption during the first 4 weeks after implantation. Clin Implant Dent Relat Res. 2018;20:82–91. doi: 10.1111/cid.12578. [DOI] [PubMed] [Google Scholar]
- 3.Albrektsson T., Jemt T., Mölne J., Tengvall P., Wennerberg A. On inflammation-immunological balance theory-A critical apprehension of disease concepts around implants: mucositis and marginal bone loss may represent normal conditions and not necessarily a state of disease. Clin Implant Dent Relat Res. 2019;21(1):183–189. doi: 10.1111/cid.12711. [DOI] [PubMed] [Google Scholar]
- 4.Albrektsson T., Dahlin C., Jemt T., Sennerby L., Turri A., Wennerberg A. Is marginal bone loss around oral implants the result of a provoked foreign body reaction? Clin Implant Dent Relat Res. 2014;16:155–165. doi: 10.1111/cid.12142. [DOI] [PubMed] [Google Scholar]
- 5.Iismaa S.E., Kaidonis X., Nicks A.M. Comparative regenerative mechanisms across different mammalian tissues. NPJ Regen Med. 2018;3:6. doi: 10.1038/s41536-018-0044-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Godwin J.W., Pinto A.R., Rosenthal N.A. Macrophages required for regeneration. Proc Natl Acad Sci U S A. 2013;110:9415–9420. doi: 10.1073/pnas.1300290110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Limmer A., Wirtz D.C. Osteoimmunology: influence of the immune system on bone regeneration and consumption. Z Orthop Unfall. 2017;155(3):273–280. doi: 10.1055/s-0043-100100. [DOI] [PubMed] [Google Scholar]
- 8.Albrektsson T., Albrektsson B. Osseointegration of bone implants: a review of an alternative mode of fixation. Acta Orthop Scand. 1987;58(5):567–577. doi: 10.3109/17453678709146401. [DOI] [PubMed] [Google Scholar]
- 9.Rahal M.D., Delorme D., Brånemark P.I., Osmond D.G. Myelointegration of titanium implants: B lymphopoiesis and hemopoietic cell proliferation in mouse bone marrow exposed to titanium implants. Int J Oral Maxillofac Implants. 2000;15(2):175–184. [PubMed] [Google Scholar]
- 10.Zhao E., Xu H., Wang L. Bone marrow and the control of immunity. Cell Mol Immunol. 2012;9:11–19. doi: 10.1038/cmi.2011.47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Amengual-Peñafiel L., Brañes-Aroca M., Marchesani-Carrasco F., Jara-Sepúlveda M.C., Parada-Pozas L., Cartes-Velásquez R. Coupling between Osseointegration and mechanotransduction to maintain foreign body equilibrium in the long-term: a comprehensive overview. J Clin Med. 2019;8(2) doi: 10.3390/jcm8020139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tsukasaki M., Takayanagi H. Osteoimmunology: evolving concepts in bone–immune interactions in health and disease. Nat Rev Immunol. 2019;19(10):626–642. doi: 10.1038/s41577-019-0178-8. [DOI] [PubMed] [Google Scholar]
- 13.Kim J., Bixel M.G. Intravital multiphoton imaging of the bone and bone marrow environment. Cytom Part A. 2019;97:496–503. doi: 10.1002/cyto.a.23937. [DOI] [PubMed] [Google Scholar]
- 14.Furuya M., Kikuta J., Fujimori S. Direct cell-cell contact between mature osteoblasts and osteoclasts dynamically controls their functions in vivo. Nat Commun. 2018;9 doi: 10.1038/s41467-017-02541-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kumar Gaurav, Roger Pierre-Marie. From crosstalk between immune and bone cells to bone erosion in infection. Int J Mol Sci. 2019;20(October (20)):5154. doi: 10.3390/ijms20205154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ponzetti M., Rucci N. Updates on osteoimmunology: what’s new on the cross-talk between bone and immune system. Front Endocrinol. 2019;10:236. doi: 10.3389/fendo.2019.00236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Blin-Wakkach C., de Vries T.J. Advances in osteoimmunology. Front Immunol. 2019;10:2595. doi: 10.3389/fimmu.2019.02595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Trindade R., Albrektsson T., Wennerberg A. Current concepts for the biological basis of dental implants: foreign body equilibrium and osseointegration dynamics. Oral Maxillofac Surg Clin North Am. 2015;27(2):175–183. doi: 10.1016/j.coms.2015.01.004. [DOI] [PubMed] [Google Scholar]
- 19.Zetao C., Travis K., Rachael M. Osteoimmunomodulation for the development of advanced bone biomaterials. Mater Today. 2016;19:304–321. [Google Scholar]
- 20.Hoellwarth Jason Shih. Osseointegration for amputees: current implants, techniques, and future directions. JBJS Rev. 2020;8(3) doi: 10.2106/JBJS.RVW.19.00043. e0043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Simkin J., Gawriluk T.R., Gensel J.C., Seifert A.W. Macrophages are necessary for epimorphic regeneration in African spiny mice. eLife. 2017;16(6) doi: 10.7554/eLife.24623. e24623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Dimitriou Rozalia, Jones Elena, McGonagle Dennis, Giannoudis Peter V. Bone regeneration: current concepts and future directions. BMC Med. 2011;9(May (66)) doi: 10.1186/1741-7015-9-66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Shrivats A.R., Mc Dermott M.C., Hollinger J.O. Bone tissue engineering: state of the union. Drug Discov Today. 2014;19(6):781–786. doi: 10.1016/j.drudis.2014.04.010. [DOI] [PubMed] [Google Scholar]
- 24.Mescher A.L. Macrophages and fibroblasts during inflammation and tissue repair in models of organ regeneration. Regeneration. 2017;4(2):39–53. doi: 10.1002/reg2.77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Oishi Y., Manabe I. Macrophages in inflammation, repair and regeneration. Int Immunol. 2018;30(11):511–528. doi: 10.1093/intimm/dxy054. [DOI] [PubMed] [Google Scholar]
- 26.Mescher A.L. Macrophages and fibroblasts during inflammation and tissue repair in models of organ regeneration. Regeneration. 2017;4:39–53. doi: 10.1002/reg2.77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Simkina J., Dawson L., Simkin M., Muneoka K. Healing power: the mammalian macrophage in skeletal regeneration, scar formation, and regenerative medicine. J Regen. 2019;2(3):93–105. [Google Scholar]
- 28.Wu C.L., Harasymowicz N.S., Klimak M.A., Collins K.H., Guilak F. The role of macrophages in osteoarthritis and cartilage repair. Osteoarthr Cartil. 2020;28(5):544–554. doi: 10.1016/j.joca.2019.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Goodman S.B., Gibon E., Pajarinen J. Novel biological strategies for treatment of wear particle-induced periprosthetic osteolysis of orthopaedic implants for joint replacement. J R Soc Interface. 2014;11(93) doi: 10.1098/rsif.2013.0962. 20130962. Published 2014 Jan 29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Trindade R., Albrektsson T., Galli S., Prgomet Z., Tengvall P., Wennerberg A. Bone immune response to materials, part I: titanium, PEEK and copper in comparison to sham at 10 days in Rabbit Tibia. J Clin Med. 2018;7:526–541. doi: 10.3390/jcm7120526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Trindade R., Albrektsson T., Galli S., Prgomet Z., Tengvall P., Wennerberg A. Bone immune response to materials, part II: copper and polyetheretherketone (PEEK) compared to titanium at 10 and 28 days in rabbit tibia. J Clin Med. 2019;8(6):814. doi: 10.3390/jcm8060814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gong Lei, Zhao Yan, Zhang Yi, Ruan Zhi. The macrophage polarization regulates MSC osteoblast differentiation in vitro. Ann Clin Lab Sci Winter. 2016;46(1):65–71. [PubMed] [Google Scholar]
- 33.Pajarinen J., Lin T., Gibon E. Mesenchymal stem cell-macrophage crosstalk and bone healing. Biomaterials. 2019;196:80–89. doi: 10.1016/j.biomaterials.2017.12.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Schlundt C., El Khassawna T., Serra A., Dienelt A., Wendler S., Schell H. Macrophages in bone fracture healing: their essential role in endochondral ossification. Bone. 2018;106:78–89. doi: 10.1016/j.bone.2015.10.019. [DOI] [PubMed] [Google Scholar]
- 35.Zhang R., Liang Y., Wei S. M2 macrophages are closely associated with accelerated clavicle fracture healing in patients with traumatic brain injury: a retrospective cohort study. J Orthop Surg Res. 2018;13:213. doi: 10.1186/s13018-018-0926-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wong A., Whited J. Parallels between wound healing, epimorphic regeneration and solid tumors. Development. 2020;147(1) doi: 10.1242/dev.181636. dev181636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wynn T.A., Chawla A., Pollard J.W. Macrophage biology in development, homeostasis and disease. Nature. 2013;496:445–455. doi: 10.1038/nature12034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Cosin-Roger J., Ortiz-Masià M.D., Barrachina M.D. Macrophages as an emerging source of wnt ligands: relevance in mucosal integrity. Front Immunol. 2019;10:2297. doi: 10.3389/fimmu.2019.02297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Li J., Yin X., Huang L. Relationships among bone quality, implant osseointegration, and wnt signaling. J Dent Res. 2017;96(7):822–831. doi: 10.1177/0022034517700131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Houschyar K.S., Tapking C., Borrelli M.R. Wnt pathway in bone repair and regeneration - what do we know so far. Front Cell Dev Biol. 2019;6:170. doi: 10.3389/fcell.2018.00170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Stoick-Cooper C.L., Weidinger G., Riehle K.J., Hubbert C., Major M.B., Fausto N., Moon R.T. Distinct Wnt signaling pathways have opposing roles in appendage regeneration. Development. 2007;134(3):479–489. doi: 10.1242/dev.001123. [DOI] [PubMed] [Google Scholar]
- 42.Wendler S., Schlundt C., Bucher C.H. Immune modulation to enhance bone Healing-A new concept to induce bone using prostacyclin to locally modulate immunity. Front Immunol. 2019;10:713. doi: 10.3389/fimmu.2019.00713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Alexander K.A., Raggatt L.J., Millard S. Resting and injury-induced inflamed periosteum contain multiple macrophage subsets that are located at sites of bone growth and regeneration. Immunol Cell Biol. 2017;95(1):7–16. doi: 10.1038/icb.2016.74. [DOI] [PubMed] [Google Scholar]
- 44.Yang D.O., Yang M.Y. The role of macrophage in the pathogenesis of osteoporosis. Int J Mol Sci. 2019;20(9):2093. doi: 10.3390/ijms20092093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Miron R.J., Bosshardt D.D. Multinucleated giant cells: good guys or bad guys. Tissue Eng Part B Rev. 2018;24(1):53–65. doi: 10.1089/ten.TEB.2017.0242. [DOI] [PubMed] [Google Scholar]
- 46.Rossi F., Lang N.P., De Santis E., Morelli F., Favero G., Botticelli D. Bone-healing pattern at the surface of titanium implants: an experimental study in the dog. Clin Oral Implants Res. 2014;25(1):124–131. doi: 10.1111/clr.12097. [DOI] [PubMed] [Google Scholar]
- 47.Ysander M., Brånemark R., Olmarker K., Myers R.R. Intramedullary osseointegration: development of a rodent model and study of histology and neuropeptide changes around titanium implants. J Rehabil Res Dev. 2001;38(2):183–190. [PubMed] [Google Scholar]
- 48.Le B.Q., Nurcombe V., Cool S.M., van Blitterswijk C.A., de Boer J., LaPointe V.L.S. The components of bone and what they can teach us about regeneration. Materials (Basel) 2017;11(1):14. doi: 10.3390/ma11010014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Li J., Liu X., Zuo B., Zhang L. The role of bone marrow microenvironment in governing the balance between Osteoblastogenesis and adipogenesis. Aging Dis. 2016;7(4):514–525. doi: 10.14336/AD.2015.1206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Morelli F., Lang N.P., Bengazi F., Baffone D., Vila Morales C.D., Botticelli D. Influence of bone marrow on osseointegration in long bones: an experimental study in sheep. Clin Oral Implants Res. 2015;26(3):300–306. doi: 10.1111/clr.12487. [DOI] [PubMed] [Google Scholar]
- 51.De Medeiros F.C.F.L., Kudo G.A.H., Leme B.G. Dental implants in patients with osteoporosis: a systematic review with meta-analysis. Int J Oral Maxillofac Surg. 2018;47:480–491. doi: 10.1016/j.ijom.2017.05.021. [DOI] [PubMed] [Google Scholar]
- 52.Srivastava R.K., Dar H.Y., Mishra P.K. Immunoporosis: immunology of osteoporosis-role of t cells. Front Immunol. 2018;9:657. doi: 10.3389/fimmu.2018.00657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Chang M., Raggatt L.J., Alexander K. Osteal tissue macrophages are intercalated throughout human and mouse bone lining tissues and regulate osteoblast function in vitro and in vivo. J Immunol. 2008;181(2):1232–1244. doi: 10.4049/jimmunol.181.2.1232. [DOI] [PubMed] [Google Scholar]
- 54.Stefanowski J., Lang A., Rauch A. Spatial distribution of macrophages during callus formation and maturation reveals close crosstalk between macrophages and newly forming vessels. Front Immunol. 2019;10:2588. doi: 10.3389/fimmu.2019.02588. https://www.frontiersin.org/article/10.3389/fimmu.2019.02588 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Batoon L., Millard S.M., Raggatt L.J., Pettit A.R. Osteomacs and bone regeneration. Curr Osteoporos Rep. 2017;15(4):385–395. doi: 10.1007/s11914-017-0384-x. [DOI] [PubMed] [Google Scholar]
- 56.Miron R.J., Bosshardt D.D. OsteoMacs: key players around bone biomaterials. Biomaterials. 2016;82:1–19. doi: 10.1016/j.biomaterials.2015.12.017. [DOI] [PubMed] [Google Scholar]
- 57.Jennissen H.J. A macrophage model of osseointegration. Curr Dir Biomed Eng. 2016;2(1):53–56. [Google Scholar]
- 58.Wang X., Li Y., Feng Y., Cheng H., Li D. The role of macrophages in osseointegration of dental implants: an experimental study in vivo. J Biomed Mater Res A. 2020 doi: 10.1002/jbm.a.36978. [DOI] [PubMed] [Google Scholar]
- 59.Milde R., Ritter J., Tennent G.A. Multinucleated giant cells are specialized for complement-mediated phagocytosis and large target destruction. Cell Rep. 2015;13(9):1937–1948. doi: 10.1016/j.celrep.2015.10.065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Anderson J.M., Rodriguez A., Chang D.T. Foreign body reaction to biomaterials. Semin Immunol. 2008;20(2):86–100. doi: 10.1016/j.smim.2007.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Dondossola E., Holzapfel B.M., Alexander S., Filippini S., Hutmacher D.W., Friedl P. Examination of the foreign body response to biomaterials by nonlinear intravital microscopy. Nat Biomed Eng. 2017;1:0007. doi: 10.1038/s41551-016-0007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Brodbeck W.G., Anderson J.M. Giant cell formation and function. Curr Opin Hematol. 2009;16(1):53–57. doi: 10.1097/MOH.0b013e32831ac52e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Albrektsson T. Hard tissue implant interface. Aust Dent J. 2008;53(Suppl. 1):S34–S38. doi: 10.1111/j.1834-7819.2008.00039.x. [DOI] [PubMed] [Google Scholar]
- 64.Miron R.J., Zohdi H., Fujioka-Kobayashi M., Bosshardt D.D. Giant cells around bone biomaterials: Osteoclasts or multi-nucleated giant cells? Acta Biomater. 2016;46:15–28. doi: 10.1016/j.actbio.2016.09.029. [DOI] [PubMed] [Google Scholar]
- 65.Anderson J.M., Cramer S. Perspectives on the inflammatory, healing, and foreign body responses to biomaterials and medical devices. In: Badylak S., editor. Host response to biomaterials. The impact of host response on biomaterial selection. Elsevier; New York, NY, USA: 2015. pp. 13–36. [Google Scholar]
- 66.Mariani E., Lisignoli G., Borzì R.M., Pulsatelli L. Biomaterials: foreign bodies or tuners for the immune response? Int J Mol Sci. 2019;20(3):636. doi: 10.3390/ijms20030636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Land W.G. The role of damage-associated molecular patterns (DAMPs) in human diseases: part II: DAMPs as diagnostics, prognostics and therapeutics in clinical medicine. Sultan Qaboos Univ Med J. 2015;15(2):e157–e170. [PMC free article] [PubMed] [Google Scholar]
- 68.Biguetti C.C., Cavalla F., Silveira E.V. HGMB1 and RAGE as essential components of Ti osseointegration process in mice. Front Immunol. 2019;10:709. doi: 10.3389/fimmu.2019.00709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Arasaki Y., Li M., Akiya T. The RNA-binding protein Cpeb4 is a novel positive regulator of osteoclast differentiation. Biochem Biophys Res Commun. 2020;528(August (4)):621–627. doi: 10.1016/j.bbrc.2020.05.089. [DOI] [PubMed] [Google Scholar]
- 70.Alvarez Carla. Osteoimmunology of oral and maxillofacial diseases: translational applications based on biological mechanisms. Front Immunol. 2019;10:1664. doi: 10.3389/fimmu.2019.01664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Hasséus B., Jontell M., Bergenholtz G. Langerhans cells from human oral epithelium are more effective at stimulating allogeneic T cells in vitro than Langerhans cells from skin. Clin Exp Immunol. 2004;136(3):483–489. doi: 10.1111/j.1365-2249.2004.02469.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Chomiczewska D., Trznadel-Budźko E., Kaczorowska A., Rotsztejn H. The role of Langerhans cells in the skin immune system. Pol Merkur Lekarski. 2009;26(153):173–177. [PubMed] [Google Scholar]
- 73.Plekhova N.G., Lyapun I.N., Shumatov V. Responses of dendritic cells to different coatings of titanium. In: Oral A., Oral Z., editors. vol 186. Springer; Cham: 2017. pp. 165–174. (3rd International multidisciplinary microscopy and microanalysis congress (InterM). Springer proceedings in physics). [Google Scholar]
- 74.Segura E., Amigorena S. Inflammatory dendritic cells in mice and humans. Trends Immunol. 2013;34:440–445. doi: 10.1016/j.it.2013.06.001. [DOI] [PubMed] [Google Scholar]
- 75.Babensee J.E. Interaction of dendritic cells with biomaterials. Semin Immunol. 2008;20(2):101–108. doi: 10.1016/j.smim.2007.10.013. [DOI] [PubMed] [Google Scholar]
- 76.Kou P.M., Babensee J.E. Macrophage and dendritic cell phenotypic diversity in the context of biomaterials. J Biomed Mater Res A. 2011;96(1):239–260. doi: 10.1002/jbm.a.32971. [DOI] [PubMed] [Google Scholar]
- 77.Lapérine O., Blin-Wakkach C., Guicheux J., Beck-Cormier S., Lesclous P. Dendritic-cell-derived osteoclasts: a new game changer in bone-resorption-associated diseases. Drug Discov Today. 2016;21(9):1345–1354. doi: 10.1016/j.drudis.2016.04.022. [DOI] [PubMed] [Google Scholar]
- 78.Rivollier A., Mazzorana M., Tebib J. Immature dendritic cell transdifferentiation into osteoclasts: a novel pathway sustained by the rheumatoid arthritis microenvironment. Blood. 2004;104(13):4029–4037. doi: 10.1182/blood-2004-01-0041. [DOI] [PubMed] [Google Scholar]
- 79.Shortman K., Liu Y.J. Mouse and human dendritic cell subtypes. Nat Rev Immunol. 2002;2:151–161. doi: 10.1038/nri746. [DOI] [PubMed] [Google Scholar]
- 80.Blum J.S., Wearsch P.A., Cresswell P. Pathways of antigen processing. Annu Rev Immunol. 2013;31:443–473. doi: 10.1146/annurev-immunol-032712-095910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Steinman R.M. The dendritic cell system and its role in immunogenicity. Annu Rev Immunol. 1991;9:271–296. doi: 10.1146/annurev.iy.09.040191.001415. [DOI] [PubMed] [Google Scholar]
- 82.Chen Z., Wu C., Xiao Y. Convergence of osteoimmunology and immunomodulation for the development and assessment of bone biomaterials. In: Corradetti B., editor. The immune response to implanted materials and devices. Springer; Cham: 2017. [Google Scholar]
- 83.McKee A.S., Fontenot A.P. Interplay of innate and adaptive immunity in metal-induced hypersensitivity. Curr Opin Immunol. 2016;42:25–30. doi: 10.1016/j.coi.2016.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Hallab N.J., Caicedo M., Finnegan A., Jacobs J.J. Th1 type lymphocyte reactivity to metals in patients with total hip arthroplasty. J Orthop Surg. 2008;3:6. doi: 10.1186/1749-799X-3-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Høl P.J., Kristoffersen E.K., Gjerdet N.R., Pellowe A.S. Novel nanoparticulate and ionic titanium antigens for hypersensitivity testing. Int J Mol Sci. 2018;19(4):1101. doi: 10.3390/ijms19041101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Chan E., Cadosch D., Gautschi O.P., Sprengel K., Filgueira L. Influence of metal ions on human lymphocytes and the generation of titanium-specific T-lymphocytes. J Appl Biomater Biomech. 2011;9(2):137–143. doi: 10.5301/JABB.2011.8567. [DOI] [PubMed] [Google Scholar]
- 87.Pogribna M., Koonce N.A., Mathew A. Effect of titanium dioxide nanoparticles on DNA methylation in multiple human cell lines. Nanotoxicology. 2020;14(4):534–553. doi: 10.1080/17435390.2020.1723730. [DOI] [PubMed] [Google Scholar]
- 88.Lucarelli M., Gatti A.M., Savarino G. Innate defence functions of macrophages can be biased by nanosized ceramic and metallic particles. Eur Cytokine Netw. 2004;15(4):339–346. [PubMed] [Google Scholar]
- 89.Teigen K., Jokstad A. Dental implant suprastructures using cobalt-chromium alloy compared with gold alloy framework veneered with ceramic or acrylic resin: a retrospective cohort study up to 18 years. Clin Oral Implants Res. 2012;23(7):853–860. doi: 10.1111/j.1600-0501.2011.02211.x. [DOI] [PubMed] [Google Scholar]
- 90.Adya N., Alam M., Ravindranath T., Mubeen A., Saluja B. Corrosion in titanium dental implants: literature review. J Indian Prosthodont Soc. 2005;5(3):126–131. [Google Scholar]
- 91.Delgado-Ruiz R., Romanos G. Potential causes of titanium particle and ion release in implant dentistry: a systematic review. Int J Mol Sci. 2018;19(11):3585. doi: 10.3390/ijms19113585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Dohan Ehrenfest D.M., Del Corso M., Kang B. Identification card and codification of the chemical and morphological characteristics of 62 dental implant surfaces. Part 3: Sand-blasted/acid-etched [SLA type] and related surfaces [Group 2A, main subtractive process] POSEIDO. 2014;2:37–55. [Google Scholar]
- 93.Christiansen R.J., Münch H.J., Bonefeld C.M. Cytokine profile in patients with aseptic loosening of total hip replacements and its relation to metal release and metal allergy. J Clin Med. 2019;8(8):1259. doi: 10.3390/jcm8081259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Takayanagi H., Ogasawara K., Hida S. T-cell-mediated regulation of osteoclastogenesis by signalling cross-talk between RANKL and IFN-γ. Nature. 2000;408:600–605. doi: 10.1038/35046102. [DOI] [PubMed] [Google Scholar]
- 95.Pajarinen J., Jamsen E., Konttinen Y.T., Goodman S.B. Innate immune reactions in septic and aseptic osteolysis around hip implants. J Long Term Eff Med Implants. 2014;24(4):283–296. doi: 10.1615/jlongtermeffmedimplants.2014010564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Kim W., Heo Y., Jeong C. Influence of transmucosal designs of dental implant on tissue regeneration in beagle dogs. Tissue Eng Regen Med. 2013;10:25–32. [Google Scholar]
- 97.Kim J.C., Lee J., Kim S., Koo K.T., Kim H.Y., Yeo I.L. Influence of implant-abutment connection structure on peri-implant bone level in a second molar: a 1-year randomized controlled trial. J Adv Prosthodont. 2019;11(3):147–154. doi: 10.4047/jap.2019.11.3.147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Kzhyshkowska J., Gudima A., Riabov V., Dollinger C., Lavalle P., Vrana N.E. Macrophage responses to implants: prospects for personalized medicine. J Leukoc Biol. 2015;98(6):953–962. doi: 10.1189/jlb.5VMR0415-166R. [DOI] [PubMed] [Google Scholar]
- 99.Weszl M., Pelyhe L., Bognár E., Kientzl I. The overview of titanium and its crystalline phases the impact in biomedical applications. In: Vrana N.E., editor. Biomaterials and immune response complications, mechanisms and immunomodulation. Taylor & Francis; Boca Raton: 2018. pp. 71–85. [Google Scholar]
- 100.Albrektsson T., Dahlin C., Reinedahl D., Tengvall P., Trindade R., Wennerberg A. An imbalance of the immune system instead of a disease behind marginal bone loss around oral implants: position paper. Int J Oral Maxillofac Implants. 2020;35(3):495–502. doi: 10.11607/jomi.8218. [DOI] [PubMed] [Google Scholar]
- 101.Aktaş B., Garipcan B., Ahi Z.H., Tuzlakoğlu K., Ergene E., Huri P.Y. Osteoimmunomodulation with biomaterials. In: Vrana N.E., editor. Biomaterials and immune response complications, mechanisms and immunomodulation. Taylor & Francis; Boca Raton: 2018. pp. 161–189. [Google Scholar]
- 102.Anderson J.M., Rodriguez A., Chang D.T. Foreign body reaction to biomaterials. Semin Immunol. 2008;20(2):86–100. doi: 10.1016/j.smim.2007.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Sridharan R., Cameron A., Kelly D.J., Kearney C.J., O’Brien F.J. Biomaterial based modulation of macrophage polarization: a review and suggested design principles. Mater Today. 2015;18(6):313–325. [Google Scholar]
- 104.Wang J., Meng F., Song W. Nanostructured titanium regulates osseointegration via influencing macrophage polarization in the osteogenic environment. Int J Nanomed. 2018;13:4029–4043. doi: 10.2147/IJN.S163956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Su E.P., Justin D.F., Pratt C.R., Sarin V.K., Nguyen V.S., Oh S., Jin S. Effects of titanium nanotubes on the osseointegration, cell differentiation, mineralisation and antibacterial properties of orthopaedic implant surfaces. Bone Joint J. 2018;100-B(1 Suppl. A):9–16. doi: 10.1302/0301-620X.100B1.BJJ-2017-0551.R1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Latha T.S., Reddy M.C.R., Durbaka P.V., Muthukonda S.V., Lomada D. Immunomodulatory properties of titanium dioxide nanostructural materials. Indian J Pharmacol. 2017;49(6):458–464. doi: 10.4103/ijp.IJP_536_16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Neacsua P., Mazareb A., Cimpeana A. Reduced inflammatory activity of RAW 264.7 macrophages on titania nanotube modified Ti surface. Int J Biochem Cell Biol. 2014;55:187–195. doi: 10.1016/j.biocel.2014.09.006. [DOI] [PubMed] [Google Scholar]
- 108.Sun S.J., Yu W.Q., Zhang Y.L., Jiang X.Q., Zhang F.Q. Effects of TiO2 nanotube layers on RAW 264.7 macrophage behaviour and bone morphogenetic protein-2 expression. Cell Prolif. 2013;46(6):685–694. doi: 10.1111/cpr.12072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Gao S., Lu R., Wang X. Immune response of macrophages on super-hydrophilic TiO2 nanotube arrays. J Biomater Appl. 2020;34(9):1239–1253. doi: 10.1177/0885328220903249. [DOI] [PubMed] [Google Scholar]
- 110.Razzi F., Fratila-Apachitei L., Fahy N. Immunomodulation of surface biofunctionalized 3D printed porous titanium implants. Biomed Mater. 2020;15(3) doi: 10.1088/1748-605X/ab7763. 035017. [DOI] [PubMed] [Google Scholar]
- 111.Hotchkiss K.M., Ayad N.B., Hyzy S.L., Boyan B.D., Olivares-Navarrete R. Dental implant surface chemistry and energy alter macrophage activation in vitro. Clin Oral Implants Res. 2017;28:414–423. doi: 10.1111/clr.12814. [DOI] [PubMed] [Google Scholar]
- 112.Loi F., Córdova L.A., Pajarinen J., Lin T.H., Yao Z., Goodman S.B. Inflammation, fracture and bone repair. Bone. 2016;86:119–130. doi: 10.1016/j.bone.2016.02.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Vasconcelos D.M., Santos S.G., Lamghari M., Barbosa M.A. The two faces of metal ions: From implants rejection to tissue repair/regeneration. Biomaterials. 2016;84:262–275. doi: 10.1016/j.biomaterials.2016.01.046. [DOI] [PubMed] [Google Scholar]
- 114.Yang C., Wang W., Zhu K. Lithium chloride with immunomodulatory function for regulating titanium nanoparticle-stimulated inflammatory response and accelerating osteogenesis through suppression of MAPK signaling pathway. Int J Nanomed. 2019;14:7475–7488. doi: 10.2147/IJN.S210834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Loi F., Córdova L.A., Zhang R. The effects of immunomodulation by macrophage subsets on osteogenesis in vitro. Stem Cell Res Ther. 2016;22:7–15. doi: 10.1186/s13287-016-0276-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Córdova L.A., Loi F., Lin T.H. CCL2, CCL5, and IGF-1 participate in the immunomodulation of osteogenesis during M1/M2 transition in vitro. J Biomed Mater Res A. 2017;105(11):3069–3076. doi: 10.1002/jbm.a.36166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Viganò M., Sansone V., d’Agostino M.C., Romeo P., Perucca Orfei C., de Girolamo L. Mesenchymal stem cells as therapeutic target of biophysical stimulation for the treatment of musculoskeletal disorders. J Orthop Surg Res. 2016;11(1):163. doi: 10.1186/s13018-016-0496-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Meirelles L.S., Fontes A.M., Covas D.T., Caplan A.I. Mechanisms involved in the therapeutic properties of mesenchymal stem cells. Cytokine Growth Factor Rev. 2009;20:419–427. doi: 10.1016/j.cytogfr.2009.10.002. [DOI] [PubMed] [Google Scholar]
- 119.English K., French A., Wood K.J. Mesenchymal stroman cell: Facilitators of successful transplantation? Cell Stem Cell. 2010;7:431–442. doi: 10.1016/j.stem.2010.09.009. [DOI] [PubMed] [Google Scholar]
- 120.Hou Y., Zhou X., Cai W.L., Guo C.C., Han Y. Regulatory effect of bone marrow mesenchymal stem cells on polarization of macrophages. Zhonghua Gan Zang Bing Za Zhi. 2017;25(4):273–278. doi: 10.3760/cma.j.issn.1007-3418.2017.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Amengual-Peñafiel L., Jara-Sepúlveda M.C., Parada-Pozas L., Marchesani-Carrasco F., Cartes-Velásquez R., Galdames-Gutiérrez B. Immunomodulation of osseointegration through extracorporeal shock wave therapy. Dent Hypotheses. 2018;9:45–50. [Google Scholar]
- 122.Shin Jae-Won, Mooney David J. Improving Stem Cell Therapeutics With Mechanobiology. Cell Stem Cell. 2016;18(January (1)):16–19. doi: 10.1016/j.stem.2015.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Ho J.C., Ueda J., Shimizu T. The impact of mechanical stress on stem cell properties: the link between cell shape and pluripotency. Histol Histopathol. 2016;31(1):41–50. doi: 10.14670/HH-11-665. [DOI] [PubMed] [Google Scholar]
- 124.Lee D.A., Knight M.M., Campbell J.J., Bader D.L. Stem cell mechanobiology. J Cell Biochem. 2011;112(1):1–9. doi: 10.1002/jcb.22758. [DOI] [PubMed] [Google Scholar]
- 125.Tummala P., Arnsdorf E.J., Jacobs C.R. The role of primary cilia in mesenchymal stem cell differentiation: a pivotal switch in guiding lineage commitment. Cell Mol Bioeng. 2010;3(3):207–212. doi: 10.1007/s12195-010-0127-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Corrigan M.A., Johnson G.P., Stavenschi E., Riffault M., Labour M.N., Hoey D.A. TRPV4-mediates oscillatory fluid shear mechanotransduction in mesenchymal stem cells in part via the primary cilium. Sci Rep. 2018;8 doi: 10.1038/s41598-018-22174-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Mennens S.F.B., van den Dries K., Cambi A. Role for mechanotransduction in macrophage and dendritic cell immunobiology. Results Probl Cell Differ. 2017;62:209–242. doi: 10.1007/978-3-319-54090-0_9. [DOI] [PubMed] [Google Scholar]
- 128.Fahy N., Menzel U., Alini M., Stoddart M.J. Shear and dynamic compression modulates the inflammatory phenotype of human monocytes in vitro. Front Immunol. 2019;10:383. doi: 10.3389/fimmu.2019.00383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Xie Y., Zhang L., Xiong Q., Gao Y., Ge W., Tang P. Bench-to-bedside strategies for osteoporotic fracture: from osteoimmunology to mechanosensation. Bone Res. 2019;7:25. doi: 10.1038/s41413-019-0066-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Albrektsson T., Wennerberg A. The impact of oral implants - past and future, 1966-2042. J Can Dent Assoc. 2005;71(5):327. PMID: 15949251. [PubMed] [Google Scholar]
- 131.Corpas Ldos S., Lambrichts I., Quirynen M. Peri-implant bone innervation: histological findings in humans. Eur J Oral Implantol. 2014;7(3):283–292. [PubMed] [Google Scholar]
- 132.Cerutti-Kopplin D., Feine J., Padilha D.M. Tooth loss increases the risk of diminished cognitive function: a systematic review and meta-analysis. JDR Clin Trans Res. 2016;1(1):10–19. doi: 10.1177/2380084416633102. [DOI] [PubMed] [Google Scholar]
- 133.Ki S., Yun J., Kim J., Lee Y. Association between dental implants and cognitive function in community-dwelling older adults in Korea. J Prev Med Public Health. 2019;52(5):333–343. doi: 10.3961/jpmph.19.163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Habre-Hallage P., Dricot L., Jacobs R., van Steenberghe D., Reychler H., Grandin C.B. Brain plasticity and cortical correlates of osseoperception revealed by punctate mechanical stimulation of osseointegrated oral implants during fMRI. Eur J Oral Implantol. 2012;5(2):175–190. [PubMed] [Google Scholar]
- 135.Mishra S.K., Chowdhary R., Chrcanovic B.R., Brånemark P.I. Osseoperception in dental implants: a systematic review. J Prosthodont. 2016;25:185–195. doi: 10.1111/jopr.12310. [DOI] [PubMed] [Google Scholar]
- 136.Enkling N., Utz K.H., Bayer S., Stern R.M. Osseoperception: active tactile sensibility of osseointegrated dental implants. Int J Oral Maxillofac Implants. 2010;25(6):1159–1167. [PubMed] [Google Scholar]
- 137.Roehling S.K., Meng B., Cochran D.L. Sandblasted and acid-etched implant surfaces with or without high surface free energy: experimental and clinical background. In: Wennerberg A., Albrektsson T., Jimbo R., editors. Implant surfaces and their biological and clinical impact. Springer; Berlin, Heidelberg: 2015. pp. 93–136. [Google Scholar]
- 138.Brazill J.M., Beeve A.T., Craft C.S., Ivanusic J.J., Scheller E.L. Nerves in bone: evolving concepts in pain and anabolism. J Bone Miner Res. 2019;34(8):1393–1406. doi: 10.1002/jbmr.3822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Jones R.E., Salhotra A., Robertson K.S. Skeletal stem cell-Schwann cell circuitry in mandibular repair. Cell Rep. 2019;28(11):2757–2766. doi: 10.1016/j.celrep.2019.08.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Korsching S. The role of nerve growth factor in the CNS. Trends Neurosci. 1986;9:570–577. [Google Scholar]
- 141.Eppley B.L., Snyders R.V., Winkelmann T.M. Efficacy of nerve growth factor in regeneration of the mandibular nerve: a preliminary report. J Oral Maxillofac Surg. 1999;49(1):61–68. doi: 10.1016/0278-2391(91)90268-q. [DOI] [PubMed] [Google Scholar]
- 142.He H., Yao Y., Wang Y., Wu Y., Yang Y., Gong P. A novel bionic design of dental implant for promoting its long-term success using nerve growth factor (NGF): utilizing nano-springs to construct a stress-cushioning structure inside the implant. Med Sci Monit. 2012;18(8) doi: 10.12659/MSM.883253. HY42-46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Zhang J., Li Y., Duan Z. The effects of the M2a macrophage-induced axonal regeneration of neurons by arginase 1. Biosci Rep. 2020;40(2) doi: 10.1042/BSR20193031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Naveau A., Shinmyouzu K., Moore C., Avivi-Arber L., Jokerst J., Koka S. Etiology and measurement of peri-implant crestal bone loss (CBL) J Clin Med. 2019;8(2):166. doi: 10.3390/jcm8020166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Wada S., Kojo T., Wang Y.H. Effect of loading on the development of nerve fibers around oral implants in the dog mandible. Clin Oral Implants Res. 2001;12(3):219–224. doi: 10.1034/j.1600-0501.2001.012003219.x. [DOI] [PubMed] [Google Scholar]
- 146.Dingle A.M., Ness J.P., Novello J., Millevolte A.X.T., Zeng W., Sanchez R. Experimental basis for creating an osseointegrated neural interface for prosthetic control: a pilot study in rabbits. Mil Med. 2020;185(Suppl. 1):462–469. doi: 10.1093/milmed/usz246. [DOI] [PubMed] [Google Scholar]
- 147.Li Y., Kulbacka-Ortiz K., Caine-Winterberger K., Brånemark R. Thumb amputations treated with osseointegrated percutaneous prostheses with up to 25 years of follow-up. J Am Acad Orthop Surg Glob Res Rev. 2019;3(1):e097. doi: 10.5435/JAAOSGlobal-D-18-00097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Gallagher P., Desmond D., MacLachlan M. Psychoprosthetics: an introduction. In: Gallagher P., Desmond D., MacLachlan M., editors. vol 1. Springer; London, UK: 2008. pp. 1–10. (Psychoprosthetics). [Google Scholar]
- 149.Ortiz-Catalan M., Håkansson B., Brånemark R. An osseointegrated human-machine gateway for long-term sensory feedback and motor control of artificial limbs. Sci Transl Med. 2014;6(257):257re6. doi: 10.1126/scitranslmed.3008933. [DOI] [PubMed] [Google Scholar]