Abstract
Heart failure symptoms, in particular dyspnea, may be difficult to frame in a patient with cancer. We report the case of an oncological patient whose dyspnea could have been attributable to various causes and whose management was challenging in the context of the coronavirus disease-2019 pandemic. (Level of Difficulty: Beginner.)
Key Words: cancer, computed tomography, echocardiography, imaging, right ventricle, shortness of breath
Abbreviations and Acronyms: COVID-19, coronavirus disease-2019; CT, computed tomography; MPNST, malignant peripheral nerve sheath tumor; RVOT, right ventricular outflow tract
Central Illustration
History of Presentation
A 45-year-old man with a history of malignant peripheral nerve sheath tumor (MPNST), status post resection, chemotherapy, and radiation therapy, with multiple subsequent resections of recurrences at different sites, began experiencing shortness of breath. After 3 weeks of worsening dyspnea, he started experiencing exertional dizziness, causing him to present to the emergency department of his local hospital.
Learning Objectives
-
•
To consider intracardiac tumors in the differential diagnosis of patients with dyspnea and a history of cancer from other sites, to avoid potentially fatal diagnostic delays.
-
•
To understand the importance of echocardiography in the diagnosis of intracardiac tumors, as noncontrast CT imaging could fail to detect them.
-
•
To remark the importance of a careful clinical examination, even in this uneasy pandemic era.
Due to the simultaneous, severe acute respiratory syndrome-coronavirus-2 epidemic in Italy, the presence of dyspnea mandated the exclusion of coronavirus disease-2019 (COVID-19). Coronavirus was not detected and no signs of pneumonia were found at noncontrast computed tomography (CT) imaging. However, a nodular formation of 18 mm, partially attached to the pleura of the left lower lobe, of possible metastatic nature was seen. The patient chose to be voluntarily discharged and after 10 days accessed the Oncology Outpatient Clinic for evaluation. The lung mass was considered too small to explain the dyspnea, and thus the oncologist referred the patient to our Cardio-Oncology outpatient clinics the same day.
At our examination, the patient was completely asymptomatic at rest, showing lack of change in symptoms with postural modifications but reported severe dyspnea and episodes of dizziness with minimal efforts. Cardiac auscultation revealed a 4/6 harsh systolic murmur in the parasternal region. His lung examination was unrevealing. There was no evidence of peripheral swelling.
Past Medical History
The patient was obese and an ex-smoker with a history of MPNST. The MPNST had first been diagnosed and surgically resected from the left popliteal fossa ∼4 years earlier.
Surgery was followed by radiotherapy and first-line chemotherapy with epidoxorubicin (4 cycles for a total dose of 140 mg/m2, the last administered 37 months before) and ifosphamide. In the last 2 years, the patient had undergone surgery 3 other times for resection of recurrences, the first located at the inguinal region and the last 2 at the scalp. In accordance with previous histological examination, the surgical specimens confirmed MPNST with disease-free resection margins.
Differential Diagnosis
The differential diagnosis included: recurrence of oncological disease; cancer therapeutics–related cardiac dysfunction; pulmonary embolism; an infectious or inflammatory process, including COVID-19; and ischemic heart disease.
Investigations
The patient’s electrocardiography showed sinus rhythm, with signs of right ventricular strain.
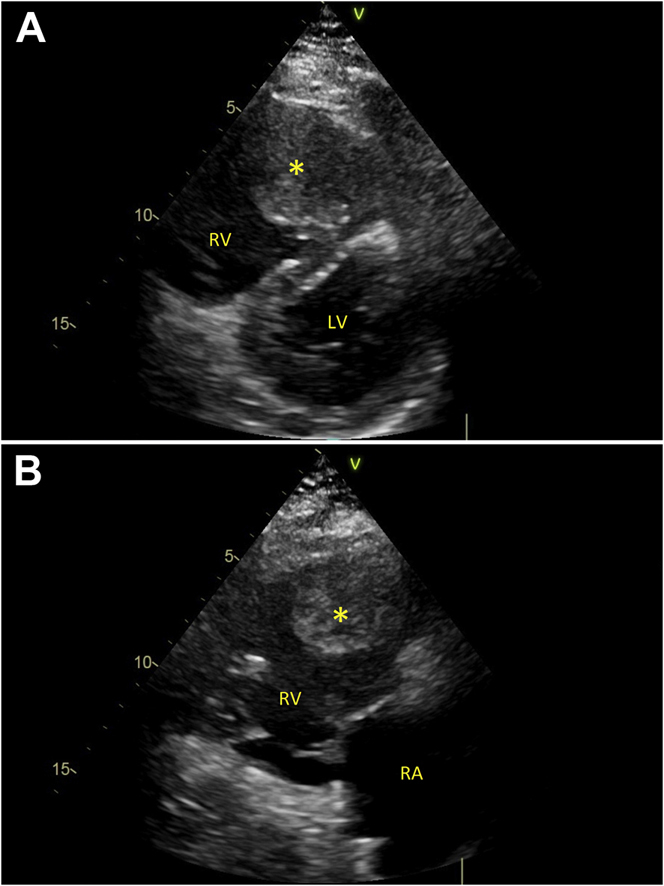
Transthoracic echocardiography revealed a large (50 × 43 mm) mass in the right ventricular outflow tract (RVOT), causing severe outflow obstruction. The right ventricle was dilated (basal diameter 58 mm) and hypokinetic. A D-shaped left ventricle with paradoxical systolic movement of interventricular septum was noted (Figure 1, Video 1).
Figure 1.
Echocardiographic Views
(A) D-shaped pattern of the left ventricle (LV). (B) The mass obstructing the right ventricular (RV) outflow tract. ∗Mass. RA = right atrium.
The patient was then hospitalized at the local Division of Cardiac Surgery. Results of blood tests revealed a high N-terminal pro–B-type natriuretic peptide (6,485 ng/l) level; troponin I values were negative in repeated measurements.
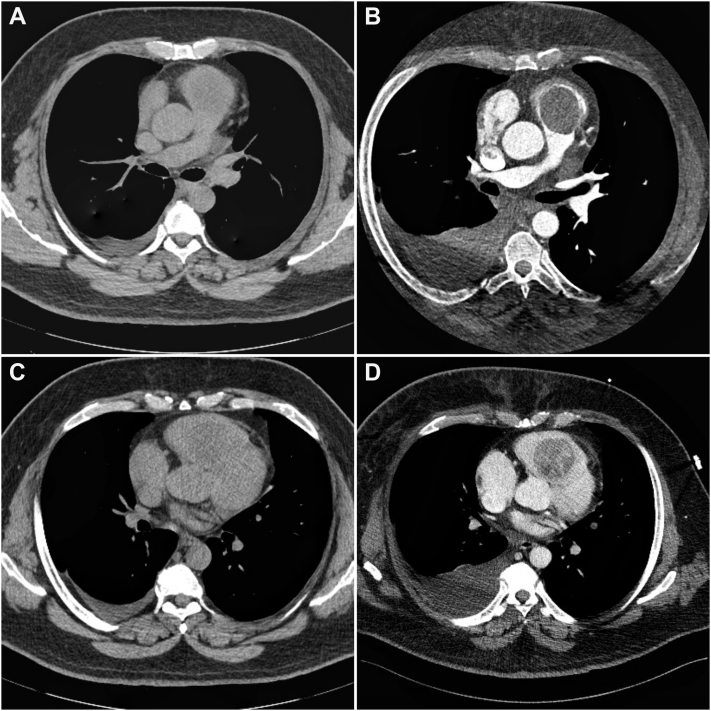
A gated CT angiography confirmed the presence of an expansive formation of 65 mm × 45 mm × 51 mm, inserted in the interventricular septum in the RVOT protruding in systole into the pulmonary valve (Figure 2).
Figure 2.
Gated Computed Tomography Angiography
An expansive mass almost completely obstructing the RV outflow tract. RV dilation and leftward deviation of the interventricular septum are evident. ∗Mass. PA = pulmonary artery; other abbreviations as in Figure 1.
Management
The patient was scheduled for tumor resection surgery, with subsequent chemotherapy once the imminent life-threatening RVOT obstruction had been resolved.
Cardiopulmonary bypass was established with bicaval cannulation, using a rigorous no-touch technique to prevent dislodgment of debris from the tumor, and placing a leukocyte filter in the bypass circuit with the purpose of entrapping potential tumor microemboli. Radical en bloc mass resection was achieved through a RV infundibular approach (Figure 3). The area of resection was meticulously cauterized.
Figure 3.
Surgical Field: Intracardiac Mass From an RV Infundibular Approach
MPA = main pulmonary artery; RV = right ventricle; RVOT = right ventricular outflow tract.
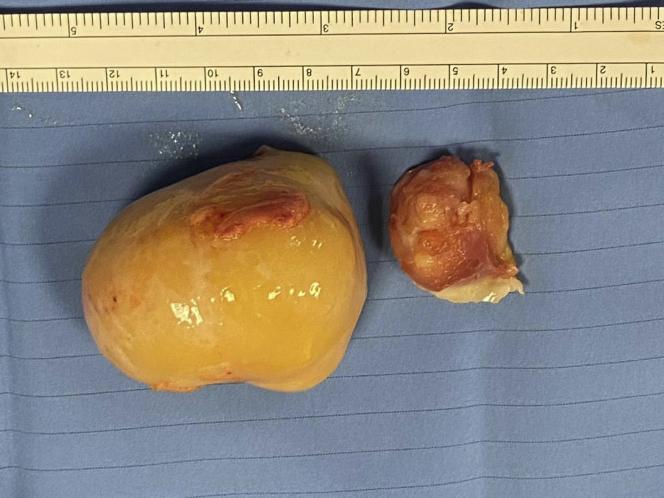
The specimen was 6 cm × 5.5 cm × 5 cm, yellow-gray in color, and of hard consistency (Figure 4). Histological examination confirmed a malignant mesenchymal neoplasm compatible with the previous diagnoses of MPNST (Figure 5).
Figure 4.
Surgical Specimen of the Resected Tumor
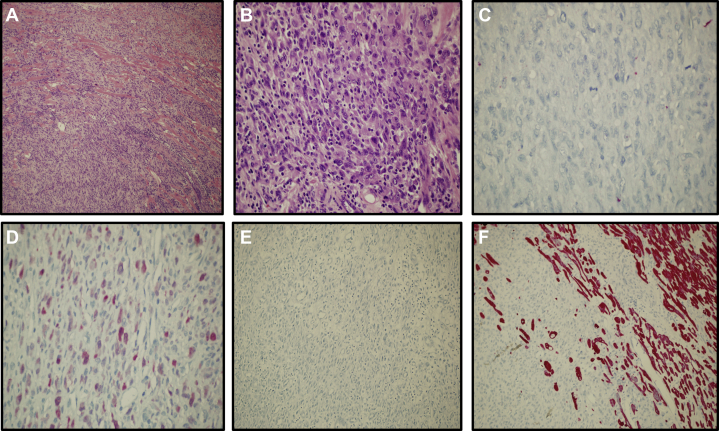
Figure 5.
Histology
(A) Malignant peripheral nerve sheath tumor infiltrates myocardial fibers (hematoxylin and eosin staining; 10×). (B) The lesion shows spindle and epithelioid cells arranged in a fascicular pattern (hematoxylin and eosin staining; 20×). (C) The neoplastic cells are negative for cytokeratin AE1 and AE3. (D and E) pS100 is focally positive in neoplastic cells, whereas HMB45 staining is negative. (F) Desmin is negative in neoplastic cells, whereas it is positive in cardiac muscle fibers.
Discussion
This clinical case offers multiple points for reflection.
First, this type of tumor and particularly its cardiac location are extremely rare. MPNSTs arise from glial cells and occur more commonly in the nerve trunk of the proximal upper and lower extremities. Almost 50% of MPNSTs are associated with neurofibromatosis type I, which in our patient, however, was not present. Although MPNST ranks sixth in terms of all soft-tissue sarcoma, its occurrence in the heart is extremely rare, with an incidence of 0.75% among all primary cardiac tumors (1,2). Moreover, primary cardiac neoplasm in the right ventricle are rare in general and usually do not interfere with the pulmonary valve. The location of this tumor within the RV infundibulum is typical of neoplasms of neurogenic origin. The prevalence of neurogenic tumors in this location has been related to the anatomic distribution of the vagus nerve plexus, giving off nerve twig branches in the infundibular and septal regions, from which these tumors may arise (3).
Only a few cases of cardiac MPNSTs have been previously reported in the literature (4,5), and only one described RVOT obstruction, in the context of suspected neurofibromatosis, which eventually led to the patient’s death (6).
Beyond the rarity of the case, another interesting aspect concerns its clinical presentation. The tumor manifested itself through expansive/extrinsic growth, instead of an infiltrative behavior, and the symptoms appeared late, when the RVOT was almost completely obstructed. The patient’s shortness of breath had peculiar characteristics; in fact, there was a clear contrast between the complete absence of symptoms at rest, even in a supine position, and severe dyspnea for minimal efforts, different from the classic symptoms of left-sided heart failure.
Finally, the clinical pathway of the case is worth some consideration. Our evaluation occurred ∼30 days from the beginning of symptoms. The delayed diagnosis should be interpreted in the historical context of the health emergency due to the COVID-19 pandemic. By now, the COVID-19 pandemic requires that the diagnostic evaluation of a patient with dyspnea begins with a mandatory exclusion of severe acute respiratory syndrome-coronavirus-2 pneumonia. Once the infection was excluded, our patient chose voluntary discharge from the hospital where he was initially admitted. His decision was motivated by the fear of being infected and by the widespread sense of guilt of “stealing” medical attention from patients with COVID-19.
In such an infectious epidemic, another aspect that must be considered is the exposure risk of health-care personnel and how exposure would limit the ability to care for patients. In patients suspected of having COVID-19, physical examination is often overlooked and the use of tools such as the phonendoscope, a potential source of virus dissemination, is probably limited to few situations. Our patient did not receive a deep physical examination at the time of first hospitalization because he was a possible COVID-19–infected patient. Nevertheless, the detection of a grade 4/6 ejection systolic murmur at the first medical contact would have probably allowed a prompter diagnosis.
Follow-Up
The patient’s dyspnea rapidly improved and N-terminal pro–B-type natriuretic peptide values decreased to 1,387 ng/l 2 days after surgery. His recovery was uneventful without complications.
Conclusions
Intracardiac tumors are rare but should be considered in the differential diagnosis of dyspnea in patients with cancer from other sites. Noncontrast CT imaging could miss cardiac masses (Figure 6), although echocardiographic examination should not overlook the right ventricle. Patients with primary malignant cardiac tumors are infrequently managed in the operative room, but a skilled surgical approach can make the difference, even in selected cases of metastases.
Figure 6.
Comparison Between Nongated Computed Tomography Scanning With and Without Contrast
Computed tomography scanning without (A and C) and with (B and D) contrast.
Funding Support and Author Disclosures
The authors have reported that they have no relationships relevant to the contents of this paper to disclose.
Footnotes
The authors attest they are in compliance with human studies committees and animal welfare regulations of the authors’ institutions and Food and Drug Administration guidelines, including patient consent where appropriate. For more information, visit the Author Center.
Appendix
For a supplemental video, please see the online version of this paper.
Appendix
Echocardiographic Imaging D-shaped pattern of the left ventricle.
References
- 1.James A.W., Shurell E., Singh A. Malignant peripheral nerve sheath tumor. Surg Oncol Clin N Am. 2016;25:789–802. doi: 10.1016/j.soc.2016.05.009. [DOI] [PubMed] [Google Scholar]
- 2.Rahman M., Cook D.S., Ellis G. Malignant peripheral nerve sheath tumor of the heart. Asian Cardiovasc Thorac Ann. 2006;14:425. doi: 10.1177/021849230601400517. [DOI] [PubMed] [Google Scholar]
- 3.Dammert K., Elfving G., Halonen P.I. Neurogenic sarcoma in the heart. Am Heart J. 1955;49:794–800. doi: 10.1016/0002-8703(55)90226-x. [DOI] [PubMed] [Google Scholar]
- 4.Li X., Liu C., Zhang R. Primary cardiac malignant peripheral nerve sheath tumor: a case report. Int J Cardiovasc Imaging. 2019;35:1615–1618. doi: 10.1007/s10554-019-01608-7. [DOI] [PubMed] [Google Scholar]
- 5.Eindhoven J.A., Loonstra E.E.G., Kik C. Atypical presentation of a primary cardiac malignant peripheral nerve sheath tumor. Int J Cardiovasc Imaging. 2018;34:903–904. doi: 10.1007/s10554-018-1302-8. [DOI] [PubMed] [Google Scholar]
- 6.Ursell P.C., Arline A., Fenoglio J.J., Jr. Malignant neurogenic tumor of the heart. Human Pathol. 1982;13:640–645. doi: 10.1016/s0046-8177(82)80007-5. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Echocardiographic Imaging D-shaped pattern of the left ventricle.