Abstract
Background
The oral health-promoting effects of green tea are attributed to its polyphenol components. Aim of this work was to systematically review the literature in search for clinical trials assessing green tea for managing periodontitis and caries.
Methods
Randomized clinical trials comparing the efficacy of green tea versus control groups in oral hygiene and gingival health; periodontitis; caries; periodontal pathogens number; Streptococcus mutans, Lactobacillus spp. Meta-analysis and meta-regression analysis were performed.
Literature searches were carried out using MedLine (PubMed), Scopus, and the Cochrane Library. Eighteen studies (870 subjects) were included.
Results
Green tea treatment had medium positive effect size in reducing GI (SMD: 0.50; 95%CI: −0.02/1.01); PI (SMD: 0.54; 95%CI: 0.14/0.95); GBI (SMD: 0.58; 95%CI: −0.41/1.56) and BOP (SMD: 0.52; 95%CI: −0.57/1.60) in respect to the control group. Splitting to subgroups, green tea showed a small negative effect in the chlorhexidine control groups. Green tea treatment had medium positive effect size in reducing CAL (SMD 0.58; 95%CI: −0.49/1.65) and large positive effect size in reducing PPD (SMD:1.02; 95%CI: 0.45/1.59).
Conclusion
Even if the results are encouraging, there is insufficient evidence to recommend the use of green tea formulation as first choice treatment for gingivitis, periodontitis and caries.
Keywords: Gingivitis, Periodontitis, Caries, Plaque control, Systematic review, Evidence-based dentistry, Meta-analysis
1. Introduction
Green tea (Camellia sinensis) represents 20% of tea's worldwide production and it is consumed in Asia, in the Middle East and parts of North America. Green tea is a non-fermented tea, that is produced by drying and steaming the fresh leaves to prevent polyphenol oxidase [1].
Green tea is the most common functional beverage consumed worldwide. Functional foods are defined as “healthful foods or food ingredients that have a potential health benefit beyond their nutrient content when consumed regularly in typical quantities as part of a varied diet” [2].
The health-promoting effects of green tea are attributed to its polyphenol components (catechins). Epigallocatechin-3 gallate (EGCG) and epicatechin-3-gallate (ECG) are the most important catechins. The polyphenol concentration in green tea is higher than in black tea, 30−40% compared to 3–10% respectively, with a greater antioxidant activity and potent anti-inflammatory, antibacterial, antiviral, antimutagenic and anti-aging properties [[3], [4], [5], [6]].
The preventive role of green tea in the development and progression of oral diseases has been shown in chronic periodontitis (effect on periodontopathogens, effect on host immune reactions) and in dental caries (effect on cariogenic enzymes, effect on bacterial biofilm, effect on F1Fo-ATPase and the agmatine deiminase systems, effect on oxidative stress) [7].
Latest systematic reviews have qualitatively summarized selected studies on either caries or periodontitis [2] without comprehensive quantitative synthesis of the evidence available from randomized controlled trials.
This study aimed to systematically review and synthesize available randomized controlled trials investigating the effect of green tea on periodontal diseases (gingivitis and periodontitis) and/or caries. The results of this review are meant to provide useful information to make clinical decisions, and to direct further research in this field.
2. Materials and methods
This systematic review was conducted according to the PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analyses) statement [8] and the guidelines from the Cochrane Handbook for Systematic Reviews of Interventions [9]. The study protocol was registered after the screening stage (PROSPERO CRD42019130629).
2.1. Eligibility criteria
The following inclusion criteria were applied for this meta-analysis: (a) randomized clinical trials (RCTs); (b) all considered participants were dentate humans who used Camellia sinensis preparations, regardless the way of use or concentration; (c) the control intervention could have been placebo or other treatments (sodium fluoride, chlorhexidine, triclosan, saline); (d) studies published in English, French, German, Spanish, Polish, Albanian and Portuguese. Broad inclusion criteria have been used to be as sensitive as possible. The followings were the exclusion criteria: (a) in vitro RCTs; (b) lack of effective statistical analysis; (c) abstract and author debates or editorials; (d) in vivo RCTs on patients with orthodontic fixed appliances.
The outcomes to be assessed are listed as follows: oral hygiene and gingival health (Gingival Index [GI], Plaque Index [PI], Bleeding on Probing [BOP], Gingival Bleeding Index [GBI]; caries (caries or caries experience prevalence [DMFT/dmft > 0 or DT/dt > 0]; caries experience or its increment); periodontitis (Probing Pocket Depths [PPD], Clinical Attachment Loss [CAL]); Bacterial Colony-Forming Unit (CFU) (Streptococcus mutans [SM], Porphyromonas gingivalis [PG], Aggregatibacter actinomycementcomitans [AA], Lactobacillus spp. [LB], Prevotella Intermedia [PI]).
2.2. Search strategy and study selection
Literature searches of free text and MeSH terms were performed using MedLine (PubMed), Scopus, and the Cochrane Library (from 1950 to December 31st 2019). All searches were conducted using a combination of subject headings and free-text terms; the final search strategy was determined through several pre-searches. The keywords used in the search strategy were as follows: [("Camellia sinensis" OR "green tea") AND ("oral health" OR "caries" OR "periodontal" OR "periodontitis" OR "gingival" OR "gingivitis" OR "gum" OR "mutans")]. There was no restriction on publication years. Reference lists of primary research reports were cross-checked in an attempt to identify additional studies. Following the inclusion criteria, two authors (MM and AN) independently selected the literature by reading the titles and abstracts. The full text of each identified article was then read to determine whether it was suitable for inclusion. Disagreements were resolved through consensus or by discussion with a third author (LO).
2.3. Data collection
For each eligible study, data was independently extracted by two authors (MM and AN) and examined by the third author (MJ) by creating a piloted spreadsheet and comparing them through it, according to the Cochrane Collaboration guidelines [9]. In case of missing data, MJ contacted via e-mail the corresponding author of the related research and excluded those ones for which no reply was given.
2.4. Data items
The following data items were recorded: study year, type and setting; age, size and recruitment sample; case and control interventions; any pre-treatment and co-intervention; vehicle, daily dose and the calculated total dose; frequency and length of consumption; wash out period in RCTs with cross-over design; follow-up, drop-out and sample size at follow-up.
2.5. Quality assessment
According to the PRISMA statements, the evaluation of the methodological quality gives an indication of the strength of evidence provided by the study because methodological flaws can result in biases. This procedure provides a total score that can range from 0 to 5, where 0 is a low-quality study, and 5 is the highest possible quality. A trial is considered having a good quality when it gets a score of at least 3.
2.6. Risk of Bias in individual studies
Selection bias (retained allocation concealment), performance and detection bias (blinding of participants and operators), attrition bias (patient dropout, wash-out period of cross over trials and missing values or participants, too short duration of follow-up) and reporting bias (selective reporting, unclear eliminations, missing results) were recorded, evaluated and allocated according to Cochrane guidelines [9].
2.7. Consistency measures and risk of Bias Across studies
Heterogeneity was assessed quantitatively using Cochrane’s Q and I 2 -statistics [10]. The high percentage of variability come from heterogeneity of samples among studies. Funnel plot analysis were performed to assess small study effects or publication bias for analyses with two or more studies being present.
2.8. Summary measures and heterogeneity
Differences in index values and no. of bacterial CFU between final and initial measurements were taken as a measure of treatment efficacy for both groups – Test group (TG) and Control group (CG). Unavailable correlation coefficients between dependent measurements were imputed at conservative value r = 0.5 when calculating variance of the difference [11,12]. Meta-analysis was performed using random-effect model via metafor and compute.es R packages, with Standardized Mean Differences (SMD) and 95% confidence intervals (95% CI) being calculated as effect estimates. Heterogeneity was assessed quantitatively using I2-statistics and Cochran’s Q [10].
For indices, for which at least 7 studies were available, meta-regression analyses were conducted with categorical moderator, that was used in CG, to examine its influence on the outcomes.
3. Results
3.1. Study selection
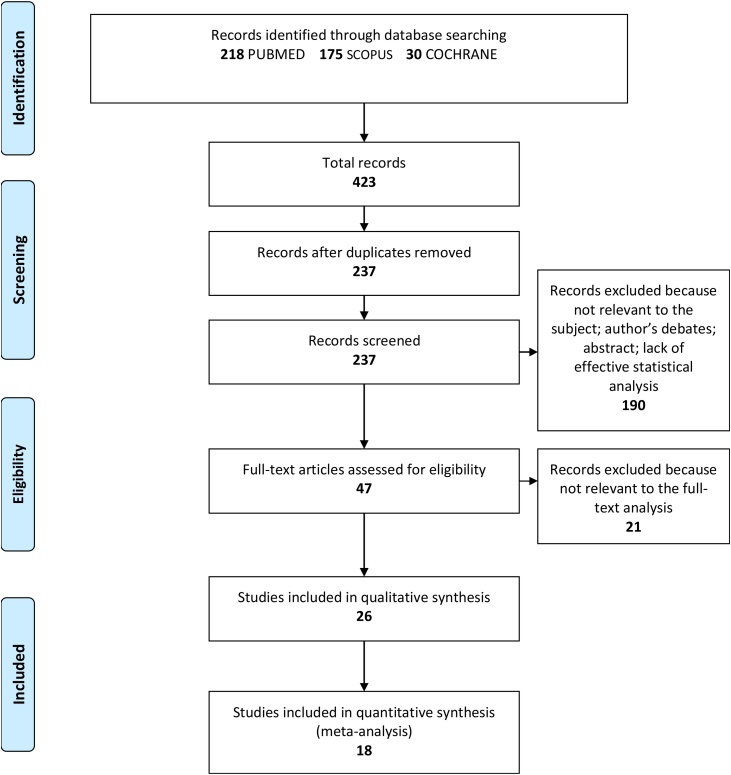
The search strategy identified 423 potential articles: 218 from PubMed, 175 from Scopus and 30 from Cochrane. After removal of duplicates, 237 articles were analyzed. Subsequently, 190 papers were excluded because they did not meet the inclusion criteria. Of the remaining 47 papers, 21 were excluded because they were not relevant to the subject of the study. The remaining 26 papers were included in the qualitative synthesis, and 18 of these were included in the meta-analysis [[13], [14], [15], [16], [17], [18], [19], [20], [21], [22], [23], [24], [25], [26], [27], [28], [29], [30]] (Fig. 1). Table A1 in Supplementary material summarizes the characteristics of each of the 18 included studies. All the included papers reported odd ratio (OR) for the study’s relevant query data. There were 18 included studies in meta-analysis with 2–12 studies for individual characteristic (bacterial number or gingival-periodontitis index). The results are shown on Fig. 2, Fig. 3, Fig. 4, Fig. 5, Fig. 6, Fig. 7, Fig. 8, Fig. 9, Fig. 10, Fig. 11, Fig. 12 and Table A1 in Supplementary material. Positive values of SMD indicate greater efficacy in TG (green tea treatment), negative ones indicate - greater efficacy in CG.
Fig. 1.
Flow of the search.
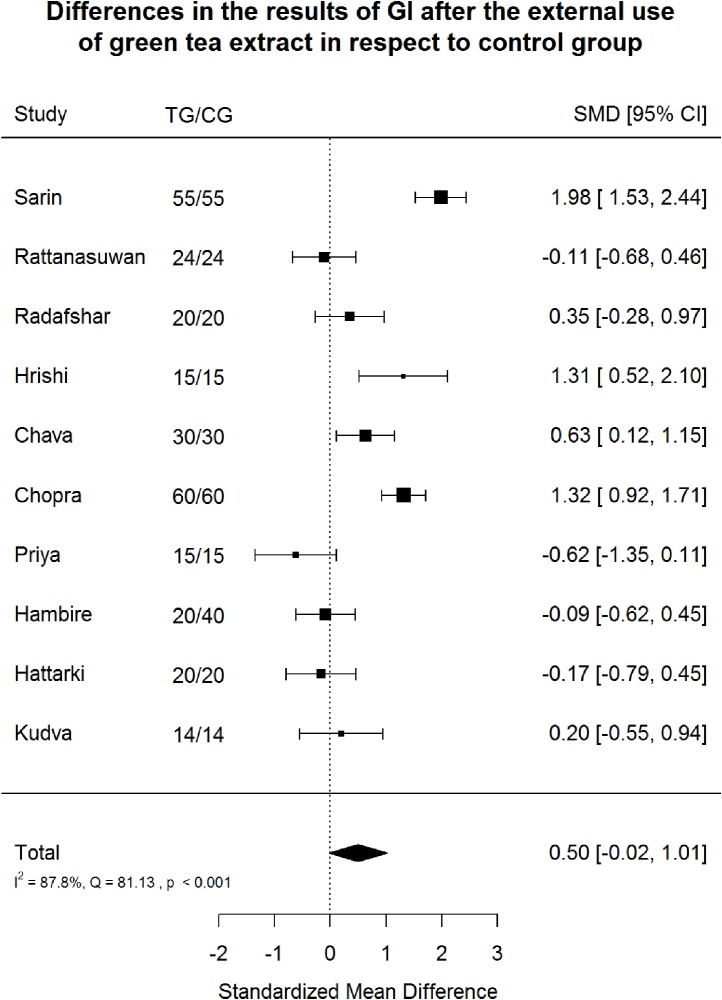
Fig. 2.
Gingiva Index (GI) after green tea treatment and control therapy. Standardized mean differences (SMD) and 95% CI are given. Values below or above 0 indicate reduced and increased values in green tea treatment versus control group, respectively.
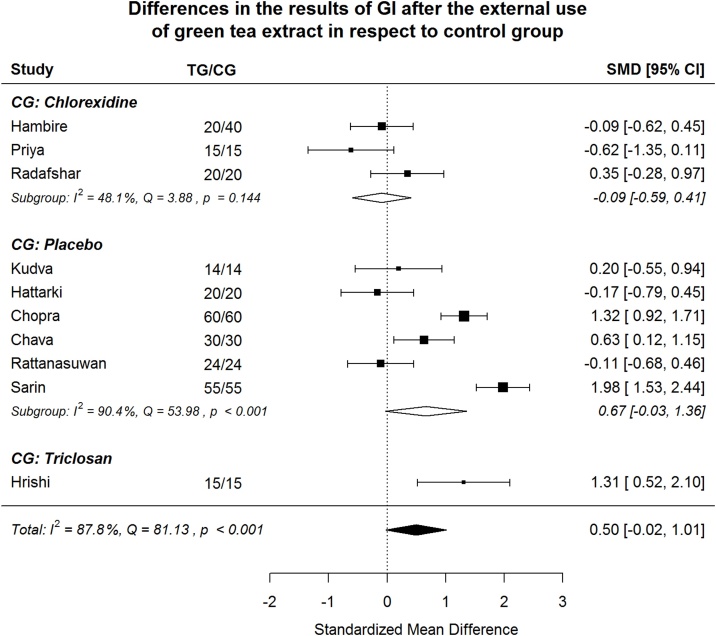
Fig. 3.
Gingiva Index (GI). After splitting to subgroups, green tea treatment has medium-large positive effect size in reducing GI in the subgroups where placebo or triclosan were used in CG, but shows very small negative effect size in the subgroup where chlorhexidine was used in CG.
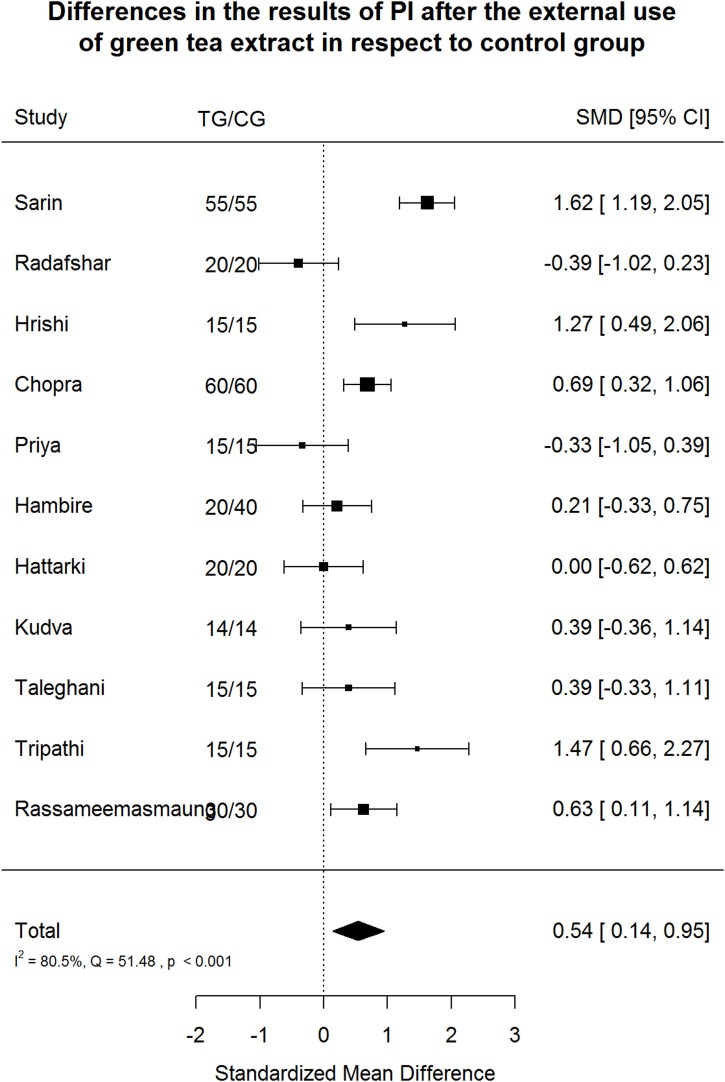
Fig. 4.
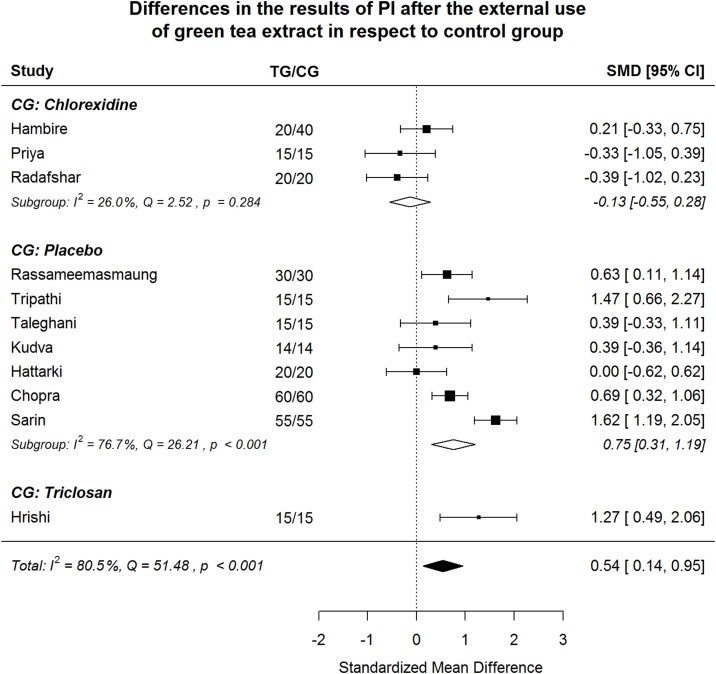
Plaque Index (PI) after green tea treatment and control therapy. Standardized mean differences (SMD) and 95% CI are given. Values below or above 0 indicate reduced and increased values in green tea treatment versus control group, respectively.
Fig. 5.
Plaque Index (PI). By subgroups, green tea treatment has large positive effect size in reducing PI in the subgroups where placebo or triclosan were used in CG, but shows small negative effect size in the chlorhexidine subgroup.
Fig. 6.
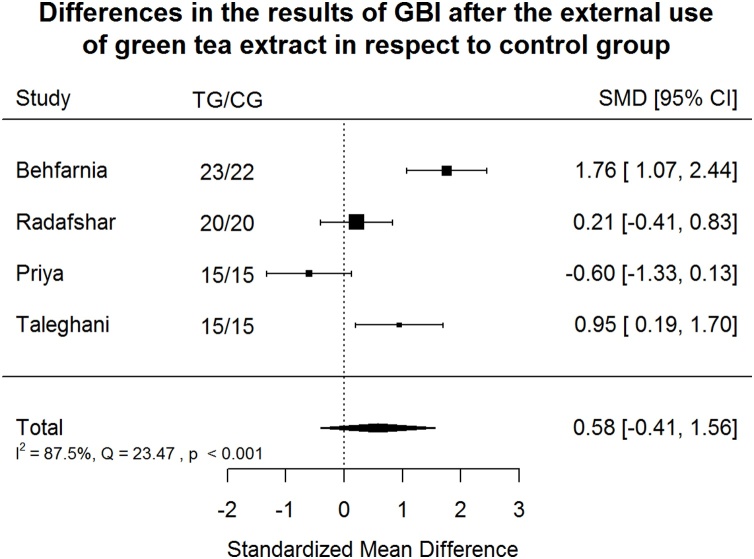
Gingival Bleeding Index (GBI) after green tea treatment and control therapy. Standardized mean differences (SMD) and 95% CI are given. Values below or above 0 indicate reduced and increased values in green tea treatment versus control group, respectively. Green tea treatment has medium positive effect size in reducing GBI in respect to the control group.
Fig. 7.
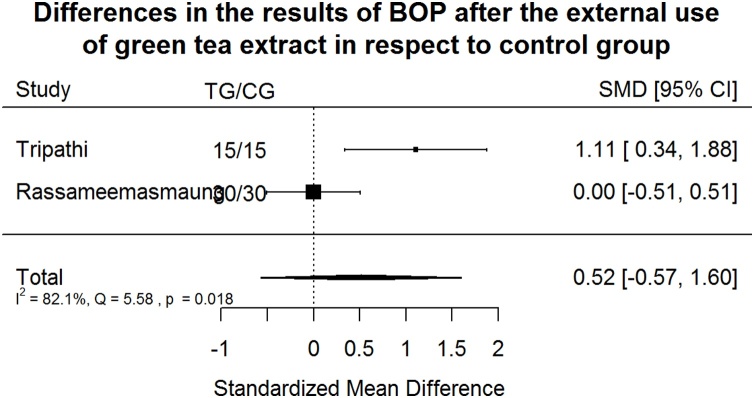
Bleeding on probing Index (BOP) after green tea treatment and control therapy. Standardized mean differences (SMD) and 95% CI are given. Values below or above 0 indicate reduced and increased values in green tea treatment versus control group, respectively. Green tea treatment has medium positive effect size in reducing BOP in respect to the control group.
Fig. 8.
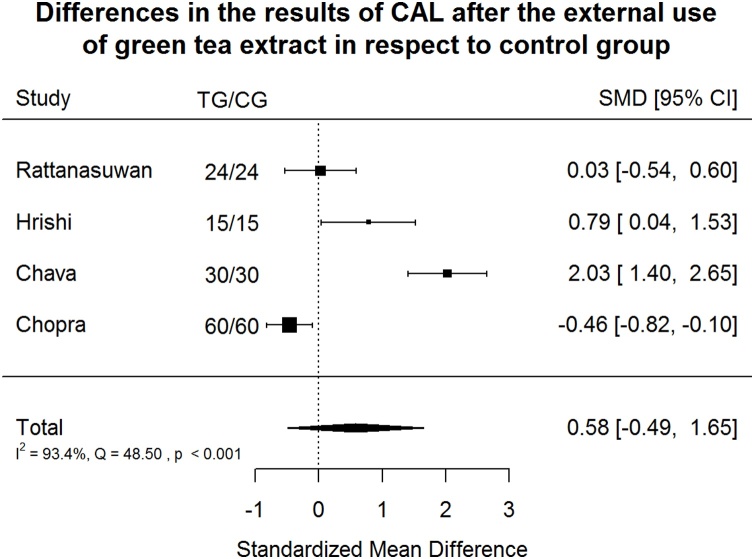
Clinical Attachment Loss Index (CAL) after green tea treatment and control therapy. Standardized mean differences (SMD) and 95% CI are given. Values below or above 0 indicate reduced and increased values in green tea treatment versus control group, respectively. Green tea treatment had medium positive effect size in reducing CAL in respect to the control group.
Fig. 9.
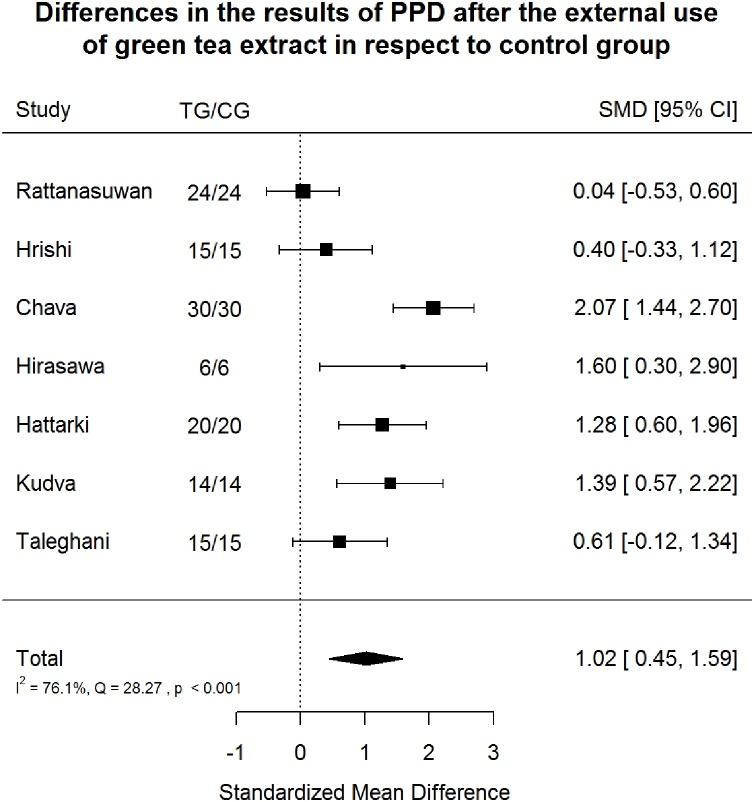
Probing Pocket Depth Index (PPD) after green tea treatment and control therapy. Standardized mean differences (SMD) and 95% CI are given. Values below or above 0 indicate reduced and increased values in green tea treatment versus control group, respectively. Green tea treatment had large positive effect size in reducing PPD in respect to the control group.
Fig. 10.
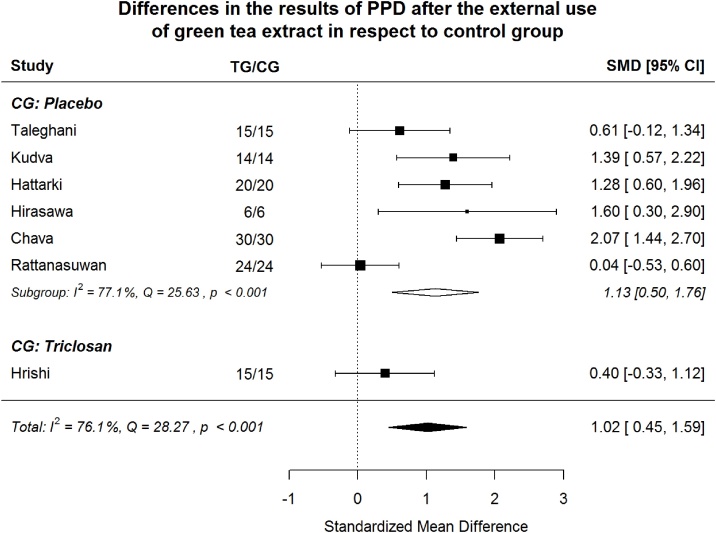
Probing Pocket Depth Index (PPD) Splitting to subgroups, green tea treatment has large positive effect size in reducing PPD in the placebo subgroup and medium positive effect size in the triclosan subgroup.
Fig. 11.
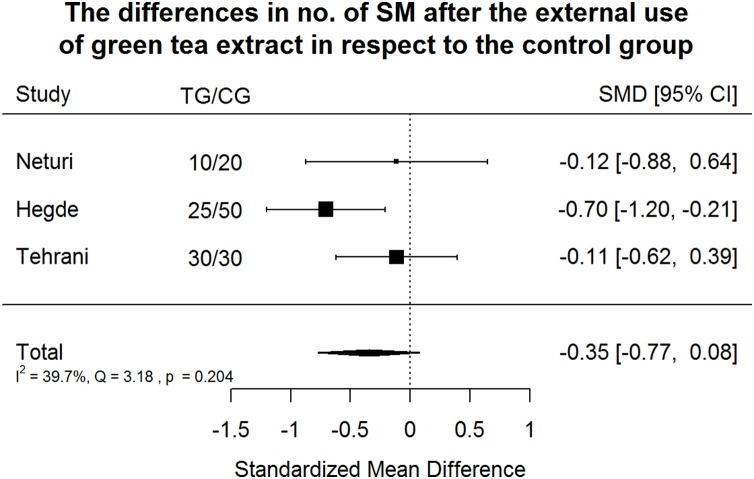
Effect of green tea treatment on SM counts. Green tea treatment appeared to have small-medium negative effect according to Cohen's interpretive guidelines size in reducing SM number in respect to the control group.
Fig. 12.
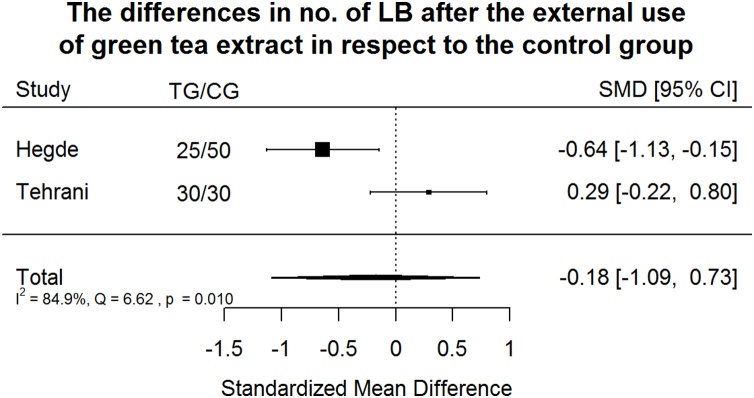
Effect of green tea treatment on SM counts. Green tea treatment had small negative effect size in reducing LB number in respect to the control group.
3.2. Study characteristics
Included studies (Table A1 in Supplementary material) were published between 2011 and 2019, and used parallel group (n:14), split-mouth (n:3) and cross-over design (n:1). Eighteen studies were performed: in children (aged < 18: 3), or adults (18−74: 15). Sample sizes ranged between 14 and 120 participants (mean: 48). The overall sample size was 870. Green tea was proposed through several vehicle products: mouth rinse (n:8), gel (n:2), chewing gum (n:1), toothpaste (n:1), drinking tea (n:2), strips (n:3) and capsule (n:1). Intervention lasted 14 days to 12 weeks (mean: 4 weeks); total trial duration (intervention plus follow-up) ranged between 14 days and 6 months (mean: 26 days).
3.3. Quality assessment
According to the Jadad scale for RCT, the authors evaluated the qualities of all 26 clinical trials included in the qualitative synthesis, based on 5 questions that analyze the randomization process, the experimental blinding, and the dropout rate, i.e., the patients lost to follow-up. In the evaluation of the quality of RCTs, cross-sectional studies, the total score of twelve studies was less or equal to 2, indicating a low-quality study, while the total score of the other studies was greater than or equal to 4, indicating high-quality studies (Table 1).
Table 1.
Jadad scale for reporting randomized controlled trials (1 = yes, 0 = no, -1 = no description).
| Author | 1) Is the study described as randomized? | 2) Is the study described as double blind? | 3) Is there a description of withdrawals and dropouts? | 4) The method of randomisation is appropriate? | 5) The method of blinding is appropriate? | Total score= |
|---|---|---|---|---|---|---|
| Krahwinkel 2000 [49] | 0 | 1 | 1 | −1 | 1 | 2 |
| Hirasawa 2002 [30] | 1 | 0 | 1 | 1 | −1 | 2 |
| Awadalla 2011 [47] | 0 | 0 | 1 | −1 | −1 | −1 |
| Ferrazzano 2011 [48] | 1 | 0 | 1 | 1 | −1 | 2 |
| Kudva 2011 [27] | 1 | 0 | 0 | 1 | −1 | 1 |
| Tehrani 2011 [26] | 1 | 1 | 1 | 1 | 1 | 5 |
| Jenabian 2012 [45] | 1 | 0 | 0 | 1 | −1 | 1 |
| Chava 2013 [44] | 1 | 0 | 1 | 1 | −1 | 2 |
| Gadagi 2013 [51] | 1 | 0 | 1 | 1 | 1 | 4 |
| Hattarki 2013 [25] | 1 | 0 | 1 | 1 | −1 | 2 |
| Rassameemasmaung 2013 [43] | 1 | 0 | 1 | 1 | 1 | 4 |
| Hrishi 2014 [19] | 1 | 0 | 1 | 1 | 1 | 4 |
| Kaur 2014 [50] | 1 | 0 | 1 | 1 | 1 | 4 |
| Neturi 2014 [14] | 1 | 0 | 1 | 1 | 1 | 4 |
| Rattanasuwan 2014 [42] | 1 | 1 | 1 | 1 | 1 | 5 |
| Hambire 2015 [24] | 1 | 1 | 1 | 1 | 1 | 5 |
| Priya 2015 [23] | 1 | 1 | 0 | 1 | 1 | 4 |
| Radafshar 2015 [18] | 1 | 1 | 1 | 1 | 1 | 5 |
| Sarin 2015 [13] | 1 | 1 | 1 | 1 | 1 | 5 |
| Behfarnia 2016 [17] | 1 | 0 | 1 | 1 | −1 | 2 |
| Chopra 2016 [21] | 1 | 0 | 1 | 1 | −1 | 2 |
| Thomas 2016 [46] | 1 | 1 | 1 | 1 | 1 | 5 |
| Hegde 2017 [22] | 1 | 1 | 1 | 1 | 1 | 5 |
| Shalini 2018 [52] | 1 | 1 | 0 | 1 | 1 | 4 |
| Taleghani 2018 [28] | 1 | 0 | 0 | 1 | −1 | 1 |
| Tripathi 2019 [29] | 1 | 0 | 0 | 1 | −1 | 1 |
3.4. Gingival and oral hygiene indices
Most of the included studies (n:15) evaluated indices of oral hygiene and gingivitis (GI, PI, GBI, BOP) in TG and CG directly after therapy and at different times of follow-up.
Green tea treatment had medium positive effect size in reducing GI (SMD: 0.50; 95%CI: −0.02/1.01); PI (SMD: 0.54; 95%CI: 0.14/0.95); GBI (SMD: 0.58; 95%CI: −0.41/1.56) and BOP (SMD: 0.52; 95%CI: −0.57/1.60) in respect to the CG. Heterogeneity was significant (p < 0.001) in all cases and in relation to GI, PI, GBI, BOP, 87.8%, 80.5%, 87.5% and 82.1% of the variability came from heterogeneity, respectively (Fig. 2). The included studies showed high heterogeneity with suspected publication bias.
3.4.1. Gingival index
Splitting to subgroups, green tea treatment has medium-large positive effect size in reducing GI in the subgroups where placebo or triclosan were used in CG, but shows very small negative effect size in the subgroup where chlorhexidine was used in CG (Fig. 3). In order to examine if between-study heterogeneity can be explained by “control group agent”, meta-regression model was estimated. Both coefficients revealing difference in effect size comparing to “chlorhexidine group” are insignificant (Table 2). The omnibus test () shows the model is insignificant (p = 0.223). Non-explained by moderator heterogeneity is significant (p < 0.001), 87.1% of the variability come from heterogeneity. In the “placebo subgroup” heterogeneity remains significant also (p < 0.001), 90.4% of the variability come from heterogeneity.
Table 2.
Meta-regression model estimates for Gingival Index (GI).
| estimate | se | zval | p | |
|---|---|---|---|---|
| intercept | −0.110 | 0.460 | −0.239 | 0.811 |
| Placebo | 0.782 | 0.559 | 1.398 | 0.162 |
| Triclosan | 1.419 | 0.950 | 1.494 | 0.135 |
3.4.2. Plaque index
Green tea treatment has medium positive effect size in reducing PI in respect to the CG (Fig. 4). Heterogeneity is significant (p < 0.001). 80.5% of the variability come from heterogeneity.
By subgroups, green tea treatment has large positive effect size in reducing PI in the subgroups where placebo or triclosan were used in CG but shows small negative effect size in the subgroup where chlorhexidine was used in CG (Fig. 5). In order to examine if between-study can be explained by “control group agent”, meta-regression model was estimated. The difference between “chlorhexidine subgroup” and two other subgroups is significant (Table 3). The omnibus test () shows the model is significant (p = 0.027). Non-explained by moderator heterogeneity is significant (p < 0.001), 87.1% of the variability come from heterogeneity. In the “placebo subgroup” heterogeneity remains significant (p < 0.001), but its share in overall heterogeneity falls from 80.5% to 71.0%.
Table 3.
Meta-regression model estimates for Plaque Index (PI).
| estimate | se | zval | p | |
|---|---|---|---|---|
| intercept | −0.155 | 0.320 | −0.483 | 0.629 |
| Placebo | 0.910 | 0.381 | 2.386 | 0.017* |
| Triclosan | 1.428 | 0.685 | 2.086 | 0.037* |
3.4.3. Gingival bleeding index
Green tea treatment has medium positive effect size in reducing GBI in respect to the CG (Fig. 6). Heterogeneity is significant (p < 0.001). 87.5% of the variability come from heterogeneity.
3.4.4. Bleeding on probing index
Green tea treatment has medium positive effect size in reducing BOP in respect to the CG (Fig. 7). Heterogeneity is significant (p < 0.001). 82.1% of the variability come from heterogeneity.
3.5. Periodontal indices
3.5.1. Clinical attachment loss index
Green tea treatment had medium positive effect size in reducing CAL in respect to the CG (Fig. 8). Results of the studies are very heterogeneous: 93.4% of the variability came from heterogeneity.
3.5.2. Probing pocket depth index
Green tea treatment had large positive effect size in reducing PPD in respect to the CG (Fig. 9). Heterogeneity was significant (p < 0.001). 76.1% of the variability came from heterogeneity.
Splitting to subgroups, green tea treatment has large positive effect size in reducing PPD in the subgroup where placebo was used in CG and medium positive effect size in the subgroup where triclosan was used in CG (Fig. 10). No study with chlorhexidine agent in CG reports PPD index. The difference between “placebo subgroup” and “triclosan study” is insignificant (Table 4). Non-explained by moderator heterogeneity remains significant (p < 0.001).
Table 4.
Meta-regression model estimates for PPD.
| estimate | se | zval | p | |
|---|---|---|---|---|
| intercept | 1.1277 | 0.3221 | 3.5009 | 0.0005 |
| Triclosan | −0.7312 | 0.8384 | −0.8721 | 0.3832 |
3.6. LB and SM CFU numbers
Green tea treatment appeared to have small-medium negative effect according to Cohen's interpretive guidelines size in reducing SM number in respect to the CG (Fig. 11) [31]. Heterogeneity is not significant at p = 0.1 level. Only 39.7% of the variability came from heterogeneity (variability in effect sizes that are due to true differences among the studies) [32].
Green tea treatment had small negative effect size in reducing LB number in respect to the CG (Fig. 12). Heterogeneity is significant (p = 0.01). The results of two available studies are very different, almost 85% of the variability came from heterogeneity.
3.7. Summarizing findings
The Table 5 contains data listed on forest plots and additional column “p-value” with significance levels of Effect Size. The fifth column indicates two characteristics that are used in “Quality of Evidence” and also have qualitative/graphical measures. Two other characteristics, i.e risk of bias and impression, are rather non-statistical.
Table 5.
Summary of findings.
| Measurements | Effect size (95% CI) | p-value for ES | No of participants TG/CG (studies) | Consistent/pub.bias |
|---|---|---|---|---|
| Streptococcus mutans CFU/mL | −0.35 (−0.77, 0.08) | 0.110 | 65/100 (3) | yes/no |
| Lactobacillus spp CFU/mL | −0.18 (−1.09, 0.73) | 0.705 | 55/80 (2) | no/no |
| GI Gingival index | 0.50 (−0.02, 1.01) | 0.059 | 273/293 (10) | no/yes |
| PI Plaque index | 0.54 (0.14, 0.95) | 0.008 | 279/299 (11) | no/yes |
| GBI Sulcus bleeding index | 0.58 (−0.41, 1.56) | 0.249 | 73/72 (4) | no/yes |
| BOP Bleeding on probing | 0.52 (−0.57, 1.60) | 0.352 | 45/45 (2) | no/no |
| CAL Clinical attachment loss | 0.58 (−0.49, 1.65) | 0.291 | 129/129 (4) | no/yes |
| PPD Probing pocket depth | 1.02 (0.45, 1.59) | <0.001 | 124/124 (7) | no/yes |
Analyses of all measurements led to inconsistent results, except for SM CFU. Even in the case of SM CFU No, consistency should be taken cautiously, because the Q-based test is known to be poor at detecting true heterogeneity when the number of studies is small.
Publication bias was detected for GI, PI, GBI, CAL, PPD indices analyzing funnel plots.
Taking “control group agent” as moderator partially explains between-study heterogeneity for PI and has no essential effect for GI and PPD indexes. Additional studies of green tea are needed to clarify the reason of inconsistency.
3.8. Risk of Bias
Most often, allocation concealment, examiner blinding, patient drop-out were not sufficiently described or not satisfyingly completed. One cross-over study used wash-out period of 7 days [14]. The qualitative analyses revealed that the effects of the risk of bias were consistent and quite serious.
4. Discussion
4.1. Review findings
This systematic review endeavored to comprehensively display the available evidence on effect and efficacy of green tea on both periodontal disease and caries using both qualitatively and quantitatively synthesis.
The majority of the included studies (n:15) evaluated gingival and oral hygiene indices, as GI, PI, GBI and BOP. Moreover, green tea showed a medium positive effect in reducing the markers of inflammation as GI, GBI and BOP, suggesting a direct major effect on the host immune system rather than on the oral pathogens. However, after splitting to subgroups, green tea treatment showed a positive effect size in those CG where triclosan and placebo were used. When compared with chlorhexidine subgroups, green tea showed a very small negative effect in reducing GI and a small negative effect in reducing PI.
Periodontal indices have been investigated by 8 included studies [15,[19], [20], [21],25,27,28,30]. Green tea showed a large positive effect in reducing PPD, large positive effect size in the placebo subgroup and medium positive effect size in the triclosan subgroup. Green tea showed a medium positive effect in reducing CAL. Both the indices reflect the status of periodontal tissue inflammation, and the smaller efficacy on CAL could be explained by the limited duration of follow up, as this parameter assesses a long-term periodontal status.
The bacterial CFU numbers of LB and SM have been investigated by few studies (n:3). Green tea vehicle was exclusively mouth rinse and it showed a small medium negative effect on SM and a small negative effect on LB. The control intervention was in two cases chlorhexidine 0.12% [14,22] and in one case sodium fluoride 0.05% [26]. Green tea mouth rinse was more effective than sodium fluoride on LB reduction [26]. The cariogenicity of SM and LB is mostly based on understanding of caries as infectious disease. Moreover, frequently found in dental research on caries is the DMFT/dmft index, i.e. “caries or caries experience prevalence”. However, none of the included studies focused on these indices.
Periodontal pathogens (AA and PI) were investigated by 1 study on green tea catechin strips as local drug delivery system in addition to scaling and root planning (SRP) in patients with chronic periodontitis with a significative reduction in periopathogens number and PPD compared to scaling and root plaining alone [27]. These results are in accordance with a recent systematic review on the positive effect of topical green tea as an adjunct to SRP in non-surgical periodontitis therapy, without negative side effects [33].
None of the studies included in this systematic review reported on adverse events. Nevertheless, the most prevalent adverse events (AEs) from human intervention studies involving different green tea preparations or EGCG were on the gastrointestinal system and also, at a very low rate, reported hepatotoxicity [34]. However, incidence of liver-related AEs is very low in published clinical trials involving green tea preparations [35]. Moreover, there is clear evidence that green tea catechins are not genotoxic or carcinogenic. The recent safety assessment by Dekant et al., proposed a 300 mg/day dosage limit for EGCG consumed in supplemental form [36]. None of the included studies evaluated the amount of EGCG in the used preparations.
The control interventions were: i) chlorhexidine (n:5); ii) placebo (n:7); iii) triclosan toothpaste (n:1); iv) sodium fluoride (n:1); v) none (n:4). Chlorhexidine is a gold-standard anti-plaque agent, because of its substantivity, retention in the oral cavity and slow release, allowing anti-plaque efficacy. However, the long-term use of chlorhexidine is associated with several side effects, as tooth discolouration, taste alterations and oral desquamation. In vitro studies have confirmed the chlorhexidine toxicity on fibroblasts, also showing cytotoxicity and genotoxicity [[37], [38], [39]].
Green tea does not exhibit side effects and shows effective anti-inflammatory and antioxidant properties when compared with placebo or triclosan agent. The present review confirms the efficacy of green tea in the management of gingival inflammation, as documented by Kushiyama [4], by lowering the oxidative stress. EGCG has shown in former in vitro studies to stop the production of toxic metabolites of P. gingivalis [40]. Moreover, EGCG reduces also the expression of matrix metalloproteinase-9 in osteoblasts and avoids the formation of osteoclasts, thus green tea might be used to prevent alveolar bone resorption in patients with periodontal disease [41].
4.2. Limitations
Many of the included studies had limited validities, as blinding of the examiners, allocation concealment and patient drop-out frequently remained unclear. Moreover, none of the included studies provided a precise dosage of EGCG in the green tea formulations used. The present analyses found high heterogeneity among studies, therefore, for future studies, it is required to consider more homogeneous designs.
4.3. Recommendations and conclusions
This review comprehensively evaluates the results of randomized control trials on the use of green tea and its effects on processes of gingivitis, periodontitis and tooth decay. For gingival inflammation and periodontitis, these parameters were found to be positively affected by green tea. Based on these results, there is sufficient evidence to support the use of green tea to prevent and treat periodontal disease. This review shows that there is currently insufficient evidence for recommending the use of green tea to manage dental caries.
Moreover, even if the results are encouraging, there is no sufficient evidence that green tea could/can fully replace chlorhexidine, therefore the latter is still the recommended solution for treating gingivitis and periodontitis.
In conclusion, there is a growing number of clinical trials investigating the potential of green tea as an adjunct to the prevention and treatment of oral diseases. In a world scenario of antibiotic resistance and growing number of side effects of agents as chlorhexidine, that is not suitable for a chronic disease management, the development of new, more patient- and eco-friendly agents, should be encouraged.
Conflicts of interest
None.
Acknowledgments
The authors have no commercial relationships to declare.
Footnotes
Supplementary data associated with this article can be found, in the online version, at https://doi.org/10.1016/j.jdsr.2020.11.003.
Appendix A. Supplementary data
The following are Supplementary data to this article:
References
- 1.Gaur S., Agnihotri R. Green tea: a novel functional food for the oral health of older adults. Geriatr Gerontol Int. 2014;14:238–250. doi: 10.1111/ggi.12194. [DOI] [PubMed] [Google Scholar]
- 2.Hasler C.M. The changing face of functional foods. J Am Coll Nutr. 2000;19:499S–506S. doi: 10.1080/07315724.2000.10718972. [DOI] [PubMed] [Google Scholar]
- 3.Phipps R.P. The second international scientific symposium on tea & human health, September 14th, 1998. Nutrition. 1999;15:968–971. doi: 10.1016/s0899-9007(99)00196-3. [DOI] [PubMed] [Google Scholar]
- 4.Kushiyama M., Shimazaki Y., Murakami M., Yamashita Y. Relationship between intake of green tea and periodontal disease. J Periodontol. 2009;80:372–377. doi: 10.1902/jop.2009.080510. [DOI] [PubMed] [Google Scholar]
- 5.Koyama Y., Kuriyama S., Aida J., Sone T., Nakaya N., Ohmori-Matsuda K. Association between green tea consumption and tooth loss: cross-sectional results from the Ohsaki Cohort 2006 Study. Prev Med. 2010;50:173–179. doi: 10.1016/j.ypmed.2010.01.010. [DOI] [PubMed] [Google Scholar]
- 6.Ide R., Fujino Y., Hoshiyama Y., Mizoue T., Kubo T., Pham T.M. A prospective study of green tea consumption and oral cancer incidence in Japan. Ann Epidemiol. 2007;17:821–826. doi: 10.1016/j.annepidem.2007.04.003. [DOI] [PubMed] [Google Scholar]
- 7.Arab H., Maroofian A., Golestani S., Shafaee H., Sohrabi K., Forouzanfar A. Review of the therapeutic effects of Camellia sinensis (green tea) on oral and periodontal health. J Med Plants Res. 2011;5:5465–5469. [Google Scholar]
- 8.Liberati A., Altman D.G., Tetzlaff J., Mulrow C., Gøtzsche P.C., Ioannidis J.P. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration, 2009. J Clin Epidemiol. 2009;6:e1–34. doi: 10.1016/j.jclinepi.2009.06.006. [DOI] [PubMed] [Google Scholar]
- 9.Higgins J., Green S. 2011. Cochrane handbook for systematic reviews of interventions.http://www.cochrane-handbook.org (accessed 03 March 2020) [Google Scholar]
- 10.Higgins J.P.T., Thompson S.G. Quantifying heterogeneity in a meta-analysis. Stat Med. 2002;21:1539–1558. doi: 10.1002/sim.1186. [DOI] [PubMed] [Google Scholar]
- 11.Del Re A.C. A practical tutorial on conducting meta-analysis in R. Quant Method Psychol. 2015;11:37–50. [Google Scholar]
- 12.Wampold B.E., Mondin G.W., Moody M., Stich F., Benson K., Ahn H.N. A meta-analysis of outcome studies comparing bona fide psychotherapies: Empirically, "all must have prizes". Psychol Bull. 1997;122:203–215. [Google Scholar]
- 13.Sarin S., Marya C., Nagpal R., Oberoi S.S., Rekhi A. Preliminary clinical evidence of the antiplaque, antigingivitis efficacy of a mouthwash containing 2% green tea - A randomised clinical trial. Oral Health Prev Dent. 2015;13:197–203. doi: 10.3290/j.ohpd.a33447. [DOI] [PubMed] [Google Scholar]
- 14.Neturi R.S., Srinivas R., Vikram Simha B., Sandhya Sree Y., Chandra Shekar T., Siva Kumar P. Effects of green tea on streptococcus mutans counts- A randomised control trail. J Clin Diagn Res. 2014;8:128–130. doi: 10.7860/JCDR/2014/10963.5211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rattanasuwan K., Rassameemasmaung S., Sangalungkarn V., Komoltri C. Clinical effect of locally delivered gel containing green tea extract as an adjunct to non-surgical periodontal treatment. Odontology. 2016;104:89–97. doi: 10.1007/s10266-014-0190-1. [DOI] [PubMed] [Google Scholar]
- 16.Rassameemasmaung S., Phusudsawang P., Sangalungkarn V. Effect of green tea mouthwash on oral malodor. Prev Med. 2012;2013 doi: 10.5402/2013/975148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Behfarnia P., Aslani A., Jamshidian F., Noohi S. The efficacy of green tea chewing gum on gingival inflammation. J Dent (Shiraz) 2016;17:149–154. [PMC free article] [PubMed] [Google Scholar]
- 18.Radafshar G., Ghotbizadeh M., Saadat F., Mirfarhadi N. Effects of green tea (Camellia sinensis) mouthwash containing 1% tannin on dental plaque and chronic gingivitis: a double-blinded, randomized, controlled trial. J Investig Clin Dent. 2017;8 doi: 10.1111/jicd.12184. [DOI] [PubMed] [Google Scholar]
- 19.Hrishi T.S., Kundapur P.P., Naha A., Thomas B.S., Kamath S., Bhat G.S. Effect of adjunctive use of green tea dentifrice in periodontitis patients - A randomized controlled pilot study. Int J Dent Hyg. 2016;14:178–183. doi: 10.1111/idh.12131. [DOI] [PubMed] [Google Scholar]
- 20.Chava V.K., Vedula B.D. Thermo-reversible green tea catechin gel for local application in chronic periodontitis: a 4-week clinical trial. J Periodontol. 2013;84:290–1296. doi: 10.1902/jop.2012.120425. [DOI] [PubMed] [Google Scholar]
- 21.Chopra A., Thomas B.S., Sivaraman K., Prasad H.K., Kamath S.U. Green tea intake as an adjunct to mechanical periodontal therapy for the management of mild to moderate chronic periodontitis: a randomized controlled clinical trial. Oral Health Prev Dent. 2016;14:293–303. doi: 10.3290/j.ohpd.a36100. [DOI] [PubMed] [Google Scholar]
- 22.Hegde R.J., Kamath S. Comparison of the Streptococcus mutans and Lactobacillus colony count changes in saliva following chlorhexidine (0.12%) mouth rinse, combination mouth rinse, and green tea extract (0.5%) mouth rinse in children. J Indian Soc Pedod Prev Dent. 2017;35:150–155. doi: 10.4103/JISPPD.JISPPD_13_17. [DOI] [PubMed] [Google Scholar]
- 23.Priya B.M., Anitha V., Shanmugam M., Ashwath B., Sylva S.D., Vigneshwari S.K. Efficacy of chlorhexidine and green tea mouthwashes in the management of dental plaque-induced gingivitis: a comparative clinical study. Contemp Clin Dent. 2015;6:505–519. doi: 10.4103/0976-237X.169845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hambire C.U., Jawade R., Patil A., Wani V.R., Kulkarni A.A., Nehete P.B. Comparing the antiplaque efficacy of 0.5% Camellia sinensis extract, 0.05% sodium fluoride, and 0.2% chlorhexidine gluconate mouthwash in children. J Int Soc Prev Community Dent. 2015;5:218–226. doi: 10.4103/2231-0762.158016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hattarki S.A., Pushpa S.P., Bhat K. Evaluation of the efficacy of green tea catechins as an adjunct to scaling and root planing in the management of chronic periodontitis using PCR analysis: a clinical and microbiological study. J Indian Soc Periodontol. 2013;17:204–209. doi: 10.4103/0972-124X.113071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tehrani M.H., Asghari G., Hajiahmadi M. Comparing Streptococcus mutans and Lactobacillus colony count changes following green tea mouth rinse or sodium fluoride mouth rinse use in children (randomized double-blind controlled clinical trial) Dent Res J (Isfahan) 2011;8:S58–S63. [PMC free article] [PubMed] [Google Scholar]
- 27.Kudva P., Tabasum S.T., Shekhawat N.K. Effect of green tea catechin, a local drug delivery system as an adjunct to scaling and root planing in chronic periodontitis patients: a clinicomicrobiological study. J Indian Soc Periodontol. 2011;15:39–45. doi: 10.4103/0972-124X.82269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Taleghani F., Rezvani G., Birjandi M., Valizadeh M. Impact of green tea intake on clinical improvement in chronic periodontitis: a randomized clinical trial. J Stomatol Oral Maxillofac Surg. 2018;119:365–368. doi: 10.1016/j.jormas.2018.04.010. [DOI] [PubMed] [Google Scholar]
- 29.Tripathi P., Blaggana V., Upadhyay P., Jindal M., Gupta S., Nishat S. Antioxidant therapy (lycopene and green tea extract) in periodontal disease: a promising paradigm. J Indian Soc Periodontol. 2019;23:25–30. doi: 10.4103/jisp.jisp_277_18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hirasawa M., Takada K., Makimura M., Otake S. Improvement of periodontal status by green tea catechin using a local delivery system: a clinical pilot study. J Periodontal Res Suppl. 2002;37:433–438. doi: 10.1034/j.1600-0765.2002.01640.x. [DOI] [PubMed] [Google Scholar]
- 31.Cohen J. second ed. Lawrence Erlbaum; 1988. Statistical power analysis for the behavioral sciences. [Google Scholar]
- 32.Hedges L.V., Olkin I. first ed. Academic Press; Orlando, FL: 1985. Statistical methods for meta-analysis. [Google Scholar]
- 33.Gartenmann S.J., Weydlich Y.V., Steppacher S.L., Heumann C., Attin T., Schmidlin P.R. The effect of green tea as an adjunct to scaling and root planing in non-surgical periodontitis therapy: a systematic review. Clin Oral Investig. 2019;23:1–20. doi: 10.1007/s00784-018-2684-7. [DOI] [PubMed] [Google Scholar]
- 34.Hu J., Webster D., Cao J., Shao A. The safety of green tea and green tea extract consumption in adults – results of a systematic review. Regul Toxicol Pharmacol. 2018;95:412–433. doi: 10.1016/j.yrtph.2018.03.019. [DOI] [PubMed] [Google Scholar]
- 35.Isomura T., Suzuki S., Origasa H. Liver-related safety assessment of green tea extracts in humans: a systematic review of randomized controlled trials. Eur J Clin Nutr. 2016;70:1221–1229. doi: 10.1038/ejcn.2016.78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Dekant W., Fujii K., Shibata E., Morita O., Shimotoyodome A. Safety assessment of green tea based beverages and dried green tea extracts as nutritional supplements. Toxicol Lett. 2017;277:104–108. doi: 10.1016/j.toxlet.2017.06.008. [DOI] [PubMed] [Google Scholar]
- 37.Prasanna V., Lakshmanan R. Characteristics, uses and side effects of chlorhexidine- a review. IOSR-J D M S. 2016;15:57–59. [Google Scholar]
- 38.Plantinga N., Wittekamp B.H.J., Leleu K. Oral mucosal adverse events with chlorhexidine 2% mouthwash in ICU. Intensive Care Med. 2016;42:620–621. doi: 10.1007/s00134-016-4217-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hidalgo E., Dominguez C. Mechanisms underlying chlorhexidine-induced cytotoxicity. Toxicol In Vitro. 2001;15:271–276. doi: 10.1016/s0887-2333(01)00020-0. [DOI] [PubMed] [Google Scholar]
- 40.Sakanaka S., Okada Y. Inhibitory effects of green tea polyphenols on the production of a virulence factor of the periodontal-disease-causing anaerobic bacterium Porphyromonas gingivalis. J Agric Food Chem. 2004;52:1688–1692. doi: 10.1021/jf0302815. [DOI] [PubMed] [Google Scholar]
- 41.Yun J.H., Pang E.K., Kim C.S. Inhibitory effects of green tea polyphenol (-)-epigallocatechin gallate on the expression of matrix metalloproteinase-9 and on the formation of osteoclasts. J Periodontal Res Suppl. 2004;39:300–307. doi: 10.1111/j.1600-0765.2004.00743.x. [DOI] [PubMed] [Google Scholar]
- 42.Rattanasuwan K., Rassameemasmaung S., Sangalungkarn V., Komoltri C. Clinical effect of locally delivered gel containing green tea extract as an adjunct to non-surgical periodontal treatment. Odontology. 2016;104:89–97. doi: 10.1007/s10266-014-0190-1. [DOI] [PubMed] [Google Scholar]
- 43.Rassameemasmaung S., Phusudsawang P., Sangalungkarn V. Effect of green tea mouthwash on oral malodor. Prev Med. 2012;2013:975148. doi: 10.5402/2013/975148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Chava V.K., Vedula B.D. Thermo-reversible green tea catechin gel for local application in chronic periodontitis: a 4-week clinical trial. J Periodontol. 2013;84:1290–1296. doi: 10.1902/jop.2012.120425. [DOI] [PubMed] [Google Scholar]
- 45.Jenabian N., Moghadamnia A.A., Karami E., Mir A.P.B. The effect of Camellia sinensis (green tea) mouthwash on plaque-induced gingivitis: a single-blinded randomized controlled clinical trial. Daru. 2012;20:39. doi: 10.1186/2008-2231-20-39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Thomas A., Thakur S.R., Shetty S.B. Anti-microbial efficacy of green tea and chlorhexidine mouth rinses against Streptococcus mutans, Lactobacilli spp. And Candida albicans in children with severe early childhood caries: a randomized clinical study. J Indian Soc Pedod Prev Dent. 2016;34:65–70. doi: 10.4103/0970-4388.175518. [DOI] [PubMed] [Google Scholar]
- 47.Awadalla H.I., Ragab M.H., Fayed M., Abbas M.O., Bassuoni M.W. Evaluation of the effect of green tea on dental caries and composite restorations. TAF Prev Med Bull. 2011;10 [Google Scholar]
- 48.Ferrazzano G.F., Roberto L., Amato I., Cantile T., Sangianantoni G., Ingenito A. Antimicrobial properties of green tea extract against cariogenic microflora: an in vivo study. J Med Food. 2011;14:907–911. doi: 10.1089/jmf.2010.0196. [DOI] [PubMed] [Google Scholar]
- 49.Krahwinkel T., Willershausen B. The effect of sugar-free green tea chew candies on the degree of inflammation of the gingiva. Eur J Med Res. 2000;5:463–467. [PubMed] [Google Scholar]
- 50.Kaur H., Jain S., Kaur A. Comparative evaluation of the antiplaque effectiveness of green tea catechin mouthwash with chlorhexidine gluconate. J Indian Soc Periodontol. 2014;18:178–182. doi: 10.4103/0972-124X.131320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Gadagi J.S., Chava V.K., Reddy V.R. Green tea extract as a local drug therapy on periodontitis patients with diabetes mellitus: a randomized case-control study. J Indian Soc Periodontol. 2013;17:198–203. doi: 10.4103/0972-124X.113069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Shalini M., Ramesh A. Comparison of anti-plaque efficacy of green tea, herbal, and chlorhexidine mouthrinse in patients undergoing orthodontic treatment. D I T. 2018;10:1323–1327. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.