Abstract
Background:
In prostate cancer, it is unknown whether stereotactic body radiation therapy (SBRT) is substituting for other radiation treatments, substituting for surgery, or expanding the pool of patients receiving treatment instead of active surveillance.
Methods:
Using SEER-Medicare, we identified men diagnosed with prostate cancer between 2007 and 2011 and developed physician-hospital networks by identifying each patient’s treating physician based on the primary treatment received and subsequently assigning each physician to a hospital. We examined the relative distribution of prostate cancer treatments stratified by whether or not a network performed SBRT by fitting logistic regression models with robust standard errors to account for clustering of patients within networks.
Results:
We identified 344 physician-hospital networks, 30 of which (8.7%) performed SBRT and 314 of which (91.3%) did not. Networks performing and not performing SBRT did not differ in their use of robotic prostatectomy, radical prostatectomy, and active surveillance over time (all p>0.05). The relationship with IMRT did not exhibit any consistent temporal pattern, with networks performing SBRT having less IMRT initially but similar rates in the later years. Trends in brachytherapy differed among networks performing and not performing SBRT with use of brachytherapy lower in networks performing SBRT (p=0.03).
Conclusions:
Networks performing and not performing SBRT did not differ in rates of surgery and active surveillance, yet networks performing SBRT had lower rates of brachytherapy. SBRT may represent an alternative to brachytherapy more so than for active surveillance.
Keywords: prostate cancer, stereotactic body radiation therapy, physician-hospital networks, brachytherapy, active surveillance
INTRODUCTION
Stereotactic body radiation therapy (SBRT) is a novel treatment for prostate cancer with potential benefits including increased precision and shorter duration of treatment.1, 2 Based on favorable early results from retrospective and non-randomized prospective trials, guidelines recommend consideration of SBRT as a treatment option for men with low- and intermediate-risk prostate cancer.3 Initially, SBRT’s adoption was slow, although its use has increased in recent years.1 Despite SBRT’s increasing role in prostate cancer, how its adoption fits into the landscape of other prostate cancer treatments remains unknown.
Several aspects of each treatment modality may affect these patterns. Given that SBRT is a low-burden form of external beam radiation requiring only 2.5 weeks of treatment, it may substitute for more burdensome external beam modalities, such as IMRT, which requires 8 weeks of treatment.2 On the other hand, under a fee-for-service reimbursement model, IMRT is more lucrative for physicians, which may deter switching to SBRT.4 Brachytherapy represents another form of radiation that is administered in one day, albeit in the operating room, making it an attractive alternative to conventional external beam radiation. However, SBRT’s shorter treatment course may be appealing in lieu of brachytherapy’s more invasive approach. SBRT also represents an alternative to active surveillance without the risks of surgery or the hassle of an 8-week regimen. Lastly, SBRT’s shorter duration may be preferred over surgery and its inherent risks.
To better understand these issues, we sought to characterize the adoption of SBRT in the context of other prostate cancer treatments using a novel approach for identifying unique treatment teams (i.e., prostate cancer-specific physician-hospital networks), which are empirically derived units of physicians who treat men with prostate cancer anchored by the hospital with which they are most frequently affiliated. The networks are designed to encompass the full spectrum of treatments.
METHODS
Study Design, Data Sources, and Population
We conducted a longitudinal cohort study of prostate cancer treatment patterns using prostate cancer-specific physician hospital networks. Physician-hospital-networks are groups of patients, their physicians, and their physicians’ hospitals with which they are most affiliated. The rationale for designing these networks is that hospital-based units more accurately group hospitals, providers, and the patients they serve than traditional geography-based units. All analyses were conducted using Surveillance, Epidemiology, and End Results (SEER)-Medicare data, which we linked to the American Hospital Association (AHA) Annual Survey to obtain additional data on hospital characteristics.
We created physician-hospital networks, categorized them by whether or not they performed SBRT in the initial treatment for localized prostate cancer in a given year, and examined SBRT adoption over time. Finally, we examined SBRT use as part of the full spectrum of prostate cancer utilization patterns, comparing treatment patterns among networks performing and not performing SBRT.
We first identified men 66 years or older diagnosed with prostate cancer between 2007 and 2011. Using the Medicare Provider Analysis and Review (MEDPAR), outpatient, and carrier files, we determined each patient’s primary treatment using Healthcare Common Procedure Coding System (HCPCS) and International Classification of Diseases—9th Revision, Clinical Modification (ICD-9-CM) procedure codes.5 Treatments included active surveillance, radical prostatectomy, robotic prostatectomy, brachytherapy, IMRT, SBRT, and other treatments (i.e., external beam radiotherapy, proton beam therapy, perineal prostatectomy, hormonal therapy, and cryotherapy) based on the primary treatment received within the first 12 months of diagnosis. Based on previous work,6 we defined active surveillance as instances in which the patient did not receive any aggressive treatment yet had at least one prostate biopsy or prostate-specific antigen (PSA) level in the first two years after diagnosis.
We excluded men who were 65 years old to ensure accurate comorbidity estimation using Medicare claims for the 12-month period prior to diagnosis.7 We included fee-for-service beneficiaries eligible for both Medicare Parts A and B from 12 months prior until 12 months after diagnosis and those who had prostate cancer as their first and only cancer.
Identifying physician-hospital networks
We developed prostate cancer-specific physician-hospital networks based on previously defined methods (Appendix 1).8 Briefly, we assigned each patient to a prostate cancer treating physician based on the primary treatment received. Then, we assigned each physician to a hospital based on their most frequent site of practice. Next, we refined the physician-hospital networks included in the cohort. First, we excluded networks that included patients from SEER regions with physicians affiliated with hospitals in non-SEER regions. Second, we excluded patients from networks with < 30 patients and networks without at least one patient in any given year to minimize concern for unstable estimates.
Identifying treatment patterns within networks
All prostate cancer patients within networks were assigned to a primary treatment. Networks were then stratified based on their use of SBRT. A network was classified as performing SBRT if the network had affiliated patients treated with SBRT in that calendar year. To avoid attributing SBRT to networks prior to their adoption, we assessed networks annually. Once a network performed SBRT, it was considered a network performing SBRT from that point until the end of the study period. We made this decision because once a network had the ability to provide SBRT, we felt it would factor into the treatment decision-making process, whether or not a patient actually received it in a subsequent year.
Covariates
Disease risk was defined using a combination of age and tumor grade since PSA levels in SEER were unreliable during the study period.9, 10 Information on bed size, teaching hospital status, and cancer program were obtained from the AHA Annual Survey.
Statistical Analyses
To understand SBRT adoption over time, we calculated the number of networks performing SBRT and the number of patients in these networks using SBRT each study year. In both cases, we distinguished networks that were existing users of SBRT from those that were new adopters (i.e., did not use SBRT in the year prior). We also calculated the annual proportion of SBRT use among networks performing SBRT.
To understand which types of networks were adopting SBRT, we examined the characteristics of the hospitals anchoring the physician-hospital networks, stratified by whether or not they performed SBRT in a given year. Hypotheses testing was performed using chi-square tests. We performed comparisons separately for each year of the data.
To understand which types of patients received care in these networks, we compared patient characteristics among networks performing or not performing SBRT. Hypotheses testing was performed using chi-square tests. For this analysis, we grouped all years together, and considered a patient to have been cared for in an SBRT performing network if they were seen in that network in the year that they adopted SBRT or any time thereafter.
To understand how the adoption of SBRT influenced overall treatment patterns, we compared the treatment distribution among networks performing and not performing SBRT. For each treatment, we fit a multivariable logistic regression model with robust standard errors to account for clustering of patients within physician-hospital networks. The dependent variable was the relative distribution of prostate cancer treatments and the primary independent variables were whether or not a network was performing SBRT in a given year, diagnosis year, and an interaction between SBRT and diagnosis year. The coefficient for the interaction term represents the differences in the trends of treatments over time between the networks performing and not performing SBRT. We also used logistic regression with cluster-robust standard errors to calculate the probability of using each treatment based on a network’s use of SBRT in the year prior.
We performed data management and analyses in SASv9.4 (SAS Institute, Cary, NC) and Rv3.5 (R Foundation for Statistical Computing, Vienna, Austria), respectively. The University of Pittsburgh institutional review board deemed this study exempt from full board review.
RESULTS
In refining the cohort, we excluded 3078 patients (6.3%) treated at networks located outside SEER regions, 2418 patients (5.3%) from networks with < 30 patients, and 123 patients (0.3%) from networks without at least one patient in any given year. Using these criteria, our cohort consisted of 42,963 patients across 344 physician-hospital networks.
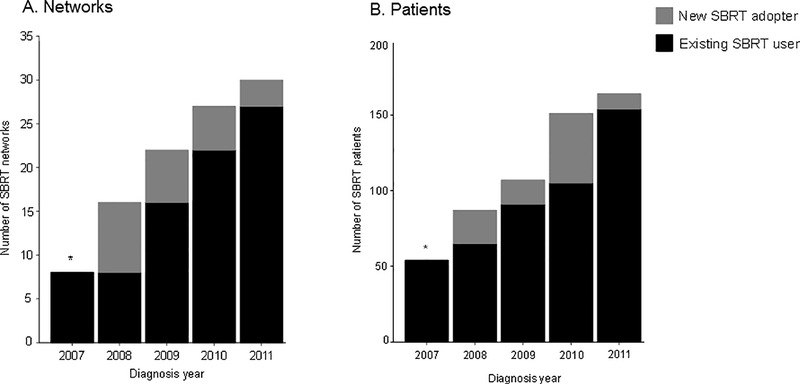
The number of networks using SBRT increased from 8 in 2007 to 30 in 2011 (Figure 1). This increase coincided with an increase in the number of patients treated with SBRT among networks performing SBRT. Newly adopting SBRT networks decreased over time whereas the number of patients treated within these newly adopting networks varied. Among networks using SBRT, the proportion of patients receiving SBRT was relatively constant over time, ranging from 12.9% to 16.9% (Appendix 2).
Figure 1.
The number of networks (A) and patients (B) using SBRT over time
Abbreviations: SBRT, stereotactic body radiation therapy
*Includes both existing and new SBRT adopters since 2007 is the first year of the study.
Since 2007 was the first year of the study period, we could not distinguish new and existing SBRT users (*). In subsequent years, we distinguished networks that were existing users of SBRT (black) from new adopters of SBRT (gray). (A) The x-axis represents the number of networks using SBRT over time. (B) The x-axis represents the number of patients who received SBRT within networks performing SBRT.
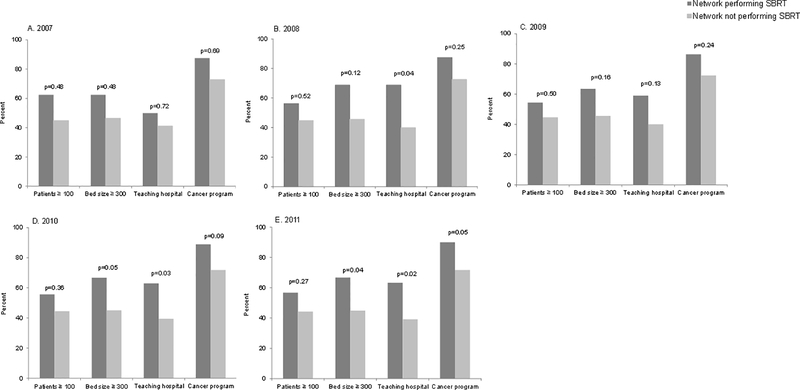
In 2011, networks performing SBRT were more likely associated with larger hospitals (p=0.04) (Figure 2). In the later years of the study (i.e., 2008, 2010, and 2011), networks performing SBRT were more likely associated with teaching hospitals (all p<0.05). Networks performing and not performing SBRT did not differ in terms of number of patients or cancer program affiliation.
Figure 2.
Characteristics of the hospitals anchoring the physician-hospital networks from 2007–2011
Abbreviations: SBRT, stereotactic body radiation therapy
P values generated from chi-square tests. Cancer programs were approved by the American College of Surgeons.
Although some differences were statistically significant due to large sample sizes, clinical characteristics were qualitatively similar between patients in networks performing and not performing SBRT (Table 1). Among the non-clinical characteristics, patients in networks performing SBRT were more likely located in highly populated areas (1 million or more people), areas with higher median incomes, and in the northeast (all p<0.001). Networks performing SBRT had lower proportions of patients receiving brachytherapy and other treatments (p<0.001).
Table 1:
Characteristics of the patient population
| Characteristics | Patients cared for in networks performing SBRT* | Patients cared for in networks not performing SBRT | P value** |
|---|---|---|---|
| Number of patients | 3437 | 39,526 | |
| Age, years (%) | 0.07 | ||
| 66–69 | 940 (27.3) | 10,448 (26.4) | |
| 70–74 | 1218 (35.4) | 13,817 (35.0) | |
| 75–79 | 813 (23.7) | 9239 (23.4) | |
| 80 or older | 466 (13.6) | 6022 (15.2) | |
| Race/ethnicity (%) | <0.001 | ||
| White | 2801 (81.5) | 32,660 (82.6) | |
| Black | 442 (12.9) | 4020 (10.2) | |
| Hispanic | 37 (1.1) | 725 (1.8) | |
| Asian | 73 (2.1) | 938 (2.4) | |
| Other | 84 (2.4) | 1183 (3.0) | |
| Marital Status (%) | <0.001 | ||
| Married | 2424 (70.5) | 26,412 (66.8) | |
| Not married | 673 (19.6) | 6800 (17.2) | |
| Unknown | 340 (9.9) | 6314 (16.0) | |
| Comorbidity (%) | 0.45 | ||
| 0 | 2126 (61.9) | 24,757 (62.6) | |
| 1 | 795 (23.1) | 9157 (23.2) | |
| 2 or more | 497 (14.5) | 5445 (13.8) | |
| Unknown | 19 (0.6) | 167 (0.4) | |
| Tumor stage (%) | <0.001 | ||
| T1 or less | 2059 (59.9) | 22,797 (57.7) | |
| T2 | 1153 (33.5) | 14,594 (36.9) | |
| T3/T4 | 125 (3.6) | 958 (2.4) | |
| Unknown | 100 (2.9) | 1177 (3.0) | |
| Disease risk classification (%) | 0.06 | ||
| Lower | 947 (27.6) | 11,490 (29.1) | |
| Higher | 2490 (72.4) | 28,036 (70.9) | |
| Population of county of residence (%) | <0.001 | ||
| 1 million or more | 2207 (64.2) | 21,182 (53.6) | |
| 250,000–999,999 | 727 (21.2) | 7541 (19.1) | |
| 250,000 or less | 503 (14.6) | 10,775 (27.3) | |
| Unknown | -- | 28 (0.1) | |
| At least a high school education in ZIP code of residence (%) | <0.001 | ||
| Low (0–75) | 292 (8.5) | 4687 (11.9) | |
| High (>75) | 3079 (89.6) | 33,934 (85.9) | |
| Unknown | 66 (1.9) | 905 (2.3) | |
| Median household income in ZIP code of residence, $ (%) | <0.001 | ||
| 40,000 or less | 411 (12.0) | 6355 (16.1) | |
| 40,001–60,000 | 819 (23.8) | 14,006 (35.4) | |
| 60,001 or more | 2141 (62.3) | 18,213 (46.1) | |
| Unknown | 66 (1.9) | 952 (2.4) | |
| Geographic region (%) | <0.001 | ||
| Northeast | 1553 (45.2) | 7570 (19.2) | |
| South | 540 (15.7) | 9363 (23.7) | |
| Central | 184 (5.4) | 7440 (18.8) | |
| West | 1160 (33.8) | 15,153 (38.3) | |
| Year of diagnosis (%) | <0.001 | ||
| 2007 | 308 (9.0) | 9148 (23.1) | |
| 2008 | 566 (16.5) | 8263 (20.9) | |
| 2009 | 758 (22.1) | 7699 (19.5) | |
| 2010 | 854 (24.8) | 7270 (18.4) | |
| 2011 | 951 (27.7) | 7146 (18.1) | |
| Treatment (%) | <0.001 | ||
| SBRT | 535 (15.6) | -- | |
| IMRT | 1230 (35.8) | 11,922 (30.2) | |
| Robotic prostatectomy | 363 (10.6) | 6013 (15.2) | |
| Radical prostatectomy | 189 (5.5) | 2435 (6.2) | |
| Brachytherapy | 363 (10.6) | 7967 (20.2) | |
| Active surveillance | 456 (13.3) | 5598 (14.2) | |
| Other treatments*** | 301 (8.8) | 5591 (14.1) |
Abbreviations: IMRT, intensity-modulated radiation therapy; SBRT, stereotactic body radiation therapy
Percentages might not sum to 100 because of rounding
Once a network performed SBRT, all patients in that network were cared for in “a network performing SBRT” from that year until the end of the study period. Patients treated in networks prior to the year of SBRT adoption were cared for in “a network not performing SBRT”.
P values generated from chi-square tests
Other treatments include external beam radiotherapy, proton beam therapy, perineal prostatectomy, hormonal therapy, and cryotherapy
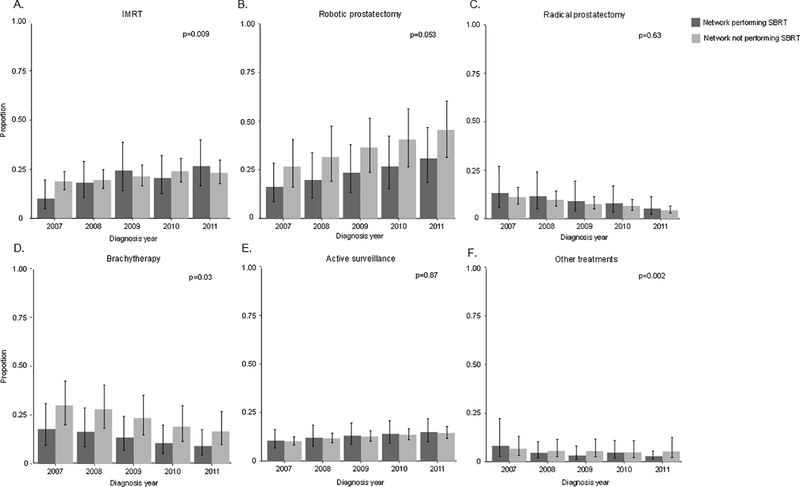
The trend in IMRT use among networks performing and not performing SBRT differed, but did not exhibit a consistent pattern (p=0.009) (Figure 3). The use of robotic prostatectomy was lower in networks performing SBRT. However, the trends in use of robotic prostatectomy (p=0.053) did not differ among networks performing and not performing SBRT, as was the case with radical prostatectomy (p=0.63) and active surveillance (p=0.87). The use of brachytherapy was lower in networks performing SBRT. In addition, trends in brachytherapy (p=0.03) and other treatments differed over time among networks performing and not performing SBRT (p=0.002). When stratified by disease risk, treatment patterns were similar. For this reason and due to less stable estimates from smaller sample sizes, we show the results for the entire cohort without disease risk stratification. The adjusted probability of brachytherapy was significantly lower in networks that used SBRT in the year prior (p=0.04). After further adjusting for baseline proportion of treatment use in 2007, this difference was no longer significant (p=0.18).
Figure 3.
Adjusted* proportions of IMRT (A), robotic prostatectomy (B), radical prostatectomy (C), brachytherapy (D), active surveillance (E), and other treatments (F), stratified by SBRT use within a physician-hospital network
Abbreviations: IMRT, intensity-modulated radiation therapy; SBRT, stereotactic body radiation therapy
*Adjusted for patient age, race, marital status, comorbidities, tumor stage, population of county of residence, education in ZIP code of residence, median household income in ZIP code of residence, geographic region, and clustering of patients.
The p-values test for differences in trends over time between networks performing and not performing SBRT and were calculated from the interaction between SBRT network and diagnosis year in a logistic regression model. Robust standard erros were used to account for clustering of patients within physician-hospital networks. The error bars represent the 95% confidence intervals for the estimated yearly proportions.
DISCUSSION
SBRT use increased steadily over time, although the effects of this increase on the use of other prostate cancer treatments is complex. Compared with networks not performing SBRT, networks performing SBRT showed no clear relationship with IMRT and did not consistently differ in trends of surgery or active surveillance. Robotic prostatectomy increased while radical prostatectomy decreased across all networks, regardless of SBRT use. Networks performing and not performing SBRT both exhibited a decrease in brachytherapy, with a more pronounced decline among networks not performing SBRT. The proportion of patients receiving brachytherapy and robotic prostatectomy was lower in networks performing SBRT.
Although brachytherapy is a well-established treatment for prostate cancer with excellent cancer control and minimal morbidity,11, 12 there are factors that may be contributing to its decline. Reimbursement for brachytherapy is decreasing and disparities between reimbursement for brachytherapy and competing modalities are widening.4, 13 Further, press releases about improperly placed brachytherapy seeds have generated negative patient perceptions.14
Brachytherapy’s use is lower in networks performing SBRT. Since patients can complete SBRT in 2.5 weeks, the advantage of completing brachytherapy in one day is not as pronounced as when comparing it to an 8-week course of standard external beam radiation, such as IMRT.2 With the advent of SBRT, the advantages of brachytherapy in terms of decreased travel burden, decreased time off work, and increased productivity are diminished. Further, SBRT represents an alternative to brachytherapy in which patients can avoid a more invasive procedure that generally occurs in the operating room and results in a hospital stay.15
Nonetheless, the rate of decline in brachytherapy is faster in networks not performing SBRT, which complicates this observation. Regardless of network type, there may be a new steady state of brachytherapy use that is low and closer to the observed rate in networks performing SBRT than in networks not performing SBRT. In networks not performing SBRT, the decrease in brachytherapy coincides with an increase in the uptake of robotic prostatectomy.
SBRT use was not related to changing rates of surgical treatment patterns. Although robotic prostatectomy was less common in networks performing SBRT, the rate of increase in the robotic approach was similar across networks while rates of radical prostatectomy uniformly decreased.
Perhaps more importantly than the associations with other radiation modalities and surgery is the finding that rates of active surveillance did not differ based on use of SBRT. Guidelines support SBRT as a treatment option for localized prostate cancer,3 which is used most frequently in patients with low-risk disease.16 Thus, patients eligible for active surveillance would also be potential candidates for SBRT, putting them at risk for overtreatment as was the case with the introduction of robotic prostatectomy and IMRT.5 SBRT’s non-invasive nature along with its decreased treatment burden and reduced cost compounds this issue.2, 17 Reassuringly, we found no indication that SBRT was drawing patients away from active surveillance.
The differing treatment patterns among networks performing and not performing SBRT have policy implications. The declining rates of brachytherapy across all networks raise some concern since it is among the most clinically effective and cost-effective treatments for localized disease.11, 18 As rates continue to decrease, training opportunities for residents will decline, which will further limit the use of brachytherapy in the future.13 At the same time, as health care transitions away from traditional fee-for-service payments towards alternative payment models, such as bundled payments, the demand for cost-effective treatments like brachytherapy will increase. Policymakers will need to consider the current decrease in training with the future increase in demand that is likely to occur as alternative payment models gain momentum.
In regards to the issue of overtreating prostate cancer, it is encouraging that the use of SBRT was not associated with decreasing rates of active surveillance. Active surveillance is the preferred approach for patients with very low-risk disease and is an attractive option for those with low-risk disease.19 The similar rate of active surveillance among networks performing and not performing SBRT suggests that SBRT, with its shorter duration than standard radiation, is not drawing patients away from active surveillance.
Our findings should be interpreted in the context of some limitations. First, SEER-Medicare only captures facilities that are hospital-based and not free-standing. Thus, we were unable to examine SBRT use by physicians who practiced exclusively in free-standing facilities. However, we were able to include physicians in free-standing facilities who were also affiliated with hospitals. Ultimately, we could assign 80% of providers who treated patients with external beam radiation to a hospital. Since SBRT is more likely associated with academic hospitals,20 we felt it was most informative to use SEER-Medicare data for this study. Second, SBRT delivery is occurring outside SEER regions. Although our proposal does not account for networks performing SBRT throughout the United States, SEER-Medicare encompasses 26% of the country’s population, which lessens the concerns regarding generalizability.
Supplementary Material
Appendix 2. The proportion of SBRT use among networks performing SBRT over time
Abbreviations: SBRT, stereotactic body radiation therapy
*Includes both existing and new SBRT adopters since 2007 is the first year of the study.
The x-axis represents the proportion of SBRT used in SBRT physician-hospital networks. Since 2007 was the first year of the study period, we could not distinguish new and existing SBRT users (*). In subsequent years, we distinguished networks that were existing users of SBRT (black) from new adopters of SBRT (gray).
Acknowledgments
Funding:
Bruce Jacobs is supported in part by the GEMSSTAR award (R03AG048091), the University of Pittsburgh Physicians Academic Foundation, P30CA047904 from the National Cancer Institute, and the Henry L. Hillman Foundation.
Jonathan Yabes is supported in part by the by the University of Pittsburgh Clinical and Translational Science Institute –Research Education and Career Development Core (UL1 TR000005).
Julie Bynum is supported in part by NIA grant (P01AG019783).
Amber Barnato is supported in part by the Levy Cluster in Health Care Delivery at Dartmouth.
Jeremy Kahn is supported in part by a career development award from the National Institutes of Health (K24HL133444).
ABBREVIATIONS
- AHA
American Hospital Association
- HCPCS
Healthcare Common Procedure Coding System
- ICD-9-CM
International Classification of Diseases—9th Revision, Clinical Modification
- IMRT
intensity-modulated radiation therapy
- MEDPAR
Medicare Provider Analysis and Review
- PSA
prostate-specific antigen
- SBRT
stereotactic body radiation therapy
- SEER
Surveillance, Epidemiology, and End Results
Footnotes
Financial disclosures:
Amber Barnato is a former board member of the Society of Medical Decision Making
REFERENCES
- 1.Jacobs BL, Yabes JG, Lopa SH et al. : The early adoption of intensity-modulated radiotherapy and stereotactic body radiation treatment among older Medicare beneficiaries with prostate cancer. Cancer, 123: 2945, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Buyyounouski MK, Price RA Jr., Harris EE et al. : Stereotactic body radiotherapy for primary management of early-stage, low- to intermediate-risk prostate cancer: report of the American Society for Therapeutic Radiology and Oncology Emerging Technology Committee. Int J Radiat Oncol Biol Phys, 76: 1297, 2010 [DOI] [PubMed] [Google Scholar]
- 3.Mohler JL, Armstrong AJ, Bahnson RR et al. : Prostate Cancer, Version 1.2016. J Natl Compr Canc Netw, 14: 19, 2016 [DOI] [PubMed] [Google Scholar]
- 4.Mitchell JM: Urologists’ use of intensity-modulated radiation therapy for prostate cancer. N Engl J Med, 369: 1629, 2013 [DOI] [PubMed] [Google Scholar]
- 5.Jacobs BL, Zhang Y, Schroeck FR et al. : Use of advanced treatment technologies among men at low risk of dying from prostate cancer. JAMA, 309: 2587, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Filson CP, Schroeck FR, Ye Z et al. : Variation in use of active surveillance among men undergoing expectant treatment for early stage prostate cancer. J Urol, 192: 75, 2014 [DOI] [PubMed] [Google Scholar]
- 7.Klabunde CN, Potosky AL, Legler JM et al. : Development of a comorbidity index using physician claims data. J Clin Epidemiol, 53: 1258, 2000 [DOI] [PubMed] [Google Scholar]
- 8.Bynum JP, Bernal-Delgado E, Gottlieb D et al. : Assigning ambulatory patients and their physicians to hospitals: a method for obtaining population-based provider performance measurements. Health Serv Res, 42: 45, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.PSA values and SEER data, 1973–2012. https://seer.cancer.gov/data/psa-values.html. Accessed December 3, 2016.
- 10.Miller DC, Gruber SB, Hollenbeck BK et al. : Incidence of initial local therapy among men with lower-risk prostate cancer in the United States. J Natl Cancer Inst, 98: 1134, 2006 [DOI] [PubMed] [Google Scholar]
- 11.Sylvester JE, Grimm PD, Wong J et al. : Fifteen-year biochemical relapse-free survival, cause-specific survival, and overall survival following I(125) prostate brachytherapy in clinically localized prostate cancer: Seattle experience. Int J Radiat Oncol Biol Phys, 81: 376, 2011 [DOI] [PubMed] [Google Scholar]
- 12.Kollmeier MA, Fidaleo A, Pei X et al. : Favourable long-term outcomes with brachytherapy-based regimens in men </=60 years with clinically localized prostate cancer. BJU Int, 111: 1231, 2013 [DOI] [PubMed] [Google Scholar]
- 13.Orio PF 3rd, Nguyen PL, Buzurovic I et al. : Prostate Brachytherapy Case Volumes by Academic and Nonacademic Practices: Implications for Future Residency Training. Int J Radiat Oncol Biol Phys, 96: 624, 2016 [DOI] [PubMed] [Google Scholar]
- 14.Bogdanich W: At V.A. Hospital, a Rogue Cancer Unit, 2009. http://www.nytimes.com/2009/06/21/health/21radiation.html. Accessed November 22, 2017.
- 15.Skowronek J: Current status of brachytherapy in cancer treatment - short overview. J Contemp Brachytherapy, 9: 581, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.King CR, Freeman D, Kaplan I et al. : Stereotactic body radiotherapy for localized prostate cancer: pooled analysis from a multi-institutional consortium of prospective phase II trials. Radiother Oncol, 109: 217, 2013 [DOI] [PubMed] [Google Scholar]
- 17.Yu JB, Cramer LD, Herrin J et al. : Stereotactic body radiation therapy versus intensity-modulated radiation therapy for prostate cancer: comparison of toxicity. J Clin Oncol, 32: 1195, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hayes JH, Ollendorf DA, Pearson SD et al. : Observation versus initial treatment for men with localized, low-risk prostate cancer: a cost-effectiveness analysis. Ann Intern Med, 158: 853, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sanda MG, Chen RC, Crispino T et al. : Clinically localized prostate cancer: AUA/ASTRO/SUO guideline, 2017. https://www.auanet.org/guidelines/clinically-localized-prostate-cancer-new-(aua/astro/suo-guideline-2017). Accessed June 8, 2017.
- 20.Pan H, Simpson DR, Mell LK et al. : A survey of stereotactic body radiotherapy use in the United States. Cancer, 117: 4566, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix 2. The proportion of SBRT use among networks performing SBRT over time
Abbreviations: SBRT, stereotactic body radiation therapy
*Includes both existing and new SBRT adopters since 2007 is the first year of the study.
The x-axis represents the proportion of SBRT used in SBRT physician-hospital networks. Since 2007 was the first year of the study period, we could not distinguish new and existing SBRT users (*). In subsequent years, we distinguished networks that were existing users of SBRT (black) from new adopters of SBRT (gray).