Abstract
Structurally unprecedented antibacterial alkaloids containing multiple electron-rich pyrrole units have recently been isolated from Curvularia sp. and Bipolaris maydis fungi. This article documents the evolution of a synthetic program aimed at accessing the flagship metabolites curvulamine and curindolizine which are presumably a dimer and trimer of a C10N biosynthetic building block, respectively. Starting with curvulamine, we detail several strategies to merge two simple, bioinspired fragments, which while ultimately unsuccessful, led us toward a pyrroloazepinone building block-based strategy and an improved synthesis of this 10π-aromatic heterocycle. A two-step annulation process was then designed to forge a conserved tetracyclic bis-pyrrole architecture and advanced into a variety of late-stage intermediates; unfortunately, however, a failed late-stage decarboxylation thwarted the total synthesis of curvulamine. By tailoring our annulation precursors, success was ultimately found through the use of a cyanohydrin nucleophile which enabled a 10-step total synthesis of curvulamine. Attempts were then made to realize a biomimetic coupling of curvulamine with an additional C10N fragment to arrive at curindolizine, the most complex family member. Although unproductive, we developed a 14-step total synthesis of this alkaloid through an abiotic coupling approach. Throughout this work, effort was made to harness and exploit the innate reactivity of the pyrrole nucleus, an objective which has uncovered many interesting findings in the chemistry of this reactive heterocycle.
Graphical Abstract

Introduction:
Owing to a variety of disparate chemical architectures and often unique chemical reactivity, pyrrole-containing alkaloids have captivated practitioners of total synthesis for many decades.1 The high acid sensitivity and oxidative fragility inherent to electron-rich pyrroles, however, places many additional constraints on the employable tactics used to access these fascinating natural products.2 In 2014, Tan and co-workers isolated the unusual, electron-rich bis-pyrrole-containing natural product curvulamine (1) from Curvularia sp. IFB-Z10 fungus isolated from the intestinal tract of the white croaker fish (Figure 1).3 The white croaker can feed on dead and decaying prey, suggesting its gut flora may harbor unique antimicrobial producing organisms, and indeed 1 was reported to possess notable antibacterial activity against a small panel of both gram positive and negative pathogens including V. parvula, B. Vulgatus, and Streptococcus and Peptostreptococcus sp. Serendipitously, when scaling up the fungal fermentation of 1, a new, even more complex alkaloid, namely curindolizine (2), was subsequently isolated.4 Interestingly, 2 did not possess the antibiotic activity of 1, but instead demonstrated anti-inflammatory properties in lipopolysaccharide-induced macrophages. More recently, genome mining efforts have culminated in the discovery of additional curvulamine-type secondary metabolites known as bipolamines (see 3-10), all produced by the fungal strain Bipolaris Maydis (Figure 1).5
Figure 1.

Fungal-derived polypyrrole alkaloids.
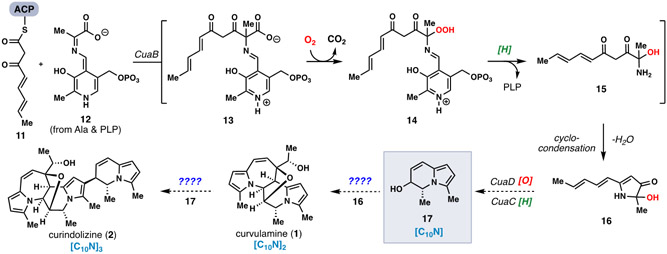
The chemical structures of 1 and 2 (and 5-10) bear little resemblance to known alkaloids and suggest the involvement of unique biosynthetic machinery. Initial isotopic feeding experiments in Curvularia sp. pointed to a mixed polyketide/amino acid origin for 1, wherein two molecules of alanine and eight acetyl-CoA units serve as building blocks.3 Recently, Tan and co-workers discovered the biosynthetic gene cluster responsible for the synthesis of C10N fragment 16, a potential monomeric building block for this family, in Bipolaris maydis (Figure 2).5 A Pyridoxal phosphate (PLP)-dependent enzyme CuaB merges polyketide fragment 11 with alanine/PLP condensation product 12 resulting in intermediate 13 after C─C bond formation. CuaB also possesses oxygenase activity, catalyzing the decarboxylative oxygenation of 13 to 14 and ultimately 15 following co-factor release and hydroperoxide reduction. Aminodiketone 15 then undergoes spontaneous cyclo-condensation to produce isolable vinylogous lactam 16. Two enzymes, an oxidase CuaD, and a reductase CuaC, are then assumed to process this material into hypothetical indolizidine 17, via an epoxidation of the polyene (via CuaD) and a postulated C─N bond-forming reductive cyclization which opens the epoxide (via CuaC).6 The latter step also requires trans to cis isomerization of the central alkene. While the structural resemblance between 17 and bipolamines A (3) and B (4) is clear, the enzymology behind the conversion of this C10N unit into higher order (C10N)2- and (C10N)3-containing natural products such as 1, 2, and 5-10 remains mysterious. Presumably 17 merges with 16 (or an oxidized variant) to produce (C10N)2 metabolites and a further reaction with 17 generates 2, the lone (C10N)3-level structure.
Figure 2.
Tan’s investigations into the early stages of polypyrrole alkaloid biosynthesis in Bipolaris maydis.
We recently reported a chemical synthesis of (−)-curvulamine, the first reported synthetic work toward any of these alkaloid natural products.7 Our successful route to 1, while concise, required intensive experimentation as we navigated multiple strategic dead-ends and detours, unanticipated chemical reactivity, and unpredictable compound stability. Herein we document the evolution of a synthetic strategy toward (−)-curvulamine as well as the first total synthesis of the most complex family member (+)-curindolizine.
Results and Discussion
Initial Synthetic Planning.
In 2015, we began our synthetic investigations with little information regarding the biosynthesis of these alkaloids, although we found it highly plausible that a building block similar to 17 might merge with a linear C10N fragment to forge the carbocyclic core of 1. We initially devised a hypothetical annulation between dianion 18 and dication 19 to construct the 7-membered ring present in higher order members (see inset, Scheme 1). In this coupling, which we evaluated in sequential fashion, the innate nucleophilicity of the pyrrole anion and an enolate would be leveraged directly. We also anticipated that dication-like reactivity could be accessible via twofold activation of a suitable indolizidine diol intermediate.
Scheme 1. First-generation approach to curvulamine via the merger of bioinspired fragments.a.
aReagents and conditions: (a) 20 (1.5 equiv), 21 (1.0 equiv), MgBr2•OEt2 (3.0 equiv), 2,6-DTBP (1.1 equiv), DCM, 0 °C, 12 h, 43% (10:1 dr); (b) SEMCl (2.0 equiv), EtN(i-Pr)2 (4.0 equiv), DCE, 40 °C, 12 h, 91%; (c) TBAF (1.0 M in THF, 1.1 equiv), THF, 50 °C, 12 h, 89%; (d) Pd(PPh3)4 (0.05 equiv), K2CO3 (2.0 equiv), MeOH, 100 °C, 4 h, 53%; (e) NBS (1.05 equiv), DMF, 0 °C, 1 h, used without purification; (f) NaH (4.0 equiv), DMF, 0 °C, 5 min, then add CDI (3.0 equiv), 25 °C, 30 min, 82% (2 steps); (g) NaH (4.0 equiv), DMF, 0 °C, 30 min then add CDI (1.1 equiv), 0 °C, 1 h, 23%; (h) 25 (1.0 equiv), 27 (1.5 equiv), SnCl4 (1.0 equiv), DCM, −78 °C, 3 h, 45% (10.6:3.4:1.5:1.0 dr); (i) MsCl (1.1 equiv), Et3N (1.2 equiv), DCM, 0 °C, 1 h, 81%; (j) NaOMe (5.0 equiv), MeOH, 0 °C, 1 h, 53%. TIPS = triisopropylsilyl, DTBP = di-tert-butylpyridine, SEM = 2-(trimethylsilyl)ethoxymethyl, DCE = 1,2-dichloroethane, TBAF = tetrabutylammonium fluoride, NBS = N-bromosuccinimide, DMF = dimethylformamide, CDI = 1,1'-carbonyldiimidazole, TES = triethylsilyl.
We began our bioinspired approach by first developing a robust route to an indolizidine coupling partner (Scheme 1). Enol ether 20 and aldehyde 21 were merged via a diastereoselective magnesium bromide-mediated Mukaiyama aldol reaction generating oxonium ion 22 that was immediately attacked by the pendant electron-rich pyrrole leading to 23 in 40% yield (10:1 dr).8 Diol 24 was obtained after sequential functional group interconversions in which the secondary alcohol was protected (SEMCl, DIPEA), the TIPS group removed (TBAF), and the allyl group cleaved (Pd(0), K2CO3, MeOH). As testament to the high reactivity of this pyrrole-containing nucleus, simple diol activation with carbonyldiimidazole (CDI) generated noticeable quantities of imidazole 26. Presumably, benzylic ionization generated an azafulvene structure which was attacked with imidazole adjacent to the nitrogen atom and further rearomatized via loss of water. This deleterious process could be averted though by first brominating the pyrrole ring (NBS) prior to activation with CDI. Through this sequence cyclic carbonate 25, whose structure was confirmed by X-ray crystallographic analysis, could be obtained in good yield.
With indolizidine 25 in hand, attempts were then made to realize the designed annulation process (Scheme 1). We found that 25 could be coupled with pyrrole-containing silyl enol ether 27 (prepared in 3-steps)9 via SnCl4-mediated activation of the cyclic carbonate (25) which delivered 28 as an inseparable mixture of diastereomers in 55% yield.10,11 With the first crucial bond formed, effort was directed toward intramolecular C-N bond formation to close the seven-membered ring. While 28, could be activated (MsCl, Et3N) and the pyrrole anion unveiled (NaOMe, MeOH), we have never elucidated a productive cyclization transformation to afford 30. In many instances, unwanted reactivity stemming from the methyl ketone was observed. For instance, treating 29 with TBAF in THF generated cyclopropane 31 and products of O-alkylation of the methyl ketone enolate. While this reactivity could be potentially averted by reduction or protection of the carbonyl, attempts to realize seven-membered ring closure of ketone analogs was also unsuccessful. Moreover, the conversion of 29 to alkene 32 also occurred under mild conditions (Cs2CO3, MeCN, 0°C).12
A Revised Indolizidine-Based Approach.
Our inability to form a carbon-nitrogen bond via intramolecular displacement led us to consider a revised orchestration of events, wherein the C─N bond would be constructed through an intermolecular coupling and the 7-membered ring constructed via a 7-endo radical cyclization (see inset, Scheme 2A). We believed the reactivity of hypothetical vinylcation 33 could be replicated by a vinyl triflate (or halide) precursor.
Scheme 2. Revised indolizidine-based route to 1.a.
aReagents and conditions: (a) 2,6-lutidine (1.0 equiv), AcOH (1.0 equiv), PhMe, 150 °C, 15 min, 50%; (b) NaHMDS (2.0 equiv), THF, −78 °C, 30 min, then add PhNTf2 (1.2 equiv), −78 °C, 1 h, 90%; (c) 2,6-dimethylpyrrole (1.5 equiv), Pd2(dba)3 (0.05 equiv), t-BuXPhos (0.1 equiv), NaOt-Bu (1.4 equiv), PhMe, 60 °C, 12 h, 60%; (d) 40 (1.0 equiv), NaHMDS (1.1 equiv), THF, −78 °C, 30 min, then add 41 (1.1 equiv), −78 °C, 1 h, 82%; (e) DIBAL (5.0 equiv), PhMe, −78 °C, 1 h, then add SiO2, DCM, 25 °C, 30 min, 31%; (f) MVK (3.0 equiv), GII (0.01 equiv), CuI (0.02 equiv), DCM, 50 °C, 1 h, 93%; (g) 1M NaOH (2.0 equiv), 50% H2O2 (2.0 equiv), MeOH, 0 to 25 °C, 3 h, 81%; (h) Cp2TiCl2 (1.0 equiv), Zn0 (8.0 equiv), THF, 25 °C, 30 min, 88%; (i) (Ir[dF(CF3)ppy]2(dtbpy))PF6 (0.01 equiv), PhSH (1.1 equiv), p-toluidine (0.5 equiv), MeCN, blue LED irradiation, 25 °C, 3 h, 74% (46:47 ~ 1:1). Tf = trifluoromethanesulfonyl, NaHMDS = sodium 1,1,1-trimethyl-N-(trimethylsilyl)silanaminide, MVK = methyl vinyl ketone, TMS = trimethylsilyl, dba = dibenzylideneacetone, t-BuXPhos = 2-di-tert-butylphosphino-2′,4′,6′-triisopropylbiphenyl, GII cat. = Grubbs 2nd generation catalyst = (1,3-Bis(2,4,6-trimethylphenyl)-2-imidazolidinylidene)dichloro(phenylmethylene)(tricyclohexylphosphine)ruthenium, (Ir[dF(CF3)ppy]2(dtbpy))PF6 = [4,4′-Bis(1,1-dimethylethyl)-2,2′-bipyridine-N1,N1′]bis[3,5-difluoro-2-[5-(trifluoromethyl)-2-pyridinyl-N]phenyl-C]Iridium(III) hexafluorophosphate.
Efforts to realize this revised plan were facilitated by the observation that previously employed diol 24 underwent a facile redox-neutral isomerization to generate ketone 34 under mildly acidic conditions via an ionization/1,2-hydride shift pathway (Scheme 2A). The ketone formed (34), could then be converted to vinyl triflate 35 (NaHMDS, PhNTf2) setting up the key pyrrole C-N bond-forming event. While a model pyrrole (i.e 2,5-dimethylpyrrole) successfully coupled with 35 under Pd-catalyzed cross-coupling conditions to generate 36,13 we could not extend this transformation to more complex pyrroles featuring extended side chains such as 37-39.14
Undeterred by these findings, we re-tooled our initial aldol-based synthesis of the indolizidine core to incorporate a fragment which already contains a pyrrole C─N bond (Scheme 2B). Deprotonation of pyrrole ester 40 (NaHMDS) followed by addition of aldehyde 41 generated adduct 42 in good yield (82%). Careful DIBAL reduction of this material then furnished an intermediate aldehyde, which then underwent cyclization and dehydration to 43 when exposed to silica gel. Finally, ruthenium-catalyzed cross metathesis of this material with methyl vinyl ketone generated cyclization precursor 44.
With 44 secured, we commenced attempts to construct the key 7-membered ring via radical cyclization.15 Reductive radical cyclizations initiated at the pendant enone with M/HSiR3 (M = Fe, Co)-based systems led to reduction without cyclization.16 While an epoxide (see 45) could be generated from 44 (H2O2, NaOH), attempted titanocene-mediated cyclizations only returned enone 44.17 The addition of a thiyl radical to initiate radical cyclization was also explored under photoredox conditions,18 but these conditions instead promoted a rather facile, yet non-stereoselective, [2+2] cycloaddition to generate cyclobutanes 46 and 47 in 74% combined yield.19
Initial Forays Into a Pyrroloazepinone Fragment-Based Synthesis.
Faced with an inability to form both of the key bonds needed for a successful indolizidine annulation-based approach, we adjusted our retrosynthesis to include a pre-existing 7-membered ring (see inset, Scheme 3). Specifically, we considered the convergence of a 5,7-fused pyrrole-containing synthetic unit (see 48) with fragment 49, an alternative retrosynthetic strategy for carbocyclic construction of curvulamine’s core, lending to a potentially more facile six-membered ring-forming annulation. Nevertheless, the precise oxidation state of 48 and the reaction types capable of realizing this annulation remained open questions; ultimately, only through significant experimentation and reactivity reconnaissance gathering did a successful route to 1 emerge along these lines.
Scheme 3. Towards a pyrroloazepinone-based route to 1.a.
aReagents and conditions: (a) Et3N (1.5 equiv), TMSOTf (1.1 equiv), DCM, 0 °C, 30 min, used without purification; (b) Pd(dba)2 (0.05 equiv), Pt-Bu3 (0.1 equiv), ZnF2 (1.5 equiv), CsF (1.5 equiv), DMF, 70 °C, 5 h, 29% (1:1 dr) (40% BRSM); (c) LiHMDS (1.2 equiv), THF, −78 °C, 30 min, then add 56 (1.1 equiv), −78 °C, 3 h, 62%; (d) nBu3SnLi (1.2 equiv), THF, −78 °C, 1 h, then add 56 (1.1 equiv), −78 °C, 3 h, 44%; (e) 57 (1.1 equiv), 58 (1.0 equiv), Pd2dba3 (0.05 equiv), TFP (0.2 equiv), CuDPP (1.1 equiv), THF, 60 °C, 1.5 h, 67% or 57 (1.1 equiv), 59 (1.0 equiv), Pd2dba3 (0.05 equiv), TFP (0.2 equiv), CuDPP (1.1 equiv), THF, 60 °C, 1.5 h, 37%; (f) 60 (1.0 equiv) , PPTS (0.5 equiv), PhMe, 60 °C, 1.5 h, 91% (4:1 dr) or 61 (1.0 equiv) , (TMS)3SiH (1.5 equiv), BEt3 (1.1 equiv), O2, DCM, −78 °C, 2 h, 43% (6:1 dr). LiHMDS = lithium 1,1,1-trimethyl-N-(trimethylsilyl)silanaminide, dba = dibenzylideneacetone, TFP = tri(2-furyl)phosphine, DPP = diphenylphosphinate.
We initiated our investigations using enone 50, a known Robinson annulation product of 2-formylpyrrole and methyl vinyl ketone.20 To construct one of the two key C─C bonds in the revised annulation, we turned toward Pd-catalyzed enolate arylation. Enone 50 could be converted to sensitive trimethylsilyl enol ether 51 and this material merged with pyrrole iodide 52 to generate 53.21 However, further manipulation of this material proved challenging; we were unable to produce dienone 54 through Saegusa-Ito or related enolate oxidation processes.
Given these setbacks, we found it prudent to investigate installation of the alternative key C─C bond first (Scheme 3). Unlike 54, enone 50 could be oxidized to pyrroloazepinone 55 using Mukaiyama’s desaturation reagent N-tert-Butylbenzenesulfinimidoyl chloride (56). Pyrroloazepinones such as 55 – uncommon 10π-aromatic heteroaromatics – are infrequently used in synthesis and their reactivity is largely unexplored.22 While we did not know it at the time, this heterocycle proved key in the development of a concise route to 1.
Pyrroloazepinone 55 underwent productive conjugate addition with a variety of nucleophiles (vide infra). Initially we found that tributylstannyllithium (LDA, HSnBu3) was readily added and that the resulting enolate could be directly oxidized with 56 to produce stannane 57 (Scheme 3). We envisioned that this fragment could be merged with a pyrrole-containing thioester via Liebeskind-Srogl coupling. Experimentally, we discovered that 56 could be merged with thioester 58 and iodo-variant 59 in 67% and 37% yield respectively.23 The products formed, namely enediones 60 and 61, lacked only a single C─C bond for advancement into the tetracyclic scaffold of 1. However, upon treating dienone 60 with a variety of Lewis acids, we observed exclusive formation spirocycle 62 as a mixture of isomers. Attempts to elicit direct, palladium-catalyzed dehydrogenative coupling of 60 were also complicated by this Lewis-acid catalyzed background reaction. Employing iodide 61 and changing the reaction manifold to one based on free radicals (i.e. Bu3SnH/AIBN or (TMS)3SiH/Et3B/O2) did not change the outcome as spirocyclic enone 62 was again generated.
While the two strategies in Scheme 3 were ultimately dead-ends, we were keen to survey additional nucleophilic partners in the coupling with a pyrroloazepinone heterocycle. An initial impediment to such endeavors was the difficulty in preparing large quantities of 55. Specifically, enone 50 (prepared using a two-step Robinson annulation), was produced in yields ranging from only ~20-25% and large quantities of reagent 56 was also required to produce 55.24
Given this backdrop, we investigated alternatives routes to this class of heterocycles (Figure 3). Inspired by the work of Flitsch,22 we developed a straightforward synthesis using Boc-protected 2-formylpyrroles, ubiquitous Vilsmeier-Haack formylation products, as starting materials (Figure 3). The enolate of vinylogous ester 64 was first added to pyrrole 63 resulting in direct formation of dienone 65 through an aldol/Boc-migration/E1cB elimination pathway. With access to dienone 65 we evaluated cyclization conditions to produce the 10π aromatic nucleus (see Table, Figure 3). We sought to avoid flash vacuum pyrolysis and other low-yielding cyclization conditions observed in the cyclization of related vinylogous amides.22
Figure 3.

Two-step synthesis of novel pyrroloazepinones.
Dienone 65 could form pyrroloazepinone 66 under mild conditions (Cs2CO3, 25 °C), but the yield of this cyclization was low (<20%, see entries 1-2). Microwave-induced thermal cyclizations employing amine bases proved more efficient, and utilizing DBU we were able to prepare 66 in 60% yield (entry 5) – notably these conditions were also scalable and enabled multi-gram procurement of this key building block.25,26
Next, we surveyed these cyclization conditions for the synthesis of novel, substituted pyrroloazepinones (see 67-73, Figure 3). Previously prepared heteroaromatic 55 was produced in comparable yield to 66 (64% cyclization yield) as was isomeric methyl-containing substrate 67. Importantly, 67 also demonstrates that ketones, and not just aldehydes, can be used in this methodology. Indole (see 68) and tetrahydroindole (see 69) units could also be incorporated although cyclization yields were somewhat diminished (40-43%). Aryl substituents were also tolerated on the pyrrole ring, leading to products 70-73.
Exploring a Dearomative Approach.
With a robust synthesis of pyrroloazepinone 66 in hand, we proceeded to evaluate a carbon-based nucleophile in the dearomative conjugate addition reaction (Scheme 4). Much to our delight, we observed that the potassium enolate of pyrrole-containing ester 74 could be prepared, and in the presence of HMPA, added to 66 to yield coupled product 75 in 54% yield and with 5:1 diastereoselectivity at C-3. While 66 possesses several sites for Michael-type attack (see C1, C9, and C5), under these conditions selectivity for the C5 position was observed.27 Notably the stereochemical relationship between the methyl group and the stereocenter on the 7-membered ring are correct for advancement into 1. We assumed (albeit incorrectly) that the C-3 ester could be easily removed later in the synthesis by decarboxylative methods (vide infra).
Scheme 4. Successful dearomative C─C bond formation leads to unexpected pyrrole reactivity.
aReagents and conditions: (a) (±)-74 (1.4 equiv), KHMDS (1.4 equiv), THF, −78 °C, 30 min, then add HMPA (5.0 equiv), 66 (1.0 equiv), −78 °C, 2 h, 54% (5:1 dr); (b) ethyl vinyl ether (7.5 equiv), t-BuLi (7.0 equiv), THF, −78 °C to 0 °C, 30 min; 75 (1.0 equiv), CeCl3 (1.0 equiv), LiCl (2.0 equiv), 0 °C, 30 min, then add lithiated ethyl vinyl ether, −78 °C, 1 h, then add 1M HCl, 25 °C, 30 min, 72% (2.6:1 dr); (c) 5M NaOH (2.0 equiv), THF, 60 °C, 1 h, used without purification; (d) MsCl (5.0 equiv), Et3N (10.0 equiv), DCM, 0 °C, 15 min, then add N-hydroxyphthalimide (2.2 equiv), 0 °C, 30 min, 35% (2 steps); (e) Cu(acac)2, (2.0 equiv), B2Pin2 (1.5 equiv), LiOH•H2O (15.0 equiv), MgCl2 (1.2 equiv), Dioxane/DMF = 5:1 (v:v), 25 °C, 2 h, used without purification; (f) NaBO3•H2O (5.0 equiv), THF/H2O = 1/1, 25 °C, 30 min, 17% for 2 steps; (g) p-TsOH (0.5 equiv), DCM, 25 °C, 85%. KHMDS = potassium bis(trimethylsilyl)amide, HMPA = hexamethylphosphoramide, acac = acetylacetonate, B2Pin2 = 4,4,4′,4′,5,5,5′,5′-Octamethyl-2,2′-bi-1,3,2-dioxaborolane.
Adopting the conditions of Knochel,28 a cerium-mediated 1,2-addition of lithiated ethyl vinyl ether into enone 75 produced methyl ketone 76 as a mixture of diastereomers (72%, 2.6:1 dr) after acidic hydrolysis (aq. HCl) of the enol ether (Scheme 4). We had hoped that treating compound 76 with base would transiently generate lactone 77 which in turn could undergo an E2 elimination, thus generating enone 78. An intramolecular addition of the pyrrole to this newly generated Michael acceptor would then produce 79, the key tetracyclic core of all (C10N)2 members. Subjecting 76 to sodium hydroxide at slightly elevated temperature followed by an acidic quench led to a much different outcome however, wherein a rather remarkable dearomatized enamine (81) containing a 5,7,5,7-fused ring system was produced. We speculate that 77 was indeed formed, but the electron-rich vinyl pyrrole nucleus expelled the carboxylate leaving group forming an extended azafulvenium ion (80). Interestingly, this electrophile was attacked by the second pyrrole at a substituted carbon center leading to pyrrole dearomatization and formation of a quaternary center. While related enamines are highly unstable,2 conjugation to the electron-withdrawing ketone stabilizes this structure. Conversion of 81 to NHPI ester 82 (MsCl, Et3N, N-Hydroxyphthalimide) then allowed for a copper-mediated decarboxylative borylation and oxidation furnishing alcohol 83 whose structure was confirmed by single Crystal X-ray diffraction.29 While these unoptimized transformations were low-yielding, this work suggested that the extraneous carbon atom added in the dearomative conjugate addition could be removed to yield a hydroxyl group.
We also briefly explored the reactivity of intermediates stemming from the minor diastereomer of 75 (see inset, Scheme 4). Compound 84 could be formed analogously to 76. Interestingly, treatment of 84 with para-toluenesulfonic acid generated putative azafulvenium ion 85 which was also quenched through intermolecular attack from the pendant pyrrole to afford 86. Fascinatingly, the sole product formed (in 85%) in this cyclization, however, was tetracycle 86 whose notable diazabicyclo[4.4.1]undecane core was confirmed crystallographically.30
While neither of these two pathways yielded the desired tetracyclic core of 1, we felt that the initial conjugate addition reaction with heterocycle 66 was a promising route for further exploration and that a decarboxylation reaction could be employed late-stage. In reality, these assumptions proved partially correct and significant experimentation remained to clarify a workable pathway forward
Successful Synthesis of the Curvulamine Core Leads to a Difficult Decarboxylation.
Our inability to close the six-membered ring via polar, pyrrole cyclization approaches led us to consider radical-based methods (Scheme 5). Specifically, our dearomative conjugate addition furnished an enolate which we believed could be oxidized to a radical. Unfortunately, the addition of common single-electron oxidants to this coupling reaction did not lead to pyrrole C-2 functionalization despite literature precedent suggesting its feasibility.31 We could however quench the coupling of 66 and 74 with NIS or I2 leading to iodide 87 (49% combined yield) and thus allowing for a broader survey of reaction conditions to be explored (Scheme 5).32 While photoredox chemistry was explored with some success,7 control experiments found that simple irradiation of 87 (390 nm light) forged compound 88 in good yield (70%) thus accomplishing our first successful synthesis of the curvulamine tetracycle. While unknown at the time, substantial challenges awaited.
Scheme 5. Successful synthesis of the curvulamine core.
aReagents and conditions: a) (±)-74 (1.4 equiv), KHMDS (1.4 equiv), THF, −78 °C, 30 min then add HMPA (5.0 equiv), 66 (1.0 equiv), −78 °C, 2 h, then add I2 (1.1 equiv), −78 °C, 30 min, 49% (5:1 dr); (b) Na2CO3 (5.0 equiv), MeOH, Kessil Lamp KSPR160 (390nm, 40 W), 25 °C, 1 h, 70%; (c) 88 (1.0 equiv), CeCl3 (2.0 equiv), THF, 0 °C, 30 min, then add vinylMgBr (2.5 equiv), 30 min, −78 °C, 1 h, 37%; (d) 88 (1.0 equiv), CeCl3 (2.0 equiv), THF, 0 °C, 30 min, then add EtMgBr (1.05 equiv), −78 °C, 1 h, 60%; (e) KHMDS (2.0 equiv), 18-crown-6 (2.0 equiv), THF, −78 °C, 30 min, then add Davis oxaziridine (1.2 equiv), −78 °C, 30 min, 45%; (f) DIBAL (1.2 equiv), DCM, −78 °C, 30 min, 52%; (g) Me3OBF4 (1.5 equiv), proton sponge (3.0 equiv), DCE, 90 °C, 12 h, 50%; (h) 1M KOH (3.0 equiv), MeOH, 90 °C, 12 h, 15%; (i) DIBAL (2.5 equiv), DCM, −78 °C, 30 min, 82%; (j) Cu[MeCN]4OTf (0.1 equiv), MeObpy (0.1 equiv), NMI (0.1 equiv), ABNO (0.05 equiv), O2, MeCN, 25 °C, 30 min, 75%; (k) MeMgBr (1.1 equiv), THF, −15 °C, 30 min, 71%; (l) Cu[MeCN]4OTf (0.1 equiv), MeObpy (0.1 equiv), NMI (0.1 equiv), ABNO (0.05 equiv), O2, MeCN, 25 °C, 30 min, 55%. 18-crown-6 = 1,4,7,10,13,16-Hexaoxacyclooctadecane, MeObpy = 4-4′-dimethoxy-2-2′-bipyridine, NMI = 1-methylimidazole, ABNO = 9-azabicyclo[3.3.1]nonane N-oxyl.
With tetracyclic enone 88 in hand, a variety of endgames were envisioned all of which required a C─C bond cleavage event to excise the extraneous C-3 carbon atom of the ester (Scheme 5). Initially, we added excess vinyl magnesium bromide to 88 producing double addition product 89. We had hoped that the array of known methods to generate alkoxy radicals would allow for facile C─C bond β-cleavage of the desired bond shown in green and further radical functionalization. Unfortunately, Suárez conditions (PIDA, I2, hv) single-electron oxidants (FeCl3, AgNO3/K2S2O8/NIS) and a variety of other reagents (Ir(III)/hv, CeBr3/O2/hv) failed to provide detectable quantities of C─C bond cleaved products – only decomposition was typically observed. Attempts to convert the tertiary alcohol into a hydroperoxide for iron-induced C─C bond cleavage were also unproductive.33,34
To promote the C─C cleavage process, we reasoned that introduction of an additional hydroxy group (thus constructing a 1,2-diol motif) might allow for a wider reactivity window to be explored. Carefully controlled addition of ethylmagnesium bromide mediated by CeCl3 allowed for the conversion of 88 into lactone 90. Remarkably, this material could be deprotonated (KHMDS, 18-crown-6) at the bridgehead position allowing for hydroxylation with Davis’ oxaziridine. The α-hydroxylactone formed (91) could then be reduced with DIBAL to generate α-hydroxy acetal 92. Much to our dismay however, standard diol cleavage conditions (NaIO4, Pb(OAc)4, H5IO6) were all incompatible with this sensitive substrate leading only to intractable mixtures.
We then decided to explore the chemistry of a conformationally distinct ester intermediate, finding that alkylative lactone opening of 90 (Me3OBF4, proton sponge, Δ) furnished methyl ester 93 in moderate yield.35 Given the breadth and renaissance in decarboxylative functionalization chemistry, we first looked toward hydrolysis of methyl ester to prepare carboxylic acid 94. The hydrolysis of this quite hindered ester proved challenging, resulting in only 15% yield of acid 94 along with extensive decomposition.36 Nevertheless, we were able to explore several strategies with this limited amount of material. Similar to substrate 89, direct decarboxylative functionalization of the free acid under radical generating conditions (i.e. Suárez chemistry, Ag(I) salts, Pb(OAc)4, photoredox) did not lead to isolable products. We had hoped that Barton’s decarboxylative oxygenation might allow for a peroxidation cascade an analogy to our prior work on terpene scaffolds;37,38 while a Barton ester could be formed in trace quantities, instant decomposition was observed during its activation in the presence of oxygen. Finally, decarboxylative borylation/oxidation, which had been used to construct 83 in low yield (Scheme 4), was unsuccessful in this setting under a variety of conditions.29,39
As a last-ditch effort, we attempted to remove this recalcitrant carbon atom from multiple different carbonyl bearing intermediates (Scheme 5). Ester 93 could be reduced cleanly with DIBAL to generate a primary alcohol which could be oxidized to an aldehyde using Stahl’s mild copper-catalyzed conditions.40 Unfortunately, attempts at performing Iwasawa’s cobalt salen-catalyzed cleavage reaction of an in-situ generated hydroperoxide led to extensive decomposition.41 In our final foray, we turned to the tried-and-true Baeyer-Villiger oxidation. While methyl ketone 96 could be synthesized from aldehyde 95 in two steps, this substrate was not compatible with peracids likely as a result of the vinyl pyrrole nucleus (vide infra).
The results in Scheme 5 were both encouraging and frustrating as is often the case during a total synthesis. While a viable strategy to the elusive curvulamine tetracycle had emerged, it was clear that alternative nucleophiles in the conjugate addition would need to be examined if we were to synthesize 1 by this strategy.
Reactivity of Alternative Nucleophiles in the Dearomative Conjugate Addition.
We undertook a fairly comprehensive study of various carbanions and their reactivity with our key pyrroloazepinone (see 66+97→98, Figure 4). Although we had not succeeded in decarboxylating acid 94, the extremely low yield in preparing this material from ester 93 made us examine free carboxylic acid 99 in the dearomative coupling (Figure 4). We attempted to generate the dianion of 99 using an excess of strong base (KHMDS) and found that a new product was formed after the addition of 66. Much to our surprise the structure of the “coupled product” turned out to be dimer 100 whose structure was secured by X-ray crystallography. Excess base apparently deprotonated the acidic methyl group of 66, forming an anion which added in a conjugate fashion to another molecule of 66 resulting in formal annulation after a second, intramolecular Michael addition.
Figure 4.

Examination of alternative nucleophiles in the conjugate addition to pyrroloazepinone 66.
A lithiated carbamate strategy was also briefly explored as this would correctly incorporate a masked hydroxyl group at the desired position.42 Deprotonation of 101 (s-BuLi) followed by addition of 66 generated several new products which where most easily analyzed following lithium aluminum hydride reduction of the carbamate. To our surprise we found that tetracycle 102, formed as a mixture of diastereomeric spirocycles, was the major product. This product ostensibly stems from lithiate 1,2-addition followed by ionization of the highly labile tertiary alcohol to form an aromatic carbocation which is trapped by the electron-rich pyrrole.
Next, we considered the use of a metalated nitrile owing to the possibility for downstream reductive decyanation/functionalization.43 The anion derived from 103 underwent coupling with 66 to generate coupled product 104 (40%) whose structure was confirmed by X-ray crystallographic analysis. Notably the stereochemical configuration of the methyl group (C2) is opposite to that found in curvulamine and would have to be corrected at some point. In addition to 104, we also observed substantial amounts (38%) of spirocycle 105 in analogy to 102. In addition to these “successful” experiments, a number of other pyrrole-containing nucleophiles (see 106-108) were examined but did not lead to C─C bond formation with 66. In addition, we attempted to generate the ketyl radical of 41 in the presence of 66, but did not observe any productive coupling.
Total Synthesis of Curvulamine.
Given the successful coupling of nitrile 103, we chose to evaluate one final nucleophile – a cyanohydrin – which are known to undergo 1,4-addition reactions.44 Ultimately this decision proved critical in enabling the first total synthesis of curvulamine (Scheme 6).7 Unlike 74, deprotonation of cyanohydrin 110 using KHMDS in the presence of HMPA lead to E1cB of the pyrrole anion, producing a variety of undesired products when reacted with 66.45 A stable carbanion could be made however using NaHMDS in the presence of LiCl; this species added to 66, giving a 64% yield of coupled product 111 (~6:1 dr) after quenching with N-iodosuccinimide. As noted when forming 104, the C2 stereocenter was incorrectly set during this process. From 111, our previously developed photochemical cyclization generated tetracyclic ketone in moderate yield (55%). The cyanohydrin could be easily removed by basic methanolysis to give diketone 113. Interestingly, this compound reacted with lithiated ethyl vinyl ether to give 114, the product of reaction at the incorrect carbonyl group. Satisfyingly, however, treatment of 112 with an excess of the same nucleophile produced lactol 115 bearing the correct connectivity 55% isolated yield.
Scheme 6. Total Synthesis of Curvulamine.

aReagents and conditions: (a) 110 (1.5 equiv), LiCl (5.0 equiv), NaHMDS (1.6 equiv), THF, −78 °C, 30 min then add 66 (1.0 equiv), THF, −78 °C, 1 h then add NIS (1.5 equiv), THF, −78 °C, 5 min (64%); (b) NaHCO3 (5.0 equiv), MeCN/t-BuOH (4:1), Kessil Lamp KSPR160 (390 nm, 40W), 23 °C, ~2 h (55%); (c) Na2CO3 (2.0 equiv), MeOH, 23 °C, 5 min (96%); (d) ethyl vinyl ether (5.5 equiv), t-BuLi (5.0 equiv), THF, −78 °C, 30 min then add 113 (1.0 equiv), −78 °C, 1 h (52%); (e) ethyl vinyl ether (5.5 equiv), t-BuLi (5.0 equiv), THF, −78 °C, 30 min then add 112 (1.0 equiv), −78 °C, 1 h (55%); (f) NaOMe (5.0 equiv.), MeOH, 90 °C, 4 h (85%); (g) KHMDS (1.6 equiv), −78 °C, 30 min; then add phenyl chlorothionoformate (2.0 equiv), DMAP (2.0 equiv), THF, −78 °C to 0 °C, 20 min (50% 117 + 21% 118); (h) HSnBu3 (2.0 equiv), BEt3 (1.0 equiv), O2, THF, 45 °C, 1 h then add aq. HCl, 0 °C, 5 min (40%); (i) (R)-2-methyl-CBS-oxazaborolidine (1.0 equiv), BH3•DMS (2.0 equiv), DCM, 23 °C, 1 h (45% (−)-1 + 45% (+)−12-epi-1; DBU = 1,8-diazabicyclo[5.4.0]undec-7-ene, DMAP = 4-dimethylaminopyridine, NIS = N-iodosuccinimide
With 115 in hand we proceeded to investigate epimerization of the C2 methyl group. We found it plausible that allylic 1,3-strain minimization between the two methyl groups (C1 and C10) might favor the desired stereochemistry. Gratifyingly, basic treatment of 115 (NaOMe, MeOH, Δ) established a 2.3:1 mixture of lactols favoring the epimerized isomer (116) in 85% combined yield.
The next obstacle in the synthesis of curvulamine entailed removal of the bridgehead hydroxyl group comprising the lactol motif. The inseparable, thermodynamic mixture of lactols (115/116) were activated (KHMDS, pyridine, ClCSOPh) gen-erating a mixture of thiocarbonate epimers 117 and 118 (71% yield) which could be separated chromatographically. Of practical importance, the undesired isomer (i.e. 118) could be recycled by one-pot, base-mediated methanolysis/epimerization (NaOMe, MeOH, Δ) redelivering the equilibrium mixture of 115 and 116. Finally, reductive deoxygenation (HSnBu3, BEt3/O2) with concomitant enol ether hydrolysis ofintermediate 119 during acidic workup (aq. HCl) afforded methyl ketone (±)-120 in 40%.
To complete the synthesis of racemic 1 we simply needed to reduce (±)-120 diastereoselectively. A variety of reducing agents (DIBAL, NaBH4, LiBH(Et)3, LiAlH4, and other simple hydrides afforded primarily the undesired secondary alcohol diastereomer, namely (±)-12-epi-1. Intrigued by a possible reagent controlled solution, we exposed our methyl ketone to CBS reduction conditions ((R)-2-Methyl-CBS-oxazaborolidine, BH3•DMS, DCM).46 Interestingly, a near perfect stereodivergent reduction ensued wherein (±)-120 was converted into an easily separable 1:1 mixture of (−)-(1) and (+)-12-epi-1 with 97% and 94% ee respectively (90% overall yield).47,48 Our journey to 1, which was filled with numerous roadblocks and unexpected surprises, was finally complete.
Total Synthesis of Curindolizine.
With a 10-step route to curvulamine in place, we turned our attention toward curindolizine (2), the most complex –and only trimeric (C10N)3 member – in the family (Figure 5). When scaling up a 300 L fermentation of Curvularia sp. IFB-Z10, Tan and co-workers found that 2 was formed in preference to 1.4 It was proposed that curindolizine is produced from the Michael addition reaction between 1 and enone 122 which could be derived from procuramine (123), a simple C10N member present in the fermentation broth. Additionally, when 1 and 123 were exposed to cell lysate containing the intracellular protein fractions, 2 could be detected implicating enzymatic assistance in this coupling. The direct coupling of 1 and 123 still requires a reduction step to generate curindolizine and thus it was not clear to us if nature’s pathway involves the sequence shown in Figure 5 or one involving allylic alcohol 17, the previously speculated biosynthetic intermediate from B. maydis and a possible Friedel-Crafts (rather than Michael) coupling partner (Figure 6). We developed simple chemistry to access both 17 and 124 based largely on our unsuccessful initial attempts to synthesize 1 via bioinspired strategies. An aldol reaction between methyl tert-butyl acetate and aldehyde 41 first generated 124 (82%, 6:1 dr). Mild Friedel-Crafts acylation of this material (TMSOTf) then generated procuramine (123) in 50% yield. Alternatively, 124 could be reduced with DIBAL generating an aldehyde which cyclized and dehydrated in the presence of silica gel to yield 123 (44%). Unfortunately attempts to merge either 17 or 123 with curvulamine (1) under acidic conditions have not yielded coupled products to date (Figure 6). Moreover, reacting 1 with other activated α,β-unsaturated systems such as acrylates or cyclohexanone has not demonstrated the feasibility of productive Michael addition chemistry at the desired pyrrole position – only recovered starting material or decomposition is observed in the presence of Brønsted or Lewis acids. Similarly, attempts at coupling 17with 1 have only led to aromatization of 17 forming 3,5-dimethylindolizine. Against this backdrop, we investigated non-biomimetic strategies to access this complex family member (Scheme 7).
Figure 5.

Tan’s biosynthetic hypothesis regarding the origins of curindolizine.
Figure 6.

Preparation and reactivity of candidate biosynthetic coupling partners.
Scheme 7. Total Synthesis of Curindolizine.

aReagents and conditions: a) NIS (1.05 equiv), acetone, 0 °C, 30 min, 88%; (b) ethyl vinyl ether (5.5 equiv), t-BuLi (5.0 equiv), THF, −78 °C to 0 °C, 30 min, then add CeCl3 (5.0 equiv), 25 °C, 1 h, then add 124, −78 °C, 1 h, then add TMSCl (5.0 equiv), −78 °C, 30 min, 79%; (c) 125 (1.0 equiv), t-BuLi (2.5 equiv), THF, −78 °C, 30 min, then add ZnCl2 (3.0 equiv), −78 °C, 1 h, then add Pd(PPh3)4 (0.03 equiv), (±)-126 (2.0 equiv), 25 °C, 6 h, 80% (127:128 = 1:1); (d) SmI2 (2.0 equiv), THF/MeOH = 9/1, 0 °C, 30 min, then add TBAF (2.0 equiv), 25 °C, 30 min, 80%; (e) NaOMe (5.0 equiv.), MeOH, 90 °C, 1 h, 57% (80% BRSM); (f) KHMDS (1.2 equiv), −78 °C, 30 min; then add DMAP (2.0 equiv), Phenyl chlorothionoformate (2.0 equiv), THF, −78 °C, 1 h, 83%); (g) HSnBu3 (2.0 equiv), BEt3 (1.0 equiv), O2, THF, 45 °C, 1 h, then add aq. HCl, 0 °C, 30 min, 41%; (h) (R)-2-methyl-CBS-oxazaborolidine (1.0 equiv), BH3•DMS (2.0 equiv), DCM, 23 °C, 1 h, 42% (−)-130 + 42% (+)-12-epi-130; (i) DIBAL (5.0 equiv), DCM, −78 °C, 30 min, then add silica gel, 5 min, 70%.
While a logical solution to the coupling problem would be to simply halogenate 1 and explore various C─C bond-forming reactions, the locus of reactivity with electrophilic reagents in this multi-heterocyclic system lies at the vinyl pyrrole group.49 Fortunately, previously prepared tetracycle 112 (prepared in 5 steps on a gram scale) underwent very clean iodination to yield iodide 124 in 88% yield (Scheme 7). Presumably this is the least-hindered position on the more electron-rich pyrrole ring. A Ce(III)-mediated addition of lithiated ethyl vinyl ether to this materialthen then gave 125 after in-situ silylation (TMSCl). Iodide 125 could be converted into an organozinc reagent (t-BuLi then ZnCl2) and subjected to Pd-catalyzed Negishi coupling with racemic triflate 126 to yield a separable mixture of diastereomeric products (see 127 and 128) in 80% combined yield.50 The desired isomer (128) could then be stereoselectively reduced by samarium iodide to generate ester 129 as essentially a single compound. Gratifyingly the relative relationship between this newly formed stereocenter and the adjacent methyl group is as found in 2.51 A similar four-step sequence as the one employed in the synthesis of 1, namely i) thermodynamic equilibration of the methyl-containing stereocenter, ii) thiocarbamate formation, iii) deoxygenation, and iv) CBS reduction proceeded uneventfully in this setting, thereby advancing (±)-129 into (−)-130. In the final step, and in analogy to the preparation of 17, careful DIBAL reduction of this material generated an aldehyde which cyclized directly to (+)-curindolizine (2) in 70% yield, thus completing the first synthesis of this complex trimeric alkaloid in 14 steps.
Discussion.
In this article, we have chronicled the evolution of a total synthetic strategy toward complex polypyrrole alkaloids from Curvularia sp. fungi resulting in a 10-step total synthesis of curvulamine (1) and 14-step route to curindolizine (2). Given their unprecedented chemical structures and mysterious biosynthetic origins, a variety of approaches were brought to bear on this synthetic problem. Throughout each planning stage though, effort was made to explore and exploit the innate reactivity of the electron-rich pyrrole nucleus.52 Indeed, we have observed the pyrrole as a nucleophile, radical acceptor, and electrophile (as part of several azafulvenium ions encountered) in the course of our studies. While many of the synthetic intermediates described herein proved challenging to work with from a technical perspective (i.e. air and acid sensitivity), we were rewarded by beholding their interesting and unexpected reactivity in a novel chemical architecture. During our studies we also developed an improved synthesis of pyrroloazepinones, which should find broader use in heterocyclic chemistry, as well as expanded the knowledge base surrounding the reactivity of this exotic 10π-electron heteroaromatic. Finally, we developed a route to curindolizine, despite being unable to elicit a direct, biomimetic coupling of (C10N)2 and C10N fragments. While not demonstrated herein, we believe our finding can also enable routes to bipolamine-type metabolites as well as facilitate a greater understanding of the antibacterial properties of these alkaloids. Such investigations are underway and will be reported in due course.
Supplementary Material
ACKNOWLEDGMENT
Financial support is acknowledged from the NIH NIGMS (R01GM136945 to T.J.M. and a diversity supplement to P.A.M.). T.J.M. acknowledges unrestricted financial support from Novartis, Bristol-Myers Squibb, Amgen, and Eli Lilly. M. S. thanks the German Research Foundation for a DFG Post-doctoral fellowship. We are grateful to Dr. Hasan Celik and Dr. Jeffrey Pelton for NMR spectroscopic assistance and NIH grant GM68933 as well as Bella Germek for technical assistance. Mr. Edward Miller is acknowledged for assistance with chiral HPLC. Dr. Nicholas Settineri and Jeffrey Derrick are acknowledged for X-ray crystallographic analysis wherein support from NIH Shared Instrument Grant (S10-RR027172) is also acknowledged.
Footnotes
The authors declare no competing financial interests.
REFERENCES
- (1).(a) For a general review, see: Young IS; Thorton PD; Thompson A Synthesis of natural products containing the pyrrolic ring. Nat. Prod. Rep 2010, 27, 1801. [DOI] [PubMed] [Google Scholar]; (b) Mal D; Shome B and Dinda BK Pyrrole and Its Derivatives. In Heterocycles in Natural Product Synthesis.; Majumdar KC and Chattopadhyay SK, Ed.; Wiley, 2011. Ch. 6, 187. [Google Scholar]
- (2).(a) For examples on the extreme end of the pyrrole reactivity scale, see: Schröder F; Franke S; Francke W; Baumann H; Kaib M; Pasteels JM; Daloze D A New Family of Tricyclic Alkaloids from Myrmicaria Ants. Tetrahedron 1996, 52, 13539; [Google Scholar]; (b) Ondrus AE; Movassaghi M Total synthesis and study of myrmicarin alkaloids. Chem Comm. 2009, 4151. [DOI] [PMC free article] [PubMed] [Google Scholar]; (c) Snyder SA; Elsohly AM; Kontes F Synthetic and Theoretical Investigations of Myrmicarin Biosynthesis. Angew. Chem. Int. Ed 2010, 49, 9693. [DOI] [PubMed] [Google Scholar]
- (3).Han WB; Lu YH; Zhang AH; Zhang GF; Mei YN; Jiang N; Lei X; Song YC; Ng SW; Tan RX Curvulamine, a New Antibacterial Alkaloid Incorporating Two Undescribed Units from a Curvularia Species Org. Lett 2014, 16, 5366. [DOI] [PubMed] [Google Scholar]
- (4).Han WB; Zhang AH; Deng XZ; Lei X; Tan RX Curindolizine, an Anti-Inflammatory Agent Assembled via Michael Addition of Pyrrole Alkaloids Inside Fungal Cells. Org. Lett 2016, 18, 1816. [DOI] [PubMed] [Google Scholar]
- (5).Dai GZ; Han WB; Mei YN; Xu K; Jiao RH; Ge HM; Tan RX Pyridoxal-5′-phosphate–dependent bifunctional enzyme catalyzed biosynthesis of indolizidine alkaloids in fungi. Proc. Natl. Acad. Sci. USA 2020, 117, 1174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (6).The nature of the nitrogen nucleophile which opens the epoxide is unclear. Whether the pyrrole is made before or after this event is not known. Compound 17 also represents a postulate, not isolated, intermediate.
- (7).Haelsig KT; Xuan J Maimone TJ Total Synthesis of (−)-Curvulamine. J. Am. Chem. Soc 2020, 142, 1206. [DOI] [PubMed] [Google Scholar]
- (8).(a) For inspiration, see: Northrup AB; Macmillan DWC Two-step Synthesis of Carbohydrates by Selective Aldol Reactions. [DOI] [PubMed] [Google Scholar]; (b) Gati W; Yamamoto H A highly diastereoselective “super silyl” governed aldol reaction: synthesis of α,β-dioxylaldehydes and 1,2,3-triols. Chem. Sci 2016, 7, 394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (9).See the SI for synthetic preparation
- (10).These compounds were quite sensitive to silica gel purification and the individual isomers could not be separated by careful chromatography.
- (11).for the addition of a silyl ketene thioacetal to an azafulvenium ion, see ref. 2b
-
(12).In a model study lacking the pyrrole C2 methyl group, we advanced compound 25 along similar lines as 28 (see SI for synthetic details). Upon unveiling the free pyrrole nitrogen, we found the product (see F1 below) exists as a stable hemiaminal that also thwarted cyclization attempts.

- (13).Movassaghi M; Ondrus AE Enantioselective Total Synthesis of Tricyclic Myrmicarin Alkaloids. Org. Lett 2005, 7, 4423. (14) Tan [DOI] [PMC free article] [PubMed] [Google Scholar]
- (14).The iodo variant of 35 could also be prepared, but this compound also failed to undergo C─N cross-coupling with these more complex pyrroles under Ullman-type conditions.
- (15).(a) for recent reviews on radical cyclizations in total synthesis, see: Romero KJ; Galliher MS; Pratt DA; Stephenson CRJ Radicals in Natural Product Synthesis, Chem. Soc. Rev 2018, 47, 7851; [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Hung K; Hu X; Maimone TJ Total Synthesis of Complex Terpenes Employing Radical Cascade Processes. Nat. Prod. Rep 2018, 35, 174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (16).For a review on this type of chemistry, see: Matos JLM; Green SA; Shenvi RA Chapter 7. Markovnikov Functionalization by Hydrogen Atom Transfer, Organic Reactions, 2019, 100, 383. [Google Scholar]
- (17).Morcillo SP; Miguel D; Campaña AG; Álvarez de Cienfuegos L; Justica J; Cuerva JM Recent applications of Cp2TiCl in natural product synthesis. Org. Chem. Front 2014, 1, 15. [Google Scholar]
- (18).(a) Tyson EL; Ament MS; Yoon TP Transition Metal Photoredox Catalysis of Radical Thiol-Ene Reactions. J. Org. Chem 2013, 78, 2046. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Guerrero-Corella A; Martinez-Gualda AM; Ahmadi F; Ming E; Fraile A; Alemán J Thiol-ene/oxidation tandem reaction under visible light photocatalysis: synthesis of alkyl sulfoxides. Chem. Commun 2017, 53, 10463. [DOI] [PMC free article] [PubMed] [Google Scholar]; (c) Xu J; Boyer C Visible Light Photocatalytic Thiol–Ene Reaction: An Elegant Approach for Fast Polymer Postfunctionalization and Step-Growth Polymerization. Macromolecules 2015, 48, 520. [Google Scholar]; (d) for an example in synthesis, see: Zhang P-P; Yan Z-M; Li Y-H; Gong J-X; Yang Z Enantioselective Total Synthesis of (−)-Pavidolide B. J. Am. Chem. Soc 2017, 139, 13989. [DOI] [PubMed] [Google Scholar]
- (19).compounds 43-45 were also prone to aromatize forming an indolizine heterocycle under slightly acidic conditions.
- (20).Jones G; Radley PM Azonia-azulene salts. Part 5. Synthesis of 5H-pyrrolo[1,2-a]azepine and of 7H-pyrrolo[1,2-a]azepin-7-one. J. Chem. Soc. Perkin Trans 1. 1982, 1123. [Google Scholar]
- (21).Su W; Raders S; Verkade JG; Liao X; Hartwig JF Pd-Catalyzed α-Arylation of Trimethylsilyl Enol Ethers with Aryl Bromides and Chlorides: a Synergistic Effect of Two Metal Fluorides as Additives. Angew. Chem. Int. Ed 2006, 45, 5852. [DOI] [PubMed] [Google Scholar]
- (22).Flitsch W; Hohenhorst M Eine einfache Synthese des 3a-Azaazulen-6-ons. Liebigs Ann. Chem 1988, 275. [Google Scholar]
- (23).Wittenberg R; Srogl J; Egi M; Liebeskind LS Ketone Synthesis Under Neutral Conditions. Cu(I) Diphenylphosphinate-Mediated, Palladium-Catalyzed Coupling of Thiol Esters and Organostannanes. Org. Lett 2003, 5, 3033. [DOI] [PubMed] [Google Scholar]
-
(24).The Robinson annulation favors 5-membered (pyrrolizine) ring formation as shown below:

-
(25).several plausible mechanisms can be envisioned for the cyclization of 65 to 66 including conjugate addition/elimination of the pyrrole anion of (Z)-65 or a 10π-electrocyclization as show below. We note that (Z)-65 has been isolated from many of the cyclization reaction mixtures.

- (26).While 63 and 64 could be converted into 66 in a single step using NaOt-Bu in THF (~30% yield on 0.5 mmol scale), this reaction afforded only ~10% yield on a gram scale.
- (27).This selectivity was only observed under conditions employing a weakly-bound potassium counterion (KHMDS) and highly polar additive (HMPA). Although somewhat unstable, products tentatively arising from C9 attack were observed in initial experiments using LHMDS as base in THF (without HMPA). C5 selectivity (over C9) cannot be rationalized by the LUMO orbital coefficients of 66, or steric effects, but the natural charge (and Mulliken charge) of C5 is positive, while the C1 and C9 carbons are negative, suggesting the importance of coulombic factors in this process.
- (28).Krasovskiy A; Kopp F; Knochel P Soluble Lanthanide Salts (LnCl3•2LiCl) for the Improved Addition of Organomagnesium Reagents to Carbonyl Compounds. Angew. Chem. Int. Ed. Engl 2006, 45, 497. [DOI] [PubMed] [Google Scholar]
- (29).Wang J; Shang M; Lundberg H; Feu KS; Hecker SJ; Qin T; Blackmond DG; Baran PS Cu-Catalyzed Decarboxylative Borylation. ACS Catal. 2018, 8, 9537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (30).It would appear this stereocenter enforces distinct pre-cyclization conformations.
- (31).(a) Baran PS; Richter JM; Lin DW Direct coupling of pyrroles with carbonyl compounds: short enantioselective synthesis of (S)-ketorolac. Angew. Chem. Int. Ed 2005, 44, 609; [DOI] [PubMed] [Google Scholar]; (b) Maimone TJ; Ishihara Y; Baran PS Scalable total syntheses of (−)-hapalindole U and (+)-ambiguine H. Tetrahedron 2015, 71, 3652; [DOI] [PMC free article] [PubMed] [Google Scholar]; (c) Richter JM; Whitefield BW; Maimone TJ; Lin DW; Castroviejo MP; Baran PS Scope and Mechanism of Direct Indole and Pyrrole Couplings Adjacent to Carbonyl Compounds: Total Synthesis of Acremoauxin A and Oxazinin 3. J. Am. Chem. Soc 2007, 129, 12857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (32).(a) for related transformations, see: Artis DR; Cho I-S; Muchowski JM Radical-based syntheses of 5-benzoyl-1,2-dihydro-3H-pyrrolo[l,2-a]pyrrole-l-carboxylic acid (ketorolac)1 Can. J. Chem 1992, 70, 1838; [Google Scholar]; (b) Baciocchi E; Muraglia E; Sleiter G Homolytic Substitution Reactions of Electron-Rich Pentatomic Heteroaromatics by Electrophilic Carbon-Centered Radicals. Synthesis of α-Heteroarylacetic Acids. J. Org. Chem 1992, 57, 6817; [Google Scholar]; (c) Baciocchi E; Muraglia E Homolytic Aromatic Substitutions of Pentatomic Heteroaromatics with Electrophilic Carbon Radicals Generated by Alkyl Halides and Triethylborane. Tetrahedron Lett. 1993, 34, 5015; [Google Scholar]; (d) Byers JH; Duff MP; Woo GW A one-step radical synthesis of pyrrol-2-acetic acids. Tetrahedron Lett. 2003, 44, 6853; [Google Scholar]; (e) Byers JH; DeWitt A; Nasveschuk CG; Swigor JE Tandem radical-electrophilic annulations to pyrrole. Tetrahedron Lett. 2004, 45, 6587. [Google Scholar]; (f) Beeson TD; Mastracchio A; Hong J-B; Ashton K; MacMillan DWC Enantioselective Organocatalysis Using SOMO Activation. Science 2007, 316, 582; [PubMed] [Google Scholar]; (g) Furst L; Matsuura BS; Narayanam JMR; Tucker JW; Stephenson CRJ Visible Light-Mediated Intermolecular C─H Functionalization of Electron-Rich Heterocycles with Malonates. Org. Lett 2010, 12, 3104; [DOI] [PubMed] [Google Scholar]; (h) Tucker JW; Narayanam JMR; Krabbe SW; Stephenson CRJ Electron Transfer Photoredox Catalysis: Intramolecular Radical Addition to Indoles and Pyrroles. Org. Lett 2010, 12, 368; [DOI] [PubMed] [Google Scholar]; (i) Boubertakh O; Goddard J-P Construction and Functionalization of Heteroarenes by Use of Photoredox Catalysis. Eur. J. Org. Chem 2017, 2072. [Google Scholar]
- (33).Hawkins EGE; Young DP Reactions of Organic Peroxides. Part V. Reaction of Ferrous Sulphate with Methylcyclopentyl and Methylcyclohexyl Hydroperoxides. J. Chem. Soc 1950, 2804. [Google Scholar]
-
(34).We also explored the use of the minor diastereomer of 87. While this compound could be advanced to diast-88 and then F2 (see below), we were unable to forge the bridging ether shown below by direct C-H etherification.

- (35).(a) Hung K; Condakes ML; Morikawa T; Maimone TJ Oxidative Entry into the Illicium Sesquiterpenes: Enantiospecific Synthesis of (+)-Pseudoanisatin. J. Am. Chem. Soc 2016, 138, 16616; [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Hung K; Condakes ML; Novaes LFT; Harwood SJ; Morikawa T; Yang Z; Maimone TJ Development of a Terpene Feedstock-based Oxidative Synthetic Approach to the Illicium Sesquiterpenes. J. Am. Chem. Soc 2019, 141, 3083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (36).A possible mechanism for decomposition under basic conditions initiates with α-deprotonation of the ester followed by E1cB elimination of the pyrrole. The first step is supported by the observation that isolated acid 94 has underwent epimerization.
- (37).Barton DHR; Crich D; Motherwell WB The Invention of New Radical Chain Reactions. Part VIII. Radical Chemistry of Thiohydroxamic Esters; A New Method for the Generation of Carbon Centered Radicals from Carboxylic Acids. Tetrahedron 1985, 41, 3901. [Google Scholar]
- (38).(a) Hu X; Maimone TJ Four-step Synthesis of the Antimalarial Cardamom Peroxide via an Oxygen Stitching Strategy. J. Am. Chem. Soc 2014, 136, 5287; [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Hu X; Lim P; Fairhurst R; Maimone TJ Synthesis and Study of the Antimalarial Cardamom Peroxide. Tetrahedron, 2018, 74, 3358; [DOI] [PMC free article] [PubMed] [Google Scholar]; (c) Hu X; Musacchio A; Shen X; Tao Y; Maimone TJ Allylative Approaches to the Synthesis of Complex Guaianolide Sesquiterpenes from Apiaceae and Asteraceae, J. Am. Chem. Soc 2019, 141, 14904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (39).(a) Li C; Wang J; Barton LM; Yu S; Tian M; Peters DS; Kumar M; Yu AW; Johnson KA; Chatterjee AK; Yan M; Baran PS Decarboxylative Borylation. Science 2017, 356, eaam7355; [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Candish L; Teders M; Glorius F Transition-Metal-Free, Visible-Light Enabled Decarboxylative Borylation of Aryl N-Hydroxyphthalimide Esters. J. Am. Chem. Soc 2017, 139, 7440. [DOI] [PubMed] [Google Scholar]; (c) Fawcett A; Pradeilles J; Wang Y; Mutsuga T; Myers EL; Aggarwal VK Photoinduced decarboxylative borylation of carboxylic acids. Science, 2017, 357, 283. [DOI] [PubMed] [Google Scholar]
- (40).Steves JE; Stahl SS Copper(I)/ABNO-Catalyzed Aerobic Alcohol Oxidation: Alleviating Steric and Electronic Constraints of Cu/TEMPO Catalyst Systems. J. Am. Chem. Soc 2013, 135, 15742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (41).Watanabe E; Kaiho A; Kusama H; Iwasawa N Cobalt–Salen Complex-Catalyzed Oxidative Generation of Alkyl Radicals from Aldehydes for the Preparation of Hydroperoxides. J. Am. Chem. Soc 2013, 135, 11744. [DOI] [PubMed] [Google Scholar]
- (42).Beak P; Reitz DB Dipole-Stabilized Carbanions: Novel and Useful Intermediates. Chem. Rev 1978, 78, 275. [Google Scholar]
- (43).for a recent review, see: Mattalia J-MR The reductive decyanation reaction: an overview and recent developments. Beilstein J. Org. Chem 2017, 13, 267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (44).(a) For seminal work on the generation of cyanohydrin anions and their use in conjugate addition chemistry, see: Stork G; Maldonado L Anions of Protected Cyanohydrins as Acyl Carbanion Equivalents and Their Use in a New Synthesis of Ketones. J. Am. Chem. Soc 1971, 93, 5286; [Google Scholar]; (b) Stork G; Maldonado L Conjugate Addition of Acyl Carbanion Equivalents via the Protected Cyanohydrin Method. J. Am. Chem. Soc 1974, 96, 5272. [Google Scholar]
- (45).Interestingly, the liberated pyrrole anion was found to add to 66. In addition, dimer 100 was also formed under these conditions.
- (46).(a) For reviews, see: Corey EJ; Helal CJ Reduction of Carbonyl Compounds with Chiral Oxazaborolidine Catalysts: A New Paradigm for Enantioselective Catalysis and a Powerful New Synthetic Method. Angew. Chem. Int. ed 1998, 37, 1986. [DOI] [PubMed] [Google Scholar]; (b) Helal CJ; Meyer MP The Corey–Bakshi–Shibata Reduction: Mechanistic and Synthetic Considerations – Bifunctional Lewis Base Catalysis with Dual Activation. In Lewis Base Catalysis in Organic Synthesis.; Vedejs E and Denmark SE, Ed.; Wiley, 2016; Ch. 11, p 387. [Google Scholar]
- (47).For a review on stereodivergent reactions of racemic mixtures, see: Miller LC; Sarpong R Chem. Soc. Rev 2011, 40, 4550. [DOI] [PubMed] [Google Scholar]
- (48).for a closely related example in total synthesis, see: Kurosu M; Kishi Y A Novel Example for Optical Resolution of Racemic Ketones Originating from Batrachotoxin Synthesis. J. Org. Chem 1998, 63, 6100. [DOI] [PubMed] [Google Scholar]
- (49).Treating 1 with NBS for example leads to dibromination of the alkene and not bromination of the desired pyrrole. These addition products are also highly unstable and could not be manipulated further.
-
(50).A number of additional reaction manifolds, including cuprate conjugate addition, Heck reaction, and Hayashi-Miyaura coupling, were explored to couple the fragments below but were uniformly unsuccessful.

-
(51).In addition to successfully completing the total synthesis of 2, an X-ray crystal structure of the samarium iodide reduction product of isomer 127 was obtained (see diast-129 below) and allowed us to deduce which isomer to carry forward.

- (52).Baran PS; Maimone TJ; Richter JM Total Synthesis of Marine Natural Products Without Using Protecting Groups. Nature 2007, 446, 404. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.