Abstract
The innate immune system plays an integral role in the brain. Synaptic pruning, a fundamental process in developmental circuit refinement, is partially mediated by neuroimmune signalling at the synapse. In particular, microglia, the major tissue-resident macrophages of the brain, and the classical complement cascade, an innate immune pathway that aids in the clearance of unwanted material, have been implicated in mediating synapse elimination. Emerging data suggest that improper signalling of the innate immune pathway at the synapse leads to pathological synapse loss in age-related neurodegenerative diseases, including Alzheimer’s disease. Now the key questions are whether synapses are targeted by complement and, if so, which synapses are vulnerable to elimination. Here, we review recent work implicating C1q, the initiator of the classical complement cascade, and surrounding glia as mediators of synapse loss. We examine how synapses could undergo apoptosis-like pathways in the Alzheimer brain, which may lead to the externalisation of phosphatidylserine on synapses. Finally, we discuss potential roles for microglia and astrocytes in this ‘synaptic apoptosis’. Critical insight into neuroimmune regulatory pathways on synapses will be key to developing effective targets against pathological synapse loss in dementia.
Keywords: Alzheimer’s disease, mitochondrial dysfunction, synapse loss, classical complement cascade, microglia, astrocyte, phosphatidylserine, synaptosis, caspase-3, MFG-E8, TAM, TREM2
Introduction
Genetic studies in Alzheimer’s disease (AD) implicate microglia, the major resident immune cells of the brain, as modulators for the risk of dementia1–5. Studies in animal models of AD suggest that microglia may contribute to the risk by acting as cellular mediators of synapse loss6–11. One proposed mechanism for the microglia-mediated synapse loss involves a region-specific reactivation of an innate immune pathway called the classical complement cascade, which has been shown to play a critical role in developmental synaptic pruning12,13. However, what triggers this reactivation of the complement-mediated synapse pruning pathway is unclear. In particular, how synapses may be lost in AD is a critical question that needs to be elucidated. Literature in AD models suggests an interesting concept of ‘synaptosis’, whereby focal apoptotic cascades at dendritic spines can occur in the absence of neuronal death14–16. This raises the intriguing questions of whether complement-mediated synapse loss by microglia in AD requires synaptosis and, if so, how. Here, we summarise emerging data from developing and diseased brains which suggest a role for phosphatidylserine (PtdSer), a canonical ‘eat me’ signal on apoptotic cells, in synapse loss. We then discuss potential links between externalised phosphatidylserine (ePtdSer), complement (C1q and C3) and receptors on microglia and astrocytes that could be involved in the recognition of ePtdSer. Furthermore, we speculate on whether and how ePtdSer may act as a signal for synaptosis in the AD brain. Synapse loss is a significant correlate of cognitive impairment in AD17–22. Therefore, critical insight into mechanisms mediating synapse loss has the potential to identify effective therapeutic targets against cognitive decline and alter AD prognosis.
Complement-mediated synapse loss
A universal hallmark of neurologic diseases is the region-specific vulnerability of neurons and neuronal networks to dysfunction and loss23. Hence, a long-standing question in neurobiology has been what contributes to the region-specific loss of synapses and neurons. In AD, synapse loss strongly correlates with cognitive impairment17–22 and appears to be present before overt neuronal loss24,25. Data from multiple laboratories collectively suggest that synaptic failure and loss in AD are likely initiated by pre-fibrillar oligomers of amyloid-beta (Aβ) and tau at synapses6,26–33. However, precise mechanisms of how these oligomers initiate synapse loss and dysfunction need further investigation.
Insight into the role of the innate immune pathway in synapse loss stemmed from post-natal circuit refinement in the developing mouse brain. Synaptic pruning in the developing brain is a normal and highly regulated process, where supernumerary synapses are removed to obtain the appropriate number of synapses34. Multiple mechanisms have been shown to mediate synaptic refinement in the developing brain, depending on brain regions and timepoints35,36. These include immune pathways such as fractalkine signalling37,38 and triggering receptor expressed on myeloid cells 2 (TREM2)39 in the hippocampus, complement (C1q/C3)12,13, MERTK-MEGF1040 and IL-3341 in the visual thalamus, MHC class I-PirB42–45 in the visual cortex, and fractalkine signalling and ADAM1046 in the barrel cortex. Among these, the classical complement cascade (C1q and C3) has been shown to be reactivated in multiple models of neurologic diseases6,8–10,47–51. Complement proteins are innate immune molecules that act as ‘eat me’ signals to promote rapid clearance of invading pathogens or cellular debris52–55. One way the complement-bound materials are eliminated from the blood is via circulating macrophages53,56. At the peak synaptic pruning period in the developing visual thalamus, microglia engulf synapses in a complement (C3-CR3)- and neuronal activity-dependent manner13. When the critical pruning window is largely over, complement (C1q and C3) activation appears to be down-regulated12,13,57. Disruption of complement pruning pathway results in sustained defects on synaptic connectivity12,13,58,59, suggesting a fundamental role for the classical complement cascade in brain wiring. Interestingly, a recent study suggested a possible role for complement and microglia in the healthy adult mouse brain involving engram-related memory processes60, raising the intriguing question of whether immune pathways critical for synaptic pruning in developing brains contribute to normal synaptic plasticity in the steady-state healthy adult brain. With normal aging, there is a region-specific vulnerability of synapses to loss and dysfunction61, and C1q and C3 have been shown to differentially affect age-dependent synaptic vulnerability57,62. Together, these studies suggest that the classical complement cascade contributes to synaptic development, maintenance and function throughout the lifespan of an animal.
In AD, complement activation was initially regarded as a secondary event related to peri-plaque neuropathology63, as C1q, C3 and C4 are often found up-regulated and localised to neuritic plaques64. Moreover, Aβ plaques have been shown to bind and regulate the expression and localisation of complement65. However, genetic data suggest that complement may be more than bystanders of AD: among the risk variants for AD are CLU, also known as complement lysis inhibitor or APOJ, and CR1, which encodes for the complement component C3b receptor66. Indeed, emerging data in both amyloid- and tau-induced mouse models of AD suggest that the classical complement cascade mediates synapse loss and dysfunction and cognitive impairment6,8–10,67. At pre-plaque ages of mouse models of AD (the J20 hAPP and APP/PS1 transgenic), C1q and C3 are reactivated in a brain region–specific manner and appear punctate and localised to synaptic proteins in vulnerable brain regions6. In addition, microglia were found to engulf synaptic proteins in a CR3-dependent manner6. Importantly, genetic or antibody means of blocking C1q, C3 or CR3 protect synapses from Aβ-induced loss and dysfunction and downstream cognitive impairment6,8,10. These findings corroborate those of an earlier study where C1qa-deficient mice crossed with the Tg2576 hAPP mouse model resulted in less plaque-related neuronal damage, synapse loss and gliosis compared with C1qa-sufficient mice67. Similarly, in the Tau-P301S model, unbiased proteomics of hippocampal post-synaptic densities (PSDs) revealed C1q as the most highly up-regulated protein relative to wild-type mice9. Injecting anti-C1q functional blocking antibody into the hippocampus of these mice attenuated the loss and microglial engulfment of synaptic proteins9. In addition, levels of C1q also positively correlated with levels of phospho-tau in PSDs from the temporal cortex of AD human brains9. Genetic deletion of C3 also rescued neurodegeneration in the Tau-P301S model10. Together, these data suggest that the classical complement cascade is reactivated in AD-like brains and mediates synapse loss and dysfunction. Interestingly, inhibiting68 or deleting69 C3 in one APP mouse model (the J20) resulted in increased plaque-related neurodegeneration whereas C3 deletion in other mouse models (APP/PS18 and PS2APP10) resulted in an amelioration of plaque-related neurodegeneration. In a tau-based model, C3 deletion was protective for neuron loss and brain atrophy10. This apparent discrepancy could have stemmed from major differences in the mouse models themselves8. However, it is important to note that, despite increased levels of plaques, synapses were still protected from loss and memory was intact in the aged APP/PS1 mice8. These studies together suggest that complement is activated in the brain in various contexts to clear what is deemed as ‘debris’ (for example, synapses as well as plaques). Therefore, understanding what on synapses reactivates complement for microglial elimination will be a critical question for the AD field to assess1.
Understanding the molecular determinants of synaptic vulnerability in Alzheimer’s disease
Apoptosis-like events on synapses in Alzheimer’s disease
Apoptosis, a process of programmed cell death involving caspase-3 activation, has an essential role in triggering the removal of damaged or dying cells by the immune system55. Interestingly, Aβ-induced synaptic impairment was ameliorated in caspase-3–deficient rodent models, suggesting that caspase-3 activation is important for Aβ-induced synaptic dysfunction70. Caspase-3 activation within hippocampal neurons has been shown to be essential for regulation of spine density and dendrite morphology71. Synaptotoxic Aβ species appear to activate local apoptotic cascades, including the cleavage of caspase-3, in synaptosomes and dendrites14. Cleaved caspase-3 levels are increased in post-synaptic densities from post-mortem AD human brains72 and in hippocampal synaptosomes of pre-plaque Tg2576 hAPP mice at the onset of memory decline and spine loss15. These findings collectively suggest that caspase-3 activity contributes to the loss and dysfunction of dendritic spines in AD models and support the notion of focal apoptotic cascades at synapses (that is, ‘synaptosis’)73,74. Furthermore, cleaved caspase-3 immunoreactivity was found in spines but not in neuronal soma or pre-synaptic terminals of the Tg2576 hAPP mice15, suggesting a potential selective vulnerability of spines in this synaptosis paradigm. Some intriguing questions are whether apoptotic synapses are specifically removed by the immune system and, if so, what mediates this.
A role for externalised phosphatidylserine at the synapse
A fundamental mechanism employed by the immune system to eliminate damaged or dying cells is the recognition by macrophages of ‘eat me’ and ‘don’t eat me’ signals expressed on the cell surface55. PtdSer is a membrane phospholipid that acts as an ‘eat me’ signal on apoptotic cell surfaces55. PtdSer is normally asymmetrically localised to the inner leaflet of the plasma membrane, but as cells undergo apoptosis, PtdSer is externalised to the outer leaflet. Cleavage of caspase-3 activates flippases such as ATP11A and ATP11C and inactivates scramblases such as Xkr8, which promote the externalisation and internalisation of PtdSer, respectively75–77. ePtdSer on the surface of apoptotic cells then is recognised as an ‘eat me’ signal by macrophages for phagocytosis55. Interestingly, ePtdSer has also been proposed to act as a ligand for C1q on apoptotic cells and this binding of C1q to apoptotic cells is inhibited with annexin V, a known PtdSer-binding protein78. Recent studies in the developing brain suggest that ePtdSer levels are increased on pre-synaptic compartments during critical periods of circuit refinement79,80. Furthermore, ePtdSer-positive neuronal terminals were found within lysosomal compartments of microglia and this localisation was ameliorated in C1qa knockout mice79. These data suggest a potential role for ePtdSer on synapses as a molecular target of C1q deposition and subsequent microglial engulfment. In the Tg2576 hAPP mouse model of AD, there was an increase of ePtdSer on hippocampal synaptosomes at the onset of hippocampal-dependent memory impairment, synaptic alterations and spine loss15. However, whether ePtdSer contributes to synapse loss in AD has yet to be shown.
Potential links between mitochondrial dysfunction and synaptosis
The activation of caspase-3 on dendritic spines of Tg2576 hAPP mice appears to be dependent on apoptosomes15, which are apoptosis-mediating protein complexes formed following the release of cytochrome c from mitochondria81. Furthermore, mitochondrial ATP synthase activity, which modulates levels of neuronal PtdSer externalisation82, has been shown to be impaired in AD mouse and human brains83–85, particularly in synaptic mitochondria85. These data suggest a possible link between synaptic mitochondria and synaptosis. In AD human brains, synaptosomes isolated from the temporal cortex have decreased levels of mitochondrial electron transport chain (ETC) complexes I, IV and V, along with an increased level of complement proteins in the same synaptosomes, relative to healthy control subjects86. Accordingly, proteomic analysis of the APP/PS1 transgenic mice showed altered levels of mitochondrial ETC proteins in C1q-associated synaptosomes87. It is unclear whether these findings are due to decreased protein expression, decreased localisation of mitochondria within synapses or due to preferential loss of mitochondria-rich synapses. However, reduction in the expression of mitochondrial oxidative phosphorylation genes in AD human brains has been shown at the mRNA level88. Furthermore, the activity of PtdSer flippases and scramblases can be modulated by ATP89–92, reactive oxygen species (ROS)93 and intracellular Ca2+ levels92–95. Mitochondria are critical for supplying ATP and ROS96,97 as well as buffering Ca2+ following synaptic activity97–99. The expression of mitochondrial Ca2+ efflux and influx genes is altered in post-mortem AD human brains100; and in hippocampal and cortical neurons from hAPP transgenic mice, the ability of mitochondria to buffer Ca2+ is impaired101,102. Furthermore, the levels of ROS are increased in synaptic mitochondria103 and synaptosomes104 of pre-plaque hAPP mice relative to wild-type mice. These studies together raise the question of whether mitochondrial dysfunction leads to synapse loss and, if so, how. Further studies are needed to strengthen the role of synaptic mitochondria in synaptosis as well as potential links between synaptic Ca2+, ATP and ROS levels with ePtdSer.
How apoptotic synapses may be recognised for elimination
Tissue-resident macrophages recognise ‘eat me’ signals, such as ePtdSer, on apoptotic cells to mediate engulfment and clearance using a plethora of receptors55. Binding of ePtdSer by these receptors can be direct (for example, T-cell immunoglobulin and mucin domain containing 4, or TIM4) or indirect (for example, TYRO, AXL and MER [TAM] receptor tyrosine kinases and α3β5 and α5β5 integrins), the latter of which require ligands to bridge the interaction between receptor and ePtdSer such as GAS6, PROS1 and milk fat globule-EGF factor 8 protein (MFG-E8)55,105.
Potential role for microglial TREM2 in synapse elimination
Of particular interest is TREM2, which has been shown to mediate the clearance of apoptotic cells by macrophages in the brain106–109. Genome-wide association studies identified mutations in TREM2, such as the R47H loss-of-function variant110, as significantly altering the risk for developing AD111,112. One mechanism proposed for TREM2 is to act as an immune sensor to detect damage109,113. Lipids that accumulate after tissue damage or become externalised on apoptotic cells, such as ePtdSer on neuronal membranes, have been shown to activate TREM2 signalling108,114,115. In line with this, multiple studies in AD mouse models suggest that microglia with dysfunctional TREM2 are unable to sense Aβ plaques and thus fail to form a putative protective barrier around plaques114–120. TREM2 has also been suggested to be a critical determinant of lipid metabolism in macrophages as well as microglial cell survival115,121. In particular, functional knockouts of Trem2 lead to the inability of microglia to adopt reactive phenotypes (the disease-associated microglia, or DAM)120–124. Hence, proper TREM2 signalling may become even more crucial for brain health and homeostasis with aging. An intriguing idea is whether with aging, when the need to clear complement (C1q)-associated synapses increases57, aged microglia with decreased lipid metabolic and phagocytic capacity125 are unable to efficiently sense or clear what the brain regards as debris.
Loss-of-function mutations in TREM2 or DAP12, an adaptor protein for TREM2 signalling, underlie the Nasu–Hakola disease, in which patients display progressive presenile dementia126,127. These findings suggest that TREM2 may have an important role in the maturation and maintenance of synaptic function and connectivity. Indeed, genetic deletion of Trem2 leads to increased synaptic density and enhanced excitatory neurotransmission in the developing mouse hippocampus39, and mice expressing mutations in DAP12 display impaired synaptic maturation128. Emerging data further suggest a role for TREM2 in microglia-mediated synapse elimination. Culturing neurons with microglia from Trem2-deficient mice prevented synapse loss compared with microglia from wild-type mice79. Introducing the humanised R47H variant of TREM2 into the TauP301S AD mouse model ameliorated C1q deposition on synapses and synaptic localisation within microglia compared with TauP301S mice with the TREM2 common variant11. A similar decrease of synaptic markers within microglial phagolysosomes was displayed in AD post-mortem human brains harbouring the R47H and R62H variants of TREM2 versus common variants11. This apparent neuroprotective role of the R47H or R62H variants, at first glance, does not concur with human genetics111,112. However, it may be in line with previous studies suggesting TREM2 as a critical immune sensor for damage and the R47H variant impairing this ability to sense113. Akin to what has been shown for the role of classical complement cascade in Aβ-induced synaptic loss versus plaque deposition6,8, whether TREM2 is beneficial versus detrimental may depend on the local milieu and the precise insult (that is, the identified ‘damage’ to be cleared)129. Future studies, including behaviour and long-term effects on cognitive function, are needed to elucidate the roles of TREM2 in synaptic and cognitive health. Furthermore, whether ePtdSer or other damage-associated lipids contribute to TREM2-mediated synapse elimination in AD and whether this involves the complement reactivation in microglia are unclear.
Astrocytic MFG-E8 as a potential phosphatidylserine interactor
Astrocytes are intimately associated with synapses, physically130–133 and functionally134, where they maintain synaptic homeostasis135. They have been shown to mediate synapse formation and maturation136–139 as well as elimination40,41,140–142. Recent data in the developing visual thalamus suggest that astrocytes can mediate synapse loss by direct engulfment of synapses via MERTK and MEGF1040,142 and by modulating microglial engulfment of synapses via secretion of IL-3341. Interestingly, astrocytes in a given region are highly specialised to meet the demands of the neurons and synapses132. This raises the questions of whether and how astrocytes contribute to region-specific synapse vulnerability in disease.
In the peripheral immune system, MFG-E8 has been identified as a bifunctional molecular linker of apoptotic cells to phagocytes143; that is, MFG-E8 binds simultaneously to ePtdSer and α5β3 or α5β5 receptors via a C2 domain and RGD motif, respectively144,145. In vitro, treating with annexin V or cyclical arginine-glycine-aspartic acid (cRGD) integrin-binding motif (which inhibit ePtdSer–MFG-E8 and MFG-E8–receptor interactions, respectively) prevents Aβ-induced engulfment of neurons by microglia146,147. In vivo, genetic deletion of Mfge8 reduces lipopolysaccharide-induced neuronal loss in the striatum148. Furthermore, tau-laden neurons cultured from P301S-tau mice externalise PtdSer and subsequently are engulfed by microglia and this can be prevented by cRGD149. Although these studies have focused on microglial MFG-E8, MFG-E8 appears to be enriched in astrocytes in the brain150–153, unlike in the periphery, where MFG-E8 is expressed by tissue-resident macrophages55,154,155. In Drosophila models, MFG-E8 is involved in the engulfment of dendrites, which display ePtdSer during developmental pruning or upon laser injury156. These data together raise the possibility of whether astrocytic MFG-E8 mediates cross-talk with microglia to facilitate synaptic engulfment.
Potential cross-talk between microglia and astrocytes in mediating synaptosis
Both microglia and astrocytes may be required for complement-mediated synapse loss. In the brain, microglia are a major cellular source of C1q6,157 and astrocytes are of C3158. Microglia have been suggested to be responsible for the ‘conversion’ of astrocytes to a reactive ‘A1’ phenotype, where C3 is a key marker, through a few factors, including C1q159. Furthermore, blocking this conversion appears neuroprotective in two models of neurodegenerative diseases: Parkinson’s160 and amyotrophic lateral sclerosis161. However, whether astrocytic C3 is required for synapse loss in AD models needs to be further elucidated. Furthermore, microglia and astrocytes both are equipped with clearance machineries, raising the question of whether these two glia cell types have complementary or redundant roles in mediating synapse loss. For example, PtdSer receptors such as TAM receptor tyrosine kinases TYRO3, AXL and MER are expressed by both microglia and astrocytes40,55,150,158. Microglial TAM has been shown to mediate the clearance of apoptotic cells in the subgranular zone of the dentate gyrus and the subventricular zone, which are neurogenic regions in the adult central nervous system162. Time-lapse in vivo imaging showed microglia and astrocytes having distinct functions in the removal of single neurons that were dying upon two-photon ablation163, such that microglia appeared to engulf large cell bodies while astrocytes engulf small diffuse debris. In vivo spinal cord imaging revealed an intimate physical interaction of astrocytes and microglia upon injury, and this interaction appears to require complement (C3) signalling164. Microglia were also suggested to instruct synaptic pruning by astrocytes in synaptic refinement, potentially via TREM2165. Together, these data suggest that cross-talk between microglia and astrocytes have important functional consequences on synaptic health and neuronal function166.
In aged and AD brains, the transcriptional profiles of microglia and astrocytes are significantly altered120,153,158,167–171. In particular, microglia up-regulate PtdSer receptors such as Trem2 and Axl120,167–170, and astrocytic expression of PtdSer-bridging molecules such as Pros1 and Mfge8 and receptors such as Megf10 becomes dysregulated149,153,158,172. Some intriguing questions are whether the changes of expression of these molecules involved in PtdSer recognition impair the ability of microglia or astrocytes to effectively respond to damaged synapses and neurons and whether they trigger the aberrant removal of otherwise healthy synapses.
Conclusions
Insight into molecular factors mediating region-specific synapse loss will be critical to changing the course of AD. Emerging data suggest that immune mechanisms involving classical complement cascade are critical for synaptic homeostasis, raising the key question of whether certain synapses are targeted for elimination by glia. To this end, recent literature highlights a potential role for ePtdSer in determining synaptic vulnerability. We postulate several pathways, including caspase-3 activation and mitochondrial dysfunction, that may lead to the externalisation of PtdSer on synapses (Figure 1). We then speculate how ePtdSer on synapses may be recognised by microglia or astrocytes (or both) for elimination (Figure 2). In particular, we focus on putative ePtdSer pathways such as TREM2 and MFG-E8. Altogether, we propose that synapses with ePtdSer may be selectively targeted by complement for deposition and subsequent engulfment by glia. However, to the best of our knowledge, no definitive link has been established between ePtdSer, complement and putative PtdSer receptors on glia. Furthermore, whether synaptic mitochondria become dysfunctional and contribute to synapse loss in AD needs further elucidation. As the classical complement cascade and microglia have been implicated in multiple models of neurologic diseases36, understanding what makes synapses vulnerable to complement-mediated engulfment and loss will be crucial to resolving neuroimmune interactions critical for brain health.
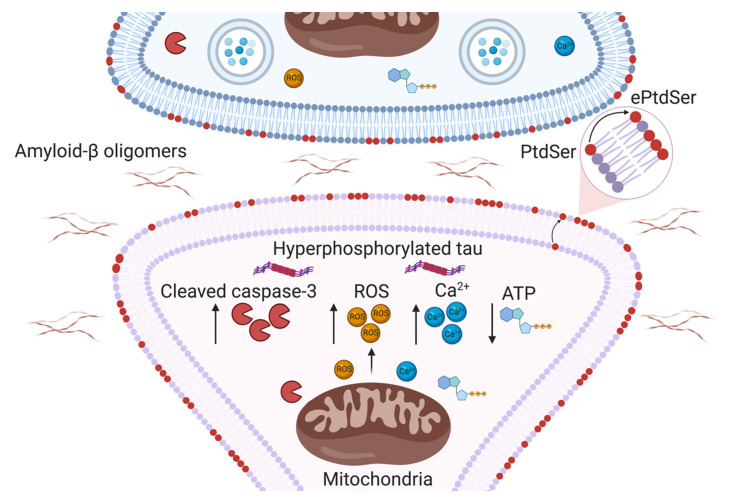
Figure 1. Potential mechanisms leading to synaptic phosphatidylserine externalisation in Alzheimer’s disease.
Schematic representation of potential pathways by which oligomeric amyloid-beta and hyperphosphorylated tau may increase the vulnerability of synapses to loss in Alzheimer’s disease. Synaptic mitochondrial dysfunction may lead to a build-up of cleaved caspase-3, reactive oxygen species (ROS) and Ca2+, accompanied by a decrease in ATP. These events can modulate the activity of flippases and scramblases, enzymes which regulate the localisation of phosphatidylserine (PtdSer), such that PtdSer is locally externalised to the outer leaflet of synaptic membranes. ePtdSer, externalised phosphatidylserine.
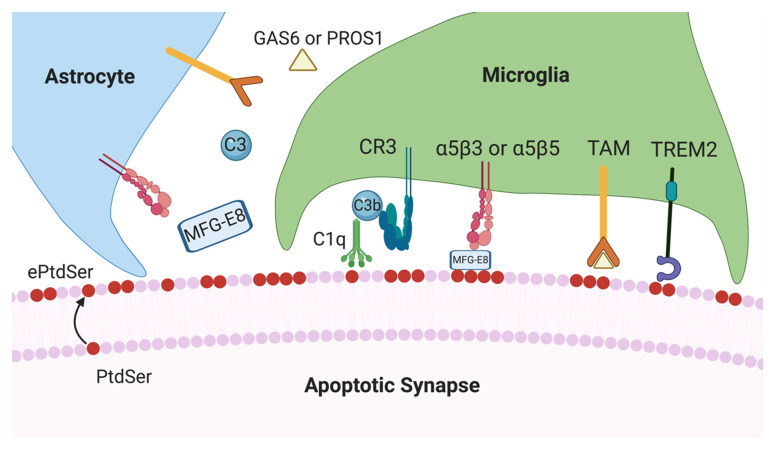
Figure 2. Potential cross-talk between microglia and astrocytes in synapse elimination in Alzheimer’s disease.
Schematic representation of proposed glial mechanisms that may mediate the clearance of synapses upon potential externalisation of phosphatidylserine (PtdSer). C1q and C3 proteins secreted by neighbouring microglia and astrocytes, respectively, may mediate engulfment by microglia upon C3b–CR3 interaction. Triggering receptor expressed on myeloid cells 2 (TREM2) may be an important determinant of synapse loss, potentially via recognition of externalised phosphatidylserine (ePtdSer). Astrocytic milk fat globule-EGF factor 8 protein (MFG-E8) may facilitate the interaction between ePtdSer and α5β3 or α5β5 glial phagocytic receptors. Other putative glial PtdSer signalling pathways, such as GAS6/PROS1 and TAM (TYRO, AXL and MER) family of receptors, may also be involved in clearing of synapses with ePtdSer.
Importantly, most of these mechanistic insights have been explored in rodent models, which can be a powerful tool to understanding the basic mechanisms of how our brain works. However, it is important to note that striking differences between mice and humans, especially in microglia170,173,174, may lead to fundamental differences in complex and chronic age-related neurodegenerative diseases such as AD. Additionally, in Aβ-induced models of AD, synapse loss has been suggested to precede overt plaque deposition6,175. However, in patients with AD, when synapses start becoming vulnerable and lost is not fully understood. Recent development of imaging markers that selectively bind to synaptic elements22 will be instrumental in better defining the timeline progression of synaptic health in AD.
Acknowledgements
We thank Morgan Sheng (Broad Institute, Cambridge, MA, USA), Cynthia Lemere (Brigham and Women’s Hospital, Boston, MA, USA) and Won-Suk Chung (KAIST, Daejeon, Republic of Korea) for critical reading of the manuscript. The figures were made with BioRender.
The peer reviewers who approve this article are:
Morgan Sheng, Stanley Center for Psychiatric Research, Broad Institute of MIT and Harvard, Cambridge, MA, 02142, USA
Cynthia Lemere, Ann Romney Center for Neurologic Diseases, Brigham and Women's Hospital, Building for Transformative Medicine, 9th Floor, 60 Fenwood Road, Boston, MA 02115, USA
Won-Suk Chung, Department of Biological Sciences, Korea Advanced Institute of Science and Technology (KAIST), Daejeon, Korea
Funding Statement
This work was supported by the UK Dementia Research Institute (SH), which receives its funding from DRI Ltd, the UK Medical Research Council, Alzheimer’s Society and Alzheimer’s Research UK (SH), the Collaborative Pairs project of the Chan Zuckerberg Initiative (SH), AstraZeneca UK Limited (DS) and the Biotechnology and Biological Sciences Research Council (DS).
The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Bartels T, De Schepper S, Hong S: Microglia modulate neurodegeneration in Alzheimer's and Parkinson's diseases. Science. 2020; 370(6512): 66–9. 10.1126/science.abb8587 [DOI] [PubMed] [Google Scholar]
- 2. Efthymiou AG, Goate AM: Late onset Alzheimer's disease genetics implicates microglial pathways in disease risk. Mol Neurodegener. 2017; 12(1): 43. 10.1186/s13024-017-0184-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Sims R, van der Lee SJ, Naj AC, et al. : Rare coding variants in PLCG2, ABI3, and TREM2 implicate microglial-mediated innate immunity in Alzheimer's disease. Nat Genet. 2017; 49(9): 1373–84. 10.1038/ng.3916 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Kunkle BW, Grenier-Boley B, Sims R, et al. : Genetic meta-analysis of diagnosed Alzheimer's disease identifies new risk loci and implicates Aβ, tau, immunity and lipid processing. Nat Genet. 2019; 51(3): 414–30. 10.1038/s41588-019-0358-2 [DOI] [PMC free article] [PubMed] [Google Scholar]; Faculty Opinions Recommendation
- 5. Jansen IE, Savage JE, Watanabe K, et al. : Genome-wide meta-analysis identifies new loci and functional pathways influencing Alzheimer's disease risk. Nat Genet. 2019; 51(3): 404–13. 10.1038/s41588-018-0311-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Hong S, Beja-Glasser VF, Nfonoyim BM, et al. : Complement and microglia mediate early synapse loss in Alzheimer mouse models. Science. 2016; 352(6286): 712–6. 10.1126/science.aad8373 [DOI] [PMC free article] [PubMed] [Google Scholar]; Faculty Opinions Recommendation
- 7. Paolicelli RC, Jawaid A, Henstridge CM, et al. : TDP-43 Depletion in Microglia Promotes Amyloid Clearance but Also Induces Synapse Loss. Neuron. 2017; 95(2): 297–308.e6. 10.1016/j.neuron.2017.05.037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Shi Q, Chowdhury S, Ma R, et al. : Complement C3 deficiency protects against neurodegeneration in aged plaque-rich APP/PS1 mice. Sci Transl Med. 2017; 9(392): eaaf6295. 10.1126/scitranslmed.aaf6295 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Dejanovic B, Huntley MA, de Mazière A, et al. : Changes in the Synaptic Proteome in Tauopathy and Rescue of Tau-Induced Synapse Loss by C1q Antibodies. Neuron. 2018; 100(6): 1322–1336.e7. 10.1016/j.neuron.2018.10.014 [DOI] [PubMed] [Google Scholar]; Faculty Opinions Recommendation
- 10. Wu T, Dejanovic B, Gandham VD, et al. : Complement C3 Is Activated in Human AD Brain and Is Required for Neurodegeneration in Mouse Models of Amyloidosis and Tauopathy. Cell Rep. 2019; 28(8): 2111–2123.e6. 10.1016/j.celrep.2019.07.060 [DOI] [PubMed] [Google Scholar]; Faculty Opinions Recommendation
- 11. Gratuze M, Leyns CE, Sauerbeck AD, et al. : Impact of TREM2R47H variant on tau pathology-induced gliosis and neurodegeneration. J Clin Invest. 2020; 130(9): 4954–68. 10.1172/JCI138179 [DOI] [PMC free article] [PubMed] [Google Scholar]; Faculty Opinions Recommendation
- 12. Stevens B, Allen NJ, Vazquez LE, et al. : The classical complement cascade mediates CNS synapse elimination. Cell. 2007; 131(6): 1164–78. 10.1016/j.cell.2007.10.036 [DOI] [PubMed] [Google Scholar]; Faculty Opinions Recommendation
- 13. Schafer DP, Lehrman EK, Kautzman AG, et al. : Microglia sculpt postnatal neural circuits in an activity and complement-dependent manner. Neuron. 2012; 74(4): 691–705. 10.1016/j.neuron.2012.03.026 [DOI] [PMC free article] [PubMed] [Google Scholar]; Faculty Opinions Recommendation
- 14. Mattson MP, Partin J, Begley JG: Amyloid beta-peptide induces apoptosis-related events in synapses and dendrites. Brain Res. 1998; 807(1–2): 167–76. 10.1016/s0006-8993(98)00763-x [DOI] [PubMed] [Google Scholar]
- 15. D'Amelio M, Cavallucci V, Middei S, et al. : Caspase-3 triggers early synaptic dysfunction in a mouse model of Alzheimer's disease. Nat Neurosci. 2011; 14(1): 69–76. 10.1038/nn.2709 [DOI] [PubMed] [Google Scholar]; Faculty Opinions Recommendation
- 16. Park G, Nhan HS, Tyan SH, et al. : Caspase Activation and Caspase-Mediated Cleavage of APP Is Associated with Amyloid β-Protein-Induced Synapse Loss in Alzheimer's Disease. Cell Rep. 2020; 31(13): 107839. 10.1016/j.celrep.2020.107839 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. DeKosky ST, Scheff SW: Synapse loss in frontal cortex biopsies in Alzheimer's disease: Correlation with cognitive severity. Ann Neurol. 1990; 27(5): 457–64. 10.1002/ana.410270502 [DOI] [PubMed] [Google Scholar]
- 18. Terry RD, Masliah E, Salmon DP, et al. : Physical basis of cognitive alterations in Alzheimer's disease: Synapse loss is the major correlate of cognitive impairment. Ann Neurol. 1991; 30(4): 572–80. 10.1002/ana.410300410 [DOI] [PubMed] [Google Scholar]
- 19. Scheff SW, Price DA, Schmitt FA, et al. : Hippocampal synaptic loss in early Alzheimer's disease and mild cognitive impairment. Neurobiol Aging. 2006; 27(10): 1372–84. 10.1016/j.neurobiolaging.2005.09.012 [DOI] [PubMed] [Google Scholar]
- 20. Scheff SW, Price DA, Schmitt FA, et al. : Synaptic alterations in CA1 in mild Alzheimer disease and mild cognitive impairment. Neurology. 2007; 68(18): 1501–8. 10.1212/01.wnl.0000260698.46517.8f [DOI] [PubMed] [Google Scholar]
- 21. Scheff SW, Price DA, Schmitt FA, et al. : Synaptic loss in the inferior temporal gyrus in mild cognitive impairment and Alzheimer's disease. J Alzheimers Dis. 2011; 24(3): 547–57. 10.3233/JAD-2011-101782 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Chen MK, Mecca AP, Naganawa M, et al. : Assessing Synaptic Density in Alzheimer Disease With Synaptic Vesicle Glycoprotein 2A Positron Emission Tomographic Imaging. JAMA Neurol. 2018; 75(10): 1215–24. 10.1001/jamaneurol.2018.1836 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Fu H, Hardy J, Duff KE: Selective vulnerability in neurodegenerative diseases. Nat Neurosci. 2018; 21(10): 1350–8. 10.1038/s41593-018-0221-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Davies CA, Mann DM, Sumpter PQ, et al. : A quantitative morphometric analysis of the neuronal and synaptic content of the frontal and temporal cortex in patients with Alzheimer's disease. J Neurol Sci. 1987; 78(2): 151–64. 10.1016/0022-510x(87)90057-8 [DOI] [PubMed] [Google Scholar]
- 25. Selkoe DJ: Alzheimer's disease is a synaptic failure. Science. 2002; 298(5594): 789–91. 10.1126/science.1074069 [DOI] [PubMed] [Google Scholar]
- 26. Lambert MP, Barlow AK, Chromy BA, et al. : Diffusible, nonfibrillar ligands derived from Abeta1-42 are potent central nervous system neurotoxins. Proc Natl Acad Sci U S A. 1998; 95(11): 6448–53. 10.1073/pnas.95.11.6448 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Walsh DM, Klyubin I, Fadeeva JV, et al. : Naturally secreted oligomers of amyloid beta protein potently inhibit hippocampal long-term potentiation in vivo. Nature. 2002; 416(6880): 535–9. 10.1038/416535a [DOI] [PubMed] [Google Scholar]; Faculty Opinions Recommendation
- 28. Spires TL, Meyer-Luehmann M, Stern EA, et al. : Dendritic spine abnormalities in amyloid precursor protein transgenic mice demonstrated by gene transfer and intravital multiphoton microscopy. J Neurosci. 2005; 25(31): 7278–87. 10.1523/JNEUROSCI.1879-05.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Shankar GM, Bloodgood BL, Townsend M, et al. : Natural oligomers of the Alzheimer amyloid-beta protein induce reversible synapse loss by modulating an NMDA-type glutamate receptor-dependent signaling pathway. J Neurosci. 2007; 27(11): 2866–75. 10.1523/JNEUROSCI.4970-06.2007 [DOI] [PMC free article] [PubMed] [Google Scholar]; Faculty Opinions Recommendation
- 30. Shankar GM, Li S, Mehta TH, et al. : Amyloid-beta protein dimers isolated directly from Alzheimer's brains impair synaptic plasticity and memory. Nat Med. 2008; 14(8): 837–42. 10.1038/nm1782 [DOI] [PMC free article] [PubMed] [Google Scholar]; Faculty Opinions Recommendation
- 31. Koffie RM, Meyer-Luehmann M, Hashimoto T, et al. : Oligomeric amyloid beta associates with postsynaptic densities and correlates with excitatory synapse loss near senile plaques. Proc Natl Acad Sci U S A. 2009; 106(10): 4012–7. 10.1073/pnas.0811698106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Zhou L, McInnes J, Wierda K, et al. : Tau association with synaptic vesicles causes presynaptic dysfunction. Nat Commun. 2017; 8: 15295. 10.1038/ncomms15295 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. McInnes J, Wierda K, Snellinx A, et al. : Synaptogyrin-3 Mediates Presynaptic Dysfunction Induced by Tau. Neuron. 2018; 97(4): 823–835.e8. 10.1016/j.neuron.2018.01.022 [DOI] [PubMed] [Google Scholar]; Faculty Opinions Recommendation
- 34. Katz LC, Shatz CJ: Synaptic activity and the construction of cortical circuits. Science. 1996; 274(5290): 1133–8. 10.1126/science.274.5290.1133 [DOI] [PubMed] [Google Scholar]
- 35. Neniskyte U, Gross CT: Errant gardeners: Glial-cell-dependent synaptic pruning and neurodevelopmental disorders. Nat Rev Neurosci. 2017; 18(11): 658–70. 10.1038/nrn.2017.110 [DOI] [PubMed] [Google Scholar]; Faculty Opinions Recommendation
- 36. de Schepper S, Crowley G, Hong S: Understanding microglial diversity and implications for neuronal function in health and disease. Dev Neurobiol. 2020. 10.1002/dneu.22777 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Paolicelli RC, Bolasco G, Pagani F, et al. : Synaptic pruning by microglia is necessary for normal brain development. Science. 2011; 333(6048): 1456–8. 10.1126/science.1202529 [DOI] [PubMed] [Google Scholar]; Faculty Opinions Recommendation
- 38. Zhan Y, Paolicelli RC, Sforazzini F, et al. : Deficient neuron-microglia signaling results in impaired functional brain connectivity and social behavior. Nat Neurosci. 2014; 17(3): 400–6. 10.1038/nn.3641 [DOI] [PubMed] [Google Scholar]; Faculty Opinions Recommendation
- 39. Filipello F, Morini R, Corradini I, et al. : The Microglial Innate Immune Receptor TREM2 Is Required for Synapse Elimination and Normal Brain Connectivity. Immunity. 2018; 48(5): 979–991.e8. 10.1016/j.immuni.2018.04.016 [DOI] [PubMed] [Google Scholar]
- 40. Chung WS, Clarke LE, Wang GX, et al. : Astrocytes mediate synapse elimination through MEGF10 and MERTK pathways. Nature. 2013; 504(7480): 394–400. 10.1038/nature12776 [DOI] [PMC free article] [PubMed] [Google Scholar]; Faculty Opinions Recommendation
- 41. Vainchtein ID, Chin G, Cho FS, et al. : Astrocyte-derived interleukin-33 promotes microglial synapse engulfment and neural circuit development. Science. 2018; 359(6381): 1269–1273. 10.1126/science.aal3589 [DOI] [PMC free article] [PubMed] [Google Scholar]; Faculty Opinions Recommendation
- 42. Huh GS, Boulanger LM, Du H, et al. : Functional requirement for class I MHC in CNS development and plasticity. Science. 2000; 290(5499): 2155–9. 10.1126/science.290.5499.2155 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Datwani A, McConnell MJ, Kanold PO, et al. : Classical MHCI molecules regulate retinogeniculate refinement and limit ocular dominance plasticity. Neuron. 2009; 64(4): 463–470. 10.1016/j.neuron.2009.10.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Kim T, Vidal GS, Djurisic M, et al. : Human LilrB2 is a β-amyloid receptor and its murine homolog PirB regulates synaptic plasticity in an Alzheimer's model. Science. 2013; 341(6152): 1399–404. 10.1126/science.1242077 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Lee H, Brott BK, Kirkby LA, et al. : Synapse elimination and learning rules co-regulated by MHC class I H2-Db. Nature. 2014; 509(7499): 195–200. 10.1038/nature13154 [DOI] [PMC free article] [PubMed] [Google Scholar]; Faculty Opinions Recommendation
- 46. Gunner G, Cheadle L, Johnson KM, et al. : Sensory lesioning induces microglial synapse elimination via ADAM10 and fractalkine signaling. Nat Neurosci. 2019; 22(7): 1075–1088. 10.1038/s41593-019-0419-y [DOI] [PMC free article] [PubMed] [Google Scholar]; Faculty Opinions Recommendation
- 47. Lui H, Zhang J, Makinson SR, et al. : Progranulin Deficiency Promotes Circuit-Specific Synaptic Pruning by Microglia via Complement Activation. Cell. 2016; 165(4): 921–35. 10.1016/j.cell.2016.04.001 [DOI] [PMC free article] [PubMed] [Google Scholar]; Faculty Opinions Recommendation
- 48. Vasek MJ, Garber C, Dorsey D, et al. : A complement-microglial axis drives synapse loss during virus-induced memory impairment. Nature. 2016; 534(7608): 538–43. 10.1038/nature18283 [DOI] [PMC free article] [PubMed] [Google Scholar]; Faculty Opinions Recommendation
- 49. Litvinchuk A, Wan Y-W, Swartzlander DB, et al. : Complement C3aR Inactivation Attenuates Tau Pathology and Reverses an Immune Network Deregulated in Tauopathy Models and Alzheimer's Disease. Neuron. 2018; 100(6): 1337–1353. e5. 10.1016/j.neuron.2018.10.031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Vukojicic A, Delestrée N, Fletcher EV, et al. : The Classical Complement Pathway Mediates Microglia-Dependent Remodeling of Spinal Motor Circuits during Development and in SMA. Cell Rep. 2019; 29(10): 3087–3100. e7. 10.1016/j.celrep.2019.11.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Werneburg S, Jung J, Kunjamma RB, et al. : Targeted Complement Inhibition at Synapses Prevents Microglial Synaptic Engulfment and Synapse Loss in Demyelinating Disease. Immunity. 2020; 52(1): 167–182. e7. 10.1016/j.immuni.2019.12.004 [DOI] [PMC free article] [PubMed] [Google Scholar]; Faculty Opinions Recommendation
- 52. Mevorach D, Mascarenhas JO, Gershov D, et al. : Complement-dependent clearance of apoptotic cells by human macrophages. J Exp Med. 1998; 188(12): 2313–20. 10.1084/jem.188.12.2313 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Gasque P: Complement: A unique innate immune sensor for danger signals. Mol Immunol. 2004; 41(11): 1089–98. 10.1016/j.molimm.2004.06.011 [DOI] [PubMed] [Google Scholar]
- 54. Martin M, Leffler J, Smoląg KI, et al. : Factor H uptake regulates intracellular C3 activation during apoptosis and decreases the inflammatory potential of nucleosomes. Cell Death Differ. 2016; 23(5): 903–11. 10.1038/cdd.2015.164 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Lemke G: How macrophages deal with death. Nat Rev Immunol. 2019; 19(9): 539–49. 10.1038/s41577-019-0167-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Ricklin D, Hajishengallis G, Yang K, et al. : Complement: A key system for immune surveillance and homeostasis. Nat Immunol. 2010; 11(9): 785–97. 10.1038/ni.1923 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Stephan AH, Madison DV, Mateos JM, et al. : A dramatic increase of C1q protein in the CNS during normal aging. J Neurosci. 2013; 33(33): 13460–74. 10.1523/JNEUROSCI.1333-13.2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Chu Y, Jin X, Parada I, et al. : Enhanced synaptic connectivity and epilepsy in C1q knockout mice. Proc Natl Acad Sci U S A. 2010; 107(17): 7975–80. 10.1073/pnas.0913449107 [DOI] [PMC free article] [PubMed] [Google Scholar]; Faculty Opinions Recommendation
- 59. Perez-Alcazar M, Daborg J, Stokowska A, et al. : Altered cognitive performance and synaptic function in the hippocampus of mice lacking C3. Exp Neurol. 2014; 253: 154–64. 10.1016/j.expneurol.2013.12.013 [DOI] [PubMed] [Google Scholar]
- 60. Wang C, Yue H, Hu Z, et al. : Microglia mediate forgetting via complement-dependent synaptic elimination. Science. 2020; 367(6478): 688–694. 10.1126/science.aaz2288 [DOI] [PubMed] [Google Scholar]; Faculty Opinions Recommendation
- 61. Morrison JH, Baxter MG: The ageing cortical synapse: Hallmarks and implications for cognitive decline. Nat Rev Neurosci. 2012; 13(4): 240–50. 10.1038/nrn3200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Shi Q, Colodner KJ, Matousek SB, et al. : Complement C3-Deficient Mice Fail to Display Age-Related Hippocampal Decline. J Neurosci. 2015; 35(38): 13029–42. 10.1523/JNEUROSCI.1698-15.2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Wyss-Coray T, Rogers J: Inflammation in Alzheimer disease-a brief review of the basic science and clinical literature. Cold Spring Harb Perspect Med. 2012; 2(1): a006346. 10.1101/cshperspect.a006346 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Eikelenboom P, Stam FC: Immunoglobulins and complement factors in senile plaques. An immunoperoxidase study. Acta Neuropathol. 1982; 57(2-3): 239–42. 10.1007/BF00685397 [DOI] [PubMed] [Google Scholar]
- 65. Morgan BP: Complement in the pathogenesis of Alzheimer's disease. Semin Immunopathol. 2018; 40(1): 113–124. 10.1007/s00281-017-0662-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Lambert J-C, Heath S, Even G, et al. : Genome-wide association study identifies variants at CLU and CR1 associated with Alzheimer's disease. Nat Genet. 2009; 41(10): 1094–9. 10.1038/ng.439 [DOI] [PubMed] [Google Scholar]
- 67. Fonseca MI, Zhou J, Botto M, et al. : Absence of C1q leads to less neuropathology in transgenic mouse models of Alzheimer's disease. J Neurosci. 2004; 24(29): 6457–65. 10.1523/JNEUROSCI.0901-04.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Wyss-Coray T, Yan F, Lin AH-T, et al. : Prominent neurodegeneration and increased plaque formation in complement-inhibited Alzheimer's mice. Proc Natl Acad Sci U S A. 2002; 99(16): 10837–42. 10.1073/pnas.162350199 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Maier M, Peng Y, Jiang L, et al. : Complement C3 deficiency leads to accelerated amyloid beta plaque deposition and neurodegeneration and modulation of the microglia/macrophage phenotype in amyloid precursor protein transgenic mice. J Neurosci. 2008; 28(25): 6333–41. 10.1523/JNEUROSCI.0829-08.2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Jo J, Whitcomb DJ, Olsen KM, et al. : Aβ(1-42) inhibition of LTP is mediated by a signaling pathway involving caspase-3, Akt1 and GSK-3β. Nat Neurosci. 2011; 14(5): 545–7. 10.1038/nn.2785 [DOI] [PubMed] [Google Scholar]
- 71. Ertürk A, Wang Y, Sheng M: Local pruning of dendrites and spines by caspase-3-dependent and proteasome-limited mechanisms. J Neurosci. 2014; 34(5): 1672–88. 10.1523/JNEUROSCI.3121-13.2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Louneva N, Cohen JW, Han L-Y, et al. : Caspase-3 is enriched in postsynaptic densities and increased in Alzheimer's disease. Am J Pathol. 2008; 173(5): 1488–95. 10.2353/ajpath.2008.080434 [DOI] [PMC free article] [PubMed] [Google Scholar]; Faculty Opinions Recommendation
- 73. Sheng M, Ertürk A: Long-term depression: A cell biological view. Philos Trans R Soc Lond B Biol Sci. 2014; 369(1633): 20130138. 10.1098/rstb.2013.0138 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Mattson MP, Keller JN, Begley JG: Evidence for synaptic apoptosis. Exp Neurol. 1998; 153(1): 35–48. 10.1006/exnr.1998.6863 [DOI] [PubMed] [Google Scholar]
- 75. Suzuki J, Denning DP, Imanishi E, et al. : Xk-related protein 8 and CED-8 promote phosphatidylserine exposure in apoptotic cells. Science. 2013; 341(6144): 403–6. 10.1126/science.1236758 [DOI] [PubMed] [Google Scholar]; Faculty Opinions Recommendation
- 76. Suzuki J, Imanishi E, Nagata S: Exposure of phosphatidylserine by Xk-related protein family members during apoptosis. J Biol Chem. 2014; 289(44): 30257–67. 10.1074/jbc.M114.583419 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Segawa K, Kurata S, Yanagihashi Y, et al. : Caspase-mediated cleavage of phospholipid flippase for apoptotic phosphatidylserine exposure. Science. 2014; 344(6188): 1164–8. 10.1126/science.1252809 [DOI] [PubMed] [Google Scholar]; Faculty Opinions Recommendation
- 78. Païdassi H, Tacnet-Delorme P, Garlatti V, et al. : C1q binds phosphatidylserine and likely acts as a multiligand-bridging molecule in apoptotic cell recognition. J Immunol. 2008; 180(4): 2329–38. 10.4049/jimmunol.180.4.2329 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Scott-Hewitt N, Perrucci F, Morini R, et al. : Local externalization of phosphatidylserine mediates developmental synaptic pruning by microglia. EMBO J. 2020; 39(16): e105380. 10.15252/embj.2020105380 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Li T, Chiou B, Gilman CK, et al. : A splicing isoform of GPR56 mediates microglial synaptic refinement via phosphatidylserine binding. EMBO J. 2020; 39(16): e104136. 10.15252/embj.2019104136 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Riedl SJ, Salvesen GS: The apoptosome: Signalling platform of cell death. Nat Rev Mol Cell Biol. 2007; 8(5): 405–13. 10.1038/nrm2153 [DOI] [PubMed] [Google Scholar]
- 82. Shacham-Silverberg V, Shalom HS, Goldner R, et al. : Phosphatidylserine is a marker for axonal debris engulfment but its exposure can be decoupled from degeneration. Cell Death Dis. 2018; 9(11): 1116. 10.1038/s41419-018-1155-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Terni B, Boada J, Portero-Otin M, et al. : Mitochondrial ATP-synthase in the entorhinal cortex is a target of oxidative stress at stages I/II of Alzheimer's disease pathology. Brain Pathol. 2010; 20(1): 222–33. 10.1111/j.1750-3639.2009.00266.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Cha MY, Cho HJ, Kim C, et al. : Mitochondrial ATP synthase activity is impaired by suppressed O-GlcNAcylation in Alzheimer's disease. Hum Mol Genet. 2015; 24(22): 6492–504. 10.1093/hmg/ddv358 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Beck SJ, Guo L, Phensy A, et al. : Deregulation of mitochondrial F1FO-ATP synthase via OSCP in Alzheimer's disease. Nat Commun. 2016; 7: 11483. 10.1038/ncomms11483 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Hesse R, Hurtado ML, Jackson RJ, et al. : Comparative profiling of the synaptic proteome from Alzheimer's disease patients with focus on the APOE genotype. Acta Neuropathol Commun. 2019; 7(1): 214. 10.1186/s40478-019-0847-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Györffy BA, Tóth V, Török G, et al. : Synaptic mitochondrial dysfunction and septin accumulation are linked to complement-mediated synapse loss in an Alzheimer's disease animal model. Cell Mol Life Sci. 2020; 77(24): 5243–5258. 10.1007/s00018-020-03468-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Sorrentino V, Romani M, Mouchiroud L: Enhancing mitochondrial proteostasis reduces amyloid-β proteotoxicity. Nature. 2017; 552(7684): 187–193. 10.1038/nature25143 [DOI] [PMC free article] [PubMed] [Google Scholar]; Faculty Opinions Recommendation
- 89. Zachowski A, Henry JP, Devaux PF: Control of transmembrane lipid asymmetry in chromaffin granules by an ATP-dependent protein. Nature. 1989; 340(6228): 75–6. 10.1038/340075a0 [DOI] [PubMed] [Google Scholar]
- 90. Tang X, Halleck MS, Schlegel RA, et al. : A subfamily of P-type ATPases with aminophospholipid transporting activity. Science. 1996; 272(5267): 1495–7. 10.1126/science.272.5267.1495 [DOI] [PubMed] [Google Scholar]
- 91. Yabas M, Teh CE, Frankenreiter S, et al. : ATP11C is critical for the internalization of phosphatidylserine and differentiation of B lymphocytes. Nat Immunol. 2011; 12(5): 441–9. 10.1038/ni.2011 [DOI] [PMC free article] [PubMed] [Google Scholar]; Faculty Opinions Recommendation
- 92. Segawa K, Kurata S, Nagata S: Human Type IV P-type ATPases That Work as Plasma Membrane Phospholipid Flippases and Their Regulation by Caspase and Calcium. J Biol Chem. 2016; 291(2): 762–72. 10.1074/jbc.M115.690727 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Schreiber R, Ousingsawat J, Wanitchakool P, et al. : Regulation of TMEM16A/ANO1 and TMEM16F/ANO6 ion currents and phospholipid scrambling by Ca2+ and plasma membrane lipid. J Physiol. 2018; 596(2): 217–229. 10.1113/JP275175 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Suzuki J, Umeda M, Sims PJ, et al. : Calcium-dependent phospholipid scrambling by TMEM16F. Nature. 2010; 468(7325): 834–8. 10.1038/nature09583 [DOI] [PubMed] [Google Scholar]; Faculty Opinions Recommendation
- 95. Suzuki J, Fujii T, Imao T, et al. : Calcium-dependent phospholipid scramblase activity of TMEM16 protein family members. J Biol Chem. 2013; 288(19): 13305–16. 10.1074/jbc.M113.457937 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Zorov DB, Juhaszova M, Sollott SJ: Mitochondrial reactive oxygen species (ROS) and ROS-induced ROS release. Physiol Rev. 2014; 94(3): 909–50. 10.1152/physrev.00026.2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Devine MJ, Kittler JT: Mitochondria at the neuronal presynapse in health and disease. Nat Rev Neurosci. 2018; 19(2): 63–80. 10.1038/nrn.2017.170 [DOI] [PubMed] [Google Scholar]
- 98. Pivovarova NB, Pozzo-Miller LD, Hongpaisan J, et al. : Correlated Calcium Uptake and Release by Mitochondria and Endoplasmic Reticulum of CA3 Hippocampal Dendrites after Afferent Synaptic Stimulation. J Neurosci. 2002; 22(24): 10653–61. 10.1523/JNEUROSCI.22-24-10653.2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Billups B, Forsythe ID: Presynaptic Mitochondrial Calcium Sequestration Influences Transmission at Mammalian Central Synapses. J Neurosci. 2002; 22(14): 5840–7. 10.1523/JNEUROSCI.22-14-05840.2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Calvo-Rodriguez M, Hou SS, Snyder AC, et al. : Increased mitochondrial calcium levels associated with neuronal death in a mouse model of Alzheimer's disease. Nat Commun. 2020; 11(1): 2146. 10.1038/s41467-020-16074-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Du H, Guo L, Fang F, et al. : Cyclophilin D deficiency attenuates mitochondrial and neuronal perturbation and ameliorates learning and memory in Alzheimer's disease. Nat Med. 2008; 14(10): 1097–105. 10.1038/nm.1868 [DOI] [PMC free article] [PubMed] [Google Scholar]; Faculty Opinions Recommendation
- 102. Lee SH, Kim KR, Ryu SY, et al. : Impaired short-term plasticity in mossy fiber synapses caused by mitochondrial dysfunction of dentate granule cells is the earliest synaptic deficit in a mouse model of Alzheimer's disease. J Neurosci. 2012; 32(17): 5953–63. 10.1523/JNEUROSCI.0465-12.2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Du H, Guo L, Yan S, et al. : Early deficits in synaptic mitochondria in an Alzheimer's disease mouse model. Proc Natl Acad Sci U S A. 2010; 107(43): 18670–5. 10.1073/pnas.1006586107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Ahmad F, Singh K, Das D, et al. : Reactive Oxygen Species-Mediated Loss of Synaptic Akt1 Signaling Leads to Deficient Activity-Dependent Protein Translation Early in Alzheimer's Disease. Antioxid Redox Signal. 2017; 27(16): 1269–1280. 10.1089/ars.2016.6860 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Rothlin CV, Carrera-Silva EA, Bosurgi L, et al. : TAM receptor signaling in immune homeostasis. Annu Rev Immunol. 2015; 33: 355–91. 10.1146/annurev-immunol-032414-112103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. Takahashi K, Rochford CDP, Neumann H: Clearance of apoptotic neurons without inflammation by microglial triggering receptor expressed on myeloid cells-2. J Exp Med. 2005; 201(4): 647–57. 10.1084/jem.20041611 [DOI] [PMC free article] [PubMed] [Google Scholar]; Faculty Opinions Recommendation
- 107. Hsieh CL, Koike M, Spusta SC, et al. : A role for TREM2 ligands in the phagocytosis of apoptotic neuronal cells by microglia. J Neurochem. 2009; 109(4): 1144–56. 10.1111/j.1471-4159.2009.06042.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108. Shirotani K, Hori Y, Yoshizaki R, et al. : Aminophospholipids are signal-transducing TREM2 ligands on apoptotic cells. Sci Rep. 2019; 9(1): 7508. 10.1038/s41598-019-43535-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109. Deczkowska A, Weiner A, Amit I: The Physiology, Pathology, and Potential Therapeutic Applications of the TREM2 Signaling Pathway. Cell. 2020; 181(6): 1207–1217. 10.1016/j.cell.2020.05.003 [DOI] [PubMed] [Google Scholar]; Faculty Opinions Recommendation
- 110. Sudom A, Talreja S, Danao J, et al. : Molecular basis for the loss-of-function effects of the Alzheimer's disease-associated R47H variant of the immune receptor TREM2. J Biol Chem. 2018; 293(32): 12634–12646. 10.1074/jbc.RA118.002352 [DOI] [PMC free article] [PubMed] [Google Scholar]; Faculty Opinions Recommendation
- 111. Guerreiro R, Wojtas A, Bras J, et al. : TREM2 variants in Alzheimer's disease. N Engl J Med. 2013; 368(2): 117–27. 10.1056/NEJMoa1211851 [DOI] [PMC free article] [PubMed] [Google Scholar]; Faculty Opinions Recommendation
- 112. Jonsson T, Stefansson H, Steinberg S, et al. : Variant of TREM2 associated with the risk of Alzheimer's disease. N Engl J Med. 2013; 368(2): 107–16. 10.1056/NEJMoa1211103 [DOI] [PMC free article] [PubMed] [Google Scholar]; Faculty Opinions Recommendation
- 113. Deczkowska A, Keren-Shaul H, Weiner A, et al. : Disease-Associated Microglia: A Universal Immune Sensor of Neurodegeneration. Cell. 2018; 173(5): 1073–1081. 10.1016/j.cell.2018.05.003 [DOI] [PubMed] [Google Scholar]; Faculty Opinions Recommendation
- 114. Wang Y, Cella M, Mallinson K, et al. : TREM2 lipid sensing sustains the microglial response in an Alzheimer's disease model. Cell. 2015; 160(6): 1061–71. 10.1016/j.cell.2015.01.049 [DOI] [PMC free article] [PubMed] [Google Scholar]; Faculty Opinions Recommendation
- 115. Ulland TK, Song WM, Huang SCC, et al. : TREM2 Maintains Microglial Metabolic Fitness in Alzheimer's Disease. Cell. 2017; 170(4): 649–663.e13. 10.1016/j.cell.2017.07.023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116. Ulrich JD, Finn MB, Wang Y, et al. : Altered microglial response to Aβ plaques in APPPS1-21 mice heterozygous for TREM2. Mol Neurodegener. 2014; 9: 20. 10.1186/1750-1326-9-20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117. Jay TR, Miller CM, Cheng PJ, et al. : TREM2 deficiency eliminates TREM2+ inflammatory macrophages and ameliorates pathology in Alzheimer's disease mouse models. J Exp Med. 2015; 212(3): 287–95. 10.1084/jem.20142322 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118. Wang Y, Ulland TK, Ulrich JD, et al. : TREM2-mediated early microglial response limits diffusion and toxicity of amyloid plaques. J Exp Med. 2016; 213(5): 667–75. 10.1084/jem.20151948 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119. Yuan P, Condello C, Keene CD, et al. : TREM2 Haplodeficiency in Mice and Humans Impairs the Microglia Barrier Function Leading to Decreased Amyloid Compaction and Severe Axonal Dystrophy. Neuron. 2016; 90(4): 724–39. 10.1016/j.neuron.2016.05.003 [DOI] [PMC free article] [PubMed] [Google Scholar]; Faculty Opinions Recommendation
- 120. Keren-Shaul H, Spinrad A, Weiner A, et al. : A Unique Microglia Type Associated with Restricting Development of Alzheimer's Disease. Cell. 2017; 169(7): 1276–1290.e17. 10.1016/j.cell.2017.05.018 [DOI] [PubMed] [Google Scholar]; Faculty Opinions Recommendation
- 121. Nugent AA, Lin K, van Lengerich B, et al. : TREM2 Regulates Microglial Cholesterol Metabolism upon Chronic Phagocytic Challenge. Neuron. 2020; 105(5): 837–854.e9. 10.1016/j.neuron.2019.12.007 [DOI] [PubMed] [Google Scholar]
- 122. Piers TM, Cosker K, Mallach A, et al. : A locked immunometabolic switch underlies TREM2 R47H loss of function in human iPSC-derived microglia. FASEB J. 2020; 34(2): 2436–2450. 10.1096/fj.201902447R [DOI] [PMC free article] [PubMed] [Google Scholar]; Faculty Opinions Recommendation
- 123. Zhou Y, Song WM, Andhey PS, et al. : Human and mouse single-nucleus transcriptomics reveal TREM2-dependent and TREM2-independent cellular responses in Alzheimer's disease. Nat Med. 2020; 26(1): 131–142. 10.1038/s41591-019-0695-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124. Hall-Roberts H, Agarwal D, Obst J, et al. : TREM2 Alzheimer's variant R47H causes similar transcriptional dysregulation to knockout, yet only subtle functional phenotypes in human iPSC-derived macrophages. Alzheimers Res Ther. 2020; 12(1): 151. 10.1186/s13195-020-00709-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125. Marschallinger J, Iram T, Zardeneta M, et al. : Lipid-droplet-accumulating microglia represent a dysfunctional and proinflammatory state in the aging brain. Nat Neurosci. 2020; 23(2): 194–208. 10.1038/s41593-019-0566-1 [DOI] [PMC free article] [PubMed] [Google Scholar]; Faculty Opinions Recommendation
- 126. Paloneva J, Kestilä M, Wu J, et al. : Loss-of-function mutations in TYROBP (DAP12) result in a presenile dementia with bone cysts. Nat Genet. 2000; 25(3): 357–61. 10.1038/77153 [DOI] [PubMed] [Google Scholar]
- 127. Paloneva J, Manninen T, Christman G, et al. : Mutations in two genes encoding different subunits of a receptor signaling complex result in an identical disease phenotype. Am J Hum Genet. 2002; 71(3): 656–62. 10.1086/342259 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128. Roumier A, Béchade C, Poncer JC, et al. : Impaired synaptic function in the microglial KARAP/DAP12-deficient mouse. J Neurosci. 2004; 24(50): 11421–8. 10.1523/JNEUROSCI.2251-04.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]; Faculty Opinions Recommendation
- 129. Rueda-Carrasco J, Hong S: The Jekyll and Hyde of TREM2. Trends Neurosci. 2020; 43(10): 739–740. 10.1016/j.tins.2020.08.002 [DOI] [PubMed] [Google Scholar]
- 130. Haber M, Zhou L, Murai KK: Cooperative astrocyte and dendritic spine dynamics at hippocampal excitatory synapses. J Neurosci. 2006; 26(35): 8881–91. 10.1523/JNEUROSCI.1302-06.2006 [DOI] [PMC free article] [PubMed] [Google Scholar]; Faculty Opinions Recommendation
- 131. Halassa MM, Fellin T, Takano H, et al. : Synaptic islands defined by the territory of a single astrocyte. J Neurosci. 2007; 27(24): 6473–7. 10.1523/JNEUROSCI.1419-07.2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132. Chai H, Diaz-Castro B, Shigetomi E, et al. : Neural Circuit-Specialized Astrocytes: Transcriptomic, Proteomic, Morphological, and Functional Evidence. Neuron. 2017; 95(3): 531–549.e9. 10.1016/j.neuron.2017.06.029 [DOI] [PMC free article] [PubMed] [Google Scholar]; Faculty Opinions Recommendation
- 133. Octeau JC, Chai H, Jiang R, et al. : An Optical Neuron-Astrocyte Proximity Assay at Synaptic Distance Scales. Neuron. 2018; 98(1): 49–66.e9. 10.1016/j.neuron.2018.03.003 [DOI] [PMC free article] [PubMed] [Google Scholar]; Faculty Opinions Recommendation
- 134. Haydon PG, Nedergaard M: How do astrocytes participate in neural plasticity? Cold Spring Harb Perspect Biol. 2014; 7(3): a020438. 10.1101/cshperspect.a020438 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135. Araque A, Parpura V, Sanzgiri RP, et al. : Tripartite synapses: Glia, the unacknowledged partner. Trends Neurosci. 1999; 22(5): 208–15. 10.1016/s0166-2236(98)01349-6 [DOI] [PubMed] [Google Scholar]
- 136. Eroglu C: The role of astrocyte-secreted matricellular proteins in central nervous system development and function. J Cell Commun Signal. 2009; 3(3–4): 167–76. 10.1007/s12079-009-0078-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137. Allen NJ, Bennett ML, Foo LC, et al. : Astrocyte glypicans 4 and 6 promote formation of excitatory synapses via GluA1 AMPA receptors. Nature. 2012; 486(7403): 410–4. 10.1038/nature11059 [DOI] [PMC free article] [PubMed] [Google Scholar]; Faculty Opinions Recommendation
- 138. Allen NJ, Eroglu C: Cell Biology of Astrocyte-Synapse Interactions. Neuron. 2017; 96(3): 697–708. 10.1016/j.neuron.2017.09.056 [DOI] [PMC free article] [PubMed] [Google Scholar]; Faculty Opinions Recommendation
- 139. Farhy-Tselnicker I, van Casteren ACM, Lee A, et al. : Astrocyte-Secreted Glypican 4 Regulates Release of Neuronal Pentraxin 1 from Axons to Induce Functional Synapse Formation. Neuron. 2017; 96(2): 428–445.e13. 10.1016/j.neuron.2017.09.053 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140. Tasdemir-Yilmaz OE, Freeman MR: Astrocytes engage unique molecular programs to engulf pruned neuronal debris from distinct subsets of neurons. Genes Dev. 2014; 28(1): 20–33. 10.1101/gad.229518.113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141. Chung WS, Allen NJ, Eroglu C: Astrocytes Control Synapse Formation, Function, and Elimination. Cold Spring Harb Perspect Biol. 2015; 7(9): a020370. 10.1101/cshperspect.a020370 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142. Chung WS, Verghese PB, Chakraborty C, et al. : Novel allele-dependent role for APOE in controlling the rate of synapse pruning by astrocytes. Proc Natl Acad Sci U S A. 2016; 113(36): 10186–91. 10.1073/pnas.1609896113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143. Hanayama R, Tanaka M, Miwa K, et al. : Identification of a factor that links apoptotic cells to phagocytes. Nature. 2002; 417(6885): 182–7. 10.1038/417182a [DOI] [PubMed] [Google Scholar]; Faculty Opinions Recommendation
- 144. Ye H, Li B, Subramanian V, et al. : NMR solution structure of C2 domain of MFG-E8 and insights into its molecular recognition with phosphatidylserine. Biochim Biophys Acta. 2013; 1828(3): 1083–93. 10.1016/j.bbamem.2012.12.009 [DOI] [PubMed] [Google Scholar]
- 145. Castellanos ER, Ciferri C, Phung W, et al. : Expression, purification, and characterization of recombinant human and murine milk fat globule-epidermal growth factor-factor 8. Protein Expr Purif. 2016; 124: 10–22. 10.1016/j.pep.2016.04.006 [DOI] [PubMed] [Google Scholar]
- 146. Neniskyte U, Neher JJ, Brown GC: Neuronal death induced by nanomolar amyloid β is mediated by primary phagocytosis of neurons by microglia. J Biol Chem. 2011; 286(46): 39904–13. 10.1074/jbc.M111.267583 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147. Neniskyte U, Brown GC: Lactadherin/MFG-E8 is essential for microglia-mediated neuronal loss and phagoptosis induced by amyloid β. J Neurochem. 2013; 126(3): 312–7. 10.1111/jnc.12288 [DOI] [PubMed] [Google Scholar]
- 148. Fricker M, Oliva-Martín MJ, Brown GC: Primary phagocytosis of viable neurons by microglia activated with LPS or Aβ is dependent on calreticulin/LRP phagocytic signalling. J Neuroinflammation. 2012; 9: 196. 10.1186/1742-2094-9-196 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149. Brelstaff J, Tolkovsky AM, Ghetti B, et al. : Living Neurons with Tau Filaments Aberrantly Expose Phosphatidylserine and Are Phagocytosed by Microglia. Cell Rep. 2018; 24(8): 1939–1948.e4. 10.1016/j.celrep.2018.07.072 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150. Cahoy JD, Emery B, Kaushal A, et al. : A transcriptome database for astrocytes, neurons, and oligodendrocytes: A new resource for understanding brain development and function. J Neurosci. 2008; 28(1): 264–78. 10.1523/JNEUROSCI.4178-07.2008 [DOI] [PMC free article] [PubMed] [Google Scholar]; Faculty Opinions Recommendation
- 151. Zeisel A, Muñoz-Manchado AB, Codeluppi S, et al. : Brain structure. Cell types in the mouse cortex and hippocampus revealed by single-cell RNA-seq. Science. 2015; 347(6226): 1138–42. 10.1126/science.aaa1934 [DOI] [PubMed] [Google Scholar]; Faculty Opinions Recommendation
- 152. Zeisel A, Hochgerner H, Lönnerberg P, et al. : Molecular Architecture of the Mouse Nervous System. Cell. 2018; 174(4): 999–1014.e22. 10.1016/j.cell.2018.06.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153. Habib N, McCabe C, Medina S, et al. : Disease-associated astrocytes in Alzheimer's disease and aging. Nat Neurosci. 2020; 23(6): 701–6. 10.1038/s41593-020-0624-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154. Hanayama R, Tanaka M, Miyasaka K, et al. : Autoimmune disease and impaired uptake of apoptotic cells in MFG-E8-deficient mice. Science. 2004; 304(5674): 1147–50. 10.1126/science.1094359 [DOI] [PubMed] [Google Scholar]; Faculty Opinions Recommendation
- 155. Miyanishi M, Tada K, Koike M, et al. : Identification of Tim4 as a phosphatidylserine receptor. Nature. 2007; 450(7168): 435–9. 10.1038/nature06307 [DOI] [PubMed] [Google Scholar]; Faculty Opinions Recommendation
- 156. Sapar ML, Ji H, Wang B, et al. : Phosphatidylserine Externalization Results from and Causes Neurite Degeneration in Drosophila. Cell Rep. 2018; 24(9): 2273–86. 10.1016/j.celrep.2018.07.095 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157. Fonseca MI, Chu SH, Hernandez MX, et al. : Cell-specific deletion of C1qa identifies microglia as the dominant source of C1q in mouse brain. J Neuroinflammation. 2017; 14(1): 48. 10.1186/s12974-017-0814-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158. Clarke LE, Liddelow SA, Chakraborty C, et al. : Normal aging induces A1-like astrocyte reactivity. Proc Natl Acad Sci U S A. 2018; 115(8): E1896–E1905. 10.1073/pnas.1800165115 [DOI] [PMC free article] [PubMed] [Google Scholar]; Faculty Opinions Recommendation
- 159. Liddelow SA, Guttenplan KA, Clarke LE, et al. : Neurotoxic reactive astrocytes are induced by activated microglia. Nature. 2017; 541(7638): 481–7. 10.1038/nature21029 [DOI] [PMC free article] [PubMed] [Google Scholar]; Faculty Opinions Recommendation
- 160. Yun SP, Kam TI, Panicker N, et al. : Block of A1 astrocyte conversion by microglia is neuroprotective in models of Parkinson's disease. Nat Med. 2018; 24(7): 931–8. 10.1038/s41591-018-0051-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161. Guttenplan KA, Weigel MK, Adler DI, et al. : Knockout of reactive astrocyte activating factors slows disease progression in an ALS mouse model. Nat Commun. 2020; 11(1): 3753. 10.1038/s41467-020-17514-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162. Fourgeaud L, Través PG, Tufail Y, et al. : TAM receptors regulate multiple features of microglial physiology. Nature. 2016; 532(7598): 240–4. 10.1038/nature17630 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163. Damisah EC, Hill RA, Rai A, et al. : Astrocytes and microglia play orchestrated roles and respect phagocytic territories during neuronal corpse removal in vivo. Sci Adv. 2020; 6(26): eaba3239. 10.1126/sciadv.aba3239 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164. Chen T, Lennon VA, Liu YU, et al. : Astrocyte-microglia interaction drives evolving neuromyelitis optica lesion. J Clin Invest. 2020; 130(8): 4025–38. 10.1172/JCI134816 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165. Jay TR, von Saucken VE, Muñoz B, et al. : TREM2 is required for microglial instruction of astrocytic synaptic engulfment in neurodevelopment. Glia. 2019; 67(10): 1873–92. 10.1002/glia.23664 [DOI] [PubMed] [Google Scholar]
- 166. Matejuk A, Ransohoff RM: Crosstalk Between Astrocytes and Microglia: An Overview. Front Immunol. 2020; 11: 1416. 10.3389/fimmu.2020.01416 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167. Mathys H, Adaikkan C, Gao F, et al. : Temporal Tracking of Microglia Activation in Neurodegeneration at Single-Cell Resolution. Cell Rep. 2017; 21(2): 366–80. 10.1016/j.celrep.2017.09.039 [DOI] [PMC free article] [PubMed] [Google Scholar]; Faculty Opinions Recommendation
- 168. Mathys H, Davila-Velderrain J, Peng Z, et al. : Single-cell transcriptomic analysis of Alzheimer's disease. Nature. 2019; 570(7761): 332–7. 10.1038/s41586-019-1195-2 [DOI] [PMC free article] [PubMed] [Google Scholar]; Faculty Opinions Recommendation
- 169. Hammond TR, Dufort C, Dissing-Olesen L, et al. : Single-Cell RNA Sequencing of Microglia throughout the Mouse Lifespan and in the Injured Brain Reveals Complex Cell-State Changes. Immunity. 2019; 50(1): 253–271.e6. 10.1016/j.immuni.2018.11.004 [DOI] [PMC free article] [PubMed] [Google Scholar]; Faculty Opinions Recommendation
- 170. Frigerio CS, Wolfs L, Fattorelli N, et al. : The Major Risk Factors for Alzheimer's Disease: Age, Sex, and Genes Modulate the Microglia Response to Aβ Plaques. Cell Rep. 2019; 27(4): 1293–1306.e6. 10.1016/j.celrep.2019.03.099 [DOI] [PMC free article] [PubMed] [Google Scholar]; Faculty Opinions Recommendation
- 171. Masuda T, Sankowski R, Staszewski O, et al. : Spatial and temporal heterogeneity of mouse and human microglia at single-cell resolution. Nature. 2019; 566(7744): 388–92. 10.1038/s41586-019-0924-x [DOI] [PubMed] [Google Scholar]; Faculty Opinions Recommendation
- 172. Boddaert J, Kinugawa K, Lambert JC, et al. : Evidence of a role for lactadherin in Alzheimer's disease. Am J Pathol. 2007; 170(3): 921–9. 10.2353/ajpath.2007.060664 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173. Geirsdottir L, David E, Keren-Shaul H, et al. : Cross-Species Single-Cell Analysis Reveals Divergence of the Primate Microglia Program. Cell. 2019; 179(7): 1609–1622.e16. 10.1016/j.cell.2019.11.010 [DOI] [PubMed] [Google Scholar]; Faculty Opinions Recommendation
- 174. Böttcher C, Schlickeiser S, Sneeboer MAM, et al. : Human microglia regional heterogeneity and phenotypes determined by multiplexed single-cell mass cytometry. Nat Neurosci. 2019; 22(1): 78–90. 10.1038/s41593-018-0290-2 [DOI] [PubMed] [Google Scholar]
- 175. Harris JA, Devidze N, Halabisky B, et al. : Many neuronal and behavioral impairments in transgenic mouse models of Alzheimer's disease are independent of caspase cleavage of the amyloid precursor protein. J Neurosci. 2010; 30(1): 372–81. 10.1523/JNEUROSCI.5341-09.2010 [DOI] [PMC free article] [PubMed] [Google Scholar]