Abstract
The purpose of this systematic review is to identify trends and extent of variability in intracranial vessel wall MR imaging (VWI) techniques and protocols. Although variability in selection of protocol design and pulse sequence type is known, data on what and how protocols vary is unknown. Three databases were searched to identify publications using intracranial VWI. Publications were screened by predetermined inclusion/exclusion criteria. Technical development publications were scored for completeness of reporting using a modified Nature Reporting Summary Guideline to assess reproducibility. From 2,431 articles, 122 met the inclusion criteria. Trends over the last 23 years (1995–2018) show increased use of 3 Tesla MR (p<0.001) and 3D volumetric T1-weighted acquisitions (p<0.001). Most (65%) clinical VWI publications report achieving a non-interpolated in-plane spatial resolution of ≤0.55 mm. In the last decade, an increasing number of technical development (n=20) and 7 Tesla (n=12) publications have been published, focused on pulse sequence development, improving cerebrospinal fluid suppression, scan efficiency, and imaging ex vivo specimen for histologic validation. Mean Reporting Summary Score for the technical development publications was high (0.87, range: 0.63–1.0) indicating strong scientific technical reproducibility. Innovative work continues to emerge to address implementation challenges. Gradual adoption into the research and scientific community was suggested by a shift in the name in the literature from “high resolution MR” to “vessel wall imaging,” specifying diagnostic intent. Insight into current practices and identifying the extent of technical variability in the literature will help direct future clinical and technical efforts to address needs for implementation.
Keywords: vessel wall MR imaging, intracranial atherosclerotic disease, magnetic resonance imaging, vasculopathy
Introduction
Intracranial vessel wall MR imaging (VWI) is a diagnostic imaging technique used to assess and differentiate intracranial vasculopathies.1 Conventional vessel imaging techniques, such as computed tomography angiography, digital subtraction angiography, and magnetic resonance angiography, show changes in lumen caliber, which are common morphologic changes in many intracranial vasculopathies and can be deemed nonspecific. Additional anatomic information about the vessel wall pathology by VWI can provide specificity to the working diagnosis and has resulted in an increasing number of publications.2 However, these publications report a wide variety of technical parameters and protocols, making it challenging to summarize the findings and compare the diagnostic technique across publications. Variability exists with the MR scanner (e.g., magnet strength, vendor, coil hardware), pulse sequence technique (e.g., contrast weighting, spatial coverage), and protocol (e.g., protocol lengths, use and timing of contrast).
One reason for the variability is the continued optimization of the technique. Increasing awareness of imaging artifacts and imaging constraints has resulted in a number of publications focused on further improving scan efficiency and minimizing artifacts.3 However, to facilitate the clinical translation of VWI for diagnostic imaging, consensus about the optimal methodology and reducing the technical variability would help with standardization across institutions and streamline multicenter diagnostic accuracy studies.
While it is known that there is variability in the selection of protocol design and pulse sequence types, data on what and how protocols vary remain unknown. We identify technical trends in intracranial VWI and examine the extent of technical variability. We also review the innovative direction of the VWI community by assessing technical development publications and assess trends in how these technical advances are incorporated into clinical research. Insight into current practices will help direct future clinical and technical efforts to address needs for implementation.
Methods
Search Strategy
This systematic review was conducted in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses guidelines. PubMed, EMBASE, and Medline were searched on September 12, 2018. To identify eligible studies, keywords covering vessel wall imaging, intracranial circulation, and vasculopathy keywords were searched using the Boolean operators “OR” and “AND” and previously reported in detail (Table 1).2 A manual review of the citations of each included article was also performed. All foreign language articles were translated. This literature search was previously used to assess vasculopathy type and funding trends of intracranial VWI publications.2 For this review, the inclusion criteria were modified and publications re-screened to assess protocols and technical trends.
Table 1:
Example Search Terms†
| For Imaging, Key Words Included: | |
| “magnetic resonance imaging” [MeSH Terms] OR (“magnetic”[All Fields] AND “resonance” [All Fields] AND “imaging”[All Fields]) OR “magnetic resonance imaging” [All Fields] OR “mri” [All Fields] OR neuroimaging [MeSH Terms] OR “neuroimaging” [All Fields] | |
| AND | |
| For Vessel Wall MR Imaging, Key Words Included: | |
| “blood vessels” [MeSH Terms] OR “blood”[All Fields] AND “vessels” [All Fields] OR “blood vessels” [All Fields] OR “vessel” [All Fields] AND wall[All Fields] AND “imaging” [All Fields] AND “blood vessels” [MeSH Terms] | |
| AND | |
| For Intracranial Circulation, Key Words Included: | |
| “cerebral arteries” [MeSH Terms] OR (“cerebral” [All Fields] AND “arteries”[All Fields]) OR “cerebral arteries” [All Fields] OR (“cerebral”[All Fields] AND “artery” [All Fields]) OR “cerebral artery” [All Fields], circle of willis [MeSH Terms] OR “circle” [All Fields] AND “willis” [All Fields] OR “circle of willis” [All Fields] | |
| AND | |
| Intracranial vascular disease, Key Words Included: | |
| “cerebral arterial diseases” [MeSH Terms] OR (“cerebral”[All Fields] AND “arterial” [All Fields] AND “diseases” [All Fields]) OR “cerebral arterial diseases” [All Fields] OR (“cerebral” [All Fields] AND “arterial”[All Fields] AND “disease” [All Fields]) OR “cerebral arterial disease” [All Fields], cerebrovascular disorders [MeSH Terms] OR (“cerebrovascular” [All Fields] AND “disorders” [All Fields]) OR “cerebrovascular disorders” [All Fields] | |
Abbreviations: MeSH, medical subject heading
Complete search terms available in Song et al 2019.3
Study selection
Two raters independently reviewed all publications for inclusion with discrepancies resolved by consensus. Inclusion criteria were (a) clinical research or technical development studies; (b) humans; (c) intracranial arteries with or without vasculopathy; (d) magnetic resonance imaging; and (e) vessel wall imaging. Studies with insufficient MR parameter information, single case reports, conference abstracts, and animal studies were excluded.
Data extraction
Data on MR vendor, magnet strength (Tesla, T), head coil, pulse sequence parameters, protocols, spatial resolution, acquisition plane and coverage, year of publication, study type, and studied vasculopathy were collected.
Acquired spatial resolution was calculated from the reported matrix size and field of view. If pulse sequence type (e.g., turbo/fast spin echo (TSE/FSE)) was not reported, the authors identified sequence type based on imaging parameters. Acquisition coverage was described as imaging the whole brain, a vessel segment (e.g., aneurysm, vessel stenosis), or the circle of Willis (e.g., anterior and posterior circulation).
To account for publications that originated from the same investigator/institution, publications were matched by corresponding author and institution. If more than 1 publication was identified from the same corresponding author/institution, MR vendor/protocol data were used from only 1 of the group’s most recent publications to avoid duplicating counts from prolific investigators. Analyses about clinical VWI protocol designs were performed only on clinical research investigations.
Technical development studies were further scored for completeness of reporting based on the MR Acquisition Section of the Nature Reporting Summary guidelines for MR studies4; the guideline was modified to include head coil, acquisition time, spatial resolution, and echo train length/turbo factor (Table 2). Sixteen criteria were scored as 1 (complete reporting), 0.5 (partial reporting), or 0 (did not fulfill reporting criteria). Scoring was performed independently by two raters and discrepancies resolved by consensus. Inter-rater agreement was calculated for the two raters.
Table 2:
Nature Reporting Summary for Magnetic Resonance Imaging, Acquisition
| Nature Reporting Summary Criteria for MR Acquisition4 | |
|---|---|
| Imaging type | Specify: functional, structural, diffusion, perfusion |
| Field strength | Specify in Tesla |
| Sequence and imaging parameters | Pulse sequence type |
| Area of acquisition | State whether a whole brain scan was used OR define the area of acquisition, describing how the region was determined |
| Additional vessel wall MR imaging relevant acquisition parameter details | |
| Acquisition time | |
| In-plane spatial resolution | |
| Echo train length or turbo factor | |
| Head coil | |
Statistical Analysis
Categorical variables are expressed in counts and percentages. Continuous variables are presented as means and standard deviations or medians and inter-quartile ranges. Agreement was calculated with an unweighted Cohen’s kappa. Logistic and linear regressions were used to test trends of categorical and continuous variables over the years, respectively. The Reporting Summary Score (RSS) was calculated by summing the points of the 16 criteria divided by total possible criteria fulfillment; the RSS ranged from 0 to 1. SPSS v19 (IBM, Chicago, IL) was used for statistical analysis.
Results
Search
From 2,431 articles, 1,635 were screened based on the inclusion/exclusion criteria (k=0.77, p<0.001) (Figure 1). Qualitative synthesis was conducted on 122 publications (κ=0.95, p<0.0001).
Figure 1: Literature search flowchart.
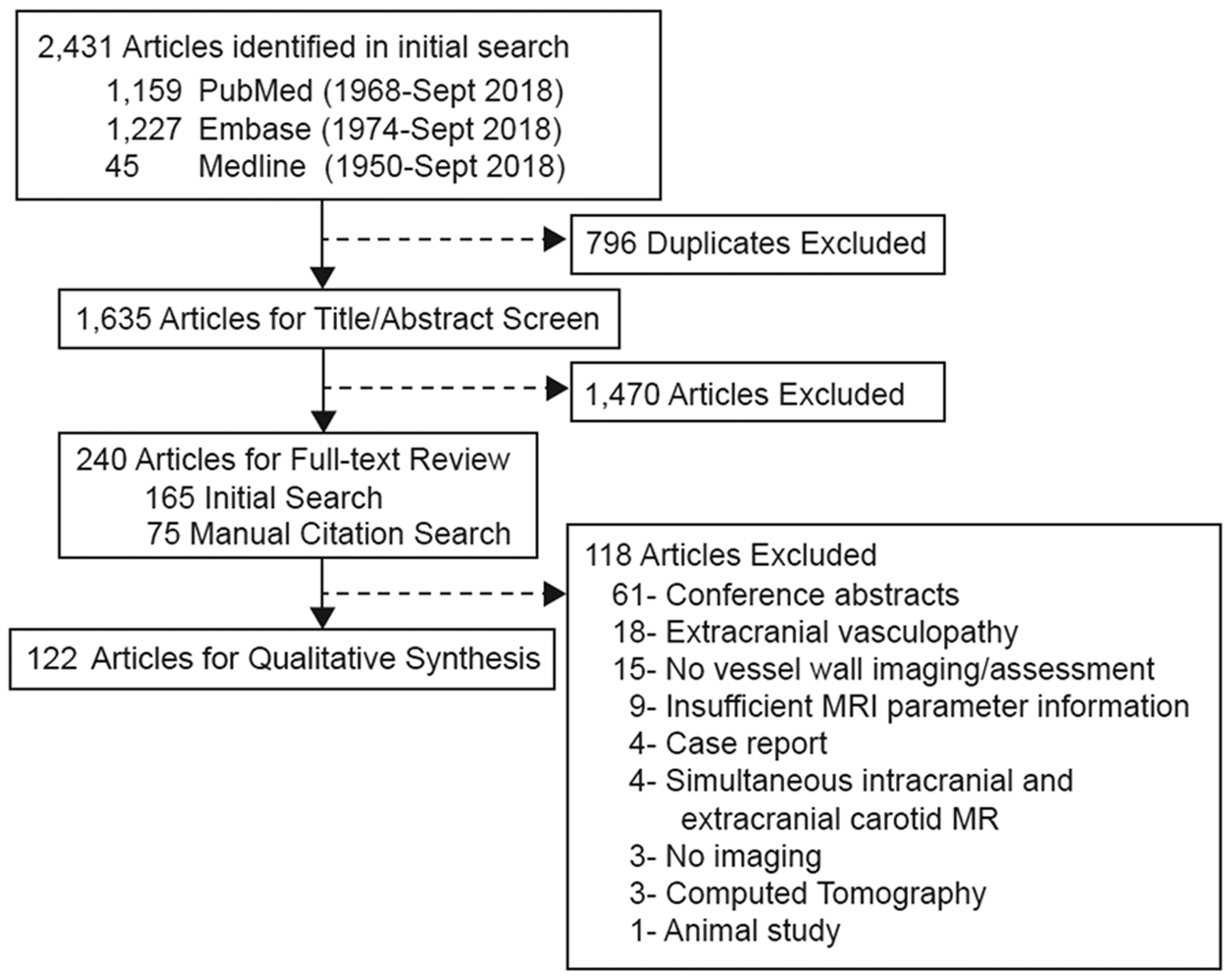
Database searches from PubMed, EMBASE and Medline identified 2,431 publications. From the initial search, 165 publications were identified for full-text review. A manual review of the citations of the 165 articles identified 807 citations, among which 75 publications met the inclusion/exclusion criteria. A total of 122 publications underwent qualitative data extraction.
Hardware Variability
To survey frequency of MRs by field strength and vendors, publications were matched by corresponding author/institution and vendor (n=104). Since 2008, a significant increase in the use of 3T for VWI emerged over time (β=5.36, 95% CI 3.34–7.12, p<0.001). By 2018, no VWI publications used 1.5T. Among publications using 3T field strength, the GE platform was the most frequently used (n=32), followed by Siemens (n=26), and Philips (n=23). For 7T, Philips was more commonly used (n=6) followed by Siemens (n=3). Among the studies using 3T, an 8 channel head coil was most frequently used (n=51) followed by 32 channel (n=8),5–12 16 channel (n=5),13–17 64 channel (n=2),18,19 12 channel (n=2),20,21 20 channel (n=2),22,23 custom-designed 36 channel (n=1)24 and 15 channel (n=1).25 Head coil was not reported in 8 publications.26–33 Two studies reported using a “standard head coil”34 and “loop coil (4 cm diameter).”35
Clinical Protocol Design Variability
Protocols from clinical research publications (n=93) varied with different combinations of T1-weighted (T1w), T2-weighted (T2w), and proton density-weighted (PDw) sequences (Figure 2A). The most common VWI protocol included 3D T1w followed by a combination of 2D T1w and 2D T2w acquisitions. Analyses by vasculopathy type showed protocol variability with 3D T1w acquisitions most frequently used to study atherosclerosis and dissection compared to 2D T1w for vasculitis, aneurysm, and reversible cerebral vasoconstriction syndrome (Figure 2B–D).
Figure 2: Intracranial vessel wall imaging (VWI) clinical protocol designs.
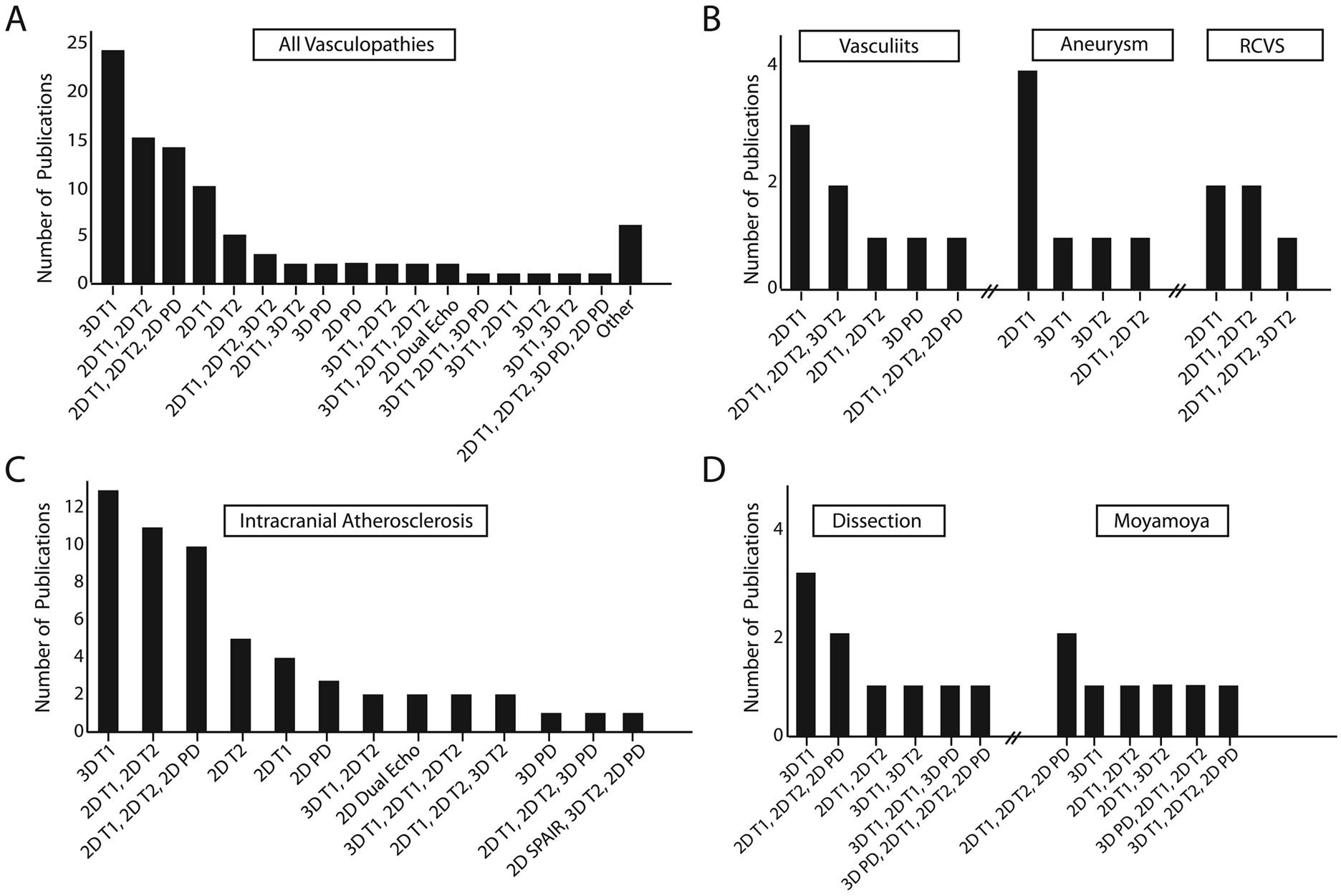
A: Protocols from 93 clinical publications using 1.5- or 3-Tesla varied with different combinations of T1-weighted (T1w), T2-weighted (T2w), and proton density-weighted (PDw) sequences. B: For intracranial atherosclerosis, 3D T1w acquisitions were the most common VWI protocol design. C: For vasculitis, aneurysms, and reversible cerebral vasoconstriction syndrome (RCVS), 2D T1w acquisitions were the most common protocol design. D: For arterial dissection, a 3D T1w acquisition protocol design was most common. Multicontrast weighting with 2D T1w, 2D T2w, and 2D PDw acquisitions was most commonly used for moyamoya syndrome and disease.
Abbreviation: SPAIR, spectral attenuated inversion recovery.
Most publications (86%) used time-of-flight magnetic resonance angiography (TOF-MRA) as the lumen-based imaging technique. No luminal imaging was reported for 8% of the publications. Both TOF-MRA and contrast-enhanced MRA (CE-MRA) were used in 3% of publications, while 1% reported using CE-MRA only.
The majority of the publications (n=67%) reported including postcontrast imaging among which 34% reported the contrast injection-to-scan time interval. The injection-to-scan time ranged from “immediate”36 to “within 20 minutes.”37 Other publications reported durations of 3 minutes (n=2),38,39 4 minutes (n=1)17, and 5 minutes (n=16).5,7,14,21,40–51 One publication evaluated only postcontrast without precontrast imaging.14
Pulse Sequence Variability
Figure 3 shows the percentages of 2D versus 3D and T1w, T2w, and PDw pulse sequences types. A trend was noted over 23 years with increased use of 3D T1w acquisitions compared to 2D acquisitions (β=2.54, 95% CI 1.29–3.80, p<0.001). The most common pulse sequence type was TSE/FSE or variable flip angle turbo spin echo (VFA-TSE; common vendor labels for VFA-TSE are sampling perfection with application optimized contrasts using different flip angle evolution (SPACE) for Siemens, volume isotropic turbo spin echo acquisition (VISTA) or volumetric isotropically reconstructed turbo spin-echo acquisition (VIRTA) for Philips, and CUBE for GE healthcare).
Figure 3: Types of pulse sequences in intracranial vessel wall imaging protocols.
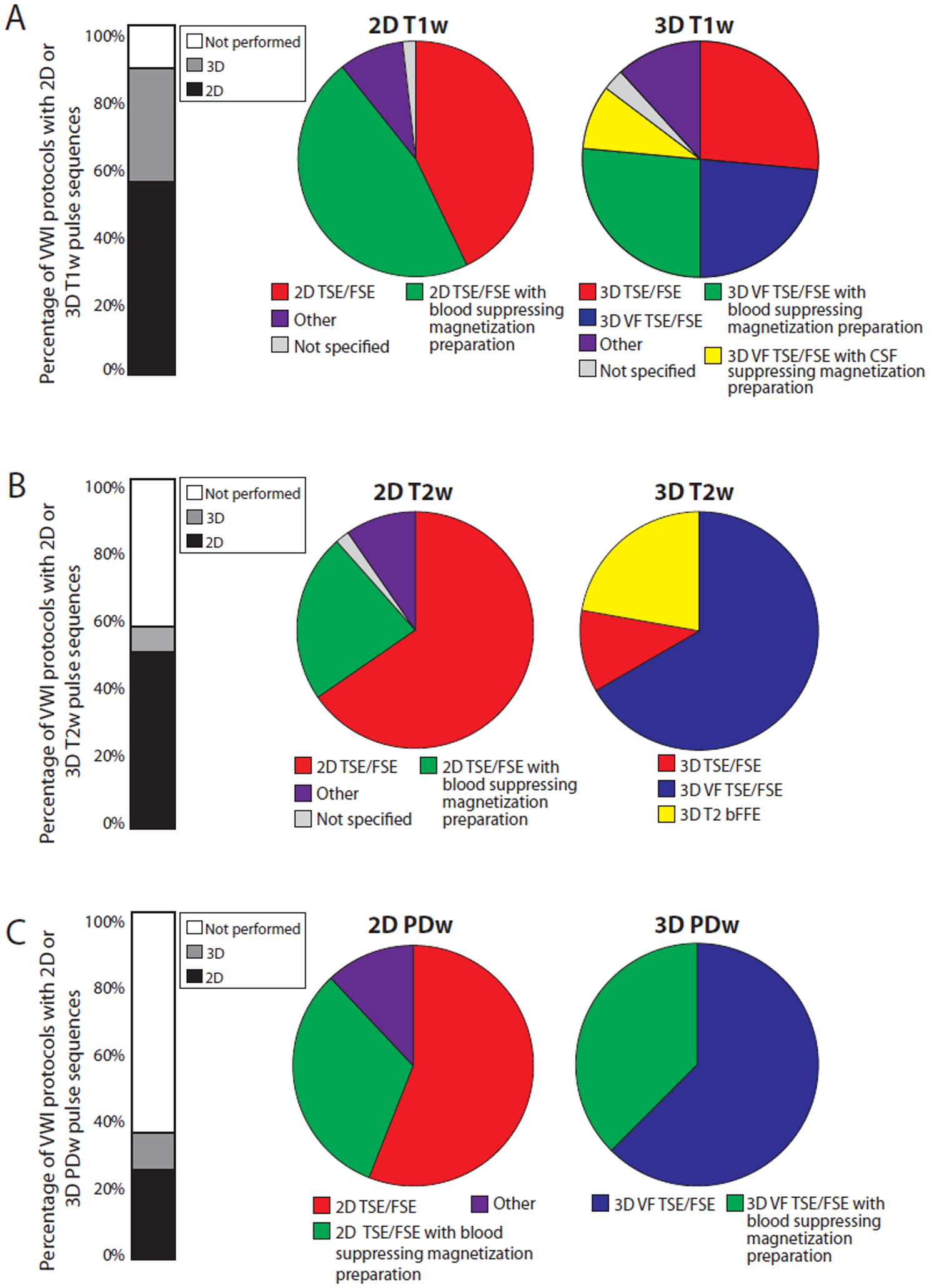
A: Types of T1-weighted (T1w) pulse sequences by 2D and 3D imaging. Other 2D T1w pulse sequences include: T1w fluid-attenuated inversion recovery with (n=1) and without (n=2) blood suppressing magnetization preparation, T1w spin echo with (n=1) and without (n=1) blood suppressing magnetization preparation. Other 3D T1w pulse sequences include: 3D magnetization prepared-rapid gradient echo, phase sensitive inversion recovery enabled 3D inversion recovery turbo field echo, and 3D TSE/FSE with blood suppressing magnetization preparation.
B: Types of T2-weighted (T2w) pulse sequences by 2D and 3D imaging. Other 2D T2w pulse sequences include: T2w spin echo with blood suppressing magnetization preparation (n=1) and dual echo with (n=2) and without (n=1) blood suppressing magnetization preparation. C: Types of proton density-weighted (PDw) pulse sequences by on 2D and 3D imaging. Other 2D PDw pulse sequences include: PDw spin echo (n=1) and dual echo with (n=2) and without (n=1) blood suppressing magnetization preparation.
Abbreviations: VFA-TSE, variable flip angle turbo spin-echo (vendor labels: SPACE, CUBE, VISTA, VIRTA); bFFE, balanced fast field echo.
The in-plane spatial resolution varied widely. Among the 82 clinical imaging publications that used T1w acquisitions on 1.5T or 3T, 46 did not report spatial resolutions and 20 reported an interpolation step. True acquired in-plane resolution was calculated by the authors. Twenty-three publications had insufficient parameter details and are not included in this analysis. Most (65%, n=38) publications achieved an in-plane resolution of ≤0.55mm with coverage focused on a vessel segment (n=22), circle of Willis (n=3) or whole brain (n=1) (Figure 4).
Figure 4: In-plane spatial resolution and anatomic coverage in intracranial vessel wall imaging.
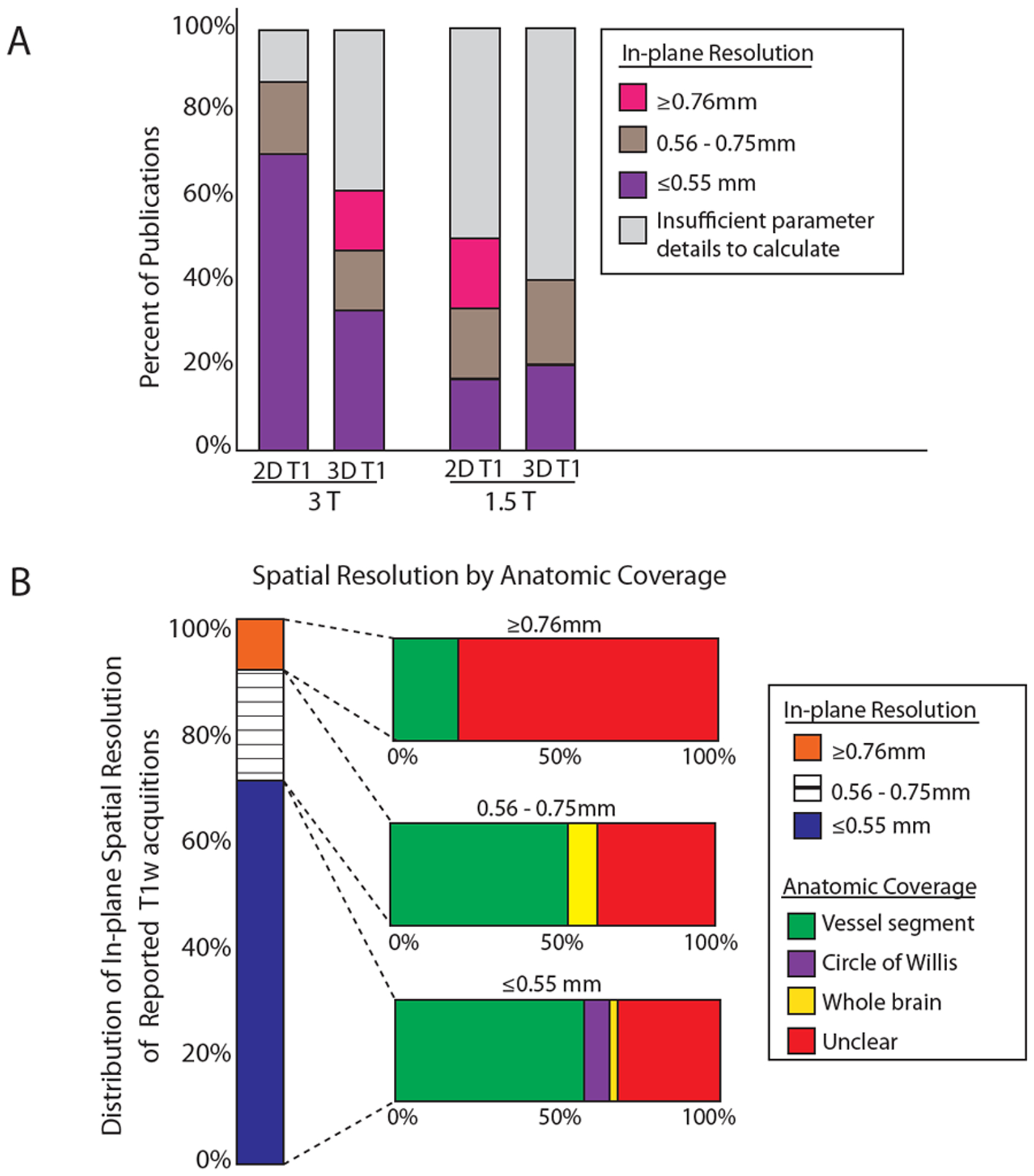
A: Acquired in-plane spatial resolution by magnet field strength. Publications from which non-interpolated spatial resolutions could be calculated were included in this analysis. The distribution of 2D and 3D T1-weighted acquisitions and the calculated in-plane spatial resolution are shown at 3 and 1.5 Tesla. B: Acquired in-plane spatial resolution by imaged anatomic coverage. The distribution of publications using T1-weighted acquisitions by calculated in-plane spatial resolution and imaged anatomic coverage is shown.
Motion and poor quality were cited as reasons for data exclusion in 40% of publications. Fifty-five articles did not report motion as a reason for exclusion, and it is unclear whether all exams were considered good quality or this information was not reported. When reported, a mean of 5% (range: 0–17%) of collected cases were excluded due to motion/poor quality. This loss of data due to motion degradation further highlights the need for improved scan efficiency.
Technical Development on 3T
The evolution of VWI has depended on technical innovations primarily focused on scan efficiency and reducing artifacts (e.g., CSF and blood suppression). Table 3 summarizes the 20 technical development articles at 3T. Study aims include pulse sequence development,6,52,53 improving scan efficiency,54–56 CSF suppression,9,11,57,58 assessment of reliability,59–63 and comparisons of 2D versus 3D acquisitions,64,65 acquisition planes,66 and 3T versus 7T imaging.22,67
Table 3:
3T Intracranial Vessel Wall MR Technical Development Publications and Reporting Summary Score
| Study* | MR Vendor, Magnet Strength, Head Coil | Study Aim | Protocol | In-plane spatial resolution (reported) | Anatomic Coverage | Acquisition Time | RSS¦¦ |
|---|---|---|---|---|---|---|---|
| Ma N, et al., 2011.37 | GE Signa, TwinSpeed, 3T, 8ch | Intra-rater and inter-rater reliability | 2D T1w DIR FSE, 2D PDw FSE and 2D T2w FSE | 2D T1w: 0.6 ×0.5 mm 2D PDw: 0.6 × 0.5 mm 2D T2w: 0.6 × 0.5 mm |
Basilar artery | NR | 0.78 |
| Qiao Y, et al., 2011.41 | Philips, Achieva, 3T, 8ch | Compare sequences (3D PD VISTA and 2D TSE) | 3D VISTA, 2D DIR TSE, 3D FLAIR VISTA | 3D VISTA: 0.5 mm and 0.4 mm 2D DIR TSE: 0.25 mm and 0.5 mm 3D FLAIR VISTA: 0.5 mm |
3D VISTA: coronal, COW|| 2D DIR TSE: basilar artery, M1 Middle cerebral artery, petrous ICA 3D FLAIR VISTA: NR |
3D VISTA: 7.9 min & 7.6 min 2D DIR TSE: 74 secs/slice & 37 secs/slice 3D FLAIR VISTA: NR |
0.94 |
| Yang WQ, et al., 2014.8 | GE Signa Excite HD, 3T, 8ch | Intra-rater and inter-rater reproducibility | 2D DIR T1w FSE, 2D T2w | T1w: 0.51 mm T2w: 0.51 mm |
Middle cerebral artery | NR | 0.72 |
| Lou X, et al., 2014.26 | GE Signa, Twin Speed, 3T, 8ch | Test sequence | 3D FDP-FSPGR | 0.6 × 0.5 mm | Basilar artery | 239 secs | 0.79 |
| Wang J, et al., 2016.35 | Philips, Achieva, 3T, 8ch | CSF and blood suppression (DANTE pre-pulse) | PDw VF-TSE with and without DANTE | 0.6 mm | NR§§ | 5 min 29 secs | 0.81 |
| Zhang L, et al., 2015.27 | Siemens, Trio, 3T, 32ch | Test sequence (3D T1w VF-TSE) | T1w VF-TSE | Two T1w SPACE pulse sequences: 0.52 mm & 0.5 mm |
Middle cerebral artery, basilar artery, petrous ICA | 10 min | 0.91 |
| Zhang X, et al., 2015.38 | GE Signa 3.0T HDxt, 8ch | Scan-rescan reproducibility | 2D T2w FSE | 0.39 × 0.31 mm | Middle cerebral artery | 3 min 45 sec | 0.88 |
| Li ML, et al., 2016.28 | GE Discovery, MR750, 3T, 8ch | Test sequence (3D VF-TSE) | T2w†, 3D T1w VF-TSE | 0.64 mm (calculated acquired) 0.4 mm (reconstructed) |
COW†† | 5 min | 0.85 |
| Fan Z, et al., 2017.33 | Siemens, Verio, 3T, 32ch | CSF suppression and increase spatial coverage to whole brain | 3D T1w VF-TSE and 3D T1w IR VF-TSE | 0.52 mm | Whole brain | 11 min 14 secs to 12 min | 0.94 |
| Zhu C, et al., 2016.43 | Siemens Skyra, 3T, 20ch | Compare 3T and 7T MR‡ | 3T: 3D VF-TSE with ETL40 and ETL60 | 3T: 3D VF-TSE with ETL40 and ELT60: 0.5 mm | COW‡‡ | 3T: 3D VF-TSE with ETL40: 10 min 14 secs 3D VF-TSE with ETL60: 9 min 58 secs |
0.94 |
| Yang H et al., 2016.34 | Philips, Achieva, 3T, 32ch | Optimize CSF suppression (anti-DRIVE) | 3D T1w VF-TSE with anti-DRIVE§ | 0.5 mm | NR | 5.4 min | 0.88 |
| Qiao Y, et al., 2016.36 | Multi-site study**, Siemens, 3T | Scan-rescan, intra-rater, inte-rrater reliability | 3D VF-TSE | 0.5 mm | COW | 8.3 min | 0.94 |
| Harteveld AA, et al., 2017.44 | Philips Achieva, 3T, 8ch | Compare 3T and 7T MR‡ | 3T: 3D T1w VIRTA with anti-DRIVE | 3T: 0.6 mm (acquired); 0.5 mm (reconstructed) | 3T: COW | 3T: 6 min 51 secs | 0.88 |
| Dieleman, N, et al., 2016.42 | Philips, Achieva, 3T, 8ch | Compare contrast weighting and acquisition planes | T1w VIRTA transverse with anti-driven Equilibrium (DRIVE) module and T1w/T2w/PDw VIRTA sagittal; | T1w VIRTA transverse and sagittal: 0.6 mm (acquired), 0.5 mm (reconstructed) T2w VIRTA sagittal: 0.7 mm (acquired), 0.6 mm (reconstructed) PDw VIRTA sagittal: 0.6 mm (acquired), 0.3 mm (reconstructed) |
COW | T1w VIRTA transverse: 6min 51 secs T1w VIRTA sagittal: 5 min 52 secs T2w VIRTA sagittal: 3 min 37 secs PDw VIRTA sagittal: 4 min 7 secs |
0.88 |
| Yang Q et al., 2017.30 | Siemens, Verio, 3T, 32ch | Optimize acquisition time | 3D T1w VF-TSE | 0.53 mm | Whole brain | 8min 7 secs | 0.94 |
| Zhu C, et al., 2018.31 | Siemens, Skyra 3T, 20ch | Optimize acquisition time | 3D T1w SPACE with compressed sensing | 0.5 mm (interpolated) |
Whole brain | 4 min 31 secs to 6 min 48 secs | 1.0 |
| Choi JW, et al., 2018.40 | Philips, Achieva, 3T, 16ch | Compare sequences | 2D PDw, 2D T2w, and 2D T1w TSE with pre-regional saturation pulse; CE-iMSDE-3D TSE MRI | CE-iMSDE-3D TSE MRI: 0.7 mm PDw, T2w, T1w TSE: NR |
Imaging range selected by clinician using CTA | PDw: 6 min T2w: 6 min 36 secs T1w: 5 min 40 secs CE-iMSDE-3D MRI: 4 min 50 secs |
0.65 |
| Cogswell PM, et al., 2018.32 | Philips, Achieva, 3T, 32ch | Optimize CSF suppression (DANTE) | 3D T1w/PDw TSE with anti-DRIVE with and without DANTE and 3D T2w TSE with DANTE | 0.5 mm | NR | 4 min 42 secs | 0.82 |
| Zhang N, et al., 2018.39 | Siemens, Verio, 3T, 32ch | Scan-rescan, intra-observer, inter-observer reliability | 3D T1 VF-TSE 2D T1 TSE |
3D T1w: 0.53 mm 2D T1w: 0.53 |
3D: Whole brain 2D: vessel segment |
3D: 7 min 10sec to 8 min 10 sec 2D: 1 min 37 sec |
0.94 |
| Lindenholz A, et al., 2018.29 | Philips, Achieva, 3T, 8ch | Optimize acquisition time | Compared 7 variants of: 3D T1w VISTA, 3D T1w VIRTA, 3D PDw VISTA Anti-DRIVE used in 3 of 7 variants |
Range of acquired resolution: 0.5 mm – 0.6 mm Range of reconstructed resolution: 0.5 mm – 0.6 mm |
COW | 4 min 39 secs to 8 min 24 secs | 0.94 |
Abbreviations: RSS, reporting summary score; Epub, electronic publication date; ch, channel; min, minutes; secs, seconds; CTA, computed tomography angiography; TSE, turbo spin echo; PD, proton density; VF-TSE, variable flip angle turbo spin-echo; DIR, double inversion recovery; VISTA, volume isotropic turbo spin echo acquisition; SPACE, sampling perfection with Application optimized contrasts using different flip angle evolution; IR, inversion recovery; VIRTA, volumetric isotropically reconstructed turbo spin-echo acquisition; DANTE, delay alternating with nutation for tailored excitation; anti-DRIVE, anti-Driven equilibrium module
Studies listed in order of online publication date to reflect precision in chronological timeline.
RRS calculated from modified criteria used in the Nature Reporting Summary.
Multi-site study, MR systems: Magnetom Skyra, 32ch; Magnetom Skyra, 20ch; Magnetom Trio, 12ch; Magnetom Verio, 12ch
Did not report parameter details for high-resolution T2WI.
Reported “to cover the major intracranial vessels as identified on the TOF MRA.”
Reported that a whole-brain MRA was used “for localizing the intracranial arteries.”
Reported as “120 coronal slices covering anterior and posterior circulation.”
7T MR parameters described in Table 3.
Reported as “prescribed to cover the major intracranial vessels.”
Tested 3D VF-TSE with anti-DRIVE (pre- and postcontrast), 3D VF-TSE (with TR of 1000 ms and 2 averages), and 3D fast field echo sequence (pre- and postcontrast).
To assess reporting completeness, a Reporting Summary Score (RSS) was calculated (κ=0.33, p<0.001) for the technical development publications. The mean RSS of the 20 publications was 0.87 (range: 0.63 to 1.0), suggesting strong technical reproducibility. Criteria that scored low included reporting the k-space sampling method (mean=0.28), matrix size (mean=0.65), and description of anatomic coverage (mean=0.75).
Ultra-high field 7T VWI
Table 4 summarizes 12 publications using 7T MR. Four publications imaged ex vivo specimens68–71 with acquisition times ranging from 1 hour 35 minutes to 11 hours 7 minutes. Publications covered topics of feasibility and sequence development,72–74 histologic validation,68–71 comparisons of 3T versus 7T22,75 and examining vasculopathies.76–78 The most commonly studied intracranial vasculopathies were atherosclerosis and aneurysms.
Table 4:
Ultra-high field 7 Tesla Vessel Wall Imaging Publications
| Study* | MR Vendor, Magnet Strength, Head coil | Pulse Sequence | Spatial resolution | Anatomic Coverage | Study Aim | Study conclusion |
|---|---|---|---|---|---|---|
| van der Kolk AG, et al., 2011.49 | Philips, 7T, 16ch | 3D T1w MPIR TSE (VF-TSE with CSF suppression MP) | 0.8 mm | COW (in vivo) | Develop sequence with goals of improving SNR, nulling CSF, and improving CNR. | Showed feasibility of visualizing vessel walls at 7T in both healthy and diseased intracranial arteries. |
| van der Kolk AG, et al., 2013.50 | Philips, 7T, 32ch | 3D T1w MPIR TSE (VF-TSE with CSF suppression MP) | 0.8 mm | Whole brain (in vivo) | Develop sequence with whole brain coverage with different image contrast weightings | Showed feasibility of a whole-brain multi-sequence vessel wall protocol at 7T. |
| Majidi S, et al., 2013.45 | Siemens, 7T, 16ch | 3D T1w VF-TSE | 0.13 mm | Vessel (ex vivo) | Identify plaque using virtual histology-intravascular ultrasound and compare a subset with 7T MR and histology ex vivo | Virtual histology intravascular ultrasonography and high-resolution MRI at 7T are reliable tools to detect plaque but have limitations with characterizing plaque components. |
| Kleinloog R, et al., 2014.46 | Philips, 7T, 32ch | 3D T1w MPIR TSE (VF-TSE with CSF suppression MP) | 0.8 mm (in vivo); 0.18 mm (ex vivo) | Whole brain (in vivo) and wall biopsy (ex vivo) | Validate in vivo and ex vivo imaging of intracranial aneurysm wall | Unruptured aneurysm wall thickness varies and can be seen as variations in signal intensity with 7T. |
| van der Kolk AG, et al., 2015.23 | Philips, 7T, 16ch or 32ch | 3D T1w MPIR TSE (VF-TSE with CSF suppression MP) | 0.8 mm | COW (in vivo) and whole brain (in vivo) | Examine preferential sites and stability of enhancing intracranial plaque. | Majority of vessel lesions were locationed in the intracranial ICA and M1-M2 MCA segments. At a mean length of 53 days (range: 23–205 days) after initial stroke/TIA presentation, 16.7% of lesions showed change in presence or enhancement characteristics. |
| Harteveld AA, et al., 2016.48 | Philips, 7T, 16ch | Driven equilibrium single pulse observation of T1w and T2w, Dual Echo 3D T2* | 0.13 mm | Circle of Willis (ex vivo) | Quantitatively characterize plaque components by obtaining MR signal characteristics and use histologic validation | Different tissue components of plaques can be distinguished with quantitative MR at 7T; T1w imaging is the most promising compared to PD, T2, T2*. |
| Blankena R, et al., 2016.52 | Philips, 7T, 32ch | 3D T1w MPIR TSE (VF-TSE with CSF suppression MP) | 0.8 mm | Whole brain (in vivo) | Compare 7T and time-resolved 3D PCMR to study aneurysm wall shear stress. | There is an inverse correlation between apparent unruptured aneurysm wall thickness and wall sheer stress. |
| Viessmann O, et al., 2017.51 | Siemens, 7T, 32ch | 3D T2w VF-TSE versus 3D T2w DANTE prepared VF-TSE | 0.5 × 0.5 × 1 mm | NR (in vivo) | Optimize sequence at 7T for sharper wall depiction and contrast | DANTE pre-pulse results in improved CSF-outer lumen wall conspicuity with a trade-off of lower wall-to-lumen contrast. |
| Zhu C, et al., 2016.43 | Siemens, 3T, 20ch and 7T, 32ch| | 7T: 3D T1w VF-TSE | 0.5 mm§ | COW or whole brain (in vivo)† | Compare 3D T1 VF-TSE at 3T and 7T of intracranial vessel walls | 3D T1 VF-TSE can be used on both 3T and 7T; there is better image quality at 7T. |
| Harteveld AA, et al., 2017.44 | Philips, 3T, 8ch and 7T, 32ch| | 7T: 3D T1w MPIR-TSE (3D T1w VF-TSE with CSF suppression MP) | 0.8 mm (acquired) and 0.49 mm (reconstructed) | 7T: Whole Brain (in vivo) | Compare 3T and 7T in elderly asymptomatic population | Vessel wall visibility is equal or significantly better at 7T and most prominent in the proximal anterior cerebral circulation and P2 PCA. The vertebral and basilar arteries were visualized equally well at 3T and 7T. |
| Harteveld AA, et al., 2017.16 | Philips, 7T, 32ch | 3D T1w MPIR TSE | 0.8 mm | Whole brain (in vivo) | Assess vessel wall lesion burden between recent posterior circulation ischemic strokes and healthy controls | Vessel wall lesions in the PCA were more frequently observed in patients than controls. No significant differences in total vessel wall lesion burden in anterior and posterior circulation or in the proportion of enhancing vessel wall lesions between patients and controls. |
| Harteveld AA, et al., 2018.47 | Philips, 7T, 16ch | 3D T1w gradient echo | 0.11 mm (specimen); 0.13 mm (histology) | COW (ex vivo) | Measure vessel wall thickness in ex vivo COW specimens using 7T | MR measurements showed excellent agreement with histology; mean wall thickness varied from 0.45–0.66 mm. |
Abbreviations: T, Tesla; Epub, electronic publication date; hr, hour; min, minutes; sec, seconds; COW, circle of Willis; NR, not reported; FOV, field of view; SNR, signal to noise ratio; CNR, contrast to noise ratio; MPIR, magnetization-prepared inversion-recovery; TSE, turbo spin echo; NR, not reported; TIA, transient ischemic attack; VF-TSE, variable flip angle turbo spin echo; CSF, cerebrospinal fluid; MP, magnetization preparation; PCMR, phase-contrast MR; DANTE, delay alternating with nutation for tailored excitation; MCA, middle cerebral artery; PCA, posterior cerebral artery
Studies listed in order of online publication date to reflect precision in chronological timeline.
Reports, “prescribed to cover the major intracranial vessels.”
Acquired resolution not reported; resolution in Table 3 is calculated from parameter details.
3 Tesla pulse sequence described in Table 2.
Clinical Adoption of 3T Technical Developments
Over the last decade, VWI pulse sequence developments have mainly focused on VFA-TSE at 3T. To reduce artifacts, magnetization preparations for blood and CSF suppression have been investigated.9,11,57,58 The impact of these ongoing improvements to VWI pulse sequences were trended by online publication dates (Figure 5). Online publication dates were used to show precision in chronological trends. After the first publication to use VFA-TSE,79 an increasing number of clinical publications used VFA-TSE from 2013 onward. Blood suppression magnetization preparation modules were previously used to study carotid vessel wall MR and the results suggest this technique was more readily incorporated into intracranial VWI clinical research investigations. A CSF suppression module was first reported by Wang et al57 using delay-alternating with nutation for tailored excitation (DANTE, a blood-suppressing magnetization preparation module). A truly dedicated CSF suppression module was subsequently published by Fan et al,58 after which an increasing number of technical development and clinical imaging publications reported VWI with different CSF suppression techniques.
Figure 5: Impact of 3-Tesla technical developments into clinical imaging publications.
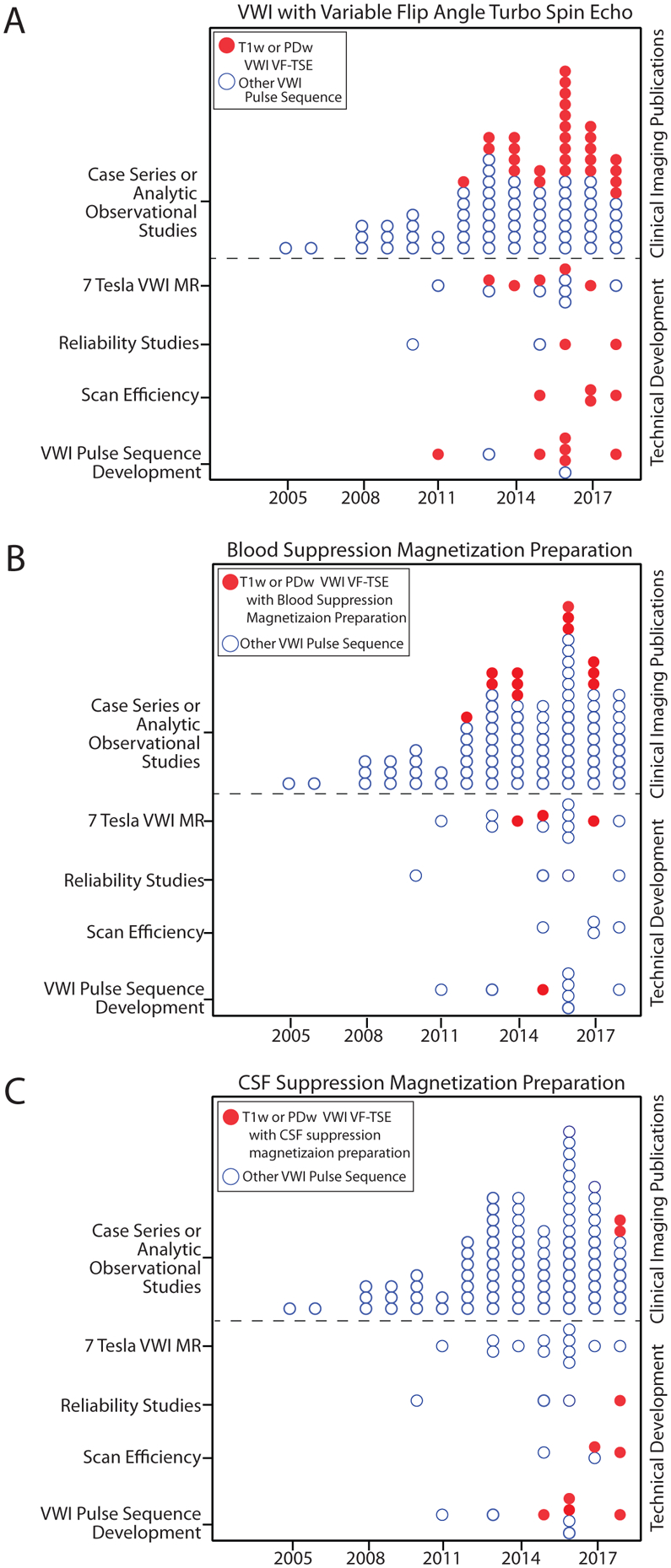
A: After the first technical development publication reported the use of VFA-TSE in 2011, an increasing number of publications using this pulse sequence emerged. This transition suggests an adoption of this pulse sequence for clinical vessel wall imaging (VWI) research investigating intracranial vasculopathies. B: Use of blood suppression magnetization preparation modules in clinical imaging publications for intracranial vasculopathies was first seen in 2012 with one dedicated technical development publication testing this module. C: CSF-suppression magnetization preparation was first tested in 2 technical development publications in 2015. A gradual increase in the number of publications in both technical development and clinical imaging publications are seen thereafter.
Abbreviations: VFA-TSE, variable flip angle turbo spin echo; T1w, T1-weighted; PDw, proton density-weighted.
Trends in Names for VWI
The name for VWI was classified into 3 categories: “vessel wall imaging or high-resolution vessel wall imaging,” “high-resolution MR” and “other.” The name “high-resolution MR” peaked in 2016. A shift was noted in 2017 with more frequent use of the specific term “vessel wall imaging” (Figure 6), indicating diagnostic intent in the name.
Figure 6:
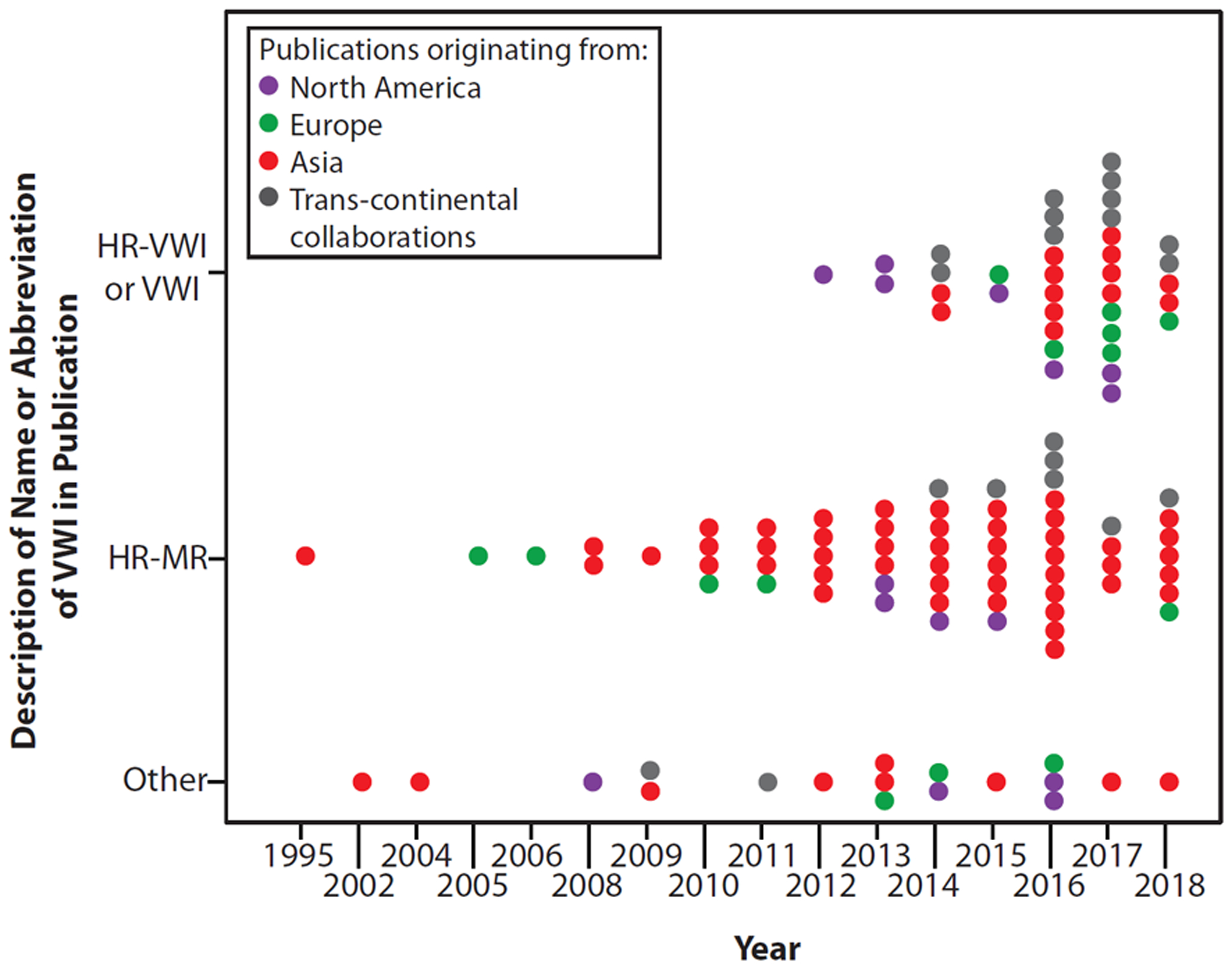
Changing trends in the name for intracranial vessel wall imaging publications from 1995 to 2018 show a shift in the name in 2016–2017.
Discussion
Considerable technical variability exists for intracranial vessel wall MR imaging. We identified changing trends and most common practices based on the literature. VWI is now primarily performed at 3T with no publications at 1.5T identified in 2018. The selection of pulse sequence and protocol design remains variable. Literature shows a shift from 2D to 3D volumetric acquisitions. There is gradual adoption of technical developments for VWI pulse sequences aimed to reduce blood and CSF flow artifacts. While advancements with technical optimization and innovative research on 7T MR continue, VWI remains clinically investigative and is in the assessment phase of the health technology assessment framework.
The American Society of Neuroradiology Vessel Wall Study Group set forth expert consensus recommendations for techniques to consider.1 Results show that 3T has become the magnet strength of choice, likely due to a higher signal-to-noise ratio than that available from 1.5T, and most studies achieve the recommended 0.5 mm in-plane spatial resolution. Results show a 3D T1w sequence to be most commonly used for VWI. However, selection of the optimal combination of tissue weightings for protocol design may vary by vasculopathy type and institutional clinical workflows. The role of 7T MR remains innovative and investigative with studies reporting on feasibility.22,75
Several insights from this study warrant attention. First, lengthy acquisition times are a barrier to implementation and is evidenced by the high reported rates of data exclusion due to motion degraded images. This loss of data highlights the need for improved scan efficiency. Second, the American Society of Neuroradiology Vessel Wall study group recommends an in-plane resolution of ≤0.5mm.1 Although, most publications are achieving this recommended resolution, spatial coverage varied widely. A possible explanation for this may be based on clinical indications. Assessing vasculitis or ICAD may be more diffuse with advantages of imaging the whole-brain with 3D imaging. By contrast, 2D acquisitions and multiple contrast weightings may be optimal to characterize a vessel segment for stenosis or an aneurysm. Indeed, our results show different protocols are reported for different intracranial vasculopathies, highlighting an area for future investigations. Third, wall enhancement signal intensity varies by the contrast injection-to-postcontrast image duration.80 The wide range of reported injection-to-scan time indicates a need for concerted efforts towards consistency and a need to identify a defined time interval in protocol design. Finally, CSF suppression techniques such as utilizing a post readout magnetization flip-down,58 anti-driven equilibrium module,11 or DANTE preparation57 are being investigated for intracranial VWI. These techniques reportedly reduce vessel wall signal-to-noise ratios leading to underestimations of imaging endpoint metrics, such as vessel wall thickness.9
The impact of technical variability for VWI hinges upon identifying reliable imaging endpoints/biomarkers for the type of intracranial vasculopathy being assessed. Questions remain as to which imaging endpoints are the strongest for diagnostic accuracy. To identify endpoints, meaningful data synthesis by meta-analyses requires reduced data heterogeneity. For example, a meta-analysis assessing intracranial atherosclerotic plaque using VWI reported wall enhancement, positive wall remodeling, and plaque surface irregularity to be significantly associated with ischemic stroke.81 However, the analysis was hampered by data heterogeneity from patient selection and technique. The development of vessel wall phantoms shows effort towards technical standardization across sites.82 Furthermore, in parallel, assessing diagnostic performance with consensus on imaging endpoints is needed to determine the necessary degree of imaging precision and measurement reliability.39,59,60,62,63,83 A future direction is to assess the diagnostic performance of imaging endpoints by vasculopathy type. Consensus on which imaging endpoint is critical for diagnosis, defining the minimum clinically achievable image quality, and showing measurement reliability and reproducibility are important steps for generalizability.
Clinical adoption is incremental. It is promising to see gradual incorporation of technical developments into clinical research. In recent years, a shift in the name from “high-resolution MR” to “vessel wall imaging” emerged suggesting universal recognition of the technique specifically for vessel wall assessment. “High-resolution” MR is vague and could reflect spatial or temporal resolution. Thus, the shift to “vessel wall imaging,” conferring diagnostic intent and specificity, is a step towards clinical adoption.
A limitation of this systematic review is the available data of MR technical details. Reporting of MR technical parameters varied widely across clinical journals. This variability may partly be explained by different journals with varying target audiences. Furthermore, a plethora of vendor specific labels adds a layer of jargon. Among the technical development publications, completeness of reporting was high when assessed by a modified version of the Nature Reporting Guideline suggesting reproducibility.
Conclusion
VWI investigations show active involvement at a medical-scientific interface among clinicians and MR physicists working to optimize technical performance to study intracranial vasculopathies. We show data on the extent of variability of intracranial VWI protocols in the literature. Trends in the literature suggest continued optimizations to address implementation challenges through technical development and innovative research. Based on the published literature, collective efforts leading to clinical adoption is on the horizon.
Acknowledgements and Disclosure:
This study was funded by the RSNA Research & Education Foundation, through grant number RSCH1929. The content is solely the responsibility of the authors and does not necessarily represent the official views of the RSNA R&E Foundation. This study was also funded by the Institute for Translational Medicine and Therapeutics/Thomas B. McCabe and Jeannette E. Laws McCabe Fund (JWS) and NIH National Heart, Lung, and Blood Institute R01HL137984 (WRW) and R01HL147355 (ZF).
References
- 1.Mandell DM, Mossa-Basha M, Qiao Y, et al. Intracranial vessel wall MRI: principles and expert consensus recommendations of the American Society of Neuroradiology. AJNR Am J Neuroradiol 2017;38:218–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Song JW, Guiry SC, Shou H, et al. Qualitative assessment and reporting quality of intracranial vessel wall MR imaging studies: a systematic review. AJNR Am J Neuroradiol 2019;40:2025–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lindenholz A, van der Kolk AG, Zwanenburg JJ, et al. The use and pitfalls of intracranial vessel wall imaging: how we do it. Radiology 2018;286:12–28. [DOI] [PubMed] [Google Scholar]
- 4.Nature research reporting summary, magnetic resonance imaging, acquisition. Nature Research Website. https://www.nature.com/nature-research/editorial-policies/reporting-standards; https://www.nature.com/documents/nr-reporting-summary-flat.pdf. Published 2019. [Google Scholar]
- 5.Wu F, Ma Q, Song H, et al. Differential features of culprit intracranial atherosclerotic lesions: A whole-brain vessel wall imaging study in patients with acute ischemic stroke. Journal of the American Heart Association 2018;7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zhang L, Zhang N, Wu J, et al. High resolution three dimensional intracranial arterial wall imaging at 3 T using T1 weighted SPACE. Magn Reson Imaging 2015;33:1026–34. [DOI] [PubMed] [Google Scholar]
- 7.Wu F, Song H, Ma Q, et al. Hyperintense plaque on intracranial vessel wall magnetic resonance imaging as a predictor of artery-to-artery embolic infarction. Stroke 2018;49:905–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zou X, Chung Y, Zhang L, et al. Middle cerebral artery atherosclerotic plaques in recent small subcortical infarction: a three-dimensional high-resolution MR study. BioMed Res Int 2015;2015:540217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cogswell PM, Siero JCW, Lants SK, et al. Variable impact of CSF flow suppression on quantitative 3.0T intracranial vessel wall measurements: CSF flow suppression in intracranial vessel wall measurements. J Magn Reson Imaging 2018;48:1120–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Matouk CC, Mandell DM, Günel M, et al. Vessel wall magnetic resonance imaging identifies the site of rupture in patients with multiple intracranial aneurysms: proof of principle. Neurosurgery 2013;72:492–6. [DOI] [PubMed] [Google Scholar]
- 11.Yang H, Zhang X, Qin Q, et al. Improved cerebrospinal fluid suppression for intracranial vessel wall MRI. J Magn Reson Imaging 2016;44:665–72. [DOI] [PubMed] [Google Scholar]
- 12.Bae YJ, Choi BS, Jung C, et al. Differentiation of deep subcortical infarction using high-resolution vessel wall MR imaging of middle cerebral artery. Korean J Radiol 2017;18:964–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Choi JW, Han M, Hong JM, et al. Feasibility of improved motion-sensitized driven-equilibrium (iMSDE) prepared 3D T1-weighted imaging in the diagnosis of vertebrobasilar artery dissection. J Neuroradiol 2018;45:186–91. [DOI] [PubMed] [Google Scholar]
- 14.Jang J, Kim T, Hwang E, et al. Assessment of arterial wall enhancement for differentiation of parent artery disease from small artery disease: comparison between histogram analysis and visual analysis on 3-dimensional contrast-enhanced T1-weighted turbo spin echo MR images at 3T. Korean J Radiol 2017;18:383–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Edjlali M, Gentric JC, Régent-Rodriguez C, et al. Does aneurysmal wall enhancement on vessel wall MRI help to distinguish stable from unstable intracranial aneurysms? Stroke 2014;45:3704–6. [DOI] [PubMed] [Google Scholar]
- 16.Yu JH, Kwak HS, Chung GH, et al. Association of intraplaque hemorrhage and acute infarction in patients with basilar artery plaque. Stroke 2015;46:2768–72. [DOI] [PubMed] [Google Scholar]
- 17.Ryu CW, Jahng GH, Shin HS. Gadolinium enhancement of atherosclerotic plaque in the middle cerebral artery: relation to symptoms and degree of stenosis. AJNR Am J Neuroradiol 2014;35:2306–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yun SY, Heo YJ, Jeong HW, et al. Spontaneous intracranial vertebral artery dissection with acute ischemic stroke: high-resolution magnetic resonance imaging findings. Neuroradiol J 2018;31:262–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jung SC, Kim HS, Choi C-, et al. Spontaneous and unruptured chronic intracranial artery dissection: high-resolution magnetic resonance imaging findings. Clin Neuroradiol 2018;28:171–81. [DOI] [PubMed] [Google Scholar]
- 20.Niu PP, Yu Y, Zhou HW, et al. Vessel wall differences between middle cerebral artery and basilar artery plaques on magnetic resonance imaging. Sci Rep 2016;6:38534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Omodaka S, Endo H, Niizuma K, et al. Quantitative assessment of circumferential enhancement along the wall of cerebral aneurysms using MR imaging. AJNR Am J Neuroradiol 2016;37:1262–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zhu C, Haraldsson H, Tian B, et al. High resolution imaging of the intracranial vessel wall at 3 and 7 T using 3D fast spin echo MRI. MAGMA 2016;29:559–70. [DOI] [PubMed] [Google Scholar]
- 23.Obusez EC, Hui F, Hajj-Ali R, et al. High-resolution MRI vessel wall imaging: spatial and temporal patterns of reversible cerebral vasoconstriction syndrome and central nervous system vasculitis. AJNR Am J Neuroradiol 2014;35:1527–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chen Z, Liu AF, Chen H, et al. Evaluation of basilar artery atherosclerotic plaque distribution by 3D MR vessel wall imaging. J Magn Reson Imaging 2016;44:1592–9. [DOI] [PubMed] [Google Scholar]
- 25.Sun L, Li Z, Tang W, et al. High resolution magnetic resonance imaging in pathogenesis diagnosis of single lenticulostriate infarction with nonstenotic middle cerebral artery, a retrospective study. BMC Neurol 2018;18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ahn SH, Lee J, Kim YJ, et al. Isolated MCA disease in patients without significant atherosclerotic risk factors: a high-resolution magnetic resonance imaging study. Stroke 2015;46:697–703. [DOI] [PubMed] [Google Scholar]
- 27.Thaler C, Kaufmann-Bühler A, Gansukh T, et al. Neuroradiologic characteristics of primary angiitis of the central nervous system according to the affected vessel size. Clin Neuroradiol 2019;29:37–44. [DOI] [PubMed] [Google Scholar]
- 28.Li F, Chen QX, Chen ZB, et al. Magnetic resonance imaging of plaque burden in vascular walls of the middle cerebral artery correlates with cerebral infarction. Curr Neurovasc Res 2016;13:263–70. [DOI] [PubMed] [Google Scholar]
- 29.Chen CY, Chen SP, Fuh JL, et al. Vascular wall imaging in reversible cerebral vasoconstriction syndrome - A 3-T contrast-enhanced MRI study. J Headache Pain 2018;19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Muraoka S, Araki Y, Taoka T, et al. Prediction of intracranial arterial stenosis progression in patients with moyamoya vasculopathy: contrast-enhanced high-resolution magnetic resonance vessel wall imaging. World Neurosurg 2018;116:e1114–21. [DOI] [PubMed] [Google Scholar]
- 31.Cheng-Ching E, Jones S, Hui FK, et al. High-resolution MRI vessel wall imaging in varicella zoster virus vasculopathy. J Neurol Sci 2015;351:168–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Niizuma K, Shimizu H, Takada S, et al. Middle cerebral artery plaque imaging using 3-Tesla high-resolution MRI. J Clin Neurosci 2008;15:1137–41. [DOI] [PubMed] [Google Scholar]
- 33.Feng C, Xu Y, Bai X, et al. Basilar artery atherosclerosis and hypertensive small vessel disease in isolated pontine infarctions: A study based on high-resolution MRI. Eur Neurol 2013;70:16–21. [DOI] [PubMed] [Google Scholar]
- 34.Mossa-Basha M, Shibata DK, Hallam DK, et al. Added value of vessel wall magnetic resonance imaging for differentiation of nonocclusive intracranial vasculopathies. Stroke 2017;48:3026–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Jiang Y, Zhu C, Peng W, et al. Ex-vivo imaging and plaque type classification of intracranial atherosclerotic plaque using high resolution MRI. Atherosclerosis 2016;249:10–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Skarpathiotakis M, Mandell DM, Swartz RH, et al. Intracranial atherosclerotic plaque enhancement in patients with ischemic stroke. AJNR Am J Neuroradiol 2013;34:299–304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Vakil P, Vranic J, Hurley MC, et al. T1 gadolinium enhancement of intracranial atherosclerotic plaques associated with symptomatic ischemic presentations. AJNR Am J Neuroradiol 2013;34:2252–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Huang B, Yang WQ, Liu XT, et al. Basilar artery atherosclerotic plaques distribution in symptomatic patients: A 3.0 T high-resolution MRI study. Eur J Radiol 2013;82:e199–203. [DOI] [PubMed] [Google Scholar]
- 39.Yang W, Huang B, Liu X, et al. Reproducibility of high-resolution MRI for the middle cerebral artery plaque at 3T. Eur J Radiol 2014;83:49. [DOI] [PubMed] [Google Scholar]
- 40.Wang M, Yang Y, Zhou F, et al. The contrast enhancement of intracranial arterial wall on high-resolution MRI and its clinical relevance in patients with moyamoya vasculopathy. Sci Rep 2017;7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lou X, Jiang W, Ma L, et al. In vivo high-resolution magnetic resonance imaging in severe intracranial stenosis. Zhonghua Nei Ke Za Zhi 2008;47:478–81. [PubMed] [Google Scholar]
- 42.Lou X, Ma N, Ma L, et al. Contrast-enhanced 3T high-resolution MR imaging in symptomatic atherosclerotic basilar artery stenosis. AJNR Am J Neuroradiol 2013;34:513–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Harteveld AA, van der Kolk AG, van der Worp HB, et al. Detecting intracranial vessel wall lesions with 7T-magnetic resonance imaging: patients with posterior circulation ischemia versus healthy controls. Stroke 2017;48:2601–4. [DOI] [PubMed] [Google Scholar]
- 44.Qiao Y, Anwar Z, Intrapiromkul J, et al. Patterns and implications of intracranial arterial remodeling in stroke patients. Stroke 2016;47:434–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Klein IF, Lavallée PC, Touboul PJ, et al. In vivo middle cerebral artery plaque imaging by high-resolution MRI. Neurology 2006;67:327–9. [DOI] [PubMed] [Google Scholar]
- 46.Qiao Y, Zeiler SR, Mirbagheri S, et al. Intracranial plaque enhancement in patients with cerebrovascular events on high-spatial-resolution MR images. Radiology 2014;271:534–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wang Y, Lou X, Li Y, et al. Imaging investigation of intracranial arterial dissecting aneurysms by using 3 T high-resolution MRI and DSA: From the interventional neuroradiologists’ view. Acta Neurochir 2014;156:515–25. [DOI] [PubMed] [Google Scholar]
- 48.Jiang W, Yu W, Ma N, et al. High resolution MRI guided endovascular intervention of basilar artery disease. J NeuroInterv Surg 2011;3:375–8. [DOI] [PubMed] [Google Scholar]
- 49.van der Kolk AG, Zwanenburg JJM, Brundel M, et al. Distribution and natural course of intracranial vessel wall lesions in patients with ischemic stroke or TIA at 7.0 tesla MRI. Eur Radiol 2015;25:1692–700. [DOI] [PubMed] [Google Scholar]
- 50.Kim TH, Choi JW, Roh HG, et al. Atherosclerotic arterial wall change of non-stenotic intracracranial arteries on high-resolution MRI at 3.0 T: Correlation with cerebrovascular risk factors and white matter hyperintensity. Clin Neurol Neurosurg 2014;126:1–6. [DOI] [PubMed] [Google Scholar]
- 51.Xu X, Wei Y, Zhang X, et al. Value of higher-resolution MRI in assessing middle cerebral atherosclerosis and predicting capsular warning syndrome. J Magn Reson Imaging 2016;44:1277–83. [DOI] [PubMed] [Google Scholar]
- 52.Lou X, Ma N, Shen H, et al. Noninvasive visualization of the basilar artery wall and branch ostia with high-resolution three-dimensional black-blood sequence at 3 Tesla. J Magn Reson Imaging 2014;39:911–6. [DOI] [PubMed] [Google Scholar]
- 53.Li M, Xu Y, Hou B, et al. High-resolution intracranial vessel wall imaging using 3D CUBE T1 weighted sequence. Eur J Radiol 2016;85:803–7. [DOI] [PubMed] [Google Scholar]
- 54.Lindenholz A, Harteveld AA, Zwanenburg JJ, et al. Comparison of 3T intracranial vessel wall MRI sequences. AJNR Am J Neuroradiol 2018;39:1112–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Zhu C, Tian B, Feng L, et al. Accelerated whole brain intracranial vessel wall imaging using black blood fast spin echo with compressed sensing (CS-SPACE). MAGMA 2018;31:457–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Yang Q, Deng Z, Bi X, et al. Whole-brain vessel wall MRI: a parameter tune-up solution to improve the scan efficiency of three-dimensional variable flip-angle turbo spin-echo. J Magn Reson Imaging 2017;46:751–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Wang J, Helle M, Zhou Z, Börnert P, Hatsukami TS, Yuan C. Joint blood and cerebrospinal fluid suppression for intracranial vessel wall MRI. Magn Reson Med 2016;75:831–8. [DOI] [PubMed] [Google Scholar]
- 58.Fan Z, Yang Q, Deng Z, et al. Whole-brain intracranial vessel wall imaging at 3 Tesla using cerebrospinal fluid-attenuated T1-weighted 3D turbo spin echo. Magn Reson Med 2017;77:1142–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Qiao Y, Guallar E, Suri FK, et al. MR imaging measures of intracranial atherosclerosis in a population-based study. Radiology 2016;280:860–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Ma N, Lou X, Zhao T, et al. Intraobserver and interobserver variability for measuring the wall area of the basilar artery at the level of the trigeminal ganglion on high-resolution MR images. AJNR Am J Neuroradiol 2011;32:E29–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Yang W, Huang B, Liu X, Liu H, Li P, Zhu W. Reproducibility of high-resolution MRI for the middle cerebral artery plaque at 3T. Eur J Radiol 2014;83:49. [DOI] [PubMed] [Google Scholar]
- 62.Zhang X, Zhu C, Peng W, et al. Scan-rescan reproducibility of high resolution magnetic resonance imaging of atherosclerotic plaque in the middle cerebral artery. PLoS ONE 2015;10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Zhang N, Zhang F, Deng Z, et al. 3D whole-brain vessel wall cardiovascular magnetic resonance imaging: a study on the reliability in the quantification of intracranial vessel dimensions. J Cardiovasc Magn Reson 2018;20:39-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Choi JW, Han M, Hong JM, et al. Feasibility of improved motion-sensitized driven-equilibrium (iMSDE) prepared 3D iation of assoT1-weighted imaging in the diagnosis of vertebrobasilar artery dissection. J Neuroradiol 2018;45:186–91. [DOI] [PubMed] [Google Scholar]
- 65.Qiao Y, Steinman DA, Qin Q, et al. Intracranial arterial wall imaging using three-dimensional high isotropic resolution black blood MRI at 3.0 Tesla. J Magn Reson Imaging 2011;34:22–30. [DOI] [PubMed] [Google Scholar]
- 66.Dieleman N, Yang W, van der Kolk AG, et al. Qualitative evaluation of a high-resolution 3D multi-sequence intracranial vessel wall protocol at 3 Tesla MRI. PloS One 2016;11:e0160781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Harteveld AA, van der Kolk AG, van der Worp HB, et al. High-resolution intracranial vessel wall MRI in an elderly asymptomatic population: comparison of 3T and 7T. Eur Radiol 2017;27:1585–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Majidi S, Sein J, Watanabe M, et al. Intracranial-derived atherosclerosis assessment: an in vitro comparison between virtual histology by intravascular ultrasonography, 7T MRI, and histopathologic findings. AJNR Am J Neuroradiol 2013;34:2259–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Kleinloog R, Korkmaz E, Zwanenburg JJ, et al. Visualization of the aneurysm wall: a 7.0-Tesla magnetic resonance imaging study. Neurosurgery 2014;75:614–22. [DOI] [PubMed] [Google Scholar]
- 70.Harteveld AA, Denswil NP, Van Hecke W, et al. Ex vivo vessel wall thickness measurements of the human circle of Willis using 7T MRI. Atherosclerosis 2018;273:106–14. [DOI] [PubMed] [Google Scholar]
- 71.Harteveld AA, Denswil NP, Siero JC, et al. Quantitative intracranial atherosclerotic plaque characterization at 7T MRI: an ex vivo study with histologic validation. AJNR Am J Neuroradiol 2016;37:802–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Viessmann O, Li L, Benjamin P, et al. T2-weighted intracranial vessel wall imaging at 7 Tesla using a DANTE-prepared variable flip angle turbo spin echo readout (DANTE-SPACE). Magn Reson Med 2017;77:655–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.van der Kolk AG, Zwanenburg JJ, Brundel M, et al. Intracranial vessel wall imaging at 7.0-T MRI. Stroke 2011;42:2478–84. [DOI] [PubMed] [Google Scholar]
- 74.van der Kolk AG, Hendrikse J, Brundel M, et al. Multi-sequence whole-brain intracranial vessel wall imaging at 7.0 tesla. Eur Radiol 2013;23:2996–3004. [DOI] [PubMed] [Google Scholar]
- 75.Harteveld AA, van der Kolk AG, van der Worp HB, et al. Detecting intracranial vessel wall lesions with 7T-magnetic resonance imaging: patients with posterior circulation ischemia versus healthy controls. Stroke 2017;48:2601–4. [DOI] [PubMed] [Google Scholar]
- 76.van der Kolk AG, Zwanenburg JJM, Brundel M, et al. Distribution and natural course of intracranial vessel wall lesions in patients with ischemic stroke or TIA at 7.0 tesla MRI. Eur Radiol 2015;25:1692–700. [DOI] [PubMed] [Google Scholar]
- 77.Blankena R, Kleinloog R, Verweij BH, et al. Thinner regions of intracranial aneurysm wall correlate with regions of higher wall shear stress: a 7T MRI study. AJNR Am J Neuroradiol 2016;37:1310–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Harteveld AA, van der Kolk AG, van der Worp HB, et al. Detecting intracranial vessel wall lesions with 7T-magnetic resonance imaging: patients with posterior circulation ischemia versus healthy controls. Stroke 2017;48:2601–4. [DOI] [PubMed] [Google Scholar]
- 79.Qiao Y, Steinman DA, Qin Q, et al. Intracranial arterial wall imaging using three-dimensional high isotropic resolution black blood MRI at 3.0 tesla. J Magn Reson Imaging 2011;34:22–30. [DOI] [PubMed] [Google Scholar]
- 80.de Havenon A, Muhina HJ, Parker DL, et al. Effect of time elapsed since gadolinium administration on atherosclerotic plaque enhancement in clinical vessel wall MR imaging studies. AJNR Am J Neuroradiol 2019;40:1709–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Lee HN, Ryu CW, Yun SJ. Vessel-wall magnetic resonance imaging of intracranial atherosclerotic plaque and ischemic stroke: a systematic review and meta-analysis. Front Neurol 2018;9:1032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Chueh JY, van der Marel K, Gounis MJ, et al. Development of a high resolution MRI intracranial atherosclerosis imaging phantom. J Neurointerv Surg 2018;10:143–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Mossa-Basha M, Watase H, Sun J, et al. Inter-rater and scan-rescan reproducibility of the detection of intracranial atherosclerosis on contrast-enhanced 3D vessel wall MRI. Br J Radiol 2019;92:20180973. [DOI] [PMC free article] [PubMed] [Google Scholar]


