Abstract
The right ventricular myocardium, much like the rest of the right side of the heart, has been consistently understudied. Presently, little is known about its mechanics, its microstructure, and its constitutive behavior. In this work, we set out to provide the first data on the mechanics of the mature right ventricular myocardium in both simple shear and uniaxial loading and to compare these data to the mechanics of the left ventricular myocardium. To this end, we tested ovine tissue samples of the right and left ventricle under a comprehensive mechanical testing protocol that consisted of six simple shear modes and three tension/compression modes. After mechanical testing, we conducted a histology-based microstructural analysis on each right ventricular sample that yielded high resolution fiber distribution maps across the entire samples. Equipped with this detailed mechanical and histological data, we employed an inverse finite element framework to determine the optimal form and parameters for microstructure-based constitutive models. The results of our study show that right ventricular myocardium is less stiff then the left ventricular myocardium in the fiber direction, but similarly exhibits non-linear, anisotropic, and tension/compression asymmetric behavior with direction-dependent Poynting effect. In addition, we found that right ventricular myocardial fibers change angles transmurally and are dispersed within the sheet plane and normal to it. Through our inverse finite element analysis, we found that the Holzapfel model successfully fits these data, even when selectively informed by rudimentary microstructural information. And, we found that the inclusion of higher-fidelity microstructural data improved the Holzapfel model’s predictive ability. Looking forward, this investigation is a critical step towards understanding the fundamental mechanical behavior of right ventricular myocardium and lays the groundwork for future whole-organ mechanical simulations.
Keywords: Hyperelasticity, Anisotropy, Material Models, Simple Shear, Uniaxial Tension and Compression, Heterogeneity, Histology
Graphical abstract
1. Introduction
The right side of the heart has historically received little attention from the medical and scientific communities. This includes not only the famously dubbed “forgotten valve [1]– the tricuspid valve –, but also the “forgotten [2], “neglected [3], “unnecessary” [4], or “dispensable chamber [5]– the right ventricle. This unbalanced effort toward understanding right ventricular function and disease has in part been due to early reports that proposed that substantial damage to the right ventricle did not significantly affect global hemodynamics [6, 7, 8]. Its anatomic location below the sternum and away from the esophagus, smaller wall thickness, and complex, crescent-shaped geometry have further inhibited its study by complicating imaging [9, 10]. Of course, we now appreciate that a healthy right ventricle is essential to a healthy cardiovascular system as exemplified in patients with pulmonary hypertension [11], myocardial infarction [12], and left ventricular dysfunction [13]. Furthermore, during the present COVID-19 pandemic, we are reminded of the right ventricle’s vital importance because the right ventricle appears to be disproportionately negatively affected in COVID-19 patients [14]. However, due to the historic neglect and the challenges associated with imaging, relatively little is known about the right ventricle.
Gaps in our knowledge about right ventricular micro- and macro-structure, kinematics, and kinetics cannot be filled by extrapolating from what is known about the left ventricle. This is because the two ventricles, albeit connected, are distinct anatomic entities. Aside from differences in location, thickness, and shape, the right ventricular myocardium has a different microstructural organization [15]. Specifically, myocardial fibers have been reported to run primarily in the circumferential direction [16]. Additionally, right ventricular cardiomyocytes may be of a different phenotype than their left-sided equivalents [17]. A sensible starting point for understanding the right ventricle and its function is to study its passive constitutive behavior; that is, to study the tissues mechanical response to deformation. Examples of noteworthy exceptions to the general neglect of right ventricular myocardial mechanics are the studies on right ventricular free wall mechanics in large animals under planar conditions [18] and under diverse loading modes but in immature animals [19] as well as the recent studies in a murine model by Sacks’ group [20, 21, 22].
Despite the differences between the left and right ventricle, we can learn from left ventricle research as we fill the gaps in right ventricle research. For example, studies on left ventricular constitutive behavior have created a comprehensive framework for myocardial tissue characterization that we will draw from in our current work. This previous work on left ventricular myocardial constitutive behavior differs in four essential aspects: i) the mechanical testing modes, ii) the form of the constitutive law, iii) the fitting procedures, and finally, iv) the inclusion of microstructural information. As to the deformation mode, while myocardium has previously been tested under planar conditions [23], those tests do not fully capture the natural deformation modes of the tissue that are dominated by shear. Thus, Dokos et al. in their seminal work performed the first simple shear experiments of left ventricular myocardium [24]. Recognizing the orthotropic nature of the material, they performed a total of six shear experiments on porcine tissue. Shear experiments have since become standard and have been repeated by Sommer et al. on human tissue [25]. With the exception of the work by Sacks et al., who have developed a complex shear device to perform experiments with non-traction-free boundary conditions, this class of experiments have implemented simple shear [26]. Shear data from Dokos et al. [24], Sommer et al.[25], and Avazmohammadi et al.[26] were subsequently fit with multiple different constitutive models. Among those models, the Holzapfel model has emerged as the de facto standard [27, 28, 29, 30], with the Guccione model as a popular alternative [31, 32, 33]. In order to identify the material parameters of these constitutive models, most previous studies employed gradient-based methods to minimize objective functions via homogeneous, analytical solutions to the simple shear problem. However, simple shear is not so simple, and thus inverse finite element methods are required to accurately capture the heterogeneous solution to a simple shear problem with traction free inclined edges [34]. Accordingly, Schmid et al. and Avazmohammadi et al. performed inverse finite element analyses to determine the parameters for their constitutive models of interest [35, 26]. Finally, myocardium is a highly heterogeneous tissue. Inclusion of material heterogeneity in previous work has ranged from ignoring it all together to detailed inclusion based on sample-specific characterization of tissue microstructure via 2-Photon imaging [25] or diffusion-tensor imaging [36].
Inspired by these previous works on the right and left ventricles, the objective of our current work is to further study the passive mechanics of right ventricular myocardium. Specifically, our work will add to these previous studies by reporting detailed, spatially-resolved mechanical and histological data, and by combining these data to test and inform different constitutive assumptions and models for right ventricular myocardium.
2. Methods
2.1. Animal model procedures
We performed all animal-related procedures according to the Principles of Laboratory Animal Care by the National Society for Medical Research and followed the Guide for Care and Use of Laboratory Animals by the National Academy of Science and published by the National Institutes of Health. Furthermore, this protocol was approved by our local Institutional Animal Care and Use Committee (IACUC #: 17–05).
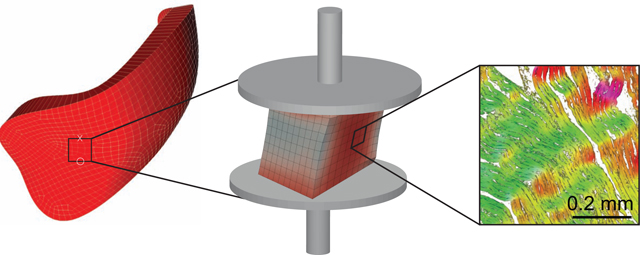
We included right and left ventricular myocardial tissue samples from twelve healthy, male, Dorsett sheep in our study. At the time of sacrifice the animals were approximately six months old and weighed 61 ± 3 kgs (mean ± SD). We sacrificed the animals via injection of sodium pentothal (100 mg/kg IV) and a potassium chloride bolus (80 mEq IV) before placing the whole hearts in physiological solution supplemented with a myosin inhibitor (2,3 Butanedione monoxime) to minimize the risk of rigor mortis. Immediately after excising the hearts, we shipped the tissue from our procurement site overnight to our testing facility on ice. Upon receipt, we placed the hearts in 4° chilled Fomblin and performed a 60 minute-long whole-heart MRI protocol (data not included here). Then, we prepared the test samples by identifying areas of the right and left ventricular free wall that were at least 8mm thick and equally far from the base, the apex, and the interventricular septum. Using a surgical blade, we removed an approximately cube-shaped sample whose principal axes aligned with the transmural (N), the fiber (F), and longitudinal (S) directions, see Figure 1A. Once the excision was completed, we measured the sample dimensions using a digital caliper. Note, we used differently colored sutures on two of the sample edges to uniquely identify the samples’ orientation throughout our experimental protocol. Next, we glued the cubes in their first orientation to sample holders and mounted the sample complex (sample plus holders) to our custom mechanical testing machine.
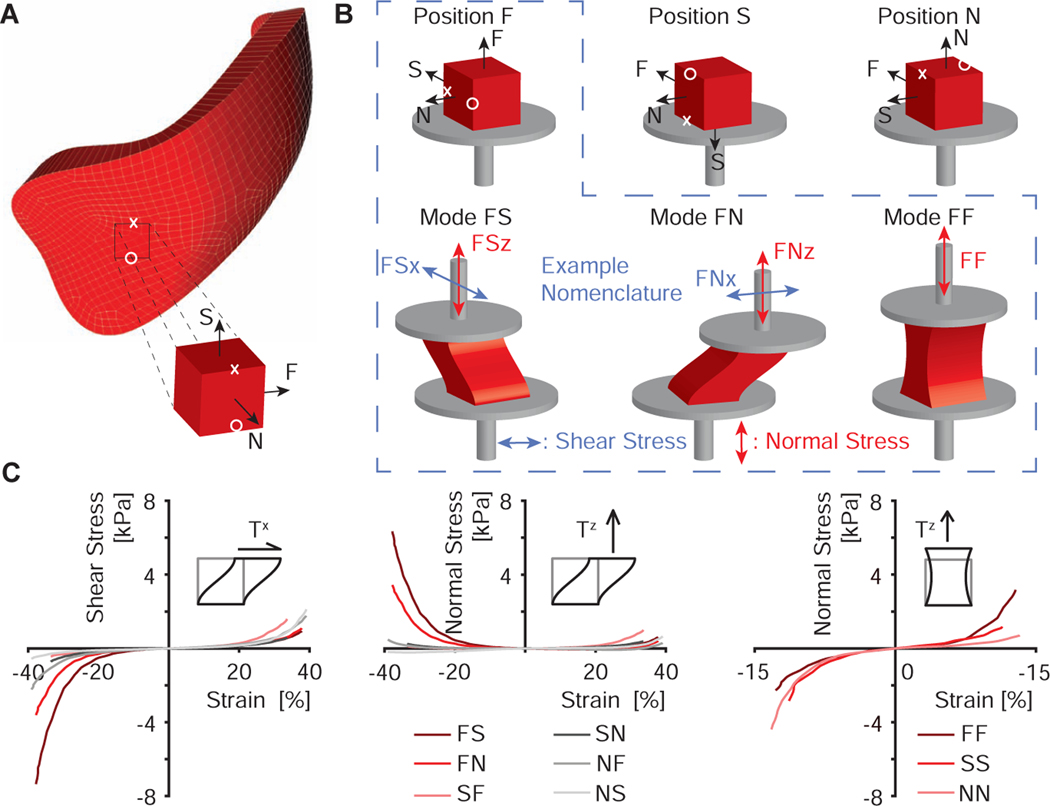
Figure 1:
Triaxial shear experiments of right ventricular myocardium. (A) Approximate anatomic location from which the samples were excised, where Fiber-Sheet-Normal (FSN) reference frame describes the anatomic directions of each sample. (B) Nomenclature for sample orientation and measurement directions. During simple shear experiments, we simultaneously measured forces, i.e., stress, in shear direction (x) and normal direction (z). Under uniaxial tension and compression, we only report stress in normal direction. In total, we conducted nine unique tests for each sample via three tests modes in three different orientations. (C) Examples for stress-strain curves for one selected sample
2.2. Simple shear and tensile/compression testing
During testing, we performed simple shear experiments in both in-plane directions as well as uniaxial tensile/compression tests, all at rates of 1mm/s. The strain magnitude varied between samples as a function of the original sample size between 28 and 57% shear strain, and from 9 to 27% normal strain for uniaxial tensile/compression tests of the right ventricular samples. The corresponding magnitudes for the left ventricular samples ranged between 10 and 51% shear strain, while the normal strain varied from 5 to 21%. All test conditions are also illustrated in Figure 1B. After one testing round was completed, we removed the sample complex, carefully dissected the tissue from the holders, and re-glued the same sample in a different orientation. Upon re-orientation of the sample, we repeated the same testing protocol. Finally, we removed the sample again, and re-orientated and re-glued the sample to perform the final testing cycle. Note, before every test, we preconditioned the samples to at least 5 cycles, see Figure 1C for a representative data set. Also note, that we permuted the order of the orientations in which we tested the samples to avoid a time/direction-dependent bias. Throughout testing, samples remained submerged at 37°C in the physiological shipping solution with myosin inhibitor. The total protocol took less than 90 minutes per sample.
2.3. Histology and microstructural organization
After concluding all mechanical tests, we removed the tissue from the holders, carefully inspected the samples, and removed all remaining glue residue. For histological preparations of right ventricular samples, we fix the tissue in formalin, and subsequently shipped all samples in 70% alcohol to a commercial histology service (Histoserv Inc., MD, USA) that divided the samples into two equal parts via separation along the plane normal to the fiber direction. For both tissue parts, they prepared Massons trichrome stains in ten sections. For the first part, they sectioned the samples from the epicardial side to the endocardial side, i.e., transmurally. For the second part, they sectioned the samples from the basal side to the apical side, i.e., longitudinally, see Figure 2A. Please note that one histological data set was not usable. Therefore, histological data and histology-based models are available only for n = 11 samples.
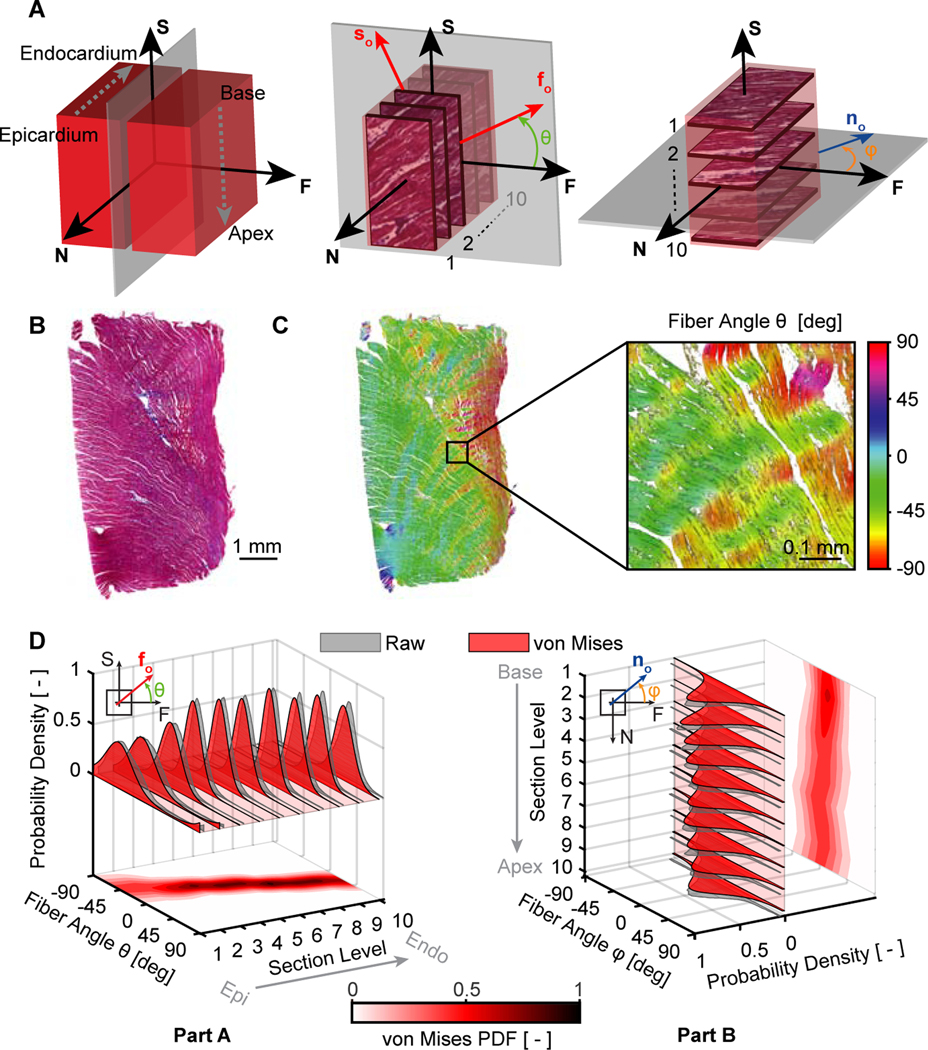
Figure 2:
Histology-based microstructural analysis of right ventricular myocardium. (A) We divided each sample into two parts along a plane normal to the fiber direction. We sectioned the first part in ten 1mm-steps in the transmural direction, and we sectioned the second part in ten 1mm-steps along the basal-apical direction, i.e, longitudinally. (B) Representative example of a histological section stained with Masson’s trichrome. (C) Identification of fiber orientation distributions via ImageJ’s OrientationJ plugin, where the color scale represents the in-plane fiber angle with respect to the horizontal axis. (D) We also fit π-periodic von Mises probability density functions (PDF) to the OrientationJ-derived normalized pixel occurrence per fiber angle for each section in both sample parts
Next, we acquired high resolution images of each stain and quantified the fiber orientation distribution for all sections via ImageJ’s OrientationJ plugin [37], see Figure 2B–C. In order to approximate the fiber distribution as a probability density function, we used non-linear least squares regression to fit the π-periodic, 2D orthotropic von Mises distribution [38],
| (1) |
where θ is the fiber angle in the sheet-plane (FS-plane) and ϕ is the fiber angle normal to the sheet-plane (NF-plane), Io the modified Bessel function, and μ and b are the von Mises location and concentration parameters, respectively. We repeated this process for each transmural and longitudinal section and subsequently interpolated the distribution functions throughout each samples’ thickness and height, see Figure 2D for this process on a representative sample.
2.4. Constitutive modeling
We modeled the right ventricle tissue as a nearly incompressible hyperelastic material according to Holzapfel et al. [27]. This strain energy function is based on the microstructural characteristics of myocardial tissue with strain energy terms accounting for the fibrous constituent of the myocardium (ostensibly muscle and/or collagen fibers), the contribution of the amorphous matrix, and their coupling interactions. Based on goodness of fit studies with an eight parameter version and a twelve parameter version of the Holzapfel model, we chose the eight parameter model because the twelve parameter model rendered only marginal improvements, see Supplementary document. Finally, as we describe in Section 2.4.2, we extended this model by including muscle fiber dispersion in the sheet-plane to take advantage of our microstructural data.
In each case below, we decompose the deformation gradient F into a volumetric, J1/3I, and isochoric part, . The corresponding deviatoric right Cauchy-Green deformation tensor thus reads . The total strain energy function W constitutes the isochoric, Wiso, and the volumetric part, U [39], i.e.,
| (2) |
where U(J) = K [ln(J)]2 /2 with K being the bulk modulus and J = det(F) [40].
We will test two general classes of material models. The first class, Class 1, represents the tissue microstructure via mean fiber direction vectors only. The second class, Class 2, accounts for dispersion of fibers around the mean fiber direction.
2.4.1. Model Class 1: Holzapfel model without fiber dispersion
Based on the (undeformed) mean fiber direction of the tissue, we defined the orthonormal material axes (fo,so,no) where fo is the in-plane fiber vector, so is the sheet-plane vector and no is the normal vector to the fo − so plane, see Figure 2A. We assumed the normal vector to be aligned with the transmural direction and, thus, normal to the epicardial surface. Considering the uncoupled formulation of the strain energy, we can write the following isochoric invariants as [27, 36]
| (3) |
while the isochoric part of the strain energy is
| (4) |
with the following individual terms
| (5) |
Of the total eight material parameters, the a’s are expressed in units of stress and the b’s are dimensionless. Fibers (Wf and Ws terms) contribute to the strain energy function only under tension or equivalently when Ī4f > 1 or Ī4s > 1. Note, the Wfs term effectively couples the material behavior in fiber and sheet direction as discussed in [41]. We implemented this form of the Holzapfel model in FEBio by utilizing the FEBio plugin development framework (FEBio, Version 2.9.1) [42].
2.4.2. Model Class 2: Holzapfel model with fiber dispersion
To expand the assumption of a single fiber vector capturing the microstructural organization of the tissue, we modified the initial constitutive models (Model Class 1) to also include fiber dispersion about the mean fiber direction. Thus, we include fiber dispersion via continuous fiber distributions (CFD). Note, the parameters of these functions were directly informed by fits to our histological data, see Section 2.3. To this end, the updated form of the strain energy function reads
| (6) |
where every term remains as stated in Section 2.4.1, except for the new term, which reads [43, 44]
| (7) |
With
| (8) |
In Equation (7), H is the Heaviside step function that enforces fibers contributing to the stored energy only if Ī4f > 1. In addition, R(n) captures the fiber dispersion according to a 2D von Mises distribution within the sheet-plane only). The function R(n) is normalized so that ∫SR(n)dA = 1. To integrate Equation (7), we used a common trapezoidal integration scheme with nθ = 31 integration points over the unit circle.
2.5. Microstructure-inclusion strategies
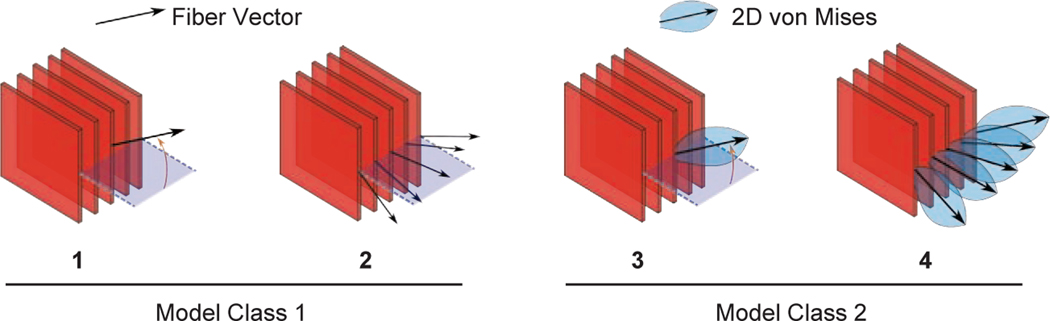
In addition to our investigation of the two model cases, we considered multiple different strategies for including microstructural information. In total we considered four different constitutive approaches which differed in their inclusion of transmural heterogeneity and assumptions about fiber distribution parameters, see Figure 3. A summary of the different cases is as follows:
Figure 3:
Microstructure-inclusion strategies. In Strategies 1–2 we used the model version without fiber dispersion (Model Class 1). In Strategies 3–4 we account for the fiber dispersion that we informed with histological data (Model Class 2)
Cases without fiber dispersion (Model Class 1):
Strategy 1: Identical fiber angle throughout the thickness based on the histology-derived average data across all sections
Strategy 2: One fiber angle per section based on the histology-derived average data for each section individually
Cases with fiber dispersion (Model Class 2):
Strategy 3: Identical fiber angle and 2D distribution throughout the thickness based on the histology-derived data across all sections
Strategy 4: One fiber angle and 2D distribution per section based on the histology-derived average data for each section individually
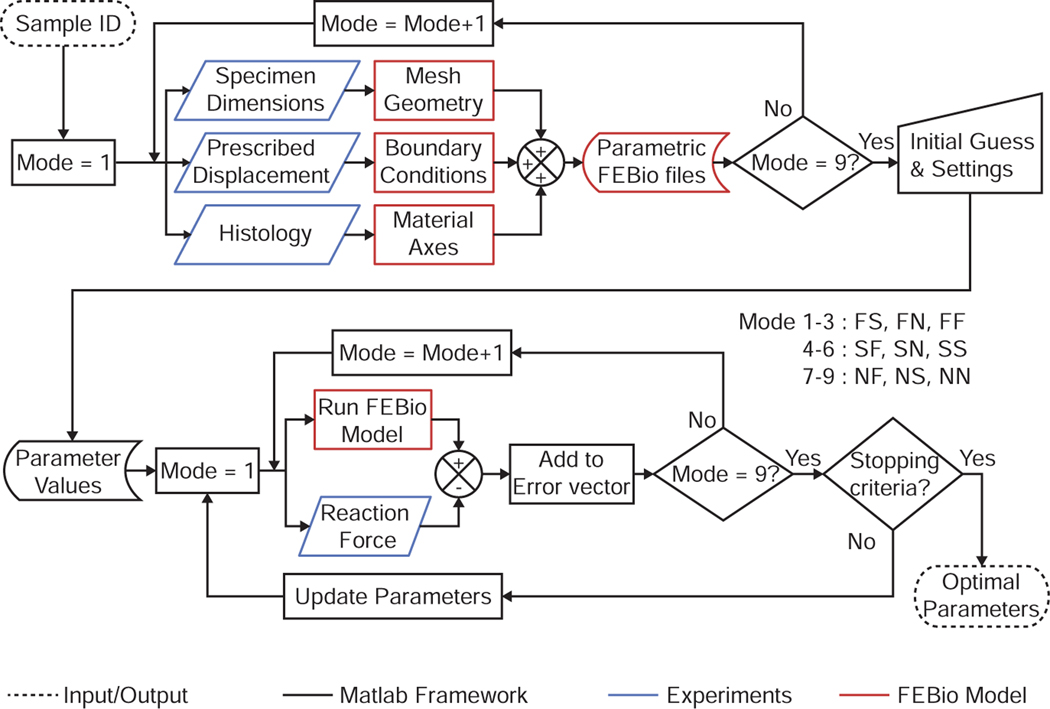
2.6. Inverse analysis
To identify the material parameters for each sample, we developed a custom inverse analysis pipeline using MATLAB (Mathworks, Version 2019) and FEBio [40], see Figure 4. Specifically, we performed a non-linear regression analysis via a trust region reflective algorithm (lsqnonlin function in MATLAB [45, 46]) to minimize following objective function:
| (9) |
where c represents the material parameter set, i refers to the loading mode index, j to the force, and sk to the displacement points (n = 100). The gradient of the objective function was evaluated numerically using forward finite differences, where each function evaluation corresponds to a finite element forward simulation of the test problem in FEBio. We evaluated the quality of our constitutive fit by computing the normalized mean square error written as:
| (10) |
where c* is the optimal set of material parameters and is the mean force over all modes and data points. According to the definition in Equation 10, e = 1 represents a perfect fit, with the poorest fit quality being e →−∞.
Figure 4:
Inverse finite element analysis pipeline
For each forward simulation, we built a finite element model for each test mode, test position, and sample. The sample geometry was approximated as a rectangular prism with dimensions corresponding to the experimental measurements described in Section 2.1. Next, we discretized the geometry isotropically via mixed linear hexahedral (Q1P0) elements. We aligned the material axes of each element with our histologically-derived, or estimated (depending on the model assumptions), fiber direction vectors. For the boundary conditions, we fixed the displacements of the bottom surface and attached the top surface to a rigid body, representing the moving parts of the testing device. Additionally, we prescribed the motion of the rigid body (actuator) as a shear- or uniaxial translation and constrained the remaining five degrees of freedom. Following a mesh refinement study based on the peak values of the rigid body reaction force and strain energy density for every loading mode, we discretized our do main with 103 elements and enforced incompressibility via the penalty method. See Figure 5 for representative finite element model simulation results. Additional studies of framework sensitivity to initial parameter guess, mesh density, bulk modulus, element type, and incompressibility enforcement strategy are included in the Supplementary document. Note, recent work has indicated that the use of the deviatoric split when modeling incompressible materials may lead to non-physical volume growth [47, 48, 49]. We specifically investigated this phenomenon in our framework and found no evidence thereof, see also the Supplementary document for additional detail.
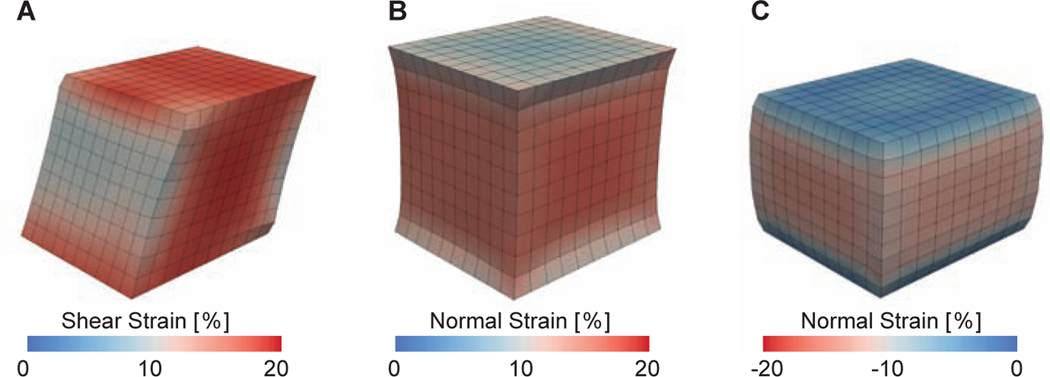
Figure 5:
Representative finite element simulation outcomes for (A) a simple shear mode, (B) a tensile mode, and (C) a compressive mode. Color corresponds to Green-Lagrange strains
2.7. Statistics
All data are reported as means ± standard error. Where we statistically compared multiple groups, we employed a linear mixed model as implemented through the afex package in R and declared tests with p-values smaller than 0.05 as statistically significant [50]. In the linear mixed model, we accounted for fixed main effects and interactions as well as random effects due to subject. If we found a statistical difference among groups, we performed multi-comparison via Tukey post-hoc analyses, also in R. The only exception to our use of linear mixed models was when we identified whether normal stress under simple shear were larger or smaller than zero to determine whether positive or negative Poynting effects were present. To this end, we used a simple Student’s t-test as implemented in Excel (Microsoft, Version 2016).
3. Results
Note, all mechanical raw data and histological imaging data are available through the Texas Data Repository as per Section 6.
3.1. Right ventricular myocardium shows strain-stiffening, anisotropy, Poynting effect, and tension-compression non-linearity
In Figure 6, we present the mean stresses among our n=12 right ventricular myocardium samples (red) with their respective standard errors. We found that the tissue response was highly non-linear and anisotropic.
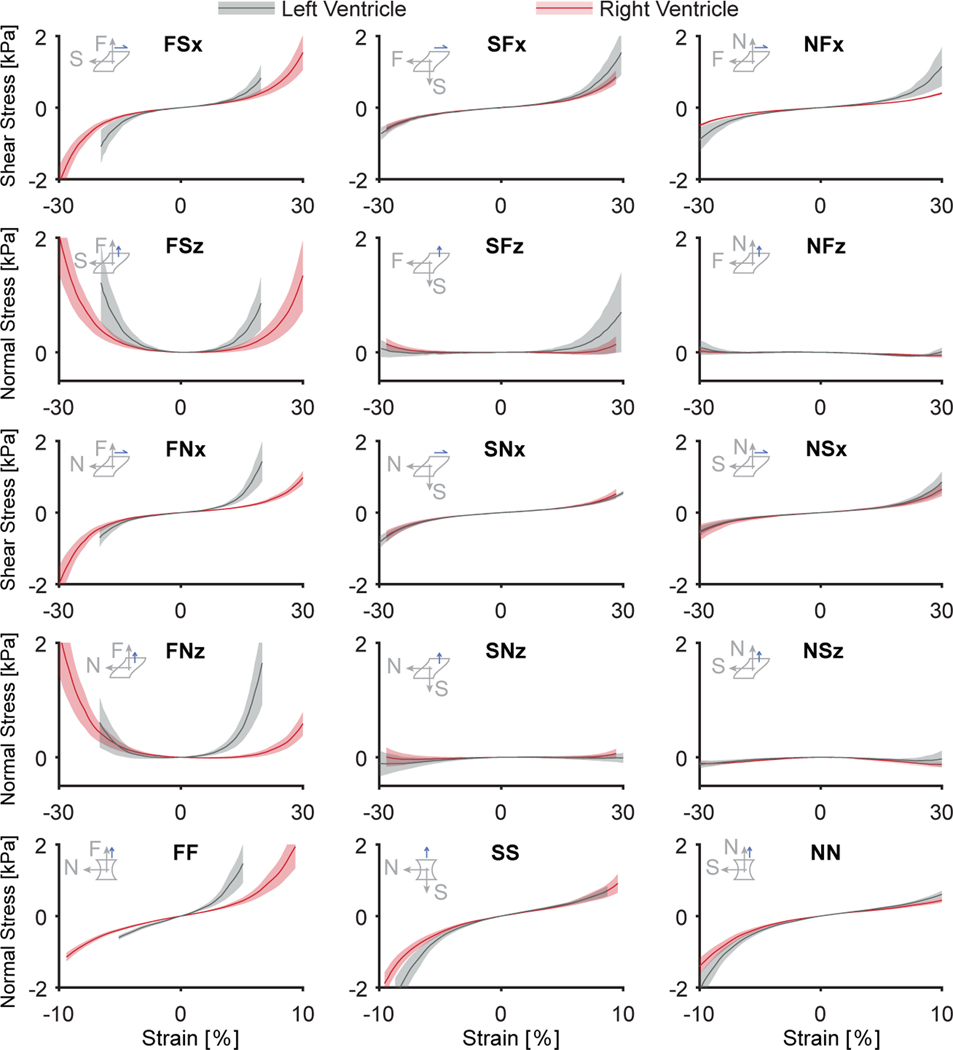
Figure 6:
Right ventricular myocardium demonstrates non-linear, strain-stiffening, and anisotropic behavior and is less stiff than left ventricular myocardium. Average stress-strain data for n = 12 right ventricular myocardium samples are shown in red, while average stress-strain data for n = 11 left ventricular myocardium samples are shown in grey for all test modes. Shaded areas represent the standard error among samples
Specifically, we found that the material was anisotropic (p < 0.0001), by determining that the shear stress in the FS mode, FSx, was higher than the shear stress in the NF, NS, SF, and SN modes, i.e., NFx, NSx, SFx, and SNx (all p < 0.01), and that the shear stress in the FN mode, FNx, was higher than in the NF, NS, SF, and SN modes, i.e., NFx, NSx, SFx, and SNx (all p < 0.05), see also Figure 7A. The normal stress in response to simple shear also indicated an anisotropic material response (p < 0.0001). Specifically, we observed the highest normal stresses for the F modes, FSz and FNz, and both were different from the other normal stresses under all other modes (p < 0.005 for all), see Figure 7B. Note, for these comparisons we evaluated stress for all modes at ±28.2% strain in shear. Further note that in our numeric results, a positive normal stress implies that the shear sample was trying to pull the sheared surfaces together rather than spread them apart. When sheared in the fiber modes FS and FN, the normal stresses, FSz and FNz, were statistically significantly larger than zero (p < 0.05). Thus, right ventricular myocardium exhibited a negative Poynting effect when the fibers were activated under shear [51]. On the other hand, when sheared under the other modes that do not correspond to the dominant fiber direction, normal stresses where either not different from zero (SFz, SNz, NFz, p > 0.05 for all), or smaller than zero (NSz, p < 0.05), i.e. the material exhibited positive Poynting effect.
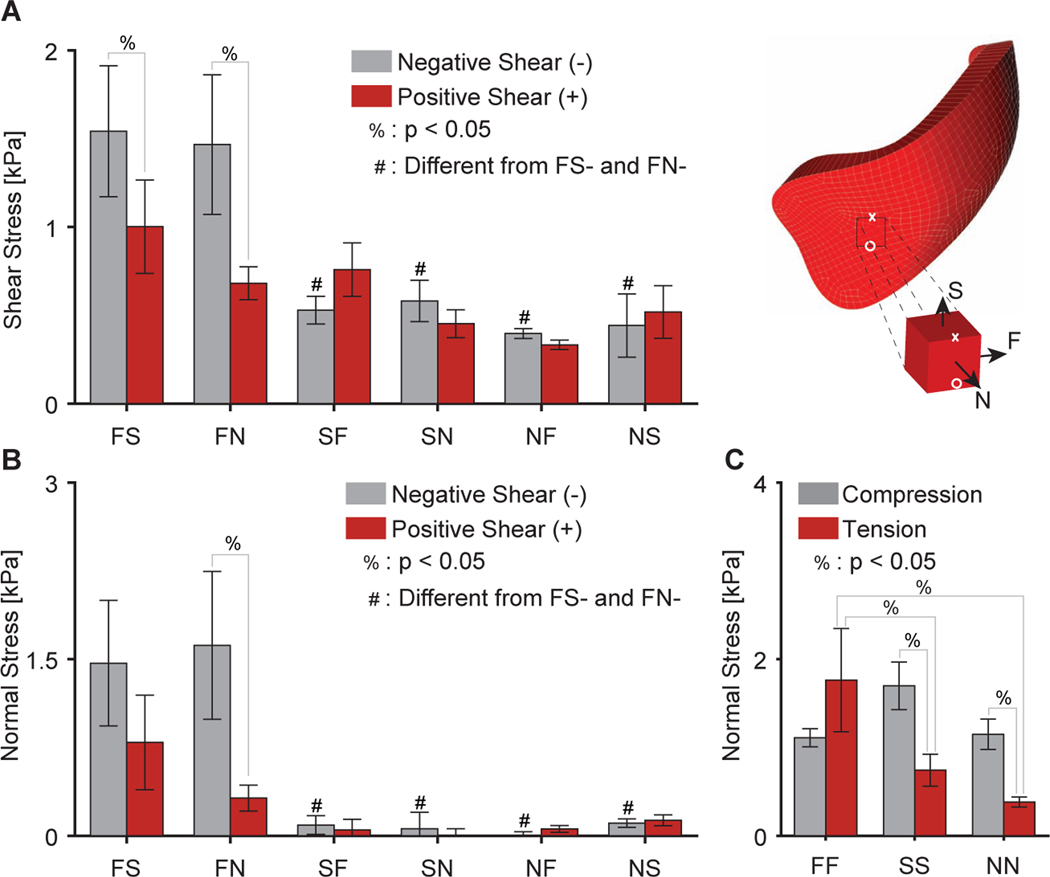
Figure 7:
Summary of differences in (A) shear stress and (B) normal stress of right ventricular myocardium under simple shear and (C) normal stress under uniaxial tension/compression. Note, stresses were evaluated for n = 12 samples at ±28.2% in shear and ±9.4% in tension/compression. Further note, that stresses are shown by their magnitude
When comparing between the tensile modes at 9.4% strain we, again, found that the material was anisotropic (p=0.023). The right ventricular myocardium demonstrated larger stresses in the FF mode than in the SS and NN modes (p = 0.011 and p = 0.001, respectively), see Figure 7C. On the other hand, comparing the compressive modes at −9.4% strain did not reveal any statistically significant anisotropy. While, we did not observe a statistically significant difference in direction-dependence as a main effect, i.e., tension-compression non-linearity (p = 0.076), we found a significant interaction between anisotropy and load-direction (p = 0.002). And, once corrected for material anisotropy, the material exhibited tension-compression non-linearity. Specifically, the material demonstrated higher stresses under compression than under tension in the SS and NN modes (p = 0.007 and p = 0.028). In the FF mode, the material showed higher stresses in tension than in compression. However, these differences did not reach statistical significance (p = 0.061).
3.2. Right ventricular myocardium is less stiff than left ventricular myocardium in the fiber direction
In Figure 6, we also present the mean stresses comparing n=12 right ventricular myocardium samples (red) with n=11 left ventricular myocardium samples (grey). Qualitatively, the right and left ventricular myocardium mechanical response was similar. However, there were marked quantitative differences between the two. First, our statistical analysis revealed a strong difference in shear stress between samples from the right and left ventricles (p < 0.001). Differences were drive primarily by lower stresses in the right ventricular myocardium than in the left ventricular myocardium when sheared in the FS mode, i.e., FSx, and FN mode, i.e., FNx (p = 0.045 and p < 0.0001, respectively). Similarly, normal stresses under simple shear also differed significantly between the right and left ventricular myocardium (p < 0.01). Specifically, these differences were due to the right ventricular myocardium producing lower normal stresses in the FN mode, i.e., FNz (p < 0.0001), and the FS mode, i.e., FSz (p < 0.05). The myocardium from both ventricles also differed in the uniaxial direction (p = 0.003), where differences were driven by a lower stresses of the right ventricular myocardium in the FF mode (p < 0.0001) but not the other modes. Note, stresses between right ventricular and left ventricular myocardium where compared at 19.7% shear strain and ±5% tensile/compressive strain.
3.3. Right ventricular myocardium fibers are dispersed in the sheet-plane and in the plane perpendicular to the sheet-plane
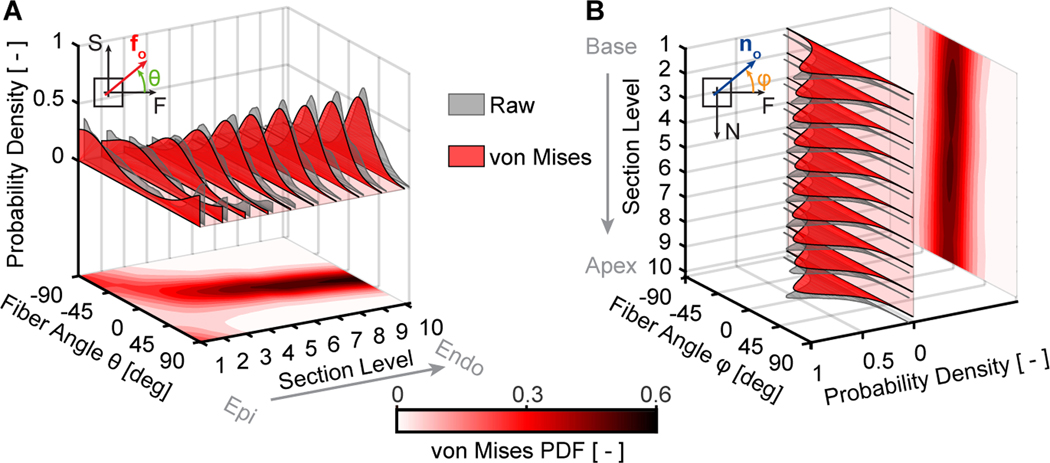
Figure 8 illustrates the results of our microstructural analysis averaged among n=11 right ventricular myocardial samples. Figure 8A illustrates the fiber dispersion transmurally. We found that fibers were dispersed according to a single-mode probability function (grey) that was well represented by a von Mises distribution (red). The mean fiber direction shifted from approximately −76.4° degrees at the epicardial side, to +22.9° degrees at the endocardial side. In other words, fibers were oriented approximately longitudinally at the epicardial surface and approximately circumferentially at the endocardial surface. In addition, the dispersion spread was the largest at the epicardial side (von Mises concentration parameter b = 0.8) and decreased toward the endocardial side (b = 2.5).
Figure 8:
Right ventricular myocardium has a spatially heterogeneous microstructure with dispersed organization within the sheet-plane and the plane perpendicular to the sheet-plane. (A) Average within sheet-plane fiber angle distributions among n=11 right ventricular myocardium samples based on Masson’s trichrome staining throughout the samples’ thickness, i.e., transmurally. (B) Average perpendicular-to-the-sheet-plane fiber angle distribution among the same samples throughout the basal-apical sample direction, i.e., longitudinally
Figure 8B illustrates the fiber dispersion in the plane perpendicular to the sheet plane. Interestingly, we found that similar to the sheet-plane, fibers were not perfectly aligned. Specifically, fibers were dispersed and could be well-represented by a von Misses distribution. However, in contrast to the fiber dispersion within the sheet-plane, the dispersion in the plane perpendicular to the sheet-plane did not shift between sections and was consistently centered around 0°. The von Mises concentration parameter, a measure of the fiber dispersion spread, was comparable to the concentration parameters of the within sheet-plane dispersion (on average b = 1.9).
3.4. Right ventricular myocardial mechanics are well represented by the Holzapfel constitutive model
In Table 1, we present the goodness of fit for all samples and all microstructure-inclusion strategies. All four microstructure-inclusion strategies yielded satisfying fits, see Figure 9 for an example. For the readers convenience, we scaled the NMSE from the value of 1 for the worst fit (minimum NMSE) to the value of 10 for the best fit (maximum NMSE). We achieved the best average scaled score with microstructure-inclusion Strategy 2 that is the model that represents the samples’ spatially heterogeneous microstructure with transmurally-varying fiber direction vectors, but without fiber dispersion. On the other hand, we observed the lowest average score for Strategy 1, which models the samples’ microstructure via only one mean fiber direction without dispersion. However, these findings should be treated with caution as the differences between the best and worst fits were marginal as indicted by the similarity between the minimum and maximum NMSE as listed in the last two columns of Table 1.
Table 1:
Quality of constitutive parameter fits for all samples and microstructure-inclusion strategies (see Figure 3). We scaled the goodness of fit from 1.0 (worst fit among the strategies) to 10.0 (best fit among the strategies). The last two columns display the best and worst Normalized Mean Square Error (NMSE) among the strategies. A higher NMSE value implies a better fit with an upper limit of NMSE=1
| Strategy | NMSE | |||||
|---|---|---|---|---|---|---|
| Sample | 1 | 2 | 3 | 4 | min | max |
| 1 | 1.0 | 3.2 | 10.0 | 9.3 | 0.913 | 0.932 |
| 2 | 1.0 | 10.0 | 8.4 | 5.8 | 0.852 | 0.901 |
| 3 | 6.1 | 4.6 | 10.0 | 1.0 | 0.858 | 0.868 |
| 4 | 10.0 | 9.9 | 1.0 | 3.0 | 0.636 | 0.734 |
| 5 | 10.0 | 9.6 | 9.1 | 1.0 | 0.713 | 0.761 |
| 6 | 1.0 | 1.2 | 10.0 | 1.3 | 0.750 | 0.765 |
| 7 | 1.0 | 9.3 | 4.9 | 10.0 | 0.692 | 0.781 |
| 8 | 7.1 | 10.0 | 1.0 | 4.7 | 0.642 | 0.683 |
| 9 | 1.0 | 4.1 | 3.5 | 10.0 | 0.569 | 0.582 |
| 10 | 1.0 | 6.0 | 8.7 | 10.0 | 0.799 | 0.893 |
| 11 | 10.0 | 9.8 | 1.5 | 1.0 | 0.813 | 0.835 |
| Mean | 4.5 | 7.1 | 6.2 | 5.2 | ||
| SE | 1.3 | 1.0 | 1.2 | 1.2 | ||
Figure 9:
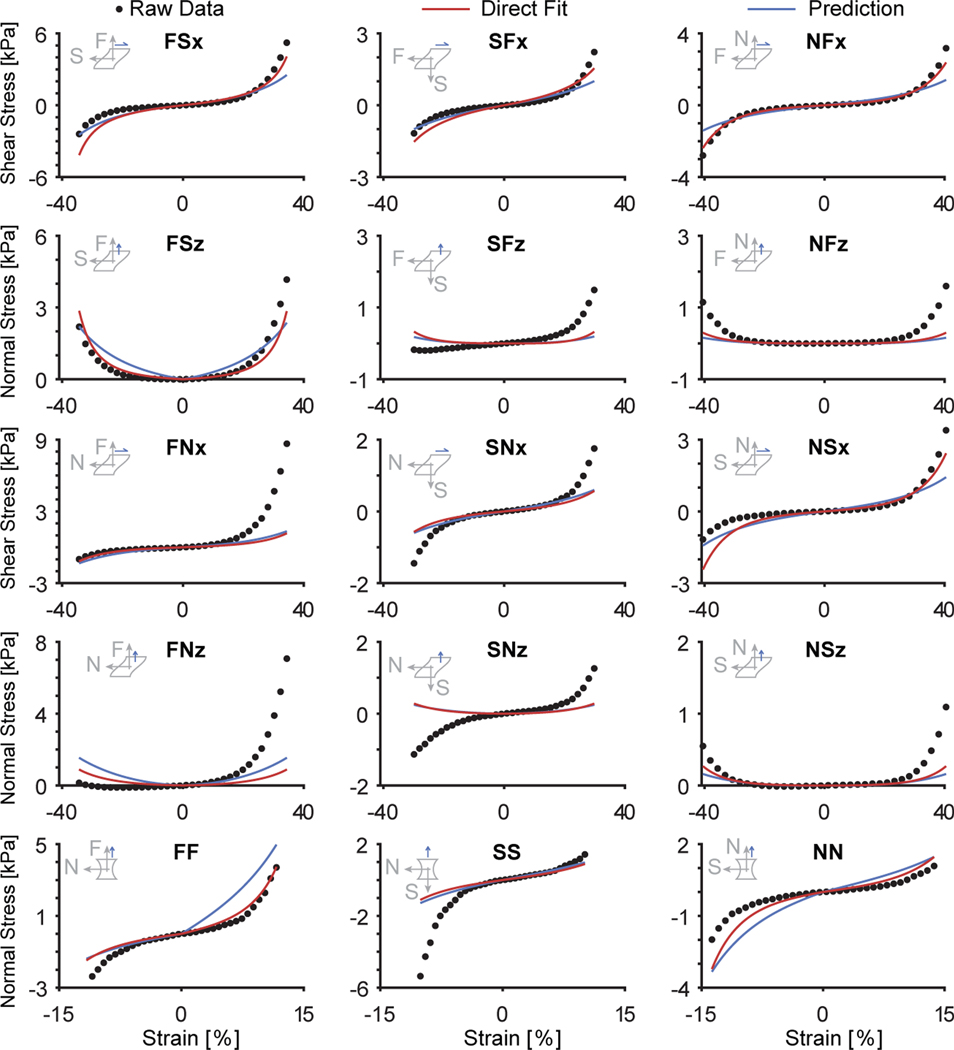
Mechanics of right ventricular myocardium can be accurately predicted based on microstructural data. Shown are raw data for a selected sample, which also serves as the “test data” (black circles). The red line shows a direct, or optimal, constitutive fit via Strategy 4, that is using the Holzapfel model that accounts for spatial variation in histology-informed fiber dispersion through the sample’s thickness. Note, this fit quality is typical in that it falls between the best and worst fits. We also show a prediction based on constitutive parameters that were informed via training on the other ten samples and the microstructural data from the sample itself
3.5. Right ventricular myocardial microstructure cannot be easily predicted from mechanical response alone
It would be convenient to estimate microstructural information from mechanical tests alone. For example, it would be convenient to estimate microstructural data in-vivo via inverse methods. To evaluate our ability to accomplish this, we used our comprehensive set of mechanical data and our inverse pipeline. Specifically, in microstructure-inclusion Strategy 3, we assumed the microstructural parameters were unknown. Thus, in addition to fitting the eight parameters of the constitutive law, we also fit the the 2D von Mises distribution parameters mu and b. Table 2 summarizes our findings. In short, there are significant discrepancies between microstructural parameters derived from histology (ground truth) and those estimated through our inverse approach.
Table 2:
Estimation of microstructural information from mechanical data alone (Est.) and comparison to histology-based measurements (Exp.). Using microstructure-inclusion Strategy 3, we inversely identified not only the eight parameters of the material model, but also the mean fiber direction (μ) and concentration parameter b of the π-periodic 2D von Mises distribution
| μ (°) | b | NMSE | ||||
|---|---|---|---|---|---|---|
| Sample | Exp. | Est. | Exp. | Est. | Exp. | Est. |
| 1 | 55.7 | −35.5 | 0.86 | 7.17 | 0.932 | 0.942 |
| 2 | −5.9 | −12.3 | 1.22 | 2.03 | 0.892 | 0.912 |
| 3 | 41.7 | −32.8 | 1.24 | 0.04 | 0.868 | 0.896 |
| 4 | 6.4 | 6.2 | 1.07 | 26.65 | 0.636 | 0.736 |
| 5 | −15.5 | −9.4 | 2.30 | 5.20 | 0.756 | 0.775 |
| 6 | −5.0 | 13.7 | 2.50 | 4.06 | 0.765 | 0.856 |
| 7 | 1.7 | 24.0 | 4.56 | 4.99 | 0.730 | 0.884 |
| 8 | −15.6 | 2.3 | 0.90 | 2.96 | 0.642 | 0.675 |
| 9 | −1.3 | 39.2 | 2.18 | 99.90 | 0.572 | 0.674 |
| 10 | 3.7 | 11.5 | 2.01 | 3.39 | 0.879 | 0.919 |
| 11 | 18.2 | 14.8 | 8.18 | 99.26 | 0.814 | 0.838 |
3.6. Right ventricular myocardial mechanics can be reliably predicted from microstructural data
After finding that all microstructure-inclusion strategies provided satisfying fits to the experimental data, we set out to identify the inclusion strategy with the best predictive ability [52, 53]. To this end, we modified our pipeline to simultaneously estimate the material parameters for ten randomly chosen samples (the “training set”). We repeated these fits for all microstructure-inclusion strategies. Subsequently, we compared their ability to predict the constitutive behavior of the eleventh, unseen sample (the “test set”).
We found that training based on ten samples led to excellent fits on the unseen data, see Table 3. We achieved fits with NMSE ranging from 0.500 to 0.515. These values correspond to 84.8% and 88.5% of the NMSE from the best individual or direct fit for this sample, see Figure 9 as an example. Compellingly, a clear pattern emerged: the more sample-specific microstructural data that we included, the better our predictions were. Strategy 1, which represented the entire microstructure via only one fiber direction vector without dispersion performed the worst. Adding spatial heterogeneity by representing the microstructure with ten fiber direction vectors throughout the thickness in Strategy 2 improved upon the prediction of Strategy 1. Further, adding dispersion to a single fiber in Strategy 3 improved upon both Strategy 1 and 2. Finally, including both spatial heterogeneity and dispersion in Strategy 4 yielded the best predictions.
Table 3:
Material parameters identified by fitting the Holzapfel model simultaneously to ten “training data sets,” for each microstructure-inclusion strategy separately. With these data, we predicted the behavior of the “test data set,” a held-out sample, based only on its microstructural data (Prediction) and compared it to a direct fit to the data (Direct Fit)
| Strategy | |||||
|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | ||
| a | (Pa) | 2088.75 | 2163.74 | 2176.85 | 2113.71 |
| b | (—) | 4.427 | 4.239 | 4.200 | 4.319 |
| af | (Pa) | 4254.81 | 3847.00 | 5402.66 | 6595.44 |
| bf | (—) | 5.027 | 10.794 | 7.174 | 4.340 |
| as | (Pa) | 966.50 | 634.37 | 78.53 | 0.82 |
| bs | (—) | 0.0 | 0.002 | 0.110 | 0.004 |
| afs | (Pa) | 1152.72 | 1119.13 | 0.0 | 393.86 |
| bfs | (—) | 9.149 | 1.263 | 0.0 | 1.154 |
| Direct Fit | NMSE | 0.569 | 0.573 | 0.572 | 0.582 |
| Prediction | NMSE | 0.500 | 0.510 | 0.512 | 0.515 |
4. Discussion
Much about the right ventricle remains to be studied and understood to make up for decades of neglect. In our current work, we report on the constitutive behavior and microstructure of ovine right ventricular myocardium. Through detailed mechanical and histological characterization we also critically evaluated the performance of the most promising constitutive framework for right ventricular myocardium introduced, modified, and popularized by Holzapfel et al., Li et al., Guan et al. and others [27, 36, 54, 55].
We chose simple shear tests for our current study as we believe they best represent the natural deformation mode of myocardium. Additionally, we included tension and compression tests as these modes enforce larger volumetric changes and may thus activate deformation modes that were not activated under simple shear. During triaxial simple shear testing, the right ventricular myocardium exhibited mechanical behavior consistent with its complex laminar and fibrous structure [24, 25]. We found that its mechanical response is non-linear, strain-stiffening, and anisotropic. Consistent with our understanding of myocardium, we found that right ventricular myocardium is stiffest in the directions that align with the myocardial fiber directions. We also found that right ventricular myocardium exhibits Poynting effect. That is, under simple shear, it produces a normal force. Interestingly, it did so in a directionally-dependent manner. Specifically, it expressed significant normal tensile stresses when sheared againstthe fiber direction (negative Poynting) while normal stresses were either insignificant or compressive when sheared in the other directions (positive Poynting). This discrepancy may indicate that the negative Poynting effect stems from the mechanical response of the muscle fibers or their collagen support. Additionally, we found that right ventricular myocardium exhibits tension-compression non-linearity under uniaxial loading. In other words, tissue stiffness was different under tensile and compressive loading. Again, this effect depended on the orientation in which the tissue was loaded with respect to the tissue microstructure. When loaded in the fiber direction, the material acted stiffer under tension than under compression. When loaded in the other two directions, the material was stiffer in compression than in tension. Consistent with this mechanism, in the absence of fiber activation, increasing stiffness under compression likely stems from the material’s incompressible behavior.
Finally, we also compared the mechanics of the right ventricular myocardium to that of the left ventricular myocardium. Our data on left ventricular myocardium reflects the findings of many before us in that the muscle of the left ventricle showed non-linear, strain-stiffening, and anisotropic behavior also [24, 25, 30, 23]. Critically, we found that the right ventricular myocardium was less stiff than the left ventricular myocardium, but only in the directions in which muscle fibers were activated. This finding is consistent with our expectation that the left ventricular myocardium, which has to resist and produce larger forces in-vivo, is stiffer in the force-producing direction. Finding that the materials don’t significantly differ in the direction in which fibers are not activated points to a preservation of the amorphous tissue structure and properties between the two sides of the heart.
Our qualitative observations on the mechanics of right ventricular myocardium are well-aligned with those of others [19, 18, 22, 20, 21], who also found that right ventricular myocardium is a non-linear, strain-stiffening, and anisotropic material. The closest comparison to our data are those by Ahmad et al. who used uniaxial, biaxial, and simple shear testing to compare the right ventricular and left ventricular myocardium in piglets [19]. Interestingly, they found, in contrast to us, and against their expectations, that the right ventricular myocardium was stiffer than the left ventricular myocardium. They argued their counter-intuitive result likely stemmed from the immaturity of the tissue in which collagen cross-linking had not yet been concluded. It is noteworthy that Sacks et al., who compared left and right ventricular myocardium using planar biaxial testing only, also found that right ventricular myocardium was stiffer in the fiber direction than left ventricular myocardium [18]. Difference in our findings are difficult to resolve as experiments were done using different test modalities, species, and age of subjects. Future studies will have to resolve this 450 apparent paradox.
In our work, we found that the fiber directions varied significantly in the transmural direction, but were highly consistent between samples. Specifically, we found that the fiber directions varied across our samples by approximately 100°. This number is well-aligned with findings by Avazmohammadi et al. [29] and Sharifi Kia et al. [56] on murine right ventricles. They found that fiber directions varied by 100° and 60° across the entire wall, respectively. Importantly, on the left ventricle it has previously been reported that rodent and large animal models show significantly different transmural fiber directions [57]. In contrast, our data indicates that microstructural data for the right ventricle appears to be well-aligned between large and small animals. In addition to mean fiber direction, we also explored fiber dispersion as a measure of fiber organization. In agreement with reports by Avazmohammadi et al. and Ahmad et al. [58], we found that fibers were dispersed within the sheet-plane. Moreover, fibers appeared to be most dispersed at the epicardial surface and least dispersed toward the endocardial surface. To date, the teleological reasons of this heterogeneous fiber dispersion are unknown but are likely driven by functional needs and natural demand for cardiac efficiency. Importantly, and surprisingly to us, fibers were also dispersed in the plane perpendicular to the sheet-plane. Interestingly, few or no current models of myocardium (either for the left or right ventricle) support asymmetric three dimensional fiber dispersion and the functional importance of such distributions have not been explored. Future studies will hopefully fill these gaps.
To obtain these detailed microstructural data, we acquired ten histological sections across our samples. This process is not only destructive and expensive, but also time-consuming. Thus, one of the questions we tried to answer in our current work was: How much microstructural data is necessary to accurately reproduce the constitutive behavior of right ventricular myocardium? Additionally, we explored the question: Can we use the inverse fits to our mechanical data to correctly identify fiber direction and dispersion? Finally, we set out to answer: How much microstructural data is necessary to predict the behavior of an unseen tissue sample?
To answer these questions, we developed an inverse analysis pipeline with microstructurally-informed finite element models. We implemented four different microstructure-inclusion strategies with increasing levels of complexity. To answer our first question, we fit our model for eleven samples individually. We found that our fits reached similar levels of goodness between the varying strategies. In short, it appears that even drastically simplifying assumptions about the tissue microstructure can yield satisfying fits. The answer to our second question is closely related to our answer to the first question. If models with vastly simplified microstructure satisfyingly fit our mechanical data, it is unlikely that an inverse approach will be able to uniquely identify the microstructure based on only the mechanical data acquired. Indeed, we found that predicted microstructural parameters compared poorly to actual measurements. This finding highlights the danger of interpreting constitutive laws as having microstructural meaning without supporting microstructural characterization. Finally, to answer our third question, we identified a set of parameters that fit ten of our data sets best. Again, we repeated this identification for each microstructure-inclusion strategy. Subsequently, we tested the predictive ability against a randomly selected eleventh, held-out data set. Here we found that increasing the information about microstructural data improves our predictions. Thus, perhaps not surprisingly, we find that the more microstructural data about a sample is known, the more confident the modeler can be in parameter predictions that are based on historic data. In more detail, we found that including fiber dispersion had a stronger, positive effect on predictive power than including spatial heterogeneity. From an entirely pragmatic perspective, this is useful for researchers whose own labs are capable of collecting histological data but may lack the equipment and computational resources to perform triaxial mechanical testing and the corresponding finite element simulations.
Of course, some limitations arise from the methodology that we followed in this study. Mechanical testing was conducted more than 12 hours after sacrificing these animals. Although, we kept tissues in solution with myosin inhibitor to avoid rigor mortis, we cannot ensure that tissue properties did not decay during transport and storage. Concerns could also arise as to the effect of Fomblin on the mechanics of our tissue samples. To address this concern, we note that Fomblin is a hydrophobic reagent. Penetration of highly hydrated soft tissues is therefore unlikely. Additionally, we did not observe any remarkable tissue characteristics during histological studies. In a similar vein, our samples were glued to the test apparatus. To ensure that gluing samples did not significantly affect our tissue properties, we checked for glue penetration in all of our histological preparations. We did not see any glue penetration under Picrosirius Red or Massons Trichrome staining, or on unstained slides. Concerns may also be raised about the boundary effects stemming from gluing the sample to our sample holders. While we cannot rule out that the stress-field of our sample may be effected, we also highlight that alternative fixation methods, such as sutures, rakes, or clamps, likely have the same or larger effects. Finally, for our histological studies, staining and sectioning the embedded tissue may have also resulted in fiber re-orientation from their fresh, untreated state. However, we expect this effect to be minimal.
5. Conclusion
Based on our comprehensive mechanical testing protocol of six simple shear modes and three tension/compression modes, we found that right ventricular myocardium exhibits non-linear, strain-stiffening, and anisotropic behavior with Poynting effect. The mechanics of right ventricular myocardium were qualitatively and quantitatively very comparable to the mechanics of the left ventricular myocardium. The exception being the sample stiffness in the fiber direction, in which the right ventricular myocardium was less stiff. When interpreting and using our mechanical data on right and left ventricular myocardium, we remind the reader of the limitations above. Additionally, we conducted detailed microstructural analyses via serial histological sections that revealed that muscle fibers in the right ventricular myocardial were dispersed in the sheet-plane and in the plane perpendicular to the sheet-plane. While fiber distributions varied in the transmural direction, they were consistent in the longitudinal direction. Additionally, we investigated fundamental questions about constitutive modeling of right ventricular myocardium and found that the Holzapfel model provides an excellent fit to our data even when limited microstructural data is provided. We also demonstrated that including more microstructural data improves the ability of this model to predict mechanical behavior. Finally, we demonstrated that inversely identifying microstructural data may provide erroneous results and should thus be done with care. In conclusion, we provide previously unreported mechanical, microstructural, and modeling data that continues to fill gaps in our knowledge of the right ventricle.
Supplementary Material
Acknowledgements
Research reported in this publication was partially supported by the National Heart, Lung, And Blood Institute of the National Institutes of Health under Award Number F31HL145976 and the American Heart Association for their support under Award Number 18CDA34120028. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health or the American Heart Association.
Footnotes
Disclosures
Dr. Rausch has a speaking agreement with Edwards Lifesciences. None of 565 the other authors have any potential conflicts of interest.
6. Supplemental Data
The mechanical raw data and histological imaging data used in this publication are available through the Texas Data Repository (Dataverse: https://dataverse.tdl.org/dataverse/RVMechanics) [59, 60, 61, 62].
Declaration of interests
The authors declare the following financial interests/personal relationships which may be considered as potential competing interests:
Dr. Rausch has a speaking agreement with Edwards Lifesciences. No other author has any conflicts of interest to declare.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- [1].Mas PT, Rodríguez-Palomares JF, Antunes MJ, Secondary tricuspid valve regurgitation: a forgotten entity, Heart 101 (22) (2015) 1840–1848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Rigolin VH, Robiolio PA, Wilson JS, Harrison JK, Bashore TM, The forgotten chamber: the importance of the right ventricle, Catheterization and Cardiovascular Diagnosis 35 (1) (1995) 18–28. [DOI] [PubMed] [Google Scholar]
- [3].Stefanadis CI, Imaging of the neglected cardiac chamber: the right ventricle, Hellenic Journal of Cardiology 51 (2010) 285. [PubMed] [Google Scholar]
- [4].Sheehan F, Redington A, The right ventricle: anatomy, physiology and clinical imaging, Heart 94 (11) (2008) 1510–1515. [DOI] [PubMed] [Google Scholar]
- [5].Sade RM, Castaneda AR, The dispensable right ventricle, Surgery 77 (5) (1975) 624–631. [PubMed] [Google Scholar]
- [6].Starr I, Jeffers WA, Meade RH Jr, The absence of conspicuous increments of venous pressure after severe damage to the right ventricle of the dog, with a discussion of the relation between clinical congestive failure and heart disease, American Heart Journal 26 (3) (1943) 291–301. [Google Scholar]
- [7].Kagan A, Dynamic responses of the right ventricle following extensive damage by cauterization, Circulation 5 (6) (1952) 816–823. [DOI] [PubMed] [Google Scholar]
- [8].Bakos AC, The question of the function of the right ventricular myocardium: an experimental study, Circulation 1 (4) (1950) 724–732. [Google Scholar]
- [9].Mertens LL, Friedberg MK, Imaging the right ventriclecurrent state of the art, Nature Reviews Cardiology 7 (10) (2010) 551. [DOI] [PubMed] [Google Scholar]
- [10].Rausch M, Dam A, oktepe SG, Abilez O, Kuhl E, Computational modeling of growth: systemic and pulmonary hypertension in the heart, Biome590 chanics and Modeling in Mechanobiology 10 (6) (2011) 799–811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Chin KM, Kim NH, Rubin LJ, The right ventricle in pulmonary hypertension, Coronary Artery Disease 16 (1) (2005) 13–18. [DOI] [PubMed] [Google Scholar]
- [12].Kinch JW, Ryan TJ, Right ventricular infarction, New England Journal of Medicine 330 (17) (1994) 1211–1217. [DOI] [PubMed] [Google Scholar]
- [13].Zornoff LA, Skali H, Pfeffer MA, Sutton MSJ, Rouleau JL, Lamas GA, Plappert T, Rouleau JR, Moyé LA, Lewis SJ, Braunwald E, Solomon SD, Right ventricular dysfunction and risk of heart failure and mortality after myocardial infarction, Journal of the American College of Cardiology 39 (9) (2002) 1450–1455. [DOI] [PubMed] [Google Scholar]
- [14].Argulian E, Sud K, Vogel B, Bohra C, Garg VP, Talebi S, Lerakis S, Narula J, Right ventricular dilation in hospitalized patients with covid-19 infection, JACC: Cardiovascular Imaging. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Haddad F, Hunt SA, Rosenthal DN, Murphy DJ, Right ventricular function in cardiovascular disease, part i: anatomy, physiology, aging, and functional assessment of the right ventricle, Circulation 117 (11) (2008) 1436–1448. [DOI] [PubMed] [Google Scholar]
- [16].Meador WD, Malinowski M, Jazwiec T, Goehler M, Quay N, Timek TA, Rausch MK, A fiduciary marker-based framework to assess heterogeneity and anisotropy of right ventricular epicardial strains in the beating ovine heart, Journal of Biomechanics 80 (26) (2018) 179–185. [DOI] [PubMed] [Google Scholar]
- [17].Christoffels VM, Habets PE, Franco D, Campione M, de Jong F, Lamers WH, Bao Z-Z, Palmer S, Biben C, Harvey RP, Moorman AF, Chamber formation and morphogenesis in the developing mammalian heart, Developmental biology 223 (2) (2000) 266–278. [DOI] [PubMed] [Google Scholar]
- [18].Sacks MS, Chuong CJ, Biaxial Mechanical Properties of Passive Right Ventricular Free Wall Myocardium, Journal of Biomechanical Engineering 115 (2) (1993) 202–205. [DOI] [PubMed] [Google Scholar]
- [19].Ahmad F, Liao J, Soe S, Jones MD, Miller J, Berthelson P, Enge D, Copeland KM, Shaabeth S, Johnston R, Maconochie I, Theobald PS, Biomechanical properties and microstructure of neonatal porcine ventricles, Journal of the Mechanical Behavior of Biomedical Materials 88 (2018) 18–28. [DOI] [PubMed] [Google Scholar]
- [20].Valdez-Jasso D, Simon MA, Champion HC, Sacks MS, A murine experimental model for the mechanical behaviour of viable right-ventricular myocardium, The Journal of Physiology 590 (18) (2012) 4571–4584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Hill MR, Simon MA, Valdez-Jasso D, Zhang W, Champion HC, Sacks MS, Structural and mechanical adaptations of right ventricle free wall myocardium to pressure overload, Annals of Biomedical Engineering 42 (12) (2014) 2451–2465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Avazmohammadi R, Hill M, Simon M, Sacks M, Transmural remodeling of right ventricular myocardium in response to pulmonary arterial hypertension, APL Bioengineering 1 (1) (2017) 016105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Novak VP, Yin F, Humphrey J, Regional mechanical properties of passive myocardium, Journal of Biomechanics 27 (4) (1994) 403–412. [DOI] [PubMed] [Google Scholar]
- [24].Dokos S, Smaill BH, Young AA, LeGrice IJ, Shear properties of passive ventricular myocardium, American Journal of Physiology-Heart and Circulatory Physiology 283 (6) (2002) H2650–H2659. [DOI] [PubMed] [Google Scholar]
- [25].Sommer G, Schriefl AJ, Andrä M, Sacherer M, Viertler C, Wolinski H, Holzapfel GA, Biomechanical properties and microstructure of human 640 ventricular myocardium, Acta Biomaterialia 24 (2015) 172–192. [DOI] [PubMed] [Google Scholar]
- [26].Avazmohammadi R, Li DS, Leahy T, Shih E, Soares JS, Gorman JH, Gorman RC, Sacks MS, An integrated inverse model-experimental approach to determine soft tissue three-dimensional constitutive parameters: Application to post-infarcted myocardium, Biomechanics and Modeling in Mechanobiology 17 (1) (2018) 31–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Holzapfel GA, Ogden RW, Constitutive modelling of passive myocardium: a structurally based framework for material characterization, Philosophical Transactions of the Royal Society A: Mathematical, Physical and Engineering Sciences 367 (1902) (2009) 3445–3475. [DOI] [PubMed] [Google Scholar]
- [28].Hunter PJ, Myocardial constitutive laws for continuum mechanics models of the heart, in: Molecular and Subcellular Cardiology, Springer, 1995, pp. 303–318. [DOI] [PubMed] [Google Scholar]
- [29].Avazmohammadi R, Hill MR, Simon MA, Zhang W, Sacks MS, A novel constitutive model for passive right ventricular myocardium: evidence for myofiber–collagen fiber mechanical coupling, Biomechanics and Modeling in Mechanobiology 16 (2) (2017) 561–581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Avazmohammadi R, Soares JS, Li DS, Raut SS, Gorman RC, Sacks MS, A contemporary look at biomechanical models of myocardium, Annual Review of Biomedical Engineering 21 (2019) 417–442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Guccione JM, McCulloch AD, Waldman LK, Passive Material Properties of Intact Ventricular Myocardium Determined From a Cylindrical Model, Journal of Biomechanical Engineering 113 (1) (1991) 42–55. [DOI] [PubMed] [Google Scholar]
- [32].Genet M, Rausch M, Lee LC, Choy S, Zhao X, Kassab GS, Kozerke S, Guccione JM, Kuhl E, Heterogeneous growth-induced prestrain in the heart, Journal of Biomechanics 48 (10) (2015) 2080–2089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Rausch MK, Zöllner AM, Genet M, Baillargeon B, Bothe W, Kuhl E, A virtual sizing tool for mitral valve annuloplasty, International Journal for Jumerical Methods in Biomedical Engineering 33 (2) (2017) e02788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Destrade M, Murphy JG, Saccomandi G, Simple shear is not so simple, International Journal of Non-Linear Mechanics 47 (2) (2012) 210–214. [Google Scholar]
- [35].Schmid H, OCallaghan P, Nash M, Lin W, LeGrice I, Smaill B, Young A, Hunter P, Myocardial material parameter estimation, Biomechanics and Modeling in Mechanobiology 7 (3) (2008) 161–173. [DOI] [PubMed] [Google Scholar]
- [36].Li DS, Avazmohammadi R, Merchant SS, Kawamura T, Hsu EW, Gorman III JH, Gorman RC, Sacks MS, Insights into the passive mechanical behavior of left ventricular myocardium using a robust constitutive model based on full 3d kinematics, Journal of the Mechanical Behavior of Biomedical Materials 103 (2020) 103508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Rezakhaniha R, Agianniotis A, Schrauwen JT, Griffa A, Sage D, Bouten CV, Van De Vosse FN, Unser M, Stergiopulos N, Experimental investigation of collagen waviness and orientation in the arterial adventitia using confocal laser scanning microscopy, Biomechanics and Modeling in Mechanobiology 11 (3–4) (2012) 461–473. [DOI] [PubMed] [Google Scholar]
- [38].Schriefl AJ, Reinisch AJ, Sankaran S, Pierce DM, Holzapfel GA, Quantitative assessment of collagen fibre orientations from two-dimensional images of soft biological tissues, Journal of The Royal Society Interface 9 (76) (2012) 3081–3093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Holzapfel GA, Nonlinear Solid Mechanics II, John Wiley & Sons, Inc., 2000. [Google Scholar]
- [40].Maas SA, Ellis BJ, Ateshian GA, Weiss JA, FEBio: Finite elements for biomechanics, Journal of Biomechanical Engineering 134 (1) (2012) 1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Melnik AV, Luo X, Ogden RW, A generalised structure tensor model for the mixed invariant i8, International Journal of Non-Linear Mechanics 107 (2018) 137–148. [Google Scholar]
- [42].Maas SA, LaBelle SA, Ateshian GA, Weiss JA, A Plugin Framework for Extending the Simulation Capabilities of FEBio, Biophysical Journal 115 (9) (2018) 1630–1637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Ateshian GA, Anisotropy of fibrous tissues in relation to the distribution of tensed and buckled fibers, Journal of Biomechanical Engineering 129 (2) (2007) 240–249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Hou C, Ateshian GA, A Gauss-Kronrod-Trapezoidal integration scheme for modeling biological tissues with continuous fiber distributions, Computer Methods in Biomechanics and Biomedical Engineering 19 (8) (2016) 883–893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Coleman TF, Li Y, On the convergence of interior-reflective newton methods for nonlinear minimization subject to bounds, Mathematical Programming 67 (1–3) (1994) 189–224. [Google Scholar]
- [46].Coleman TF, Li Y, An interior trust region approach for nonlinear minimization subject to bounds, SIAM Journal on Optimization 6 (2) (1996) 418–445. [Google Scholar]
- [47].Helfenstein J, Jabareen M, Mazza E, Govindjee S, On non-physical response in models for fiber-reinforced hyperelastic materials, International Journal of Solids and Structures 47 (16) (2010) 2056–2061. [Google Scholar]
- [48].Gültekin O, Dal H, Holzapfel GA, On the quasi-incompressible finite element analysis of anisotropic hyperelastic materials, Computational Mechanics 63 (3) (2019) 443–453. [Google Scholar]
- [49].Sansour C, On the physical assumptions underlying the volumetric-isochoric split and the case of anisotropy, European Journal of Mechanics-A/Solids 27 (1) (2008) 28–39. [Google Scholar]
- [50].Singmann H, Bolker B, Westfall J, Aust F, afex: Analysis of Factorial Experiments, r package version 0.16–1 (2016). URL https://CRAN.R-project.org/package=afex
- [51].Mihai LA, Goriely A, Positive or negative poynting effect? the role of adscititious inequalities in hyperelastic materials, Proceedings of the Royal Society A: Mathematical, Physical and Engineering Sciences 467 (2136) (2011) 3633–3646. [Google Scholar]
- [52].Lejeune E, Linder C, Interpreting stochastic agent-based models of cell death, Computer Methods in Applied Mechanics and Engineering 360 (2020) 112700. [Google Scholar]
- [53].Lejeune E, Sacks MS, Analyzing valve interstitial cell mechanics and geometry with spatial statistics, Journal of biomechanics 93 (2019) 159–166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].Göktepe S, Acharya SNS, Wong J, Kuhl E, Computational modeling of passive myocardium, International Journal for Numerical Methods in Biomedical Engineering 27 (1) (2011) 1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55].Guan D, Ahmad F, Theobald P, Soe S, Luo X, Gao H, On the aic-based model reduction for the general holzapfel–ogden myocardial constitutive law, Biomechanics and Modeling in Mechanobiology 18 (4) (2019) 1213–1232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [56].Sharifi Kia D, Benza E, Bachman TN, Tushak C, Kim K, Simon MA, Angiotensin Receptor-Neprilysin Inhibition Attenuates Right Ventricular Remodeling in Pulmonary Hypertension, Journal of the American Heart Association (2020) e015708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [57].Healy LJ, Jiang Y, Hsu EW, Quantitative comparison of myocardial fiber structure between mice, rabbit, and sheep using diffusion tensor cardiovascular magnetic resonance, Journal of Cardiovascular Magnetic Resonance 13 (1) (2011) 74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [58].Ahmad F, Soe S, White N, Johnston R, Khan I, Liao J, Jones M, Prabhu R, Maconochie I, Theobald P, Region-specific microstructure in the neonatal ventricles of a porcine model, Annals of Biomedical Engineering 46 (12) (2018) 2162–2176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [59].Rausch M, Kakaletsis S, Lejeune E, RV Histology, low resolution images (2020). doi: 10.18738/T8/VGPTWB.URL [DOI]
- [60].Rausch M, Kakaletsis S, Lejeune E, RV Mechanical Data (2020). doi: 10.18738/T8/SGWSNZ.URL [DOI] [PMC free article] [PubMed]
- [61].Rausch M, Kakaletsis S, Lejeune E, LV Mechanical Data (2020). doi: 10.18738/T8/KN3K9S.URL [DOI] [PMC free article] [PubMed]
- [62]. Rausch M, Kakaletsis S, Lejeune E, RV Histology, high resolution images (2020). doi: 10.18738/T8/IOB1G7.URL [DOI]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.