Abstract
Background
Bacteria possessing extended-spectrum beta-lactamase (ESBL), especially E. coli and Klebsiella species, are problematic, particularly in hospitalized patients. Poultry meat vendors are at risk of carrying ESBL-producing bacteria when processing and handling meat products in an unhygienic environment. There is limited information on the carriage rate of ESBL-producing pathogens among poultry meat vendors that necessitated the conduction of the study.
Method
A cross-sectional study was conducted among poultry meat vendors in Dar es Salaam, Tanzania. Participants provided rectal swabs in transport media upon instruction. The primary isolation of ESBL-producing bacteria was carried out using MacConkey agar supplemented with ceftazidime. Identification of isolates relied on conventional methods. Double-disk synergy was the method used to confirm ESBL-producing isolates. We performed descriptive statistics using Statistical Package for Social Sciences version 23. A p value < 0.05 was considered statistically significant.
Results
A total of 300 participants were recruited from five districts, with a mean age of 27.2 ± 6.7 years. The majority was male (67.3%), and 74.7% worked as poultry meat vendors for more than one year. Out of 300 participants, 107 (35.7%) had confirmed ESBL-producing E. coli and Klebsiella spp. The majority of confirmed ESBL-producing isolates was E. coli (78.5%). Participants from Ubungo District had significantly higher carriage of ESBL-producing Escherichia coli and Klebsiella spp. (48.0%, 95% CI: 34.8–47.7) than Temeke District (21.4%, 95% CI: 13.4–32.4). Only 28.0% of participants had access to latrines at the workplace, and all working areas lacked access to running water.
Conclusion
The study revealed a relatively high fecal carriage rate of ESBL-producing E. coli and Klebsiella spp. among poultry meat vendors. Poor working environments and hygienic practices are risks for spread of these multidrug-resitant pathogens.
1. Introduction
Extended-spectrum β-lactamase- (ESBL-) producing pathogens are among the common causes of bacterial infections with increased antibiotic resistance [1]. Infection due to ESBL-producing pathogens is associated with high morbidity and mortality [2, 3]. There is evidence of growing antimicrobial resistance in animals related to varying ESBL alleles [4, 5]. Of concern is the spread of ESBL-producing pathogens to humans through food substances, particularly animal food products [6, 7].
The irrational use of antimicrobial agents in the animal influence the emergency of antibiotic-resistant strains [8, 9] and subsequently spread to humans through consumption of animal products contaminated with resistant stains [10, 11]. The use of antibiotics in poultry is a common practice in several countries [12–14]. Hence, poultry meat may serve as a source of spread of ESBL strains to humans [15–17]. In particular, studies have shown that more than 90% of chicken meat carry ESBL-producing pathogens [16, 18]. In this regard, poultry meat vendors have a high chance of contact with ESBL-producing pathogens [19]. Poultry meat vendors expose during slaughtering, preparation, and selling of meat; considerably, their working environments in low-income countries are relatively not clean and unsafe [20]. The association between poultry meat and human health is becoming an essential issue in the poultry industry versus food safety in the fight against antimicrobial resistance [21].
Most studies in Tanzania on the carriage rate of ESBL-producing pathogens in humans were conducted in hospital settings [22, 23]. E. coli and K. pneumoniae are reported in these studies as the common ESBL-producing pathogens [22, 23]. On the contrary, the ESBL carriage rate among poultry meat vendors in the community has not been comprehensively studied. The current study investigated the fecal carriage rate of ESBL-producing E. coli and Klebsiella spp. among the poultry meat vendors in Dar es Salaam, Tanzania. The findings provide baseline information to guide strategies for the control of infection and antimicrobial resistance in community settings.
2. Materials and Methods
2.1. Study Design, Setting, and Population
This was a cross-sectional study conducted in Dar es Salaam, the largest city in Tanzania. The study involved poultry slaughterhouses and poultry meat-selling centers from selected wards in five districts of the Dar es Salaam region. The study recruited healthy poultry meat vendors involved in either poultry slaughtering, washing, or selling raw meat for more than three months. The study excluded vendors with signs of illness and those who failed to provide written informed consent.
2.2. Sample Size and Sampling Procedure
We determined the sample size using the Kish Leslie formula [24], considering a 24.3% prevalence of ESBL in Dar es Salaam [25], 95% confidence interval, and 5% margin of error. The minimum sample size required for the study was 283. Our approach to the selection of wards and participants in five districts used a multistage sampling technique. The wards with poultry houses and meat-selling centers per district were obtained. After that, the total number of wards from five districts was used as the denominator to calculate the sample size to be drawn per each district. We based on a proportion to size sampling technique to obtain the number of wards per district to get 23 out of 107 wards in Dar es Salaam. Two to seven wards per district and about 10 to 20 participants from each ward were recruited by simple random sampling at the poultry meat vendor centers.
2.3. Data Collection
The investigator visited poultry meat vendors at their working station for data collection. The interviewer provided a detailed explanation about the study to every study participant who provided written informed consent before recruitment. The data were collected using a structured questionnaire. The collected information includes sociodemographic characteristics (age, sex, location, and duration in business), working environments, and hygienic practice conditions.
2.4. Sample Collection and Transportation
The research assistant clearly instructed each participant and illustrated the rectal swab's self-collection and how to insert the swab onto Cary-Blair transport media. The samples were transported daily in a cool box with ice packs within six hours after collection to the microbiology and immunology laboratory at Muhimbili University of Health and Allied Sciences (MUHAS) for processing.
2.5. Laboratory Procedure
Each swab was inoculated on MacConkey agar, supplemented with 1.0 mg/L ceftazidime, within 24 hours after collection. The inoculated plates were incubated aerobically at 37°C for 18 to 24 hours. The presence of ceftazidime in the media inhibits the growth of non-ESBL-producing bacteria; therefore, isolated colonies were presumptively considered as ESBL-producing pathogens. We first suspected E. coli and Klebsiella spp. based on colonial characteristics and Gram stain reaction. Colonies with distinctive morphological appearance of dry or mucoid lactose-positive (pink) and Gram-negative rods were selected. Suspected colonies were subcultured into nutrient agar (Oxoid Ltd., UK) to get enough pure colonies for further identification. Conventional biochemical tests of Kligler's iron agar, sulfide-indole-motility test, and citrate test were used (Table 1).
Table 1.
Biochemical characteristics used for identification of E. coli and Klebsiella spp.
| Isolate | KIA | H2S | Indole | Motility | Citrate test |
|---|---|---|---|---|---|
| E. coli | A/A | − | + | + | − |
| Klebsiella spp. | A/A | − | − | − | + |
KIA: Kligler's iron agar; H2S: hydrogen sulfide; A/A: acid/acid; (−): negative reaction; (+): positive reaction.
As previously described, it was confirmed that all isolates were ESBL-producing pathogens by the double-disk synergy method [26]. In brief, Muller Hinton agar plates were inoculated with suspension of isolates matching 0.5 McFarland turbidity standards. Ceftazidime (30 μg) and cefotaxime (30 μg) disks were placed at a distance of 20 mm around the amoxicillin-clavulanic acid (30 μg) disk. The incubation environment for inoculated media was 37°C for 18 to 24 hours. Any distortion or increase in the inhibition zone towards the disk of amoxicillin-clavulanic acid indicated positive for ESBL production. Klebsiella pneumoniae ATCC 700603 represented a positive control strain for ESBL production. The laboratory workflow for isolation, identification, and confirmation of ESBL-producing E. coli and Klebsiella spp. is summarized in Figure 1.
Figure 1.
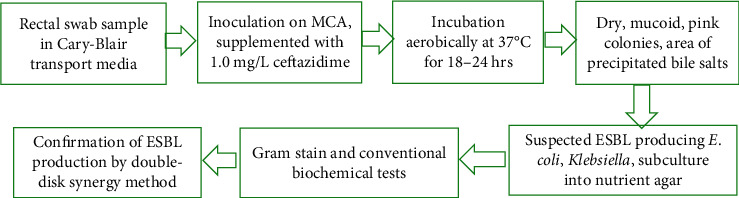
Laboratory workflow for isolation, identification, and confirmation of ESBL-producing E. coli and Klebsiella spp.
2.6. Data Analysis
Data were analyzed using Statistical Package for Social Sciences version 23.0. Categorical variables were summarized as proportions, while continuous variables were summarized as mean and standard deviation. A chi-square test was calculated to determine the differences between proportions. A 95% confidence interval of proportion was calculated to provide more information on the upper and lower bound of proportion estimates. The level of significance was specified at 0.05.
3. Results
3.1. Demographic Characteristics of Study Participants
The study enrolled 300 poultry meat vendors with a mean age of 27.2 ± 6.7 years. The majority, 202 (67.3%), was males, and 138 (46.0%) were below 25 years. More than a quarter, 80 (26.7%), were recruited from the Kinondoni District, followed by Temeke District 70 (23.3%). Of all, 224 (74.7%) worked as poultry meat vendors for more than one year. Around half, 151 (50.3%), reported a preference for self-medication with antibiotics before going to the hospital (Table 2).
Table 2.
Demographic distribution of study participants.
| Variables | Frequency/mean ± SD | Percentage (%) |
|---|---|---|
| Mean age (years) | 27.2 ± 6.7 | |
| Age group (years) | ||
| <25 | 138 | 46.0 |
| 25–30 | 85 | 28.3 |
| >30 | 77 | 25.6 |
| Sex | ||
| Male | 202 | 67.3 |
| Female | 98 | 32.7 |
| Districts of business | ||
| Ubungo | 50 | 16.7 |
| Ilala | 60 | 20.0 |
| Temeke | 70 | 23.3 |
| Kigamboni | 40 | 13.3 |
| Kinondoni | 80 | 26.7 |
| Duration in the business | ||
| Three months to one year | 76 | 25.3 |
| More than one year | 224 | 74.7 |
| Preference for self-medication | ||
| Yes | 151 | 50.3 |
| No | 149 | 49.7 |
SD: standard deviation.
3.2. Working Environment and Hygiene Practice
Most of the study participants, 216 (72%), had no latrines at their workplace, and all (100%) reported having access to water for washing hands, clearing the environment, cleaning knives, washing poultry, bowel, and plates. However, all working areas lack access to running water. No participant used hand protection when handling poultry meat (data not presented).
3.3. Distribution of ESBL Isolates
A total of 130 (43.3%) bacterial isolates were suspected to be ESBL-producing E. coli and Klebsiella spp. E. coli, 101 (77.7%), were the predominant bacteria. Of 130 ESBL-suspected isolates, 107 (82.3%) were confirmed as ESBL-producing E. coli and Klebsiella spp. Out of 107 ESBL producers, 84 (78.5%) were E. coli isolates, and 23 (21.5%) were Klebsiella spp. (Table 3).
Table 3.
Distribution of ESBL-producing Escherichia coli and Klebsiella species.
| Frequency | Percentage (%) | |
|---|---|---|
| Primary ESBL isolation (n = 130) | ||
| E. coli | 101 | 77.7 |
| Klebsiella spp. | 29 | 22.3 |
| ESBL confirmation (n = 107) | ||
| E. coli | 84 | 78.5 |
| Klebsiella spp. | 23 | 21.5 |
3.4. The Proportion of ESBL-Producing Bacteria among Study Participants
The overall carriage rate of ESBL-producing E. coli and Klebsiella spp. was 35.7% (95% CI: 30.5–41.2). The frequency of carriage was high (38.4%) among participants aged less than 25 years, but the observed difference in age groups was not significant. There was a significant difference in the proportion of participants with carriage of ESBL-producing E. coli and Klebsiella spp. in the district of business. Ubungo District had higher proportion of fecal carriage (48.0%, 95% CI: 34.8–47.7) than Temeke District (21.4%, 95% CI: 13.4–32.4) (p=0.030). The proportion of fecal carriage of ESBL-producing E. coli and Klebsiella spp. observed in sex, duration in business, and preference for self-treatment was not significantly different (p > 0.05) (Table 4).
Table 4.
Proportion of confirmed ESBL producers in study participants.
| Variable | Total population | Confirmed ESBL producer | ∗ p value | |
|---|---|---|---|---|
| N | % (95% CI) | |||
| Overall | 300 | 107 | 35.7 (30.5–41.2) | |
| Age group (years) | 0.565 | |||
| <25 | 138 | 53 | 38.4 (30.7–46.7) | |
| 25–30 | 85 | 26 | 30.6 (21.8–41.1) | |
| >30 | 77 | 28 | 36.4 (26.5–47.5) | |
| Sex | 0.599 | |||
| Male | 202 | 70 | 34.7 (28.4–41.4 | |
| Female | 98 | 37 | 37.8 (28.8–47.7) | |
| Districts of business | 0.030 | |||
| Ubungo | 50 | 24 | 48.0 (34.8–61.5) | |
| Ilala | 60 | 25 | 41.7 (30.1–54.3) | |
| Temeke | 70 | 15 | 21.4 (13.4–32.4) | |
| Kigamboni | 40 | 13 | 32.5 (20.1–48.0) | |
| Kinondoni | 80 | 30 | 37.5 (27.7–48.5) | |
| Duration in business | 0.804 | |||
| ≥3months to 1 year | 76 | 28 | 36.8 (26.9–48.1) | |
| >1 year | 224 | 79 | 32.4 (29.3–41.7) | |
| Preference for self-medication | 0.836 | |||
| Yes | 151 | 53 | 35.1 (27.9–43.0) | |
| No | 149 | 54 | 36.2 (27.0–44.2) | |
∗ p value according to Pearson's chi-square test. CI = confidence interval.
4. Discussion
The present study demonstrates a high carriage rate of ESBL-producing E. coli and Klebsiella spp. among poultry meat vendors in Dar es Salaam. Although there is no study done in a similar population in Tanzania, the finding is relatively higher than the fecal carriage of ESBL-producing bacteria in the community among people sharing latrines in Dar es Salaam [25]. The study also revealed poor working environment and hygienic practices in the poultry meat vendor. Based on these findings, the high fecal carriage of ESBL-producing E. coli and Klebsiella spp. may be due to direct contact with poultry meat that may be carrying or contaminated with ESBL strains, poor hygiene practices in their working environment, and lack of safe and running water for washing hands and other uses.
In the current study, E. coli was the most common ESBL producer isolated among poultry meat vendors than Klebsiella spp.; the finding agrees with the study reported in Spain [27]. The predominance of E. coli was also reported in another study conducted in Tanzania, where E. coli was the most common cause of community-acquired infection [28]. Furthermore, a study done in Mwanza on companion and domestic animal carriage of ESBL-producing bacteria reported the predominance of E. coli at a rate of 21.7% [5]. The study from Gabon, sub-Saharan Africa, reported a 23.0% rate of chicken contamination with ESBL-producing E. coli [29]. Thus, the predominance of ESBL-producing E. coli among poultry meat vendors indicates a community problem as these poultry meat vendors are part of the community.
Our study revealed the lack of running water in the working area for all poultry meat vendors. Instead, they used water kept in the buckets or tins for washing hands, poultry meat, and cleaning tables. Hand washing from the same bucket may increase the contamination rate, thus spreading the ESBL-producing bacteria. Studies in France reported the prevalence of 10.7% of ESBL-producing E. coli in humans related to contamination during slaughtering and in contact with food animal products, including poultry meat [30]. A Dutch study reported an 18% contribution of poultry to ESBL-producing E. coli exposure of the Dutch population through chicken meat consumption [31]. Compared to our findings, the difference in prevalence could be contributed by the level of personal hygienic practices [30]. Handling contaminated animal food products increases the risk of transmission of ESBL bacteria [15, 20]. In this study, all poultry meat vendors did not use hand protective gear such as gloves when processing poultry meat and serving customers, hence directly contacted poultry meat. The study enrolled participants from five districts in Dar es Salaam; the fecal carriage rate of ESBL showed significant difference between some districts. The prevalence between Ubungo and Temeke Districts cannot be explained with available data for demographic and hygienic practice. The findings call for a comprehensive and detailed comparative study on the demographic characteristics, working environment, and hygienic practice.
To the best of our knowledge, this is the first report from the Dar es Salaam region investigating the prevalence of ESBL-producing bacteria in poultry meat vendors. Therefore, our study presents the baseline information on the prevalence of ESBL-producing E. coli and Klebsiella spp. among poultry meat vendors. The present study's findings suggest the need for a regular screen for colonization with ESBL-producing bacteria for a specific group of poultry meat vendors and improving the working environment and hygienic practices. The government should have a policy that requires the screening of food handlers before engaging in business to control the spread of ESBL-producing bacteria and antimicrobial resistance in the community. Based on the laboratory methods used to identify E. coli and Klebsiella spp., we might have underestimated these organisms' magnitude. Some strains may have an opposite reaction with the conventional biochemical tests used for identification, which advanced identification technique.
5. Conclusion
The study demonstrates a relatively high fecal carriage rate of ESBL-producing E. coli and Klebsiella spp. among poultry meat vendors. For food handlers, the availability of clean running water is essential as washing hands in the same water as where the chickens are washed is a risk for public health as well as for the poultry workers.
Acknowledgments
The authors are grateful to all the poultry meat vendors who participated in the study. They thank all laboratory personnel at MUHAS Microbiology Laboratory for their technical support. The World Bank financially supported the study's data collection through the Ministry of Health, Development, Gender, Elders and Children, Forensic Bureau, Dar es Salaam, Tanzania.
Abbreviations
- ESBL:
Extended-spectrum beta-lactamase
- KIA:
Kligler's iron agar
- MUHAS:
Muhimbili University of Health and Allied Sciences.
Data Availability
All the relevant data generated and analyzed during this study are included within this manuscript. However, the dataset can be accessed from the corresponding author upon reasonable request.
Ethical Approval
Ethical approval was obtained from the Senate Research and Publications Committee, the Institutional Review Board of Muhimbili University of Health and Allied Sciences, Dar es Salaam, Tanzania. Permission to conduct the study was obtained from regional and district authorities.
Consent
Each participant provided written informed consent before being recruited.
Conflicts of Interest
The authors declare that they have no conflicts of interest.
Authors' Contributions
LWM and AJ conceived and designed the study. LWM participated in data collection and laboratory testing and wrote the initial draft of the manuscript. LWM, AJ, and MM were involved in data analysis and interpretation. MM, DCK, and AJ participated in the write-up and critically revising the manuscript. All authors read and approved the final version of the manuscript.
References
- 1.Dhillon R. H.-P., Clark J. ESBLS: a clear and present danger? Critical Care Research and Practice. 2012;2012 doi: 10.1155/2012/625170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kayange N., Kamugisha E., Mwizamholya D. L., Jeremiah S., Mshana S. E. Predictors of positive blood culture and deaths among neonates with suspected neonatal sepsis in a tertiary hospital, Mwanza-Tanzania. BMC Pediatrics. 2010;10(1):p. 39. doi: 10.1186/1471-2431-10-39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mshana S. E., Gerwing L., Minde M., et al. Outbreak of a novel Enterobacter sp. carrying blaCTX-M-15 in a neonatal unit of a tertiary care hospital in Tanzania. International Journal of Antimicrobial Agents. 2011;38(3):265–269. doi: 10.1016/j.ijantimicag.2011.05.009. [DOI] [PubMed] [Google Scholar]
- 4.Lupindu A. M., Olsen J. E., Ngowi H. A., et al. Occurrence and characterization of shiga toxin-ProducingEscherichia coliO157:H7 and other non-sorbitol-FermentingE. Coliin cattle and humans in urban areas of morogoro, Tanzania. Vector-Borne and Zoonotic Diseases. 2014;14(7):503–510. doi: 10.1089/vbz.2013.1502. [DOI] [PubMed] [Google Scholar]
- 5.Seni J., Falgenhauer L. Simeo N., et al. “Multiple ESBL-producing Escherichia coli sequence types carrying quinolone and aminoglycoside resistance genes circulating in companion and domestic farm animals in Mwanza, Tanzania, harbor commonly occurring plasmids. Frontiers in Microbiology. 2016;7:p. 142. doi: 10.3389/fmicb.2016.00142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Johnson J. R., Kuskowski M. A., Menard M., Gajewski A., Xercavins M., Garau J. Similarity between human and ChickenEscherichia coliIsolates in relation to ciprofloxacin resistance status. The Journal of Infectious Diseases. 2006;194(1):71–78. doi: 10.1086/504921. [DOI] [PubMed] [Google Scholar]
- 7.Mesa R. J. Extended-spectrum -lactamase-producing Enterobacteriaceae in different environments (humans, food, animal farms and sewage) Journal of Antimicrobial Chemotherapy. 2006;58(1):211–215. doi: 10.1093/jac/dkl211. [DOI] [PubMed] [Google Scholar]
- 8.Organization W. H. WHO Global Strategy for Containment of Antimicrobial Resistance. Geneva, Switzerland: World Health Organization; 2001. [Google Scholar]
- 9.Smith D. L., Harris A. D., Johnson J. A., Silbergeld E. K., Morris J. G. Animal antibiotic use has an early but important impact on the emergence of antibiotic resistance in human commensal bacteria. Proceedings of the National Academy of Sciences. 2002;99(9):6434–6439. doi: 10.1073/pnas.082188899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Levy S. B., Marshall B. Antibacterial resistance worldwide: causes, challenges and responses. Nature Medicine. 2004;10(12):S122–S129. doi: 10.1038/nm1145. [DOI] [PubMed] [Google Scholar]
- 11.Tschudin-Sutter S., Frei R., Stephan R., Hächler H., Nogarth D., Widmer A. F. Extended-spectrum β-lactamase (ESBL)-Producing enterobacteriaceae: a threat from the kitchen. Infection Control & Hospital Epidemiology. 2014;35(5):581–584. doi: 10.1086/675831. [DOI] [PubMed] [Google Scholar]
- 12.Nonga H. E., Mariki M., Karimuribo E. D., Mdegela R. H. Assessment of antimicrobial usage and antimicrobial residues in broiler chickens in Morogoro Municipality, Tanzania. Pakistan Journal of Nutrition. 2009;8(3):203–207. doi: 10.3923/pjn.2009.203.207. [DOI] [Google Scholar]
- 13.Nonga H. E., Simon C., Karimuribo E. D., Mdegela R. H. Assessment of antimicrobial usage and residues in commercial chicken eggs from smallholder poultry keepers in Morogoro municipality, Tanzania. Zoonoses and Public Health. 2010;57(5):339–344. doi: 10.1111/j.1863-2378.2008.01226.x. [DOI] [PubMed] [Google Scholar]
- 14.Nonga H., Sungura K., Ngowi H. Assessment of veterinary drug use and determination of antimicrobial residues in broiler chicken meat in urban district, zanzibar, tanzania. Pakistan Journal of Nutrition. 2013;8(3):203–207. doi: 10.3923/pjn.2009.203.207. [DOI] [Google Scholar]
- 15.Rasmussen M. M., Japheth A. O., Frimodt-Møller N., Styrishave B. Beta-lactamase producing Escherichia coli isolates in imported and locally produced chicken meat from Ghana. PloS One. 2015;10(10) doi: 10.1371/journal.pone.0139706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Stuart J. C., van den Munckhof T., Voets G., Scharringa J., Fluit A., Hall M. L. -V. Comparison of ESBL contamination in organic and conventional retail chicken meat. International Journal of Food Microbiology. 2012;154(3):212–214. doi: 10.1016/j.ijfoodmicro.2011.12.034. [DOI] [PubMed] [Google Scholar]
- 17.Tham J., Odenholt I., Walder M., Melander E. Prevalence of extended-spectrum beta-lactamase-producing bacteria in food. Infection and Drug Resistance. 2012;5:p. 143. doi: 10.2147/idr.s34941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nguyen D. P., Nguyen T. A. D., Le T. H., et al. Dissemination of extended-spectrum β-lactamase-and AmpC β-lactamase-producing Escherichia coli within the food distribution system of Ho Chi minh city, Vietnam. BioMed Research International. 2016;2016 doi: 10.1155/2016/8182096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Maron D., Smith T. J., Nachman K. E. Restrictions on antimicrobial use in food animal production: an international regulatory and economic survey. Globalization and Health. 2013;9(1):p. 48. doi: 10.1186/1744-8603-9-48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Songe M. M., Hang’ombe B., Jones T. K., Grace D. Antimicrobial resistant enteropathogenic Escherichia coli and Salmonella spp. in houseflies infesting fish in food markets in Zambia. International Journal of Environmental Research and Public Health. 2017;14(1):p. 21. doi: 10.3390/ijerph14010021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kariuki S., Odwar J., Kikuvi G., Kariuki J. A cross-sectional study on the microbiological quality and safety of raw chicken meats sold in nairobi, kenya. BioMed Central Journals. 2017;7 doi: 10.1186/1756-0500-7-627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tellevik M. G., Blomberg B., Kommedal Ø., Maselle S. Y., Langeland N., Moyo S. J. High prevalence of faecal carriage of ESBL-producing Enterobacteriaceae among children in Dar es Salaam, Tanzania. PloS One. 2016;11(12) doi: 10.1371/journal.pone.0168024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Moremi N., Claus H., Rutta L., Frosch M., Vogel U., Mshana S. E. High carriage rate of extended-spectrum beta-lactamase-producing Enterobacteriaceae among patients admitted for surgery in Tanzanian hospitals with a low rate of endogenous surgical site infections. Journal of Hospital Infection. 2018;100(1):47–53. doi: 10.1016/j.jhin.2018.05.017. [DOI] [PubMed] [Google Scholar]
- 24.Israel G. D. Determining Sample Size. Gainesville, FL, USA: University of Florida IFAS Extension; 1992. [Google Scholar]
- 25.Erb S., D’Mello-Guyett L., Malebo H. M., et al. High prevalence of ESBL-Producing E. coli in private and shared latrines in an informal urban settlement in Dar es Salaam, Tanzania. Antimicrobial Resistance & Infection Control. 2018;7(1):p. 3. doi: 10.1186/s13756-017-0292-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Dhara M. Comparison of various methods for the detection of extended spectrum beta-lactamase in Klebsiella pneumoniae isolated from neonatal intensive care unit. National Journal of Medical Research. 2012;2:348–353. [Google Scholar]
- 27.Lavilla S., Gonzalez-Lopez J. J., Miro E., et al. Dissemination of extended-spectrum -lactamase-producing bacteria: the food-borne outbreak lesson. Journal of Antimicrobial Chemotherapy. 2008;61(6):1244–1251. doi: 10.1093/jac/dkn093. [DOI] [PubMed] [Google Scholar]
- 28.Mshana S. E., Matee M., Rweyemamu M. Antimicrobial resistance in human and animal pathogens in Zambia, Democratic Republic of Congo, Mozambique and Tanzania: an urgent need of a sustainable surveillance system. Annals of Clinical Microbiology and Antimicrobials. 2013;12(1):p. 28. doi: 10.1186/1476-0711-12-28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Schaumburg F., Alabi A. S., Frielinghaus L., et al. The risk to import ESBL-producing Enterobacteriaceae and Staphylococcus aureus through chicken meat trade in Gabon. BMC Microbiology. 2014;14(1):p. 286. doi: 10.1186/s12866-014-0286-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Girlich D., Poirel L., Carattoli A., et al. Extended-spectrum β-lactamase CTX-M-1 in Escherichia coli isolates from healthy poultry in France. Applied and Environmental Microbiology. 2007;73(14):4681–4685. doi: 10.1128/aem.02491-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Evers E. G. Comparative exposure assessment of ESBL-producing Escherichia coli through meat consumption. PloS One. 2017;12(1) doi: 10.1371/journal.pone.0169589. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All the relevant data generated and analyzed during this study are included within this manuscript. However, the dataset can be accessed from the corresponding author upon reasonable request.


