Abstract
Objective
The incidence of chronic heart failure (CHF) is likely to keep increasing in Japan as the population ages, placing increased burdens on medical facilities, particularly on the limited numbers of rural hospitals. We explored the appropriateness of CHF treatment in rural areas in Japan.
Methods
We compared rates of adherence to therapeutic guidelines for CHF between residents with a left ventricular ejection fraction <35% living in urban areas (n = 207) and those in rural areas (n = 180). Treatments included pharmacological [beta-blockers, angiotensin-converting enzyme inhibitors (ACEi)/angiotensin II receptor blocker (ARB), mineralocorticoid receptor antagonist (MRA) and anticoagulants for atrial fibrillation] and non-pharmacological [implantable cardioverter defibrillator (ICD)/cardiac resynchronization therapy (CRT), cardiac rehabilitation and HF education] approaches.
Patients
This study included 387 patients with CHF, prior myocardial infarction or cardiomyopathy, and a left ventricular ejection fraction (LVEF) <35% as determined by echocardiography.
Results
The respective rates of treatments administered in urban and rural areas were as follows: beta-blockers, 91.3% vs. 61.7% (p<0.05); ACEi/ARB, 86.5% vs. 68.3% (p<0.05); MRA, 74.4% vs. 59.4% (p<0.01); anticoagulants, 100% vs. 86.5%, (p<0.05); ICD/CRT, 45.4% vs. 5.0% (p<0.05); cardiac rehabilitation, 32.4% vs. 13.3% (p<0.05) and HF education, 33.3% vs. 32.8% (p=0.75).
Conclusion
Regional disparities in treatment for CHF persist, even in Japan. Improvements in the use of guideline-directed treatment in rural areas might improve the outcomes for CHF patients.
Keywords: heart failure, regional disparity, guidelines
Introduction
Heart failure (HF) indicates cardiac dysfunction caused by organic and/or functional abnormalities occurring in the heart and a lack of a compensatory cardiac pump function. Symptoms such as dyspnea, fatigue and edema appear and are collectively defined as a clinical syndrome of reduced exercise tolerance (1). HF may occur due to factors other than the heart, and 32.5% of HF patients show non-cardiovascular diseases as the cause (2).
The prognosis for patients with chronic HF (CHF) worsens when left ventricular contractility declines compared with when it is maintained (3). However, the prognosis of CHF with reduced left ventricular contractility (HFrEF) (4) has been improved, thanks in large part to pharmacotherapies, such as beta blockers (5, 6), and cardiac implantable electric devices (CIEDs), such as implantable cardioverter defibrillators (ICDs) and cardiac resynchronization therapy (CRT) (7, 8). According to the guidelines of the Japanese Circulation Society (9), American Heart Association (10) and European Society of Cardiology (11), specific choices of essential therapies to improve the patient prognosis have been recommended according to the clinical status of the individual patient.
Under the National Health Insurance system of Japan, which covers almost all people at relatively low expense, residents of Japan are supposed to have access to a uniform quality of medical care. Thus, the quality of medical treatment for CHF in rural and urban areas is expected to be of a similarly high standard. However, epidemiological studies have predicted that approximately 1.3 million Japanese will develop HF by 2030 in parallel with the decline in the population and the rapidly increasing numbers of elderly persons (12). In particular, the number of aging rural individuals will far exceed the capacity of medical facilities and doctors. Thus, many patients with HF may only be able to receive limited medical treatment in rural areas.
Given this situation, we aimed to determine whether or not patients with CHF do indeed receive the same standards of care in rural and urban areas.
Materials and Methods
Patient selection
The study included 387 patients admitted for congestive HF, with the exception of cases associated with acute myocardial infarction. Patients who had a history of myocardial infarction or cardiomyopathy and showed a left ventricular ejection fraction (LVEF) <35% on echocardiography were registered. HF was diagnosed according to the criteria in the guidelines [symptoms and physical examinations as described above, pleural effusion and/or lung congestion in chest X-ray, brain natriuretic peptide (BNP) >100 pg/mL or N-terminal pro-brain natriuretic peptide (NT-proBNP) >400 pg/mL in blood test] (9).
The urban institution was Niigata University Hospital (Niigata City), and the rural institutions were Niigata Prefectural Tokamachi (Tokamachi City), Uonuma City Koide (Uonuma City), Niigata Prefectural Matsudai (Tokamachi City) and Tsunan Town (Tsunan Town) Hospitals. The study periods were from 2006 to 2016 and from 2015 to 2018 in the urban and rural hospitals, respectively. Patients who died after hospitalization due to initial acute HF, had unknown prior therapies due to moving or other reasons or no history of hospitalization were excluded. All patients enrolled in this study had a history of hospitalization for HF other than acute myocardial infarction.
Treatments recommended by guidelines
We compared the rates at which treatments for HFrEF recommended by the guidelines of the Japanese Circulation Society (9), American Heart Association (10) and European Society of Cardiology (11) were implemented between urban and rural areas. Comparisons were performed both in all patients and in the subgroup of patients 70-85 years old. The latter subgroup was established in order to minimize the effects of differences in the age of both groups on this comparison. Treatments included medical [beta blocker, angiotensin-converting enzyme inhibitor (ACEi)/angiotensin II receptor blockers (ARB), mineralocorticoid receptor antagonist (MRA) and anticoagulants for atrial fibrillation] and non-medical (ICD/CRT, cardiac rehabilitation and HF education) approaches. The necessity of each treatment in each patient was decided according to the stage of HF, cardiac function and presence of atrial/ventricular arrhythmia.
We also compared the administered doses of the beta blockers carvedilol and bisoprolol and of the diuretic furosemide between the two groups.
Data analyses
Between-group differences in clinical characteristics were determined using unpaired t-tests, χ2 and Fisher's exact test for continuous and categorical variables, respectively. All data were statistically analyzed using the SPSS software program, version 25.0 (SPSS, IBM, USA). Two-sided p values of <0.05 were considered statistically significant. Results are expressed as the mean ± standard deviation (SD) or n (%).
Results
Patients' characteristics
Tables 1, 2 show the backgrounds of the 207 urban and 180 rural residents (mean age, 64±15 vs. 80±15 years old; p<0.05). Women accounted for 38% and 27% (p<0.05), and the mean EF was 26% ±6.9% vs. 29% ±5.2% (p<0.05) in the urban and rural areas, respectively. The proportion of patients with atrial fibrillation was 49% in both areas (p=0.89). The administered doses (urban vs. rural) of the beta-blockers bisoprolol and carvedilol were 3.6±2.0 vs. 2.3±1.3 and 9.3±6.3 vs. 6.8±6.9 mg, respectively (both p<0.05), and those of the diuretic furosemide were 39±34 vs. 28±23 mg (p<0.05). The major underlying pathologies in the urban and rural areas comprised ischemic heart disease (IHD; 30% and 17%, respectively) and dilated cardiomyopathy (DCM; 27% and 28%, respectively), whereas IHD, DCM and valvular heart disease accounted for 60% of the total in both areas. Secondary cardiomyopathy, including cardiac sarcoidosis, was more prevalent in the urban than in the rural areas. Notably, the types of heart disease had not been clearly diagnosed in many patients in the rural areas.
Table 1.
Backgrounds of 207 Urban and 180 Rural Residents.
| Urban areas (n=207) |
Rural areas (n=180) |
p value | ||
|---|---|---|---|---|
| Age | 64±12 | 80±15 | p<0.05 | |
| >75 yrs (n, %) | 57 (28) | 126 (72) | p<0.05 | |
| Male (n, %) | 151 (62) | 111 (73) | p<0.05 | |
| EF (%) | 26±6.9 | 29±5.2 | p<0.05 | |
| Atrial fibrillation (n, %) | 101 (49) | 89 (49) | p=0.89 | |
| Dose of β-blocker (mg) | Bisoprolol | 3.6±2.0 | 2.3±1.3 | p<0.05 |
| Carvedilol | 9.3±6.3 | 6.8±6.9 | p<0.05 | |
| Dose of furosemide (mg) | 39±34 | 28±23 | p<0.05 | |
Results are presented as means±SD or n (%). EF: ejection fraction
Table 2.
Types of Heart Disease of 207 Urban and 180 Rural Residents.
| Types of heart disease(n,%) | Urban areas (n=207) |
Rural areas (n=180) |
|---|---|---|
| Ischemic heart disease | 62(30) | 30(17) |
| Dilated cardiomyopathy | 59(27) | 51(28) |
| Valvular heart disease | 21(10) | 26(14) |
| Dilated phase of hypertrophic cardiomyopathy | 13(6.3) | 2(1.1) |
| Tachycardia induced cardiomyopathy | 9(4.3) | 13(7.2) |
| Cardiac sarcoidosis | 7(3.4) | 0(0) |
| Left ventricular non-compaction | 5(2.4) | 0(0) |
| Mitochondrial cardiomyopathy | 5(2.4) | 0(0) |
| Hypertensive cardiomyopathy | 4(1.9) | 0(0) |
| Cardiac amyloidosis | 3(1.4) | 2(1.1) |
| Drug-induced cardiomyopathy | 3(1.4) | 0(0) |
| Congenital heart disease | 3(1.4) | 0(0) |
| Lamin cardiomyopathy | 2(1.0) | 0(0) |
| Peripartum cardiomyopathy | 1(1.0) | 0(0) |
| Lupus cardiomyopathy | 1(0.5) | 0(0) |
| Fabry disease | 1(0.5) | 0(0) |
| Eosinophilic myocarditis | 1(0.5) | 0(0) |
| Idiopathic ventricular aneurysm | 1(0.5) | 0(0) |
| Unkown/not examined | 8(3.9) | 60(33) |
Results are presented as n (%).
Rates of recommended treatment implementation
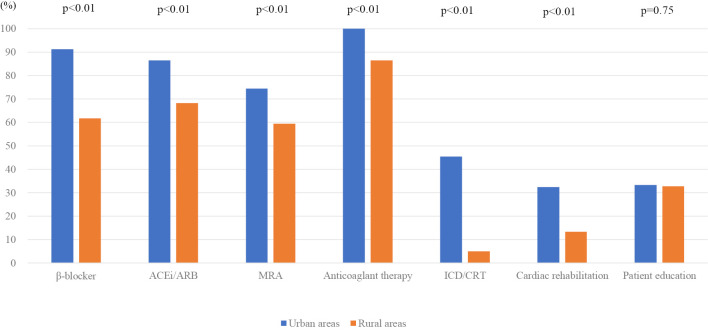
Fig. 1 shows the main findings. The implementation rates for most treatments were higher in urban than in rural areas (beta-blockers, 91.3% vs. 61.7%, p<0.05; ACEi/ARB, 86.5% vs. 68.3%, p<0.05; MRA, 74.4% vs. 59.4%, p<0.05; anticoagulant therapy, 100% vs. 86.5%, p<0.05; ICD/CRT, 45.4% vs. 5.0%, p<0.05). Rates for cardiac rehabilitation (urban vs. rural) were 32.4% vs. 13.3% (p<0.05), and those for HF education were 33.3% vs. 32.8% (p=0.75; not significant).
Figure 1.
Comparison of GDMT implementation rates among all patients selected from urban and rural areas. Therapies included beta-blockers, ACEi/ARB, MRA, anticoagulants, ICD/CRT, cardiac rehabilitation and HF education. MRA: mineral corticoid receptor antagonist, ACEi: angiotensin-converting enzyme inhibitors, ARB: angiotensin II receptor blockers, CRT: cardiac resynchronization therapy, GDMT: guideline-directed medical therapy, HF: heart failure, ICD: implantable cardioverter defibrillator (s)
We also conducted comparisons between groups among subjects 70-85 years old. Tables 3, 4 show the background characteristics of the 73 urban and 82 rural residents (mean age, 78±4.6 years old vs. 79±4.6 years old; p=0.13). No significant differences were apparent in major characteristics, such as sex, EF, or prevalence of atrial fibrillation, or age between the urban versus rural areas (women, 25% vs. 35%; p=0.15; EF, 27±6.0% vs. 28±5.3%; p=0.31; atrial fibrillation, 49% vs. 61%; p=0.15). The administered doses (urban vs. rural) of the beta-blockers bisoprolol and carvedilol were 3.0±1.6 mg vs. 1.9±1.0 mg (p<0.05) and 6.6±4.6 mg vs. 6.6±7.1 mg (p=0.67), respectively, and those of the diuretic furosemide were 30±23 mg vs. 29±22 mg (p=0.67). The major underlying pathologies showed a similar tendency among all age groups (IHD, 46% and 37%; DCM, 18% and 13%; valvular heart disease, 12% and 15%). Secondary cardiomyopathy was rare because this subgroup was restricted to elderly subjects. However, in this subgroup as well, the underlying diseases were not identified for many cases in rural areas.
Table 3.
Backgrounds of 73 Urban and 82 Rural Residents among 70-85 Years Old.
| Urban areas (n=73) |
Rural areas (n=82) |
p value | ||
|---|---|---|---|---|
| Age | 78±4.6 | 79±4.6 | p=0.13 | |
| >75yrs (n,%) | 50 (69) | 62 (76) | p=0.24 | |
| Male (n,%) | 55 (75) | 53 (65) | p=0.15 | |
| EF (%) | 27±6.0 | 28±5.3 | p=0.31 | |
| Atrial fibrillation (n,%) | 36 (49) | 50 (61) | p=0.15 | |
| Doze of β-blocker (mg) | Bisoprolol | 3.0±1.6 | 1.9±1.0 | p<0.05 |
| Carvedilol | 6.6±4.6 | 6.6±7.1 | p=0.98 | |
| Doze of furosemide (mg) | 30±23 | 29±22 | p=0.67 | |
Results are presented as means±SD or n (%). EF: ejection fraction
Table 4.
Types of Heart Disease of 73 Urban and 82 Rural Residents among 70-85 Years Old.
| Types of heart disease (n,%) | Urban areas (n=73) |
Rural areas (n=82) |
|---|---|---|
| Ischemic heart disease | 35(46) | 30(37) |
| Dilated cardiomyopathy | 14(18) | 11(13) |
| Valvular heart disease | 9 (12) | 12(15) |
| Dilated phase of hypertrophic cardiomyopathy | 6 (7.9) | 1 (1.2) |
| Tachycardia induced cardiomyopathy | 4 (5.3) | 6 (7.3) |
| Cardiac sarcoidosis | 1 (1.3) | 0 (0) |
| Hypertensive cardiomyopathy | 2 (2.6) | 0 (0) |
| Cardiac amyloidosis | 0 (0) | 2 (2.4) |
| Idiopathic ventricular aneurysm | 1 (1.3) | 0 (0) |
| Unkown/not examined | 4 (5.3) | 23(28) |
Results are presented as n (%).
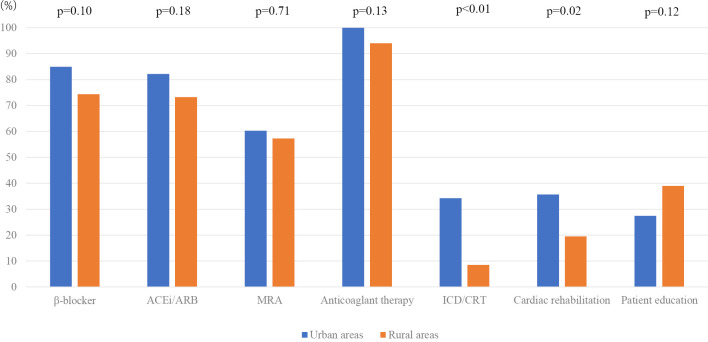
Fig. 2 shows the implementation rates of treatments at 70-85 years old. In this comparison (urban vs. rural), the difference in the introduction rate of ICD/CRT was clear (beta-blockers, 84.9% vs. 74.4%, p=0.10; ACEi/ARB, 82.2% vs. 73.2%, p=0.18; MRA, 60.3% vs. 57.3%, p=0.71; anticoagulant therapy, 100% vs. 94%, p=0.13; ICD/CRT, 34.2% vs. 8.5%, p<0.01). Rates of cardiac rehabilitation (urban vs. rural) were 35.6% vs. 19.5% (p=0.02), and those for HF education were 27% vs. 38.6% (p=0.12).
Figure 2.
Comparison of GDMT implementation rates among patients of 70-85years old selected from urban and rural areas. Therapies included β-blockers, ACEi/ARB, MRA, anticoagulants, ICD/CRT, cardiac rehabilitation and HF education. MRA: mineralcorticoid receptor antagonist, ACEi: angiotensin-converting enzyme inhibitors, ARB: angiotensin II receptor blockers, CRT: cardiac resynchronization therapy, GDMT: guideline-directed medical therapy, HF: heart failure, ICD: implantable cardioverter defibrillator (s)
Discussion
In this study, we found considerable disparity between urban and rural areas in the treatment of CHF with contraction disorders. However, considering age differences, the difference in the ICD introduction rate was noticeable, and a difference was also apparent in non-pharmacological therapy.
In numerous clinical trials on the treatment of CHF, some therapeutic options have been shown to have robust favorable effects. Several pharmacological agents can improve the prognosis of CHF and are collectively termed “cardioprotective” agents. The CONSENSUS Trial Study found that adding enalapril to conventional therapy for patients with severe CHF reduced mortality and improved symptoms (13). Bisoprolol and carvedilol also reduced the risk of death and of hospitalization for cardiovascular causes in patients with HF and reduced contractility (5, 6, 14). Thus, the clinical guidelines (9-11) recommend adding the above drugs to therapy for an HF patient to improve their prognosis. In our study, the implementation rates for cardioprotective agents (beta-blockers, ACEi/ARBs and MRAs) were considerably low in rural areas. Even though patients in rural areas tend to have some limitations concerning their access to medical therapies, such as advanced age and other organ (e.g., kidney) dysfunctions, the low implementation rate of cardioprotective agents may have further reduced the prognosis of HF patients in rural areas.
In addition to pharmacological therapy, CIEDs, such as ICDs and CRT, have been shown to be among the essential treatments for HF patients (15, 16). ICDs improve the prognosis more than antiarrhythmic agents among patients with persistent ventricular tachycardia and ventricular fibrillation associated with underlying heart disease (17). A meta-analysis summarizing these 3 trials showed that ICDs significantly reduced mortality compared with amiodarone, with a 27% reduction in relative mortality over 6 years (18). The MADIT-1 and MADIT-2 ICD trials concerning the ability of ICDs to prevent sudden death found an improved mortality among patients with coronary artery disease and a reduced left ventricular contractility (19, 20). The SCD-HeFT and DEFINITE trials found that ICDs tend to reduce sudden death even among patients with non-ischemic heart disease (21, 22). The prognosis-improving effects of CRT have also been proven in various clinical trials (COMPANION, CARE-HF) (7, 8). CRT is more effective in patients with advanced cardiac dysfunction and conduction disturbances, such as left bundle branch block, than in others. The patients who receive treatment with CRT and show normalization of the cardiac function are called “super-responders”. CRT is thus an essential treatment for HF patients in combination with pharmacological therapy.
However, the implantation and management of CIEDs requires specialized skills that are often available only in tertiary hospitals located in urban areas. Difficulty accessing tertiary hospitals may make patients in rural areas hesitant to receive CIED therapy. In our study, the difference in implementation rates between urban and rural areas was greatest for CIEDs (45.4% vs. 5.0%, p<0.05). The increasing use of CIEDs in rural areas may have substantial positive impacts on the general prognosis of HF patients. In recent years, remote monitoring systems for CIEDs have been widely implemented, and some clinical studies [such as IN-TIME (23)] have shown prognosis-improving effects of monitoring systems. Such systems may make it easier for patients in rural areas to access CIED treatment by reducing the need to visit doctors directly in tertiary hospitals.
Although not investigated in the present study, catheter interventions for structural heart disease, such as severe aortic stenosis and mitral regurgitation, have been becoming common options for managing HF patients. Transcatheter aortic valve replacement (TAVR) and transcatheter repair of functional mitral regurgitation (Mitraclip) are representative methods (24, 25). These therapies are characterized by minimal invasiveness and can be performed in patients who are unable to undergo conventional open-chest operations due to high risk or high age. However, these therapies are often provided in quite specialized hospitals in urban areas. In our study, HF patients tended to be older in rural areas than in urban areas, and those patients who might benefit the most from these novel techniques seem to predominantly live in rural areas. We feel it is important to provide opportunities to access the latest technologies to HF patients living in not only urban areas but also rural areas.
These pharmacological and non-pharmacological treatments are being combined and applied in clinical practice, and the prognosis of patients is thus improving. However, with the impending pandemic of HF, the prognosis needs to be further improved. Nationwide standard care should be accessible in developed countries such as Japan, but this has never been verified in terms of actual practice. The present study is unique in focusing on regional disparities in guideline adherence, revealing a significant disparity in “real-world” clinical practice. Efforts to improve this might help improve the prognosis of all patients with HF. The IMPROVE HF study on the rates of guideline-directed medical therapy (GDMT) implementation in HFrEF outpatient care in the USA found that the baseline implementation rates of ACEi/ARB, beta blockers and MRA were 36.1%, 20.5% and 74.4%, respectively (26). The Change the Management of Patients with Heart Failure (CHAMP-HF) study found that the target doses of recommended medications were prescribed to only 1.1% of participants (27). In the treatment of HF, the duration of hospital stay also has a significant impact on the clinical courses of older individuals. Long hospital stays lead to a poor cognitive function and disuse. In the Acute Decompensated Heart Failure Syndromes (ATTEND), a registry of acute HF in Japan, the mean length of stay was 31 days (median, 21 days), which was longer than that of Western countries (28). Providing high-quality, efficient medical care for patients with HF is important in Japan, where the population is rapidly aging. The Chronic Heart Failure Analysis and Registry in the Tohoku District (CHART)-2 and Japanese Cardiac Registry of Heart Failure in Cardiology (JCARE-CARD) are observational studies of chronic HF in Japan. Both registries included many elderly individuals, and many patients had multiple comorbidities, such as diabetes, hypertension, atrial fibrillation and chronic kidney disease. The clinical backgrounds were more severe in elderly HF patients than in young ones. The readmission rate due to worsening HF was 36.3% at 2.1 years' follow-up after discharge (29, 30). In addition to the treatment of underlying diseases with pharmacological therapy, patient education and care support were also shown to be important to prevent exacerbation of HF in the elderly. This is because many patients are hospitalized due to preventable issues, such as inadequate salt/water restrictions, overwork, inadequate use of therapeutic drugs and mental or physical stress (31).
The present study found that aging was more rapid in rural areas than in urban areas. Basic heart disease is often diagnosed in urban areas, whereas many patients in rural areas have undiagnosed underlying heart disease. In recent years, some kinds of cardiomyopathy, such as cardiac amyloidosis and Fabry disease, have become treatable with specific medicines (32, 33). In addition to the low rates of guideline adherence, the absence of the diagnosis of underlying heart disease may deteriorate the prognosis of HF patients in rural areas.
Rates of adherence to guidelines were lower in rural areas for almost all therapies, but considering the age differences, the difference in the ICD introduction rate was marked. Combining the present findings with those of the BIOlogy Study to TAilored Treatment in Chronic Heart Failure (BIOSTAT-CHF) study (34) on the target dose administration for GDMT in patients HFrEF, patient-related factors also influence the outcomes of target dose administration. Treatment could not be administered due to the high possibility of adverse events, such as bradycardia, renal injury and hyperkalemia, in some patients in rural areas, probably due to their advanced age. Doses of beta-blockers were also lower in rural areas than in urban areas in the present study. Although the adherence to guidelines for pharmacological therapies was relatively good in both areas, the adherence to guidelines for non-pharmacological therapies had room for improvement, even when the effect of age was reduced. Promoting the placement of physical therapists engaged in rehabilitation in rural areas is thus necessary. Fewer devices (ICD/CRT) were implanted in rural areas than in urban areas, indicating an opportunity to improve the outcomes of patients with HF. Implantation of an ICD can prevent sudden death among patients with a reduced left ventricular contractility who are likely to have fatal arrhythmias (35-37). The active introduction of ICD/CRT may help improve the prognosis of patients with CHF in rural areas.
With the looming HF pandemic, a limited number of doctors and hospitals will have to manage the growing cohort of HF patients in rural areas. In fact, only a few cardiologists were present in rural areas in this study. Regarding the use of drugs for HF, it is important to pay attention to side effects and to refrain from administering drugs that can worsen HF [particularly non-steroidal anti-inflammatory drugs (NSAIDs)] when treating HF in the elderly (38). To improve the prognosis of HF patients, we need to maintain robust contact with non-cardiologists in rural areas by sharing the latest information about managing HF patients. In addition, improving the infrastructure, such as access to helicopter emergency medical services (“Doctor-Heli”), also appears to be important. Many patients in rural areas had unknown underlying heart disease, even after excluding the effects of age. We need to establish more effective and efficient systems of managing HF before the HF pandemic becomes serious. Enacting nationwide improvements to the implementation rate of essential treatments without regional disparity may be one practical option for combatting the HF pandemic.
Study limitations
This study included only five facilities and a small cohort of residents in a single prefecture in Japan. More elderly persons live in rural than in urban areas. Differences in age would clearly have some effect on the treatment content, but whether this is acceptable or not requires further study.
The background also differed between the two groups in terms of the collection period, which may have affected the results (even in advanced cases, the introduction of treatment was partially insufficient in rural areas).
Some pharmacotherapeutic approaches might not have been attempted in rural areas due to concerns about adverse events associated with a high age and deteriorated organ (kidney, liver, etc.) function in rural areas. Nonetheless, why pharmacological and non-pharmacological strategies were not implemented remains obscure. We also did not assess whether or not differences in implementation rates would affect the prognosis. A larger prospective study that focuses on regional disparities and the prognosis is thus needed.
Conclusion
We found that regional disparities in treatment for HF persist in Japan. Improvements in the use of GDMT, especially ICD/CRT, in rural areas might improve outcomes for patients with HF. A prospective study is needed to prove this hypothesis.
The authors state that they have no Conflict of Interest (COI).
Acknowledgement
The authors thank the medical staff at Niigata Prefectural Tokamachi Hospital, Uonuma City Koide Hospital, Niigata Prefectural Matsudai Hospital, Tsunan Town Hospital and the Department of Cardiovascular Biology and Medicine, Niigata University Graduate School of Medical and Dental Sciences.
References
- 1.Taylor CJ, Hobbs FD, Marshall T, Leyva-Leon F, Gale N. From breathless to failure: symptom onset and diagnostic meaning in patients with heart failure-a qualitative study. BMJ Open 7: e013648, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kabutoya T, Sato H, Aramaki E, et al. Clinical characteristics of heart failure from case reports presented at reginal meeting of the Japanese society of internal medicine. Intern Med 58: 2145-2150, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Vasan RS, Benjamin EJ, Levy D. Prevalence, clinical features and prognosis of diastolic heart failure: an epidemiologic perspective. J Am Coll Cardiol 26: 1565-1574, 1995. [DOI] [PubMed] [Google Scholar]
- 4.Hsu JJ, Ziaeian B, Fonarow GC. Heart failure with mid-range (borderline) ejection fraction: clinical implications and future directions. JACC Heart Fail 5: 763-771, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.CIBIS-II Investigators Committees. The Cardiac Insufficiency Bisoprolol Study II (CIBIS-II): a randomized trial. Lancet 353: 9-13, 1999. [PubMed] [Google Scholar]
- 6.Packer M, Bristow MR, Cohn JN, et al. The effect of carvedilol on morbidity and mortality in patients with chronic heart failure. U.S. Carvedilol Heart Failure Study Group. N Engl J Med 334: 1349-1355, 1996. [DOI] [PubMed] [Google Scholar]
- 7. Bristow MR, Saxon LA, Boehmer J, et al. ; Comparison of Medical Therapy, Pacing, and Defibrillation in Heart Failure (COMPANION) Investigators. Cardiac-resynchronization therapy with or without an implantable defibrillator in advanced chronic heart failure. N Engl J Med 350: 2140-2150, 2004. [DOI] [PubMed] [Google Scholar]
- 8. Cleland JG, Daubert JC, Erdmann E, et al. ; Cardiac Resynchronization-Heart Failure (CARE-HF) Study Investigators. The effect of cardiac resynchronization on morbidity and mortality in heart failure. N Engl J Med 352: 1539-1549, 2005. [DOI] [PubMed] [Google Scholar]
- 9.Guidelines for Diagnosis and Treatment of Acute and Chronic Heart Failure (JCS 2017/JHFS 2017) [Internet]. [cited 2019 Jul 14]. Available from: http://www.j-circ.or.jp/guideline/pdf/JCS2017_tsutsui_h.pdf (in Japanese).
- 10.2017 ACC/AHA/HFSA Focused Update of the 2013 ACCF/AHA Guideline for the Management of Heart Failure. A report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines and the Heart Failure Society of America. Circulation 136: e137-e161, 2017. [DOI] [PubMed] [Google Scholar]
- 11.2016 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure: The Task Force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC). Developed with the special contribution of the Heart Failure Association (HFA) of the ESC. European Heart Journal 37: 2129-2200, 2016. [DOI] [PubMed] [Google Scholar]
- 12.Okura Y, Ramadan MM, Ohno Y, et al. Impending epidemic - future projection of heart failure in Japan to the year 2055. Circ J 72: 489-491, 2008. [DOI] [PubMed] [Google Scholar]
- 13. CONSENSUS Trial Study Group. Effects of enalapril on mortality in severe congestive heart failure. Results of the Cooperative North Scandinavian Enalapril Survival Study (CONSENSUS). N Engl J Med 316: 1429-1435, 1987. [DOI] [PubMed] [Google Scholar]
- 14. Packer M, Coats AJ, Fowler MB, et al. ; Carvedilol Prospective Randomized Cumulative Survival Study Group. Effect of carvedilol on survival in severe chronic heart failure. N Engl J Med 344: 1651-1658, 2001. [DOI] [PubMed] [Google Scholar]
- 15.Connolly SJ, Gent M, Roberts RS, et al. Canadian implantable defibrillator study (CIDS) : a randomized trial of the implantable cardioverter defibrillator against amiodarone. Circulation 101: 1297-1302, 2000. [DOI] [PubMed] [Google Scholar]
- 16.Connolly SJ, Hallstrom AP, Cappato R, et al. Meta-analysis of the implantable cardioverter defibrillator secondary prevention trials. AVID, CASH and CIDS studies. Antiarrhythmics vs. Implantable Defibrillator study. Cardiac Arrest Study Hamburg. Canadian Implantable Defibrillator Study. Eur Heart J 21: 2071-2078, 2000. [DOI] [PubMed] [Google Scholar]
- 17.Antiarrhythmics versus Implantable Defibrillators (AVID) Investigators. A comparison of antiarrhythmic-drug therapy with implantable defibrillators in patients resuscitated from near-fatal ventricular arrhythmias. N Engl J Med 337: 1576-1583, 1997. [DOI] [PubMed] [Google Scholar]
- 18.Kuck KH, Cappato R, Siebels J, Rüppel R. Randomized comparison of antiarrhythmic drug therapy with implantable defibrillators in patients resuscitated from cardiac arrest : the Cardiac Arrest Study Hamburg (CASH). Circulation 102: 748-754, 2000. [DOI] [PubMed] [Google Scholar]
- 19. Moss AJ, Hall WJ, Cannom DS, et al. ; Multicenter Automatic Defibrillator Implantation Trial Investigators. Improved survival with an implanted defibrillator in patients with coronary disease at high risk for ventricular arrhythmia. N Engl J Med 335: 1933-1940, 1996. [DOI] [PubMed] [Google Scholar]
- 20. Moss AJ, Zareba W, Hall WJ, et al. ; Multicenter Automatic Defibrillator Implantation Trial II Investigators. Prophylactic implantation of a defibrillator in patients with myocardial infarction and reduced ejection fraction. N Engl J Med 346: 877-883, 2002. [DOI] [PubMed] [Google Scholar]
- 21. Bardy GH, Lee KL, Mark DB, et al. ; Sudden Cardiac Death in Heart Failure Trial (SCD-HeFT) Investigators. Amiodarone or an implantable cardioverter-defibrillator for congestive heart failure. N Engl J Med 352: 225-237, 2005. [DOI] [PubMed] [Google Scholar]
- 22. Kadish A, Dyer A, Daubert JP, et al. ; Defibrillators in Non-Ischemic Cardiomyopathy Treatment Evaluation (DEFINITE) Investigators. Prophylactic defibrillator implantation in patients with nonischemic dilated cardiomyopathy. N Engl J Med 350: 2151-2158, 2004. [DOI] [PubMed] [Google Scholar]
- 23.Hindricks G, Taborsky M, Glikson M, et al. Implant-based multiparameter telemonitoring of patients with heart failure (IN-TIME): a randomised controlled trial. Lancet 384: 583-590, 2014. [DOI] [PubMed] [Google Scholar]
- 24. Smith CR, Leon MB, Mack MJ, et al. ; PARTNER Trial Investigator. Transcatheter versus surgical aortic-valve replacement in high-risk patients. N Engl J Med 364: 2187-2198, 2011. [DOI] [PubMed] [Google Scholar]
- 25.Feldman T, Foster E, Glower DD, et al. Percutaneous repair or surgery for mitral regurgitation. N Engl J Med 364: 1395-1406, 2011. [DOI] [PubMed] [Google Scholar]
- 26.Fonarow GC, Yancy CW, Albert NM, et al. Improving the use of evidence-based heart failure therapies in the outpatient setting: the IMPROVE HF performance improvement registry. Am Heart J 154: 12-38, 2007. [DOI] [PubMed] [Google Scholar]
- 27.Greene SJ, Butler J, Albert NM, et al. Medical therapy for heart failure with reduced ejection fraction The CHAMP-HF registry. J Am Coll Cardiol 72: 351-366, 2018. [DOI] [PubMed] [Google Scholar]
- 28.Sato N, Kajimoto K, Asai K, et al. Acute decompensated heart failure syndromes (ATTEND) registry. A prospective observational multicenter cohort study : rationale, design, and preliminary data. Am Heart J 159: 949-955.e1, 2020. [DOI] [PubMed] [Google Scholar]
- 29.Sato M, Sakata Y, Sato K, et al. Clinical characteristics and prognostic factors in elderly patients with chronic heart failure -a report from the CHART-2 study-. Int J Cardiol Heart Vasc 27: 100497, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hamaguchi S, Kinugawa S, Goto D, et al. Predictors of long-term adverse outcomes in elderly patients over 80 years hospitalized with heart failure. - a report from the Japanese Cardiac Registry of Heart Failure in Cardiology (JCARE-CARD)-. Circ J 75: 2403-2410, 2011. [DOI] [PubMed] [Google Scholar]
- 31.Tsuchihashi M, Tsutsui H, Kodama K, et al. Medical and socioenvironmental predictors of hospital readmission in patients with congestive heart failure. Am Heart J 142: 20A-26A, 2001. [DOI] [PubMed] [Google Scholar]
- 32.Maurer MS, Schwartz JH, Gundapaneni B, et al. Tafamidis treatment for patients with transthyretin amyloid cardiomyopathy. N Engl J Med 379: 1007-1016, 2018. [DOI] [PubMed] [Google Scholar]
- 33.Schiffmann R, Kopp JB, Austin HA 3rd, et al. Enzyme replacement therapy in Fabry disease: a randomized controlled trial. JAMA 285: 2743-2749, 2001. [DOI] [PubMed] [Google Scholar]
- 34.Ouwerkerk W, Zwinderman AH, Ng LL, et al. Biomarker-guided versus guideline-based treatment of patients with heart failure: Results From BIOSTAT-CHF. J Am Coll Cardiol 71: 386-398, 2018. [DOI] [PubMed] [Google Scholar]
- 35.Popović AD, Nesković AN, Pavlovski K, et al. Association of ventricular arrhythmias with left ventricular remodelling after myocardial infarction. Heart 77: 423-427, 1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Grimm W, Glaveris C, Hoffmann J, et al. Noninvasive arrhythmia risk stratification in idiopathic dilated cardiomyopathy: results of the Marburg Cardiomyopathy Study. Circulation 108: 2883-2891, 2003. [DOI] [PubMed] [Google Scholar]
- 37.Kadish A, Dyer A, Daubert JP, et al. Prophylactic defibrillator implantation in patients with nonischemic dilated cardiomyopathy. N Engl J Med 350: 2151-2158, 2004. [DOI] [PubMed] [Google Scholar]
- 38.Yamasaki N, Kitaoka Y, Matsumura Y, et al. Heart failure in elderly. Intern Med 42: 383-388, 2003. [DOI] [PubMed] [Google Scholar]