Abstract
A 23-year-old man had an 8-day history of fatigue and dry cough and papulo-nodular reactions on his extensive tattoos. Chest radiography revealed several small granular shadows, and a transbronchial lung biopsy showed non-caseating epithelioid cell granuloma. A skin biopsy of the tattooed area showed histiocytic infiltrates with phagocytized tattoo pigment. Antibody tests for hepatitis C virus were positive. The patient was successfully treated with corticosteroid therapy, and after inflammation was suppressed, he received delayed anti-viral therapy. Sarcoidosis should be considered as a concurrent condition if papules are presented on the tattoos of patients with hepatitis C.
Keywords: hepatitis C, sarcoidosis, tattoo, therapeutic dilemma
Introduction
Sarcoidosis is an autoimmune disease characterized by the presence of non-caseating epithelioid cell granulomas in multiple organs (1). The skin is one of the most commonly affected organs, and known signs of sarcoidosis include papulo-nodular reactions on tattoos (2). A Th1-mediated inflammatory process appears to be essential for the formation of granuloma in sarcoidosis (3).
Sarcoidosis has been reported to develop in individuals with hepatitis C virus (HCV) infection and during the course of therapy for chronic hepatitis C (4,5). Treatment-related sarcoidosis in chronic hepatitis C is mediated by an augmentation of the Th1 immune response (5); for example, interferon has potent immune-stimulating activities, especially on the Th1 immune response (6). Ribavirin, a direct antiviral agent (DAA), is also known to enhance the Th1 cytokine response while at the same time inhibiting the Th2 cytokine response (7). However, cases of sarcoidosis that are unrelated to antiviral therapies have also been reported in patients with chronic hepatitis C infection (8).
Cutaneous sarcoid/foreign body granulomatous reactions at the site of old tattoos have been reported in patients with hepatitis C after interferon therapy (9), but these are scarce in patients with no connection to therapies for hepatitis C.
The present case was challenging regarding the choice and timing of the treatments for the two entities, as therapy targeting hepatitis C may have led to exacerbation of the sarcoidosis, and vice versa. To our knowledge, there have been no reports on the completion of a DAA course after corticosteroid therapy for systemic sarcoidosis and concurrent chronic hepatitis C.
We herein report a unique case of treatment-naïve chronic hepatitis C with concurrent sarcoidosis that manifested as a reaction on the patient's tattoos.
Case Report
A 23-year-old man with no significant medical history visited our hospital with an 8-day history of fatigue and dry cough and with papulo-nodular reactions on his tattoos. He had developed a fever and night sweats three days before the visit. He had received no blood transfusions, nor had he ever been checked for hepatitis C. He had acquired extensive tattoos from 18 years old, and they now covered his entire back and both upper arms. He worked as a metal welder with a history of fume inhalation.
On an examination, his general appearance was alert and oriented with no acute distress. His weight was 74.1 kg, and his height was 171 cm. His temperature was 37.4°C, blood pressure 143/69 mmHg, heart rate 93 beats/min, respiratory rate 20 beats/min and SpO2 96% (room air). There was no evidence of redness of the conjunctiva. A cardiovascular examination was normal, the lungs were clear on auscultation, and abdominal examination findings were unremarkable, with no hepatosplenomegaly. A neurological examination was completely unremarkable. Notably, a skin examination revealed papules throughout his tattoos on the back and upper limbs (Fig. 1A). No lymphadenopathy was found. Laboratory findings were hemoglobin 15.5 g/dL, white blood cell count 9,830/mm3, and platelet count 28.3×104/mm3. The levels of serum calcium, blood urea nitrogen (BUN) and creatinine were 8.6 mg/dL, 12.4 mg/dL and 0.92 mg/dL, respectively. The albumin level was 3.6 g/dL. The levels of aspartate aminotransferase (AST) and alanine aminotransferase (ALT) were at 27 IU/L and 73 IU/L (normal range: 8-38 IU/L and 4-44 IU/L), respectively. The level of lactate dehydrogenase (LD) was elevated at 705 IU/L (normal range: 106-211 IU/L). The prothrombin time was 12.7 seconds, and the international normalized ratio was 1.08. C-reactive protein (CRP) level was elevated at 5.2 mg/dL.
Figure 1.
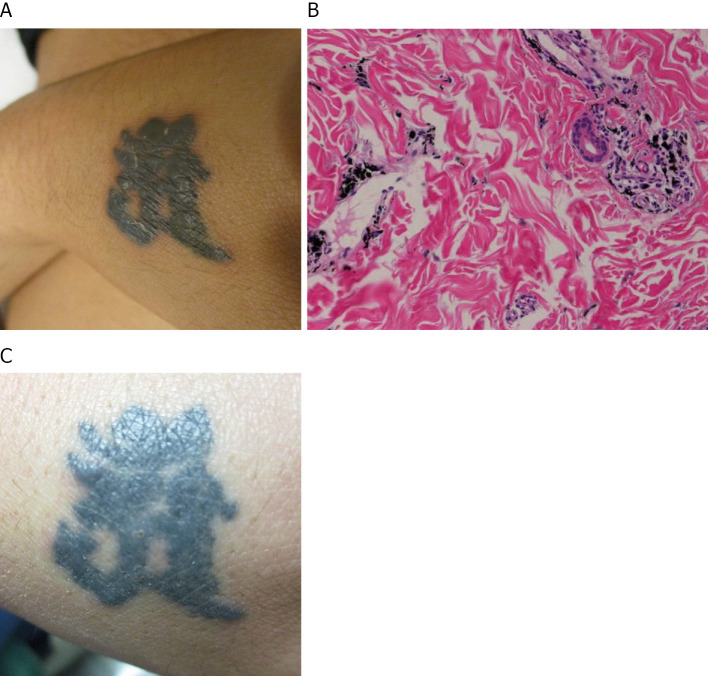
A skin examination showing papules on the tattoos of the upper limbs (A). Histopathology of the skin biopsy specimen of the tattooed area revealing perivascular lymphocytic and histiocytic infiltrates with phagocytized tattoo pigment (Hematoxylin and Eosin staining) (B). A skin examination showing the disappearance of papules on the tattooed area five months after the initiation of the corticosteroid therapy (C).
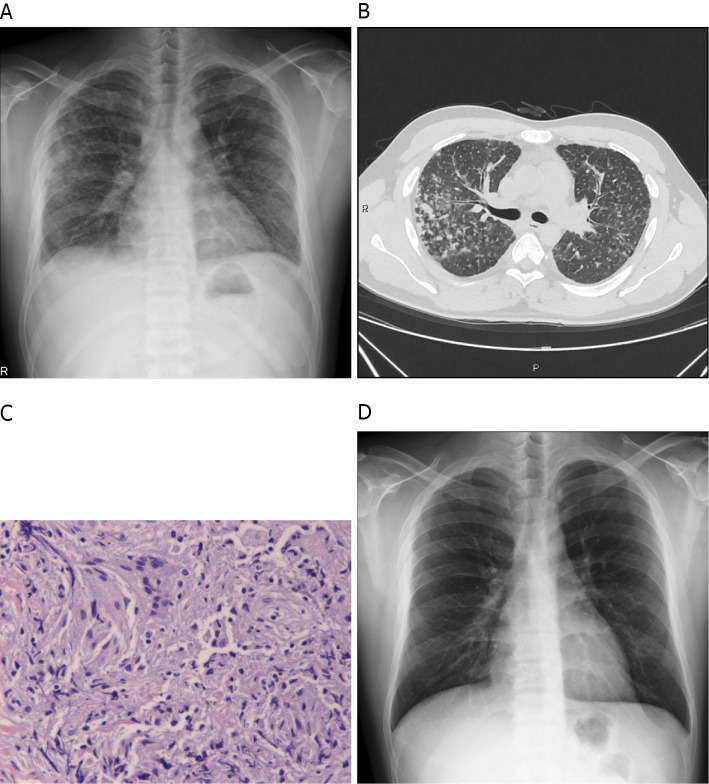
The patient had multiple small granular shadows on chest radiography. Chest computed tomography (CT) showed thickening of the bronchial wall and multiple granular shadows in both lungs with no enlargement of mediastinal or hilar lymph nodes (Fig. 2A, B). No uveitis was noted by an ophthalmologist. Smear and culture tests for bacteria and mycobacterium obtained from sputum and bronchial lavage fluid and transcription reserve transcription concerted reaction (TRC) were negative. A transbronchial lung biopsy revealed a non-caseating epithelioid cell granuloma (Fig. 2C). A skin biopsy of the tattooed area showed perivascular lymphocytic and histiocytic infiltrates with phagocytized tattoo pigment (Fig. 1B). After one month of watchful waiting, the patient returned and reported right-side ocular pain. An ophthalmology examination revealed mutton-fat keratic precipitates (Fig. 3). Gallium scintigraphy showed the accumulation in the lacrimal and salivary glands (Fig. 4).
Figure 2.
Chest radiography shows multiple small granular shadows in both lungs (A). Chest computed tomography shows thickening of the bronchial wall and multiple granular shadows in both lungs with no enlargement of the mediastinal or hilar lymph nodes (B). Histopathology of the transbronchial lung biopsy specimen shows a non-caseating epithelioid cell granuloma (Hematoxylin and Eosin staining) (C). Chest radiography shows the disappearance of small granular shadows (D).
Figure 3.
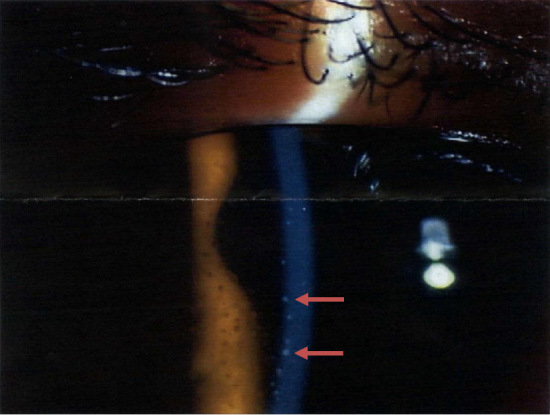
An ophthalmology examination shows mutton-fat keratic precipitates (arrows).
Figure 4.
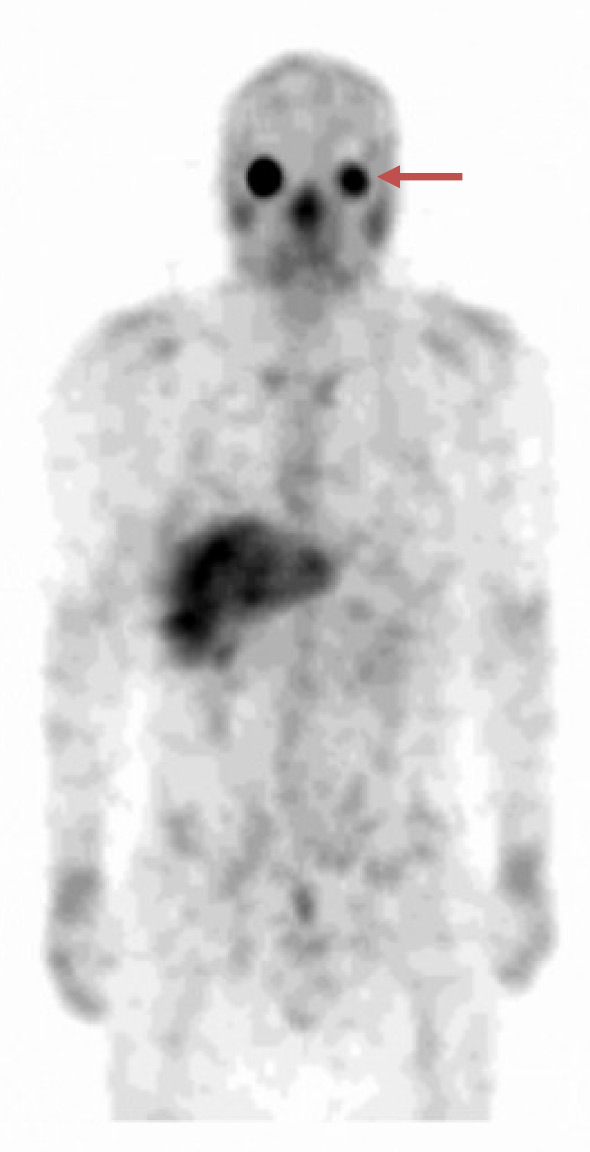
Gallium scintigraphy showing the accumulation in the lacrimal and salivary glands (arrow).
In addition, soluble interleukin-2 receptor (sIL-2R) was elevated at 2,530 U/mL, although the serum angiotensin-converting enzyme (ACE) level was normal at 9.9 IU/L; this, combined with the physical presentation, led us to a diagnosis of sarcoidosis.
Mycobacterium tuberculosis antigen-specific interferon (IFN)-g release assays (T-SPOT.TB) were negative. Antibody tests for HCV were positive with a titer of 39.43 s/co for HCV-RNA genotype 2. The viral load was 4.6 LogIU/mL.
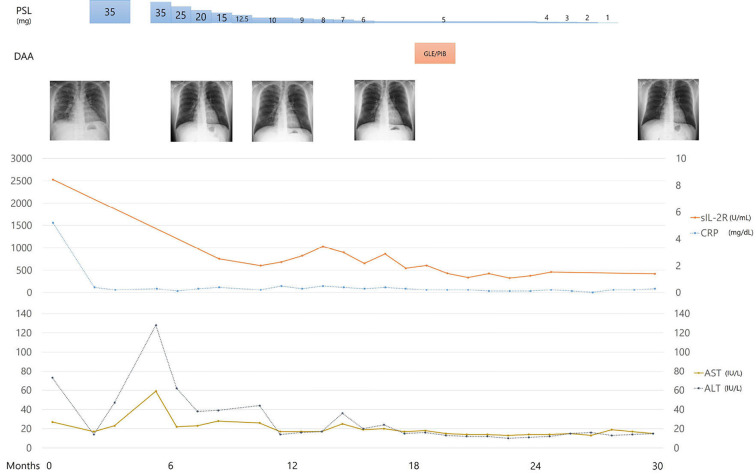
The patient's course of therapy is shown in Fig. 5. We began treatment with prednisolone (PSL; 35 mg/day) for sarcoidosis, which was equivalent to 0.5 mg/kg body weight (10). The patient's fever subsided, and the papules on the tattooed areas and small granular shadows on chest radiography soon disappeared (Fig. 1C, D). One-month after the initiation of the corticosteroid therapy, we began the additional administration of sofosbuvir plus ribavirin combination therapy for hepatitis C. However, the patient stopped visiting the office after one month without taking anti-viral therapy, which was intended to be taken for three months. He reported that there had been some improvement to his condition, and the anti-viral tablets were too large for him to take, so he decided to refrain from the therapy. One month after discontinuation, he revisited our office with recurrence of uveitis and skin reactions on his tattoos with an elevation in the transaminase level. We reinitiated PSL at 35 mg/day with gradual tapering. The patient had no subsequent recurrence of uveitis or skin tattoo reactions. After one year, he completed a two-month course of antiviral therapy with glecaprevir hydrate and pibrentasvir while PSL was tapered to 5 mg/day; the HCV viral load values were undetectable, and he achieved a sustained virologic response. He completed a 22-month course of PSL therapy and had no subsequent relapse of sarcoidosis.
Figure 5.
The patient's course of therapy. PSL: prednisolone, DAA: direct antiviral agent, GLE/PIB: glecaprevir hydrate and pibrentasvir, sIL-2R: soluble interleukin-2 receptor, CRP: C-reactive protein, AST: aspartate aminotransferase, ALT: alanine aminotransferase
Discussion
Skin lesions associated with tattoos in sarcoidosis are known to include skin rash phenomena and a papulo-nodular pattern that occurs sometimes with a significant delay after the individual is tattooed (1). The pattern of lesions varies from papules to nodules, plaques or infiltrations on the tattoos (11). A histological study showed a sarcoidosis reaction as sarcoid granuloma, typically non-necrotic epithelioid cell granulomas, but with giant cells and epithelioid cells with inflammation. Non-sarcoidosis reactions in tattoos are considered to be simple inflammation (12). However, histopathological assessments may vary among pathologists, depending on their ability to distinguish sarcoid granulomas from granulomatous inflammation (12). The clinical presentation should therefore be considered as a whole when interpreting the pathological findings of skin lesions involving tattoos.
Clinical manifestations of sarcoidosis differ depending on race and gender (13). Uveitis, for example, is reported to occur at a higher rate in Japan than in the United States and Europe (13,14). The present case also developed uveitis in a delayed manner. The patient did not have uveitis on the first presentation, so we were faced with the diagnostic challenge of differentiating pulmonary sarcoidosis from other causes of a fever and reasons for multiple pulmonary granular shadows, such as tuberculosis. Under these circumstances, the tattoo reaction was the key finding that enabled an appropriate diagnosis. Acute pulmonary damage due to metal allergy is also another possible diagnosis of multiple pulmonary shadows. Sarcoidosis is characterized by an activation of the helper T cell type 1 (Th1) immune response, whereas the helper T cell type 2 (Th2) immune response plays a critical role in the development of metal allergies (15). Our patient showed high levels of sIL-2R, which is known to reflect a Th1-type reaction (16). In addition, the titer of sIL-2R decreased during the course of corticosteroid therapy.
Several explanations have been made regarding the pathogenesis of skin lesions associated with tattoos in sarcoidosis. The chronology of “tattoo first-sarcoidosis after” supports the hypothesis that the tattoo itself might be the trigger of sarcoidosis (11). Conversely, the tattoo might be the target of sarcoidosis, and hepatitis C might play a role as a precipitating factor. There have been reports of HCV isolated in sarcoid tissue (17). In our patient, incidentally found hepatitis C may have been a potentiating factor in the development of sarcoidosis.
Particular tattoo ink colors have been reported to cause skin reactions; commonly-affected tattoo colors are red and black (11). Black ink is composed of carbon, while red and green inks contain mercury and titanium, respectively (14). Carbon black tends to agglomerate and form larger bodies (18), although precisely why some tattoo pigments more readily induce skin lesions than others is unclear. In our patient, black dye was phagocytized in the biopsy specimen and may have played a role as a trigger or as a target for sarcoidosis.
Although there have been reports of sarcoidosis along with skin rashes on tattoos following treatment of chronic hepatitis C (11), this is the first report of sarcoidosis occurring prior to, rather than as a result of, treatment. Antiviral therapy has the potential to enhance the Th1 cytokine response leading to treatment-related sarcoidosis (19), whereas corticosteroid therapy can enhance viral replication (20). Spontaneous resolution of sarcoidosis was reported in a patient with treatment-naïve chronic hepatitis C (5). In the current case, we allowed for a period of spontaneous resolution. The patient presented with uveitis after a delay, which led us to a therapeutic dilemma. We chose to initiate corticosteroid therapy first to suppress the systematic inflammation, and while this was being tapered, we began antiviral therapy. The patient eventually achieved remission of sarcoidosis while maintaining a sustained virologic response.
In conclusion, our rare case of systemic sarcoidosis presented with papulo-nodular tattoo reactions in treatment-naïve chronic hepatitis C, showing that other organ manifestations can be delayed. Our patient was successfully treated by corticosteroid and DAA therapy. Sarcoidosis should be considered as a concurrent condition if papules are present on the tattoos of patients with hepatitis C.
The authors state that they have no Conflict of Interest (COI).
Acknowledgement
We thank Benjamin Phillis from Akashi Medical Center for proofreading and editing the manuscript.
References
- 1. Iannuzzi MC, Rybicki BA, Teirstein AS. Sarcoidosis. N Engl J Med 357: 2153-2165, 2007. [DOI] [PubMed] [Google Scholar]
- 2. Madden JF. Reactions in tattoos. Arch Dermatol Syphilol 40: 256-262, 1939. [Google Scholar]
- 3. Chen ES, Moller DR. Etiologies of sarcoidosis. Clin Rev Allergy Immunol 49: 6-18, 2015. [DOI] [PubMed] [Google Scholar]
- 4. Hurst EA, Mauro T. Sarcoidosis associated with pegylated interferon alfa and ribavirin treatment for chronic hepatitis C: a case report and review of the literature. Arch Dermatol 141: 865-868, 2005. [DOI] [PubMed] [Google Scholar]
- 5. Kim TH, Joo JE. Spontaneous resolution of systemic sarcoidosis in a patient with chronic hepatitis C without interferon therapy. World J Gastroenterol 12: 150-153, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Hoffmann RM, Jung MC, Motz R, et al. Sarcoidosis associated with interferon-alpha therapy for chronic hepatitis C. J Hepatol 28: 1058-1063, 1998. [DOI] [PubMed] [Google Scholar]
- 7. Ning Q, Brown D, Parodo J, et al. Ribavirin inhibits viral-induced macrophage production of TNF, IL-1, the procoagulant fgl2 prothrombinase and preserves Th1 cytokine production but inhibits Th2 cytokine response. J Immunol 160: 3487-3493, 1998. [PubMed] [Google Scholar]
- 8. Ramos-Casals M, Mana J, Nardi N, et al. Sarcoidosis in patients with chronic hepatitis C virus infection: analysis of 68 cases. Medicine (Baltimore) 84: 69-80, 2005. [DOI] [PubMed] [Google Scholar]
- 9. Nawras A, Alsolaiman MM, Mehboob S, Bartholomew C, Maliakkal B. Systemic sarcoidosis presenting as a granulomatous tattoo reaction secondary to interferon-alpha treatment for chronic hepatitis C and review of the literature. Dig Dis Sci 47: 1627-1631, 2002. [DOI] [PubMed] [Google Scholar]
- 10. Grutters JC, van den Bosch JMM. Corticosteroid treatment in sarcoidosis. Eur Respir J 28: 627, 2006. [DOI] [PubMed] [Google Scholar]
- 11. Kluger N. Sarcoidosis on tattoos: a review of the literature from 1939 to 2011. Sarcoidosis Vasc Diffuse Lung Dis 30: 86-102, 2013. [PubMed] [Google Scholar]
- 12. Sepehri M, Hutton Carlsen K, Serup J. Papulo-nodular reactions in black tattoos as markers of sarcoidosis: study of 92 tattoo reactions from a hospital material. Dermatology 232: 679-686, 2016. [DOI] [PubMed] [Google Scholar]
- 13. Morimoto T, Azuma A, Abe S, et al. Epidemiology of sarcoidosis in Japan. Eur Respir J 31: 372-379, 2008. [DOI] [PubMed] [Google Scholar]
- 14. Ohtsuka M, Natsuko M, Toshiyuki Y. Sarcoidal granuloma presenting on tattoo: a report of a Japanese female patient and a review of Japanese published work. Sarcoidosis Vasc Diffuse Lung Dis 33: 83-89, 2016. [PubMed] [Google Scholar]
- 15. Niiyama S, Tamauchi H, Amoh Y, et al. Th2 immune response plays a critical role in the development of nickel-induced allergic contact dermatitis. Int Arch Allergy Immunol 153: 303-314, 2010. [DOI] [PubMed] [Google Scholar]
- 16. Ogata-Suetsugu S, Hamada N, Takayama K, Tsubouchi K, Arimura-Omori M, Nakanishi Y. The clinical value of serum soluble interleukin-2 receptor in pulmonary sarcoidosis. Sarcoidosis Vasc Diffuse Lung Dis 34: 41-47, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Pereira EG, Guimaraes TF, Bottino CB, D'Acri AM, Lima RB, Martins CJ. Sarcoidosis and chronic hepatitis C: treatment with prednisone and colchicine. An Bras Dermatol 91: 231-234, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Serup J. From technique of tattooing to biokinetics and toxicology of injected tattoo ink particles and chemicals. Curr Probl Dermatol 52: 1-17, 2017. [DOI] [PubMed] [Google Scholar]
- 19. Pérez Parente D, Suárez Santamaría M, Suárez Ordóñez S, Morano Amado LE. Pulmonary sarcoidosis in the context of a telaprevir-based triple therapy for hepatitis C. Rev Port Pneumol (2006) 22: 57-59, 2016. [DOI] [PubMed] [Google Scholar]
- 20. Gruber A, Lundberg LG, Björkholm M. Reactivation of chronic hepatitis C after withdrawal of immunosuppressive therapy. J Intern Med 234: 223-225, 1993. [DOI] [PubMed] [Google Scholar]