Abstract
We herein report the case of a 75-year-old man with asymptomatic immune checkpoint inhibitor (ICI)-associated myocarditis diagnosed on the basis of elevated levels of creatine kinase (CK), CK-myocardial band and troponin I (TNI). He was suspected of being complicated with myasthenia gravis (MG). High-dose prednisolone (PSL) is associated with a risk of MG exacerbation; therefore, PSL therapy was gradually increased from 5 mg/day to 20 mg/day, which resulted in the normalization of the TNI level, and no PSL-related side effects occurred. MG easily complicates myocarditis as an immune-related adverse event; thus, the treatment plan should be carefully considered.
Keywords: myocarditis, immune checkpoint inhibitor, immune-related adverse event, myasthenia gravis, prednisolone
Introduction
Immune checkpoint inhibitors (ICIs) are currently used in many types of cancer, and their use is expected to increase in the future. Immune-related adverse events (irAEs) have been reported to occur as adverse events specific to ICIs. Although most of these irAEs are rare, they can occur in a wide variety of body parts; furthermore, their timing is difficult to predict and they may become serious if the response is delayed. Although cardiovascular adverse events due to ICIs are relatively rare, there have been reports of fatal cardiac complications (1,2).
We herein report a case of asymptomatic ICI-associated myocarditis with the suspected complication of myasthenia gravis (MG).
Case Report
A 75-year-old man was diagnosed with liver, lung, pericardial, and cervical spine metastases, of the right renal cell carcinoma (Fig. 1A-C); consequently, first-line treatment with combined nivolumab [anti programmed cell death (PD)-1] and ipilimumab [anti cytotoxic T-lymphocyte antigen (CTLA)-4] was started. Seven weeks later (Day 53), just before the third cycle of treatment, he started complaining of posterior neck pain and neck drop. On Day 53, a laboratory investigation revealed elevated levels of creatine kinase (CK) to 1,396 U/L, CK-myocardial band to 91.9 ng/mL, and troponin I (TNI) to 2.15 ng/mL. His white blood cell count was 6,800/μL and his eosinophil count was 834/μL. His creatinine level was 0.98 mg/dL, and his estimated glomerular filtration rate was 57.4 mL/min/body surface area.
Figure 1.
Computed tomography. The patient was diagnosed with right renal cell carcinoma with liver (A, arrows), pericardial (B, arrow), and cervical spine (C, arrow) metastases (Day 0). Both the primary and metastatic sites of the tumor showed a significant reduction (D, E, F) (Day 58).
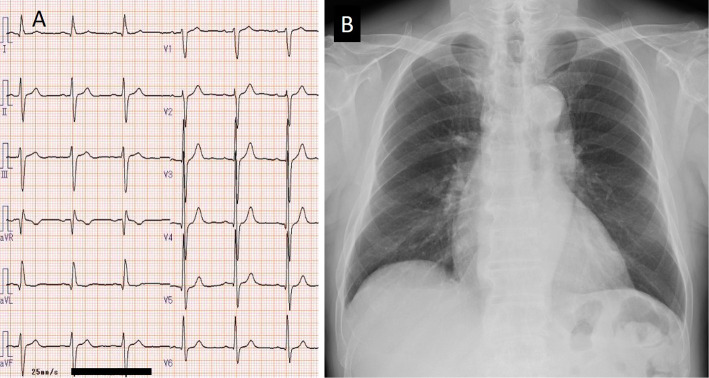
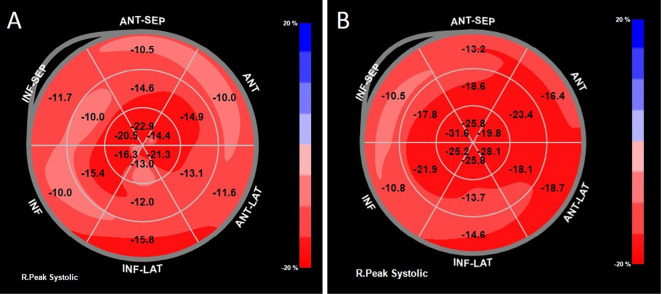
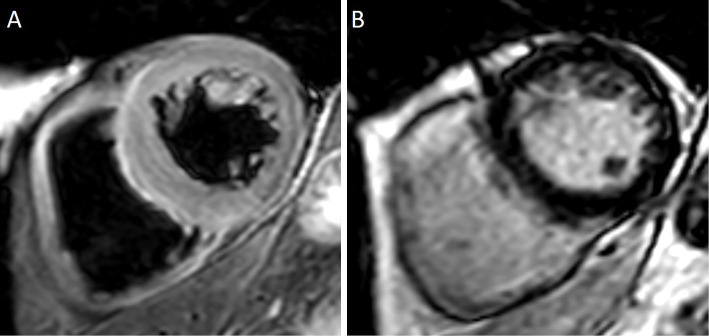
Although he did not complain of chest pain, myocardial ischemia and myocarditis were suspected, and he was admitted to our hospital. Twelve-lead electrocardiography (ECG) performed on admission (Day 53) revealed a normal sinus rhythm and left anterior hemiblock, with no marked changes in comparison with that before the start of ICI (Fig. 2A). Chest radiography revealed a cardiothoracic ratio of 51%, no pulmonary congestion, and no pleural effusion (Fig. 2B). Transthoracic echocardiography (TTE) revealed a left ventricular ejection fraction (LVEF) of 59% and no apparent wall motion abnormality. The LV wall thickness was increased (intraventricular septum, 13 mm; posterior wall thickness, 13 mm). Doppler echocardiography revealed LV diastolic dysfunction, as supported by the ratio of mitral peak early diastolic velocity (E=66 cm/s) and late diastolic velocity (A=111 cm/s) of 0.6 and the ratio of E velocity and mitral septal annulus velocity (e'=3.7 cm/s) of 17.8. The LV global longitudinal strain (GLS) was -14.4% (Fig. 3A). The right ventricular (RV) size and systolic function (RV fractional area change=46.4%, tricuspid annular plane systolic excursion=23.8 mm, peak systolic velocity of tricuspid annulus by pulsed wave Doppler tissue imaging=15.6 cm/s) were normal. Coronary angiography revealed no evidence of acute coronary syndrome (Fig. 4). On computed tomography (Day 56), both the primary and metastatic sites of the tumor showed a significant reduction (Fig. 1D-F). On cardiac magnetic resonance imaging (MRI) (Day 58), a global increase in the T2 signal intensity was detected (Fig. 5A); late gadolinium enhancement imaging revealed no abnormal findings (Fig. 5B), but did indicate the existence of myocardial edema, which was consistent with myocarditis in the acute phase. The drug-induced lymphocyte stimulation test (DLST) was positive for both ICI treatments. The third ICI treatment was discontinued; however, 1 week after admission, his TNI level had increased to 6.5 ng/mL Although MG was suspected owing to posterior neck pain and dropped head, the patient had no evidence of muscle weakness and was negative for autoantibodies.
Figure 2.
Twelve-lead electrocardiography revealed a normal sinus rhythm and left anterior hemiblock (A). Chest radiography revealed a cardiothoracic ratio of 51%, no pulmonary congestion, and no pleural effusion (B).
Figure 3.
On admission (Day 53), the LV global longitudinal strain was -14.4% (A). Three months after discharge (Day 170), the LV global longitudinal strain improved to -19.5% (B).
Figure 4.
Coronary angiography (A: right coronary artery, B: left coronary artery) reveals no evidence of acute coronary syndrome.
Figure 5.
Cardiac magnetic resonance imaging. A global increase in the T2 signal intensity can be observed (A), but the late gadolinium enhancement (LGE) imaging finding was normal (B), indicating the presence of myocardial edema.
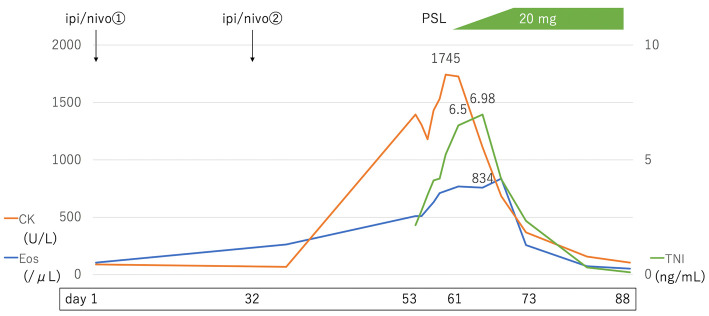
High-dose prednisolone (PSL) is associated with a risk of MG exacerbation; therefore, PSL therapy was started at 5 mg/day. The CK level peaked on Day 64, and the TNI level peaked on Day 67 when the dose was gradually increased by 5 mg every 3 days. On Day 73, the PSL dose was increased to 20 mg/day. The patient was discharged after confirmation of no further increases in the TNI level and no side effects of PSL. On Day 88, the symptoms of posterior neck pain and dropped head also improved, and the patient's eosinophil count and TNI level were normalized on Day 102 (Fig. 6). Three months after discharge (Day 170), TTE revealed that the LVEF was 64% and the LV GLS improved from -14.4% (Day 53) to -19.5% (Fig. 3B). The LV wall thickness was slightly decreased (intraventricular septum, 12 mm; posterior wall thickness, 12 mm).
Figure 6.
Clinical course. Prednisolone (PSL) administration was started at 5 mg/day on Day 61. CK peaked on Day 64, and TNI peaked on Day 67 when the dose was gradually increased by 5 mg every 3 days. On Day 73, the PSL dose was increased to 20 mg/day, and the patient's eosinophil count and TNI levels were normalized on Day 102. CK: creatine kinase (normal range: 59-248 U/L), TNI: troponin I (normal range: <0.04 ng/mL), Eos: eosinophil count (normal range: <500/μL)
Discussion
PD-1 and CTLA-4, which are present on T cells, are collectively referred to as immune checkpoints and act as inhibitors of the development of excessive immune responses against self-tissues. ICIs are antibody drugs against ligands, such as PD-1 and CTLA-4, that exert anti-tumor effects by enhancing the immune response to tumors by blocking inhibitory signals from the immune checkpoint system.
The indications forz ICI therapy have been expanded from malignant melanoma to non-small cell lung cancer, kidney cancer, Hodgkin lymphoma, and head and neck tumors. Although cardiovascular adverse events due to ICI administration are relatively rare, sporadic reports of fatal cardiac complications have emerged (1,2). Physiologically, PD-1 and CTLA-4 protect the heart against immune-mediated damage, and CTLA-4 knockout mice have been reported to develop lethal autoimmune myocarditis mediated by CD8 and T cells (3). In a study of cardiovascular irAEs due to ICI administration, the incidence was 0.31% for ICI monotherapy and 1.3% for ICI combination therapy, and the mortality rate was 44.4% for monotherapy and 65.6% for combination therapy, indicating that many of these events were serious (2). The median timing to the onset of events was 30 days after the first dose. No screening or diagnostic criteria have been established for ICI-induced cardiovascular irAEs, but baseline tests, such as a troponin I analysis, ECG and TTE, are recommended for patients who are preparing to receive ICIs.
Immediate glucocorticoid and immunosuppressive therapies are required for the treatment of cardiovascular irAEs (3). One was proposed by the American Heart Association as a diagnostic criterion for ICI-related myocarditis (4). Although an endomyocardial biopsy is the gold standard for the diagnosis of underlying myocarditis, it is rarely performed. Recently, cardiac MRI has become important in the diagnosis of myocarditis. The Lake Louise criteria are well-known diagnostic criteria, and cardiac MRI is expected to improve the diagnostic accuracy for myocardial inflammation (5). Data from a North American Registry revealed a high proportion of patients with elevated troponin, and about half of patients with ICI-related myocarditis have been found to have an EF within normal limits (6). Aside from acute myocardial infarction, there are several potential diseases that show troponin release, according to various etiologies of myocardial damage (myocarditis, acute pulmonary embolism, heart failure and end-stage renal disease) (7). In the present case, other than myocarditis were definitively or clinically excluded. Because the LVEF and RV systolic function were preserved and the severity of myocarditis was considered to be mild, myocardial biopsy was not performed. Myocarditis is an uncommon but often fulminant and severe side effect of ICI (6,8). An endomyocardial biopsy should be considered with suspected fulminant myocarditis in order to select an adequate treatment strategy as well as doses of PSL. There has been increasing interest in the measurement of LV GLS to detect chemotherapy-related cardiac dysfunction because it is a sensitive and robust index for detecting subclinical myocardial dysfunction (9,10). In the present case, a decrease in the LV GLS was found before the LVEF dropped, and PSL administration was initiated. Three months later, the LVEF remained stable, and the LV GLS recovered.
Among patients with preexisting autoimmune diseases, 40% relapsed with ICI therapy. Evidence concerning the timing and severity of irAE is limited, and in theory, irAEs can occur in any tissue in the body (1). ICI-related myocarditis is associated with MG, myositis, and rhabdomyolysis, and particularly close attention should be paid to MG. Recent literature has suggested a relationship between MG and myocarditis as irAEs (11). A systematic review of ICI-induced neuromuscular complications reported that 10% of patients with ICI-induced MG had concomitant myocarditis (12). High doses of steroid therapy may result in MG exacerbation after administration. This is closely related to the treatment plan for myocarditis.
Mahmood et al. show that in cases of ICI-related myocarditis, the dose of steroids may affect outcomes (6). Lower doses of steroid were associated with higher troponin levels and increased rates of major adverse cardiac events. Myocarditis has a wide spectrum of presentations according to the etiology and variable patterns of tissue involvement. Patients with autoimmunity can show subclinical or subacute myocarditis. Because our patient's blood tests revealed elevated eosinophil levels, and PSL escalation therapy was effective in this case, the cause of the observed myocarditis may involve the allergic mechanism. With the increasing recognition of ICI-related myocarditis, it will be important to identify less-severe cases in order to appropriately document the full spectrum of the condition and ascertain the true long-term outcomes. Establishing a uniform definition of myocarditis for application in clinical trials of cancer immunotherapies will enable a greater understanding of these events.
In conclusion, we herein report a case of ICI-induced myocarditis that was successfully treated with PSL therapy. As ICI-associated myocarditis is a potentially fatal condition, its early diagnosis and therapeutic intervention are required. However, MG easily complicates myocarditis as an irAE, and the treatment plan should be carefully formulated.
The authors state that they have no Conflict of Interest (COI).
References
- 1. Postow MA, Sidlow R, Hellmann MD. Immune-related adverse events associated with immune checkpoint blockade. N Engl J Med 378: 158-168, 2018. [DOI] [PubMed] [Google Scholar]
- 2. Salem JE, Manouchehri A, Moey M, et al. Cardiovascular toxicities associated with immune checkpoint inhibitors: an observational, retrospective, pharmacovigilance study. Lancet Oncol 19: 1579-1589, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Love VA, Grabie N, Duramad P, et al. CTLA-4 ablation and interleukin-12 driven differentiation synergistically augment cardiac pathogenicity of cytotoxic T lymphocytes. Circ Res 101: 248-257, 2007. [DOI] [PubMed] [Google Scholar]
- 4. Bonaca MP, Olenchock BA, Salem JE, et al. Myocarditis in the setting of cancer therapeutics. Proposed case definitions for emerging clinical syndromes in cardio-oncology. Circulation 140: 80-91, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Ferreira VM, Schulz-Menger J, Holmvang G, et al. Cardiovascular magnetic resonance in nonischemic myocardial inflammation. Expert recommendations. J Am Coll Cardiol 72: 3158-3176, 2018. [DOI] [PubMed] [Google Scholar]
- 6. Mahmood SS, Fradley MG, Cohen JV, et al. Myocarditis in patients treated with immune checkpoint inhibitors. J Am Coll Cardiol 71: 1755-1764, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Korff S, Katus HA, Giannitsis E. Differential diagnosis of elevated troponins. Heart 92: 987-993, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Tocchetti CG, Galdiero MR, Varricchi G. Cardiac toxicity in patients treated with immune checkpoint inhibitors: it is now time for cardio-immuno-oncology. J Am Coll Cardiol 71: 1765-1767, 2018. [DOI] [PubMed] [Google Scholar]
- 9. Negishi T, Negishi K. Echocardiographic evaluation of cardiac function after cancer chemotherapy. J Echocardiogr 16: 20-27, 2018. [DOI] [PubMed] [Google Scholar]
- 10. Tanaka H. Utility of strain imaging in conjunction with heart failure stage classification for heart failure patient management. J Echocardiogr 17: 17-24, 2019. [DOI] [PubMed] [Google Scholar]
- 11. Suzuki S, Ishikawa N, Konoeda F, et al. Nivolumab-related myasthenia gravis with myositis and myocarditis in Japan. Neurology 89: 1127-1134, 2017. [DOI] [PubMed] [Google Scholar]
- 12. Puwanant A, Isfort M, Lacomis D, et al. Clinical spectrum of neuromuscular complications after immune checkpoint inhibition. Neuromuscul Disord 29: 127-133, 2019. [DOI] [PubMed] [Google Scholar]