Abstract
Background
Extracorporeal membrane oxygenation (ECMO) has become increasingly accepted as a life-saving procedure for patients with severe acute respiratory distress syndrome (ARDS). This study investigated the relationship between cumulative fluid balance (CFB) and outcomes in adult ARDS patients treated with ECMO.
Methods
We retrospectively analyzed the data of adult ARDS patients who received ECMO between December 2009 and December 2019 at Korea University Anam Hospital. CFB was calculated during the first 7 days after ECMO initiation. The primary endpoint was 28-day mortality.
Results
The 74 patients were divided into survivor (n=33) and non-survivor (n=41) groups based on 28-day survival. Non-survivors showed a significantly higher CFB at 1–7 days (p<0.05). Cox multivariable proportional hazard regression revealed a relationship between CFB on day 3 and 28-day mortality (hazard ratio, 3.366; 95% confidence interval, 1.528–7.417; p=0.003).
Conclusion
In adult ARDS patients treated with ECMO, a higher positive CFB on day 3 was associated with increased 28-day mortality. Based on our findings, we suggest a restrictive fluid strategy in ARDS patients treated with ECMO. CFB may be a useful predictor of survival in ARDS patients treated with ECMO.
Keywords: Acute respiratory distress syndrome, Extracorporeal membrane oxygenation, Fluid balance
Introduction
In recent years, extracorporeal membrane oxygenation (ECMO) has made remarkable progress to become an accepted treatment option for patients refractory to conventional therapy [1]. Depending on the organ being supported, there are 3 indications for ECMO: (1) cardiac support, (2) respiratory support, and (3) both cardiac and respiratory support. Although both venovenous (VV) ECMO and venoarterial (VA) ECMO can be used for respiratory support, VV ECMO is generally used for respiratory support [2].
Acute respiratory distress syndrome (ARDS) is an acute inflammatory form of lung injury that is associated with significant mortality and morbidity. The incidence of ARDS was reported to be 78.9 per 100,000 person-years in the United States; furthermore, ARDS was observed in 10.4% of all intensive care unit (ICU) admissions and in 23.4% of mechanically ventilated patients [3,4]. Although the mortality of ARDS has decreased over time, recent studies have still reported high mortality rates in patients with ARDS (30%–43%) [5-7]. According to the Berlin definition, ARDS is an acute-onset (within 1 week) condition characterized by bilateral lung opacities on chest radiography, with no evidence of cardiac failure-related hydrostatic edema on echocardiography and moderate to severe impairment of oxygenation [8].
The principle of treatment for ARDS patients is to treat the underlying cause, while providing supportive therapy to prevent further lung injury through lung-protective ventilator management and conservative fluid therapy [4,9]. In patients with severe ARDS, defined as a ratio of the partial pressure of oxygen to the fraction of inspired oxygen (PaO2/FiO2) <100 mm Hg with a positive end-expiratory pressure ≥5 cmH2O [10], ECMO has become increasingly accepted as a rescue therapy to avoid the potentially injurious aspects of mechanical ventilation [11,12], since the 2009 H1N1 influenza pandemic [13] and the “Conventional Ventilatory Support versus Extracorporeal Membrane Oxygenation for Severe Adult Respiratory Failure” trial [14]. The benefits of ECMO for severe ARDS was once again proven in the “ECMO to Rescue Lung Injury in Severe ARDS” study [15].
Daily fluid balance is the daily sum of all intake and output, and cumulative fluid balance (CFB) is the sum total of fluid accumulation within a set period of time. A higher CFB leads to an increased risk of death, longer time on mechanical ventilation, and longer length of ICU stay in ARDS patients [16-18]. Although restrictive fluid therapy in ARDS patients reduces mortality and shortens the duration of mechanical ventilation and the length of ICU stay [19], an inappropriate fluid restriction strategy can lead to hemodynamic aggravation and dysfunction of multiple organs [20].
Adequate fluid resuscitation is essential for initiating and achieving sufficient extracorporeal blood flow in patients treated with ECMO, and fluid overload commonly occurs. Although restrictive fluid therapy in patients with ARDS can reduce mortality, the most appropriate fluid balance in ARDS patients treated with ECMO remains controversial. CFB is widely used as a surrogate marker of intravenous fluid management. The present study investigated the association between CFB and outcomes in adult ARDS patients treated with ECMO. To the best of our knowledge, this is the first study to analyze the association between CFB and outcomes in ARDS patients treated with ECMO.
Methods
Patients and data collection
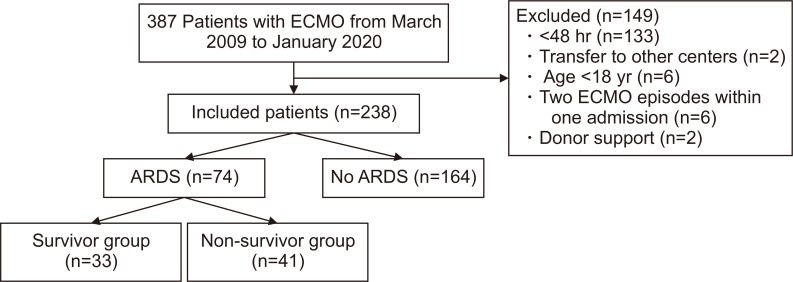
The data were collected retrospectively, and this study included ARDS patients treated with ECMO between December 2009 and December 2019 at Korea University Anam Hospital, Korea University College of Medicine. The exclusion criteria for this study were as follows: (1) age <18 years old; (2) ECMO support for less than 48 hours; (3) repeated ECMO applied within 1 admission; (4) transfer to other centers after ECMO application; and (5) ECMO support for organ donation (Fig. 1). Initially, 387 adult patients treated during the study period were included in the analysis, of whom 149 were excluded (133 received ECMO for less than 48 hours, 2 were transferred to other centers, 6 were younger than 18 years old, 6 had 2 ECMO episodes within 1 admission, and 2 were organ donors). ECMO-treated patients who did not have ARDS (i.e., those who received ECMO for cardiac support and extracorporeal cardiopulmonary resuscitation) were also excluded (n=164). The remaining 74 patients were included in the final sample and subdivided into survivor (n=33) and non-survivor (n=41) groups based on mortality within 28 days of ECMO initiation.
Fig. 1.
Flowchart of participant enrollment. ECMO, extracorporeal membrane oxygenation; ARDS, acute respiratory distress syndrome.
Patient data included demographic characteristics, hospital course, daily input, daily output, basic blood testing, pre-ECMO ventilator settings, pre-ECMO arterial blood gas analysis (ABGA), post-ECMO ventilator settings, post-ECMO ABGA, ECMO complications, survival to hospital discharge, successful ECMO weaning, and ICU scoring systems, such as Acute Physiology And Chronic Health Evaluation II (APACHE II), Sequential Organ Failure Assessment (SOFA), and Respiratory ECMO Survival Prediction (RESP), which were calculated on the basis of patients’ medical records. Screening of the underlying disease was mostly based on the patients’ previous medical history. Monitoring lung function is essential for evaluating changes in a patient’s condition and includes regular assessments of the patient’s clinical status (vital signs; particularly the respiratory rate, respiratory muscle effort, and level of consciousness), lung mechanics, periodic ABGA, and lung imaging. Bleeding complications were defined according to the Extracorporeal Life Support Organization definition. Bleeding was defined as clinically overt bleeding recorded in the patient’s medical charts associated with a decrease in hemoglobin of at least 2 g/dL in 24 hours, or a transfusion requirement of 1 or more 10-mL/kg red blood cell transfusions over the same time period.
The primary endpoint was 28-day mortality. The secondary endpoints included successful ECMO weaning, the duration of ECMO, the length of ICU stay, and complications of ECMO, such as acute kidney injury (AKI), bleeding, and neurological complications.
Extracorporeal membrane oxygenation protocol
ECMO is indicated for patients with severe respiratory failure that is unresponsive to optimal mechanical ventilation and medical treatment. The specific indications of ECMO are severe hypoxia (PaO2/FiO2 ratio <70 mm Hg), uncompensated respiratory acidosis (pH <7.15), or high plateau pressure (≥35 cmH2O). Patients on mechanical ventilation for over 7 days prior to ECMO initiation are relatively contraindicated for ECMO.
For respiratory support, VV ECMO is commonly used, while VA ECMO is used to provide respiratory and circulatory support. The decision of which patients with ARDS should be initiated on ECMO was made by the ECMO team, which consisted of cardiologists, pulmonologists, and cardiovascular surgeons. In addition to routine ICU monitoring, monitoring of the ECMO device and potential risk was also performed. At our center, the target oxygen saturation (SaO2) was >85% and the target PaO2 was >50 mm Hg. If a Swan-Ganz catheter was inserted, a target of mixed venous saturation was >75%. To reach adequate oxygenation, the initial ECMO flow was 3–5 L/min, corresponding to 100% of the cardiac index (2.4 L/min/m2) in VA ECMO and about 80% of the cardiac index (2.4 L/min/m2) in VV ECMO. The initial sweep gas flow rate was set to 3 L/min, and was then changed according to the PaCO2 level on ABGA to maintain a PaCO2 of 45–50 mm Hg. Unfractionated heparin (UFH) was given as a bolus injection (50 U/kg) before cannulation, and then UFH was continuously infused. The activated partial thromboplastin time (aPTT), which was used to monitor the patient’s response to heparin therapy, was checked every 6 hours for a 3-day period and every 12 hours thereafter. The aPTT target range was 45 to 65 seconds in VV ECMO and 60 to 80 seconds in VA ECMO.
Transthoracic echocardiography (TTE) prior to ECMO initiation was not routinely performed in all patients. VA ECMO was indicated when the ejection fraction (EF) was below 30%. At our center, TTE was routinely performed after 7 days after ECMO initiation to detect right ventricular (RV) failure. If RV failure was confirmed, conversion to veno-arterio-venous mode was actively considered. The target mean arterial pressure (MAP) was 60 mm Hg to maintain optimal organ perfusion. Vasopressors were often used to maintain the MAP above 60 mm Hg; norepinephrine was the first drug of choice and continuously infused (0.02–0.2 μg/kg/min). If the EF was reduced, dobutamine was continuously infused (2–20 μg/kg/min).
If hemodynamics and oxygen delivery were adequate on zero sweep gas and a FiO2 of 21%, weaning was considered. Either the Emergency Bypass System (Terumo, Tokyo, Japan) or the Permanent Life Support system (Getinge, Gothenberg, Sweden) was used.
Cumulative fluid balance
CFB was calculated as the sum of total fluid accumulation during the first 7 days after ECMO initiation based on daily fluid balance (i.e., the daily sum of all intake and output). Fluid intake included oral intake, tube feeding, intravenous fluids, medications, blood products, parenteral nutrition, and dialysis influent-dialysate fluid. Fluid output included urine output, drainage from drains or chest tubes, stools, and dialysis effluent-dialysate from continuous renal replacement therapy (CRRT).
Statistical analyses
Categorical variables were analyzed using the chi-square test or the Fisher test. Continuous variables were represented as mean values and standard deviations, and the t-test was used for between-group comparisons. Receiver operating characteristic (ROC) curves were used to determine the cut-off value for the maximum sensitivity and specificity to evaluate CFB as a predictive factor of 28-day mortality. Patients were subdivided into 2 groups using the cut-off value for CFB at 3 days. In order to analyze the relationship between outcomes and CFB, a Cox proportional hazards model was used, and the data were expressed as hazard ratios (HRs) with 95% confidence intervals (CIs). The data were analyzed with IBM SPSS ver. 20.0 (IBM Corp., Armonk, NY, USA) and p-values <0.05 were considered to indicate statistical significance.
The study was approved by the Institutional Review Board of Korea University (IRB approval no., 2020AN0424). The requirement for informed consent from individual patients was omitted because of the retrospective design of this study.
Results
The baseline characteristics of survivors and non-survivors are summarized in Table 1. No statistically significant differences were found in patient characteristics between the 2 groups. There were no significant differences in the APACHE II score (p=0.240), SOFA score (p=0.848), or RESP score (p=0.189) between the 2 groups. VV ECMO was performed in 93.9% of survivors and 82.9% of non-survivors (p=0.283), and the mean duration of mechanical ventilation support prior to ECMO initiation was 4.42± 7.018 days in survivors and 2.68±3.745 days in non-survivors (p=0.176). Pneumonia was the most common cause of ARDS in both groups (81.8% in survivors, 78% in non-survivors, p=0.688). The ventilation parameters during ECMO support are shown in Table 2. No statistically significant differences were found in ventilation parameters between the 2 groups.
Table 1.
Patient characteristics in the survivor and non-survivor groups
| Characteristic | Survivors (n=33) | Non-survivors (n=41) | p-value |
|---|---|---|---|
| Age (yr) | 53.24±2.633 | 59.32±2.490 | 0.100 |
| Sex (male) | 16 (48.5) | 17 (41.5) | 0.546 |
| Body mass index (kg/m2) | 25.3±0.645 | 24.44±0.654 | 0.356 |
| Comorbidities | |||
| Hypertension | 14 (42.4) | 20 (48.8) | 0.585 |
| Diabetes mellitus | 6 (18.2) | 9 (22.0) | 0.688 |
| Chronic kidney disease | 3 (9.1) | 4 (9.8) | 1.000 |
| Malignancy | 5 (15.2) | 10 (24.4) | 0.326 |
| Immunosuppression | 8 (24.2) | 13 (31.7) | 0.479 |
| Intensive care unit score | |||
| APACHE II | 13.27±5.642 | 14.83±5.603 | 0.240 |
| SOFA | 6.48±3.938 | 6.63±2.300 | 0.848 |
| RESP | -0.55±3.133 | -1.51±3.107 | 0.189 |
| ECMO mode (venovenous mode) | 31 (93.9) | 34 (82.9) | 0.283 |
| Duration of MV support prior to ECMO initiation (day) | 4.42±7.018 | 2.68±3.745 | 0.176 |
| Cause of ARDS | |||
| Sepsis | 2 (6.1) | 6 (14.6) | 0.286 |
| Pneumonia | 27 (81.8) | 32 (78.0) | 0.688 |
| Aspiration | 1 (3.0) | 0 | 0.446 |
| Massive transfusion | 3 (9.1) | 3 (7.3) | 1.000 |
| Trauma | 0 | 1 (2.4) | 1.000 |
| Already on CRRT before ECMO initiation | 2 (6.1) | 7 (17.1) | 0.283 |
| New start of CRRT | 12 (36.4) | 17 (42.5) | 0.594 |
Values are presented as mean±standard deviation or number (%).
APACHE, Acute Physiology And Chronic Health Evaluation; SOFA, Sequential Organ Failure Assessment; RESP, Respiratory Extracorporeal Membrane Oxygenation Survival Prediction; ECMO, extracorporeal membrane oxygenation; MV, mechanical ventilation; ARDS, acute respiratory distress syndrome; CRRT, continuous renal replacement therapy.
Table 2.
Ventilation parameters during ECMO support in the survivor and non-survivor groups
| Variable | Before ECMO initiation | After ECMO initiation (immediate) | After 24 hr | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Survivors (n=33) | Non-survivors (n=41) | p-value | Survivors (n=33) | Non-survivors (n=41) | p-value | Survivors (n=33) | Non-survivors (n=41) | p-value | |||
| Vital signs | |||||||||||
| MAP (mm Hg) | 81.67±20.907 | 74.66±17.187 | 0.118 | 77.21±17.453 | 108.36±21.627 | 0.516 | 69.42±24.021 | 64.10±27.300 | 0.382 | ||
| Heart rate (/min) | 108.45±27.602 | 115.20±25.417 | 0.279 | 108.36±21.627 | 111.58±22.191 | 0.532 | 93.21±21.177 | 102.32±19.757 | 0.060 | ||
| Respiratory rate (/min) | 22.94±6.519 | 22.34±5.448 | 0.669 | 19.03±5.457 | 20.02±5.875 | 0.458 | 15.55±4.024 | 16.93±4.891 | 0.196 | ||
| Arterial blood gases | |||||||||||
| pH | 7.26±0.126 | 7.24±0.173 | 0.532 | 7.40±0.088 | 7.38±0.129 | 0.375 | 7.42±0.063 | 7.39±0.108 | 0.198 | ||
| PaO2 (mm Hg) | 63.34±13.155 | 89.34±101.551 | 0.149 | 112.13±89.362 | 109.69±73.128 | 0.897 | 102.22±61.686 | 91.03±93.610 | 0.348 | ||
| PaCO2 (mm Hg) | 58.38±39.804 | 62.09±40.038 | 0.692 | 33.91±12.966 | 35.13±9.500 | 0.644 | 39.04±12.129 | 38.40±8.137 | 0.786 | ||
| HCO3 (mEq/L) | 24.53±8.491 | 26.51±14.242 | 0.484 | 21.09±5.809 | 20.81±7.636 | 0.865 | 24.30±4.443 | 23.69±5.497 | 0.607 | ||
| SaO2 (%) | 86.43±8.899 | 85.73±13.576 | 0.799 | 95.75±4.435 | 94.78±4.744 | 0.371 | 96.16±6.102 | 93.29±8.471 | 0.068 | ||
| Ventilation parameters | |||||||||||
| FiO2 (%) | 97.12±7.184 | 92.8±15.250 | 0.114 | 45.90±13.372 | 52.439±18.678 | 0.096 | 42.27±8.577 | 44.43±17.033 | 0.508 | ||
| PEEP (cmH2O) | 9.70±2.767 | 9.32±2.832 | 0.564 | 8.39±2.370 | 8.39±2.574 | 0.945 | 8.21±1.691 | 8.37±2.129 | 0.717 | ||
| PIP (cmH2O) | 26.24±4.931 | 26.80±5.460 | 0.647 | 22.03±5.323 | 23.37±4.930 | 0.263 | 21.51±4.055 | 22.35±4.894 | 0.432 | ||
Values are presented as mean±standard deviation.
ECMO, extracorporeal membrane oxygenation; MAP, mean arterial pressure; Pa, partial pressure; SaO2, oxygen saturation; FiO2, fraction of inspired oxygen; PEEP, positive end-expiratory pressure; PIP, peak inspiratory pressure.
Table 3 compares the clinical outcomes of survivors and non-survivors. The rate of successful ECMO weaning was 63.6% (n=21) in survivors and 24.3% (n=10) in non-survivors. Survivors had a significantly longer length of ICU stay (p=0.001), length of hospital stay (p=0.001), duration of ECMO support (p=0.001), and duration of mechanical ventilation support (p=0.001). The most common cause of death within 28 days after ECMO initiation was sepsis (n=21, 51.2%). One patient died with respiratory failure, as pneumonia worsened after successful ECMO weaning. No statistically significant differences were observed in the complications of ECMO, except for AKI (p=0.032). The causes of in-hospital death are shown in Supplementary Table 1. No statistically significant differences were observed in sepsis (p=0.201), bleeding/disseminated intravascular coagulation (p=0.373), respiratory failure (p=1.000), or anoxic brain injury (p=0.499). Non-survivors had a significantly higher rate of multiple organ failure (MOF) than survivors as the cause of in-hospital death (p=0.006). In this study, 9 patients received VA ECMO, of whom 2 patients survived until 28 days after ECMO initiation and 1 patient was discharged alive from the hospital.
Table 3.
Clinical outcomes of patients in the survivor and non-survivor groups
| Variable | Survivors (n=33) | Non-survivors (n=41) | p-value |
|---|---|---|---|
| Successful ECMO weaning | 21 (63.6) | 10 (24.3) | 0.001 |
| Survival to hospital discharge | 17 (51.5) | - | |
| Length of intensive care unit stay (day) | 47.12±28.178 | 17.46±11.272 | 0.001 |
| Length of hospital stay (day) | 71.61±51.701 | 27.02±27.432 | 0.001 |
| Duration of ECMO support (day) | 28.67±24.441 | 9.27±6.527 | 0.001 |
| ECMO mode change (veno-arterio-venous mode) | 2 (6.0) | 3 (7.3) | 1.000 |
| Duration of mechanical ventilation support | 39.64±23.231 | 17.76±14.896 | 0.001 |
| Cause of death (within 28 days of ECMO initiation)a) | |||
| Sepsis | - | 21 (51.2) | |
| Multiple organ failure | - | 13 (31.7) | |
| Bleeding/disseminated intravascular coagulation | - | 4 (9.7) | |
| Respiratory failure | - | 1 (2.4) | |
| Anoxic brain injury | - | 2 (4.8) | |
| Complications of ECMO (in hospital) | |||
| Bleeding | 15 (45.4) | 10 (24.3) | 0.057 |
| Acute kidney injury | 3 (9.0) | 12 (29.2) | 0.032 |
| Brain hemorrhage | 3 (9.0) | 1 (2.4) | 0.208 |
| Leg ischemia | 1 (3.0) | 2 (4.8) | 1.000 |
Values are presented as number (%) or mean±standard deviation.
ECMO, extracorporeal membrane oxygenation.
a)Causes of in-hospital death are shown in Supplementary Table 1.
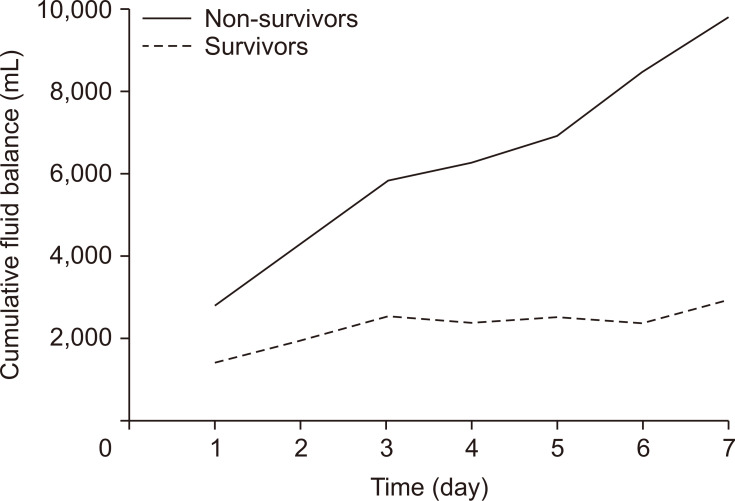
The mean CFB in both groups during 7 days after ECMO initiation was calculated (Fig. 2). Non-survivors had a higher CFB than survivors during 7 days after ECMO initiation. The mean CFB in survivors plateaued between 3 and 6 days, while the CFB in non-survivors continued to increase throughout the 7-day period. Statistically significant differences in CFB between non-survivors and survivors were found on days 1–7 (p<0.05) (Table 4). The sample size of CFB on day 3 was 74 patients, which was the largest sample size. In addition, the p-value of CFB on day 3 was 0.015, which was the smallest value. Therefore, we used CFB on day 3 for Cox regression analysis.
Fig. 2.
Comparison of mean cumulative fluid balance between survivors and non-survivors.
Table 4.
Cumulative fluid balance during 7 days after extracorporeal membrane oxygenation initiation in the survivor and non-survivor groups
| Variable | Overall no. | Survivors | Non-survivors | p-value | |||
|---|---|---|---|---|---|---|---|
| No. | Mean±SD | No. | Mean±SD | ||||
| CFB on day 1 (mL) | 74 | 33 | 1,433.82±1,869.363 | 41 | 2,792.59±3,439.949 | 0.045 | |
| CFB on day 2 (mL) | 74 | 33 | 1,918.03±2,979.214 | 41 | 4,290.46±5,196.105 | 0.016 | |
| CFB on day 3 (mL) | 74 | 33 | 2,558.58±3,992.746 | 41 | 5,843.49±7,113.176 | 0.015 | |
| CFB on day 4 (mL) | 65 | 31 | 2,372.52±4,667.463 | 34 | 6,280.12±9,038.247 | 0.035 | |
| CFB on day 5 (mL) | 62 | 31 | 2,502.71±5,126.720 | 31 | 6,913.61±10,474.581 | 0.039 | |
| CFB on day 6 (mL) | 58 | 30 | 2,344.10±5,806.386 | 28 | 8,507.54±12,427.716 | 0.022 | |
| CFB on day 7 (mL) | 55 | 29 | 3,123.07±6,107.004 | 26 | 9,843.54±13,966.551 | 0.030 | |
SD, standard deviation; CFB, cumulative fluid balance.
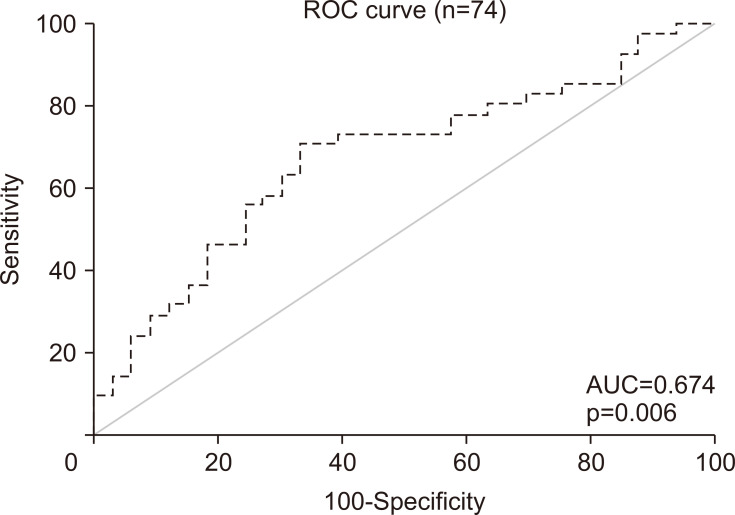
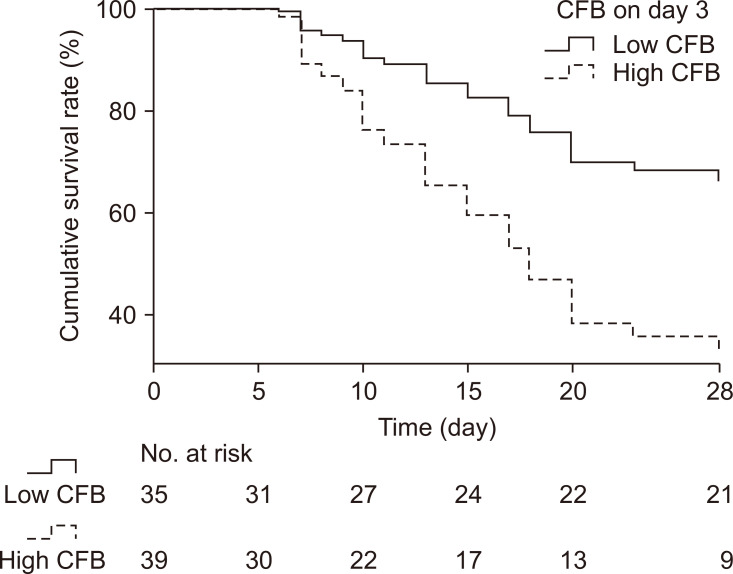
The ROC curve of CFB on day 3 associated with 28-day mortality was used to determine the cut-off value for CFB on day 3 as a predictor of 28-day mortality (n=74) (Fig. 3). The optimal cut-off value was 2629 mL, which showed sensitivity and specificity of 70.7% and 66.7%, respectively (Fig. 3). In order to obtain a clear evaluation in terms of HRs, the patients were subdivided into a low-CFB group (CFB on day 3 <the cut-off value) and a high-CFB group (CFB on day 3 ≥the cut-off value). The 28-day cumulative survival curve for the low-CFB group and high-CFB group showed that the 28-day mortality rate was 37% in the low-CFB group and 72% in the high-CFB group (p<0.05) (Fig. 4).
Fig. 3.
ROC curve of cumulative fluid balance on day 3 associated with 28-day mortality. The optimal cut-off value was 2,632 mL, which showed sensitivity and specificity of 70.7% and 66.7%, respectively. ROC, receiver operating characteristic; AUC, area under the curve.
Fig. 4.
Cox regression model for 28-day survival between the low-CFB and high-CFB groups. Low-CFB group: CFB on day 3 <the cut-off value (2,629 mL), n=35; high-CFB group: CFB on day 3 ≥the cut-off value (2,629 mL), n=39. The 28-day mortality rate was 37% in the low-CFB group and 72% in the high-CFB group (p=0.002). CFB, cumulative fluid balance.
To determine the HR for 28-day mortality, CFB on day 3 (low versus high) and other confounding variables (age, ICU scoring systems [APACHE II, SOFA, and RESP], ECMO type, duration of mechanical ventilation support prior to ECMO initiation, use of CRRT, and complications [AKI and bleeding]) were analyzed using Cox multivariable proportional hazard regression. In the Cox multivariable proportional hazard regression model of factors associated with 28-day mortality (Table 5), the factors independently related to 28-day mortality were high CFB on day 3 (HR, 3.366; 95% CI, 1.528–7.417; p=0.003), and bleeding (HR, 3.157; 95% CI, 1.434–6.953; p=0.004). Higher CFB on day 3 was an independent predictor of 28-day mortality.
Table 5.
Cox multivariable proportional hazard regression of factors associated with 28-day mortality (n=74)
| Variable | HR (95% CI) | p-value |
|---|---|---|
| CFB on day 3 | 3.366 (1.528–7.417) | 0.003 |
| Age | 1.005 (0.981–1.030) | 0.669 |
| APACHE II score | 1.000 (0.933–1.072) | 0.997 |
| SOFA score | 1.062 (0.918–1.228) | 0.417 |
| RESP score | 0.916 (0.798–1.052) | 0.214 |
| ECMO type: venovenous ECMO | 0.748 (0.292–1.914) | 0.545 |
| Cause of ARDS: sepsis | 1.859 (0.575–6.012) | 0.301 |
| Duration of MV support prior to ECMO initiation | 0.913 (0.834–1.001) | 0.052 |
| Use of CRRT | 0.482 (0.210–1.107) | 0.085 |
| Complication: acute kidney injury | 1.394 (0.544–3.568) | 0.489 |
| Complication: bleeding | 3.157 (1.434–6.953) | 0.004 |
HR, hazard ratio; CI, confidence interval; CFB, cumulative fluid balance; APACHE II, Acute Physiology And Chronic Health Evaluation II; SOFA, Sequential Organ Failure Assessment; RESP, Respiratory Extracorporeal Membrane Oxygenation Survival Prediction; ECMO, extracorporeal membrane oxygenation; ARDS, acute respiratory distress syndrome; MV, mechanical ventilation; CRRT, continuous renal replacement therapy.
Discussion
Appropriate fluid management to maintain adequate organ perfusion is a controversial issue that remains challenging. The relationship of a negative fluid balance with clinical outcomes in critically ill patients has been described in several studies, and fluid overload has been reported to be strongly associated with negative outcomes [21-23]. A restricted fluid strategy has been widely adopted in clinical practice if a patient has stable hemodynamics.
ECMO has become widespread and its outcomes have improved in recent years, as advances have been made both in ECMO devices themselves and in the management of patients treated with ECMO. However, excessive fluid overload still remains a common characteristic of ECMO patients. Despite the short history of ECMO, the potential impact of fluid balance on outcomes in ECMO patients has been recognized. For instance, Kim et al. [24] reported a significantly increased risk of 90-day mortality in patients with higher CFB. Furthermore, Schmidt et al. [25] reported that survivors had a lower fluid balance on ECMO days 3–5.
In our study, we showed that CFB on day 3 was associated with 28-day mortality. However, the mechanism by which positive fluid balance causes negative outcomes in ARDS patients remains unknown. A possible explanation is that positive fluid balance causes aggravation of extravascular and interstitial edema in the lungs and other organs. Aggravation of interstitial edema in organs such as the lungs and kidneys leads to impaired oxygenation and perfusion, which cause MOF. Therefore, fluid restriction may help patients with ARDS by reducing edema [16,26].
Some factors other than fluid balance have been shown to be associated with mortality. Specifically, while previous studies showed that positive CFB was associated with mortality in ARDS patients, other potential confounding factors, such AKI and sepsis, can also play a role in mortality [17,27-29]. The combination of ECMO and CRRT can effectively improve fluid balance and electrolyte disturbances. A systematic review showed that in ECMO survivors receiving CRRT, the overall fluid balance was lower than that in non-CRRT survivors [27]. In our study, the use of CRRT was not statistically significantly associated with 28-day mortality (HR, 0.482; 95% CI, 0.210–1.107; p=0.085). This result may be due to the small sample size. A previous study reported that red blood cell transfusions were associated with increased mortality in ARDS patients [30]. Bleeding is a common complication of ECMO and sometimes requires massive transfusion. In our results, bleeding had a positive association with 28-day mortality (HR, 3.157; 95% CI, 1.434–6.953; p=0.005).
Limitations
The present study has several limitations. First, this was a retrospective single-center study. Second, the sample size of patients was small. Therefore, a larger-scale study would be needed to more appropriately evaluate the 3-day CFB cut-off value and to find significant relationships in the data. Third, cardiac failure was not validated, as TTE was not routinely performed in all patients.
Conclusions
In adult ARDS patients treated with ECMO, a higher positive CFB on day 3 was found to be associated with a higher 28-day mortality risk. Based on our findings, we suggest restrictive fluid management in ARDS patients treated with ECMO. CFB may be a useful predictor of survival in ARDS patients treated with ECMO.
Supplementary materials
Supplementary materials can be found via https://doi.org/10.5090/kjtcs.20.123. Supplementary Table 1. Causes of in-hospital death.
Footnotes
Conflict of interest
No potential conflict of interest relevant to this article was reported.
References
- 1.Makdisi G, Wang IW. Extra Corporeal Membrane Oxygenation (ECMO) review of a lifesaving technology. J Thorac Dis. 2015;7:E166–76. doi: 10.3978/j.issn.2072-1439.2015.07.17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sorokin V, MacLaren G, Vidanapathirana PC, Delnoij T, Lorusso R. Choosing the appropriate configuration and cannulation strategies for extracorporeal membrane oxygenation: the potential dynamic process of organ support and importance of hybrid modes. Eur J Heart Fail. 2017;19 Suppl 2:75–83. doi: 10.1002/ejhf.849. [DOI] [PubMed] [Google Scholar]
- 3.Bellani G, Laffey JG, Pham T, et al. Epidemiology, patterns of care, and mortality for patients with acute respiratory distress syndrome in intensive care units in 50 countries. JAMA. 2016;315:788–800. doi: 10.1001/jama.2016.0291. [DOI] [PubMed] [Google Scholar]
- 4.Diamond M, Peniston Feliciano HL, Sanghavi D, Mahapatra S. Acute respiratory distress syndrome. StatPearls Publishing; Treasure Island (FL): 2020. [PubMed] [Google Scholar]
- 5.Zambon M, Vincent JL. Mortality rates for patients with acute lung injury/ARDS have decreased over time. Chest. 2008;133:1120–7. doi: 10.1378/chest.07-2134. [DOI] [PubMed] [Google Scholar]
- 6.Davidson TA, Caldwell ES, Curtis JR, Hudson LD, Steinberg KP. Reduced quality of life in survivors of acute respiratory distress syndrome compared with critically ill control patients. JAMA. 1999;281:354–60. doi: 10.1001/jama.281.4.354. [DOI] [PubMed] [Google Scholar]
- 7.Erickson SE, Martin GS, Davis JL, Matthay MA, Eisner MD NIH NHLBI ARDS Network, author. Recent trends in acute lung injury mortality: 1996-2005. Crit Care Med. 2009;37:1574–9. doi: 10.1097/CCM.0b013e31819fefdf. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ranieri VM, Rubenfeld GD, et al. ARDS Definition Task Force, author. Acute respiratory distress syndrome: the Berlin definition. JAMA. 2012;307:2526–33. doi: 10.1001/jama.2012.5669. [DOI] [PubMed] [Google Scholar]
- 9.Thompson BT, Chambers RC, Liu KD. Acute respiratory distress syndrome. N Engl J Med. 2017;377:562–72. doi: 10.1056/NEJMra1608077. [DOI] [PubMed] [Google Scholar]
- 10.Villar J, Perez-Mendez L, Blanco J, et al. A universal definition of ARDS: the PaO2/FiO2 ratio under a standard ventilatory setting: a prospective, multicenter validation study. Intensive Care Med. 2013;39:583–92. doi: 10.1007/s00134-012-2803-x. [DOI] [PubMed] [Google Scholar]
- 11.Hardin CC, Hibbert K. ECMO for severe ARDS. N Engl J Med. 2018;378:2032–4. doi: 10.1056/NEJMe1802676. [DOI] [PubMed] [Google Scholar]
- 12.Ma DS, Kim JB, Jung SH, Choo SJ, Chung CH, Lee JW. Outcomes of venovenous extracorporeal membrane oxygenation support for acute respiratory distress syndrome in adults. Korean J Thorac Cardiovasc Surg. 2012;45:91–4. doi: 10.5090/kjtcs.2012.45.2.91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Davies A, Jones D, et al. Australia and New Zealand Extracorporeal Membrane Oxygenation (ANZ ECMO) Influenza Investigators, author. Extracorporeal membrane oxygenation for 2009 influenza A(H1N1) acute respiratory distress syndrome. JAMA. 2009;302:1888–95. doi: 10.1001/jama.2009.1535. [DOI] [PubMed] [Google Scholar]
- 14.Peek GJ, Clemens F, Elbourne D, et al. CESAR: conventional ventilatory support vs extracorporeal membrane oxygenation for severe adult respiratory failure. BMC Health Serv Res. 2006;6:163. doi: 10.1186/1472-6963-6-163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Combes A, Hajage D, Capellier G, et al. Extracorporeal membrane oxygenation for severe acute respiratory distress syndrome. N Engl J Med. 2018;378:1965–75. doi: 10.1056/NEJMoa1800385. [DOI] [PubMed] [Google Scholar]
- 16.Van Mourik N, Metske HA, Hofstra JJ, et al. Cumulative fluid balance predicts mortality and increases time on mechanical ventilation in ARDS patients: an observational cohort study. PLoS One. 2019;14:e0224563. doi: 10.1371/journal.pone.0224563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rosenberg AL, Dechert RE, Park PK, Bartlett RH NIH NHLBI ARDS Network, author. Review of a large clinical series: association of cumulative fluid balance on outcome in acute lung injury: a retrospective review of the ARDSnet tidal volume study cohort. J Intensive Care Med. 2009;24:35–46. doi: 10.1177/0885066608329850. [DOI] [PubMed] [Google Scholar]
- 18.Zinter MS, Spicer AC, Liu KD, et al. Positive cumulative fluid balance is associated with mortality in pediatric acute respiratory distress syndrome in the setting of acute kidney injury. Pediatr Crit Care Med. 2019;20:323–31. doi: 10.1097/PCC.0000000000001845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wiedemann HP, Wheeler AP, et al. National Heart, Lung, and Blood Institute Acute Respiratory Distress Syndrome (ARDS) Clinical Trials Network, author. Comparison of two fluid-management strategies in acute lung injury. N Engl J Med. 2006;354:2564–75. doi: 10.1056/NEJMoa062200. [DOI] [PubMed] [Google Scholar]
- 20.Finfer S, Liu B, Taylor C, et al. Resuscitation fluid use in critically ill adults: an international cross-sectional study in 391 intensive care units. Crit Care. 2010;14:R185. doi: 10.1186/cc9293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Shen Y, Huang X, Zhang W. Association between fluid intake and mortality in critically ill patients with negative fluid balance: a retrospective cohort study. Crit Care. 2017;21:104. doi: 10.1186/s13054-017-1692-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Boyd JH, Forbes J, Nakada TA, Walley KR, Russell JA. Fluid resuscitation in septic shock: a positive fluid balance and elevated central venous pressure are associated with increased mortality. Crit Care Med. 2011;39:259–65. doi: 10.1097/CCM.0b013e3181feeb15. [DOI] [PubMed] [Google Scholar]
- 23.Malbrain ML, Marik PE, Witters I, et al. Fluid overload, de-resuscitation, and outcomes in critically ill or injured patients: a systematic review with suggestions for clinical practice. Anaesthesiol Intensive Ther. 2014;46:361–80. doi: 10.5603/AIT.2014.0060. [DOI] [PubMed] [Google Scholar]
- 24.Kim H, Paek JH, Song JH, et al. Permissive fluid volume in adult patients undergoing extracorporeal membrane oxygenation treatment. Crit Care. 2018;22:270. doi: 10.1186/s13054-018-2211-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Schmidt M, Bailey M, Kelly J, et al. Impact of fluid balance on outcome of adult patients treated with extracorporeal membrane oxygenation. Intensive Care Med. 2014;40:1256–66. doi: 10.1007/s00134-014-3360-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Roch A, Guervilly C, Papazian L. Fluid management in acute lung injury and ARDS. Ann Intensive Care. 2011;1:16. doi: 10.1186/2110-5820-1-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chen H, Yu RG, Yin NN, Zhou JX. Combination of extracorporeal membrane oxygenation and continuous renal replacement therapy in critically ill patients: a systematic review. Crit Care. 2014;18:675. doi: 10.1186/s13054-014-0675-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Vlaar AP, Binnekade JM, Prins D, et al. Risk factors and outcome of transfusion-related acute lung injury in the critically ill: a nested case-control study. Crit Care Med. 2010;38:771–8. doi: 10.1097/CCM.0b013e3181cc4d4b. [DOI] [PubMed] [Google Scholar]
- 29.Murphy CV, Schramm GE, Doherty JA, et al. The importance of fluid management in acute lung injury secondary to septic shock. Chest. 2009;136:102–9. doi: 10.1378/chest.08-2706. [DOI] [PubMed] [Google Scholar]
- 30.Gong MN, Thompson BT, Williams P, Pothier L, Boyce PD, Christiani DC. Clinical predictors of and mortality in acute respiratory distress syndrome: potential role of red cell transfusion. Crit Care Med. 2005;33:1191–8. doi: 10.1097/01.CCM.0000165566.82925.14. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.